Abstract
The spread of multidrug-resistant Acinetobacter baumannii (MDRAB) has led to the renaissance of colistin (COL), often the only agent to which MDRAB remains susceptible. Effective therapy with COL is beset with problems due to unpredictable pharmacokinetics, toxicity, and the rapid selection of resistance. Here, we describe a potent synergistic interaction when COL was combined with fusidic acid (FD) against A. baumannii. Synergy in vitro was assessed against 11 MDRAB isolates using disc diffusion, checkerboard methodology (fractional inhibitory concentration index [FICI] of ≤ 0.5, susceptibility breakpoint index [SBPI] of >2), and time-kill methodology (≥2 log10 CFU/ml reduction). The ability of FD to limit the emergence of COL resistance was assessed in the presence and absence of each drug alone and in combination. Synergy was demonstrated against all strains, with an average FICI and SBPI of 0.064 and 78.85, respectively. In time-kill assays, COL-FD was synergistic and rapidly bactericidal, including against COL-resistant strains. Fusidic acid prevented the emergence of COL resistance, which was readily selected with COL alone. This is the first description of a novel COL-FD regimen for the treatment of MDRAB. The combination was effective at low concentrations, which should be therapeutically achievable while limiting toxicity. Further studies are warranted to determine the mechanism underlying the interaction and the suitability of COL-FD as an unorthodox therapy for the treatment of multidrug-resistant Gram-negative infections.
INTRODUCTION
Infections due to the Gram-negative bacterium Acinetobacter baumannii are increasingly challenging to treat and control. The organism has emerged worldwide as a major nosocomial pathogen in critical care units responsible for bloodstream, respiratory, skin and soft tissue, and device-related infections (1). Clinical isolates are often resistant to multiple antimicrobial drugs and belong to successful epidemiologically defined clones that, once established, are extremely difficult to eradicate from the hospital environment (2). As a result, outbreaks are common, typically last for months, and may cost institutions in excess of $500,000 to curtail (3). Treatment of infected individuals is equally hampered by a seemingly endless capacity of the organism to acquire and maintain large numbers of antimicrobial resistance genes (4). Carbapenems, once considered the treatment of choice, are increasingly found to be ineffective, leaving polymyxins (polymyxin B and colistin [COL]) as the treatment of last resort (5).
Although polymyxins have been widely employed in the treatment of A. baumannii infections, there are still concerns about their efficacy and safety. These include the unreliable methods for performing susceptibility testing, inadequate population pharmacokinetic data, uncertainties around appropriate dosing regimens, and the availability of the licensed formulations of colistin only as the inactive prodrug colistimethate sodium (6).
Despite this, polymyxins have frequently been shown to enhance the activity of other antimicrobial agents against resistant Gram-negative pathogens in vitro. Synergy has been shown not only with β-lactams, carbapenems, aminoglycosides, quinolones, and tetracyclines but also with agents with little intrinsic Gram-negative activity (macrolides, rifamycins, fosfomycin, glycopeptides, and oxazolidones) (7, 8). Given that any new drugs with unique modes of action are unlikely to become available for clinical use within the next decade, the use of unorthodox combinations of existing agents may be a rational approach for the therapy of resistant Gram-negative infections. Although a meta-analysis of data from historical studies has not yet revealed a clear benefit for polymyxin combination regimens, a number of prospective trials have recently been initiated (9).
Here, we describe a highly active and potent combination of COL and fusidic acid (FD) against multidrug-resistant A. baumannii (MDRAB).
(Part of this work was presented at the 54th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, 5 to 9 September 2014.)
MATERIALS AND METHODS
Bacterial isolates.
Characteristics of all isolates used in this study are listed in Table 1. Antimicrobial resistance determinants were identified by sequencing of PCR products obtained using a number of multiplex PCRs, as described previously (10). Isolates were classified as multidrug-resistant (MDR), extensively drug-resistant (XDR), or pandrug-resistant (PDR) A. baumannii according to the classification of Magiorakos et al. (11). Molecular typing of all A. baumannii strains was performed by pulsed-field gel electrophoresis (PFGE) and variable-number tandem-repeat (VNTR) analysis at the Antimicrobial Resistance and Healthcare Associated Infections Reference Unit (by J. Turton, Public Health England).
TABLE 1.
Characteristics of A. baumannii isolates studied, antimicrobial susceptibility testing, disc diffusion, and checkerboard synergy assay results
| Isolate | Characteristic(s)a | MICb (μg/ml) of: |
FD-COL 0.5× MIC zone diam difference (mm) | FD-COL synergy |
||
|---|---|---|---|---|---|---|
| FD | COL | FICIc | SBPIc | |||
| ATCC 19606 | Antibiotic-susceptible type strain | 128 | 0.25 | 16 | 0.006 | 155 |
| AB 12 | MDR PFGE-defined UK South East Clone; OXA-51 producer | 64 | 1 | 16 | 0.1 | 43 |
| AB 14 | XDR PFGE-defined UK OXA-23 clone 1; OXA-51 and OXA-23 producer; IMP R | 128 | 0.5 | 15 | 0.23 | 42 |
| AB 16 | XDR PFGE defined UK OXA-23 clone 2; OXA-51 and OXA-23 producer; IMP R | 512 | 0.5 | 9.5 | 0.11 | 37 |
| AB 184 | MDR PFGE-defined UK “T strain”; OXA-51 producer | 32 | 0.5 | 6.5 | 0.04 | 158 |
| AB 186 | MDR PFGE-defined UK “Burn strain”; OXA-51 producer | 32 | 0.5 | 17 | 0.06 | 82 |
| AB 205 | PDR PFGE-defined UK OXA-23 clone 1; OXA-51 and OXA-23 producer; IMP R and TGC R | 32 | 512 (R) | 21.5 | 0.02 | 8 |
| AB 210 | XDR PFGE-defined UK OXA-23 clone 1; OXA-51 and OXA-23 producer; IMP R | 64 | 0.5 | 18 | 0.04 | 101 |
| AB 211 | PDR PFGE defined UK OXA-23 clone 1; OXA-51 and OXA-23 producer; IMP R and TGC R | 256 | 4 (R) | 16 | 0.03 | 35 |
| AB 219 | PDR PFGE-defined UK OXA-23 clone 1; OXA-51 and OXA-23 producer; IMP R and TGC R | 64 | 512 (R) | 21.5 | 0.02 | 4 |
| AB 315 | XDR PFGE-defined UK OXA-23 clone 1; OXA-51 and OXA-23 producer; IMP R and TGC R | 256 | 1 | 10 | 0.2 | 42 |
PFGE, pulsed-field gel electrophoresis; IMP, imipenem; R, resistant; TGC, tigecycline; MDR, multidrug resistant; XDR, extensively drug resistant; PDR, pandrug resistant.
COL, colistin; FD, fusidic acid. MIC results shown are averages from experiments done in triplicate. MICs have been rounded up to the higher 2-fold dilution when falling between dilutions.
FICI, fractional inhibitory concentration index; SBPI, susceptible breakpoint index. FICI and SBPI results shown are averages from experiments done in triplicate.
Antimicrobial susceptibility and synergy tests.
Colistin sulfate (COL; Sigma-Aldrich, St. Louis, MO) and fusidic acid (FD; Sigma-Aldrich, St. Louis, MO) were dissolved in sterile distilled water to obtain stock solutions of 50 mg/ml. The MICs of COL and FD were determined by broth microtiter dilution (BMD) and by agar dilution (for COL) according to the Clinical and Laboratory Standards Institute (CLSI) (12) and the British Society for Antimicrobial Chemotherapy (BSAC) (13) methodologies. Breakpoints used to interpret MICs were based on published CLSI (14) and European Clinical Antimicrobial Susceptibility Testing (EUCAST) (15) guidelines (Acinetobacter spp. for COL; Staphylococcus spp. for FD).
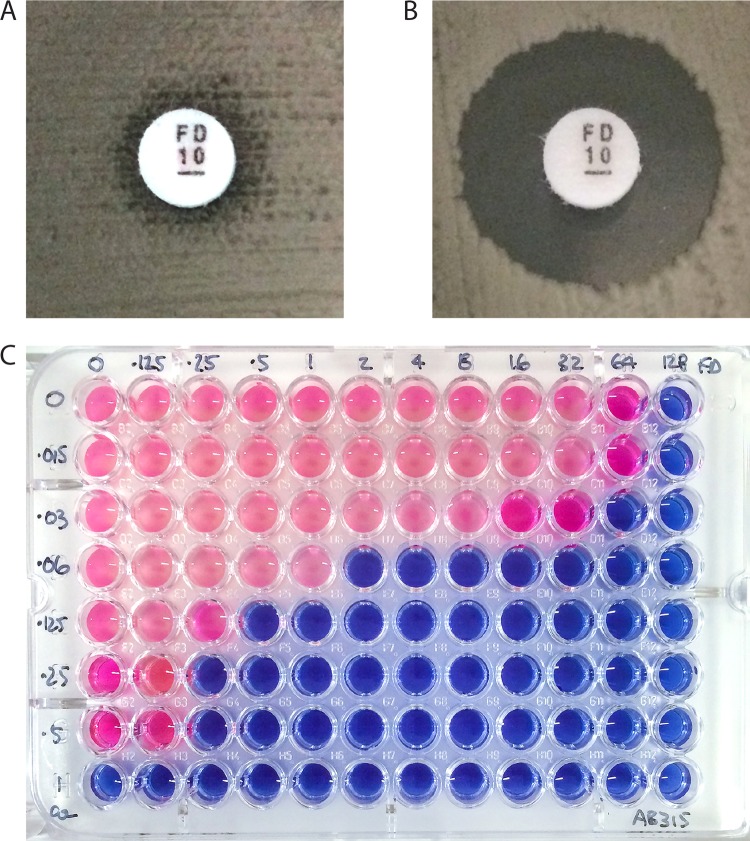
A screening test for potential synergy between COL and FD was performed using a disk diffusion assay. Bacterial suspensions of each isolate were prepared in a phosphate-buffered saline (PBS) solution adjusted to 0.5 McFarland standard. An even lawn was spread onto 2 Iso-Sensitest agar plates (ISA; Oxoid, Basingstoke, United Kingdom), one supplemented with 0.5× the MIC of COL. After application of FD 10-μg discs (Oxoid, Basingstoke, United Kingdom), the plates were incubated in air at 37°C for 18 h. Potential synergy was inferred when the zone of inhibition around the FD disc was ≥5 mm on the COL-supplemented plate (Fig. 1).
FIG 1.
(A and B) Disc diffusion synergy screen for isolate AB 205. (A) FD 10-μg disc applied onto an unsupplemented ISA plate. (B) Same assay performed on an ISA plate supplemented with 0.5× the MIC of COL. (C) COL-FD checkerboard for isolate AB 315.
Synergy using the BMD method was assessed in checkerboard assays (16) using Iso-Sensitest broth (ISB; Oxoid, Basingstoke, United Kingdom). The plates were set up with serial doubling dilutions of COL and FD at concentrations ranging from 0.008 to 512 mg/liter. Following incubation, inhibition of growth in each well was confirmed by recording turbidity and by the addition of 20 μl of alamarBlue (Invitrogen Corporation, San Diego, CA, USA) as a marker of bacterial viability. The fractional inhibitory concentration indices (FICI) [which equals (MIC of A in combination/MIC of A) + (MIC of B in combination/MIC of B)] were calculated using the wells with the lowest fractional inhibitory concentration (FIC), and synergy was defined according to standard criteria (≤0.5, synergy; >0.5 to ≤1, additive; >1 to ≤4, indifference; >4, antagonism). The susceptible breakpoint index (SBPI; >2, useful synergy) [which equals (susceptible breakpoint of A/MIC of A in combination) + (susceptible breakpoint of B/MIC of B in combination)] was also calculated as a means to assess the likely clinical relevance of any synergistic activity observed (17, 18).
Time-kill studies.
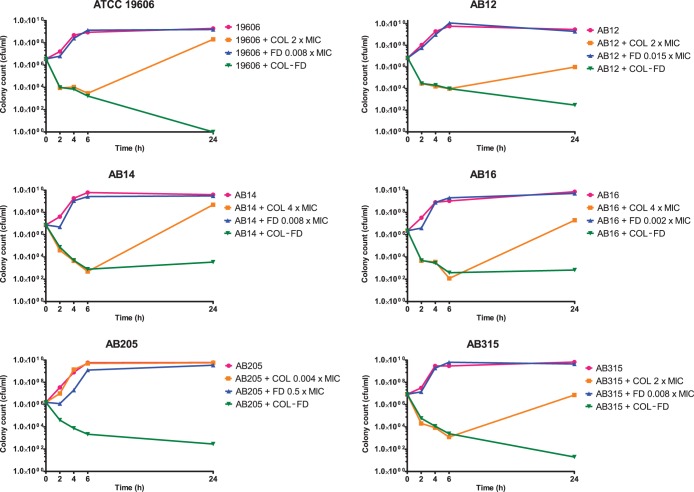
Six representative A. baumannii isolates were selected for further assessment in time-kill assays to investigate the bactericidal activity of the combination. COL was used at a final concentration at or below the clinical pharmacodynamic breakpoint (2 mg/liter), and FD was added at 1 mg/liter or 0.5× the MIC (in the case of isolate AB 205). These concentrations were chosen to reflect clinically achievable plasma levels using standard dosing regimens. Each experiment was performed in 10 ml of ISB supplemented with COL and FD (alone and in combination) as well as with an unsupplemented growth control. A starting inoculum of 106 CFU/ml was used in each broth and incubated aerobically at 37°C in a shaking incubator at 224 rpm for 24 h. At 2, 4, 6, and 24 h time points, 100-μl aliquots were taken and serially diluted prior to plating onto ISA with overnight incubation to obtain viable colony counts for each condition at the selected time points. A ≥2 log10 CFU/ml reduction in the colony count in the COL-FD condition at 24 h compared to the most active single agent was used to define synergy. Additionally, a >3 log10 CFU/ml reduction in the COL-FD colony count compared to the starting inoculum denoted bactericidal activity with the combination (19).
Colistin heteroresistance was determined by population analysis profiling (PAP). Briefly, 5 ml of ISB containing colistin sulfate (concentration range, 0.5 to 512 mg/liter) and a drug-free control were inoculated with 106 CFU/ml of each isolate. Following incubation, 100-μl aliquots were spread onto ISA to obtain colony counts. The interpretation of heteroresistance was made according to the criteria proposed by El-Halfawy et al. (20).
Mutational resistance to colistin-fusidic acid.
The potential for rapid emergence of resistance to COL and FD in vitro was assessed by serial passage in increasing concentrations of the drugs alone and in combination. These experiments were performed using 3 COL-susceptible (ATCC 19606, AB 14, AB 315) and one COL-resistant (AB 205) A. baumannii strains, using previously published methods with some modifications (21, 22). Briefly, overnight cultures in ISB were diluted to obtain inocula of 105 CFU/ml and then used in COL-FD checkerboard assays. Following aerobic incubation at 37°C for 24 h with continuous shaking (160 rpm), the MICs of COL and FD were recorded together with the well with the lowest FIC for the combination. Aliquots from wells containing 0.5× the MIC of the single agents and from wells with growth at 0.5× the MIC of the COL-FD well with the lowest FIC were diluted 1:1,000 in ISB and used as the inoculum in the next serial passage experiment for a total of 7 days. The number of days required for either a 4-fold or 8-fold increase in the MIC of COL and FD above baseline was used as a measure of the likelihood that resistance would readily emerge.
RESULTS
Synergy was observed between COL and FD against all A. baumannii isolates examined. In disc screening assays, the average increase in zone diameter around FD discs was 15.7 mm (range, 6.5 to 21.5 mm) (Table 1; Fig. 1A and B). Of note, the largest increases in zone diameters on COL-supplemented plates were seen against PDR isolates with COL resistance (MIC, 4 to 512 mg/liter). Synergy was subsequently confirmed in checkerboard assays for every strain, and the interaction appeared to be particularly strong, with an average FICI of 0.07 (range, 0.02 to 0.23) recorded (Table 1). The potency of the combination was reflected in very high SBPI values (mean, 66.5; range, 4 to 158) and the potential to achieve efficacy against COL-resistant strains (MIC, 512 mg/liter; SBPI, 4). As no correlation was seen between zone sizes observed in disc diffusion assays and FICI or SBPI values derived from checkerboards, a cutoff value (mm) for predicting likely synergy in a disc diffusion test could not be established.
Time-kill assays identified that the COL-FD combination was rapidly bactericidal, again versus both COL-susceptible and COL-resistant strains of MDRAB (Fig. 2). With the exception of AB 205 (a colistin-resistant isolate), all tested isolates exhibited heteroresistant properties (23), with regrowth at 24 h despite exposure to COL at concentrations in excess of the MIC (2 to 4× the MIC) in time-kill studies. This was confirmed by PAP analysis for ATCC 19606, AB 12, AB 14, and AB 16. This phenomenon was abolished when FD was added in combination. Resistance to either COL or FD was easily selected when A. baumannii was exposed to increasing concentrations of either drug alone, with 4-fold to 8-fold increases in COL and FD MICs reached after just 1 to 4 days of passage (Tables 2 and 3). In contrast, there was little increase in the MIC above baseline after 7 days of serial passage in the presence of both drugs compared with the single-agent selection pressure. Prevention of mutational resistance was particularly marked in those isolates with a COL-heteroresistant phenotype.
FIG 2.
Time-kill assays conducted with COL and FD versus 5 A. baumannii isolates. Strains (concentrations of COL) used were as follows: ATCC 19606 (0.5 μg/ml), AB 12 (2 μg/ml), AB 14 (2 μg/ml), AB 16 (2 μg/ml), AB 205 (2 μg/ml), and AB 315 (2 μg/ml). Strains (concentrations of FD) used were as follows: ATCC 19606 (1 μg/ml), AB 12 (1 μg/ml), AB 14 (1 μg/ml), AB 16 (1 μg/ml), AB 205 (8 μg/ml), and AB 315 (1 μg/ml).
TABLE 2.
Effects of exposure to COL and FD alone and in combination on susceptibility (MIC) of A. baumannii in serial passage experiments
| Day | Passage condition | MIC (mg/liter) of: |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| ATCC 19606 |
AB 14 |
AB 205 |
AB 315 |
||||||
| COL | FD | COL | FD | COL | FD | COL | FD | ||
| 0 | Unexposed | 0.25 | 256 | 0.25 | 128 | 512 | 64 | 1 | 128 |
| 7 | Single-agent pressure | 4 | 2,048 | 2,048 | 2,048 | 2,048 | 8,192 | 1,024 | >16,384 |
| Combined selection pressure | 0.5 | 512 | 4 | 256 | 512 | 2,048 | 1 | 128 | |
TABLE 3.
Effects of exposure to COL and FD alone and in combination on preventing the emergence of resistance (time to MIC increase) of A. baumannii in serial passage experiments
| Increase in MIC | Passage condition | Time to MIC increase (days) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| ATCC 19606 |
AB 14 |
AB 205 |
AB 315 |
||||||
| COL | FD | COL | FD | COL | FD | COL | FD | ||
| ≥4× | Single-agent pressure | 3 | 4 | 2 | 5 | 4 | 2 | 1 | 2 |
| Combined selection pressure | NRa | NR | 7 | NR | NR | 6 | NR | NR | |
| ≥8× | Single-agent pressure | 4 | 7 | 3 | 7 | NR | 2 | 1 | 4 |
| Combined selection pressure | NR | NR | 7 | NR | NR | 7 | NR | NR | |
NR, not reached by the final day (day 7) of experiment.
DISCUSSION
A. baumannii is a member of the ESKAPE (Enterobacter, Staphylococcus aureus, Klebsiella, A. baumannii, Pseudomonas, Enterococcus) group of bacterial pathogens for which there is an urgent need to find new or repurpose existing treatments (24). Finding innovative ways to tackle Gram-negative members of this group is a priority, given the current trajectory and predicted importance of these infections over the next decade (25). Many strains of A. baumannii are already XDR, with polymyxins often the only drug with any useful activity demonstrated in vitro. Clinical experience with polymyxin monotherapy in the treatment of MDRAB and other ESKAPE organisms has been mixed, with the rapid emergence of resistance (26) and frequent reports of clinical failures. While there remains uncertainty over the optimal treatment of MDR, XDR, and PDR infections, clinicians frequently resort to the use of antibiotics in combination.
There are numerous data supporting enhanced antimicrobial activity when polymyxins are added to other antimicrobial agents in vitro. A recent meta-analysis concluded that, versus A. baumannii, this activity was most pronounced when polymyxins were partnered with a carbapenem, rifampin, or glycopeptides (7). Here, we identified COL and FD as another unorthodox combination with potent activity against MDRAB. We consistently observed synergy against strains with MDR, XDR, and PDR characteristics belonging to important epidemic MDRAB clones using a range of assays (agar, microtiter dilution, time-kill). Using static methods, synergy could be readily shown in a simple disc diffusion assay, and FIC indices denoting the strength of the interaction were noted to be particularly low. The combination also promoted sustained bacterial killing when assessed using a time-kill methodology. Due to the degree of heterogeneity in methodology and the interpretation of in vitro synergy studies, there is still debate about the relevance of these data to clinical practice (27). This applies also to our findings, although it should be noted that, when using SBPI as a marker for useful synergy, values many magnitudes above the theoretical pharmacodynamic breakpoint were observed.
The mechanism of synergy between COL and FD remains to be determined. In Gram-positive bacteria, FD acts to inhibit protein synthesis, binding to elongation factor G (EF-G) and locking it to the ribosome (28). Resistance may arise due to point mutations in the gene encoding EF-G (fusA) and/or by acquisition and expression of genes (fusB-fusF) encoding proteins able to act as alternative substrates (29). Gram-negative bacteria are considered to be intrinsically resistant due to impermeability (30). Polymyxins act via an electrostatic interaction with lipopolysaccharide (LPS), disrupting the integrity of the Gram-negative outer membrane and promoting cell lysis. This may increase permeability to compounds that are usually excluded and is hypothesized to be the mechanism behind the synergy observed with glycopeptides and other hydrophobic antimicrobials (31). An inverse relationship between resistance to polymyxins and susceptibility to other antimicrobials has been reported (32). Among the COL-resistant strains studied here, slightly lower MICs were observed for FD (16 to 256 mg/liter). Although overcoming permeability may be important for the activity of FD against A. baumannii, it is notable that the combination retained, and even had enhanced, activity against all the COL-resistant strains. Acquired resistance to COL is a complex adaptive response involving multiple regulatory pathways leading to modifications, loss/alteration of LPS, and changes to the net charge on the outer membrane (33). High-level resistance in clinical isolates is rare, often unstable, and accompanied by fitness costs (34), while heteroresistance is frequently observed and easily maintained with ongoing selective pressure (26).
The potential for FD to limit the emergence of COL resistance when used in combination was therefore investigated by us. In time-kill studies, FD prevented the regrowth of COL-heteroresistant strains and, in serial passage experiments, limited the emergence of COL-resistant mutants. This suggests that a COL-FD combination might limit the emergence of resistance during therapy and be an effective treatment. As the EF-G target is essential and highly conserved among bacterial species (see Fig. S1 in the supplemental material), FD-containing combination treatments might also be useful in combating infections with other resistant Gram-negative pathogens.
Clinical outcome data on combination therapies for XDR and PDR A. baumannii strains are sparse and often conflicting. A recent retrospective review of MDR Gram-negative infections treated with COL in combination with glycopeptides found that this combination improved survival (35), while another linked it with only an increased risk of renal impairment (36). Fusidic acid is licensed in the United Kingdom and Europe, where it is available as an oral preparation, sodium fusidate. It is widely used as an adjunctive agent in the treatment of Staphylococcal infections of the skin and soft tissue, bone and joint, and bloodstream (including for infective endocarditis) and against methicillin-resistant Staphylococcus aureus pneumonia. In the United States, an FD preparation, CEM-102 (Taksta; Cempra Pharmaceuticals), has undergone phase II trials for the treatment of Staphylococcal skin and soft tissue and prosthetic joint infections. The development of a unique loading-dose regimen has passed a number of regulatory and financial hurdles, and CEM-102 may be available as a licensed product in the near future (37).
There may be a number of other advantages, in addition to the antimicrobial effects we have shown in vitro, in partnering FD with polymyxins for the treatment of MDRAB. The drug has excellent bioavailability and widespread tissue penetration into skin, bone, and the respiratory tract. Unlike polymyxins, it is metabolized in the liver and excreted in the bile (38), potentially reducing the risk of nephrotoxicity with coadministration. Although FD is highly protein bound, the free concentrations of both FD and COL required for synergy appear to be very low and, therefore, are likely achievable at sites of infection. Adequate penetration into respiratory tissue and epithelial lining fluid gives the possibility of combining it with aerosolized COL for the treatment of ventilator-associated pneumonia. FD has also been reported to have some immunomodulatory effects in terms of cytokine and interleukin production, which may be advantageous in critically ill or septic patients (39).
The need to administer multiple antimicrobial agents is well recognized in a number of chronic infectious diseases (mycobacterial diseases, bacterial endocarditis, HIV, hepatitis) but less so with acute bacterial infections. Dual therapy has not often been shown to be superior to monotherapy when given empirically for the treatment of Gram-negative sepsis (9). However, in the setting of MDR, XDR, and PDR infections where the organism is already known, combinations as a targeted definitive therapy may be more appropriate. A fuller understanding of the properties and efficacy of FD combinations in dynamic (hollow fiber) and animal models, along with more clinical data, is needed before this approach can be recommended. However, FD is another example of an old antimicrobial that may be fit for repurposing and development as an adjunctive agent for use in the treatment of MDR Gram-negative infections.
Supplementary Material
ACKNOWLEDGMENTS
L.M.P. was supported by a clinical training fellowship from Barts and The London Charity to undertake studies on novel antimicrobial combination therapies. J.W.B., B.B., and D.W.W. were supported by internal funds from Queen Mary University, London; this work was generated as part of routine work. L.M.P., J.W.B., and D.W.W. have received research funding from Pfizer/Wyeth, Basilea, and Astellas in the preceding 5 years for unrelated projects which do not represent any conflict of interest.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00753-15.
REFERENCES
- 1.Howard A, O'Donoghue M, Feeney A, Sleator RD. 2012. Acinetobacter baumannii: an emerging opportunistic pathogen. Virulence 3:243–250. doi: 10.4161/viru.19700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fournier PE, Richet H. 2006. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin Infect Dis 42:692–699. doi: 10.1086/500202. [DOI] [PubMed] [Google Scholar]
- 3.Ayraud-Thévenot S, Huart C, Mimoz O, Taouqi M, Laland C, Bousseau A, Castel O. 2012. Control of multidrug-resistant Acinetobacter baumannii outbreaks in an intensive care unit: feasibility and economic impact of rapid unit closure. J Hosp Infect 82:290–292. doi: 10.1016/j.jhin.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 4.Poirel L, Bonnin RA, Nordmann P. 2011. Genetic basis of antibiotic resistance in pathogenic Acinetobacter species. IUBMB Life 63:1061–1067. doi: 10.1002/iub.532. [DOI] [PubMed] [Google Scholar]
- 5.Maragakis LL, Perl TM. 2008. Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis 46:1254–1263. doi: 10.1086/529198. [DOI] [PubMed] [Google Scholar]
- 6.Nation RL, Li J, Cars O, Couet W, Dudley MN, Kaye KS, Mouton JW, Paterson DL, Tam VH, Theuretzbacher U, Tsuji BT, Turnidge JD. 2015. Framework for optimisation of the clinical use of colistin and polymyxin B: the Prato polymyxin consensus. Lancet Infect Dis 15:225–234. doi: 10.1016/S1473-3099(14)70850-3. [DOI] [PubMed] [Google Scholar]
- 7.Ni W, Shao X, Di X, Cui J, Wang R, Liu Y. 2015. In vitro synergy of polymyxins with other antibiotics for Acinetobacter baumannii: a systematic review and meta-analysis. Int J Antimicrob Agents 45:8–18. doi: 10.1016/j.ijantimicag.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Claeys KC, Fiorvento AD, Rybak MJ. 2014. A review of novel combinations of colistin and lipopeptide or glycopeptide antibiotics for the treatment of multidrug-resistant Acinetobacter baumannii. Infect Dis Ther 3:69–81. doi: 10.1007/s40121-014-0051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paul M, Carmeli Y, Durante-Mangoni E, Mouton JW, Tacconelli E, Theuretzbacher U, Mussini C, Leibovici L. 2014. Combination therapy for carbapenem-resistant Gram-negative bacteria. J Antimicrob Chemother 69:2305–2309. doi: 10.1093/jac/dku168. [DOI] [PubMed] [Google Scholar]
- 10.Hornsey M, Phee L, Stubbings W, Wareham DW. 2013. In vitro activity of the novel monosulfactam BAL30072 alone and in combination with meropenem versus a diverse collection of important Gram-negative pathogens. Int J Antimicrob Agents 42:343–346. doi: 10.1016/j.ijantimicag.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 13.Andrews JM. 2001. Determination of minimum inhibitory concentrations. J Antimicrob Chemother 48(Suppl 1):S5–S16. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- 14.Clinical and Laboratory Standards Institute. 2014. Performance standards for antimicrobial susceptibility testing; 24th informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 15.EUCAST. 2014. Breakpoint tables for interpretation of MICs and zone diameters. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/Breakpoint_table_v_4.0.pdf.
- 16.Moody J. 2004. Synergism testing: broth microdilution checkerboard and broth macrodilution methods, p 5.1.2.1–5.12.23. In Isenberg HD (ed), Clinical microbiology procedures handbook, 2nd ed, vol 2 ASM Press, Washington, DC. [Google Scholar]
- 17.Karlowsky JA, Hoban DJ, Zhanel GG, Goldstein BP. 2006. In vitro interactions of anidulafungin with azole antifungals, amphotericin B, and 5-fluorocytosine against Candida species. Int J Antimicrob Agents 27:174–177. doi: 10.1016/j.ijantimicag.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Milne KE, Gould IM. 2010. Combination testing of multidrug-resistant cystic fibrosis isolates of Pseudomonas aeruginosa: use of a new parameter, the susceptible breakpoint index. J Antimicrob Chemother 65:82–90. doi: 10.1093/jac/dkp384. [DOI] [PubMed] [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. 1999. Methods for determining bactericidal activity of antimicrobial agents. National Committee for Clinical Laboratory Standards, Wayne, PA. [Google Scholar]
- 20.El-Halfawy OM, Valvano MA. 2015. Antimicrobial heteroresistance: an emerging field in need of clarity. Clin Microbiol Rev 28:191–207. doi: 10.1128/CMR.00058-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drago L, De Vecchi E, Nicola L, Tocalli L, Gismondo MR. 2005. In vitro selection of resistance in Pseudomonas aeruginosa and Acinetobacter spp. by levofloxacin and ciprofloxacin alone and in combination with beta-lactams and amikacin. J Antimicrob Chemother 56:353–359. doi: 10.1093/jac/dki204. [DOI] [PubMed] [Google Scholar]
- 22.Friedman L, Alder JD, Silverman JA. 2006. Genetic changes that correlate with reduced susceptibility to daptomycin in Staphylococcus aureus. Antimicrob Agents Chemother 50:2137–2145. doi: 10.1128/AAC.00039-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Rayner CR, Nation RL, Owen RJ, Spelman D, Tan KE, Liolios L. 2006. Heteroresistance to colistin in multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 50:2946–2950. doi: 10.1128/AAC.00103-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 25.de Kraker ME, Davey PG, Grundmann H. 2011. Mortality and hospital stay associated with resistant Staphylococcus aureus and Escherichia coli bacteremia: estimating the burden of antibiotic resistance in Europe. PLoS Med 8:e1001104. doi: 10.1371/journal.pmed.1001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hawley JS, Murray CK, Jorgensen JH. 2008. Colistin heteroresistance in Acinetobacter and its association with previous colistin therapy. Antimicrob Agents Chemother 52:351–352. doi: 10.1128/AAC.00766-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doern CD. 2014. When does 2 plus 2 equal 5? A review of antimicrobial synergy testing. J Clin Microbiol 52:4124–4128. doi: 10.1128/JCM.01121-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y, Koripella RK, Sanyal S, Selmer M. 2010. Staphylococcus aureus elongation factor G: structure and analysis of a target for fusidic acid. FEBS J 277:3789–3803. doi: 10.1111/j.1742-4658.2010.07780.x. [DOI] [PubMed] [Google Scholar]
- 29.O'Neill AJ, McLaws F, Kahlmeter G, Henriksen AS, Chopra I. 2007. Genetic basis of resistance to fusidic acid in staphylococci. Antimicrob Agents Chemother 51:1737–1740. doi: 10.1128/AAC.01542-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verbist L. 1990. The antimicrobial activity of fusidic acid. J Antimicrob Chemother 25(Suppl B):1–5. [DOI] [PubMed] [Google Scholar]
- 31.Liu B, Liu Y, Di X, Zhang X, Wang R, Bai Y, Wang J. 2014. Colistin and anti-Gram-positive bacterial agents against Acinetobacter baumannii. Rev Soc Bras Med Trop 47:451–456. doi: 10.1590/0037-8682-0081-2014. [DOI] [PubMed] [Google Scholar]
- 32.Li J, Nation RL, Owen RJ, Wong S, Spelman D, Franklin C. 2007. Antibiograms of multidrug-resistant clinical Acinetobacter baumannii: promising therapeutic options for treatment of infection with colistin-resistant strains. Clin Infect Dis 45:594–598. doi: 10.1086/520658. [DOI] [PubMed] [Google Scholar]
- 33.Olaitan AO, Morand S, Rolain JM. 2014. Mechanisms of polymyxin resistance: acquired and intrinsic resistance in bacteria. Front Microbiol 5:643. doi: 10.3389/fmicb.2014.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pournaras S, Poulou A, Dafopoulou K, Chabane YN, Kristo I, Makris D, Hardouin J, Cosette P, Tsakris A, De E. 2014. Growth retardation, reduced invasiveness, and impaired colistin-mediated cell death associated with colistin resistance development in Acinetobacter baumannii. Antimicrob Agents Chemother 58:828–832. doi: 10.1128/AAC.01439-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petrosillo N, Giannella M, Antonelli M, Antonini M, Barsic B, Belancic L, Inkaya AC, De Pascale G, Grilli E, Tumbarello M, Akova M. 2014. Clinical experience of colistin-glycopeptide combination in critically ill patients infected with Gram-negative bacteria. Antimicrob Agents Chemother 58:851–858. doi: 10.1128/AAC.00871-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garnacho-Montero J, Amaya-Villar R, Gutierrez-Pizarraya A, Espejo-Gutierrez de Tena E, Artero-Gonzalez ML, Corcia-Palomo Y, Bautista-Paloma J. 2013. Clinical efficacy and safety of the combination of colistin plus vancomycin for the treatment of severe infections caused by carbapenem-resistant Acinetobacter baumannii. Chemotherapy 59:225–231. doi: 10.1159/000356004. [DOI] [PubMed] [Google Scholar]
- 37.Fernandes P, Pereira D. 2011. Efforts to support the development of fusidic acid in the United States. Clin Infect Dis 52(Suppl 7):S542–S546. doi: 10.1093/cid/cir170. [DOI] [PubMed] [Google Scholar]
- 38.Turnidge J. 1999. Fusidic acid pharmacology, pharmacokinetics, and pharmacodynamics. Int J Antimicrob Agents 12(Suppl 2):S23–S34. doi: 10.1016/S0924-8579(98)00071-5. [DOI] [PubMed] [Google Scholar]
- 39.Nicoletti F, Zaccone P, Di Marco R, Magro G, Grasso S, Morrone S, Santoni A, Tempera G, Meroni PL, Bendtzen K. 1995. Effects of sodium fusidate in animal models of insulin-dependent diabetes mellitus and septic shock. Immunology 85:645–650. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.