Abstract
GSK2140944 is a novel bacterial type II topoisomerase inhibitor with in vitro activity against key causative respiratory pathogens, including methicillin-resistant Staphylococcus aureus (MRSA). We described the pharmacodynamics of GSK2140944 against MRSA in the neutropenic murine lung infection model. MICs of GSK2140944 were determined by broth microdilution. Plasma and epithelial lining fluid (ELF) pharmacokinetics were evaluated to allow determination of pulmonary distribution. Six MRSA isolates were tested. GSK2140944 doses of 1.56 to 400 mg/kg of body weight every 6 h (q6h) were utilized. Efficacy as the change in log10 CFU at 24 h compared with 0 h controls and the area under the concentration-time curve for the free, unbound fraction of a drug (fAUC)/MIC required for various efficacy endpoints were determined. GSK2140944 MICs were 0.125 to 0.5 mg/liter against the six MRSA isolates. ELF penetration ratios ranged from 1.1 to 1.4. Observed maximal decreases were 1.1 to 3.1 log10 CFU in neutropenic mice. The mean fAUC/MIC ratios required for stasis and 1-log-unit decreases were 59.3 ± 34.6 and 148.4 ± 83.3, respectively. GSK2140944 displayed in vitro and in vivo activity against MRSA. The pharmacodynamic profile of GSK2140944, as determined, supports its further development as a potential treatment option for pulmonary infections, including those caused by MRSA.
INTRODUCTION
While a recent CDC surveillance study reports a decrease in health care-associated invasive methicillin-resistant Staphylococcus aureus (MRSA) infections in the United States from 2005 through 2011 (1), it remains a prevalent pathogen causing hospital-acquired (HAP) and ventilator-associated pneumonia (VAP) (2) and represents a major health threat with significant mortality rates (3, 4). Until lately, most cases of health care-associated pneumonia (HCAP), HAP, and VAP were thought to be caused by the hospital-acquired MRSA (HA-MRSA) strains, but a second variant of MRSA, community-acquired MRSA (CA-MRSA), has emerged in health care settings as a cause of severe pneumonia (2, 4). While the significance of this shift in epidemiology remains unknown (2), CA-MRSA has exhibited more rapid replication (5) and greater efficiency of transfer than HA-MRSA isolates (6). Additionally, virulence factors such as Panton-Valentine leukocidin (PVL), mostly associated with CA-MRSA strains, can cause severe necrotizing pneumonia even in young, otherwise healthy individuals (4).
To cope with the emergence of these hard-to-treat multidrug-resistant organisms, the development of new classes of antibacterial agents targeting type IIA topoisomerase was prompted in recent years (7). GSK2140944 is one such agent. GSK2140944 is a novel bacterial type II topoisomerase inhibitor with a mode of action distinguished from that of fluoroquinolones currently marketed or under development (8); instead of stabilizing the DNA double-strand breaks like fluoroquinolones, it stabilizes the precleavage topoisomerase-DNA complex prior to DNA cleavage and generates single-strand breaks (7, 9). The potent inhibition of supercoiling by DNA gyrase confers GSK2140944 in vitro activity against key causative respiratory pathogens, including MRSA (10).
In this study, we aimed to evaluate the pharmacokinetic and pharmacodynamic properties of GSK2140944 against diverse MRSA isolates using the murine lung infection model to guide its development as a potential treatment option for pulmonary infections caused by MRSA.
MATERIALS AND METHODS
Antimicrobial test agents.
GSK2140944 mesylate (powder of batch 2140944E-B-01P; GlaxoSmithKline, Collegeville, PA, USA) was used throughout these experiments. For in vitro studies, GSK2140944 was first dissolved in dimethyl sulfoxide (DMSO) and further diluted in sterile water followed by cation-adjusted Muller-Hinton broth to yield DMSO concentrations of ≤0.5%, consistent with Clinical and Laboratory Standards Institute (CLSI) guidelines (11). In in vivo studies, the GSK2140944 powder was weighed in a quantity required to achieve the desired concentration and dissolved in 0.9% sterile saline. GSK2140944 was administered by subcutaneous (s.c.) injections of 0.2 ml.
For in vitro MIC studies, levofloxacin analytical standard powder (Sigma-Aldrich, St. Louis, MO) was utilized, while commercially available levofloxacin for injection (Akorn, Inc., Lake Forest, IL; 25-mg/ml stock) was utilized for in vivo analyses. Levofloxacin was administered by s.c. injections of 0.2 ml. Linezolid was prepared from analytical standard powder (Sigma-Aldrich, St. Louis, MO) in both in vitro MIC studies and in vivo efficacy studies and was administered by s.c. injections of 0.3 ml.
Bacterial isolates.
S. aureus 508 (GSK WCUH29) and 509 (GSK PVL-2) were provided by GlaxoSmithKline (Collegeville, PA, USA), and four out of the six MRSA isolates were provided by the Center for Anti-Infective Research and Development (Hartford, CT, USA), which included two CA-MRSA isolates, two isolates producing PVL, and one vancomycin-resistant strain (Table 1).
TABLE 1.
In vitro activity of GSK2140944 and comparators against MRSA isolates utilized in the murine lung infection model
| S. aureus isolate | Characteristic(s)a | MIC (mg/liter) |
||
|---|---|---|---|---|
| GSK2140944 | Levofloxacin | Linezolid | ||
| 456 | VRSA | 0.125 | 32 | 1 |
| 508 | MRSA | 0.25 | 0.125 | 4 |
| F40-14 | MRSA | 0.25 | >32 | 1 |
| 146 | USA300, PVL(+), SCCmec IV, CA-MRSA | 0.5 | 8 | 2 |
| 156 | USA300, PVL(−), spa type 1, SCCmec IV, CA-MRSA | 0.5 | 8 | 4 |
| 509 | USA300, PVL(+), CA-MRSA | 0.5 | 0.125–0.25 | 2–4 |
VRSA, vancomycin-resistant S. aureus; MRSA, methicillin-resistant S. aureus; PVL(+), produces Panton-Valentine leukocidin; PVL(−), does not produce PVL; SCCmec IV, staphylococcal cassette chromosome mec type IV; CA-MRSA, community-acquired methicillin-resistant S. aureus.
MICs for GSK2140944, levofloxacin, and linezolid were determined in triplicates (three times using three replicate samples) by broth microdilution in accordance with Clinical and Laboratory Standards Institute guidelines (11). The modal MICs were reported. Isolates were stored in skim milk (BD Biosciences, Sparks, MD) at −80°C and were subcultured twice onto Trypticase soy agar with 5% sheep blood (BAP; BD Biosciences) within 48 h prior to use.
Neutropenic murine lung infection models.
Specific-pathogen-free, female BALB/c mice 7 to 8 weeks old were obtained from Harlan Sprague Dawley, Inc. (Indianapolis, IN). The protocol was reviewed and approved by the Institutional Animal Care and Use Committee at Hartford Hospital, and studies were conducted in accordance with GlaxoSmithKline policy on the care, welfare, and treatment of laboratory animals. Neutropenia was induced by intraperitoneal (i.p.) injections (0.2 ml in normal saline) of cyclophosphamide (Baxter Healthcare Corp., Deerfield, IL) at doses of 250 mg/kg of body weight 4 days prior to infection and 100 mg/kg 1 day prior to infection. Pneumonia was induced by instillation of 0.05 ml suspension in 3% hog gastric mucin in normal saline containing approximately 107 CFU/ml of the S. aureus isolate into the isoflurane-anesthetized animal's oral cavity while blocking the nares and holding the mouse in a vertical position until it aspirated the fluid inoculum (12); this took 2 to 4 inhalations, and the mouse was held in this position for 5 to 10 additional inhalations. After allowing the mice to fully recover from anesthesia in an oxygen-enriched chamber, they were randomized into treatment and control groups. For randomization, individual mice were arbitrarily selected from a box containing the group of inoculated animals such that each animal had an equal likelihood of receiving control or active therapies.
Pharmacokinetic studies. (i) Blood pharmacokinetic studies.
For neutropenic pharmacokinetic studies, at 3 h postinoculation (0 h), groups of 48 infected mice were administered GSK2140944 s.c. in single doses of 6.25, 50, or 200 mg/kg. Blood samples were collected from groups of six mice at 5 min and 0.25, 0.5, 1, 1.5, 2, 3, and 4 h postdose for 6.25- or 50-mg/kg doses and 5 min and 0.25, 0.5, 1, 1.5, 2, 4, and 6 h postdose for the 200-mg/kg dose via cardiac puncture; animals were euthanized by CO2 followed by cardiac puncture and ultimately cervical dislocation. All blood samples were collected in K2 EDTA Microtainer tubes (BD, Franklin Lakes, NJ, USA) and centrifuged to obtain plasma, which was stored at −80°C until analysis.
(ii) Pulmonary distribution.
Lavage fluid was collected from both neutropenic infected mice subsequent to terminal blood collection via bronchoalveolar lavage (BAL) at 0.5, 1, 2, and 3 h postdose for mice receiving 6.25 or 50 mg/kg GSK2140944 and at 0.5, 1, 2, and 4 h postdose for mice receiving 200 mg/kg GSK2140944. BAL fluid was performed by inserting a catheter in the trachea and instilling four aliquots of 0.4 ml normal saline followed by immediate removal of this fluid as previously described (13). BAL fluid samples were centrifuged to remove blood and cellular debris. Supernatants were stored at −80°C.
(iii) GSK2140944 concentration determination.
Plasma and BAL fluid concentrations of GSK2140944 were analyzed by GlaxoSmithKline (Collegeville, PA, USA) using a validated liquid chromatography-tandem mass spectrometry (LC/MS/MS) assay. A linear regression weighted by 1/concentration gave the best fit for the calibration curve over the concentration ranges of 10 to 100,000 ng/ml for plasma (coefficient of determination [r2] = 0.997) and 1 to 5,000 ng/ml for BAL fluid (r2 = 0.997). The accuracy of check samples (10 to 50,000 ng/ml for plasma and 5 ng/ml for BAL fluid) was within 8%.
(iv) Urea concentration analysis.
Aliquots of plasma and BAL fluid were taken for the determination of urea to derive drug levels in epithelial lining fluid (ELF) (14). Urea concentrations were analyzed using a colorimetric enzymatic assay (Teco Diagnostics, Anaheim, CA, USA) via a spectrophotometric detection method (Cary 50 Series; Varian, Walnut Creek, CA, USA). The assay was linear with an r2 of ≥0.999 for both BAL fluid and plasma urea concentrations over the range of 0.1 to 2.0 mg/dl. Quality control samples of 0.15 and 1.5 mg/dl had intraday and interday coefficients of variation of 1.68 and 0.81% and 7.3 and 3.7%, respectively.
In vivo efficacy.
A total of six MRSA isolates were tested in in vivo efficacy studies (Table 1). Treatment and control regimens were initiated 3 h postinoculation (0 h) with the test strain. GSK2140944 doses of 1.56 to 400 mg/kg were administered as 0.2-ml injections every 6 h (q6h) for 24 h. Two comparators were employed throughout the studies, levofloxacin and linezolid. A previously described levofloxacin dose of 10.6 mg/kg every 8 h (32 mg/kg/day) was utilized that simulated the area under the concentration-time curve for the free, unbound fraction of a drug (fAUC) profile of 500 mg once daily given in healthy volunteers (15, 16); the target fAUC of 42 to 53 mg · h/liter observed in healthy volunteers (15) was achieved in a mouse lung infection model with a resultant fAUC of 44 mg · h/liter (14) from a 32-mg/kg dose. A 5-mg/kg dose of uranyl nitrate was administered to levofloxacin-treated mice intraperitoneally 3 days prior to inoculation to predictably impair renal function of mice so that the elimination of levofloxacin in mice mimicked the human profile (15). Linezolid was administered to simulate a fAUC in ELF of 960 mg · h/liter, which was observed in the ELF after a 600-mg q12h dose (12, 17, 18) in healthy volunteers. A regimen of 240 mg/kg q12h was given to mimic this exposure (12, 18). Control animals received 0.2 ml of normal saline s.c. every 6 h for 24 h.
At 0 h, a group of untreated control mice (n = 6) was sacrificed to establish the initial bacterial burden. At 24 h postinitiation of dosing (24 h), all groups of control and treatment mice (n = 6 per each group) were euthanized via CO2 inhalation, followed by cervical dislocation. The lungs were then removed and homogenized for bacterial enumeration. Dilutions in normal saline of each lung homogenate were plated on Columbia CNA agar with 5% sheep blood (BD Biosciences, Sparks, MD) using spiral plating techniques (Spiral Biotech Auto plate 4000, model AP4000) and incubated overnight at 37°C.
Data analysis. (i) Pharmacokinetics.
Pharmacokinetic parameters for single doses of GSK2140944 were calculated using first-order input and elimination, by nonlinear least-squares techniques (Phoenix 32 WinNonlin version 6.3; Pharsight, Mountain View, CA). Compartment model selection was based on visual inspection of the profile and use of the correlation between the observed and calculated concentrations. The mean pharmacokinetic parameters derived from single-dose studies were used to construct concentration-time profiles for all multidose regimens evaluated in the in vivo efficacy. The AUC was calculated using the linear trapezoidal rule formula. Free, unbound drug concentrations in plasma were calculated using a murine protein binding value of 22.46% (data on file at GlaxoSmithKline).
(ii) Pulmonary distribution.
The concentration of GSK2140944 in ELF was determined using the urea dilution method:
where GSK2140944BAL is the concentration of GSK2140944 in the BAL fluid sample and UreaBAL and UreaPlasma are the concentrations of urea in the paired BAL fluid and plasma samples, respectively. The penetration of GSK2140944 into the ELF was estimated by the ratio of the AUC in ELF and fAUC in plasma calculated by the linear trapezoidal rule formula from observed data. For doses of 0.78, 6.25, and 50 mg/kg, area under the concentration-time curve from 0 to 3 h (AUC0–3) in both plasma and ELF was used, while AUC0–4 was used for 200 mg/kg as directed by the different sampling times for each regimen.
(iii) In vivo efficacy.
Antibacterial efficacy was calculated as the mean change in lung bacterial density in treated or control animals at 24 h compared with the average bacterial density at the initiation of dosing (0 h). The lung bacterial density was expressed as log10 CFU and reported as the mean for each treatment group ± standard deviation (SD).
(iv) Pharmacodynamic assessment.
fAUC/MIC was previously determined to be the pharmacodynamic parameter most closely associated with GSK2140944 activity (19); therefore, data analyses were performed only for this parameter. The relationship between fAUC/MIC and efficacy was determined using the sigmoidal Emax inhibitory model (Phoenix 32 WinNonlin version 6.3; Pharsight, Mountain View, CA, USA) for each isolate utilized in efficacy studies, in addition to a composite data set of all six strains evaluated. The exposure indices required for stasis, 1-log10-unit decrease, and 2-log10-unit decrease were calculated from each of these models.
RESULTS
Bacterial isolates.
The MICs of GSK2140944 against the six isolates studied ranged from 0.125 to 0.5 mg/liter (Table 1). S. aureus 456, F40-14, 146, and 156 were clinical isolates, while it is not known whether S. aureus 508 and 509 were clinical isolates. Four out of six strains were resistant to levofloxacin, whereas all six strains were susceptible to linezolid.
Pharmacokinetics.
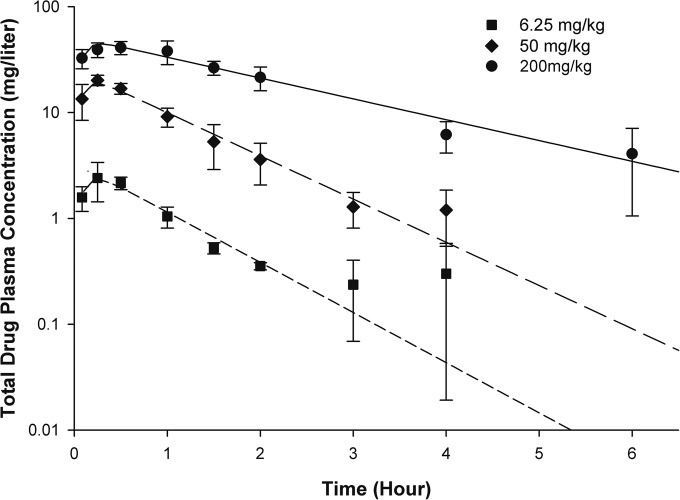
The pharmacokinetic profile of GSK2140944 was best described using a one-compartment model with first-order input and elimination. The pharmacokinetic parameters are shown in Table 2. GSK2140944 showed relative linearity across the dose range, allowing for extrapolation of all doses using mean pharmacokinetic parameters (Fig. 1).
TABLE 2.
Pharmacokinetics of GSK2140944 after a single subcutaneous dose in a murine (BALB/c) S. aureus lung infection modela
| Model | Dose (mg/kg) | V (liter/kg) | K01 (h−1) | K10 (h−1) | AUC0–∞ (mg · h/liter) | t1/2 (h) | Tmax (h) | Cmax (mg/liter) |
|---|---|---|---|---|---|---|---|---|
| I− | 6.25 | 2.055 | 10.000 | 1.091 | 2.788 | 0.636 | 0.249 | 2.319 |
| 50 | 2.130 | 11.338 | 0.940 | 24.965 | 0.737 | 0.239 | 18.739 | |
| 200 | 3.959 | 11.953 | 0.453 | 111.417 | 1.529 | 0.285 | 44.406 | |
| I+ | 0.78 | 3.172 | 10.156 | 0.515 | 0.478 | 1.345 | 0.309 | 0.210 |
| 6.25 | 2.262 | 4.500 | 0.688 | 4.018 | 1.008 | 0.493 | 1.969 |
I−, neutropenic; I+, immunocompetent; V, volume of distribution; K01, transfer rate constant into the central compartment; K10, transfer rate constant out of the central compartment; AUC0–∞, AUC from 0 h to infinity; t1/2, elimination half-life; Tmax, time to maximal concentration; Cmax, maximal concentration.
FIG 1.
Total drug plasma concentrations of GSK2140944 following single subcutaneous doses in a murine neutropenic S. aureus-infected lung model. Values are means ± standard deviations (error bars).
Pulmonary distribution.
GSK2140944 distributed well into the ELF of infected animals. The mean penetration of GSK2140944 into ELF was 1.27 ± 0.09 with the range of 1.14 to 1.37.
In vivo efficacy/pharmacodynamics.
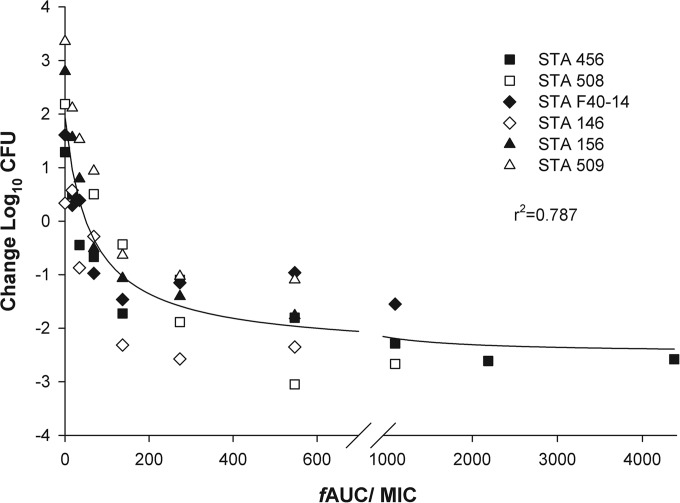
Initial lung bacterial density at 0 h averaged between 5.6 and 6.3 log10 CFU, which increased by 0.3 to 3.4 log10 CFU in untreated mice after 24 h. The fAUC/MIC values required to achieve various efficacy endpoints against six isolates are presented in Table 3. When calculated as a composite, fAUC/MIC required for stasis and 1 log10 CFU were 49 and 130 (r2 = 0.79), which are graphically displayed in Fig. 2. The activities of the comparators, levofloxacin and linezolid, were predictive given their phenotypic profiles; linezolid showed 0.8 to 2.6 log10 CFU decrease against all six isolates (MIC, 1 to 4 mg/liter), whereas levofloxacin showed 1.6 to 2.8 log10 CFU increase for the four isolates with a MIC of ≥8 mg/liter and 1.5 to 2.6 log10 CFU decrease for the two isolates with a MIC of ≤0.25 mg/liter.
TABLE 3.
Pharmacodynamic profile for GSK2140944 in a neutropenic murine S. aureus-infected lung modela
| Isolate or measure | MIC (mg/liter) |
fAUC/MIC |
Max Δ log CFU | ||
|---|---|---|---|---|---|
| r2 | Stasis | 1-Log-unit decrease | |||
| S. aureus isolates | |||||
| 456 | 0.125 | 0.95 | 24 | 107 | −2.87 |
| 508 | 0.25 | 0.98 | 99 | 162 | −3.14 |
| F40-14 | 0.25 | 0.92 | 31 | 104 | −1.37 |
| 146 | 0.5 | 0.89 | 45 | 87 | −2.48 |
| 156 | 0.5 | 1.00 | 53 | 121 | −1.82 |
| 509 | 0.5 | 0.98 | 105 | 310 | −1.60 |
| Statistical measures | |||||
| Mean | 59 | 148 | |||
| SD | 35 | 83 | |||
| Median | 49 | 114 | |||
r2, coefficient of determination; Max Δ log CFU, maximum change in the log CFU.
FIG 2.
Composite free drug pharmacodynamic profile (fAUC/MIC) of GSK2140944 versus six S. aureus (STA) isolates in the murine neutropenic lung infection model.
DISCUSSION
GSK2140944 is a novel bacterial type II topoisomerase inhibitor under development in both oral and intravenous formulations with in vitro activity against key causative pathogens of respiratory infections, including MRSA. We evaluated the pharmacokinetic and pharmacodynamic properties of GSK2140944 against diverse MRSA isolates using the neutropenic murine lung infection model. GSK2140944 was found to possess in vitro activity against a phenotypically and genotypically diverse group of MRSA isolates that translated into efficacy in vivo.
When selecting isolates for the purposes of early pharmacodynamic analyses, it is important to target organisms that are relevant to the likely distribution anticipated in clinical practice and in the range of expected in vitro activity for the compound under study. In a recent study of 201 clinical S. aureus isolates, GSK2140944 exhibited a rather narrow MIC distribution where 98% of organisms had MICs of 0.125 to 0.5 mg/liter with MIC50 and MIC90 values of 0.25 and 0.5 mg/liter, respectively (10). Moreover, another surveillance study of 1,008 clinical isolates of S. aureus collected in 2011 through 2012 reported the same MIC50 and MIC90 values (20) with 97% of isolates with MICs of ≤0.5 mg/liter. GSK2140944 was similarly active in vitro against the six strains studied herein, for which MIC values were between 0.125 and 0.5 mg/liter.
In dose fractionation studies using 2 to 512 mg/kg/day with one MRSA isolate and one Streptococcus pneumoniae isolate, Bulik et al. demonstrated that fAUC/MIC was closely correlated with the efficacy of GSK2140944 using a neutropenic murine thigh infection model (19). Moreover, while GSK2140944 is a novel bacterial topoisomerase inhibitor whose mode of action is distinguished from available fluoroquinolones, it shares the same target enzymes (i.e., DNA gyrase and topoisomerase IV) (7) and could reasonably be expected to follow traditional fluoroquinolone pharmacodynamics.
In our study, the fAUC/MIC target required for stasis and a 1 log10 CFU decrease varied across the six isolates tested; this variability is not uncommon in this model and emphasizes the reasoning for testing multiple strains. Similar degrees of variability were also noted in the aforementioned study by Bulik et al. which evaluated the pharmacodynamics of GSK2140944 against 2 methicillin-susceptible S. aureus (MSSA) isolates and 3 MRSA isolates (MIC = 0.5 to 2 mg/liter) with the doses ranging from 2 to 2,048 mg/kg/day in a neutropenic murine thigh infection model (19). In their study, fAUC/MIC targets for stasis and 1 log10 CFU decrease ranged from 3.97 to 35.5 and 12.6 to 103.2, respectively. When considering this in the context of identified median fAUC/MIC required for a 1-log-unit decrease, the median identified in our lung infection model was similar to the upper end of the range identified in the thigh model (i.e., 114 versus 103), but nearly double that of the median thigh value, 59. While the reason for this difference is unclear, certainly these data sets were derived from two distinctive groups of isolates using varied models of infection, and factors such as drug distribution to the site of infection and strain variability could be contributing. Of note, the Bulik et al. study also reported fAUC/MIC targets for a 2 log10 CFU decrease. Similar targets in our model were not calculated, as only three of six isolates achieved such a response. The inability to reach a 2 log10 CFU reduction has been encountered among other currently available bactericidal and bacteriostatic anti-MRSA antimicrobials in the neutropenic and immunocompetent in vivo lung infection model (18, 21–24).
It is clearly of importance to understand our identified targets in the context of clinical pharmacokinetic data. Tiffany et al. performed a pharmacokinetic study in 48 healthy volunteers with repeated doses of GSK2140944 from 400 to 1,500 mg infused over 2 or 3 h two or three times a day for 7 to 10 days (25). Repeated intravenous doses of 1,000 mg twice daily achieved an average steady-state AUC0–12 of 26.1 mg · h/liter with 24.0% coefficient of variation (CV). If not considering the variability among patients versus healthy volunteers studied, this value would translate to an average fAUC0–24 of 35.0 mg · h/liter after applying 33% protein binding of GSK2140944 in humans. Therefore, it could be inferred that at the MIC90 of GSK2140944 against S. aureus (i.e., 0.5 mg/liter), this dose would achieve a fAUC/MIC of 70.0, which falls in between our stasis and 1 log10 CFU targets from our neutropenic model.
Another important consideration is the relative pulmonary distribution of GSK2140944 in humans and mice. A study by Hossain et al. generated a population pharmacokinetic model using plasma and ELF concentrations derived from 22 healthy volunteers after giving a single dose of GSK2140944 (1,000 mg) intravenously over 2 h (26). Plasma samples were drawn at 0.5 h, 1 h, 2 h (at the end of infusion), 3 h, 4 h, 6 h, 8 h, and 12 h, while BAL fluid samples were drawn at 2 h, 4 h, 8 h, and 12 h. Their mean ELF penetration estimate was 1.88 (%CV, 17.9; 95% confidence interval, 1.22 to 2.54) compared with 1.27 identified in mice. While the presence of lung infection (12, 27–30) could alter pulmonary distribution, it is possible that the fAUC/MIC blood targets of GSK2140944 in patients may be lower than the targets identified from mice in our study. Namely, secondary to enhanced pulmonary distribution in humans, a similar fAUC in blood between humans and mouse could result in a greater ELF AUC in humans.
In conclusion, GSK2140944 demonstrated in vitro activity and in vivo efficacy against all six MRSA isolates tested, and these data provide a pharmacodynamic understanding of GSK2140944 that should help guide further development as a potential treatment option for pulmonary infections, including those caused by MRSA.
ACKNOWLEDGMENTS
We thank Mary Anne Banevicius, Henry Christensen, Jennifer Hull, Lucinda Lamb, Sara Robinson, Debora Santini, Shawn MacVane, Christina Sutherland, and Pamela Tessier for their assistance with the animal experimentation and in vitro testing.
This study was supported by GlaxoSmithKline (Collegeville, PA, USA) and has been funded in whole or in part with federal funds from the Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority, under OTA agreement no. HHSO100201300011C.
REFERENCES
- 1.Dantes R, Mu Y, Belflower R, Aragon D, Dumyati G, Harrison LH, Lessa FC, Lynfield R, Nadle J, Petit S, Ray SM, Schaffner W, Townes J, Fridkin S, Emerging Infections Program-Active Bacterial Core Surveillance MRSA Surveillance Investigators . 2013. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med 173:1970–1978. doi: 10.1001/jamainternmed.2013.10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubinstein E, Kollef MH, Nathwani D. 2008. Pneumonia caused by methicillin-resistant Staphylococcus aureus. Clin Infect Dis 46(Suppl 5):S378–S385. doi: 10.1086/533594. [DOI] [PubMed] [Google Scholar]
- 3.Hanberger H, Walther S, Leone M, Barie PS, Rello J, Lipman J, Marshall JC, Anzueto A, Sakr Y, Pickkers P, Felleiter P, Engoren M, Vincent JL, EPIC II Group of Investigators . 2011. Increased mortality associated with methicillin-resistant Staphylococcus aureus (MRSA) infection in the intensive care unit: results from the EPIC II study. Int J Antimicrob Agents 38:331–335. doi: 10.1016/j.ijantimicag.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Diederen BMW, Kluytmans JAJW. 2006. The emergence of infections with community-associated methicillin resistant Staphylococcus aureus. J Infect 52:157–168. doi: 10.1016/j.jinf.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Okuma K, Iwakawa K, Turnidge JD, Grubb WB, Bell JM, O'Brien FG, Coombs GW, Pearman JW, Tenover FC, Kapi M, Tiensasitorn C, Ito T, Hiramatsu K. 2002. Dissemination of new methicillin-resistant Staphylococcus aureus clones in the community. J Clin Microbiol 40:4289–4294. doi: 10.1128/JCM.40.11.4289-4294.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fey PD, Said-Salim B, Rupp ME, Hinrichs SH, Boxrud DJ, Davis CC, Kreiswirth BN, Schlievert PM. 2003. Comparative molecular analysis of community- or hospital-acquired methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 47:196–203. doi: 10.1128/AAC.47.1.196-203.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bax BD, Chan PF, Eggleston DS, Fosberry A, Gentry DR, Gorrec F, Giordano I, Hann MM, Hennessy A, Hibbs M, Huang J, Jones E, Jones J, Brown KK, Lewis CJ, May EW, Saunders MR, Singh O, Spitzfaden CE, Shen C, Shillings A, Theobald AJ, Wohlkonig A, Pearson ND, Gwynn MN. 2010. Type IIA topoisomerase inhibition by a new class of antibacterial agents. Nature 466:935–940. doi: 10.1038/nature09197. [DOI] [PubMed] [Google Scholar]
- 8.Singh SB. 2014. Confronting the challenges of discovery of novel antibacterial agents. Bioorg Med Chem Lett 24:3683–3689. doi: 10.1016/j.bmcl.2014.06.053. [DOI] [PubMed] [Google Scholar]
- 9.Smart DJ, Lynch AM. 2012. Evaluating the genotoxicity of topoisomerase-targeted antibiotics. Mutagenesis 27:359–365. doi: 10.1093/mutage/ger089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flamm RK, Rhomberg PR, Huynh HK, Farrell DJ, Sader HS, Scangarella-Oman NE, Jones RN. 2014. Activity of the novel bacterial topoisomerase II inhibitor, GSK2140944, against select Gram-positive and Gram-negative bacteria. Poster C-1421. Abstr 54th Intersci Conf Antimicrob Agents Chemother, Washington, DC. [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. 2011. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 8th ed CLSI publication M07-A8. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 12.Keel RA, Crandon JL, Nicolau DP. 2012. Pharmacokinetics and pulmonary disposition of tedizolid and linezolid in a murine pneumonia model under variable conditions. Antimicrob Agents Chemother 56:3420–3422. doi: 10.1128/AAC.06121-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laohavaleeson S, Tessier PR, Nicolau DP. 2008. Pharmacodynamic characterization of ceftobiprole in experimental pneumonia caused by phenotypically diverse Staphylococcus aureus strains. Antimicrob Agents Chemother 52:2389–2394. doi: 10.1128/AAC.01422-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Housman ST, Pope JS, Russomanno J, Salerno E, Shore E, Kuti JL, Nicolau DP. 2012. Pulmonary disposition of tedizolid following once daily oral 200 milligram tedizolid phosphate in healthy adult volunteers. Antimicrob Agents Chemother 56:2627–2634. doi: 10.1128/AAC.05354-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Onyeji CO, Bui KQ, Owens RC Jr, Nicolau DP, Quintiliani R, Nightingale CH. 1999. Comparative efficacies of levofloxacin and ciprofloxacin against Streptococcus pneumoniae in a mouse model of experimental septicaemia. Int J Antimicrob Agents 12:107–114. doi: 10.1016/S0924-8579(98)00087-9. [DOI] [PubMed] [Google Scholar]
- 16.Child J, Mortiboy D, Andrews JM, Chow AT, Wise R. 1995. Open-label crossover study to determine pharmacokinetics and penetration of two dose regimens of levofloxacin into inflammatory fluid. Antimicrob Agents Chemother 39:2749–2751. doi: 10.1128/AAC.39.12.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conte JE Jr, Golden JA, Kipps J, Zurlinden E. 2002. Intrapulmonary pharmacokinetics of linezolid. Antimicrob Agents Chemother 46:1475–1480. doi: 10.1128/AAC.46.5.1475-1480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tessier PR, Keel RA, Hagihara M, Crandon JL, Nicolau DP. 2012. Comparative in vivo efficacies of epithelial lining fluid exposures of tedizolid, linezolid, and vancomycin for methicillin-resistant Staphylococcus aureus in a mouse pneumonia model. Antimicrob Agents Chemother 56:2342–2346. doi: 10.1128/AAC.06427-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bulik CC, Okusanya OO, Bhavnani SM, Lepak A, Forrest A, Ambrose PG, Hoover JL, Andes DR. 2014. Evaluation of the pharmacokinetics-pharmacodynamics (PK-PD) of GSK2140944 against Staphylococcus aureus and Streptococcus pneumoniae in a murine thigh-infection model. Poster A-680. Abstr 54th Intersci Conf Antimicrob Agents Chemother, Washington, DC. [Google Scholar]
- 20.Bouchillon S, Hackel M, Miller LA, Scangarella-Oman NE. 2013. In vitro activity of GSK2140944, a novel topoisomerase inhibitor, against isolates associated with lower respiratory tract and skin infections. Poster F-1216. Abstr 53rd Intersci Conf Antimicrob Agents Chemother, Denver, CO. [Google Scholar]
- 21.Koomanachai P, Crandon JL, Banevicius MA, Peng L, Nicolau DP. 2009. Pharmacodynamic profile of tigecycline against methicillin-resistant Staphylococcus aureus in an experimental pneumonia model. Antimicrob Agents Chemother 53:5060–5063. doi: 10.1128/AAC.00985-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crandon JL, Kuti JL, Nicolau DP. 2010. Comparative efficacies of human simulated exposures of telavancin and vancomycin against methicillin-resistant Staphylococcus aureus with a range of vancomycin MICs in a murine pneumonia model. Antimicrob Agents Chemother 54:5115–5119. doi: 10.1128/AAC.00062-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhalodi AA, Crandon JL, Biek D, Nicolau DP. 2012. Efficacy of ceftaroline fosamil in a staphylococcal murine pneumonia model. Antimicrob Agents Chemother 56:6160–6165. doi: 10.1128/AAC.01078-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hegde SS, Reyes N, Skinner R, Difuntorum S. 2008. Efficacy of telavancin in a murine model of pneumonia induced by methicillin-susceptible Staphylococcus aureus. J Antimicrob Chemother 61:169–172. [DOI] [PubMed] [Google Scholar]
- 25.Tiffany CA, Hossain M, McDonald M, Lerman S, Dumont EF. 2014. Safety and pharmacokinetics of repeat escalating IV doses of GSK2140944, a novel bacterial topoisomerase inhibitor. Poster F-278. Abstr 54th Intersci Conf Antimicrob Agents Chemother, Washington, DC. [Google Scholar]
- 26.Hossain M, Dumont EF. 2014. Population pharmacokinetic modeling of plasma and epithelial lining fluid data for a novel antimicrobial compound. Poster A-681. Abstr 54th Intersci Conf Antimicrob Agents Chemother, Washington, DC. [Google Scholar]
- 27.Crandon JL, Kim A, Nicolau DP. 2009. Comparison of tigecycline penetration into the epithelial lining fluid of infected and uninfected murine lungs. J Antimicrob Chemother 64:837–839. doi: 10.1093/jac/dkp301. [DOI] [PubMed] [Google Scholar]
- 28.Drusano GL, Preston SL, Gotfried MH, Danziger LH, Rodvold KA. 2002. Levofloxacin penetration into epithelial lining fluid as determined by population pharmacokinetic modeling and Monte Carlo simulation. Antimicrob Agents Chemother 46:586–589. doi: 10.1128/AAC.46.2.586-589.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boselli E, Breilh D, Rimmelé T, Djabarouti S, Saux MC, Chassard D, Allaouchiche B. 2005. Pharmacokinetics and intrapulmonary diffusion of levofloxacin in critically ill patients with severe community-acquired pneumonia. Crit Care Med 33:104–109. doi: 10.1097/01.CCM.0000150265.42067.4C. [DOI] [PubMed] [Google Scholar]
- 30.Kuti JL, Nicolau DP. 2012. Levofloxacin penetration into epithelial lining fluid differs between infected and uninfected patients. Abstr A-1964 Abstr 52nd Intersci Conf Antimicrob Agents Chemother, San Francisco, CA. [Google Scholar]