Abstract
The WHO recommends that children living in areas of highly seasonal malaria transmission in the Sahel subregion should receive seasonal malaria chemoprevention (SMC) with sulfadoxine-pyrimethamine plus amodiaquine (SPAQ). We evaluated the use of dihydroartemisinin-piperaquine (DHAPQ) as an alternative drug that could be used if SPAQ starts to lose efficacy. A total of 1,499 children 3 to 59 months old were randomized to receive SMC with SPAQ or DHAPQ over 3 months. The primary outcome measure was the risk of clinical malaria (fever or a history of fever with a parasite density of at least 3,000/μl). A cohort of 250 children outside the trial was followed up as a control group. Molecular markers of drug resistance were assessed. The risk of a malaria attack was 0.19 in the DHAPQ group and 0.15 in the SPAQ group, an odds ratio of 1.33 (95% confidence interval [CI], 1.02 to 1.72). Efficacy of SMC compared to the control group was 77% (67% to 84%) for DHAPQ and 83% (74% to 89%) for SPAQ. pfdhfr and pfdhps mutations associated with antifolate resistance were more prevalent in parasites from children who received SPAQ than in children who received DHAPQ. Both regimens were highly efficacious and well tolerated. DHAPQ is a potential alternative drug for SMC. (This trial is registered at ClinicalTrials.gov under registration no. NCT00941785.)
INTRODUCTION
Substantial progress has been made in the control of malaria (1), but in most of sub-Saharan Africa, the disease remains a major public health problem. In Burkina Faso, malaria is still a leading cause of severe illness and mortality, accounting for 63% of hospital admissions and 71% of all deaths in hospital among children under 5 years of age in 2011 (2). Results from research studies indicate that the burden remains very high. In Boussé, in the Sahelian zone of the country, in 2011, 1,232 episodes of malaria were recorded over one transmission season in 1,500 children under 5 years of age who were using an insecticide-treated net (3). In such areas, new strategies for malaria control are needed. The WHO now recommends seasonal malaria chemoprevention (SMC) with sulfadoxine pyrimethamine plus amodiaquine (SPAQ) as a new strategy for malaria control in children in areas of highly seasonal transmission (4, 5), defined as areas where at least 60% of malaria cases occur during 4 months of the year and where SP and AQ retain good efficacy. Most parts of Burkina Faso fit these criteria, and implementation of SMC in Burkina Faso started in 2014 in seven districts.
Resistance to both SP and AQ is common in much of Africa, but in most areas of seasonal transmission in the Sahel these drugs retain their antimalarial efficacy (5). However, alternative drug regimens may be needed in these areas in the future, and they are needed now if SMC is to be deployed in areas of eastern or southern Africa where antifolate resistance makes SP an unsuitable drug for SMC. Dihydroartemisinin-piperaquine (DHAPQ) is a potential alternative. Piperaquine (PQ) is a long-acting antimalarial; administration daily for 3 days results (in adults) in a 3- to 7-fold accumulation and a long terminal half-life (6, 7), making it suitable for chemoprevention. PQ has been used extensively for chemoprophylaxis in China and is now available in a fixed combination with dihydroartemisinin. Two studies have investigated the use of DHAPQ for SMC (8, 9). When DHAPQ and SPPQ (sulfadoxine-pyrimethamine plus piperaquine) were compared with SPAQ, efficacy was similar for all three regimens, but the incidence of malaria was low, limiting the power to differentiate between regimens. DHAPQ and SPPQ were better tolerated than SPAQ, and DHAPQ was associated with lower selection of dhfr and dhps mutations, strongly associated with antifolate resistance in Plasmodium falciparum, compared to the SP-containing drug combinations (9). The hemoglobin concentration at the end of the transmission season was slightly lower in children who had received DHAPQ than in the other groups. In Uganda, Nankabirwa et al. (10) compared the efficacy of single preventive treatments with SP alone, SPAQ, and DHAPQ in schoolchildren and found DHAPQ to be the most effective with a substantially reduced prevalence of parasitemia assessed 42 days after treatment. In adults in Thailand, the protective efficacy of DHAPQ was 98% when DHAPQ was administered monthly and 86% when it was administered bimonthly (11). Despite extensive clinical evaluation and use of DHAPQ in Southeast Asia (12, 13) and Africa (14–16), few studies have addressed pharmacokinetics of PQ in children (17–19). However, one of these studies suggested that children are underdosed with current regimens (17), which was also supported by a recent meta-analysis (20). The primary objective of this study was to determine whether DHAPQ is as effective as SPAQ for SMC in an area where SPAQ is highly efficacious and to compare the tolerability and safety of two regimens when used for SMC in children.
(Preliminary results of this study were presented at the 6th MIM Pan-African Malaria Conference, Durban, South Africa, 2013.)
MATERIALS AND METHODS
Study site.
The study was conducted between August 2009 and January 2010 in a rural area served by three health centers (Satiri, Kadomba, and Balla) in the district of Lena, approximately 30 miles from the city of Bobo-Dioulasso in western Burkina Faso. The climate of the area is typical of the Sudan savannah, with a long dry season and a shorter rainy season (July to October). Transmission of malaria is highly seasonal.
Recruitment of participants.
Before the trial started, meetings were held in the community to explain the study aims. A population census was done in July 2009. Households with a child under 5 years of age were then visited to explain the procedures of the study and to seek signed consent from parents. If parents were unable to read, a witness signed to indicate that the study details had been explained correctly. The inclusion criteria were as follows: the child's age was between 3 and 59 months; the family expected to remain in the study area over the study period; the child had no history of allergy to the study medications and no chronic condition requiring hospitalization (for example, severe malnutrition); and parental consent was obtained. The presence of malaria at enrollment was not an exclusion criterion; if malaria was diagnosed, the patient was enrolled and treated with artemether-lumefantrine (AL; Coartem), and SMC was not given, but the child was eligible to receive subsequent monthly doses of SMC.
Enrollment and randomization procedures.
On the day of enrollment, a clinical assessment was made, including the measurement of weight, height, and axillary temperature. A physical examination was done and questions asked about the use of insecticide-treated bed nets (ITNs) and medical history. After a further check of eligibility, children were assigned a randomization envelope bearing a randomization number. Allocations (generated using permuted blocks of 10 in Stata version 10) were sealed in opaque envelopes which were assigned in a strict numerical sequence. A finger-prick blood sample was taken for preparation of thick and thin blood smears and for blood spots on filter paper (Whatman no. 3) for molecular analyses. Participants were then referred to the study nurse, who opened the envelope to determine the treatment allocation and administered the first dose of medication. This was an open trial, as blinding was not feasible due to the difference in the appearance of the study drugs, but steps were taken to ensure concealed randomization, and staff who performed laboratory analyses were not aware of the child's treatment group.
Study drugs and SMC administration.
Children in the SPAQ arm received one dose of SP (Fansidar [Roche]; tablets of 500 mg sulfadoxine and 25 mg pyrimethamine) in a dosage of 25 mg sulfadoxine and 1.25 mg pyrimethamine per kg of body weight and three doses of AQ (Camoquin [Parke-Davis] syrup; 60 ml, 50 mg/5 ml) in a dosage of 10 mg/kg each day for three consecutive days. Children in the DHAPQ group received Duocotexin (Holley Cotec, China) tablets (40 mg DHA and 320 mg PQ phosphate) in a dosage of 4 mg/kg DHA and 18 mg/kg PQ daily for three consecutive days. Children were weighed each month to determine dosage, which was rounded to the nearest quarter tablet or the nearest 5 ml of AQ syrup. Three rounds of preventive treatment were given (August, September, and October 2009). The single dose of SP and the first dose of AQ or DHAPQ were given at the study clinic observed by the study nurse. A field worker visited the child at home on each of the next 2 days to administer the two remaining doses of AQ or DHAPQ and to ask about adverse events. Children were observed for 30 min after each dose and a repeat dose was given if the child vomited.
Follow-up visits and malaria diagnosis and treatment.
Parents were asked to bring their child to the clinic whenever the child was unwell. A field worker visited each family 2 weeks after each SMC round to check that the child was well and to refer any children who were unwell to the clinic, where a study physician was available. Children who presented with a history of fever had a rapid diagnostic test for malaria (SD Bioline; Standard Diagnostics, South Korea), and if this was positive they were treated with AL. A blood smear was taken to be read later. If a child was diagnosed with malaria on the day SMC was scheduled to be given, SMC was withheld that month and the child was treated with AL. Medications commonly used to treat other illnesses included antibiotics (amoxicillin and oxacillin tablets or syrup) and paracetamol.
Control group.
To estimate the incidence of malaria in untreated children, a separate cohort was enrolled in part of the study area (one of the three areas used for the main trial) with the same inclusion criteria as those used for the main trial cohorts, but 1 month later, at the time of the second round of SMC administration. These children were followed up in a manner similar to that used for the other study children.
Cross-sectional surveys.
At the end of the malaria transmission season, a survey of all study children was undertaken (one month after the last administration of SMC in the randomized groups and 1 month later in the control group) to determine the prevalence of parasitemia and gametocytemia and the concentration of hemoglobin using a Haemocue (Angelholm, Sweden).
Laboratory methods.
Thick and thin blood smears were stained with 2% Giemsa for 30 min and double read by experienced laboratory technicians. For parasite isolates sampled during the first episode of malaria following the initiation of SMC, mutations in pfmdr1 (N86Y, F184Y, and D1246Y), pfdhfr (N51I, C59R, and S108N), and pfdhps (A436S, A436F, A437G, K540E, and A613S) were detected by dideoxy sequencing and mutations in pfcrt (K76T) by qPCR as described previously (21, 22). A subset of 45 children was identified at randomization for assessment of biochemical and hematological parameters, 15 to be sampled each month, and a subset of 210 children in the DHAPQ group (70 each month) gave additional blood samples for evaluation of the pharmacokinetic properties of piperaquine (full details of these results will be published separately; here we present only the day 7 concentrations).
Statistical methods.
The primary outcome measure of the trial was the risk of clinical malaria (axillary temperature ≥ 37.5°C or history of fever in the last 24 h and P. falciparum density of at least 3,000 parasites/μl). Secondary outcome measures included the incidence of clinical malaria with any parasitemia, the prevalence of asexual parasitemia and gametocyte carriage, and the presence of anemia at the end of the malaria transmission season; the presence of molecular markers of resistance to study drugs among patients diagnosed with malaria during the trial or with parasitemia at the end of the transmission season; and the pharmacokinetics of PQ. Sample size was chosen to give adequate power to demonstrate that SMC with DHAPQ was noninferior to SMC with SPAQ with respect to the risk of malaria with a parasite density of 3,000/μl or more. The noninferiority margin was specified as an odds ratio of 1.64, equivalent to a risk difference of 4% if the risk in the comparator group was 7%. A sample size of 1,500 children was needed to give a study with 80% power using a one-sided 2.5% significance level, allowing for up to 10% of subjects being excluded from the according-to-protocol analysis due to loss to follow-up or nonadherence to the protocol. The intention-to-treat (ITT) analysis (considered primary) included all randomized children, in the group they were assigned to at randomization. For according-to-protocol (ATP) analysis, we excluded children who did not attend for an SMC treatment round, but we included children who attended but did not receive SMC because they had malaria and were treated with AL. Analysis of noninferiority was based on the 95% confidence interval on the odds ratio for malaria, obtained from the Kaplan-Meier estimate of the risk and its standard error.
Further details of methods are given in the supplemental material.
Ethics.
The study protocol was approved by the ethics committee of Centre Muraz (Comité d'Ethique Institutionnel du Centre Muraz) and by the ethics committee of the London School of Hygiene & Tropical Medicine. A data safety monitoring board was appointed to oversee the trial, and an independent monitor provided oversight of the conduct of the trial.
RESULTS
Characteristics of study children.
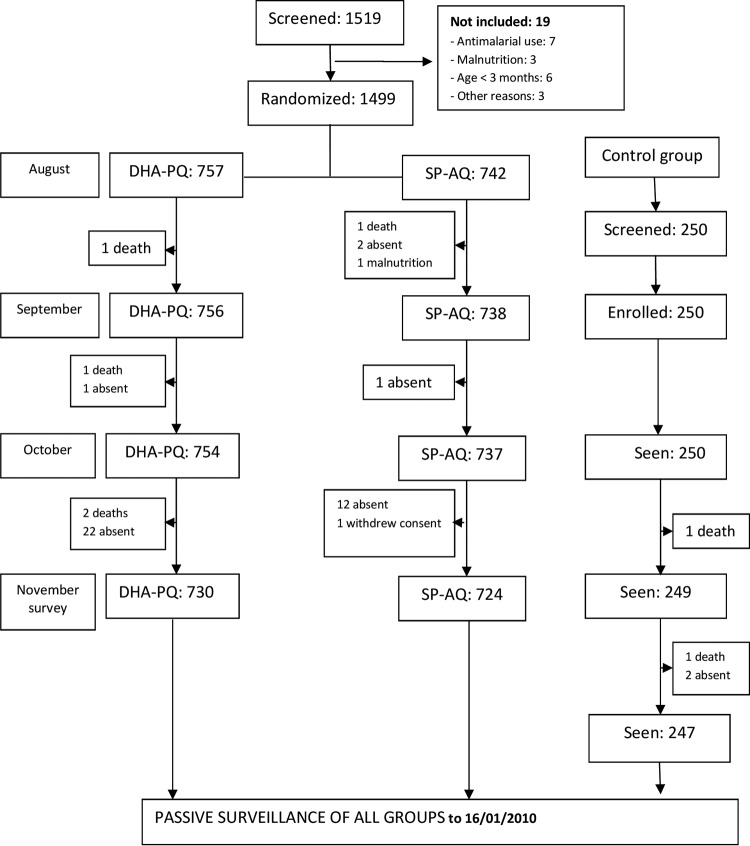
The trial profile is shown in Fig. 1. A total of 1,499 children were randomized, 750 to the DHAPQ group and 749 to the SPAQ group. The randomized groups were similar in terms of baseline characteristics. The cohort of 250 children who did not receive SMC, recruited at the time the main cohorts received their second round of SMC, was similar to the trial cohorts in terms of age but were more malnourished, with a higher prevalence of being underweight and higher prevalence of parasitemia (Table 1).
FIG 1.
Trial profile. Twelve allocation errors occurred (7 children randomized to SPAQ received DHAPQ in error, 2 randomized to SPAQ received mixed treatments, and 3 randomized to DHAPQ received mixed treatments), leaving 754 who received DHAPQ and 740 who received SPAQ in the ATP analysis. At enrollment, 9.4% (70/742) and 9.5% (72/757) of children in the SPAQ and DHAPQ groups, respectively, did not receive SMC because they had clinical malaria. These proportions were 7.5% (55/738) and 8.1% (61/756) in September and 8.3% (61/737) and 8.8% (66/754) in October. Ninety-seven percent (1,454/1,499) of randomized children were seen at the survey at the end of the transmission season.
TABLE 1.
Characteristics of children in the randomized groups and the untreated cohort at enrollment
| Variable | SMC randomized groups |
Untreated cohort (n = 250) | |
|---|---|---|---|
| SPAQ (n = 742) | DHAPQ (n = 757) | ||
| Date enrolled | 11–20 Aug | 11–20 Aug | 17–19 Sep |
| No. (%) of participants at study site | |||
| Kadomba | 323 (44) | 325 (43) | 0 |
| Balla | 151 (20) | 150 (20) | 250 (100) |
| Satiri | 268 (36) | 282 (37) | 0 |
| % male:% female | 49:51 | 50:50 | 49:51 |
| No. (%) in age group | |||
| <12 mo | 129 (17) | 153 (20) | 47 (19) |
| 12–23 mo | 155 (21) | 158 (22) | 63 (25) |
| 24–35 mo | 155 (21) | 152 (20) | 56 (22) |
| 36–47 mo | 147 (20) | 138 (18) | 45 (18) |
| 48–59 mo | 156 (21) | 156 (21) | 39 (16) |
| Wt (kg) [mean (SD)] | 10.9 (3.22) | 10.7 (3.13) | 10.1 (2.72) |
| No. (%) underweight | 193 (26) | 189 (25) | 95 (38) |
| No. (%) with stunting | 186 (25) | 182 (24) | 35 (14) |
| No. (%) with wasting | 163 (22) | 167 (22) | 105 (42) |
| No. (%) reporting use of bed nets | 267 (36) | 273 (36) | 79 (32) |
| No. (%) who slept under ITN the night before | 204 (27) | 186 (25) | 77 (31) |
| No. (%) with fevera | 213 (29) | 216 (29) | 179 (72) |
| No. (%) with malariab | 72 (9.5) | 70 (9.4) | 160 (64) |
| Geometric mean parasite density (/μl) (range) | 2,655 (16–185,000) | 2,216 (12–180,000) | 2,950 (12–111,000) |
| Prevalence of parasitema [no. (%)] | 336 (45) | 323 (43) | 152 (61) |
| Prevalence of gametocyte carriage [no. (%)] | 80 (11) | 80 (11) | 75 (30) |
Axillary temperature of >37.5°C or history of fever in the past 24 h.
Fever with any parasitemia, measured in August for the randomized groups and a month later in September for the untreated cohort.
Adherence to daily doses.
All daily doses of SMC were supervised. Three children in the SPAQ group did not complete the course in August, two children in the DHAPQ group did not complete the course in August, and one child in the DHAPQ group did not complete the course in October. Between 7% and 9% of children missed SMC doses each month because they required treatment for malaria (Fig. 1).
Efficacy of SPAQ and DHAPQ against clinical malaria.
The incidence of malaria in the SMC treatment groups and that in the untreated group were compared during the 2 months following the second round of SMC. There were 229 episodes of malaria, defined as fever with a parasite density of ≥3,000/μl, in the untreated cohort, a mean of 0.92 episode per child, compared with 108 episodes (mean, 0.14 per child) in the DHAPQ group and 78 (mean, 0.11 per child) in the SPAQ group, giving an efficacy against malaria (adjusted for the covariates site, age, and ITN use) of 79% (95% CI, 70% to 85%) and 84% (76% to 90%), respectively. Efficacy against malaria, defined as fever with any parasitemia and adjusted for the same covariates, was 74% (65% to 81%) for DHAPQ and 80% (72% to 86%) for SPAQ (Fig. 2). To estimate the duration of protection provided by SMC, the incidence of clinical malaria after the last round of SMC in each treatment group was compared with the incidence in controls over the same period, adjusted for covariates. In both groups protection persisted at a high level for 3 to 4 weeks and decreased rapidly thereafter (Fig. 3).
FIG 2.
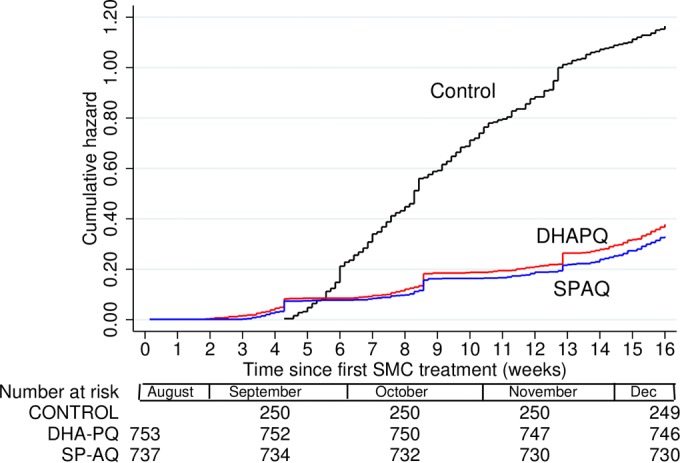
Cumulative hazard of malaria (fever or history of fever with any parasitemia) in children who received SMC with DHA-PQ or SP-AQ on three occasions in August, September, and October. A cohort of untreated children were recruited as a control group, at the time the main cohorts received the second round of SMC. The y axis shows the mean number of episodes per child since the start of surveillance. Malaria episodes were detected by passive detection, and at cross-sectional surveys performed just before each round of SMC.
FIG 3.
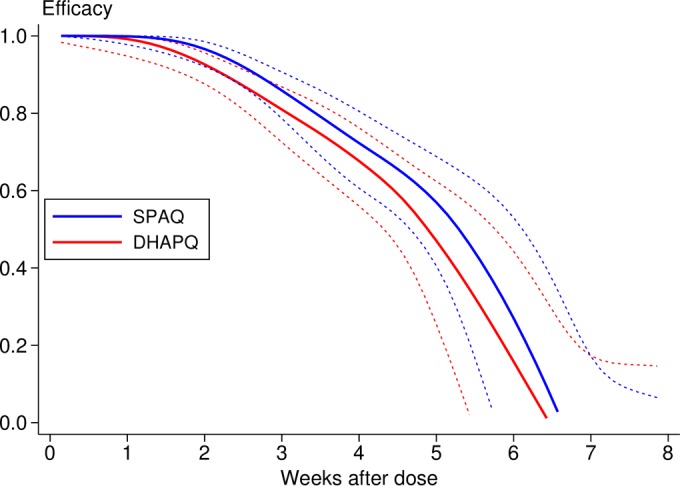
Duration of protection (malaria with any parasitemia). A smoothed estimate of the hazard ratio was obtained using regression splines using the method of Lambert and Royston (32), and the efficacy (1-hazard ratio) with 95% confidence band is plotted against time since the final round of SMC.
Analysis of the noninferiority of DHAPQ to SPAQ.
Over the 3 months of the trial, there were 281 episodes of malaria (fever with parasite density ≥ 3,000/μl), 122 in children in the SPAQ group and 159 in children in the DHAPQ group. The Kaplan-Meier estimate of the risk of malaria during the 3 months was 0.15 for the SPAQ group and 0.19 for the DHAPQ group (odds ratio, 1.33; 95% CI, 1.02 to 1.72) (Fig. 4). This confidence interval is above 1, indicating superiority of SPAQ, the upper limit just exceeding the margin for noninferiority. The cumulative hazard at 3 months was 0.16 (SPAQ) and 0.21 (DHAPQ), and the hazard ratio was 1.29 (95% CI, 0.97,1.71) (Table 2). Similar results were obtained by ATP analysis (Table 3).
FIG 4.
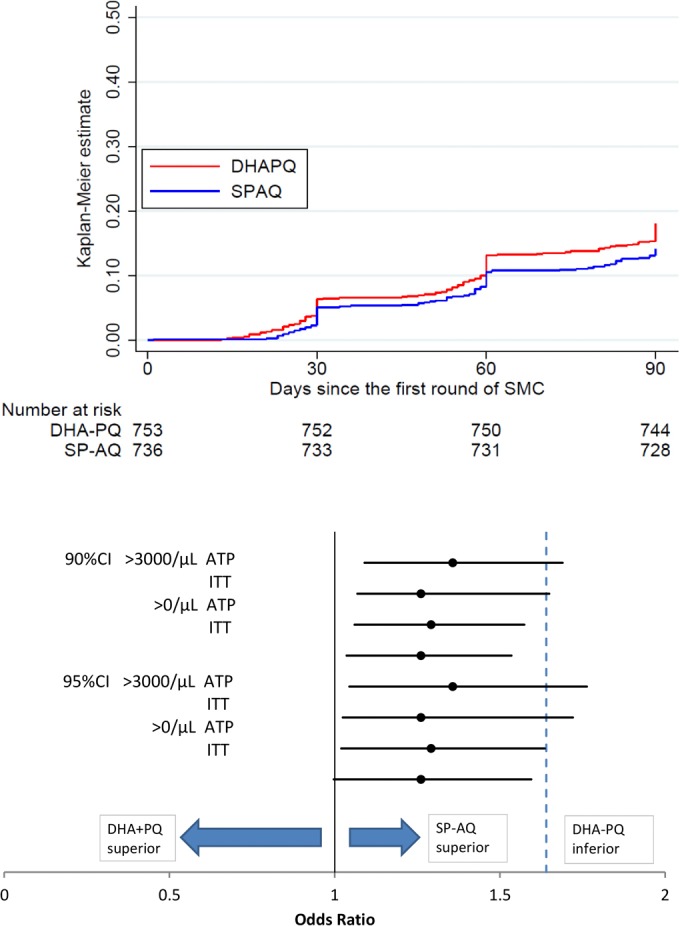
(Top) Kaplan-Meier estimates of the proportion of children with an episode of malaria. (Bottom) Diagram showing the 90% and 95% confidence intervals for the odds ratios for ATP and ITT analyses for the primary endpoint (malaria with parasitemia above 3,000/μl) and for the secondary endpoint (malaria with parasitemia at any density). An odds ratio of 1.64 was specified as the noninferiority margin. The 90% and 95% CIs cross this margin for some analyses but are entirely above 1, so we are confident that SPAQ is superior to DHAPQ and are somewhat less confident in our conclusion that the DHAPQ is not inferior to SPAQ.
TABLE 2.
ITT analysisa of malaria incidence during a 3-month period from the time of the first round of SMC
| Group and treatment | No. of participants | No. of cases | Person-months | Rate (no./1,000/month) | Proportion with malaria (K-M estimate) | SE | OR (95% CI) | Cumulative hazard | SE | HR (95% CI) [P] |
|---|---|---|---|---|---|---|---|---|---|---|
| Fever with parasitemia ≥ 3,000/μl | ||||||||||
| SPAQ | 749 | 122 | 2,202.5 | 56.1 | 0.151 | 0.0126 | 1 | 0.163 | 0.0148 | 1 |
| DHAPQ | 750 | 159 | 2,216.6 | 71.3 | 0.191 | 0.0137 | 1.33 (1.02–1.72) | 0.210 | 0.0168 | 1.29 (0.97–1.71) [0.075] |
| Fever with any parasitemia | ||||||||||
| SPAQ | 749 | 161 | 2,202.5 | 73.1 | 0.195 | 0.0138 | 1 | 0.215 | 0.017 | 1 |
| DHAPQ | 750 | 199 | 2,216.6 | 89.8 | 0.234 | 0.0146 | 1.26 (1.00–1.59) | 0.264 | 0.0188 | 1.22 (0.95–1.58) [0.122] |
Analysis of noninferiority was based on the 95% confidence interval of the odds ratio (OR) for malaria, obtained from the Kaplan-Meier (K-M) estimate of the risk and its standard error, using the delta method. The cumulative hazard function (an estimate of the average number of malaria episodes per child) was estimated using the Nelson-Aalen method. The hazard ratio (HR) was obtained using Cox regression, with confidence intervals calculated using a robust standard error to account for repeated malaria episodes in the same child.
TABLE 3.
ATP analysis of malaria incidence during a 3-month period from the time of the first round of SMCa
| Group and treatment | No. of participants | No. of cases | Person-months | Rate (no./1,000/month) | Proportion with malaria (K-M estimate) | SE | OR (95% CI) | Cumulative hazard | SE | HR (95% CI) [P] |
|---|---|---|---|---|---|---|---|---|---|---|
| Fever with parasitemia ≥ 3,000/μl | ||||||||||
| SPAQ | 740 | 119 | 2,175.4 | 54.7 | 0.149 | 0.0127 | 1 | 0.161 | 0.0148 | 1 |
| DHAPQ | 754 | 161 | 2,228.9 | 72.2 | 0.192 | 0.0137 | 1.36 (1.04–1.76) | 0.212 | 0.0168 | 1.31 (0.99–1.74) [0.072] |
| Fever with any parasitemia | ||||||||||
| SPAQ | 740 | 156 | 2,175.4 | 71.7 | 0.193 | 0.0139 | 1 | 0.213 | 0.017 | 1 |
| DHAPQ | 754 | 200 | 2,228.9 | 89.7 | 0.236 | 0.0146 | 1.29 (1.02–1.64) | 0.266 | 0.0188 | 1.25 (0.97–1.62) [0.090] |
Abbreviations are as defined for Table 2.
Concentration of piperaquine on day 7 and its relationship to efficacy against malaria.
Piperaquine plasma concentration was measured in capillary samples in 159 children on day 7 after treatment with DHAPQ. The mean concentration was 48 ng/ml (standard deviation [SD], 21) in August, 52 ng/ml (SD, 26) in September, and 60 ng/ml (SD, 31) in October. To assess the association between piperaquine concentration measured on day 7 and protection against clinical malaria during the month that the concentration was measured, these children were divided into three equal groups according to the tertiles of the day 7 concentration. The incidence of malaria that month decreased with increasing concentration (log rank test for trend, stratified by month; χ2 = 5.10 [1df]; P = 0.024) (Table 4).
TABLE 4.
Incidence of malaria cases in children whose piperaquine concentration in plasma was measured in capillary samples on day 7
| Piperaquine concn (ng/ml) |
No. of: |
Person-months at risk | Rate (no./month) | Ratio (95% CI) | ||
|---|---|---|---|---|---|---|
| Mean (SD) | Range | Children | Malaria cases | |||
| 27.7 (8.40) | 7.4–40.5 | 53 | 10 | 78.81 | 0.127 | 1 |
| 50.4 (6.14) | 40.6–63.0 | 53 | 8 | 94.55 | 0.085 | 0.67 (0.23–1.9) |
| 85.2 (21.8) | 63.1–163 | 53 | 4 | 99.11 | 0.040 | 0.32 (0.07–1.1) |
In these children, the mean estimated dose of piperaquine administered was 50 mg/kg (SD, 7.95; range, 28.6 to 67.9). In linear regression analysis, a 10-mg/kg increase in dose of PQ administered was associated with an increase of 4.7 ng/ml (95% CI, −1.2 to 11) in the day 7 plasma concentration of PQ in August, 5.7 ng/ml (0.3 to 11) in September, and 7.7 ng/ml (2.0 to 13) in October (Fig. 5).
FIG 5.
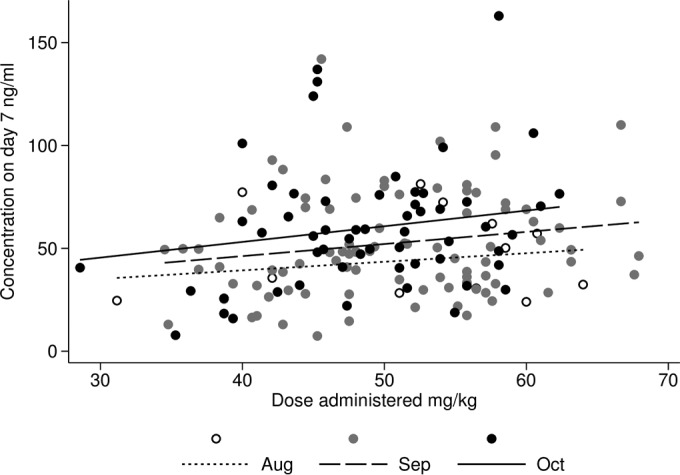
Relationship between dose of piperaquine administered and plasma concentration on day 7.
To illustrate the relationship between the dose of piperaquine administered and the incidence of malaria in the subsequent month, children who received DHAPQ were divided into three groups according to the tertiles of the dose administered (<45 mg/kg, 45 to 55 mg/kg, and >55 mg/kg), and the timing of malaria episodes in each of these groups in that month was shown in a plot of the cumulative hazards (Fig. 6). In Cox regression analysis, an increase in piperaquine dose administered was associated with a reduction in the incidence of malaria, with a hazard ratio of 0.62 (95% CI, 0.43 to 0.90) for a 10-mg/kg increase in dose administered in August, 0.52 (0.31 to 0.89) in September, and 0.85 (0.43 to 1.7) in October.
FIG 6.
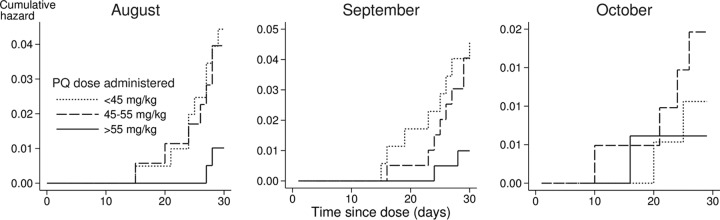
Cumulative hazard of malaria in children who received DHAPQ, according to the dose of piperaquine administered.
Efficacy against parasite and gametocyte prevalence and anemia at the end of the malaria transmission season.
At the end of transmission season, the prevalence of parasitemia by microscopy was 12% in each group of treated children (88/731 and 88/722 in the SPAQ and DHAPQ groups, respectively) and 36% (88/247) in the control group (efficacy for each group compared with the control value of 34% (95% CI, 26% to 44%). The prevalence of gametocytemia measured by microscopy was 0.8% in each treatment group (6/727 and 6/721 in the SPAQ and DHAPQ groups, respectively) and 1.6% (4/243) in the control group (efficacy for the combined SMC groups compared to controls was 50%; 95% CI, −55% to 84%).
The prevalence of anemia (hemoglobin < 8 g/dl) was 14% (35/243) in the control group, 15% (108/719) in the DHAPQ group, and 16% (117/713) in the SPAQ group. The difference in prevalence between the two SMC groups was 1.4% (95% CI, 2.3% to 5.2%), and the difference from controls was 0.6% (−4.5% to 5.7%) (DHAPQ) and 2.0% (−3.2% to 7.2%) (SPAQ) (see Tables S3 and S4 in the supplemental material).
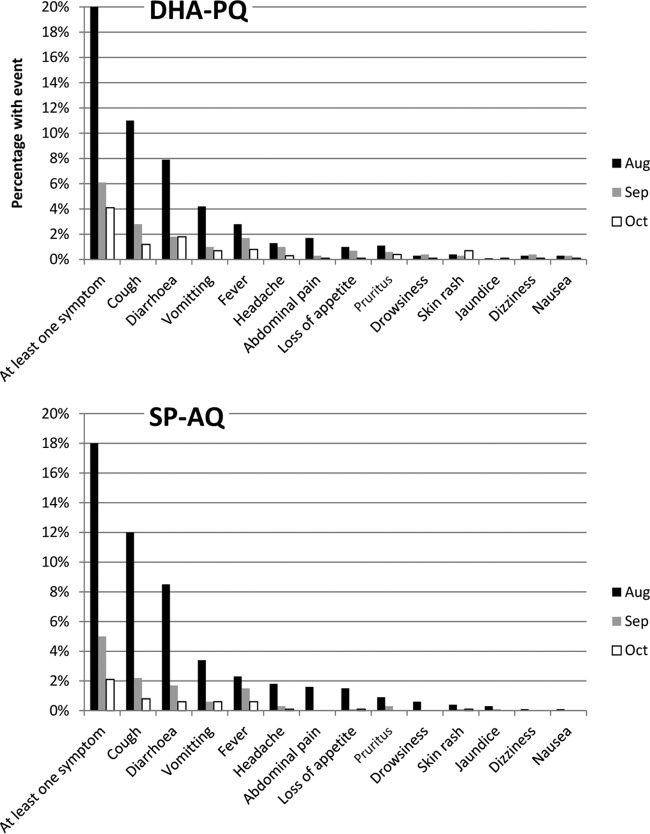
Adverse events.
The most commonly reported mild adverse events were cough, diarrhea, vomiting, and fever. The incidence of these adverse events was higher after the first round of SMC than in subsequent rounds, and in each round it was similar in both treatment groups (Fig. 7). Four cases of severe anemia were recorded (one in the SPAQ group, two in the DHAPQ group, and one in the untreated group), and 7 deaths occurred (two in the SPAQ group, four in the DHAPQ group, and one in the untreated group); three of these deaths (one in each group) occurred at home. None of these deaths was considered related to SMC. In the untreated cohort, a 3-year-old girl died in November, with malnutrition. Further details of these adverse events are provided in the supplemental material. Biochemical and hematological parameters were similar in the two treated groups on day 7, apart from the hemoglobin concentration, which was slightly lower in children who received DHAPQ (10.4 g/dl compared to 11.3 g/dl in those who received SPAQ), with an adjusted difference between groups of 1.03 (0.51 to 1.55). A small number of children had values outside the normal range (see the supplemental material), but these were not associated with clinical symptoms.
FIG 7.
Incidence of mild adverse events during each SMC round.
Drug resistance markers.
The pfdhfr 51I, pfdhfr 59R, and dhps437 mutations and the pfdhfr I51/R59/S108 and pfdhfr I51/R59/S108 plus pfdhps G437 haplotypes were more common in samples from children who had received SPAQ than in samples from children who had received DHAPQ and those in samples from the untreated children (Table 5). The prevalence of these mutations in samples from children who had received DHAPQ was similar to the prevalence in samples from untreated children. The pfdhps S613 mutation was detected in the study area for the first time, but its prevalence was similar among the study groups.
TABLE 5.
Prevalence of molecular markers of resistance among samples from malaria cases
| Genotype | % (no./total) in group |
Odds ratio (95% CI) [P] |
||||
|---|---|---|---|---|---|---|
| DHAPQ | SPAQ | No SMC | SPAQ vs. DHAPQ | DHAPQ vs. no SMC | SPAQ vs. no SMC | |
| pfcrt CVIET T76 | 62.9 (66/105) | 61 (50/82) | 61.5 (24/39) | 0.9 (0.5 to 1.7) [0.79] | 1.1 (0.5 to 2.4) [0.88] | 0.98 (0.4 to 2.3) [0.97] |
| pfmdr1 Y86 | 33 (30/91) | 44.4 (28/63) | 30.4 (24/79) | 1.6 (0.8–3.3) [0.14] | 0.7 (0.3–1.5) [0.33] | 1.8 (0.9–3.9) [0.08] |
| pfmdr1 Y184 | 36.4 (32/88) | 32.3 (20/62) | 39.2 (31/79) | 1.2 (0.6–2.5) [0.6] | 0.9 (0.5–1.7) [0.7] | 0.7 (0.3–1.6) [0.3] |
| pfdhfr i51 | 40.6 (54/133) | 64 (64/100) | 51.3 (41/80) | 2.6 (1.5–4.6) [<0.001] | 0.7 (0.4–1.2) [0.1] | 1.6 (0.9–3.2) [0.08] |
| pfdhfr s108 | 58.3 (77/132) | 27 (27/100) | 70 (56/80) | 0.3 (0.1–1.5) [<0.001] | 0.6 (0.3–1.1) [0.08] | 0.2 (0.1–0.3) [<0.001] |
| pfdhfr r59 | 43.6 (58/133) | 71 (71/100) | 53.75 (43/80) | 3.2 (1.8–5.7) [<0.001] | 0.7 (0.4–1.2) [0.15] | 2.1 (1.1–4.1) [0.01] |
| pfdhps g437 | 63.4 (85/134) | 84 (84/100) | 75 (48/64) | 3 (1.5–6.1) [<0.001] | 0.6 (0.3–1.2) [0.10] | 1.8 (0.7–4.1) [0.15] |
| pfdhps s613 | 6.5 (19/138) | 6.9 (7/101) | 13.6 (9/66) | 1.1 (0.3–3.3) [0.9] | 0.4 (0.1–1.3) [0.09] | 0.5 (0.4–1.5) [0.1] |
| pfmdr1 Y86 + pfcrt T76 (CVIET) | 3.3 (5/149) | 6 (7/116) | 1.2 (1/81) | 1.8 (0.5–7.6) [0.29] | 2.8 (0.3–133) [0.33] | 5.3 (0.6–234) [0.09] |
| pfmdr1 Y184 + pfcrt T76 (CVIET) | 3.3 (5/149) | 3.4 (4/116) | 6.2 (5/81) | 1 (0.2–4.9) [0.96] | 0.5 (0.1–2.4) [0.31] | 0.5 (0.1–2.6) [0.36] |
| pfdhfr I51/R59/S108 | 30.9 (38/123) | 53.2 (50/94) | 33.9 (20/59) | 2.5 (1.4–4.6) [0.001] | 1.1 (0.6–2.4) [0.68] | 2.1 (1.1–4.6) [0.01] |
| pfdhfr I51/R59/S108 + pfdhps G437 | 19.5 (24/123) | 41.5 (39/94) | 23.7 (14/59) | 2.9 (1.5–5.6) [<0.001] | 0.8 (0.3–1.8) [0.5] | 2.3 (1.1–5.1) [0.02] |
DISCUSSION
There is increasing recognition of the potential importance of drugs for malaria prevention in countries where malaria is endemic, but the choice of drug regimens remains limited. SPAQ, the regimen used for SMC, remains highly effective in the areas of seasonal transmission, where its use is recommended, but resistance to SP is likely to spread, so alternative regimens will be needed. We have shown that DHAPQ is highly effective for SMC, and similar in efficacy to SPAQ, in an area where P. falciparum is still sensitive to SP and AQ. Both regimens had an efficacy over 70%. The duration of protection was similar with both regimens, with a high level of protection for about 4 weeks followed by a rapid decrease, highlighting the importance of strict timing in SMC programs to ensure that children receive treatment at monthly intervals. These results are consistent with those of previous studies in children (8, 9) and a study in adults in Thailand (11), which showed that DHAPQ was well tolerated and highly effective when used for monthly prophylaxis, and with a study in schoolchildren in Uganda, which showed that monthly DHPAQ was well tolerated and reduced malaria incidence by 96% and the prevalence of anemia by 40% (23). In another study in Uganda in younger children, the efficacy of monthly DHAPQ was only 58%, possibly due to poor adherence and underdosing (24). In our study, the efficacy of DHAPQ was related to the circulating concentration of piperaquine; there was a steady reduction in incidence of malaria with increasing day 7 concentration. This is consistent with results from two previous studies of recurrence of malaria after treatment, that of Price et al. (25), who found that patients with day 7 concentrations of piperaquine less than 30 ng/ml had an increased risk of recurrence of malaria, and that of Creek et al. (26), who found a similar figure (≤27.3 ng/ml). In our study, children whose concentrations were in the upper third had a substantially lower risk of malaria than children with concentrations in the lower third (rate ratio, 0.32), highlighting the importance of choosing dosing schemes carefully, balancing efficacy with tolerability, in order to maximize protection.
Both treatment regimens were well tolerated. As seen in other studies, the incidence of mild adverse events decreased in successive rounds of treatment. There was a slight drop in hemoglobin after treatment with DHAPQ that was not seen after SPAQ, but the prevalence of anemia at the end of the transmission season was similar in all groups. In a previous SMC study (8), anemia was more common in children who received DHAPQ than in those receiving other treatments, but other studies have not reported anemia associated with DHAPQ, although artemisinins may reduce reticulocyte count (27).
When isolates from the first incident malaria cases were typed, the triple dhfr mutation and the dhps g437 mutation were more frequent in children who received SPAQ than in the malaria cases in the DHAPQ group and in the control group, but the frequencies of pfcrt CVIET T76, pfmdr1 Y86, and pfmdr1 Y184, associated with resistance to AQ, were similar in all three groups. Analysis of molecular markers of resistance in a subset of samples from children in this study taken at enrollment and at the end of the transmission season have been reported separately (28). Among children who received SPAQ who had parasitemia at the end of the transmission season, the frequency of pfcrt 76T, pfdhfr 59R, and pfdhfr 108N was greater than at baseline. In children who received DHAPQ, there was no evidence of an increase in frequency of the markers that were investigated, but it is possible other factors may be involved in resistance to PQ (29).
SPAQ has the disadvantage that being a loose combination, tablets could be used separately. DHAPQ is a fixed-dose combination, but the rapid elimination of DHA means that parasites are exposed to PQ monotherapy. This is a concern, given the potential for selection of parasites with decreased sensitivity to PQ. However, selection for resistance to artemisinins, which is a growing concern (30), is less likely, as the combination ensures that parasites are exposed to DHA only when the concentration of PQ is high (31). However, there is a need for new long-acting antimalarial combinations to be developed for prevention. Monitoring of the emergence of resistance to these drugs where SMC is used will require continued clinical and molecular surveillance.
A limitation of this study was the use of a nonrandomized control group. As SMC with SPAQ had been shown to be highly effective at the time the trial was planned, it was considered unethical to randomize children to a placebo. An untreated control group outside the trial but living under circumstances similar to those of the study children was recruited. Adjusted analyses were used for comparisons to the untreated group, to control for potential confounding, but some residual confounding may have remained. A second limitation is that collection of data on the use of ITNs relied on caregivers' affirmation, which may not reflect real use.
This study has confirmed a continued high burden of malaria in Burkina Faso, with 338 episodes of malaria in 250 control children over 2 months. New malaria control tools are needed urgently, and the potential of SMC with SPAQ to reduce the burden in countries such as Burkina Faso needs to be fully realized by scaling up access to this intervention. In situations where SPAQ cannot be used, we have shown that DHAPQ appears to offer an effective alternative.
Supplementary Material
ACKNOWLEDGMENTS
We thank the patients and their parents or guardians, the clinical study teams at the health centers, and the DSMB members N. Alexander (chair), C. Merle, and H. Tamboura.
This study was funded by Holley Cotec Pharmaceutical Company Ltd., Beijing. The funder played no role in analysis and interpretation of study findings and did not participate in writing of the manuscript. The Wellcome Trust-Mahidol University-Oxford Tropical Medicine Research Programme is supported by the Wellcome Trust of Great Britain.
We have no conflicts of interest to declare.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.04923-14.
REFERENCES
- 1.WHO. 2011. World malaria report. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Ministère de la Santé, Burkina Faso. 2011. Annuaire statistique. Ministère de la Santé, Ougadougou, Burkina Faso. [Google Scholar]
- 3.Konate AT, Yaro JB, Ouedraogo AZ, Diarra A, Gansane A, Soulama I, Kangoye DT, Kabore Y, Ouedraogo E, Ouedraogo A, Tiono AB, Ouedraogo IN, Chandramohan D, Cousens S, Milligan PJ, Sirima SB, Greenwood B, Diallo DA. 2011. Intermittent preventive treatment of malaria provides substantial protection against malaria in children already protected by an insecticide-treated bednet in Burkina Faso: a randomised, double-blind, placebo-controlled trial. PLoS Med 8:e1000408. doi: 10.1371/journal.pmed.1000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. 2012. WHO policy recommendation: seasonal malaria chemoprevention (SMC) for Plasmodium falciparum malaria control in highly seasonal transmission areas of the Sahel sub-region in Africa. WHO, Geneva, Switzerland: http://www.who.int/malaria/publications/atoz/who_smc_policy_recommendation/en/index.html. [Google Scholar]
- 5.WHO. 2012. Seasonal malaria chemoprevention with sulfadoxine-pyrimethamine plus amodiaquine in children, a field guide. WHO, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/85726/1/9789241504737_eng.pdf. [Google Scholar]
- 6.Ahmed T, Sharma P, Gautam A, Varshney B, Kothari M, Ganguly S, Moehrle JJ, Paliwal J, Saha N, Batra V. 2008. Safety, tolerability, and single- and multiple-dose pharmacokinetics of piperaquine phosphate in healthy subjects. J Clin Pharmacol 48:166. doi: 10.1177/0091270007310384. [DOI] [PubMed] [Google Scholar]
- 7.Tarning J, Lindegardh N, Annerberg A, Singtoroj T, Day NP, Ashton M, White NJ. 2005. Pitfalls in estimating piperaquine elimination. Antimicrob Agents Chemother 49:5127–5128. doi: 10.1128/AAC.49.12.5127-5128.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cisse B, Cairns M, Faye E, NDiaye O, Faye B, Cames C, Cheng Y, NDiaye M, Lô AC, Simondon K, Trape J-F, Faye O, NDiaye JL, Gaye O, Greenwood B, Milligan P. 2009. Randomized trial of piperaquine with sulfadoxine-pyrimethamine or dihydroartemisinin for malaria intermittent preventive treatment in children. PLoS One 4:e7164. doi: 10.1371/journal.pone.0007164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bojang K, Akor F, Bittaye O, Conway D, Bottomley C, Milligan P, Greenwood B. 2010. A randomised trial to compare the safety, tolerability and efficacy of three drug combinations for intermittent preventive treatment in children. PLoS One 5:e11225. doi: 10.1371/journal.pone.0011225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nankabirwa J, Cundill B, Clarke S, Kabatereine N, Rosenthal PJ, Dorsey G, Brooker S, Staedke SG. 2010. Efficacy, safety, and tolerability of three regimens for prevention of malaria: a randomized, placebo-controlled trial in Ugandan schoolchildren. PLoS One 5:e13438. doi: 10.1371/journal.pone.0013438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lwin KM, Phyo AP, Tarning J, Hanpithakpong W, Ashley EA, Lee SJ, Cheah P, Singhasivanon P, White NJ, Lindegardh N, Nosten F. 2012. Randomized, double-blind, placebo-controlled trial of monthly versus bimonthly dihydroartemisinin-piperaquine chemoprevention in adults at high risk of malaria. Antimicrob Agents Chemother 56:1571–1577. doi: 10.1128/AAC.05877-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayxay M, Thongpraseuth V, Khanthavong M, Lindegardh N, Barends M, Keola S, Pongvongsa T, Phompida S, Phetsouvanh R, Stepniewska K, White NJ, Newton PN. 2010. A phase III, randomized, non-inferiority trial to assess the efficacy and safety of dihydroartemisinin-piperaquine in comparison with artesunate-mefloquine in patients with uncomplicated Plasmodium falciparum malaria in southern Laos. Am J Trop Med Hyg 83:1221–1229. doi: 10.4269/ajtmh.2010.10-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song J, Socheat D, Tan B, Seila S, Xu Y, Ou F, Sokunthea S, Sophorn L, Zhou C, Deng C, Wang Q, Li G. 2011. Randomized trials of artemisinin-piperaquine, dihydroartemisinin-piperaquine phosphate and artemether-lumefantrine for the treatment of multi-drug resistant falciparum malaria in Cambodia-Thailand border area. Malar J 10:231. doi: 10.1186/1475-2875-10-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zongo I, Dorsey G, Rouamba N, Dokomajilar C, Lankoande M, Ouedraogo JB, Rosenthal PJ. 2007. Randomized comparison of amodiaquine plus sulfadoxine-pyrimethamine, artemether-lumefantrine, and dihydroartemisinin-piperaquine for the treatment of uncomplicated Plasmodium falciparum malaria in Burkina Faso. Clin Infect Dis 45:1453–1461. doi: 10.1086/522985. [DOI] [PubMed] [Google Scholar]
- 15.Menan H, Faye O, Same-Ekobo A, Oga AS, Faye B, Kiki Barro CP, Kuete T, N′diaye JL, Vicky AM, Tine R, Yavo W, Kane D, Kassi KF, Kone M. 2011. Comparative study of the efficacy and tolerability of dihydroartemisinin-piperaquine-trimethoprim versus artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in Cameroon, Ivory Coast and Senegal. Malar J 10:185. doi: 10.1186/1475-2875-10-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nambozi M, Van Geertruyden J-P, Hachizovu S, Chaponda M, Mukwamataba D, Mulenga M, Ubben D, D'Alessandro U. 2011. Safety and efficacy of dihydroartemisinin-piperaquine versus artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in Zambian children. Malar J 10:50. doi: 10.1186/1475-2875-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tarning J, Zongo I, Somé FA, Rouamba N, Parikh S, Rosenthal PJ, Hanpithakpong W, Jongrak N, Day NP, White NJ, Nosten F, Ouedraogo JB, Lindegardh N. 2012. Population pharmacokinetics and pharmacodynamics of piperaquine in children with uncomplicated falciparum malaria. Clin Pharmacol Ther 91:497–505. doi: 10.1038/clpt.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salman S, Page-Sharp M, Batty KT, Kose K, Griffin S, Siba PM, Ilett KF, Mueller I, Davis TM. 2012. Pharmacokinetic comparison of two piperaquine-containing artemisinin combination therapies in Papua New Guinean children with uncomplicated malaria. Antimicrob Agents Chemother 56:3288–3297. doi: 10.1128/AAC.06232-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hung TY, Davis TM, Ilett KF, Karunajeewa H, Hewitt S, Denis MB, Lim C, Socheat D. 2004. Population pharmacokinetics of piperaquine in adults and children with uncomplicated falciparum or vivax malaria. Br J Clin Pharmacol 57:253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WorldWide Antimalarial Resistance Network (WWARN) DP Study Group. 2013. The effect of dosing regimens on the antimalarial efficacy of dihydroartemisinin-piperaquine: a pooled analysis of individual patient data. PLoS Med 10:e1001564. doi: 10.1371/journal.pmed.1001564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gadalla NB, Abdallah TM, Atwal S, Sutherland CJ, Adam I. 2013. Selection of pfdhfr/pfdhps alleles and declining artesunate/sulphadoxine-pyrimethamine efficacy against Plasmodium falciparum eight years after deployment in eastern Sudan. Malar J 12:255. doi: 10.1186/1475-2875-12-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gadalla NB, Adam I, Elzaki S-E, Bashir S, Mukhtar I, Oguike M, Gadalla A, Mansour F, Warhurst D, El-Sayed B, Sutherland CJ. 2011. Increased pfmdr1 copy number and sequence polymorphisms in Plasmodium falciparum isolates from Sudanese malaria patients treated with artemether lumefantrine. Antimicrob Agents Chemother 55:5408–5411. doi: 10.1128/AAC.05102-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nankabirwa JI, Wandera B, Amuge P, Kiwanuka N, Dorsey G, Rosenthal PJ, Brooker SJ, Staedke SG, Kamya MR. 2014. Impact of intermittent preventive treatment with dihydroartemisinin-piperaquine on malaria in ugandan schoolchildren: a randomized, placebo-controlled trial. Clin Infect Dis 58:1404–1412. doi: 10.1093/cid/ciu150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bigira V, Kapisi J, Clark TD, Kinara S, Mwangwa F, Muhindo MK, Osterbauer B, Aweeka FT, Huang L, Achan J, Havlir DV, Rosenthal PJ, Kamya MR, Dorsey G. 2014. Protective efficacy and safety of three antimalarial regimens for the prevention of malaria in young Ugandan children: a randomized controlled trial. PLoS Med 11:e1001689. doi: 10.1371/journal.pmed.1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Price RN, Hasugian AR, Ratcliff A, Siswantoro H, Purba HLE, Kenangalem E, Lindegardh N, Penttinen P, Laihad F, Ebsworth EP, Anstey NM, Tjitra E. 2007. Clinical and pharmacological determinants of the therapeutic response to dihydroartemisinin-piperaquine for drug-resistant malaria. Antimicrob Agents Chemother 51:4090–4097. doi: 10.1128/AAC.00486-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Creek DJ, Bigira V, McCormack S, Arinaitwe E, Wanzira H, Kakuru A, Tappero JW, Sandison TG, Lindegardh N, Nosten F, Aweeka FT, Parikh S. 2013. Pharmacokinetic predictors for recurrent malaria after dihydroartemisinin-piperaquine treatment of uncomplicated malaria in Ugandan infants. J Infect Dis 207:1646–1654. doi: 10.1093/infdis/jit078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO. 1998. The use of artemisinin & its derivatives as anti-malarial drugs. Report of a Joint CTD/DMP/TDR Informal Consultation Geneva, 10-12 June 1998. WHO, Geneva, Switzerland: http://www.who.int/malaria/publications/atoz/who_mal_98_1086/en/. [Google Scholar]
- 28.Somé AF, Zongo I, Compaoré Y-D, Sakandé S, Nosten F, Ouédraogo J-B, Rosenthal PJ. 2014. Selection of drug resistance-mediating Plasmodium falciparum genetic polymorphisms by seasonal malaria chemoprevention in Burkina Faso. Antimicrob Agents Chemother 58:3660–3665. doi: 10.1128/AAC.02406-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eastman RT, Dharia NV, Winzeler EA, Fidock DA. 2011. Piperaquine resistance is associated with a copy number variation on chromosome 5 in drug-pressured Plasmodium falciparum parasites. Antimicrob Agents Chemother 55:3908–3916. doi: 10.1128/AAC.01793-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tun KM, Imwong M, Lwin KM, Win AA, Hlaing TM, Hlaing T, Lin K, Kyaw MP, Plewes K, Faiz MA, Dhorda M, Cheah PY, Pukrittayakamee S, Ashley EA, Anderson TJC, Shalini Nair S, McDew-White M, Flegg JA, Grist EPM, Guerin P, Maude RJ, Smithuis F, Dondorp AM, Day NPJ, Nosten F, White NJ, Woodrow CJ. 2015. Spread of artemisinin-resistant Plasmodium falciparum in Myanmar: a cross-sectional survey of the K13 molecular marker. Lancet Infect Dis. 15:415–421. doi: 10.1016/S1473-3099(15)70032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White NJ, Olliari PL. 1996. Strategies for the prevention of antimalarial drug resistance: rationale for combination chemotherapy for malaria. Parasitol Today 12:399–401. doi: 10.1016/0169-4758(96)10055-7. [DOI] [PubMed] [Google Scholar]
- 32.Lambert PC, Royston P. 2009. Further development of exible parametric models for survival analysis. Stata J 9:265–290. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.