Abstract
In 2002 and 2006, respectively, miltefosine (MIL) and paromomycin (PMM) were licensed in the Indian subcontinent for treatment of visceral leishmaniasis; however, their future routine use might become jeopardized by the development of drug resistance. Although experimental selection of resistant strains in vitro has repeatedly been reported using the less relevant promastigote vector stage, the outcome of resistance selection on intracellular amastigotes was reported to be protocol and species dependent. To corroborate these in vitro findings, selection of resistance in Leishmania donovani and Leishmania infantum was achieved by successive treatment/relapse cycles in infected Syrian golden hamsters. For PMM, resistant amastigotes were already obtained within 3 treatment/relapse cycles, while their promastigotes retained full susceptibility, thereby sharing the same phenotypic characteristics as in vitro-generated PMM-resistant strains. For MIL, even five treatment/relapse cycles failed to induce significant susceptibility changes in either species, which also corresponds with the in vitro observations where selection of an MIL-resistant phenotype proved to be quite challenging. In conclusion, these results argue for cautious use of PMM in the field to avoid rapid emergence of primary resistance and highlight the need for additional research on the mechanisms and dynamics of MIL resistance selection.
INTRODUCTION
In the Indian subcontinent, the spread of antimony resistance has enforced a shift in visceral leishmaniasis (VL) therapy. Miltefosine (MIL) was licensed for VL in 2002 and is now being used as a first-line therapy within the Kala-azar elimination program in India, Nepal, and Bangladesh (1). Quite recently, increased MIL treatment failure rates have been reported (2) that have been endorsed by the first reports of laboratory-confirmed primary field resistance (3, 4). Paromomycin (PMM), an aminoglycoside antibiotic with a confirmed effectivity against VL, was licensed in 2006 mainly for use in combination therapy (5). For now, its use is still limited and widespread field resistance has not yet been reported, although some naturally PMM-resistant strains have already been documented (4). Given the paucity of other affordable VL therapeutic options and the increasing pressure on MIL therapy, more widespread use of PMM may logically ensue. Conversely, laboratory studies already demonstrated that MIL and PMM resistance can be selected in vitro using axenic promastigotes (6–8). Considering the debatable relevance of promastigote-based studies, our group developed an in vitro resistance selection protocol on intracellular amastigotes, revealing a process-dependent outcome (4, 9). Rapid generation of PMM-resistant amastigotes for several Leishmania donovani and Leishmania infantum strains was obtained, while the derived promastigotes remained fully PMM susceptible. In contrast, selection of MIL resistance consistently failed as reflected by the unchanged MIL susceptibilities at the promastigote and amastigote levels (4). To validate these unexpected in vitro findings and in an alternative attempt to obtain MIL-resistant strains, the present study in hamsters established a resistance selection process on in vivo amastigotes. This model was chosen based on the previous observations that Syrian golden hamsters do not fully clear Leishmania infection after MIL treatment at 40 mg/kg of body weight orally for 5 days (10) and that intraperitoneal PMM treatment at 150 mg/kg for 5 days only resulted in 80% reduction of amastigote burdens (S. Hendrickx, unpublished data); this is in contrast to the study performed by Sane et al. (11). Since these two treatment regimens resulted in incomplete parasite clearance, this treatment approach was exploited in the present study to monitor relapse and recurrence of disease in treated animals and assess the effect of successive treatment cycles on drug susceptibility, thereby fully mimicking clinical drug use in the patient. Using this strategy, strains generated in vivo and in vitro can be compared phenotypically, validating our resistance selection procedures.
MATERIALS AND METHODS
Ethics statement.
The use of laboratory rodents was carried out in strict accordance to all mandatory guidelines (European Union directives, including the Revised Directive 2010/63/EU on the protection of animals used for scientific purposes that went into effect on 1 January 2013 and the Declaration of Helsinki in its latest version) and was approved by the ethical committee of the University of Antwerp, Belgium (UA-ECD 2010-17 [18 August 2010]).
Animals.
Female golden hamsters (body weight, 80 to 100 g) were purchased from Janvier (France) and kept in quarantine for at least 5 days before infection. Food for laboratory rodents (Carfil, Arendonk, Belgium) and drinking water were available ad libitum. The animals were randomly allocated to experimental units of 3 animals each.
Leishmania parasites and infection.
Leishmania infantum (MHOM/MA[BE]/67/ITMAP263) and L. donovani (MHOM/ET/67/L82) ex vivo amastigotes were obtained from the spleen of heavily infected donor hamsters and purified using two centrifugation steps. After determination of the Stauber index (12), the amastigote suspension was diluted to prepare an infection inoculum containing 2 × 107 amastigotes/100 μl phosphate-buffered saline (PBS). Hamsters were anesthetized by isoflurane inhalation and infected by intracardial injection. Promastigotes were cultured in promastigote hemoflagellate minimal essential medium (HOMEM) supplemented with 20% inactivated fetal calf serum (Invitrogen, Ghent, Belgium) and 20% spent promastigote medium (13).
Treatment relapse schedule and evaluation parameters.
Two treatment groups were set up for each Leishmania species: one orally treated with MIL (Sigma, Diegem, Belgium) at 20 mg/kg body weight for 5 consecutive days and one intraperitoneally treated with PMM (Sigma, Diegem, Belgium) at 350 mg/kg body weight for 5 consecutive days. MIL (molecular weight [MW], 407.57) was formulated in distilled water at 20 mg/ml, and PMM (MW, 713.71) was formulated in distilled water at 150 mg/ml. Infected animals were treated starting from 21 days postinfection (dpi). Animals were then closely monitored, since the time-to-relapse would most likely vary between individual hamsters and might shorten upon subsequent selection cycles owing to decreasing parasite susceptibility. Their general condition and body weight were checked twice weekly. When an animal presented clinical signs of recurrent disease (body weight loss and deteriorating overall appearance), a liver biopsy specimen was taken to confirm the presence of adequate parasite burden, and a next round of treatment was initiated. Whenever possible, a promastigote back-transformation assay was performed on the biopsy specimens to evaluate the PMM-MIL susceptibility in vitro. To minimize animal suffering, hamsters were euthanized with a CO2 overdose after the second relapse. At that time, they were used to collect spleen-derived ex vivo amastigotes to allow infection of a new hamster (Fig. 1). Subsequent selection rounds were terminated either when drug susceptibility values indicated that a resistant endpoint was reached or after 3 successive treatment/relapse cycles.
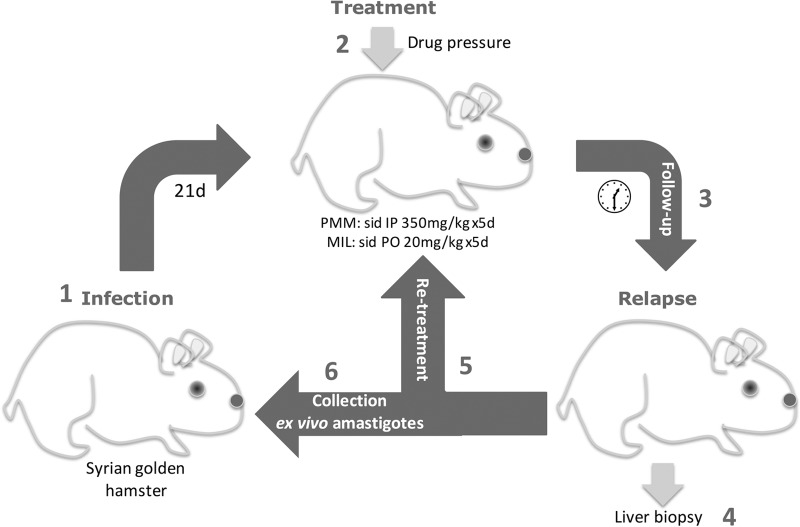
FIG 1.
Schematic representation of the in vivo drug resistance selection protocol in the Syrian golden hamster. (1) Hamsters were infected with ex vivo amastigotes. (2) After 21 days, infected hamsters were treated with PMM or MIL. (3) Upon treatment, hamsters were closely monitored until they relapsed showing clinical signs. (4) A liver biopsy specimen was taken to assess the presence of substantial amastigote burdens. (5 and 6) Hamsters were treated again with MIL or PMM (5) or they were euthanized for harvesting ex vivo spleen-derived amastigotes to be used to infect a new hamster, thereby initiating the next selection cycle (6). Sid, once daily; IP, intraperitoneal; PO, per oral; d, day.
Susceptibility determination.
Amastigote and promastigote susceptibility was determined as described previously (14). For promastigote susceptibility testing, procyclic promastigotes were exposed to 2-fold serial drug dilutions. After 4 days incubation at 25°C, promastigote viability was measured using resazurin and 50% inhibitory concentration (IC50) values were determined. For amastigote susceptibility testing, primary peritoneal macrophages were infected with metacyclic promastigotes. After 24 h, the medium was discarded and replaced by 2-fold drug dilutions in medium. Cells were incubated at 37°C in the presence of 5% CO2 for 4 days, upon which the plates were Giemsa-stained for microscopic determination of the IC50 values. Cutoff values for resistance were set at 150 μM for PMM and 15 μM for MIL, as described earlier (4). The activity index (AI) as introduced by Yardley et al. (15) was used to normalize IC50 values between different strains by direct comparison with the susceptible reference strain. Based on previous studies on antimonials, strains with an AI of >4 can be considered fully resistant (16).
Statistical analysis.
Statistical differences between the different passages were analyzed using two-way analysis of variance (ANOVA). Results were considered statistically significant if P was <0.05.
RESULTS
Promastigote back-transformation assay.
On the liver biopsy specimens and at autopsy, promastigote back-transformation was performed in an attempt to recover residual parasites that could be used for in vitro susceptibility determination. However, due to difficulties of adaptation of the amastigotes derived in vivo to adjust to the in vitro conditions, this assay unfortunately was not always successful. When the promastigote back-transformation did become positive, promastigotes were collected and transferred to routine promastigote culture with modifications to the promastigote medium to contain a higher concentration of fetal calf serum (20%) and spent medium to boost promastigote growth.
Amastigote and promastigote susceptibility data.
Already after one treatment cycle, L. infantum amastigotes from relapsed PMM-treated hamsters were considered PMM-resistant according to the preset IC50 cutoff value of >150 μM (4), while the promastigotes remained fully PMM susceptible. The marginally increased AI values for promastigotes were not significant (Table 1). Since selection of PMM resistance was immediately obtained, no further selection was performed. As for the L. donovani-infected PMM-treated hamsters, a significant shift in PMM susceptibility was observed on the amastigote level after 3 selection rounds, although the cutoff value for resistance was merely reached. Therefore, two additional treatment/relapse cycles were carried out, finally resulting in a generation of fully PMM-resistant amastigotes and PMM-susceptible promastigotes (Table 2). Although the selection of PMM resistance proved to be straightforward, successive MIL treatment of L. infantum and L. donovani did not result in the anticipated shift in MIL IC50 values toward an MIL-resistant phenotype (Tables 3 and 4). Moreover, determination of MIL susceptibility after successive MIL treatment rounds was relatively challenging due to the lack of promastigote growth capability after amastigote-to-promastigote back-transformation.
TABLE 1.
Amastigote and promastigote susceptibility of L. infantum upon PMM selection rounds
| L. infantum typea | PMM (μM) amastigote susceptibility (mean ± SEM) | AIb | PMM (μM) promastigote susceptibility (mean ± SEM) | AI |
|---|---|---|---|---|
| WT | 65.7 ± 5.9 | 17.3 ± 2.9 | ||
| P1 | 397.7 ± 30.4 | 6.0c | 49.5 ± 7.2 | 2.9 (nsd) |
| P2 | 430.9 ± 24.5 | 6.5c | 31.2 ± 8.1 | 1.8 (ns) |
WT, wild type; P1, after 1 drug treatment round; P2, after 2 drug treatment rounds.
IC50 values and corresponding AI were the result of at least three independent in vitro assays run in duplicate.
P is <0.001.
ns, nonsignificant.
TABLE 2.
Amastigote and promastigote susceptibility of L. donovani upon PMM selection rounds
| L. donovani typea | PMM (μM) amastigote susceptibility (mean ± SEM) | AIb | PMM (μM) promastigote susceptibility (mean ± SEM) | AI |
|---|---|---|---|---|
| WT | 73.7 ± 8.2 | 28.1 ± 2.3 | ||
| P1 | NDc | ND | ||
| P2 | 184.9 ± 27.6 | 2.5d | 25.7 ± 3.6 | 0.9 (nse) |
| P3 | ND | ND | ||
| P4 | 294.6 ± 26.9 | 4.0d | 46.0 ± 1.8 | 1.6 (ns) |
WT, wild type; P1, after 1 drug treatment round; P2, after 2 drug treatment rounds; P3, after 3 drug treatment rounds; P4, after 4 drug treatment rounds.
IC50 values and corresponding AI were the result of at least three independent assays run in duplicate.
ND, not done.
P < 0.001.
ns, nonsignificant.
TABLE 3.
Amastigote and promastigote susceptibility of L. infantum upon MIL selection rounds
| L. infantum typea | MIL (μM) amastigote susceptibility (mean ± SEM) | AIb | MIL (μM) promastigote susceptibility (mean ± SEM) | AI |
|---|---|---|---|---|
| WT | 0.4 ± 0.1 | 0.8 ± 0.1 | ||
| P1 | 2.3 ± 0.3 | 5.8c | NDd | |
| P2 | 1.9 ± 0.6 | 4.7c | ND | |
| P3 | 3.0 ± 0.3 | 7.5c | 3.2 ± 0.2 | 4.0c |
WT, wild type; P1, after 1 drug treatment round; P2, after 2 drug treatment rounds; P3, after 3 drug treatment rounds.
IC50 values and corresponding AI were the result of at least three independent in vitro assays run in duplicate.
P < 0.001.
ND, not done.
TABLE 4.
Amastigote and promastigote susceptibility of L. donovani upon MIL selection rounds
| L. donovani typea | MIL (μM) amastigote susceptibility (mean ± SEM) | AIb | MIL (μM) promastigote susceptibility (mean ± SEM) | AI |
|---|---|---|---|---|
| WT | 1.8 ± 0.4 | 1.7 ± 0.2 | ||
| P1 | 1.7 ± 0.2 | 0.9 (nsc) | NDd | |
| P2 | ND | ND | ||
| P3 | 3.6 ± 0.5 | 2.0e | 5.0 ± 0.5 | 2.9e |
WT, wild type; P1, after 1 drug treatment round; P2, after 2 drug treatment rounds; P3, after 3 drug treatment rounds.
IC50 values and corresponding AI were the result of at least three independent assays run in duplicate.
ns, nonsignificant.
ND, not done.
P < 0.001.
DISCUSSION
Due to the spread of primary antimony resistance in the Indian subcontinent, alternative drugs like MIL and PMM are now recommended for the treatment of VL (17). Considering some intrinsic traits of the two drugs, repetitive use in areas where VL is endemic will ultimately lead to the development of primary resistance (5, 17). For example, MIL has a long elimination half-life and requires a 4-week treatment course affecting proper compliance, hence causing lasting suboptimal drug levels in the patient (17). A drop in clinical efficacy has been reported worldwide, resulting in increasing numbers of MIL treatment failure with levels reaching up to 20% in the Indian subcontinent (2). Surprisingly, very few of these relapse isolates actually were MIL resistant in the standard intracellular amastigote laboratory assay. Although a decreased MIL susceptibility was observed in a number of Brazilian L. infantum relapse isolates, susceptibility results of Indian relapse isolates still yield controversy (2, 18, 19). Since the first MIL-resistant isolate was reported in 2012, a second naturally resistant strain was found by our research group (3, 4). As for PMM resistance, this was already reported to develop fairly readily (4, 7, 9, 20). Moreover, numerous in vitro studies demonstrated quick selection of MIL and PMM resistance on promastigotes (6–8). To target the more relevant amastigote stage in mammalian infection, our research group previously developed an in vitro resistance selection protocol on intracellular amastigotes (9). For PMM, several strains with PMM-resistant amastigotes but still fully PMM-susceptible promastigotes were obtained. In contrast, selection on promastigotes caused the two stages to become resistant, showing that in vitro selection data should be interpreted with some caution. For MIL, in vitro selection on intracellular amastigotes failed to induce a susceptibility shift in most L. donovani and L. infantum isolates (4).
Previous experiments already indicated that treatment relapses can be evoked in the Syrian golden hamster, which provides an ideal tool to validate our in vitro selection results in vivo, hereby more closely mimicking the actual field situation (10). Since the Syrian hamster model also has fairly good predictive value for human VL, it was logically selected for our drug resistance selection design in vivo (21, 22). For PMM, implementation of this protocol resulted in a prompt reduction of the PMM susceptibility for L. infantum and L. donovani (Tables 1 and 2). Although amastigote susceptibility declined based on the predetermined susceptibility cutoff value of 150 μM, a drug-susceptible phenotype was conserved at promastigote level, which fully corresponded to the phenotypic outcome after in vitro selection (4). Hence, the present study endorses the applicability and predictivity of our in vitro selection protocol on intracellular amastigotes to generate PMM-resistant strains and once more emphasizes the need to use intracellular amastigotes for the selection of drug resistance. After successive cycles of MIL exposure in vivo and adopting the predetermined susceptibility cutoff IC50 value of 15 μM, no resistant phenotype was revealed on either parasite species or stage (Tables 3 and 4), despite the observation that posttreatment relapse did occur systematically. Using statistical analysis, calculation of the activity index for each passage suggested the appearance of a “resistant” phenotype. However, considering that substantial susceptibility variations may occur between different “susceptible” strains, hence influencing the actual calculated AI value, it may be more rational to use the predetermined susceptibility cutoff values to define “clinical resistance” (4). Conversely, using AI values may still provide a useful tool to indicate more subtle nuances for fundamental drug susceptibility research purposes. After the initial selection cycles, the anticipated MIL relapses mainly resulted in a rapid increase of amastigote burdens in the liver, while the spleen-derived ex vivo amastigotes remained rather scarce. However, adequate purification of liver-derived amastigotes proved to be difficult likely due to the presence of metabolizing enzymes, and only spleen-derived amastigotes were used for subsequent infection. For PMM, this was never an issue, as subtoxic doses resulted in an 80% clearance rate at best, leading to equally rapid increases in parasite burdens in the two target organs.
A remarkable finding was the reduced promastigote growth rate observed after MIL treatment, which conflicts with the higher metacyclogenesis and in vitro infection potential observed for L. donovani MIL relapse isolates from the Indian subcontinent (23), and the increase in parasite fitness observed in promastigote-selected MIL-resistant L. donovani (24). It is evident that additional research is still needed on the impact of MIL resistance on parasite fitness. Although the present in vivo protocol may indeed avoid adaptive phenomena to the in vitro culture system with subsequent virulence loss, in vitro susceptibility testing was practically hampered by the low conversion level of amastigotes into promastigotes. Remarkably, promastigote back-transformation and subsequent growth appeared more problematic after MIL exposure, leaving susceptibility determination mostly entirely dependent on the use of ex vivo amastigotes upon necropsy of MIL-treated animals, which explains the lack of susceptibility data for several passages (Tables 1 to 4). Although the present study offers convincing arguments to investigate phenotypic and genotypic strain-specific alterations by repeated drug exposure, such research will unfortunately be restrained by this problem of in vitro adaptation and will, therefore, be limited to studies on ex vivo amastigotes, if available.
In conclusion and until fully characterized resistant field isolates become available, “laboratory-selected” resistant strains may be a good proxy to study features associated with drug resistance and become an asset in uncovering the underlying dynamics and mechanisms of PMM or MIL resistance. It also needs to be reemphasized that in vitro resistance selection should run on intracellular amastigotes rather than on extracellular promastigotes, while in vivo selection models may have even better predictive value. The present study also highlights why PMM must be avoided in monotherapy and strongly recommends more in-depth research on the underlying mechanisms of MIL relapse to clarify the missing link between treatment failure and the unintentional evolution toward intrinsic MIL resistance.
ACKNOWLEDGMENTS
This work was funded by the Research Fund Flanders (FWO) (projects G051812N and 11V4315N).
We thank Pim-Bart Feijens and Mandy Vermont for their excellent technical assistance with the laboratory and animal work. LMPH is a partner of the Antwerp Drug Discovery Network (ADDN) (www.addn.be).
REFERENCES
- 1.Dhillon GP, Sharma SN, Nair B. 2008. Kala-azar elimination programme in India. J Indian Med Assoc 106(10):664, 666–668. [PubMed] [Google Scholar]
- 2.Rijal S, Ostyn B, Uranw S, Rai K, Bhattarai NR, Dorlo TP, Beijnen JH, Vanaerschot M, Decuypere S, Dhakal SS, Das ML, Karki P, Singh R, Boelaert M, Dujardin JC. 2013. Increasing failure of miltefosine in the treatment of kala-azar in Nepal and the potential role of parasite drug resistance, reinfection, or noncompliance. Clin Infect Dis 56:1530–1538. doi: 10.1093/cid/cit102. [DOI] [PubMed] [Google Scholar]
- 3.Cojean S, Houze S, Haouchine D, Huteau F, Lariven S, Hubert V, Michard F, Bories C, Pratlong F, Le Bras J, Loiseau PM, Matheron S. 2012. Leishmania resistance to miltefosine associated with genetic marker. Emerg Infect Dis 18:704–706. doi: 10.3201/eid1804.110841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hendrickx S, Boulet G, Mondelaers A, Dujardin JC, Rijal S, Lachaud L, Cos P, Delputte P, Maes L. 2014. Experimental selection of paromomycin and miltefosine resistance in intracellular amastigotes of Leishmania donovani and L. infantum. Parasitol Res 113:1875–1881. doi: 10.1007/s00436-014-3835-7. [DOI] [PubMed] [Google Scholar]
- 5.Davidson RN, den Boer M, Ritmeijer K. 2009. Paromomycin. Trans R Soc Trop Med Hyg 103:653–660. doi: 10.1016/j.trstmh.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Seifert K, Matu S, Javier Perez-Victoria F, Castanys S, Gamarro F, Croft SL. 2003. Characterisation of Leishmania donovani promastigotes resistant to hexadecylphosphocholine (miltefosine). Int J Antimicrob Agents 22:380–387. doi: 10.1016/S0924-8579(03)00125-0. [DOI] [PubMed] [Google Scholar]
- 7.Maarouf M, Adeline MT, Solignac M, Vautrin D, Robert-Gero M. 1998. Development and characterization of paromomycin-resistant Leishmania donovani promastigotes. Parasite 5:167–173. doi: 10.1051/parasite/1998052167. [DOI] [PubMed] [Google Scholar]
- 8.Perez-Victoria FJ, Castanys S, Gamarro F. 2003. Leishmania donovani resistance to miltefosine involves a defective inward translocation of the drug. Antimicrob Agents Chemother 47:2397–2403. doi: 10.1128/AAC.47.8.2397-2403.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hendrickx S, Inocencio da Luz RA, Bhandari V, Kuypers K, Shaw CD, Lonchamp J, Salotra P, Carter K, Sundar S, Rijal S, Dujardin JC, Cos P, Maes L. 2012. Experimental induction of paromomycin resistance in antimony-resistant strains of L. donovani: outcome dependent on in vitro selection protocol. PLoS Negl Trop Dis 6:e1664. doi: 10.1371/journal.pntd.0001664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fortin A, Hendrickx S, Yardley V, Cos P, Jansen H, Maes L. 2012. Efficacy and tolerability of oleylphosphocholine (OlPC) in a laboratory model of visceral leishmaniasis. J Antimicrob Chemother 67:2707–2712. doi: 10.1093/jac/dks273. [DOI] [PubMed] [Google Scholar]
- 11.Sane SA, Shakya N, Gupta S. 2011. Immunomodulatory effect of picroliv on the efficacy of paromomycin and miltefosine in combination in experimental visceral leishmaniasis. Exp Parasitol 127:376–381. doi: 10.1016/j.exppara.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Stauber L. 1955. Leishmaniasis in the hamster, p 76–90. In Cole WH. (ed), Some physiological aspects and consequences of parasitism. Rutgers University Press, New Brunswick, NJ. [Google Scholar]
- 13.Maes L, Cos P, Croft S. 2013. The relevance of susceptibility tests, breakpoints and markers, p 407–429. In Ponte-Sucre A, Diaz E, Padŕon-Nieves M (ed), Drug resistance in Leishmania parasites. Springer, Vienna, Austria. [Google Scholar]
- 14.Vermeersch M, da Luz RI, Tote K, Timmermans JP, Cos P, Maes L. 2009. In vitro susceptibilities of Leishmania donovani promastigote and amastigote stages to antileishmanial reference drugs: practical relevance of stage-specific differences. Antimicrob Agents Chemother 53:3855–3859. doi: 10.1128/AAC.00548-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yardley V, Ortuno N, Llanos-Cuentas A, Chappuis F, Doncker SD, Ramirez L, Croft S, Arevalo J, Adaui V, Bermudez H, Decuypere S, Dujardin JC. 2006. American tegumentary leishmaniasis: is antimonial treatment outcome related to parasite drug susceptibility? J Infect Dis 194:1168–1175. doi: 10.1086/507710. [DOI] [PubMed] [Google Scholar]
- 16.da Luz RI, Vermeersch M, Dujardin JC, Cos P, Maes L. 2009. In vitro sensitivity testing of Leishmania clinical field isolates: preconditioning of promastigotes enhances infectivity for macrophage host cells. Antimicrob Agents Chemother 53:5197–5203. doi: 10.1128/AAC.00866-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dorlo TP, Balasegaram M, Beijnen JH, de Vries PJ. 2012. Miltefosine: a review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J Antimicrob Chemother 67:2576–2597. doi: 10.1093/jac/dks275. [DOI] [PubMed] [Google Scholar]
- 18.Bhandari V, Kulshrestha A, Deep DK, Stark O, Prajapati VK, Ramesh V, Sundar S, Schonian G, Dujardin JC, Salotra P. 2012. Drug susceptibility in Leishmania isolates following miltefosine treatment in cases of visceral leishmaniasis and post kala-azar dermal leishmaniasis. PLoS Negl Trop Dis 6:e1657. doi: 10.1371/journal.pntd.0001657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prajapati VK, Sharma S, Rai M, Ostyn B, Salotra P, Vanaerschot M, Dujardin JC, Sundar S. 2013. In vitro susceptibility of Leishmania donovani to miltefosine in Indian visceral leishmaniasis. Am J Trop Med Hyg 89:750–754. doi: 10.4269/ajtmh.13-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jhingran A, Chawla B, Saxena S, Barrett MP, Madhubala R. 2009. Paromomycin: uptake and resistance in Leishmania donovani. Mol Biochem Parasitol 164:111–117. doi: 10.1016/j.molbiopara.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melby PC, Chandrasekar B, Zhao W, Coe JE. 2001. The hamster as a model of human visceral leishmaniasis: progressive disease and impaired generation of nitric oxide in the face of a prominent Th1-like cytokine response. J Immunol 166:1912–1920. doi: 10.4049/jimmunol.166.3.1912. [DOI] [PubMed] [Google Scholar]
- 22.Gupta S. 2011. Visceral leishmaniasis: experimental models for drug discovery. Indian J Med Res 133:27–39. [PMC free article] [PubMed] [Google Scholar]
- 23.Rai K, Cuypers B, Bhattarai NR, Uranw S, Berg M, Ostyn B, Dujardin JC, Rijal S, Vanaerschot M. 2013. Relapse after treatment with miltefosine for visceral leishmaniasis is associated with increased infectivity of the infecting Leishmania donovani strain. mBio 4:e00611-13. doi: 10.1128/mBio.00611-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mishra J, Singh S. 2013. Miltefosine resistance in Leishmania donovani involves suppression of oxidative stress-induced programmed cell death. Exp Parasitol 135:397–406. doi: 10.1016/j.exppara.2013.08.004. [DOI] [PubMed] [Google Scholar]