Abstract
Objective The current study investigated whether factors associated with quality of life (QOL) in children with asthma (e.g., family functioning, asthma routines, asthma severity) differed by child age. Methods Participants included 192 children with asthma (5–12 years) and their caregivers. Both children and caregivers completed questionnaires at an initial research session. Family functioning was determined from a mealtime observation that occurred in family homes. Results Child age moderated the association between asthma severity and child QOL and between routine burden and QOL in children with asthma. Post hoc probing analyses revealed that among older children, QOL levels were lower in the presence of worse asthma severity and more routine burden. Conclusions Findings suggest that associations between asthma severity, routine burden, and QOL may differ by child age. Treatment programs and health-care recommendations addressing QOL in children with asthma may need to be tailored to address differences in factors associated with QOL by child age.
Keywords: asthma, family functioning, quality of life
Pediatric asthma is a major public health concern that affects both children and their caregivers. Reports estimate that one in five children with asthma are treated in the emergency department (ED) for asthma-related care (Centers for Disease Control and Prevention [CDC], 2012) and approximately 50% of children with asthma miss at least 1 day of school yearly owing to asthma (CDC, 2012). Quality of life (QOL) is often used as an outcome measure in pediatric asthma to describe how a child’s asthma is impacting his or her daily life (Juniper, 1997). Assessments of asthma-related QOL measure a different component of health status than clinical measures (e.g., spirometry readings); for instance, correlations between QOL measures and objective measures of lung health are often inconsistent (Juniper et al., 2004; van Gent et al., 2008). Children experiencing lower levels of QOL may be distressed by limited activities or frustrated by symptoms during the school day (Juniper, 1997). Treatment decisions may also be made or medications adjusted based on a child’s current level of QOL.
Child Age and Child QOL
An emerging body of research has investigated separate factors that may be associated with child QOL, including asthma medication routines (Fiese, Wamboldt, & Anbar, 2005), family rituals and climate (Santos, Crespo, Silva, & Canavarro, 2012), household income (Erickson et al., 2002), caregiver QOL (Garro, 2011), and asthma-related ED visits (Garro, 2011). To date, however, research has not considered whether factors associated with child QOL may differ based on child age. Research suggests that QOL is higher among younger children with asthma as compared with older children with asthma (Moreira et al., 2013). Better understanding as to how factors associated with QOL may differ across child age has particular importance in intervention research focused on improving child QOL in pediatric asthma. As a child develops, she/he may change priorities, goals, and values, which can influence disease-related behaviors (Drotar, 2004). In adolescence, youth responsibility for medical care often increases, which may prompt a decline in adherence to treatment regimens and may even contribute to poorly controlled asthma (Blaakman, Cohen, Fagnano, & Halterman, 2014; Mosnaim et al., 2014). Although this study focused on school-aged children (5–12 years), it is important to note that as a child matures into adolescence, factors that influence their QOL (e.g., transition in regimen responsibility) may differ from factors influencing QOL in younger children.
Oftentimes, research studies control for child age in associations with child QOL (Goldbeck, Koffmane, Lecheler, Thiessen, & Fegert, 2007; Josie, Greenley, & Drotar, 2007; Santos et al., 2012) instead of considering whether age may moderate associations between specific factors that can influence child QOL. Furthermore, most research on QOL in young children with asthma (<7 years) has included parent report of child QOL (Garro, 2011; Petsios et al., 2013). This can be problematic given that parent and child perceptions of child QOL may differ (van Gent et al., 2008). Few measures exist for children <7 years of age that allow children to report on their own QOL. Our study improved on previous research by including a validated measure of child QOL (Everhart & Fiese, 2009b) that can be completed by children as young as 5 years of age.
Previous Research on Specific Factors Associated With Child QOL
Based on existing QOL literature and Bronfenbrenner’s (1979) social ecological model of development, we examined associations between child QOL and both individual (e.g., asthma severity) and family-based factors (e.g., asthma routines, family functioning). Our study was designed to determine whether child age moderated associations between QOL and factors that have proven to be associated with child QOL in past studies. For instance, research suggests that given their developmental capacities, younger children may rely more on their caregivers and other family members for support than older children (Kaugars, Klinnert, & Bender, 2004). On the other hand, older children may be more independent and may be given increased responsibility in completing disease-specific tasks (Duncan et al., 2013).
Findings from previous research studies suggest that asthma severity is linked to child QOL, in that more severe asthma has been associated with worse child QOL in children 2–18 years of age (Everhart & Fiese, 2009a; Petsios et al., 2013; Schmier, Chan, & Kline-Leidy, 1998; Williams et al., 2000). However, not all studies have found consistent associations between asthma severity and child QOL (Annett, Bender, DuHamel, & Lapidus, 2003; Goldbeck et al., 2007). Further, few studies have specifically considered whether child age influences the association between asthma severity and QOL.
At the family level, Fiese and colleagues (2005) found that family asthma medication routines were associated with medical adherence, and a family’s perceived medication burden was associated with caregiver and child QOL. Given that pediatric asthma is managed within families (Kaugars et al., 2004), considering how families accomplish routines related to daily asthma care has important consequences for child QOL. Additionally, caregiver-perceived burden of asthma routine management may influence treatment behaviors within the family, with potential effects on child QOL. Further, as family relationships vary across child age, family functioning and its associations with child QOL may also differ depending on child age. Family functioning has been positively associated with QOL in children with asthma (7–12 years old) even after controlling for frequency of asthma symptoms (Sawyer, Spurrier, Kennedy, & Martin, 2001). Higher levels of cohesion and expressiveness, used to assess family functioning, have also been related to better QOL in children with asthma (8–18 years old; Crespo, Carona, Silva, Canavarro, & Dattilio, 2011). In sum, research is needed to further investigate how associations between child QOL and asthma severity, family functioning, and asthma routines may differ across child age.
The current study improved on existing asthma-related child QOL research in two ways. First, this study considered whether child age served as a moderator between specific factors and child QOL. Second, the current study incorporated an observational measure of family functioning in considering child age as a moderator in the association between family functioning and child QOL. Previous studies considering family functioning and child QOL in children with asthma have primarily included self-report measures of family functioning (Annett et al., 2003; Crespo et al., 2011; Garro, 2011; Sawyer, Spurrier, Kennedy, et al., 2001). Observational measures of family functioning often have the advantage of being able to simultaneously assess complex family characteristics (e.g., communication) within a relatively short time frame (Fiese, Winter, & Botti, 2011). In particular, mealtime observations provide an opportunity for researchers to observe the whole family completing a daily routine together and can serve as a microcosm for family life and social interactions (Fiese, Foley, & Spagnola, 2006).
Current Study
Given the emotional and cognitive developmental differences of young children compared with older children, the current study investigated whether child age moderated the association between child QOL and asthma severity, family functioning, medication routines, and routine burden. Based on previous research on child QOL in pediatric asthma (Fiese et al., 2005; Petsios et al., 2013; Sawyer, Spurrier, Whaites, et al., 2001), we hypothesized that worse asthma severity would be associated with worse child QOL and that this association would be stronger among older children. Second, we hypothesized that poorer family functioning and poorer asthma routines (poorer medication routines, more routine burden) would be associated with worse child QOL and that this association would be stronger among younger children.
Methods
Participants
Data were obtained from a larger study investigating family life and asthma targeting children between 5 and 12 years of age (Fiese, Winter, Wamboldt, Anbar, & Wamboldt, 2010). Participants in the larger study included 215 children (63% boys) with asthma (44% intermittent, 18% mild persistent, 29% moderate persistent, 5% severe) between the age of 5 and 12 years (M = 7.86 years, SD = 2.18) and their primary caregivers. Fifty-three percent of children were White, 31% Black, 3% Hispanic, and 13% “Other,” as reported by their caregivers. Family size ranged from 2 to 11 members, with a median of four members. Socioeconomic status (SES) as measured by the Hollingshead Index ranged from 8 to 66 (M = 38.58, SD = 16.33). Although 215 families completed the study, 192 families had complete data on all measures and were included in this study (91 families with younger children and 101 with older children).
Families were recruited through a pediatric pulmonary clinic (31%), an ambulatory clinic at a teaching hospital (37%), and surrounding private pediatric practices in a mid-size city (32%). Approximately 460 families were approached to participate in the study and about 47% were excluded owing to a range of reasons (i.e., families not reachable by phone, decline to participate at enrollment, ineligible during the screening process, missed three or more scheduled initial visits). Children were included if they were between 5 and 12 years old, had an asthma diagnosis, and had a daily controller medication prescription for at least 6 months. Children were excluded if they had another chronic medical condition for which they were taking daily medication, were in foster care, were not able to read English, or if they had exercise-induced asthma.
Procedures
Institutional review board approvals were obtained for this study. Children and their caregivers completed an initial research session in a laboratory setting. Written informed consent was obtained from caregivers and assent from children. During the initial session, caregivers completed a series of questionnaires including a background information questionnaire and a measure of asthma-related family routines. Children were interviewed separately and completed a measure of asthma-related QOL. Children also completed a spirometry test. A home visit occurred within a week of the initial research session. Families were given video equipment and instructed on how to record their family having dinner together. Study staff was not present for the meal and retrieved the video equipment after the family finished dinner. Families reported on how representative the observed mealtime was on a scale from 1 (not at all typical) to 4 (very typical). Ninety percent of families reported that the videotaped meal was typical or very typical for their family. Families were compensated for their time.
Measures
Demographic Information
Caregivers completed a demographic questionnaire including child’s race/ethnicity, family size, and income. Caregivers also reported on their level of education, occupation, and relationship status.
Hollingshead Index
SES was measured using the Hollingshead Index from parent educational level and occupation (Hollingshead, 1975). Educational level was ranked on a 7-point scale and then multiplied by three, and occupation was ranked on a 9-point scale and multiplied by five. These two values were added together to obtain the SES level. If based on a two-parent household, parents’ scores were averaged.
Pediatric Asthma Quality of Life Questionnaire
The Pediatric Asthma Quality of Life Questionnaire (PAQLQ; Juniper et al., 1996) was completed by children aged ≥8 years. This asthma-specific QOL measure consists of 23 items, which assess physical, emotional, and social impairment due to asthma over the past week. Responses are rated on a 7-point scale that ranges from 1 (extremely bothered/all of the time) to 7 (not at all bothered/none of the time). An overall score of QOL is determined from the mean of all responses. Clinical asthma control outcomes have been used to validate the PAQLQ, including β-agonist use and morning peak flow rates (Juniper et al., 1996). Cronbach’s α of .87 was reported for the current study.
Pictorial Version of the PAQLQ
Children 5–7 years of age completed the 16-item pictorial version of the PAQLQ (Everhart & Fiese, 2009b). Children are presented with a line anchored by three thermometers ranging from empty to half full to full to indicate how bothered they have been by their asthma in the past week. An empty thermometer indicates “not at all” and a full thermometer indicates “a lot” bothered. Children mark where they fall along the line. Markings are converted into scores from 1 to 7, with higher scores indicating better QOL.
The pictorial PAQLQ is a downward extension of the established PAQLQ (Juniper et al., 1996) that has demonstrated adequate psychometric properties (Everhart & Fiese, 2009b). Both factors of the pictorial measure, symptoms (Cronbach’s α = .83) and emotions (Cronbach’s α = .71) had good internal consistency. During development of the pictorial scale, children were administered the pictorial version and then once the child turned 8 years old, they completed the established PAQLQ at a subsequent research visit. Children completed both measures to determine whether the pictorial PAQLQ assessed the same underlying construct as the established PAQLQ. After controlling for child age, symptom scores on the pictorial PAQLQ were associated with symptom scores on the established PAQLQ; emotion scores on the pictorial PAQLQ were also associated with emotion scores on the established PAQLQ (Everhart & Fiese, 2009b). We acknowledge that age is confounded with the use of both versions of the scale; we have provided specific findings from the validity study of the pictorial PAQLQ to suggest that both measures are assessing the same construct. Cronbach’s α of .86 for overall QOL on the pictorial PAQLQ was calculated for this sample.
Mealtime Interaction Coding System
Family functioning was assessed by the Mealtime Interaction Coding System (MICS; Dickstein, Hayden, Schiller, Seifer, & San Antonio, 1994), which was adapted from the McMaster Model of Family Functioning (Epstein, Baldwin, & Bishop, 1983). Videotaped observations of family meals were coded by trained and reliable coders. Two graduate students were trained to a reliability of ≥.80 using archival videotapes. Videotapes for the current study were coded separately by each rater and then codes were reviewed together with the principal investigator for consensus. Twenty percent of the interactions from the videotaped meals were double-coded by raters. Validity of the MICS has been supported by correlations with other family functioning measures including the McMaster Structured Interview of Family Functioning (r = .52), Family Assessment Device (r = .33), and the Parent/Caregiver Involvement Scale (r values = .38–.56; Hayden et al., 1998).
The MICS consists of six subscales that cover the dimensions of task accomplishment, communication, affect management, interpersonal involvement, behavior control, and role allocation (Table I). In addition, an overall family functioning score is derived from the strengths and weaknesses of the family. Each dimension is scored from 1 (very unhealthy) to 7 (very healthy). Ratings <5 are considered unhealthy; ratings of ≥5 indicate adequate/good functioning. In children with chronic conditions, the MICS has been used to discern their patterns of interactions from healthy controls (Janicke, Mitchell, & Stark, 2005; Spieth et al., 2001). MICS cutoff scores have also been found to distinguish between families that have a family member with a clinical illness (e.g., medical illness, mental health illness) and families that do not (Dickstein et al., 1998; Mitchell, Powers, Byars, Dickstein, & Stark, 2004). In the current study, intraclass correlation coefficients indicated good interrater reliability: task accomplishment = .88, communication = .83, affect management = .88, interpersonal involvement = .80, behavior control = .86, role allocation = .83, and overall functioning = .90. We used overall family functioning in our analyses.
Table I.
Dimensions of the Family Mealtime Interaction Coding System (MICS) With Sample Means and Standard Deviations
| MICS subscales mean, SD of our sample | Description |
|---|---|
| Task accomplishment (M = 4.78, SD = 1.33) | Rates the structure and orderliness of the family during the meal. “Unhealthy” scores indicate chaotic and disrupted behavior (e.g., members leaving the table). “Healthy” scores indicate orderly and flexible responses to interruptions. |
| Communication (M = 4.34, SD = 1.51) | Assesses communication among family members, taking note of the quality of conversation. “Unhealthy” scores indicate ineffective communication such as excluding certain members or discussing inappropriate topics. “Healthy” scores indicate clear and direct communication. |
| Affect management (M = 4.47, SD = 1.41) | Rates the emotion expressed by family members during the meal. “Unhealthy” scores include members who display inappropriate affect and members who react inappropriately. “Healthy” scores indicate appropriate affect and appropriate responses to others’ emotions. |
| Interpersonal involvement (M = 4.51, SD = 1.72) | Assesses family members’ interest and value of others’ daily activities and feelings. “Unhealthy” scores include members who are uninvolved with each other. “Healthy” scores indicate empathetic actions toward other members. |
| Behavior control (M = 4.54, SD = 1.43) | Rates a family’s standards of behavior in regard to physical danger, psychobiological needs, and rules for social behavior. Families are rated on four dimensions: chaotic, laissez-faire, rigid, and flexible. “Unhealthy” scores include mostly chaotic or rigid interactions. “Healthy” range indicates more flexible or mildly rigid interactions. |
| Roles (M = 4.85, SD = 1.16) | Assesses family fulfillment of responsibilities. “Unhealthy” scores indicate inefficient or inappropriate task delegation. “Healthy” scores indicate appropriate assignment of tasks and capability to complete them by members. |
| Overall family functioning (M = 4.54, SD = 1.56) | Reflects the overall clinical impression of the family. “Unhealthy” range indicates uncoordinated and unpredictable functioning. “Healthy” scores indicate high-quality interactions. |
Asthma Routines Questionnaire
Medication routines and routine burden were measured using the Asthma Routines Questionnaire (ARQ), which was developed as part of the Family Ritual Questionnaire-Asthma Version (Fiese et al., 2005). Fiese et al. (2005) found a two-factor structure of the measure after dropping one item from the original eight items of the ARQ. The final scale consisted of the following dimensions: medication routines (e.g., regularity, predictability, and planning of medication use; four items) and routine burden (e.g., degree to which asthma management is perceived as a chore or hassle; three items). Participants respond to items on a 1–4 scale. Higher medication routine scores indicate better medication routines; higher routine burden scores indicate more burdensome asthma management. The ARQ has been associated with medical adherence (Fiese et al., 2005; Fiese, Winter, Anbar, Howell, & Poltrock, 2008). In this sample, Cronbach’s α of .58 was calculated for the medication routines factor. Cronbach’s α of .59 was calculated for the routine burden factor in this sample.
Asthma Severity
Asthma severity was determined from a spirometry test administered by a trained respiratory therapist during the laboratory visit. A PDS 313100-WSU KOKO spirometer was used to obtain measurements of forced vital capacity (FVC), forced expiratory flow in 1 s (FEV1), and forced expiratory flow, 25–75% of vital capacity (FEV25–75). Each child performed three FVC maneuvers to ensure reproducibility. Spirometry measurements were repeated 10 min later after albuterol administration. Asthma severity-level classifications were made using an average of the three ratings conducted at the initial session by a board-certified pulmonologist based on standard guidelines (NHLBI, 2007).
Data Analysis
Analyses were conducted using PASW Statistics version 18.0 software (SPSS Inc., Chicago, IL, USA). Given that we used the established PAQLQ measure (Juniper et al., 1996) and the pictorial PAQLQ measure (Everhart & Fiese, 2009b), we standardized child QOL scores by calculating z-scores for each child. For older children completing the established PAQLQ, the mean and standard deviation from children completing this measure were used to calculate z-scores. Similarly, for younger children, the mean and standard deviation from the sample of younger children completing the pictorial version were used to calculate z-scores. By calculating z-scores for each group of children, we were able to combine QOL scores into one continuous variable for analyses.
An analysis of variance (ANOVA) was first conducted to test child race/ethnicity and SES as potential covariates in associations with QOL. Children from Hispanic, Native American, and mixed-race backgrounds were classified as “other” owing to the low number of children in each group. A post hoc comparison using Tukey honest significant difference was conducted to pinpoint differences between groups. In addition, an ANOVA was conducted to test for differences in asthma severity by child race/ethnicity and post hoc comparisons were conducted.
Bivariate correlations were conducted to assess whether potential independent variables (i.e., asthma severity, family functioning, medication routines, routine burden) were correlated with child QOL. Age was tested as a moderator in associations between independent variables and child QOL. Before moderation analyses, independent and moderator variables were centered and a product term was created from each centered variable and the moderator (Baron & Kenny, 1986). Covariates were entered as the first step, the independent variable was entered in the second step, the moderator (age) was entered in the third step, and the interaction term was entered in the last step. Post hoc probing analyses as described by Holmbeck (2002) were conducted for all significant interactions to determine which of the simple slopes differed from zero. Given that two QOL measures were used (which was confounded with child age), significant moderation analyses were rerun in the older group of children who had completed the established PAQLQ (8–12 years). Finally, moderation analyses involving the MICS were rerun in families who reported the meal was typical or very typical for them (90% of the sample).
Results
Descriptive Statistics
Mean scores were calculated separately for the established PAQLQ scale that older children completed (M = 5.02, SD = 1.22) and the pictorial PAQLQ scale that younger children completed (M = 4.72, SD = 1.45). Using the standardized QOL scores for the full sample, a t test indicated that there were no differences on child QOL scores between the younger age-group and the older group, t(190) = −.43, p > .05. All mean scores and standard deviations for the independent variables are presented in Table II. In addition, all independent variables were significantly correlated with child QOL (Table II).
Table II.
Bivariate Correlations of Independent Variables and Child Quality of Life
| Asthma severity M = 1.92, SD = 0.99 | Family functioning M = 4.54, SD = 1.56 | Medication routines M = 3.42, SD = 0.64 | Routine burden M = 2.70, SD = 0.82 | |
|---|---|---|---|---|
| Child quality of life | −.16* | .26*** | .18* | −.19** |
Note. Means and standard deviations of the independent variables included in table.
*p < .05, **p < .01, ***p < .001.
Using a one-way ANOVA, child QOL was found to differ across child race/ethnicity, F(2, 189) = 15.15, p < .001. Post hoc analyses indicated that Black children reported worse QOL (M = −0.57, SD = 0.99) than both White children (M = 0.28, SD = 0.91, p < .001) and children of other races/ethnicities (M = −0.003. SD = 0.93, p = .03). In addition, an ANOVA revealed that asthma severity differed by racial/ethnic group, F(2, 189) = 18.09, p < .001. Post hoc analyses indicated that Black children had more severe asthma than both White children (p < .001) and children of other races/ethnicities (p = .01). Child race/ethnicity was controlled for in all subsequent analyses. An ANOVA testing SES as a covariate was not significant.
Moderation Analyses
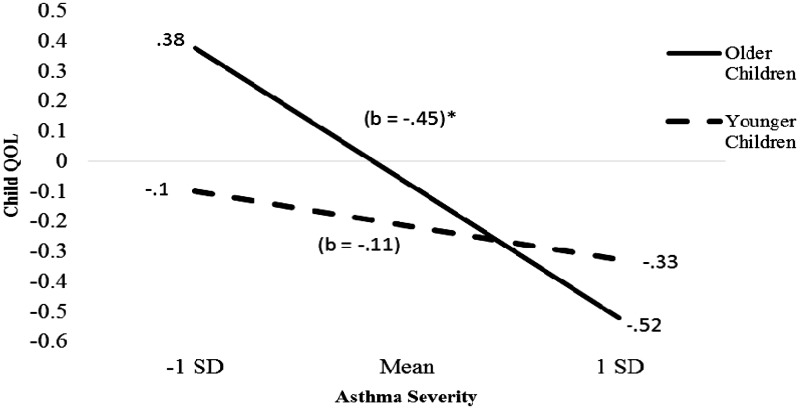
Child age significantly moderated the association between asthma severity and child QOL (β = −.17, t(187) = −2.42, p = .02; ΔR2 = 0.03, ΔF (187) = 5.86, p = .02). Children who were older showed a greater decrease in QOL as their asthma severity increased than younger children (Figure 1). The simple slope for the older children regression line was significant, t(187) = −4.85, p < .001, but was not for the younger children regression line.
Figure 1.
Regression lines for association between asthma severity and child quality of life (QOL) moderated by child age.
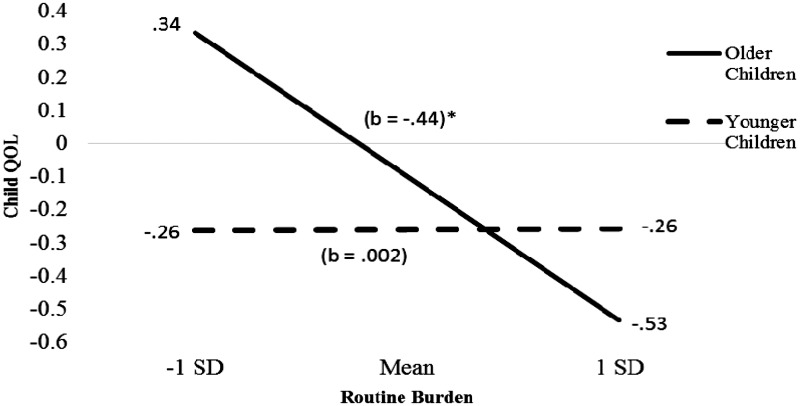
In addition, child age significantly moderated the association between routine burden and child QOL (β = −.17, t(187) = −2.43, p = .02; ΔR2 = 0.03, ΔF (187) = 5.92, p = .02). Children who were older showed a greater decrease in QOL as routine burden increased than younger children (Figure 2). The simple slope for older children was significant, t(187) = −3.52, p = .001. Child age did not moderate the association between family functioning and child QOL, or the association between medication routines and child QOL. Analyses considering child age as a moderator in the association between family functioning and child QOL remained nonsignificant when only families reporting their mealtime as typical or very typical were included.
Figure 2.
Regression lines for association between routine burden and child quality of life (QOL) moderated by child age.
Because age was found to significantly moderate the association between child QOL and asthma severity and between child QOL and routine burden, these analyses were rerun in the older group of children (8–12 years) who had completed the original PAQLQ. Age did not significantly moderate the association between asthma severity and child QOL (β = −.18, t(96) = −2.0, p = .052; ΔR2 = 0.03, ΔF(96) = 3.88, p = .052). However, model statistics indicated effect sizes similar to that of the full sample and a trend toward significance. In addition, age did not significantly moderate the association between routine burden and child QOL in the 8–12-year-old group (β = −.18, t(96) = −1.85, p = .07; ΔR2 = .03, ΔF(96) = 3.41, p = .07). Again, effect sizes were similar and the analysis was trending toward significance.
Discussion
This study was designed to investigate the association between individual and family-based factors (asthma severity, family functioning, and asthma routines) and QOL in children with asthma between the ages of 5 and 12 years from a developmental perspective. Child age was found to moderate the association between asthma severity and child QOL. We also found that child age moderated the association between routine burden and child QOL. Our study adds to existent literature on child QOL in pediatric asthma by suggesting that factors contributing to child QOL differ across child age.
As hypothesized, child age moderated the association between asthma severity and child QOL. This association was significant for older children, such that worse asthma severity was associated with poorer QOL in older children. Our findings are consistent with previous studies suggesting an inverse association between asthma severity and child QOL (Everhart & Fiese, 2009a; Petsios et al., 2013; Sawyer, Spurrier, Whaites, et al., 2001; Williams et al., 2000). Children with more severe asthma may find that they are limited by their asthma or do not feel well overall, which may negatively affect their QOL (Sawyer, Spurrier, Whaites, et al., 2001). Our findings also suggest that factors at the individual level, such as asthma severity, may be more proximal to child QOL in older children. This is consistent with literature, suggesting that as children begin to enter adolescence, peer relationships gain importance and adolescents may find their asthma treatments interfere with opportunities to interact with peers (Drotar, 2004). Given that older children may be engaging in more asthma management behaviors, children with severe asthma may be performing more daily tasks related to their asthma care. Further, older children may better understand their asthma symptoms and what it means to have severe asthma.
Additionally, child age was found to significantly moderate the association between routine burden and child QOL. Interestingly, the moderation was only significant for older children with asthma, such that more routine burden was associated with lower QOL. This finding is consistent with previous research that has found an association between parent reports of routine burden and child-reported QOL (Fiese et al., 2005). Given that we found this association only among older children, our finding may suggest that asthma management routines become more burdensome for caregivers as they begin to incorporate the child’s responsibility for their asthma care into daily management as well.
As two different QOL measures were used, we reran the significant moderation analyses in the older age-group (8–12 years) that completed the original PAQLQ. Age did not significantly moderate the association between asthma severity and child QOL or between routine burden and child QOL. However, both of these analyses were trending toward significance and effect sizes were similar to that of the whole group analyses. Our findings may not have reached significance in the older group because of the smaller age range and/or sample size. It may also be that age was confounded by measurement scale; therefore, future research is needed to determine whether child age serves as a moderator in samples of children from a range of ages that have completed the same measure of QOL.
On the other hand, we did not find that child age moderated associations between QOL and family functioning assessed by the MICS or between QOL and medication routines. This might suggest that both family functioning and medication routines are important factors for child QOL regardless of child age. For instance, a previous study found that parental involvement in child asthma treatment was associated with better outcomes (e.g., adherence, lower functional severity scores) among older children (Duncan et al., 2013). Our findings may suggest that although the role of families in a child’s daily life may change with age, some sort of familial involvement may be an important determinant of QOL in children with asthma. On average, level of family functioning in this sample was considered “unhealthy” based on the mean rating of the MICS overall scale. The limited range in family functioning ratings offers another possible reason as to why child age may not have moderated the association between family functioning and child QOL. It is important to note that although the level of family functioning for families in our study fell in the unhealthy range, this is consistent with other research studies measuring family functioning in pediatric chronic conditions (Mitchell et al., 2004; Spieth et al., 2001).
Limitations
Our study had several limitations, including the possibility that a more accurate picture of family functioning may come from viewing more than one videotaped mealtime. Although evidence supports the notion that the established PAQLQ and the pictorial PAQLQ are assessing the same construct (Everhart & Fiese, 2009b), younger and older children did complete two different QOL measures, which may confound results. Further, measurement differences between the pictorial PAQLQ and established PAQLQ may exist in that social impairment was not found to be a subscale of the pictorial PAQLQ (Everhart & Fiese, 2009b). Given that scale means from the pictorial PAQLQ have not been published in other samples, we can only compare the 8–12-year-old scores on the established PAQLQ in our sample (M = 5.02, SD = 1.22) with other studies. There are no clinical cutoff scores for high versus low QOL, but published mean QOL scores have been approximately 5 in similar studies (Annett et al., 2003; Erikson et al., 2002). Given the few number of items on the ARQ, the medication routines and routine burden factors had poor internal consistency and should be considered a limitation. However, low alpha levels may be an underestimate due to the small number of items on the scale. These alpha levels are comparable with those published by Fiese and colleagues (2008). The current study also used an average of three spirometry readings to measure asthma severity at a single time point. Future studies should include spirometry readings at multiple time points to more accurately assess asthma severity. Additionally, child clinical status (e.g., sick vs. well) was not assessed at the research session. Therefore, it is possible that the child’s clinical status may have affected their lung function during the spirometry test.
Another limitation is that adolescents were not included because the larger study was focused on family life in children with asthma between 5 and 12 years of age. Family-based issues regarding asthma management may differ among families with adolescents and should be studied in future research. In addition, the current study sample included primarily White and Black children and may not generalize to children of other races/ethnicities. Although rates of asthma are higher in boys versus girls, it is important to note that our study sample was 63% male and may not generalize to other samples of children with asthma.
Children had to be prescribed a controller medication to participate in our study; therefore, results may not generalize to children with exercise-induced asthma or those not prescribed a daily controller medication. Another limitation is that participants were recruited through several clinics, suggesting that our participants had been actively seeking and receiving treatment and may have had higher asthma control. Finally, our study was cross-sectional and we are unable to determine directionality. We have conceptualized child QOL as an outcome variable; however, it is possible that associations between factors in our models and QOL are bidirectional. Longitudinal studies should be used to confirm the direction of effects between family functioning, asthma severity, and child QOL.
Future Research and Clinical Implications
Future research should replicate findings from this study with larger, diverse samples of children to determine racial/ethnic differences in factors associated with child QOL by child age. Consistent with previous research (Josie et al., 2007), we found that Black children in our sample had both lower levels of child QOL and worse asthma severity. Future research should consider focusing specifically on Black families to determine whether culturally based factors (e.g., use of alternative treatments, acculturation) may influence child QOL in these families. Our study built on existing child QOL literature in considering how child age may moderate associations between QOL and family functioning, asthma routines, and asthma severity in children between 5 and 12 years of age. Differences in asthma management and adherence may also be key variables that explain differences between these groups and should be considered in future studies. Future research should also include structural equation modeling to determine how processes related to child QOL may work together in predicting QOL in children with asthma.
Our study also has important clinical implications for health-care providers and clinicians treating children with asthma. Depending on the child’s age, different factors may be more important to QOL in children with asthma. In older children, more severe asthma and more caregiver-perceived burden from asthma management routines may place a child at risk for worse QOL. Physicians may wish to provide more psychoeducation to older children and their families regarding the importance of preventative treatment (e.g., controller medications). We suggest that health-care providers tailor their discussions with families around these factors (e.g., severity, routine burden) and highlight the potential importance of each in its association with child QOL. Strategies tailored by child age may serve to improve a child’s overall experience with their asthma and improve child QOL.
Funding
This study was supported by a grant from the National Institute of Mental Health (R01 MH51771; to B.H.F., PI).
Conflicts of interest: None declared.
References
- Annett R. D., Bender B. G., DuHamel T. R., Lapidus J. (2003). Factors influencing parent reports on quality of life for children with asthma. Journal of Asthma, 40, 577–587. [DOI] [PubMed] [Google Scholar]
- Baron R. M., Kenny D. A. (1986). The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology, 51, 1173–1182. [DOI] [PubMed] [Google Scholar]
- Blaakman S. W., Cohen A., Fagnano M., Halterman J. S. (2014). Asthma medication adherence among urban teens: A qualitative analysis of barriers, facilitators and experiences with school-based care. The Journal of Asthma, 51, 522–529. doi: 10.3109/02770903.2014.885041 [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner U. (1979). The ecology of human development: Experiments in nature and design. Cambridge, MA: Harvard University Press. [Google Scholar]
- Center for Disease Control. (2012). Asthma’s impact on the nation: Data from the CDC national asthma control program. Retrieved from http://www.cdc.gov/asthma/impacts_nation/asthmafactsheet.pdf. Retrieved December 1, 2013.
- Crespo C., Carona C., Silva N., Canavarro M. C., Dattilio F. (2011). Understanding the quality of life for parents and their children who have asthma: Family resources and challenges. Contemporary Family Therapy, 33, 179–196. [Google Scholar]
- Dickstein S., Hayden L. C., Schiller M., Seifer R., San Antonio W. (1994). Providence family study mealtime family interaction coding system. Adapted from the McMaster clinical rating scale. East Providence, RI: E.P. Bradley Hospital. [Google Scholar]
- Dickstein S., Seifer R., Hayden L. C., Schiller M., Sameroff A. J., Keitner G., Miller I., Rasmussen S., Matzko M., Magee K. D. (1998). Levels of family assessment II: Impact of maternal psychopathology on family functioning. Journal of Family Psychology, 12, 23–40. [Google Scholar]
- Drotar D. (2004). Validating measures of pediatric health status, functional status, and health-related quality of life: Key methodological challenges and strategies. Ambulatory Pediatrics, 4, 358–364. [DOI] [PubMed] [Google Scholar]
- Duncan C. L., Hogan M. B., Tien K. J., Graves M. M., Chorney J. M., Zettler M. D., Portnoy J. (2013). Efficacy of a parent-youth teamwork intervention to promote adherence in pediatric asthma. Journal of Pediatric Psychology, 38, 617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein N. B., Baldwin L. M., Bishop D. S. (1983). The McMaster family assessment device. Journal of Marital and Family Therapy, 9, 171–180. [Google Scholar]
- Erickson S. R., Munzenberger P. J., Plante M. J., Kirking D. M., Hurwitz M. E., Vanuya R. Z. (2002). Influence of sociodemographics on the health-related quality of life of pediatric patients with asthma and their caregivers. Journal of Asthma, 39, 107–117. [DOI] [PubMed] [Google Scholar]
- Everhart R. S., Fiese B. H. (2009a). Asthma severity and child quality of life in pediatric asthma: A systematic review. Patient Education and Counseling, 75, 162–168. [DOI] [PubMed] [Google Scholar]
- Everhart R. S., Fiese B. H. (2009b). Development and initial validation of a pictorial quality of life measure for young children with asthma. Journal of Pediatric Psychology, 34, 966–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiese B. H., Foley K. P., Spagnola M. (2006). Routine and ritual elements in family mealtimes: Contexts for child well-being and family identity. New Directions for Child and Adolescent Development, 111, 67–89. [DOI] [PubMed] [Google Scholar]
- Fiese B. H., Wamboldt F. S., Anbar R. D. (2005). Family asthma management routines: Connections to medical adherence and quality of life. The Journal of Pediatrics, 146, 171–176. [DOI] [PubMed] [Google Scholar]
- Fiese B. H., Winter M., Anbar R., Howell K., Poltrock S. (2008). Family climate of routine asthma care: Associating perceived burden and mother-child interaction patterns to child well-being. Family Process, 47, 63–79. [DOI] [PubMed] [Google Scholar]
- Fiese B. H., Winter M. A., Botti J. C. (2011). The ABCs of family mealtimes: Observational lessons for promoting healthy outcomes for children with persistent asthma. Child Development, 82, 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiese B. H., Winter M. A., Wamboldt F. S., Anbar R. D., Wamboldt M. Z. (2010). Do family mealtime interactions mediate the association between asthma symptoms and separation anxiety? Journal or Child Psychology and Psychiatry and Allied Disciplines, 51, 144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garro A. (2011). Health-related quality of life (HRQOL) in Latino families experiencing pediatric asthma. Journal of Child Health Care, 15, 350–357. [DOI] [PubMed] [Google Scholar]
- Goldbeck L., Koffmane K., Lecheler J., Thiessen K., Fegert J.M. (2007). Disease severity, mental health, and quality of life in children and adolescents with asthma. Pediatric Pulmonology, 42, 15–22. [DOI] [PubMed] [Google Scholar]
- Hayden L., Schiller M., Dickstein S., Seifer R., Sameroff S., Miller I., Keitner G., Rasmussen S. (1998). Levels of family assessment I: Family, martial, and parent-child interaction. Journal of Family Psychology, 12, 7–22. [Google Scholar]
- Hollingshead A. B. (1975). A four-factor classification of social status . New Haven, CT: Yale University. [Google Scholar]
- Holmbeck G. N. (2002). Post-hoc probing of significant moderational and mediational effects in studies of pediatric populations. Journal of Pediatric Psychology, 27, 87–96. [DOI] [PubMed] [Google Scholar]
- Janicke D. M., Mitchell M. J., Stark L. J. (2005). Family functioning in school-age children with cystic fibrosis: An observational assessment of family interactions on the mealtime environment. Journal of Pediatric Psychology, 30, 179–186. [DOI] [PubMed] [Google Scholar]
- Josie K. L., Greenley R. N., Drotar D. (2007). Cumulative risk and asthma outcomes in inner-city African-American youth. The Journal of Asthma, 44, 535–541. [DOI] [PubMed] [Google Scholar]
- Juniper E. F. (1997). How important is quality of life in pediatric asthma? Pediatric Pulmonology, 15, 17–21. [PubMed] [Google Scholar]
- Juniper E. F., Guyatt G. H., Feeny D., Ferrie P. J., Griffith L. E., Townsend M. (1996). Measuring quality of life in children with asthma. Quality of Life Research, 5, 35–46. [DOI] [PubMed] [Google Scholar]
- Juniper E. F., Wisniewski M. E., Cox F. M., Emmett A. H., Nielsen K. E., O’Byrne P. M. (2004). Relationship between quality of life and clinical status in asthma: A factor analysis. European Respiratory Journal, 23, 287–291. [DOI] [PubMed] [Google Scholar]
- Kaugars A. S., Klinnert M. D., Bender B. G. (2004). Family influences on pediatric asthma. Journal of Pediatric Psychology, 29, 475–491. [DOI] [PubMed] [Google Scholar]
- Mitchell M. J., Powers S. W., Byars K. C., Dickstein S., Stark L. J. (2004). Family functioning in young children with cystic fibrosis: Observations of interactions at mealtime. Journal of Developmental and Behavioral Pediatrics, 25, 335–346. [DOI] [PubMed] [Google Scholar]
- Moreira H., Carona C., Silva N., Frontini R., Bullinger M., Canavarro M. C. (2013). Psychological and quality of life outcomes in pediatric populations: A parent-child perspective. The Journal of Pediatrics, 163, 1471–1478. [DOI] [PubMed] [Google Scholar]
- Mosnaim G., Li H., Martin M., Richardson D., Belice P. J., Avery E., Ryan N., Bender B., Powell L. (2014). Factors associated with levels of adherence to inhaled corticosteroids in minority adolescents with asthma. Annals of Allergy, Asthma, & Immunology, 112, 116–20. doi: 10.1016/j.anai.2013.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Heart, Lung, and Blood Institute. (2007). Expert panel report 3: Guidelines for the diagnosis and management of asthma. Bethesda, MD: National Institutes of Health. [Google Scholar]
- Petsios K. T., Priftis K. N., Hatziagorou E., Tsanakas J. N., Antonogeorgos G., Matziou V. N. (2013). Determinants of quality of life in children with asthma. Pediatric Pulmonology, 48, 1171–1180. [DOI] [PubMed] [Google Scholar]
- Santos S., Crespo C., Silva N., Canavarro M. C. (2012). Quality of life and adjustment in youths with asthma: The contributions of family rituals and the family environment. Family Processes, 51, 557–569. [DOI] [PubMed] [Google Scholar]
- Sawyer M. G., Spurrier N., Kennedy D., Martin J. (2001). The relationship between the quality of life of children with asthma and family functioning. Journal of Asthma, 38, 279–284. [DOI] [PubMed] [Google Scholar]
- Sawyer M. G., Spurrier N., Whaites L., Kennedy D., Martin A. J., Baghurst P. (2001). The relationship between asthma severity, family functioning and the health-related quality of life of children with asthma. Quality of Life Research, 9, 1105–1115. [DOI] [PubMed] [Google Scholar]
- Schmier J. K., Chan K. S., Kline-Leidy N. (1998). The impact of asthma on health-related quality of life. Journal of Asthma, 35, 585–597. [DOI] [PubMed] [Google Scholar]
- Spieth L. E., Stark L. J., Mitchell M. J., Schiller M., Cohen L. L., Mulvihill M., Hovell M. F. (2001). Observational assessment of family functioning at mealtime in preschool children with cystic fibrosis. Journal of Pediatric Psychology, 26, 215–224. [DOI] [PubMed] [Google Scholar]
- van Gent R., van Essen-Zandvliet E. E. M., Klijn P., Brackel H. J. L., Kimpen J. L. L., van Der Ent C.K. (2008). Participation in daily life of children with asthma. Journal of Asthma, 45, 807–813. [DOI] [PubMed] [Google Scholar]
- Williams S., Sehgal M., Falter K., Dennis R., Jones D., Boudreaux J., Homa D., Raskin-Hood C., Brown C., Griffith M., Redd S. (2000). Effect of asthma on the quality of life among children and their caregivers in the Atlanta empowerment zone. Journal of Urban Health, 77, 268–279. [DOI] [PMC free article] [PubMed] [Google Scholar]