Abstract
Three hybrid CTX-M β-lactamases, CTX-M-64, CTX-M-123, and CTX-M-132, with N and C termini matching CTX-M-1 group enzymes and centers matching CTX-M-9 group enzymes, have been identified. The hybrid gene sequences suggested recombination between blaCTX-M-15 and blaCTX-M-14, the two most common blaCTX-M variants worldwide. However, blaCTX-M-64 and blaCTX-M-123 are found in an ISEcp1-blaCTX-M transposition unit with a 45-bp “spacer,” rather than the 48 bp usually associated with blaCTX-M-15, and 112 bp of IncA/C plasmid backbone. This is closer to the context of blaCTX-M-55, which has one nucleotide difference from blaCTX-M-15, on IncI2 plasmid pHN1122-1. Here, we characterized an IncI2 plasmid carrying blaCTX-M-15 with a 45-bp spacer (pHNY2-1) by complete sequencing and also sequenced IncI2 plasmids carrying blaCTX-M-64 (pHNAH46-1) or blaCTX-M-132 (pHNLDH19) and an IncI1 plasmid carrying blaCTX-M-123 (pHNAH4-1). pHNY2-1 has the same ISEcp1-blaCTX-M-IncA/C insertion as pHN1122-1, pHNAH46-1, and pHNLDH19, and all four plasmid backbones are almost identical. pHNAH4-1 (IncI1 sequence type 108 [ST108]) carries a transposition unit that includes a 2,720-bp fragment of the IncI2 backbone, suggesting ISEcp1-mediated transfer of blaCTX-M-IncA/C-IncI2 to an IncI1 plasmid. All three hybrid blaCTX-M genes may have resulted from recombination between blaCTX-M-14 and blaCTX-M-15 with a 45-bp spacer on an IncI2 plasmid. Five additional Escherichia coli isolates of different sequence types from different provinces, farms, and/or animals had blaCTX-M-64 on a pHNAH46-1-like IncI2 plasmid and 9 had blaCTX-M-123 on a pHNAH4-1-like IncI1 ST108 plasmid. Thus, epidemic IncI plasmids may be responsible for the spread of blaCTX-M-64 and blaCTX-M-123 between different animals and different locations in China.
INTRODUCTION
CTX-M-type extended-spectrum β-lactamases (ESBLs) that exhibit potent activity against extended-spectrum cephalosporins are widespread, not only in human clinical settings but also in the community and in animals worldwide. The CTX-M family has been considered a model example of the evolution of drug resistance, and their worldwide spread represents a success story for antimicrobial resistance (1, 2). More than 160 CTX-M variants have been identified (http://www.lahey.org/Studies/other.asp#table1), and these can be divided into six groups (CTX-M-1, CTX-M-2, CTX-M-9, CTX-M-8, CTX-M-25, and KLUC) with an intergroup amino acid identity of ≤90% (2, 3). Evolution within different CTX-M groups is the result of gradual accrual of mutations under the selective pressure exerted by the presence of antibiotics (1, 2). However, recombination between genes from different CTX-M groups has also accelerated this evolution (1).
Four CTX-M enzymes (CTX-M-64, CTX-M-123, CTX-M-132, and CTX-M-137) that are hybrids of members of the CTX-M-1 and CTX-M-9 groups have emerged in recent years (4–6). CTX-M-137 is a simple hybrid, with the N terminus matching CTX-M-14 and the C terminus matching CTX-M-15 (6). The other three hybrids match CTX-M-1 group enzymes at the start and end but CTX-M-9 group enzymes in the middle, suggesting different double crossover events (4). Interestingly, these four CTX-M hybrids have all been found in China, where three of them were first reported, and have been detected in animals and in animal food (4, 6–11).
These hybrid blaCTX-M genes have all been suggested to be the result of recombination between blaCTX-M-15 and blaCTX-M-14, the most dominant variants detected worldwide (2, 4–6). However, analysis of the genetic environment surrounding blaCTX-M-123 and blaCTX-M-64 showed that ISEcp1 lies 45 bp upstream of these genes, rather than the usual 48 bp seen with blaCTX-M-15, and orf477Δ downstream of blaCTX-M is followed by a 112-bp fragment matching IncA/C plasmid backbones (4). This genetic environment resembles that of blaCTX-M-55 in the IncI2 plasmid pHN1122-1, from an Escherichia coli isolate from a dog in Guangzhou (12), but blaCTX-M-55 has a nucleotide substitution at position 239 (resulting in A77V) compared with blaCTX-M-15 (4). One possibility was that the blaCTX-M-1/9/1 hybrids resulted from recombination with blaCTX-M-15 with a 45-bp spacer on an IncI2 plasmid (4), an arrangement which had only been reported in a single isolate from Russia in 2010 (13). Here we identified and characterized a plasmid with blaCTX-M-15 and a 45-bp spacer by complete sequencing and also completely sequenced plasmids carrying blaCTX-M-64, blaCTX-M-132, or blaCTX-M-123, for which the immediate contexts had already been identified (12), to try to define how these hybrid genes may have arisen.
MATERIALS AND METHODS
E. coli isolates.
AHC4, carrying blaCTX-M-123, and AHC46, carrying blaCTX-M-64, were found in chicken samples submitted to a veterinary diagnostic center in Anhui Province, China, and the contexts of their blaCTX-M genes had already been partially characterized (4). JC2, from a chicken sample from Shandong Province, was identified as carrying blaCTX-M-15 in a previous study (14). LDH19 was from a urine sample collected in 2013 from a female patient at a community hospital in Guangzhou. PCR with published primers (see Table S1 in the supplemental material) was used to amplify blaCTX-M-1 group and blaCTX-M-1/9/1 hybrid genes in LDH19 and to determine the spacer length in both isolates. Five more E. coli isolates carrying blaCTX-M-64 and 15 carrying blaCTX-M-123 were available from a set of isolates collected from animals in China in 2010-2012 (Table 1) (14). Multilocus sequence typing (MLST) was performed according to http://mlst.warwick.ac.uk.
TABLE 1.
Isolates and plasmids carrying blaCTX-M-15, blaCTX-M-64, blaCTX-M-123, or blaCTX-M-132
| Isolatea | blaCTX-M | Date of isolation (mo/yr) | Source | Region/farmb | E. coli MLST | Plasmid markerc | IncI2/orf477d | IncI2/ISEcp1e | Plasmid size (kb)f |
|---|---|---|---|---|---|---|---|---|---|
| IncI2 | |||||||||
| JC2g | 15 | 11/2006 | Chicken | SD17 | 224 | pHNY2-1 | P | P | 65,358 |
| AHC46h | 64 | 6/2011 | Chicken feces | AH14 | 1011 | pHNAH46-1 | P | P | 62,194 |
| LDH19i | 132 | 1/2013 | Human urine | GD- | 4528 | pHNLDH19 | P | P | 62,194 |
| SG0514-2c | 64 | 3/2012 | Chicken heart | GD07 | 117 | P | P | P | ∼62 |
| ACH5i | 64 | 6/2011 | Chicken feces | AH03 | 162 | P | P | P | ∼62 |
| SG0532-2i | 64 | 3/2012 | Chicken heart | GD07 | 3851 | P | P | P | ∼62 |
| GDC16i | 64 | 8/2010 | Chicken feces | GD15 | 4474 | P | P | P | ∼62 |
| BSC1i | 64 | 1/2010 | Chicken feces | GD13 | 4346 | P | P | P | ∼62 |
| IncI1 ST108 | |||||||||
| AHC4i | 123 | 6/2011 | Chicken feces | AH04 | 746 | pHNAH4-1 | P | NA | 109,194 |
| AHC13h | 123 | 6/2011 | Chicken feces | AH06 | 746 | P | P | NA | ∼113 |
| AHC14i | 123 | 6/2011 | Chicken feces | AH06 | 746 | P (no MRR1) | P | NA | ∼113 |
| AHC2h | 123 | 6/2011 | Chicken feces | AH03 | 746 | P (no MRR1) | P | NA | ∼113 |
| AHC54i | 123 | 7/2011 | Chicken feces | AH02 | 155 | P | P | NA | ∼113 |
| NKSC61h | 123 | 1/2011 | Chicken liver | GD07 | 155 | P | P | NA | ∼109 |
| AHC55h | 123 | 7/2011 | Chicken feces | AH02 | 162 | P | P | NA | ∼113 |
| FKP358i | 123 | 11/2010 | Pig feces | GD05 | 165 | P | P | NA | ∼113 |
| FKD567h | 123 | 8/2012 | Duck feces | GD01 | 1437 | P | P | NA | ∼113 |
| NKSC1i | 123 | 1/2011 | Chicken liver | GD07 | 2309 | P | P | NA | ∼105 |
| Inc group not determined | |||||||||
| FKP614Fj | 64 | 11/2011 | Pig feces | GD16 | 746 | ND | ND | ND | ND |
| TP36g | 123 | 6/2012 | Chicken heart | GD12 | 93 | ND | ND | ND | ND |
| FKP587j | 123 | 1/2011 | Pig feces | GD09 | 156 | ND | ND | ND | ND |
| FKD453j | 123 | 1/2011 | Duck feces | GD10 | 205 | ND | ND | ND | ND |
| FKP97j | 123 | 8/2010 | Pig feces | GD11 | 1437 | ND | ND | ND | ND |
| FKP745j | 123 | 8/2012 | Pig liver | GD08 | 1771 | ND | ND | ND | ND |
Bold type indicates isolates from which plasmids were completely sequenced. Plasmid names are shown in the “Plasmid marker” column.
AH, Anhui Province; GD, Guangdong Province; SD, Shandong Province. Farms are numbered. LDH19 is from a human clinical isolate.
Transconjugants (Tc) or transformants (Tx) were screened for IncI2 or IncI1 (pHNAH4-1) markers, as appropriate, using primers in Table S1 in the supplemental material. P, positive; ND, not determined.
PCR with primers CHP1-F and Orf477-R (see Table S1), linking the IncI2 backbone fragment to orf477.
PCR with HP2-R and ISEcp1-F (see Table S1) linking the IncI2 backbone to ISEcp1. NA, not applicable.
Approximate plasmid sizes were estimated from S1 gels.
Gave Tx carrying blaCTX-M but more than one plasmid.
Gave Tx carrying a single plasmid with blaCTX-M.
Gave Tc carrying a single plasmid with blaCTX-M.
No Tc/Tx carrying the relevant blaCTX-M gene were obtained.
Conjugation experiments.
Transconjugants of AHC4 carrying pHNAH4-1 (blaCTX-M-123) and transformants of AHC46 carrying pHNAH46-1 (blaCTX-M-64) were obtained previously (4). LDH19, JC2, and the 20 isolates carrying blaCTX-M-123 or blaCTX-M-64 were conjugated by filter mating with streptomycin-resistant E. coli C600 and selection on Luria-Bertani agar supplemented with 2 μg/ml cefotaxime and 2,000 μg/ml streptomycin. Where multiple plasmids were cotransferred by conjugation, transformation was performed to try to obtain a single plasmid carrying the relevant blaCTX-M gene, as verified by S1 nuclease pulsed-field gel electrophoresis (PFGE) (15). The presence of blaCTX-M genes in transconjugants/transformants was confirmed by PCR and sequencing using published primers (7).
Plasmid analysis.
PCR-based replicon typing (PBRT) (16) was performed on all transconjugants/transformants carrying a single plasmid plus the transformant from JC2 carrying two plasmids. An IncI2-type replicon was screened for with published primers (see Table S1 in the supplemental material) (12). IncI1 plasmid multilocus sequence typing (pMLST) was performed as described previously (17), and alleles were assigned by www.pubmlst.org/plasmid/. IncI2 plasmids carrying hybrid blaCTX-M genes were compared by restriction digestion with ApaLI (TaKaRa Biotechnology, Dalian, China), according to the manufacturer's instructions. The sizes of the plasmids were estimated by S1 nuclease PFGE.
Plasmid sequencing.
Plasmids pHNY2-1 and pHNY2-2 from the only isolate with blaCTX-M-15 and a 45-bp spacer, the partially characterized pHNAH4-1 (IncI1, blaCTX-M-123) and pHNAH46-1 (IncI2, blaCTX-M-64), both from chickens in the same province, and pHNLDH19 (IncI2, blaCTX-M-132), from the only isolate with blaCTX-M-132, were selected for sequencing. Plasmid DNA purified from a transformant or transconjugant using a Qiagen plasmid midi kit (Qiagen, Hilden, Germany) was sequenced using the GS-FLX system (454 Life Sciences). Contigs were assembled with the 454 GS de novo assembler (Newbler) v2.8. Gaps between contigs (average coverage, ∼100-fold) were closed by PCR and sequencing. PCR (see Table S1 in the supplemental material) and sequencing across the shufflon region gave mixed sequences for all plasmids, suggesting active shufflon rearrangement. As a contig covering the whole region was obtained during automated assembly of each IncI2 plasmid, these arrangements were used to assemble the final sequences. For the IncI1 plasmid pHNAH4-1, additional cloning of a PCR product in pMD19T (TaKaRa Biotechnology) and sequencing revealed that shufflon segment B, initially missing from the assembled sequence, was actually present in the plasmid population. In this plasmid, shufflon segments were assembled in the order of the cloned fragment to close the sequence. Gene prediction and annotation were performed using Glimmer 3.02 (http://ccb.jhu.edu/software/glimmer/index.shtml) and the BLASTp program (http://blast.ncbi.nlm.nih.gov/Blast.cgi). IncI1 plasmid R64 (GenBank accession no. AP005147) and IncI2 plasmid pHN1122-1 (JN797501) were used as reference plasmids for annotation.
PCR screening for pHNAH4-1-like and pHNAH46-1-like plasmids.
Published primers designed to amplify selected regions (see Table S1 in the supplemental material) of the IncI2 backbone (12) were used to characterize IncI2 plasmids. The insertion site of the ISEcp1 transposition unit in IncI1 or IncI2 plasmids and junctions between the IncI1 backbone and the other two insertions found in pHNAH4-1 were also determined by PCR (see Table S1).
Nucleotide sequence accession numbers.
The nucleotide sequences of pHNY2-1, pHNAH46-1, pHNAH4-1, and pHNLDH19 have been deposited in GenBank under accession numbers KF601686, KJ020576, KJ125070, and KM207012, respectively.
RESULTS AND DISCUSSION
IncI plasmids carrying hybrid blaCTX-M genes.
PCR and sequencing revealed that LDH19 carries blaCTX-M-132 with a 45-bp spacer and that the same spacer separates blaCTX-M-15 from ISEcp1 in JC2. pHNAH46-1, carrying blaCTX-M-64, had previously been identified as IncI2 and pHNAH4-1, carrying blaCTX-M-123, as IncI1 sequence type 108 (ST108) (4). A single IncI2 plasmid (pHNLDH19) carrying blaCTX-M-132 was transferred from LDH19 by conjugation. Transconjugants from JC2 carried two plasmids (∼65 kb and ∼55 kb, designated pHNY2-1 and pHNY2-2, respectively) and both an IncI2 and an IncN replicon. Transformants with a single plasmid carrying blaCTX-M-15 could not be obtained, despite repeated attempts. The IncI2 replicons in pHNAH46-1, pHNLDH19, and the JC2 transconjugant were all 100% identical to that of pHN1122-1 carrying blaCTX-M-55 (GenBank accession no. JN797501) (12). pHNAH46-1 and pHNLDH19 gave ApaLI patterns indistinguishable from those of pHN1122-1, while pHNY2-1 plus pHNY2-2 gave a similar pattern (see Fig. S1 in the supplemental material). Plasmids from all four isolates were completely sequenced.
IncI2 plasmids pHN1122-1, pHNY2-1, pHNAH46-1, and pHNLDH19 are almost identical.
Sequencing and assembly revealed that blaCTX-M-15 is carried by the 65,358-bp plasmid pHNY2-1, which is IncI2. pHNY2-1, pHNAH46-1 (blaCTX-M-64; 62,194 bp), and pHNLDH19 (blaCTX-M-132; 62,194 bp) are almost identical to each other and to pHN1122-1(blaCTX-M-55; 62,196 bp) (12). These plasmids are also closely related to p1081-CTXM (GenBank accession no. KJ460501; blaCTX-M-55; 62,194 bp) from a clinical Shigella sonnei isolate (18), pSTH21 (LN623683; blaCTX-M-55; 62,139 bp) from a Salmonella enterica isolate from China, and pCTXM64_C0967 (KP091735; blaCTX-M-64; 62,194 bp) and pCTXM132_P0421 (KP198615; blaCTX-M-123; 63,124 bp) from chicken and swine E. coli isolates, respectively, from Hong Kong (11). They all have a typical IncI2 backbone, including a replicon region, plasmid stability functions, and genes encoding two types of pili (tra and pil operons), with only minor sequence differences (see Table S2 in the supplemental material).
Automated assembly suggested that, like pHN1122-1, the three plasmids sequenced here were missing segment C of the shufflon, which generates variation in the C terminus of the PilV tip adhesin compared with the archetypal IncI2 plasmid R721 (19) and had different arrangements of the remaining two shufflon segments. PCR across the shufflon gave a product of the size expected if segment C is missing (1,650 bp), but sequencing suggested multiple arrangements of shufflon segments in each sample. The sequences of the remaining A and B′/D′ segments are identical for all plasmids except for pCTXM132_P0421, which has differences in segment A.
All of these plasmids have a 3,080-bp ISEcp1-blaCTX-M transposition unit containing 112 bp of IncA/C backbone inserted in the same position flanked by the same 5-bp direct repeats, differing only by the expected variations in the blaCTX-M genes. pHNY2-1 only also has IS150 (inserted in the same position as in R721; GenBank accession no. AP002527) and IS1294b (Fig. 1A) (19). This common context suggests that both blaCTX-M-64 and blaCTX-M-132 were generated in an IncI2 plasmid. IncI2 plasmids were not included in the original PBRT panel (16) and have not been well studied but have recently been found to harbor several other clinically important resistance genes, such as blaKPC (20) and blaCMY-2 (21).
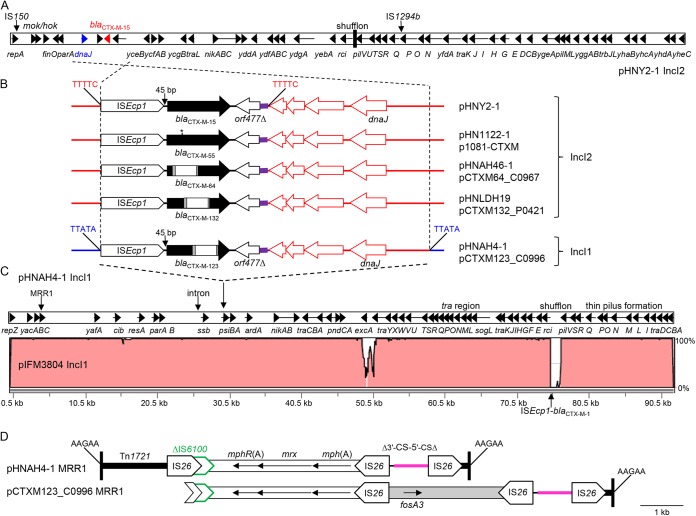
FIG 1.
(A) Organization of IncI2 plasmids carrying blaCTX-M-15, blaCTX-M-55, or blaCTX-M-1/9/1 hybrids, as represented by pHNY2-1 (GenBank accession no. KF601686) carrying blaCTX-M-15. Arrows indicate the positions and directions of different genes, with blaCTX-M-15 shown in red and the shufflon region shown as a black box. The positions of IS150 and IS1294b, found in pHNY2-1 only, are indicated by vertical arrows. (B) Comparison of the genetic environments of blaCTX-M-15 (pHNY2-1), blaCTX-M-55 (pHN1122-1, JN797501; p1081-CTXM, KJ460501; pSTH21, LN623683), and blaCTX-M-1/9/1 hybrids blaCTX-M-64 (pHNAH46-1, KJ125070; pCTXM64_C0907, KP091735) and blaCTX-M-132 (pHNLDH19, KM207012; pCTXM-132_P0421, KP198615) on IncI2 plasmids with the genetic environments of blaCTX-M-123 on IncI1 plasmids (pHNAH4-1, KC160505; pCTXM123_C0996, KP198616). The IncI2 backbone is shown in red, the IncI1 backbone in blue, and the IncA/C backbone fragment in purple. The asterisk in blaCTX-M-55 indicates the nucleotide that differs from blaCTX-M-15. Within the blaCTX-M genes, black corresponds to regions matching blaCTX-M-15, white to regions matching blaCTX-M-14, and gray to short sections matching both of these genes. The 5-bp direct repeats (DR) flanking the transposition unit in IncI2 plasmids are shown for pHNY2-1 only but are present in all IncI2 plasmids shown. The 5-bp direct repeats flanking the blaCTX-M-123 transposition unit are shown. (C) Comparison of the pHNAH4-1 and pIFM3804 (CP006657) backbones, with insertions removed. The percent identity is displayed on the right of the diagram. The pCTXM123_C0996 backbone is almost identical to pHNAH4-1 except for missing shufflon segments and a deletion extending into the left end of MRR1, which may both be due to assembly issues, and this plasmid also has the intron insertion. (D) MRR1 in pHNAH4-1. The 38 bp of Tn1721 are shown by black bars and the sequences of the flanking 5-bp DR are given. The pink segment and adjacent black line represent the truncated class 1 integron 5′-CS and 3′-CS, respectively. IS6100 is truncated by IS26. pCTXM123_C0996 has an additional segment carrying fosA3.
Transfer of ISEcp1-blaCTX-M from IncI2 to IncI1 plasmids.
pHNAH4-1 (IncI1 ST108), carrying blaCTX-M-123, is 109,194 bp and is made up of a typical IncI1 backbone with three insertions. As predicted from examination of a cloned fragment (4), an ISEcp1 transposition unit containing blaCTX-M-123, the 112-bp IncA/C backbone fragment, and a fragment of IncI2 backbone was identified. This 5,800-bp insertion includes 2,720 bp of the IncI2 backbone found adjacent to the IncA/C fragment in IncI2 plasmids and is flanked by a 5-bp duplication (TTATA) (Fig. 1B). This suggests ISEcp1-mediated transposition of the ISEcp1-blaCTX-M--IncA/C region found in IncI2 plasmids plus the adjacent 2,720 bp of IncI2 backbone into an IncI1 plasmid backbone.
The second insertion in pHNAH4-1 (MRR1, 8,587 bp) (Fig. 1D) is bounded by the ends of transposon Tn1721, including the 38-bp terminal inverted repeats, and is located in the yafA gene flanked by 5-bp direct repeats characteristic of this transposon. The central part of Tn1721 has been replaced by a region containing three copies of IS26, the mph(A)-mrx-mphR(A) macrolide resistance region and fragments of the class 1 integron 5′-conserved sequence (CS), 3′-CS, and IS6100. The third insertion in pHNAH4-1 is a 2,343-bp putative group II intron located upstream of the ssb gene.
pHNAH4-1 has differences from another ST108 plasmid.
pHNAH4-1 has a typical IncI1 backbone that includes the tra and trb gene clusters and the nikAB (DNA processing functions) and pil genes (type IV pilus) required for conjugation plus genes involved in plasmid partitioning and stability (parAB), plasmid addiction (pndCA), and inhibition of the bacterial SOS response (psiAB). Automated sequence assembly suggested that the shufflon was missing segments B and D compared with the archetypal IncI1 plasmid R64 (22). Additional PCRs across the shufflon region, cloning, and sequencing indicated that only segment D is missing and suggested multiple arrangements of the remaining three segments.
pHNAH4-1 is closely related to pCTXM123-C0996, which is also IncI1 ST108 and carries blaCTX-M-123 (11) (Fig. 1), and its backbone is similar to that of IncI1 ST108 plasmid pIFM3804 (GenBank accession no. KF787110), which carries blaCTX-M-1 and has disseminated across multiple genera at a United Kingdom pig farm (Fig. 1C) (23). Like pHNAH4-1, pIFM3804 is apparently missing shufflon segment D, but despite identity at the pMLST target sites, two other regions display low identity, suggesting variability within pMLST ST108, as previously observed for IncI1 ST2 plasmids (20). The main differences are in a region including the traY and excA genes, where pHNAH4-1 is 100% identical to the prototype IncI1 plasmid R64 (ST13; GenBank accession no. AP005147, Salmonella Typhimurium, Japan), while pIFM3804 matches the IncI1 ST3 plasmid (e.g., pC49-108; KJ484638, E. coli, Switzerland). Interactions between the products of these genes influence entry exclusion of one plasmid by another (24).
Related plasmids are spreading between different E. coli sequence types and locations.
Transconjugants or transformants carrying a single IncI2 plasmid were obtained from five of six other E. coli isolates carrying blaCTX-M-64 from chicken samples (Table 1). These had all of the IncI2 backbone markers and the same ISEcp1-blaCTX-M-64-IncA/C transposition unit in the same position as the IncI2 plasmids sequenced here (Table 1). Four gave the same ApaLI pattern as these plasmids, while in pHNBSC1, from BSC1, a large ApaLI fragment seen in the other IncI2 plasmids seems to have been replaced by a slightly smaller band and an ∼4.4-kb band (see Fig. S1 in the supplemental material), suggesting a rearrangement/and or insertion. Ho et al. (11) also identified an additional five related IncI2 plasmids carrying blaCTX-M-64 from chicken isolates from Hong Kong.
Transconjugants or transformants carrying a single IncI1 ST108 plasmid were identified in 9 of 14 additional isolates with blaCTX-M-123 from chickens, a pig, and a duck (Table 1). All had both the intron and the ISEcp1 transposition unit containing the IncI2 fragment inserted in the same positions as in pHNAH4-1, but only seven had MRR1 (Table 1). All plasmids were similar in size to pHNAH4-1, although there was some variation (Table 1). Ho et al. (11) also identified an additional four IncI1 ST108 plasmids carrying blaCTX-M-64 from chickens, a pig, and a dog in Hong Kong.
Sixteen different E. coli sequence types were identified among the 23 isolates (Table 1). Two isolates each of ST155 and ST1437 carried blaCTX-M-123 and two ST162 isolates carried either blaCTX-M-123 or blaCTX-M-64. ST746 was the most prevalent type, corresponding to four isolates carrying blaCTX-M-123 from three different chicken farms and one carrying blaCTX-64 on an untyped plasmid from a pig. An E. coli ST746 strain carrying blaCTX-M-132 on an untyped plasmid was also recovered from cattle in Hong Kong (11). It is also interesting that AHC46 and four isolates from Hong Kong (11) are all ST1011, and single isolates of ST93 and ST117 were identified in each location, with all nine carrying an IncI2/blaCTX-M-64 plasmid and isolated from chickens. JC2 (blaCTX-M-15 IncI2, human isolate) and a Hong Kong blaCTX-M-64 IncI2 chicken isolate are also both ST224. Thus, although IncI2 and IncI1 plasmid transfer seems to play a major role in the epidemiology of blaCTX-M-1/9/1 hybrid genes, some clonal dissemination of E. coli strains carrying these plasmids between locations and host species also appears to have occurred.
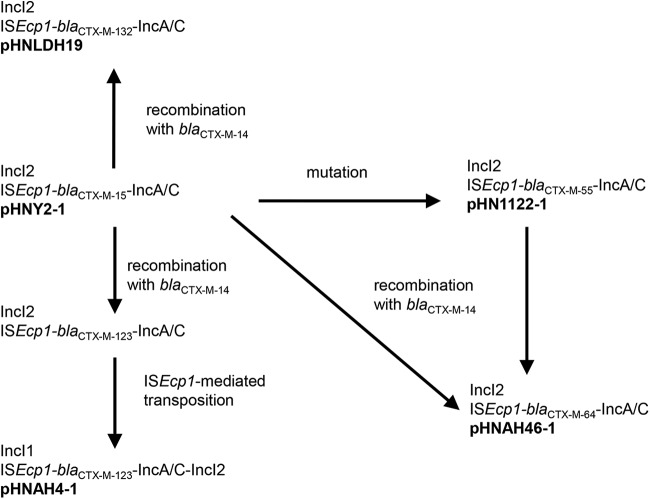
In conclusion, the almost identical sequences of IncI2 plasmids carrying blaCTX-M-15, blaCTX-M-55, blaCTX-M-64, and blaCTX-M-132, all with a 45-bp spacer, suggest that the hybrid genes could have been created on an IncI2 plasmid by a recombination with blaCTX-M-14. In the case of blaCTX-M-64, the nucleotide that differs between blaCTX-M-15 and blaCTX-M-55 is not present, due to the position of the first crossover with blaCTX-M-14 (Fig. 1B), so either blaCTX-M-15 or blaCTX-M-55 could be the ancestor (Fig. 2). In the case of blaCTX-M-132, the final sequence could result directly either from recombination of blaCTX-M-14 with blaCTX-M-15 or from recombination of blaCTX-M-14 with blaCTX-M-55 followed by reversion of the mutation leading to A77V. Although blaCTX-M-15 with a 45-bp spacer sequence appears rare, reversion of the mutation in blaCTX-M-55 seems less likely given the proposed evolutionary trajectory of these genes: blaCTX-M-15 has a single nucleotide change from blaCTX-M-3, the ancestral gene found on the Kluyvera ascorbata chromosome (25), and the resultant amino acid change (D240G) gives a smaller increase in ceftazidime hydrolysis (26). Another single nucleotide change gives blaCTX-M-55 and the A77V mutation leads to a marked increase in ceftazidime hydrolysis (26). blaCTX-M-123 has only been seen on Inc1 or untyped plasmids to date but could have been generated on an IncI2 plasmid and then transferred to an IncI1 plasmid, along with 2,720 bp of IncI2 backbone, by ISEcp1-mediated transposition, as suggested in Fig. 2.
FIG 2.
Suggested scheme for the formation of the observed hybrids and plasmids. blaCTX-M-64 on an IncI2 plasmid could have been created by either of two different pathways, as the nucleotide that differs between blaCTX-M-15 and blaCTX-M-55 is not present in this hybrid gene.
All three of the blaCTX-M-1/9/1 hybrid genes have been found in both mainland China, where two were first reported, and Hong Kong (11). It seems that these three hybrids might have been created in China, with blaCTX-M-64 and blaCTX-M-123 apparently spreading on epidemic plasmids (IncI2 and IncI1) between E. coli types, different animals, and different geographical regions in this country, while blaCTX-M-132 has been identified more rarely. Only blaCTX-M-64 has been reported in other countries: to date in Japan (5), the Netherlands (27), and the Lao People's Democratic Republic (28). Further studies are required to understand the distribution of these hybrid genes in other geographic regions and bacterial sources.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by the National Key Basic Research Program of China (no. 2013CB127200), the Foundation for High-level Talents in Higher Education of Guangdong, and the National Natural Science Foundation and Natural Science Foundation of Guangdong Province, China (no. U1031004).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00501-15.
REFERENCES
- 1.Cantón R, Gonzalez-Alba JM, Galan JC. 2012. CTX-M enzymes: origin and diffusion. Front Microbiol 3:110. doi: 10.3389/fmicb.2012.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D'Andrea MM, Arena F, Pallecchi L, Rossolini GM. 2013. CTX-M-type β-lactamases: a successful story of antibiotic resistance. Int J Med Microbiol 303:305–317. doi: 10.1016/j.ijmm.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Bonnet R. 2004. Growing group of extended-spectrum β-lactamases: the CTX-M enzymes. Antimicrob Agents Chemother 48:1–14. doi: 10.1128/AAC.48.1.1-14.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He D, Partridge SR, Shen J, Zeng Z, Liu L, Rao L, Lv L, Liu JH. 2013. CTX-M-123, a novel hybrid of the CTX-M-1 and CTX-M-9 group β-lactamases recovered from Escherichia coli isolates in China. Antimicrob Agents Chemother 57:4068–4071. doi: 10.1128/AAC.00541-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagano Y, Nagano N, Wachino J, Ishikawa K, Arakawa Y. 2009. Novel chimeric β-lactamase CTX-M-64, a hybrid of CTX-M-15-like and CTX-M-14 β-lactamases, found in a Shigella sonnei strain resistant to various oxyimino-cephalosporins, including ceftazidime. Antimicrob Agents Chemother 53:69–74. doi: 10.1128/AAC.00227-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian GB, Huang YM, Fang ZL, Qing Y, Zhang XF, Huang X. 2014. CTX-M-137, a hybrid of CTX-M-14-like and CTX-M-15-like β-lactamases identified in an Escherichia coli clinical isolate. J Antimicrob Chemother 69:2081–2085. doi: 10.1093/jac/dku126. [DOI] [PubMed] [Google Scholar]
- 7.Sun Y, Zeng Z, Chen S, Ma J, He L, Liu Y, Deng Y, Lei T, Zhao J, Liu JH. 2010. High prevalence of blaCTX-M extended-spectrum β-lactamase genes in Escherichia coli isolates from pets and emergence of CTX-M-64 in China. Clin Microbiol Infect 16:1475–1481. doi: 10.1111/j.1469-0691.2010.03127.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhao WD, Yan P, Guan HN, Zhang QZ. 2014. Characterization of CTX-M-type extended-spectrum β-lactamase in clinical clones of Escherichia coli in Southwest China. J Basic Microbiol 54:247–252. doi: 10.1002/jobm.201200313. [DOI] [PubMed] [Google Scholar]
- 9.Xia S, Fan X, Huang Z, Xia L, Xiao M, Chen R, Xu Y, Zhuo C. 2014. Dominance of CTX-M-type extended-spectrum β-lactamase (ESBL)-producing Escherichia coli isolated from patients with community-onset and hospital-onset infection in China. PLoS One 9:e100707. doi: 10.1371/journal.pone.0100707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu YY, Cai JC, Zhou HW, Chi D, Zhang XF, Chen WL, Zhang R, Chen GX. 2013. Molecular typing of CTX-M-producing Escherichia coli isolates from environmental water, swine feces, specimens from healthy humans, and human patients. Appl Environ Microbiol 79:5988–5996. doi: 10.1128/AEM.01740-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho PL, Liu MC, Lo WU, Lai EL, Lau TC, Law OK, Chow KH. 2015. Prevalence and characterization of hybrid blaCTX-M among Escherichia coli isolates from livestock and other animals. Diagn Microbiol Infect Dis 82:148–153. doi: 10.1016/j.diagmicrobio.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Lv L, Partridge SR, He L, Zeng Z, He D, Ye J, Liu JH. 2013. Genetic characterization of IncI2 plasmids carrying blaCTX-M-55 spreading in both pets and food animals in China. Antimicrob Agents Chemother 57:2824–2827. doi: 10.1128/AAC.02155-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fursova NK, Pryamchuk SD, Abaev IV, Kovalev YN, Shishkova NA, Pecherskikh EI, Korobova OV, Astashkin EI, Pachkunov DM, Svetoch EA, Sidorenko SV. 2010. Genetic environments of blaCTX-M genes located on conjugative plasmids of Enterobacteriaceae nosocomial isolates collected in Russia within 2003-2007. Antibiot Khimioter 55:3–10. (In Russian.) [PubMed] [Google Scholar]
- 14.Rao L, Lv L, Zeng Z, Chen S, He D, Chen X, Wu C, Wang Y, Yang T, Wu P, Liu Y, Liu JH. 2014. Increasing prevalence of extended-spectrum cephalosporin-resistant Escherichia coli in food animals and the diversity of CTX-M genotypes during 2003-2012. Vet Microbiol 172:534–541. doi: 10.1016/j.vetmic.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 15.Barton BM, Harding GP, Zuccarelli AJ. 1995. A general method for detecting and sizing large plasmids. Anal Biochem 226:235–240. doi: 10.1006/abio.1995.1220. [DOI] [PubMed] [Google Scholar]
- 16.Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ. 2005. Identification of plasmids by PCR-based replicon typing. J Microbiol Methods 63:219–228. doi: 10.1016/j.mimet.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 17.García-Fernández A, Chiaretto G, Bertini A, Villa L, Fortini D, Ricci A, Carattoli A. 2008. Multilocus sequence typing of IncI1 plasmids carrying extended-spectrum β-lactamases in Escherichia coli and Salmonella of human and animal origin. J Antimicrob Chemother 61:1229–1233. doi: 10.1093/jac/dkn131. [DOI] [PubMed] [Google Scholar]
- 18.Qu F, Ying Z, Zhang C, Chen Z, Chen S, Cui E, Bao C, Yang H, Wang J, Liu C, Mao Y, Zhou D. 2014. Plasmid-encoding extended-spectrum β-lactamase CTX-M-55 in a clinical Shigella sonnei strain, China. Future Microbiol 9:1143–1150. doi: 10.2217/fmb.14.53. [DOI] [PubMed] [Google Scholar]
- 19.Kim SR, Komano T. 1992. Nucleotide sequence of the R721 shufflon. J Bacteriol 174:7053–7058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen L, Chavda KD, Al Laham N, Melano RG, Jacobs MR, Bonomo RA, Kreiswirth BN. 2013. Complete nucleotide sequence of a blaKPC-harboring IncI2 plasmid and its dissemination in New Jersey and New York hospitals. Antimicrob Agents Chemother 57:5019–5025. doi: 10.1128/AAC.01397-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bortolaia V, Hansen KH, Nielsen CA, Fritsche TR, Guardabassi L. 2014. High diversity of plasmids harbouring blaCMY-2 among clinical Escherichia coli isolates from humans and companion animals in the upper Midwestern USA. J Antimicrob Chemother 69:1492–1496. doi: 10.1093/jac/dku011. [DOI] [PubMed] [Google Scholar]
- 22.Sampei G, Furuya N, Tachibana K, Saitou Y, Suzuki T, Mizobuchi K, Komano T. 2010. Complete genome sequence of the incompatibility group I1 plasmid R64. Plasmid 64:92–103. doi: 10.1016/j.plasmid.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Freire Martín I, Abuoun M, Reichel R, La Ragione RM, Woodward MJ. 2014. Sequence analysis of a CTX-M-1 IncI1 plasmid found in Salmonella 4,5,12:i:-, Escherichia coli and Klebsiella pneumoniae on a UK pig farm. J Antimicrob Chemother 69:2098–2101. doi: 10.1093/jac/dku098. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi H, Shao M, Furuya N, Komano T. 2011. The genome sequence of the incompatibility group Iγ plasmid R621a: evolution of IncI plasmids. Plasmid 66:112–121. doi: 10.1016/j.plasmid.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Rodríguez MM, Power P, Radice M, Vay C, Famiglietti A, Galleni M, Ayala JA, Gutkind G. 2004. Chromosome-encoded CTX-M-3 from Kluyvera ascorbata: a possible origin of plasmid-borne CTX-M-1-derived cefotaximases. Antimicrob Agents Chemother 48:4895–4897. doi: 10.1128/AAC.48.12.4895-4897.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Novais A, Comas I, Baquero F, Canton R, Coque TM, Moya A, Gonzalez-Candelas F, Galan JC. 2010. Evolutionary trajectories of β-lactamase CTX-M-1 cluster enzymes: predicting antibiotic resistance. PLoS Pathog 6:e1000735. doi: 10.1371/journal.ppat.1000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kluytmans JA, Overdevest IT, Willemsen I, Kluytmans-van den Bergh MF, van der Zwaluw K, Heck M, Rijnsburger M, Vandenbroucke-Grauls CM, Savelkoul PH, Johnston BD, Gordon D, Johnson JR. 2013. Extended-spectrum β-lactamase-producing Escherichia coli from retail chicken meat and humans: comparison of strains, plasmids, resistance genes, and virulence factors. Clin Infect Dis 56:478–487. doi: 10.1093/cid/cis929. [DOI] [PubMed] [Google Scholar]
- 28.Stoesser N, Xayaheuang S, Vongsouvath M, Phommasone K, Elliott I, Del Ojo Elias C, Crook DW, Newton PN, Buisson Y, Lee SJ, Dance DA. 2015. Colonization with Enterobacteriaceae producing ESBLs in children attending preschool childcare facilities in the Lao People's Democratic Republic. J Antimicrob Chemother 70:1893–1897. doi: 10.1093/jac/dkv021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.