Abstract
Carbapenem-resistant Enterobacteriaceae (CRE) usually infect patients with significant comorbidities and health care exposures. We present a case of a pregnant woman who developed community-acquired pyelonephritis caused by KPC-producing Klebsiella pneumoniae. Despite antibiotic treatment, she experienced spontaneous prolonged rupture of membranes, with eventual delivery of a healthy infant. This report demonstrates the challenge that CRE may pose to the effective treatment of common infections in obstetric patients, with potentially harmful consequences to maternal and neonatal health.
CASE REPORT
Pyelonephritis in pregnancy confers a high risk of maternal complications and preterm birth (1). Carbapenem-resistant Enterobacteriaceae (CRE) threaten patients throughout the continuum of health care but do not commonly cause community-associated infections. Extensive resistance and the limited efficacy of existing alternatives limit the treatment of infections caused by CRE (2). We review a case of pyelonephritis caused by CRE acquired in the community by an unexpected host, a pregnant woman, which led to obstetric complications and therapeutic challenges.
CASE PRESENTATION
A 27-year-old woman (G3P0020) presented at 18 weeks of pregnancy to the emergency department of a metropolitan hospital in northeastern Ohio with fever, rigors, nausea, vomiting, dysuria, and flank pain. She had a history of recurrent urinary tract infections (UTIs) since childhood. The most recent episode occurred at the onset of pregnancy (8 weeks), was caused by Escherichia coli susceptible to ampicillin and all tested cephalosporins, and was treated with cephalexin. Previously, she had been prescribed nitrofurantoin and trimethoprim-sulfamethoxazole to treat a UTI caused by similarly susceptible bacteria. She had not traveled outside the region and had not been admitted to the hospital in the past 2 years. Recently, the patient had cared for her mother, who received chemotherapy for breast and ovarian cancer; the mother had not been treated at a long-term-care facility.
On admission, the patient's temperature was 102.4°F, her pulse was 110 beats/min, and her blood pressure was within the normal range. On physical examination, dry mucous membranes were noted; pulmonary auscultation did not reveal any rales, and her abdomen was gravid and nontender, with a fetal heart rate measured at 140 beats/min. Right costovertebral angle tenderness was demonstrated. Urine analysis showed pyuria; empirical antibiotic treatment with ceftriaxone was administered at 2 g intravenously (i.v.) every 24 h for 3 days. The patient's fever persisted, and a urine culture eventually yielded >100,000 CFU/ml of Klebsiella pneumoniae. Antimicrobial susceptibility testing (AST) demonstrated resistance to all beta-lactams, including carbapenems, as well as aminoglycosides and quinolones (Table 1). Blood cultures remained sterile, and ultrasound imaging did not reveal abscess or anatomical anomalies of the urinary tract.
TABLE 1.
Antimicrobial susceptibility testing of KPC-2-producing K. pneumoniaea
| Antibiotic(s) | MIC (μg/ml) | Interpretation |
|---|---|---|
| Ampicillin | ≥32 | Resistant |
| Ciprofloxacin | ≥4 | Resistant |
| Ceftriaxone | ≥64 | Resistant |
| Cefepime | 32 | Resistant |
| Meropenem | ≥16 | Resistant |
| Imipenem | 32 | Resistant |
| Gentamicin | ≥16 | Resistant |
| Amikacin | 32 | Intermediate |
| Colistin | 0.5 | Susceptible |
| Nitrofurantoin | 256 | Resistant |
| Piperacillin-tazobactam | ≥128/4 | Resistant |
| Fosfomycin | 32 | Susceptible |
According to guidelines issued by the Clinical and Laboratory Standards Institute.
CHALLENGE QUESTION
Which antimicrobial(s) would be appropriate for the patient presented in the case?
A. Colistin (i.v.)
B. Oral fosfomycin
C. Oral fosfomycin and extended-infusion meropenem
D. Oral fosfomycin and extended-infusion cefepime
E. Ceftazidime-avibactam (i.v.)
F. Meropenem and ertapenem (i.v.)
TREATMENT AND OUTCOME
After the results of AST became available, antimicrobial therapy was administered; it consisted of one dose of meropenem (2 g standard infusion), followed by two doses of meropenem (2 g i.v. over 3 h every 8 h), and fosfomycin (3 g per os [p.o.] every 72 h for a total of three doses). Meropenem was switched to cefepime (6 g i.v. administered over 24 h by continuous infusion for 6 days). Urine cultures at days 4 and 12 were sterile.
At week 26 of pregnancy, the patient relapsed with fever, rigors, and costovertebral angle tenderness. K. pneumoniae with antibiotic susceptibilities identical to those of the first organism was isolated from the urine. The patient again received fosfomycin at 3 g p.o. (one dose) and cefepime at 6 g i.v. over 24 h by continuous infusion for 14 days, followed by cefepime at 2 g i.v. every 8 h by standard infusion administered for approximately 10 weeks until delivery. Urine cultures obtained every week or every other week remained sterile. At week 38, the patient experienced spontaneous prolonged rupture of membranes and arrest of labor, leading to cesarean section with delivery of a healthy infant. During labor, she received four doses of cefepime at 2 g i.v. every 8 h by standard infusion. Microbiological cure and resolution of symptoms were documented at a follow-up visit 6 weeks after delivery (at which time she was off antibiotics). The infant remained healthy during the same period.
As this was an unexpected pathogen, we sought to characterize the strain and its mechanism of resistance to carbapenems and searched for the presence of blaIMP, blaKPC, blaNDM, and blaVIM. The blaKPC gene was detected and sequenced as blaKPC-2 (2). Multilocus sequence typing (MLST), following the method of Diancourt et al., demonstrated that the KPC-2-harboring K. pneumoniae isolate from this case belonged to sequence type 258 (ST258), which is the predominant strain of CRE found in northeastern Ohio (2, 3). Further genetic typing using repetitive-sequence-based PCR (rep-PCR) revealed that the KPC-2-harboring K. pneumoniae from this patient shares <80% similarity with other K. pneumoniae ST258 isolates from northeastern Ohio and represents a strain that was distinct from the two predominant clades found in the region (Fig. 1). Additionally, PCR amplification and sequencing of the wzi gene of the capsular polysaccharide cps cluster (according to the method described by Brisse et al. [4]) identified sequence 83, which predicts capsular type K23. In contrast, the two predominant KPC-producing K. pneumoniae ST258 clades in northeastern Ohio belong to wzi sequences 29 and 81, which predict capsular types K41 and K81, respectively.
FIG 1.
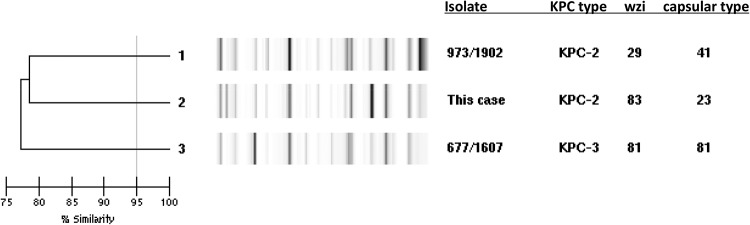
Genetic typing of Klebsiella pneumoniae from the present case with rep-PCR and sequencing of wzi demonstrate that the KPC-2-harboring K. pneumoniae ST258 isolate from this case differs from the two KPC-2- and KPC-3-producing K. pneumoniae ST258 isolates representative (973/1902 and 677/1607) of the two clades that predominate in northeastern Ohio.
Here we present a case of complicated urinary tract infection (cUTI) caused by KPC-producing K. pneumoniae in a pregnant woman without recent health care exposures or travel history; this is a very unexpected host for this pathogen. This report highlights the potential emergence of CRE in community-associated infections and echoes reports of New Delhi metallo-beta-lactamase (NDM)-producing Enterobacteriaceae causing cystitis among community dwellers (5). Molecular typing revealed that the KPC-producing K. pneumoniae isolate from this case, although belonging to the widespread ST258, is of a genotype and capsular type different from those of other isolates found in northeastern Ohio. Therefore, this represents the introduction of a novel strain with an unusual clinical behavior in our community. The establishment in our region of a network for the molecular surveillance of CRE allowed us to detect this sentinel event (2). To our knowledge, no other community-acquired CRE infections have occurred in our region.
Infection with KPC-producing K. pneumoniae in this case proved difficult to treat due to extensive drug resistance and the potential adverse impact of antibiotics on fetal health. We chose fosfomycin as the “backbone” of the antibiotic regimen because of its effectiveness against KPC-producing K. pneumoniae and its safety when given for bacteriuria in pregnancy (category B) (6, 7). We refrained from using colistin, a mainstay of therapy against CRE infection, because the existing evidence is inadequate to determine the risk of fetal harm when polymyxins are used in pregnancy (category C), and we would have used this agent only if the maternal condition had justified the potential risk to the fetus. Tigecycline was not considered since it may cause fetal harm (category D); furthermore, tigecycline has limited effectiveness in the treatment of UTIs. Rather, we added cefepime, which is regarded as safe in pregnancy (category B) and offers potential synergistic activity with fosfomycin, also an inhibitor of cell wall synthesis (8). Cefepime, a zwitterion, achieves high concentrations in the periplasm and is a relatively poor substrate for hydrolysis by KPC-2 beta-lactamase (9). Since cefepime is stable over time, it is feasible to administer it in continuous infusion in order to maximize the time that the concentration of the drug exceeds the MIC of the organism, the most relevant pharmacodynamic parameter. By using 6 g/day as a continuous infusion, we estimate that levels in plasma of 20 to 30 μg/ml were maintained; moreover, cefepime achieves a very high concentration in the urine, averaging 3,120 μg/ml after the administration of a 2-g dose. We therefore reasoned that concentrations of cefepime sufficient to inhibit the growth of K. pneumoniae (MIC = 32 μg/ml) would be maintained in the urine and genitourinary tract. Given that its half-life is 2 h (in subjects with normal renal function), we estimate that the concentration of cefepime in urine 2 days after stopping treatment is negligible at <0.00002 μg/ml (http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/050679s036lbl.pdf). In this case, treatment was prolonged for several weeks until delivery with the intent of preventing further relapses of pyelonephritis and sepsis, considered seriously detrimental to the fetus and justifying the risk of “collateral damage” from antibiotics. This approach was successful, as proven by sterile urine cultures (obtained weekly while the patient was on cefepime and 6 weeks after the end of therapy) and the absence of symptoms.
Meropenem (also category B) administered as a prolonged infusion in combination with fosfomycin may have achieved a similar effect. Indeed, meropenem administered by continuous infusion was used successfully to treat a KPC-producing K. pneumoniae bloodstream infection arising from a urinary source. In that case, fresh solutions of meropenem had to be prepared every 8 h to overcome the potential instability of meropenem after several hours at room temperature, posing an additional logistical challenge (10). This, and our limited experience with the use of carbapenems in pregnancy (relative to that with cephalosporins), justified the choice of cefepime over meropenem or dual-carbapenem therapy.
Ceftazidime coformulated with avibactam, a novel beta-lactamase inhibitor that inactivates KPC, recently received approval by the U.S. FDA for the treatment of cUTI but was not available when this patient was treated. Registration trials included one female patient with cUTI proven to be caused by KPC-producing K. pneumoniae (meropenem MIC, >8 μg/ml) who was successfully treated with ceftazidime-avibactam. Of note, female patients recruited in these trials had a negative serum pregnancy test; reproductive toxicology studies performed with avibactam during early pregnancy in animals did not raise any safety concerns (http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM425459.pdf.).
In conclusion, this case illustrates an attempt to devise an antibiotic regimen to overcome a molecularly defined mechanism of resistance that is a challenge to clinicians worldwide. At the same time, it underscores the obstacles for the delivery of precise and effective treatment for infections in the era of extensively drug-resistant organisms, such as genetic heterogeneity of bacteria, host factors that affect treatment response and recurrence, and limited knowledge regarding drug combinations.
COMMENTARY
The development of an antibiotic regimen for any given patient is no easy matter. In the accompanying paper, Khatri and colleagues describe their decision-making when posed with a pregnant patient who developed pyelonephritis due to KPC-producing Klebsiella pneumoniae. Three issues became relevant when deliberating on the drug regimen: (i) extensive drug resistance, (ii) the type and site of the infection (pyelonephritis), and (iii) pregnancy. We will examine each of these sequentially.
The term “extensive drug resistance” (XDR) refers to loss of susceptibility to all but one or two antibiotic classes. Although Khatri and colleagues do not list susceptibility to all antibiotics which comprise the XDR definition (11), it is clear that KPC production severely compromised the antibiotic choice for this patient. A number of observational studies have suggested that combination antibiotic therapy leads to a superior clinical outcome in patients with severe infections due to KPC producers than monotherapy does (12). It is important to point out that the superiority of combination antibiotic therapy for KPC producers has not yet been shown in randomized controlled trials, although such trials are under way.
Interestingly, Khatri and colleagues chose to use cefepime in their antibiotic regimen, despite in vitro testing indicating a MIC of 32 mg/liter, which is defined as “resistant” using current breakpoints. A number of authors have now used prolonged infusions of beta-lactam antibiotics to optimize pharmacodynamic parameters and potentially achieve prolonged antibiotic concentrations above the MIC (13), even if such a MIC may be defined as “resistant.” In this era of XDR, breakpoints should be regarded as “guidelines” rather than as absolute determinants of the efficacy of an antibiotic regimen against any given organism.
Increasingly, concentrations of beta-lactam antibiotics can be measured in clinical practice to determine whether such concentrations actually exceed the MIC for a considerable part of the dosing interval. In a study of 384 critically ill patients receiving beta-lactams who had drug concentrations measured (14), 16% did not achieve a target of 50% fT>MIC (free antibiotic concentrations above the MIC at 50% of the dosing interval), and these patients were 32% less likely to have a positive clinical outcome. A positive clinical outcome was associated with increasing 50% fT>MIC and 100% fT>MIC ratios (14). This underscores the importance of infectious disease clinicians interacting with clinical pathologists at their hospitals in order to facilitate introduction of beta-lactam drug concentrations into routine clinical practice.
The site of infection is also an important consideration in choosing an antibiotic regimen. The issue of utility of orally administered fosfomycin for infections other than cystitis is vexed. Current guidelines for the treatment of pyelonephritis do not include fosfomycin as a recommended agent (15). Serum and renal tissue concentrations of fosfomycin, when administered orally, are substantially lower than urinary concentrations (16). In one study, clinical cure of patients with pyelonephritis due to fosfomycin-susceptible E. coli was only 44% in patients treated with oral fosfomycin (8 g twice daily for 1 week) (16). These results send a clear message to drug developers – there is an urgent need for new orally administered antibiotics active against multidrug-resistant (MDR) and XDR Gram-negative organisms. While the situation is being improved somewhat with intravenous therapy, few new options exist for orally administered therapy (with the possible exception of eravacycline).
The final consideration in the case presented by Khatri and colleagues is that of the use of antibiotics in pregnancy. It is estimated that between 19 and 44% of women are prescribed antibiotics during pregnancy (17). In the case of critically ill patients, concerns about the pregnant woman's well-being typically outweigh the potential risk of teratogenicity. However, in most clinical situations, such as the one described, the pregnant state substantially reduces treatment options. Increasing antibiotic resistance and the need for classes other than “safe” penicillins and cephalosporins mandate exploration of the safety in pregnancy of the antibiotics most likely to be used for XDR organisms.
This case highlights the alarming prospect of a community-acquired XDR K. pneumoniae infection in an otherwise healthy host. Unfortunately, it is likely that clinicians will increasingly face such therapeutic dilemmas in the future.
ACKNOWLEDGMENTS
The work related to the case article was supported by the Cleveland Department of Veterans Affairs (VA), by the Geriatric Research Education and Clinical Center VISN 10, and by funds from the VA Merit Review Program (award 1I01BX001974), the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (NIH) (awards R01AI063517 and R01AI10056), the Antibiotic Resistance Leadership Group (award UM1AI104681 from NIH), and the Clinical and Translational Science Collaborative of Cleveland (award UL1TR000439 from the National Center for Advancing Translational Sciences). The content is the responsibility of the authors and does not represent the official views of the NIH or the VA.
This Journal section presents a real, challenging case involving a multidrug-resistant organism. The case authors present the rationale for their therapeutic strategy and discuss the impact of mechanisms of resistance on clinical outcome. An expert clinician then provides a commentary on the case.
REFERENCES
- 1.Dotters-Katz SK, Heine RP, Grotegut CA. 2013. Medical and infectious complications associated with pyelonephritis among pregnant women at delivery. Infect Dis Obstet Gynecol 2013:124102. doi: 10.1155/2013/124102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Duin D, Perez F, Rudin SD, Cober E, Hanrahan J, Ziegler J, Webber R, Fox J, Mason P, Richter SS, Cline M, Hall GS, Kaye KS, Jacobs MR, Kalayjian RC, Salata RA, Segre JA, Conlan S, Evans S, Fowler VG Jr, Bonomo RA. 2014. Surveillance of carbapenem-resistant Klebsiella pneumoniae: tracking molecular epidemiology and outcomes through a regional network. Antimicrob Agents Chemother 58:4035–4041. doi: 10.1128/AAC.02636-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diancourt L, Passet V, Verhoef J, Grimont PA, Brisse S. 2005. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J Clin Microbiol 43:4178–4182. doi: 10.1128/JCM.43.8.4178-4182.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brisse S, Passet V, Haugaard AB, Babosan A, Kassis-Chikhani N, Struve C, Decre D. 2013. wzi gene sequencing, a rapid method for determination of capsular type for Klebsiella strains. J Clin Microbiol 51:4073–4078. doi: 10.1128/JCM.01924-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nordmann P, Couard JP, Sansot D, Poirel L. 2012. Emergence of an autochthonous and community-acquired NDM-1-producing Klebsiella pneumoniae in Europe. Clin Infect Dis 54:150–151. doi: 10.1093/cid/cir720. [DOI] [PubMed] [Google Scholar]
- 6.Endimiani A, Patel G, Hujer KM, Swaminathan M, Perez F, Rice LB, Jacobs MR, Bonomo RA. 2010. In vitro activity of fosfomycin against blaKPC-containing Klebsiella pneumoniae isolates, including those nonsusceptible to tigecycline and/or colistin. Antimicrob Agents Chemother 54:526–529. doi: 10.1128/AAC.01235-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keating GM. 2013. Fosfomycin trometamol: a review of its use as a single-dose oral treatment for patients with acute lower urinary tract infections and pregnant women with asymptomatic bacteriuria. Drugs 73:1951–1966. doi: 10.1007/s40265-013-0143-y. [DOI] [PubMed] [Google Scholar]
- 8.Kastoris AC, Rafailidis PI, Vouloumanou EK, Gkegkes ID, Falagas ME. 2010. Synergy of fosfomycin with other antibiotics for Gram-positive and Gram-negative bacteria. Eur J Clin Pharmacol 66:359–368. doi: 10.1007/s00228-010-0794-5. [DOI] [PubMed] [Google Scholar]
- 9.Lamoureaux TL, Frase H, Antunes NT, Vakulenko SB. 2012. Antibiotic resistance and substrate profiles of the class A carbapenemase KPC-6. Antimicrob Agents Chemother 56:6006–6008. doi: 10.1128/AAC.01338-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho VP, Jenkins SG, Afaneh CI, Turbendian HK, Nicolau DP, Barie PS. 2011. Use of meropenem by continuous infusion to treat a patient with a bla(kpc-2)-positive Klebsiella pneumoniae blood stream infection. Surg Infect (Larchmt) 12:325–327. doi: 10.1089/sur.2010.072. [DOI] [PubMed] [Google Scholar]
- 11.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 12.Doi Y, Paterson DL. 2015. Carbapenemase-producing Enterobacteriaceae. Semin Respir Crit Care Med 36:74-84. doi: 10.1055/s-0035-1544208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacVane SH, Kuti JL, Nicolau DP. 2014. Prolonging beta-lactam infusion: a review of the rationale and evidence, and guidance for implementation. Int J Antimicrob Agents 43:105–113. doi: 10.1016/j.ijantimicag.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 14.Roberts JA, Paul SK, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, Kaukonen KM, Koulenti D, Martin C, Montravers P, Rello J, Rhodes A, Starr T, Wallis SC, Lipman J, DALI Study. 2014. DALI: defining antibiotic levels in intensive care unit patients: are current beta-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis 58:1072–1083. doi: 10.1093/cid/ciu027. [DOI] [PubMed] [Google Scholar]
- 15.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, Moran GJ, Nicolle LE, Raz R, Schaeffer AJ, Soper DE. 2011. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 52:e103–e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 16.Ode B, Haidl S, Hoffstedt B, Walder M, Ursing J. 1988. Fosfomycin versus ampicillin in the treatment of acute pyelonephritis. Chemioterapia 7:96–100. [PubMed] [Google Scholar]
- 17.Meeraus WH, Petersen I, Gilbert R. 2015. Association between antibiotic prescribing in pregnancy and cerebral palsy or epilepsy in children born at term: a cohort study using the Health Improvement Network. PLoS One 10:e0122034. doi: 10.1371/journal.pone.0122034. [DOI] [PMC free article] [PubMed] [Google Scholar]


