Abstract
In this study, we investigated the in vitro antifungal activities, cytotoxicities, and membrane-disruptive actions of amphiphilic tobramycin (TOB) analogues. The antifungal activities were established by determination of MIC values and in time-kill studies. Cytotoxicity was evaluated in mammalian cell lines. The fungal membrane-disruptive action of these analogues was studied by using the membrane-impermeable dye propidium iodide. TOB analogues bearing a linear alkyl chain at their 6″-position in a thioether linkage exhibited chain length-dependent antifungal activities. Analogues with C12 and C14 chains showed promising antifungal activities against tested fungal strains, with MIC values ranging from 1.95 to 62.5 mg/liter and 1.95 to 7.8 mg/liter, respectively. However, C4, C6, and C8 TOB analogues and TOB itself exhibited little to no antifungal activity. Fifty percent inhibitory concentrations (IC50s) for the most potent TOB analogues (C12 and C14) against A549 and Beas 2B cells were 4- to 64-fold and 32- to 64-fold higher, respectively, than their antifungal MIC values against various fungi. Unlike conventional aminoglycoside antibiotics, TOB analogues with alkyl chain lengths of C12 and C14 appear to inhibit fungi by inducing apoptosis and disrupting the fungal membrane as a novel mechanism of action. Amphiphilic TOB analogues showed broad-spectrum antifungal activities with minimal mammalian cell cytotoxicity. This study provides novel lead compounds for the development of antifungal drugs.
INTRODUCTION
The frequency of invasive fungal infections, such as candidiasis, has dramatically increased due to the rising populations of immunocompromised patients as a result of AIDS and cancer therapy, as well as bone marrow and organ transplantations (1). Candida spp. are known to cause the majority of fungal infections and are the fourth most common cause of nosocomial blood infection in the United States (2). However, other fungal pathogens, such as Candida glabrata, Candida parapsilosis, Candida tropicalis, Candida lusitaniae, Crytococcus neoformans, and Aspergillus spp., are also on the rise and are causing threats to human and animal health (3). Currently, a number of antifungal drugs, such as azoles, echinocandins, and amphotericin B (AmB), are used to treat invasive fungal infections, including candidiasis, in humans. Growing fungal resistance and host side effects, however, limit their therapeutic efficacies. There is therefore a growing need to develop novel antifungal drugs.
Aminoglycosides (AGs) are compounds that consist of two or more amino sugars that are connected to a 2-deoxystreptamine scaffold via glycosidic bonds. AGs, such as tobramycin (TOB), are well-known antibiotics that are used to treat bacterial infections in humans, but they do not inhibit the growth of fungi. They bind to the prokaryotic 16S rRNA in the decoding A-site region, which induces codon misreading and inhibits translocation (4, 5). Although AGs are predominantly known for their antibacterial activities, some conventional AGs have been reported to have antifungal activities against fungal oomycetes and other true fungi (6). Despite being potent antibiotics, emerging bacterial resistance against AGs has compromised their therapeutic use. However, there has been a growing interest in structural modifications of AGs that could revive the efficacy of these drugs against resistant bacterial strains. We recently demonstrated that modifying TOB at the 6″-position by incorporating linear alkyl chains (C6 to C22) in a thioether linkage resulted in chain length-dependent antibacterial activities against vancomycin-resistant enterococci (VRE) and TOB-resistant Escherichia coli, with resistance to the parent drug TOB itself (7). However, the antifungal activities of these analogues are yet to be determined. It was recently reported that the incorporation of a C8 linear alkyl chain at the O-4″-position and of an octanesulfonyl chain at the O-6′-position of kanamycins A and B, respectively, led to the discovery of new applications of these AGs as antifungal agents that lack bacterial activity (8–11). This prompted us to investigate the antifungal properties of our 6″-thioether TOB analogues with linear alkyl chains with chain lengths between C4 and C14 (here, we are referring to these as C4, C6, C8, C10, C12, and C14) (Fig. 1) and to further investigate the extent of the antibacterial spectra of these analogues against resistant clinical bacterial isolates that we had not previously tested. We report the antibacterial and antifungal activities as well as the cytotoxicities of our C4 to C14 TOB analogues.
FIG 1.
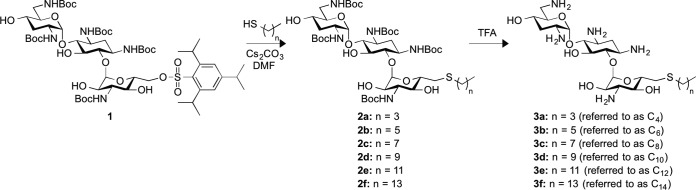
Scheme for the synthesis of the 6″-thioether TOB analogues C4 to C14 used in this study.
MATERIALS AND METHODS
Materials.
TOB was purchased from AK Scientific (Union City, CA). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO) and used without further purification. Compound 1 (Fig. 1) was prepared as previously described (7). Chemical reactions were monitored via thin-layer chromatography (TLC; with silica gel 60, F250; Merck). Visualization was achieved by using one of the following methods: H2SO4 stain (5% in methanol) or KMnO4 stain (1.5 g KMnO4, 10 g K2CO3, 1.25 ml 10% NaOH, 200 ml H2O). Compounds were purified by SiO2 flash chromatography (Flash silica gel, 32 to 63 μm; Dynamic Adsorbents Inc.). 1H and 13C nuclear magnetic resonance (NMR) spectra were recorded on a Varian 400-MHz spectrometer. TOB analogues (C4 to C14) were dissolved in double-distilled water (ddH2O) at a final concentration of 10 g/liter.
The Eis (12), AAC(6′)-Ib′ (13), AAC(6′)-Ie/APH(2″)-Ia [used only for its AAC(6′) activity] (14), AAC(3)-IV (14), AAC(2′)-Ic (12), and ANT(4′) (15) resistance enzymes were purified as previously described. 5,5′-Dithiobis(2-nitrobenzoic acid) (DTNB), ATP, acetyl coenzyme A, and inorganic pyrophosphatase were bought from Sigma-Aldrich and used without further purification. The determinations of the activities of these resistance enzymes against C4 were performed as previously reported for a group of C6 to C14 antimicrobial agents (Fig. 2) (7). 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide was purchased from TCI America (Portland, OR, USA). Spectrophotometric and colorimetric assays were performed on a multimode SpectraMax M5 plate reader (Molecular Devices, Sunnyvale, CA) using 96-well plates (Fisher Scientific).
FIG 2.
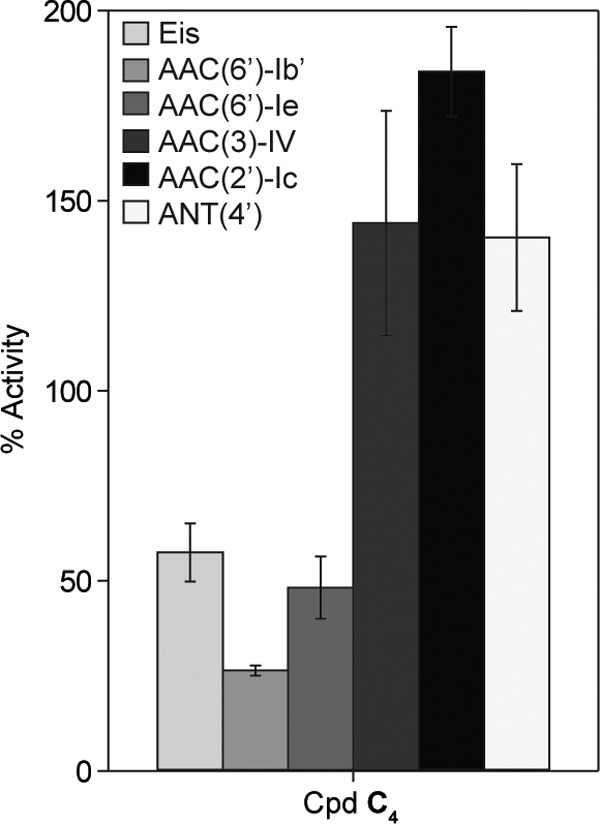
Bar graph showing the relative activities of the listed AMEs against C4, normalized to that of TOB (100%).
The antifungal agents posaconazole (POS), itraconazole (ITC), and fluconazole (FLC) were obtained from AK Scientific, Inc. (Union City, CA). POS, ITC, and FLC were dissolved in dimethyl sulfoxide (DMSO) at a final concentration of 5 g/liter.
The yeast strains Candida albicans ATCC 10231 (designated strain A here), C. albicans ATCC 64124 (B), and C. albicans ATCC MYA-2876 (C) were kindly provided by Jon Y. Takemoto (Utah State University, Logan, UT, USA). The yeast strains C. albicans ATCC MYA-90819 (D), C. albicans ATCC MYA-2310 (E), C. albicans ATCC MYA-1237 (F), C. albicans ATCC MYA-1003 (G), and Cryptococcus neoformans MYA-895 (H) were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). The filamentous fungal strains Aspergillus nidulans ATCC 38163 (I) and Fusarium graminearum 053 (J) were kindly provided by Jon S. Thorson and Lisa J. Vaillancourt (University of Kentucky, Lexington, KY, USA), respectively. Filamentous fungi and yeasts were cultivated at 35°C (except for F. graminearum 053 [J], which was grown at room temperature [RT]) in RPMI 1640 (catalog number R6504; Sigma-Aldrich Chemical Co., St. Louis, MO) buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid (MOPS) buffer (Sigma-Aldrich Chemical Co.).
The bacterial strains used in this study were obtained from various sources. Mycobacterium parascrofulaceum ATCC BAA-614 (D+) and Haemophilus influenzae ATCC 51907 (F−) were purchased from ATCC. Methicillin-resistant Staphylococcus aureus (MRSA; C+) and S. aureus NorA (F+) were a gift from David H. Sherman (University of Michigan, Ann Arbor, MI, USA). Mycobacterium smegmatis MC2 155 (E+) was a gift from Sabine Ehrt (Weill Cornell Medical College, New York, NY, USA). Enterococcus faecium BM4105-RF (A+), Listeria monocytogenes ATCC 19115 (B+), S. aureus NRS22 (G+), E. coli MC1061 (E−), Klebsiella pneumoniae ATCC 27736 (G−), and Pseudomonas aeruginosa PAO1 (I−) were gifts from Paul J. Hergenrother (University of Illinois at Urbana-Champaign, Champaign, IL, USA). Staphylococcus epidermidis ATCC 12228 (H+), Streptococcus pyogenes ATCC 12384 (I+), Escherichia coli ATCC 25922 (A−), and P. aeruginosa ATCC 27853 (H−) were generously provided by Dev P. Arya (Clemson University, Clemson, SC, USA). E. coli Δ7 wild type (wt; B−), E. coli Δ7 A1408G (C−), and E. coli Δ7 G1491U (D−) were gifts from Kurt Frederick (Ohio State University, Columbus, OH, USA). Shigella flexneri 2475 pgm-24 (J−) was obtained from Anthony T. Maurelli (Uniformed Services University of the Health Sciences, Bethesda, MD, USA).
The human lung carcinoma epithelial cell line A549 (ATCC CCL-185) and the normal human bronchial epithelial cell line Beas 2B (ATCC CRL-9609) were kindly provided by David K. Orren (University of Kentucky, Lexington, KY, USA).
Protocol for formation of the Boc-protected thioether TOB analogues.
The amphiphilic tert-butyloxycarbonyl (Boc)-protected TOB analogues C6 to C14 (compounds 2b to 2f) were synthesized from TOB as previously described (Fig. 1) (7). For the preparation of the novel analogue 2a (Boc-protected C4), 1-butanethiol (0.13 ml, 1.22 mmol) was added to a solution of analogue 1 (0.30 g, 0.24 mmol) and Cs2CO3 (0.12 g, 0.36 mmol) in freshly distilled dimethylformamide (3 ml). The mixture was stirred at RT overnight. Progress of the reaction was monitored by TLC (hexanes:ethyl acetate [EtOAc] ratio, 2:3; Rf, 0.60). Upon completion, the reaction mixture was diluted with EtOAc (10 ml) and washed with H2O (5 ml). The aqueous layer was extracted with EtOAc (twice, 10 ml). The combined organic layers were washed again with H2O (twice, 10 ml) and brine (10 ml), dried over anhydrous MgSO4, and filtered. The solvents were removed under reduced pressure, and the crude product obtained was purified by flash column chromatography (SiO2; pure hexanes to hexanes:EtOAc in a 2:3 ratio) to afford compound 2a (0.19 g; 76%) as a white solid: 1H NMR (400 MHz, CD3OD) δ 5.07 (br s, 1H, H-1′), 5.02 (br s, 1H, H-1″), 4.05 (m, 1H, H-5″), 3.70 to 3.28 (m, 13H, H-1, H-3, H-4, H-5, H-6, H-2′, H-4′, H-5′, H-6′ (2H), H-2″, H-3″, H-4″), 2.97 (br dd, J1 = 14.4 Hz, J2 = 2.2 Hz, 1H, H-6″), 2.62 (m, 1H, H-6″), 2.58 [t, J = 7.6 Hz, 2H, SCH2(CH2)2CH3], 2.12 (m, 1H, H-2eq), 1.99 (m, 1H, H-3′eq), 1.66 to 1.24 [m, 51H, H-2ax, H-3′ax, 5 × CO2(CH3)3, SCH2(CH2)2CH3], 0.90 [t, J = 7.6 Hz, 3H, SCH2(CH2)2CH3]; 13C NMR (100 MHz, CD3OD) δ 158.0, 157.9, 156.5, 156.4 (2 carbons), 98.6 (anomeric carbon), 98.1 (anomeric carbon), 82.9, 81.1, 79.3, 79.0 (2 carbons), 78.9, 78.7, 75.8, 72.5 (2 carbons), 72.1, 70.7, 65.0, 55.7, 50.0, 49.8, 49.6, 40.5, 34.4, 33.3, 32.9, 32.4, 31.6, 27.4 to 27.3 (15 carbons), 21.6, 12.7.
Protocol for Boc deprotection [e.g., synthesis of compound 3a (C4)].
The amphiphilic TOB analogues C6 to C14 (compounds 3b to f) were synthesized from 2b to f as previously described (Fig. 1) (7). For the preparation of the novel analogue 3a (C4), compound 2a (46 mg, 0.044 mmol) was treated at RT with neat trifluoroacetic acid (TFA; 1 ml) for 3 min. The TFA was removed under reduced pressure, and the residue was dissolved in a minimal volume of H2O and freeze-dried to afford the novel 6″-thioether TOB derivative 3a (C4; 49 mg, 98%) as a white foam: 1H NMR (400 MHz, D2O) δ 5.74 (d, J = 3.6 Hz, 1H, H-1′), 5.10 (d, J = 4.0 Hz, 1H, H-1″), 4.05 to 3.93 (m, 4H, H-4, H-5′, H-2″, H-5″), 3.89 (app. t, J1 = J2 = 9.2 Hz, 1H, H-5), 3.82 to 3.54 (m, 6H, H-1, H-3, H-6, H-2′, H-4′, H-4″), 3.46 (app. t, J1 = J2 = 10.4 Hz, 1H, H-3″), 3.43 (dd, J1 = 13.6 Hz, J2 = 3.6 Hz, 1H, H-6′), 3.28 (dd, J1 = 13.6 Hz, J2 = 6.4 Hz, 1H, H-6′), 3.05 (dd, J1 = 14.4 Hz, J2 = 2.4 Hz, 1H, H-6″), 2.78 (dd, J1 = 14.4 Hz, J2 = 8.0 Hz, 1H, H-6″), 2.63 [t, J = 7.6 Hz, 2H, SCH2(CH2)2CH3], 2.57 (app. dt, J1 = 12.4 Hz, J2 = J3 = 4.4 Hz, 1H, H-2eq), 2.57 (app. dt, J1 = 12.0 Hz, J2 = J3 = 4.4 Hz, 1H, H-3′eq), 2.03 (app. q, J1 = J2 = J3 = 12.4 Hz, 1H, H-2ax), 1.95 (app. q, J1 = J2 = J3 = 12.8 Hz, 1H, H-3′ax), 1.58 (p, J = 7.6 Hz, 2H, SCH2CH2CH2CH3), 1.39 (sextet, J = 7.6 Hz, 2H, SCH2CH2CH2CH3), 0.89 [t, J = 7.6 Hz, 3H, SCH2(CH2)2CH3]; 13C NMR (100 MHz, D2O) δ 100.5 (anomeric carbon), 94.5 (anomeric carbon), 83.8, 77.6, 74.1, 72.5, 70.2, 68.0, 67.8, 64.2, 54.5, 49.3, 48.1, 47.8, 39.7, 32.2, 32.0, 30.9, 29.2, 27.7, 21.1, 12.7. Note that compounds 3a to f were stored at −20°C as a 10-g/liter stock solution in ddH2O.
Antibacterial susceptibility testing.
MIC values of TOB analogues (C4 to C14) were determined by using the microdilution broth method for aerobic and anaerobic organisms according to CLSI standardized methodology (16, 17) with minor modifications. A variety of Gram-positive and Gram-negative bacteria were diluted 1:1,000 from overnight cultures into fresh medium and grown to an optical density of ∼0.4 at 600 nm, or on average, for 4 h. To prepare bacteria for MIC determinations, an additional 1:1,000 dilution of turbid culture was performed. Briefly, solutions of the studied compounds were added to the optimal medium for each bacterial strain (i.e., LB for MRSA [B+], S. aureus NorA [F+], S. epidermidis ATCC 12228 [H+], S. pyogenes ATCC 12384 [I+], E. coli Δ7 wt [B−], E. coli Δ7 A1408G [C−], E. coli Δ7 G1491U [D−], E. coli MC1061 [E−], K. pneumoniae ATCC 27736 [G−], and S. flexniri 2475T pgm-24 [J−]; brain heart infusion (BHI) for E. faecium BM4105-RF [A+], L. monocytogenes ATCC 19115 [B+], and S. aureus NRE22 USA600 [G+]; supplemented BHI for H. influenzae ATCC 51907 [F−]; tryptic soy medium for E. coli ATCC 25922 [A−], P. aeruginosa ATCC 27583 [H−], and P. aeruginosa PAO1 [I−]; 7H9 for M. smegmatis MC2 155 [E+]; 7H9 with ADC for M. parascrofulaceum ATCC BAA-614 [D+]), and a double dilution was performed (100 μl) in a microtiter plate. The diluted bacterial cultures were then added to these medium-drug mixtures (100 μl). The microtiter plate cultures were grown for 16 to 20 h and visually inspected for growth. MIC values were defined as the concentration of the drug in the last well showing no bacterial growth.
Antifungal susceptibility testing.
In vitro MIC values for the C4 to C14 TOB analogues against yeast cells were evaluated in 96-well plates as described in CLSI document M27-A3 (18) with minor modifications. Yeast cells were grown in RPMI 1640 for 48 h, counted using a hemocytometer, and diluted to a concentration of 5 × 104 cells/ml in fresh RPMI 1640. Cell suspensions (200 μl) containing 0.97 to 125 mg/liter of TOB analogue, 0.97 to 62.5 mg/liter of POS, 0.97 to 62.5 mg/liter of ITC, or 0.97 to 125 mg/liter of FLC were added to the wells of a 96-well microtiter plate and incubated for 48 h at 35°C. The final concentration of DMSO was ensured to be <2% in all experiments. Growth of C. albicans 10231 (strain A) was not affected by this concentration of DMSO. The MIC values of TOB analogues were defined as the minimum drug concentration that yielded complete inhibition, or the MIC-0. MIC values of azoles for yeasts were determined as the lowest drug concentration that produced at least 50% growth inhibition (i.e., MIC-2) compared with the growth control well. It is important to note that at the lowest concentration tested, the growth of C. neoformans MYA-895 (H) was completely inhibited. For testing azoles against molds, on the other hand, the MIC-0 endpoint was used.
The minimal fungicidal concentration (MFC) values for the C12 and C14 TOB analogues were determined as previously described (19, 20) with minor modifications. To determine MFCs, yeast cells of 1 × 104 CFU/ml were used to perform broth microdilution assays as described above. After 48 h of incubation, all of the MIC wells with no visible growth were homogenized with a micropipette, and aliquots of 20 μl cell content were spread on Sabouraud's dextrose agar (SDA; Difco, BD, Franklin Lakes, NJ, USA). The plates were incubated for 24 to 48 h at 35°C for colony counts. The MFC was defined as the lowest drug concentration that killed 99% of the final inoculum (with ≤3 colonies on SDA plates, in agreement with a previous report [21]). Each test was performed in triplicate.
In vitro MIC values for these analogues against A. nidulans ATCC 38163 (I) and F. graminearum 053 (J) were conducted as previously described in CLSI document M38-A2 (22). Spores were collected from sporulating cultures growing in potato dextrose agar (PDA) by filtration through sterile glass wool and enumerated using a hemocytometer to obtain the desired inoculum size. Serial dilutions of TOB analogues as well as POS, ITC, and FLC were made in sterile 96-well plates in the ranges of 0.97 to 125 mg/liter (for TOB analogues), 0.097 to 12.5 mg/liter (for POS and ITC), and 0.97 to 62.5 mg/liter (for FLC) using RPMI, and spore suspensions were added to make a final concentration of 5 × 105 CFU/ml. The plates were incubated at 35°C for 48 h (except for F. graminearum 053 [J], which was incubated at room temperature). The MIC values of azoles and TOB analogues against molds were based on the complete inhibition of growth compared to the growth control, or the MIC-0 (18, 22). Each test was performed in triplicate.
Antifungal carryover and time-kill studies.
Prior to performing time-kill studies, antifungal carryover effects were evaluated as previously described (19). C. albicans ATCC 64124 (B) cell suspensions were prepared to achieve an inoculum of approximately 1 × 105 to 4 × 105 CFU/ml. An aliquot of 100 μl of each suspension was added to 900 μl of sterile ddH2O (control) or to sterile ddH2O plus TOB analogue (C12 or C14) at concentrations of 8, 16, or 32 mg/liter and 2, 4, or 8 mg/liter, respectively. Immediately after addition of the fungal suspension, tubes were vortexed and 100 μl of suspension was removed and spread on PDA for colony count determinations. Antifungal carryover was defined as a reduction in colony counts of a sample by >25% compared to controls. Time-kill curve studies were performed against the azole-resistant C. albicans strain ATCC 64124 (B) as previously described (23) with modifications. The initial inoculum used for this assay was 1 × 105 CFU/ml in liquid RPMI 1640 medium at 35°C in 50 ml Falcon tubes (10-ml volume) with continuous agitation (200 rpm). TOB analogues (C12 and C14) at concentrations of 8, 16, or 32 mg/liter and 2, 4, or 8 mg/liter, respectively, were tested against C. albicans ATCC 64124 (B). Test solutions were incubated at 200 rpm at 35°C. At 0, 3, 9, 12, and 24 h, 100-μl aliquots were removed from each solution and serially diluted in sterile ddH2O. Fifty microliters of each dilution was spread onto a potato dextrose agar plate and incubated at 35°C. FLC (16 mg/liter) and AmB (1 mg/liter) were used as controls in this assay. Colony counts were determined after 48 h of incubation. The experiment was performed in triplicate. The lower limit for accurate and reproducible quantification was 50 CFU/ml (23).
In vitro cytotoxicity assay.
The in vitro cytotoxicity assay was performed as previously described (24) with minor modifications. Human lung carcinoma epithelial A549 cells and normal human bronchial epithelial cells Beas 2B were grown in F-12K and Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% antibiotics, respectively. The confluent cells were then trypsinized with 0.05% trypsin–0.53 mM EDTA and resuspended in fresh medium (F-12K or DMEM). The cells were transferred into 96-well microtiter plates at a density of 3,000 cells/well and were grown overnight. The following day, the media were replaced with 100 μl of fresh culture medium containing serially diluted TOB analogues at final concentrations of 0.97 to 125 mg/liter or sterile ddH2O (negative control). The cells were incubated for an additional 24 h at 37°C with 5% CO2 in a humidified incubator. To evaluate cell survival, each well was treated with 10 μl (25 mg/liter) of resazurin sodium salt (Sigma-Aldrich) for 3 h. Metabolically active cells can convert the blue nonfluorescent dye resazurin to the pink and highly fluorescent dye resorufin, which can be detected at wavelengths of 560 nm (excitation) and 590 nm (emission) by using a SpectraMax M5 plate reader. Triton X-100 (1%, vol/vol) resulted in complete loss of cell viability and was used as the positive control. The percent cell survival was calculated as follows: [(control value – test value) × 100]/(control value), where the control value represents results for cells plus resazurin but without drug and the test value represents cells plus resazurin plus the drug.
Propidium iodide staining.
A single colony of C. albicans ATCC 64124 (B) was used to inoculate 5 ml of potato dextrose broth (PDB) in a Falcon tube and was grown overnight at 35°C at 200 rpm. The overnight culture was further diluted by transferring 200 μl of the cell suspension to 800 μl of RPMI 1640 medium. Fifty microliters of cell suspension was then added to the RPMI medium containing no drug (negative control) or C12 or C14 TOB analogues at concentrations of 15.6 (0.5× MIC), 31.2 (1× MIC), and 62.5 mg/liter (2× MIC), or 3.9 (0.5× MIC), 7.8 (1× MIC), and 15.6 (2× MIC), respectively. TOB (125 mg/liter) and POS (62.5 mg/liter) were used as controls. The cell suspensions were then treated for 1 h at 35°C with continuous agitation (200 rpm). After incubation, treated cells were centrifuged and resuspended in 500 μl of phosphate-buffered saline (PBS; pH 7.2). Subsequently, cells were treated with propidium iodide (PI; 9 μM, final concentration) and incubated for 5 min at room temperature in the dark (25). Glass slides prepared with 15 μl of each mixture were observed in bright-field and fluorescence modes (using a Texas Red filter set, with excitation and emission wavelengths of 535 and 617 nm, respectively) using a Zeiss Axiovert 200M fluorescence microscope. Data were obtained from at least two independent experiments.
Annexin V and PI staining.
We chose one of the potent TOB analogues, C14, to perform the assay with Annexin V and PI staining. C. albicans ATCC 64124 (B) was treated with C14 at a subinhibitory concentration (4 mg/liter) for 1 h. Cells were washed in PBS and digested at 30°C for 30 min in 0.1 M potassium phosphate buffer that contained 150 mg/liter Zymolyase 20T, 40 mM 2-mercaptoethanol, and 2.4 M sorbitol at pH 7.2 (26). Protoplasts were washed in modified Annexin binding buffer (10 mM HEPES-NaOH [pH 7.4], 40 mM NaCl, 50 mM CaCl2, and 1.2 M sorbitol), resuspended in the same buffer along with 5 μl of fluorescein isothiocyante (FITC)-Annexin V, 2 μl of PI (20 mg/liter), and RNase (250 mg/liter), and incubated at 37°C for 30 min. Cells were analyzed in bright-field and fluorescence modes (using Texas Red and FITC filter sets) using a Zeiss Axiovert 200M fluorescence microscope.
RESULTS
In vitro antibacterial assay.
The new C4 compound along with the C6 to C14 compounds previously evaluated (7) were tested for antibacterial activity (MIC value determinations) against several strains of Gram-positive and Gram-negative bacteria (Table 1). The strains tested ranged from completely resistant to TOB (>150 mg/liter, strains E. faecium BM4105-RF [A+], MRSA [C+], S. aureus NRS22 USA600 [G+], E. coli Δ7 A1408G [C−], E. coli Δ7 G1491U [D−], and P. aeruginosa ATCC 27853 [H−]) to very susceptible to TOB (<0.3 mg/liter; strain M. smegmatis MC2 155 [E+]). The C4 analogue showed excellent antibacterial activity (2.3 to 4.7 mg/liter) against four Gram-positive strains (L. monocytogenes ATCC 19115 [B+], M. smegmatis MC2 155 [E+], S. aureus NorA [F+], and S. epidermidis ATCC 12228 [H+]) and one Gram-negative strain (H. influenzae ATCC 51907 [F−]), and there were only 10 strains (E. faecium BM4105-RF [A+], MRSA [C+], M. parascrofulaceum ATCC BAA-614 [D+], S. aureus NRS22 USA600 [G+], E. coli ATCC 25922 [A−], E. coli Δ7 A1408G [C−], E. coli Δ7 G1491U [D−], K. pneumoniae ATCC 27736 [G−], P. aeruginosa ATCC 27853 [H−], and P. aeruginosa PAO1 [I−]) against which it was ineffective (MIC, 75 to >150 mg/liter). For the C6 analogue, MIC values ranged from >150 mg/liter to 18.8 mg/liter, and for the C8 compound the values ranged from >150 mg/liter to 37.5 mg/liter against all Gram-positive and Gram-negative strains tested. Compounds with the C10, C12, or C14 modification had none to very good activity, with MIC values ranging from >150 mg/liter to 2.3 mg/liter (C10), >150 mg/liter to 0.6 mg/liter (C12), and >150 mg/liter to <0.3 mg/liter (C14). On a broader scale, all compounds displayed better MIC values against Gram-positive strains than against Gram-negative strains.
TABLE 1.
MICs for TOB and its analogues
| Bacterial straina | MIC (mg/liter) for compoundb |
||||||
|---|---|---|---|---|---|---|---|
| C4 | C6 | C8 | C10 | C12 | C14 | TOB | |
| Gram-positive strains | |||||||
| A+ | >150 | >150 | >150 | 75 | 9.4 | 4.7 | >150 |
| B+ | 2.3 | 37.5 (75) | 75 (>150) | 18.8 (37.5) | 4.7 (18.8) | 2.3 (9.4) | 1.2 (4.7)a |
| C+ | >150 | >150 | >150 | >150 | 18.8 | 2.3 | >150 |
| D+ | 75 | 150 | 150 | 37.5 | 75 | 9.4 | 1.2 |
| E+ | 2.3 | 18.8 | >150 | >150 | >150 | >150 | <0.3 |
| F+ | 4.7 | 150 (>150) | >150 (150) | 18.8 (37.5) | 4.7 (9.4) | 0.6 (9.4) | 1.2 (9.4) |
| G+ | >150 | >150 | 150 | 9.4 | 2.3 | 1.2 | >150 |
| H+ | 2.3 | 75 (75) | 150 (75) | 9.4 (9.4) | 1.2 (2.3) | 0.6 (1.2) | <0.3 (0.3) |
| I+ | 37.5 | 150 | 37.5 | 2.3 | 0.6 | <0.3 | 9.4 |
| Gram-negative strains | |||||||
| A− | 75 | >150 | >150 | 150 | 18.8 | 37.5 | 9.4 |
| B− | 18.8 | >150 | 150 | 75 | >150 | 4.7 | 2.3 |
| C− | >150 | >150 | >150 | 75 | >150 | 4.7 | >150 |
| D− | >150 | >150 | >150 | 75 | 9.4 | 4.7 | >150 |
| E− | 9.4 | 150 (150) | 75 (75) | 18.8 (9.4) | 9.4 (18.8) | 9.4 (4.7) | 2.3 (9.4) |
| F− | 2.3 | 18.8 | >150 | 75 | >150 | >150 | 1.2 |
| G− | 150 | >150 | >150 | >150 | 75 | 18.8 | 2.3 |
| H− | >150 | >150 | >150 | 150 | 18.8 | 18.8 | >150 |
| I− | >150 | >150 | 150 | 37.5 | 18.8 | 37.5 | 1.2 |
| J− | 18.8 | 150 | 150 | 37.5 | 2.3 | 2.3 | 4.7 |
Gram-positive strains: A+, E. faecium BM4105-RF; B+, L. monocytogenes ATCC 19115; C+, MRSA; D+, M. parascrofulaceum ATCC BAA-614; E+, M. smegmatis MC2 155; F+, S. aureus NorA; G+, S. aureus NRS22 USA600; H+, S. epidermidis ATCC 12228; I+, S. pyogenes ATCC 12384. Gram-negative strains: A−, E. coli ATCC 25922; B−, E. coli Δ7 wt; C−, E. coli Δ7 A1408G; D−, E. coli Δ7 G1491U, E−, E. coli MC1061; F−, H. influenzae ATCC 51907; G−, K. pneumoniae ATCC 27736; H−, P. aeruginosa ATCC 27853; I−, P. aeruginosa PA01; J−, S. flexneri 2475T pgm-24.
MICs were determined for TOB and its analogues with various alkyl chain lengths (C4 to C10) against a variety of Gram-positive and Gram-negative bacterial strains. The values in parentheses were previously reported in reference 7 for a different batch of these molecules.
The main mechanism of resistance to aminoglycoside antibiotics is their modification by resistance enzymes termed aminoglycoside-modifying enzymes (AMEs) that can be acquired by bacteria (27). There are three types of AMEs: the aminoglycoside N-acetyltransferases (AACs), the aminoglycoside O-phosphotransferases (APHs), and the aminoglycoside O-nucleotidyltransferases (ANTs). Recently, a unique AAC, the enhanced intracellular survival (Eis) protein from a variety of bacterial species, was found capable of multiacetylating aminoglycosides (12, 28–36). Knowing that our compounds had good antibacterial activity, we tested the novel C4 compound to see if it became modified by AMEs (Fig. 2). All other compounds in this study had previously been tested against these AMEs (7). The rates at which AAC(6′)-Ib′, AAC(6′)-Ie, and Eis modified C4 were only 50% or less of the rate at which TOB was modified by these enzymes. The converse was true for the AAC(3)-IV, AAC(2′)-Ic, and ANT(4′) enzymes, as C4 was modified at a rate 1.5 to 2 times that of TOB with the same enzymes.
In vitro antifungal assay.
The MIC values for TOB and its C4 to C14 analogues as well as those of three known antifungal azoles (POS, ITC, and FLC) were determined against various fungal strains (Table 2). TOB analogues C12 and C14 showed broad-spectrum antifungal activities against yeast and filamentous fungi. The MIC values for C12 and C14 against tested fungal strains ranged from 1.95 to 31.2 mg/liter and 1.95 to 7.8 mg/liter, respectively. The C10 TOB analogue exhibited good to no activity against various fungal strains, with MICs ranging from 3.9 to 125 mg/liter. The parent TOB and its analogues C4, C6, and C8 exhibited no antifungal activities except for C10 against F. graminearum 053 (strain J; 3.9 mg/liter) and only low activity against C. neoformans MYA-895 (H; 15.6 mg/liter). It should be noted that the fungal strains used in this study showed better sensitivity to C12 and C14 than all three azoles, with the exception of two yeast strains, C. neoformans MYA-895 (H) and C. albicans 10231 (A), and two filamentous strains, A. nidulans 38163 (I) and F. graminearum 053 (J) (Table 2). However, only C. neoformans MYA-895 (H) showed higher sensitivity to C8, C12, and C14, with low MIC values of 1.95 mg/liter.
TABLE 2.
MICs determined for TOB and its analogues with various alkyl chain lengths (C4 to C14) and for three control antifungal agents (POS, ITC, and FLC) against various yeast strains and filamentous fungi
| Yeast or fungal straina | MIC (mg/liter) for compoundb |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C4 | C6 | C8 | C10 | C12 | C14 | TOB | POS | ITC | FLC | |
| Yeast strains | ||||||||||
| A | >125 | >125 | >125 | 31.2 | 31.2 | 7.8 | >125 | 0.5 | 0.5 | 62.5 |
| B | >125 | >125 | >125 | 62.5 | 31.2 | 7.8 | >125 | >62.5 | >62.5 | >125 |
| C | >125 | >125 | >125 | 62.5 | 15.6 | 7.8 | >125 | 7.8 | 7.8 | 15.6 |
| D | >125 | >125 | >125 | 125 | 31.2 | 7.8 | >125 | 31.2 | 31.2 | >125 |
| E | >125 | >125 | >125 | 125 | 31.2 | 7.8 | >125 | 31.2 | 31.2 | >125 |
| F | >125 | >125 | >125 | 125 | 15.6 | 7.8 | >125 | 15.6 | 31.2 | 62.5 |
| G | >125 | >125 | >125 | 125 | 31.2 | 7.8 | >125 | 15.6 | 31.2 | 62.5 |
| H | 31.25 | 7.8 | 1.95 | 15.6 | 1.95 | 1.95 | 62.5 | <0.975 | <0.975 | <0.975 |
| Filamentous fungi | ||||||||||
| I | >125 | 125 | 125 | 62.5 | 7.8 | 7.8 | >125 | 0.195 | 0.195 | 62.5 |
| J | 62.5 | 31.2 | 31.2 | 3.9 | 3.9 | 3.9 | 62.5 | ≤1.56 | <1.95 | >125 |
Yeast strains: A, C. albicans ATCC 10231; B, C. albicans ATCC 64124; C, C. albicans ATCC MYA-2876(S); D, C. albicans ATCC 90819(R); E, C. albicans ATCC MYA-2310(S); F, C. albicans ATCC MYA-1237(R); G, C. albicans ATCC MYA-1003(R); H, C. neoformans MYA-895. Note that here the S or R following a strain name indicates that ATCC reports the strain susceptible (S) or resistant (R) to ITC and FLC. Filamentous fungi: I, Aspergillus nidulans ATCC 38163; J, Fusarium graminearum 053.
For yeast strains, MIC-0 values are reported for TOB and its analogues, whereas MIC-2 values are reported for azoles. For filamentous fungi, MIC-0 values are reported for all compounds.
The MFC values were also determined for the most active C12 and C14 compounds and were found to be 62.5 mg/liter and 15.6 mg/liter, respectively, in almost all strains tested, with the exception of C14 against C. albicans ATCC 64124, for which the MFC was 7.8 mg/liter (Table 3). A trailing growth effect was observed for all three azoles against all yeast strains except against C. albicans 10231 (A) and C. neoformans (H). Since the majority of azoles did not completely inhibit the growth of yeast at the tested concentrations, MFC values were not determined for them.
TABLE 3.
MFCs determined for TOB analogues with an alkyl chain length of C12 or C14 against various yeast strains
| Yeast straina | MFC (mg/liter) for analogue |
|
|---|---|---|
| C12 | C14 | |
| A | 62.5 | 15.6 |
| B | 62.5 | 15.6 |
| C | 62.5 | 7.8 |
| D | 62.5 | 15.6 |
| E | 62.5 | 15.6 |
| F | 62.5 | 15.6 |
| G | 62.5 | 15.6 |
| H | 3.9 | 3.9 |
Yeast strains: A, C. albicans ATCC 10231; B, C. albicans ATCC 64124; C, C. albicans ATCC MYA-2876 (S); D, C. albicans ATCC 90819 (R); E, C. albicans ATCC MYA-2310 (S); F, C. albicans ATCC MYA-1237 (R); G, C. albicans ATCC MYA-1003 (R); H, C. neoformans MYA-895. The S or R following a strain name indicates that ATCC reports the strain susceptible (S) or resistant (R) to ITC and FLC.
Antifungal carryover and time-kill studies.
An antifungal carryover effect was not observed for TOB analogues with linear alkyl chain lengths of C12 and C14 at 1, 2, or 4× MICs against C. albicans ATCC 64124 (B). The time-kill course of the most potent TOB analogues, C12 and C14, and of FLC against the representative strain C. albicans ATCC 64124 (B) over a 24-h period is shown in Fig. 3. C12 at 32 mg/liter (MIC) and C14 at 8 mg/liter (MIC) rapidly reduced the CFU of C. albicans ATCC 64124 (B) by ≥2 log10 after 6 and 9 h of treatment, but complete killing was observed only after 24 h for C12 and after 12 h for C14 (Fig. 3). However, FLC at 16 mg/ml showed a fungistatic effect against C. albicans ATCC 64124 (B) for the first 6 h of growth, and after that the trend of cell growth was similar to that of control cells. Likewise, AmB at a subinhibitory concentration (1 mg/liter) maintained its fungistatic activity against C. albicans ATCC 64124 (B) for 24 h.
FIG 3.
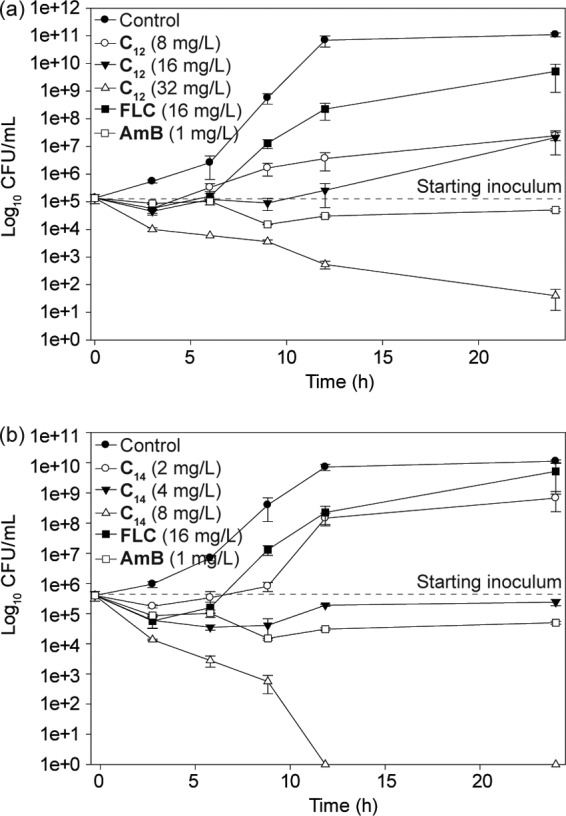
Representative time-kill studies of 6″-thioether TOB analogues with C12 and C14 linear alkyl chains against azole-resistant C. albicans ATCC 64124 (strain B). (a) Cultures were exposed to C12 at 8, 16, or 32 mg/liter. (b) Cultures were exposed to C14 at 2, 4, or 8 mg/liter. In both panels, cultures were exposed to FLC at 16 mg/liter and AmB at 1 mg/liter or to a no-drug control.
In vitro cytotoxicity assay.
The cytotoxicity assay results with TOB analogues with linear alkyl chain lengths of C4 to C14 against A549 and Beas 2B cells are shown in Fig. 4. The IC50s of C14 against the A549 and Beas 2B cell lines were >62.5 mg/liter and 125 mg/liter, respectively (Fig. 4). This was a 32- to 64-fold-higher concentration than the antifungal MICs against fungi (Table 2). Similarly, the IC50s of C12 against both cell lines were ≥125 mg/liter, 4- to 64-fold higher than its antifungal MICs. However, C4, C6, C8, C10, and TOB itself show little to no toxicity against these cell lines.
FIG 4.
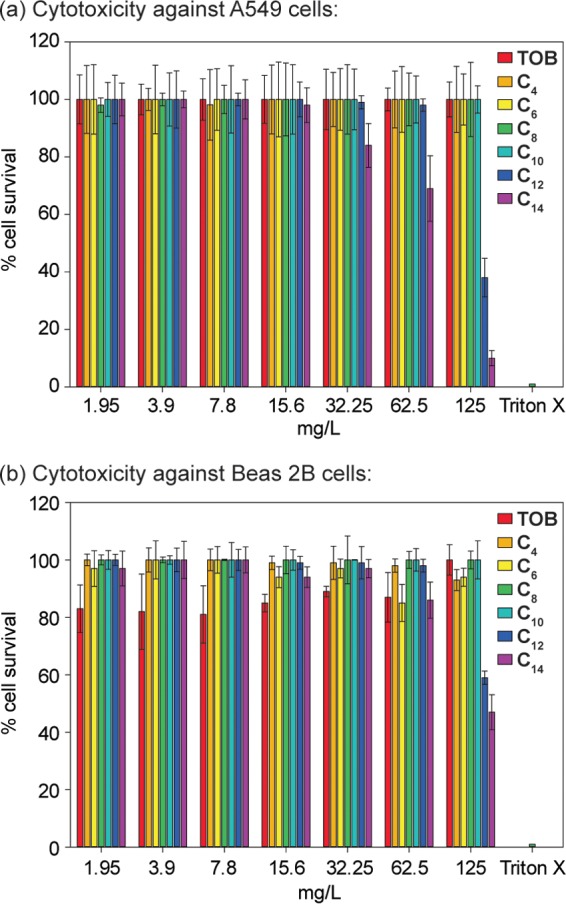
Cytotoxicity of 6″-thioether C4 to C14 TOB analogues against mammalian cells. A549 cells (a) and Beas 2B cells (b) were incubated at 37°C for 24 h in a CO2 incubator with various concentrations of TOB or its 6″-thioether analogues with C4, C6, C8, C10, C12, and C14 linear alkyl chains.
Effects of C12 and C14 on fungal membrane integrity.
PI was used to investigate the effects of TOB analogues (C12 and C14) on the fungal membrane integrity of C. albicans ATCC 64124 (strain B). PI, a membrane-impermeable dye, cannot enter an intact cell unless the cell membrane is compromised, and it fluoresces upon binding to nucleic acids (37). When yeast cells were exposed to TOB analogues C12 and C14 at 15.6, 31.2, or 62.5 mg/liter and 3.9, 7.8, or 15.6 mg/liter, respectively, 6, 46, or 93% and 10, 36, or 92% staining of the yeast cells was observed, respectively. However, cells treated with TOB (2% staining) or POS (5%) or untreated (1%) showed negligible staining (Fig. 5).
FIG 5.
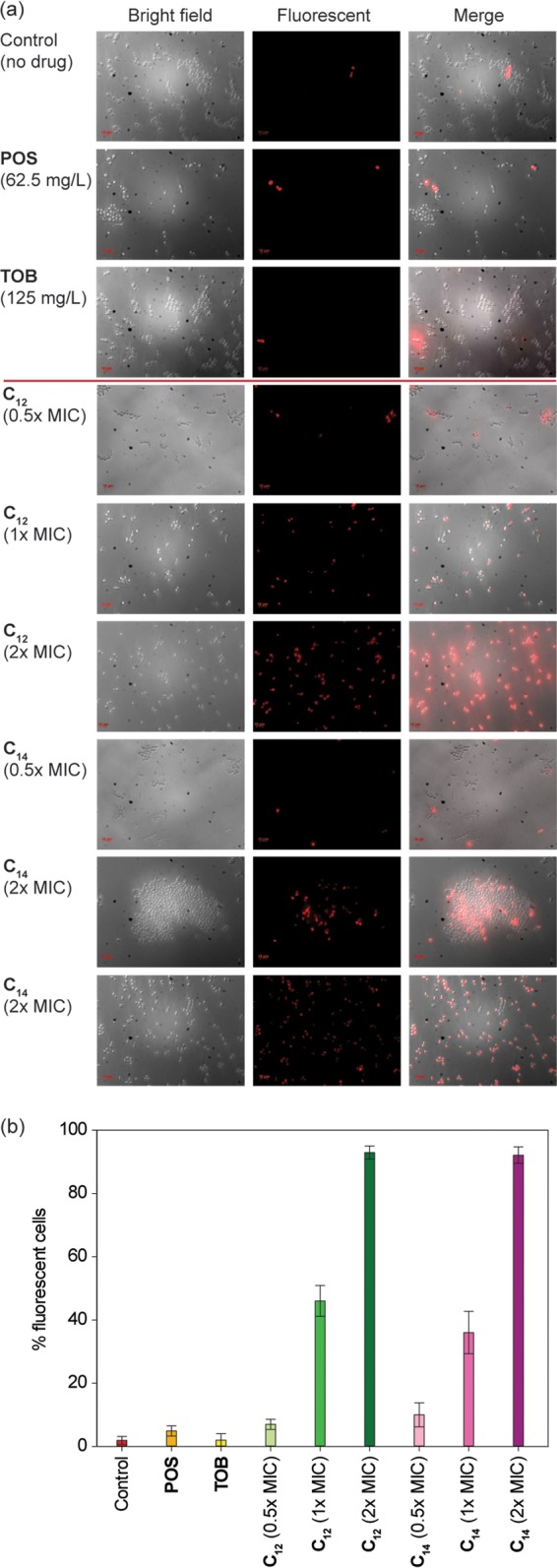
(a) Representative dose-dependent membrane permeabilization effects of 6″-thioether TOB analogues with C12 or C14 linear alkyl chains on azole-resistant C. albicans ATCC 64124 (strain B). From top to bottom: PI dye uptake by yeast cells without drug, with POS (62.5 mg/liter), with TOB (125 mg/liter), with C12 (0.5×, 1×, or 2× MIC) or with C14 (0.5×, 1×, or 2× MIC). (b) Quantitative representation of the images shown in panel a.
Annexin V and PI staining analysis.
Annexin V and PI staining assays were performed to determine whether the killing effect of TOB analogues involves apoptosis or necrosis in yeast cells. Phosphatidylserine (PS) is distributed asymmetrically in the inner leaflet of the lipid bilayer of plasma membranes of yeast cells (38). Externalization of PS on the outer surface of the plasma membrane is an early symptom of apoptosis or programmed cell death that can be detected by FITC-conjugated Annexin V staining (green) in yeast cells. Similarly, PI dye only stains necrotic cells, as intact fungal membranes are impermeable. As shown in Fig. 6, C14, at subinhibitory doses, induced both early apoptosis (∼13%) and late apoptosis/necrosis (∼40%) of C. albicans ATCC 64124 (strain B) after 1 h of exposure. However, untreated yeast cells did not show any Annexin V or PI staining.
FIG 6.
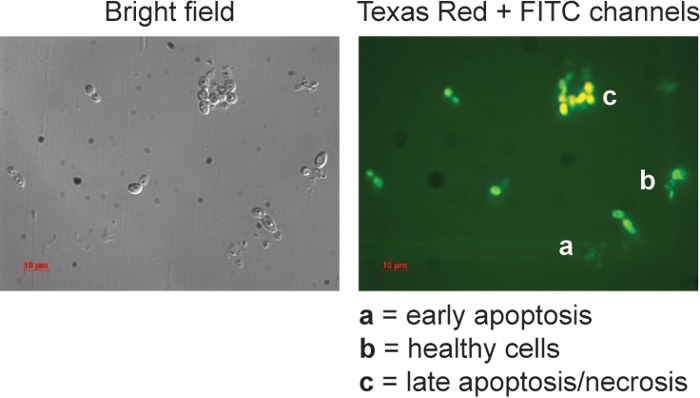
Annexin V and propidium iodide stains of C. albicans ATCC 64124 (strain B) cells treated with 6″-thioether C14 TOB analogue (4 mg/liter) for 1 h (right panel) and the bright-field image (left panel). Note: in the right panel, the letter a indicates early apoptotic cells that stained only green, the letter b indicates healthy cells that did not stain, and the letter c indicates late apoptotic/necrotic cells that stained green and orange.
DISCUSSION
Previously, we showed chain length-dependent antibacterial activities of TOB analogues derivatized at the 6″-position with linear alkyl chains (C6 to C22) against VRE and E. coli with resistance to the parent compound TOB itself (7). In the current study, we further expanded the antibacterial spectra of these analogues along with that of a newly synthesized C4 TOB analogue against various clinically important bacterial isolates. Unlike TOB analogues with longer alkyl chains, C4 demonstrated strong antibacterial activities against L. monocytogenes ATCC 19115 (strain B+; 2.3 mg/liter) and H. influenzae ATCC 51907 (F−; 2.3 mg/liter) that were also comparable to the activity of TOB itself (1.2 mg/liter) against these strains (Table 1). Among all of these analogues, C12 and C14 exhibited moderate to strong antibacterial activities against most of the bacterial strains, except against M. smegmatis MC2 155 (E+; >150 mg/liter) and H. influenzae ATCC 51907 (F−; >150 mg/liter) (Table 1). The MIC values of C6 to C14 determined in this study were mostly consistent with previously reported MIC values of these analogues against the specific strains of bacteria (7).
Recently, the amphiphilic kanamycin A and B derivatives K20 and FG08, respectively, with C8 linear alkyl chains were shown to have promising antifungal properties with no or mild antibacterial properties, respectively (8–10). This report has motivated us to explore the potential antifungal activities of our C4 to C14 TOB analogues that were yet to be determined. The C4 to C14 analogues demonstrated a parabolic pattern of chain length-dependent antifungal activity, and C12 and C14 were found to be the most potent among them all. A similar pattern was also observed for the antibacterial properties of these analogues (7). Both C12 and C14 showed moderate growth-inhibitory effects against all tested fungal strains, with a relatively high degree of inhibition against C. neoformans MYA-895 (strain H) and against two filamentous fungi, A. nidulans 38163 (I) and F. graminearum 053 (J). Intriguingly, the C8 TOB analogue showed no activity against fungi tested in this study, except against C. neoformans MYA-895 (H). In contrast to kanamycin derivatives FG08 and K20, our TOB analogues did not require an optimal chain length of C8 to show antifungal properties (9, 19). Not only were the MIC values of C12 and C14 at least 1- to 2-fold lower than amphiphilic kanamycin derivatives FG08 and K20, but also C12 and C14 were active against A. nidulans 38163 (I) while FG08 and K20 were completely inactive against Aspergillus strains (Table 2) (9, 19). It is also worth noting that the MIC values of C12 and C14 against resistant fungal strains, except for C. albicans 10231 (strain A), C. neoformans MYA-895 (H), and A. nidulans 38163 (I), were lower than those of POS, ITC, and FLC (Table 2). These results not only indicate that C12 and C14 may have different modes of action than the conventional azoles against fungi, but also it appears that they may circumvent the azole resistance mechanisms of resistant fungi such as C. albicans ATCC 64124 (B), which has mutations in its ERG11 sequences (39, 40).
Another noteworthy feature of the C12 and C14 analogues is that they showed a fungicidal effect on the azole-resistant strain C. albicans ATCC 64124 (strain B), inhibiting it completely after 24 h (Fig. 3). However, as expected, FLC was fungistatic against C. albicans ATCC 64124 (B) and completely lost its activity after 6 h, which was consistent with previous reports (41). Also, C12 and C14 lysed 50% of mouse erythrocytes at concentrations that were 4- to 32-fold and 8- to 32-fold higher than their antifungal MICs, respectively (data not shown), and these results were consistent with the results published previously for these analogues (7). However, in this study, we expanded the reports of effects of C12 and C14 on nucleated mammalian cells. C12 and C14 did not affect A549 (human lung carcinoma epithelial cells) or Beas 2B (normal human bronchial epithelial cells) proliferation even at concentrations higher than their antifungal MIC values, thus suggesting a selective antifungal activity (Fig. 4).
It has been shown that C14 targets bacterial membranes over the ribosomes, as does the parent drug (7), but the effect of C14 on fungal membranes was yet to be determined. We chose the most potent TOB analogues, C12 and C14, to explore their abilities to affect fungal membrane integrity by measuring PI dye influx into C12- or C14-treated C. albicans ATCC 64124 (strain B). Both C12 and C14, at their MIC and at 2× MIC, triggered rapid influx of PI by yeast cells after 1 h of exposure (Fig. 5). It is noteworthy that C12 and C14, even at 0.5× MIC, caused more PI staining of yeast cells than POS (62.5 mg/liter) after 1 h of exposure, which further supports our proposed statement of different modes of action of TOB analogues than that of azoles against fungi. The rapidity of PI dye influx into C12- or C14-treated yeast cells, however, suggests that the possible cause of killing may be associated with a direct effect on fungal membranes. The membrane primary or secondary effects of TOB analogues against fungi are not known. PS externalization on the outer surface of the lipid bilayer of plasma membranes is a sign of early apoptosis in yeast cells. The yeast cells that were treated with C14 at a subinhibitory concentration did show both early apoptosis (Fig. 6, indicated by the letter a), late apoptosis/necrosis (indicated by the letter c), and no apoptosis (as indicated by the letter b), as demonstrated in the Annexin V and PI assay. We speculate that the induction of early apoptosis by C14 causes externalization of phosphatidylserine on the plasma membrane by making the surface highly negatively charged, which could then react with the polycationic amphiphilic aminoglycosides generated. However, thorough experimental analysis, outside the scope of this study, is desirable for the future to elucidate the exact target of these analogues on fungal membranes.
In conclusion, C12 and C14 showed broad-spectrum antifungal activities against yeasts and filamentous fungi as they targeted fungal plasma membranes. They were less hemolytic and showed low mammalian cell toxicities. This study provides novel lead compounds for the development of antifungal drugs, which we are currently following by synthesizing and investigating the properties of additional AGs derivatized in 6″-thioether form with long linear alkyl chains.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health (NIH) grant AI090048 (S.G.-T.) and by startup funds from the College of Pharmacy at the University of Kentucky (S.G.-T.).
We thank Gregory A. Graf and Markos Leggas (University of Kentucky) for letting us use their fluorescence microscope and cell culture facilities, respectively. We thank David H. Sherman (University of Michigan), Sabine Ehrt (Weill Cornell Medical College), Paul J. Hergenrother (University of Illinois at Urbana-Champaign), Dev P. Arya (Clemson University), Kurt Frederick (Ohio State University), and Anthony T. Maurelli (Uniformed Services University of Health Sciences) for providing some of the bacterial strains used in our work. We also thank Lisa J. Vaillancourt and Jon S. Thorson (University of Kentucky), as well as Jon Y. Takemoto (Utah State University), for providing some of the fungal strains used in our study.
REFERENCES
- 1.Fridkin SK, Jarvis WR. 1996. Epidemiology of nosocomial fungal infections. Clin Microbiol Rev 9:499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jarvis WR. 1995. Epidemiology of nosocomial fungal infections, with emphasis on Candida species. Clin Infect Dis 20:1526–1530. doi: 10.1093/clinids/20.6.1526. [DOI] [PubMed] [Google Scholar]
- 3.Pfaller MA, Diekema DJ. 2004. Rare and emerging opportunistic fungal pathogens: concern for resistance beyond Candida albicans and Aspergillus fumigatus. J Clin Microbiol 42:4419–4431. doi: 10.1128/JCM.42.10.4419-4431.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldman MB, Terry DS, Altman RB, Blanchard SC. 2010. Aminoglycoside activity observed on single pre-translocation ribosome complexes. Nat Chem Biol 6:54–62. doi: 10.1038/nchembio.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galimand M, Courvalin P, Lambert T. 2003. Plasmid-mediated high-level resistance to aminoglycosides in Enterobacteriaceae due to 16S rRNA methylation. Antimicrob Agents Chemother 47:2565–2571. doi: 10.1128/AAC.47.8.2565-2571.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee HB, Kim Y, Kim JC, Choi GJ, Park SH, Kim CJ, Jung HS. 2005. Activity of some aminoglycoside antibiotics against true fungi, Phytophthora and Pythium species. J Appl Microbiol 99:836–843. doi: 10.1111/j.1365-2672.2005.02684.x. [DOI] [PubMed] [Google Scholar]
- 7.Herzog IM, Green KD, Berkov-Zrihen Y, Feldman M, Vidavski RR, Eldar-Boock A, Satchi-Fainaro R, Eldar A, Garneau-Tsodikova S, Fridman M. 2012. 6″-Thioether tobramycin analogues: towards selective targeting of bacterial membranes. Angew Chem 51:5652–5656. doi: 10.1002/anie.201200761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang CW, Fosso M, Kawasaki Y, Shrestha S, Bensaci MF, Wang J, Evans CK, Takemoto JY. 2010. Antibacterial to antifungal conversion of neamine aminoglycosides through alkyl modification. Strategy for reviving old drugs into agrofungicides. J Antibiot 63:667–672. doi: 10.1038/ja.2010.110. [DOI] [PubMed] [Google Scholar]
- 9.Shrestha S, Grilley M, Fosso MY, Chang CW, Takemoto JY. 2013. Membrane lipid-modulated mechanism of action and non-cytotoxicity of novel fungicide aminoglycoside FG08. PLoS One 8:e73843. doi: 10.1371/journal.pone.0073843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang CW, Takemoto JY. 2014. Antifungal amphiphilic aminoglycosides. MedChemComm 5:1048–1057. doi: 10.1039/C4MD00078A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fosso MY, Li Y, Garneau-Tsodikova S. 2014. New trends in aminoglycosides use. MedChemComm 5:1075–1091. doi: 10.1039/C4MD00163J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen W, Biswas T, Porter VR, Tsodikov OV, Garneau-Tsodikova S. 2011. Unusual regioversatility of acetyltransferase Eis, a cause of drug resistance in XDR-TB. Proc Natl Acad Sci U S A 108:9804–9808. doi: 10.1073/pnas.1105379108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green KD, Chen W, Garneau-Tsodikova S. 2011. Effects of altering aminoglycoside structures on bacterial resistance enzyme activities. Antimicrob Agents Chemother 55:3207–3213. doi: 10.1128/AAC.00312-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green KD, Chen W, Houghton JL, Fridman M, Garneau-Tsodikova S. 2010. Exploring the substrate promiscuity of drug-modifying enzymes for the chemoenzymatic generation of N-acylated aminoglycosides. ChemBioChem 11:119–126. doi: 10.1002/cbic.200900584. [DOI] [PubMed] [Google Scholar]
- 15.Porter VR, Green KD, Zolova OE, Houghton JL, Garneau-Tsodikova S. 2010. Dissecting the cosubstrate structure requirements of the Staphylococcus aureus aminoglycoside resistance enzyme ANT(4′). Biochem Biophys Res Commun 403:85–90. doi: 10.1016/j.bbrc.2010.10.119. [DOI] [PubMed] [Google Scholar]
- 16.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard, 9th ed CLSI document M07-A9. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 17.Clinical and Laboratory Standards Institute. 2012. Methods for antimicrobial susceptibility testing of anaeorbic bacteria. Approved standard, 8th ed CLSI document M11-A8. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 18.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard. CLSI document M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 19.Shrestha SK, Chang CW, Meissner N, Oblad J, Shrestha JP, Sorensen KN, Grilley MM, Takemoto JY. 2014. Antifungal amphiphilic aminoglycoside K20: bioactivities and mechanism of action. Front Microbiol 5:671. doi: 10.3389/fmicb.2014.00671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hazirolan G, Canton E, Sahin S, Arikan-Akdagli S. 2013. Head-to-head comparison of inhibitory and fungicidal activities of fluconazole, itraconazole, voriconazole, posaconazole, and isavuconazole against clinical isolates of Trichosporon asahii. Antimicrob Agents Chemother 57:4841–4847. doi: 10.1128/AAC.00850-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Espinel-Ingroff A, Fothergill A, Peter J, Rinaldi MG, Walsh TJ. 2002. Testing conditions for determination of minimum fungicidal concentrations of new and established antifungal agents for Aspergillus spp.: NCCLS collaborative study. J Clin Microbiol 40:3204–3208. doi: 10.1128/JCM.40.9.3204-3208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi, 2nd ed CLSI document M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 23.Klepser ME, Malone D, Lewis RE, Ernst EJ, Pfaller MA. 2000. Evaluation of voriconazole pharmacodynamics using time-kill methodology. Antimicrob Agents Chemother 44:1917–1920. doi: 10.1128/AAC.44.7.1917-1920.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lafleur MD, Sun L, Lister I, Keating J, Nantel A, Long L, Ghannoum M, North J, Lee RE, Coleman K, Dahl T, Lewis K. 2013. Potentiation of azole antifungals by 2-adamantanamine. Antimicrob Agents Chemother 57:3585–3592. doi: 10.1128/AAC.00294-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park C, Lee DG. 2009. Fungicidal effect of antimicrobial peptide arenicin-1. Biochim Biophys Acta 1788:1790–1796. doi: 10.1016/j.bbamem.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 26.Phillips AJ, Sudbery I, Ramsdale M. 2003. Apoptosis induced by environmental stresses and amphotericin B in Candida albicans. Proc Natl Acad Sci U S A 100:14327–14332. doi: 10.1073/pnas.2332326100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Labby KJ, Garneau-Tsodikova S. 2013. Strategies to overcome the action of aminoglycoside-modifying enzymes for treating resistant bacterial infections. Future Med Chem 5:1285–1309. doi: 10.4155/fmc.13.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Green KD, Biswas T, Chang C, Wu R, Chen W, Janes BK, Chalupska D, Gornicki P, Hanna PC, Tsodikov OV, Joachimiak A, Garneau-Tsodikova S. 2015. Biochemical and structural analysis of an Eis family aminoglycoside acetyltransferase from Bacillus anthracis. Biochemistry 54:3197–3206. doi: 10.1021/acs.biochem.5b00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Green KD, Pricer RE, Stewart MN, Garneau-Tsodikova S. 2015. Comparative study of Eis-like enzymes from pathogenic and nonpathogenic bacteria. ACS Infect Dis doi: 10.1021/acsinfecdis.b00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsodikov OV, Green KD, Garneau-Tsodikova S. 2014. A random sequential mechanism of aminoglycoside acetylation by Mycobacterium tuberculosis Eis protein. PLoS One 9:e92370. doi: 10.1371/journal.pone.0092370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Houghton JL, Biswas T, Chen W, Tsodikov OV, Garneau-Tsodikova S. 2013. Chemical and structural insights into the regioversatility of the aminoglycoside acetyltransferase Eis. ChemBioChem 14:2127–2135. doi: 10.1002/cbic.201300359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jennings BC, Labby KJ, Green KD, Garneau-Tsodikova S. 2013. Redesign of substrate specificity and identification of the aminoglycoside binding residues of Eis from Mycobacterium tuberculosis. Biochemistry 52:5125–5132. doi: 10.1021/bi4002985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Houghton JL, Green KD, Pricer RE, Mayhoub AS, Garneau-Tsodikova S. 2013. Unexpected N-acetylation of capreomycin by mycobacterial Eis enzymes. J Antimicrob Chemother 68:800–805. doi: 10.1093/jac/dks497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pricer RE, Houghton JL, Green KD, Mayhoub AS, Garneau-Tsodikova S. 2012. Biochemical and structural analysis of aminoglycoside acetyltransferase Eis from Anabaena variabilis. Mol Biosyst 8:3305–3313. doi: 10.1039/c2mb25341k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen W, Green KD, Garneau-Tsodikova S. 2012. Cosubstrate tolerance of the aminoglycoside resistance enzyme Eis from Mycobacterium tuberculosis. Antimicrob Agents Chemother 56:5831–5838. doi: 10.1128/AAC.00932-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen W, Green KD, Tsodikov OV, Garneau-Tsodikova S. 2012. Aminoglycoside multiacetylating activity of the enhanced intracellular survival protein from Mycobacterium smegmatis and its inhibition. Biochemistry 51:4959–4967. doi: 10.1021/bi3004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pina-Vaz C, Sansonetty F, Rodrigues AG, Costa-Oliveira S, Tavares C, Martinez-de-Oliveira J. 2001. Cytometric approach for a rapid evaluation of susceptibility of Candida strains to antifungals. Clin Microbiol Infect 7:609–618. doi: 10.1046/j.1198-743x.2001.00307.x. [DOI] [PubMed] [Google Scholar]
- 38.Cerbon J, Calderon V. 1991. Changes of the compositional asymmetry of phospholipids associated to the increment in the membrane surface potential. Biochim Biophys Acta 1067:139–144. doi: 10.1016/0005-2736(91)90035-7. [DOI] [PubMed] [Google Scholar]
- 39.Favre B, Didmon M, Ryder NS. 1999. Multiple amino acid substitutions in lanosterol 14alpha-demethylase contribute to azole resistance in Candida albicans. Microbiology 145:2715–2725. [DOI] [PubMed] [Google Scholar]
- 40.Kakeya H, Miyazaki Y, Miyazaki H, Nyswaner K, Grimberg B, Bennett JE. 2000. Genetic analysis of azole resistance in the Darlington strain of Candida albicans. Antimicrob Agents Chemother 44:2985–2990. doi: 10.1128/AAC.44.11.2985-2990.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calabrese EC, Castellano S, Santoriello M, Sgherri C, Quartacci MF, Calucci L, Warrilow AG, Lamb DC, Kelly SL, Milite C, Granata I, Sbardella G, Stefancich G, Maresca B, Porta A. 2013. Antifungal activity of azole compounds CPA18 and CPA109 against azole-susceptible and -resistant strains of Candida albicans. J Antimicrob Chemother 68:1111–1119. doi: 10.1093/jac/dks506. [DOI] [PubMed] [Google Scholar]


