Abstract
Immunocompromised individuals are at increased risk of Staphylococcus aureus pneumonia. Neutralization of alpha-toxin (AT) with the monoclonal antibody (MAb) MEDI4893* protects normal mice from S. aureus pneumonia; however, the effects of the MAb in immunocompromised mice have not been reported. In this study, passive immunization with MEDI4893* increased survival rates and reduced bacterial numbers in the lungs in an immunocompromised murine S. aureus pneumonia model. Lungs from infected mice exhibited alveolar epithelial damage, protein leakage, and bacterial overgrowth, whereas lungs from mice passively immunized with MEDI4893* retained a healthy architecture, with an intact epithelial barrier. Adjunctive therapy or prophylaxis with a subtherapeutic MEDI4893* dose combined with subtherapeutic doses of vancomycin or linezolid improved survival rates, compared with the monotherapies. Furthermore, coadministration of MEDI4893* with vancomycin or linezolid extended the antibiotic treatment window. These data suggest that MAb-mediated neutralization of AT holds promise in strategies for prevention and adjunctive therapy among immunocompromised patients.
INTRODUCTION
Staphylococcus aureus is a leading cause of pneumonia among hospitalized patients (1, 2). These infections are difficult to treat and can be complicated by a high prevalence of methicillin-resistant S. aureus (MRSA) (2). Patients with risk factors such as advanced age, broad-spectrum antibiotic exposure, prolonged ventilation, and immunosuppression are most susceptible to these infections (3). To combat the increase in antibiotic-resistant strains, passive immunization with a monoclonal antibody (MAb) targeting the invading pathogen or its virulence factor(s) is being explored as an alternative strategy for protecting at-risk populations (4–7).
One virulence factor under investigation as a target for new therapeutic options against S. aureus disease is alpha-toxin (AT). AT is a secreted protein that binds ADAM10 (a disintegrin and metalloproteinase 10) on cell membranes and oligomerizes to form heptameric transmembrane pores (8, 9). AT can directly lyse cells, and it has been demonstrated to exert other toxic effects at sublytic concentrations. For example, AT pore formation on macrophage membranes activates the NLRP3 inflammasome, which, along with staphylococcal pathogen-associated molecular patterns (PAMPs), induces interleukin 1β (IL-1β) secretion and promotes cell death (10, 11). AT also activates ADAM10-mediated proteolysis of E-cadherin in cell-cell adhesive contacts, contributing to epithelial and endothelial damage (9, 12, 13). Therefore, targeted AT inhibition may neutralize multiple S. aureus pathogenic mechanisms, effectively disarming the S. aureus and enabling the host to combat the infection.
MEDI4893 is an extended-half-life, high-affinity, AT-neutralizing MAb under development for the prevention of S. aureus nosocomial pneumonia in high-risk patients (in a study registered at www.clinicaltrialsregister.eu under registration no. 2014-001097-34). MEDI4893 was generated by introducing the YTE mutations into the previously reported anti-AT MAb LC10, to extend the antibody half-life (4, 14, 15). LC10, also known as MEDI4893*, is identical to MEDI4893 except for the absence of the YTE mutations in the Fc domain (16). While the YTE mutations increase IgG half-lives in humans, they significantly reduce serum exposure in mice and preclude the use of MEDI4893 in murine models (17, 18). Therefore, preclinical animal testing is conducted with MEDI4983*.
MEDI4893* was shown to neutralize AT and to promote survival in an acute S. aureus pneumonia model when administered prophylactically to mice (4). To date, all reported preclinical testing with MEDI4893* has been conducted in immunocompetent animals. However, it is likely that some of the high-risk patients targeted in the MEDI4893 clinical studies will be immunocompromised. In the present study, we tested MEDI4893* in an immunocompromised murine pneumonia model. Herein, we report that MEDI4893* prophylaxis preserves airway structure and the air-liquid barrier, ultimately leading to increased survival rates in this model. MEDI4893* also provides benefits over linezolid or vancomycin monotherapy and extends the therapeutic treatment window of both drugs, making it a promising candidate for prophylaxis or adjunctive treatment of S. aureus pneumonia in immunocompromised patients.
MATERIALS AND METHODS
Bacterial strains and chemicals.
NRS382 (type USA100, clonal complex 5 [CC5]) and NRS261 (CC30) were obtained from the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA). SF8300 (type USA300) was generously provided by Binh An Diep (University of California, San Francisco). All strains produced AT, as measured in overnight culture supernatants (at 0.730, 1.273, and 3.44 μg/ml, respectively). NRS261 and SF8300 contain the genes encoding Panton-Valentine leucocidin. Bacteria were grown to an optical density at 600 nm (OD600) of ∼0.8 in trypticase soy broth (TSB) (VWR International), washed twice in ice-cold phosphate-buffered saline (PBS) (Life Technologies), and frozen as aliquots in TSB with 10% glycerol. Challenge inocula were prepared from one frozen vial for each experiment, diluted in ice-cold PBS, and placed on ice until used for infection.
Vancomycin (Sigma-Aldrich) was prepared in 5% dextrose, and linezolid (Tecoland Corp.) was dissolved in 5% aqueous hydroxypropyl-β-cyclodextrin (Sigma-Aldrich). Anti-AT MAb MEDI4893* and isotype control R347 were diluted in sterile PBS (pH 7.2).
Immunocompromised pneumonia model.
Specific-pathogen-free, 7- to 9-week-old, female C57BL/6J mice (The Jackson Laboratory) were used for all in vivo experiments. All animal studies were approved by the MedImmune Institutional Animal Care and Usage Committee and were conducted in an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-accredited facility, in compliance with U.S. regulations governing the housing and use of animals.
Mice were rendered immunocompromised by intraperitoneal injection of 150 and 100 mg/kg of cyclophosphamide monohydrate (CYP) (Sigma-Aldrich), suspended in 0.9% saline solution, on days −4 and −1, respectively. Circulating white blood cells (WBCs) in CYP-treated mice were enumerated using a XT-2000i automated hematology analyzer (Sysmex Corp.), and results were compared with those for untreated controls to confirm CYP-mediated white blood cell depletion. Mice were anesthetized with 3% isofluorane (Butler Schein Animal Health) and maintained with oxygen at 3 liters/min before inoculation with a S. aureus suspension (50 μl; dose range, 1 × 107 to 2 × 107 CFU) in the nares. The animals were placed in a cage in a supine position for recovery, and survival was monitored for 7 days. Animals appearing moribund were euthanized and were counted as nonsurvivors in the census. For the passive immunization studies, MEDI4893* and R347 were administered intraperitoneally at the described doses and times. In studies involving antibiotics, vancomycin or linezolid was delivered by subcutaneous injection 1 h postinfection. Vancomycin was then dosed at 12, 24, 36, 48, and 60 h postinfection and linezolid at 12, 24, and 36 h.
For CFU determinations, mice were sacrificed in CO2 chambers 48 h following bacterial challenge, and their lungs were harvested into 1.5-ml Lysing Matrix A tubes (MP Biomedicals) and homogenized for 30 to 60 s using a FastPrep-24 homogenizer (MP Biomedicals). Lung homogenates were serially diluted in PBS and plated on trypticase soy agar (TSA) plates (VWR International) for CFU enumeration.
Epithelial integrity was monitored by measuring protein levels in bronchoalveolar lavage (BAL) fluid. The trachea was cannulated immediately following euthanasia, and 3 ml of sterile PBS was flushed through the airways in 1-ml increments. Protein concentrations in the recovered fluid were measured with the Pierce 660-nm protein assay, according to the manufacturer's instructions (Thermo Scientific). Briefly, 10 μl of BAL fluid was incubated with the protein assay reagent, and the absorbance of light at 660 nm was measured. Protein concentrations were measured by comparing absorbance values for experimental samples to those for standards provided with the kit.
Histopathology.
Fourteen hours after bacterial challenge, mice (n = 5) were euthanized and their lungs were removed and inflated with 10% neutral buffered formalin (VWR). The lungs were processed and embedded in a frontal plane in molten paraffin. A 4-μm section from each animal was stained with Gill's hematoxylin (Mercedes Medical) and eosin (Surgipath). All stained sections were analyzed using a Nikon 80i microscope with 10× and 40× objectives and were reviewed by a pathologist who was blinded to the treatment groups.
Flow cytometry.
Cells in BAL fluid or lungs homogenized through a 40-μm filter (Corning, Inc.) were pelleted (500 × g for 5 min) and washed twice in fluorescence-activated cell sorting (FACS) buffer (PBS with 5% fetal bovine serum and 0.1% sodium azide). Red blood cells were removed with ACK (ammonium-chloride-potassium) lysing buffer (Life Technologies), Fc receptors were blocked with anti-mouse CD16/CD32 (eBioscience), and epithelial cells were stained with fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD326 (EpCAM; eBioscience). Cells were imaged using a LSR II flow cytometer (BD Biosciences), and data were analyzed with FlowJo software (Tree Star). A known concentration of counting beads (Bang Laboratories) was added to each sample for calculation of the number of cells. Epithelial cell numbers are presented as the fold differences of the R347 and MEDI4893* samples in comparison with the R347 group mean.
Statistical analyses.
Data were analyzed using t tests, the Mann-Whitney test, analysis of variance (ANOVA) followed by Dunnett's test, the Kruskal-Wallis test followed by Dunn's test, or the log rank test, as appropriate. All statistical analyses were performed using GraphPad Prism v.5.0. P values of <0.05 were considered statistically significant.
RESULTS
MEDI4893* increases survival rates in an immunocompromised murine pneumonia model.
We previously described the AT-neutralizing MAb LC10 (MEDI4893*), which significantly improved disease outcomes when delivered prophylactically and as adjunctive therapy with antibiotics in a S. aureus pneumonia model with immunocompetent mice (4). To characterize MEDI4893* in a pneumonia model with immunocompromised mice, mice were depleted of their white blood cells (WBCs) with cyclophosphamide (CYP) prior to intransal infection with community-acquired methicillin-resistant S. aureus (CA-MRSA) (type USA300, strain SF8300). CYP-treated mice exhibited ∼99% reductions in circulating neutrophil levels for up to 4 days (see Fig. S1 in the supplemental material). The 100% lethal dose (LD100) was determined to be 2 × 107 CFU (data not shown) in neutropenic mice, which is ∼1 log10 unit lower than that in healthy mice (4).
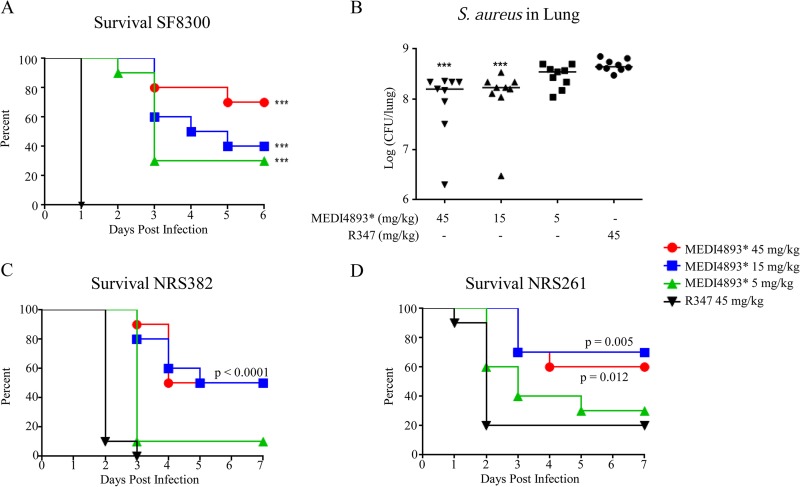
To assess the protective effects of MEDI4893*, immunocompromised mice were passively immunized with MEDI4893* (45, 15, or 5 mg/kg) or the IgG1 isotype control R347 (45 mg/kg) at the time of the final CYP treatment (day −1) and 24 h prior to intranasal challenge with SF8300. MEDI4893* prophylaxis resulted in dose-dependent increases in survival rates (P < 0.0001 at the dose of 5 mg/kg) and reductions in bacterial CFU, relative to R347 (P < 0.0001 for 15 and 45 mg/kg) (Fig. 1A and B). Similarly, passive immunization with MEDI4893* protected immunocompromised mice from intranasal challenge with diverse methicillin-sensitive S. aureus (MSSA) and methicillin-resistant S. aureus (MRSA) clinical isolates, i.e., NRS382 (MRSA, type USA100, CC5) and NRS261 (MSSA, type USA200, CC30) (Fig. 1C and D), indicating that MEDI4893* can provide protection against diverse S. aureus clinical isolates, irrespective of antibiotic resistance status, in an immunocompromised pneumonia model.
FIG 1.
MEDI4893* prophylaxis increases survival rates and reduces bacterial burdens in an immunocompromised murine pneumonia model. (A) Survival of CYP-treated C57BL/6J mice (n = 10) injected with MEDI4893* or R347 control 24 h prior to infection with S. aureus SF8300 (2 × 107 CFU). (B) Enumeration of SF8300 recovered 24 h postinfection from the lungs of mice passively immunized with MEDI4893* or R347 (45 mg/kg). The horizontal lines represent the geometric means for each group. (C and D) Survival of CYP-treated C57BL/6J mice (n = 10) passively immunized with MEDI4893* or R347 and infected with NRS382 (type USA100) (C) or NRS261 (type USA200) (D). Results are representative of three independent experiments. ***, P < 0.0001, compared to R347.
MEDI4893* prophylaxis reduces pulmonary damage.
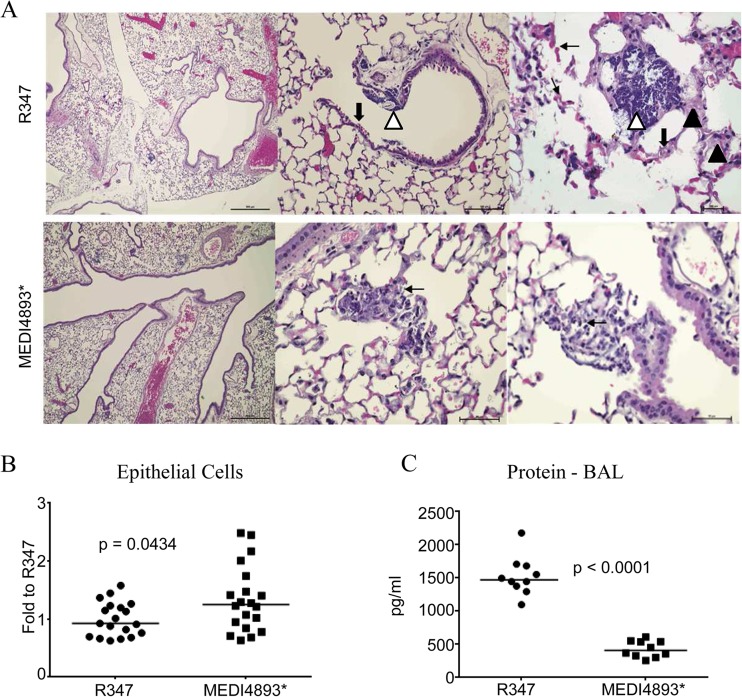
In immunocompetent animals, AT neutralization reduced inflammation and pulmonary damage associated with S. aureus pneumonia (4, 19). To determine whether MEDI4893* had similar effects in immunocompromised animals, mice were rendered neutropenic with CYP and treated with MEDI4893* or R347 (IgG1 isotype control MAb) 24 h before intranasal challenge, and lung pathology was examined 14 h postinfection. It was shown previously that lungs from immunocompetent mice that had been passively immunized with R347 and then infected with S. aureus strain SF8300 exhibited the hallmarks of bacterial pneumonia, including necrosis, edema, increased neutrophilic infiltration, and alveolar consolidation (4). In contrast, immunocompromised mice that had been passively immunized with R347 and challenged with SF8300 lacked acute inflammatory infiltration in response to the bacterial infection, due to depletion of their circulating neutrophils (Fig. 2A). Lungs from mice that had been passively immunized with R347 exhibited areas of bronchial epithelial damage, as indicated by cytolytic effects, an absence of cilia, and bacterial colonization. In all sections examined, there were focal areas of bacterial colonization with destruction of alveolar architecture and fluid leakage into the alveoli, as evidenced by the presence of proteinaceous material. Areas of hemorrhage were represented by the presence of red blood cells and lysed red blood cells in the alveolar sac (Fig. 2A). In contrast, lungs from mice passively immunized with MEDI4893* exhibited minimal epithelial damage, smaller areas of hemorrhage, and no obvious alveolar or bronchial epithelial damage (Fig. 2A). Enumeration of epithelial cells by FACS confirmed the histological observations that MEDI4893* preserved epithelial cell numbers (Fig. 2B). Consistent with these data, there were significantly lower protein levels (P < 0.0001) in the BAL fluid from mice passively immunized with MEDI4893* than in that from R347-treated mice (Fig. 2C). The lower BAL fluid protein levels in MEDI4893*-treated mice were associated with minimal alveolar damage, consistent with the lung histological findings. These results indicate that MEDI4893* prophylaxis protects the lungs of S. aureus-infected immunocompromised mice from AT-mediated damage.
FIG 2.
MEDI4893* prophylaxis reduces pulmonary damage and increases macrophage phagocytosis. (A) Hematoxylin- and eosin-stained sections of lungs from mice (14 h postinfection) that had been passively immunized with R347 (upper row) or MEDI4893* (lower row) (15 mg/kg) 24 h prior to infection with SF8300 (2 × 107 CFU). The higher-magnification histomicrographs for sections from R347-treated mice show epithelial damage (thick black arrows), bacterial colonization (white arrowheads), areas of hemorrhage and red blood cell debris in the alveolar sac (thin black arrows), and alveoli filled with eosinophilic proteinaceous material (black arrowheads), indicating epithelial damage. Limited alveolar damage is noted in sections from MEDI4893*-treated mice. MEDI4893*-treated mice had mononuclear cell foci with intracellular bacteria (arrow). Magnifications: left column, ×40; middle column, ×100; right column, ×400. (B) Epithelial cell numbers in the lungs of mice treated with MEDI4893* or R347 (15 mg/kg), as measured by flow cytometry 14 h postinfection. The data are presented as fold changes relative to the R347 mean. The horizontal lines represent the geometric means for each group. (C) Protein levels in BAL fluid from mice treated with MEDI4893* or R347 (15 mg/kg), as measured 14 h postinfection. The horizontal lines represent the geometric means for each group. Data are representative of 3 independent experiments.
MEDI4893* adjunctive therapy provides therapeutic benefits and extends the antibiotic treatment window.
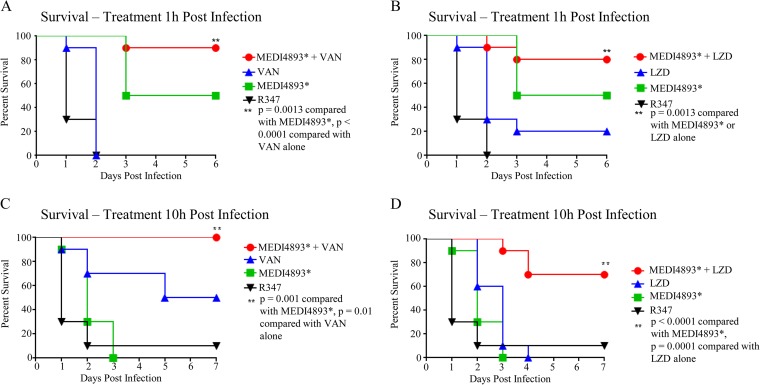
To determine whether MEDI4893* adjunctive therapy with antibiotics provides benefits over antibiotic monotherapy in the immunocompromised pneumonia model, treatment of infected animals was initiated 1 h postinfection with subtherapeutic doses of vancomycin (25 mg/kg/day twice a day [BID] for 3 days) or linezolid (12.5 mg/kg/day BID for 2 days) (see Fig. S2 in the supplemental material) plus a single subtherapeutic dose of MEDI4893* (15 mg/kg). Subtherapeutic doses were used to mimic inadequate antibiotic exposure for an antibiotic-resistant strain. Animals that received MEDI4893* plus vancomycin or linezolid exhibited survival rates of 90% or 80%, respectively, whereas only 30 to 40% of animals survived following the antibiotic monotherapies (Fig. 3A and B). These results indicate that MEDI4893* adjunctive therapy improves disease outcomes, compared with antibiotic monotherapy.
FIG 3.
MEDI4893* improves survival rates over antibiotic monotherapy and extends the antibiotic treatment window. (A) Survival of CYP-treated C57BL/6J mice treated 1 h postinfection with MEDI4893* (15 mg/kg) and vancomycin (25 mg/kg/day for 2 days), alone or in combination. (B) Survival of CYP-treated C57BL/6J mice treated 1 h postinfection with MEDI4893* (15 mg/kg) and linezolid (12.5 mg/kg/day for 2 days), alone or in combination. (C) Survival of CYP-treated C57BL/6J mice treated 10 h postinfection with MEDI4893* (15 mg/kg) and vancomycin (75 mg/kg/day for 2 days), alone or in combination. (D) Survival of CYP-treated C57BL/6J mice treated 10 h postinfection with MEDI4893* (15 mg/kg) and linezolid (50 mg/kg/day for 2 days), alone or in combination. Data are representative of 3 independent experiments. VAN, vancomycin; LZD, linezolid.
To understand how adjunctive therapy with MEDI4893* affects treatment with an effective antibiotic dose, we determined the vancomycin or linezolid dose required to provide 100% protection (i.e., 100% effective dose [ED100]) when treatment was initiated 1 h postinfection (see Fig. S2 in the supplemental material), and then we extended the time of administration postinfection to determine the point at which antibiotics were no longer fully effective (10 h) (see Fig. S3 in the supplemental material). Next, immunocompromised mice were treated with MEDI4893* (15 mg/kg) 10 h postinfection, as treatment with vancomycin (75 mg/kg/day) or linezolid (50 mg/kg/day) was initiated. As adjunctive therapy, MEDI4893* resulted in significant increases in survival rates, relative to treatment with vancomycin or linezolid (Fig. 3C and D). These results indicate that MEDI4893* adjunctive therapy extended the effective treatment window for both vancomycin and linezolid in this model.
MEDI4893* prophylaxis improves the effectiveness of antibiotic therapy.
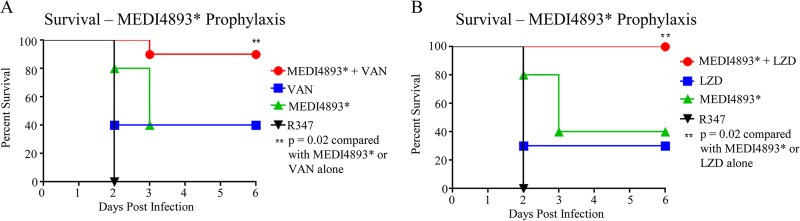
MEDI4893 is currently under development for the prevention of nosocomial pneumonia in high-risk ventilated patients, who may have multiple comorbidities. Given that no immunoprophylactic strategy is likely to be 100% effective, we wanted to investigate the impact of immunoprophylaxis on subsequent antibiotic treatment of S. aureus breakthrough infections. To model this, immunocompromised mice were passively immunized with a subtherapeutic MEDI4893* dose or with R347 (15 mg/kg) (Fig. 4) 24 h prior to an intranasal S. aureus challenge. Antibiotic therapy with subtherapeutic doses of vancomycin (25 mg/kg/day) or linezolid (12.5 mg/kg/day) was then initiated 1 h postinfection. Animals that received MEDI4893* prophylaxis plus vancomycin or linezolid treatment exhibited survival rates of 90% or 100%, respectively, whereas only 30 to 40% of the animals that received the control MAb survived with the antibiotic monotherapies (Fig. 4A and B). These results indicate that even a marginally effective dose of MEDI4893* can be beneficial, as it can complement the effectiveness of antibiotic therapy, resulting in more complete resolution of breakthrough infections.
FIG 4.
MEDI4893* prophylaxis improves the effectiveness of antibiotic treatment. The survival of CYP-treated C57BL/6J mice that were treated prophylactically with MEDI4893* (15 mg/kg) or R347 control (15 mg/kg) 24 h prior to infection and then were treated 1 h postinfection with either vancomycin (25 mg/kg/day BID for 3 days) (A) or linezolid (12.5 mg/kg/day BID for 2 days) (B) was monitored. Data are representative of 3 independent experiments. VAN, vancomycin; LZD, linezolid.
DISCUSSION
S. aureus, a leading cause of bacterial pneumonia, is notoriously difficult to treat even when the infecting strain is drug susceptible; consequently, it is associated with high mortality rates (2, 20, 21). Two risk factors associated with increased mortality rates among patients with pneumonia are a compromised immune system and antibiotic resistance in the infecting pathogen (20). One alternative to empirical antibiotic therapy that is under investigation is the use of pathogen-specific monoclonal antibodies (MAbs) in prophylaxis or adjunctive therapy with antibiotics (4, 6, 7, 22). One such MAb, MEDI4893, which targets S. aureus AT, is in clinical development for the prevention of S. aureus pneumonia among high-risk ventilated patients. Similar to experiments in immunocompetent mice, prophylaxis with MED4893* in an immunocompromised S. aureus pneumonia model resulted in reduced bacterial numbers and limited damage to the lung epithelium, accompanied by dose-dependent increases in survival rates relative to an IgG1 isotype control.
The airway epithelium often represents the initial cells that come into contact with airway pathogens, and it is an active contributor to innate immune responses in the lungs (23). S. aureus AT binds directly to ADAM10 on the apical surface of epithelial cells, resulting in cell lysis at high concentrations and disruption of epithelial junctions at low concentrations (9, 24). Loss of epithelial integrity via cell death or degradation of junctional proteins results in alveolar edema and reduced lung function. Treatment with MEDI4893* preserved both epithelial cell numbers and junctional integrity, allowing bacterial clearance.
In adjunctive treatment, MEDI4893* also provided benefits over linezolid or vancomycin monotherapy in a model in which mice were treated with subtherapeutic antibiotic doses plus a subtherapeutic MED4893* dose. These experiments are similar to in vitro checkerboard analyses for the assessment of antibiotic combinations and were designed to simulate infection with an isolate with an elevated MIC or insufficient antibiotic exposure at the infection site (25). This is particularly relevant for drugs such as vancomycin, for which S. aureus clinical isolates with reduced susceptibility have emerged (26). Also, vancomycin has relatively poor lung bioavailability, and it can be difficult to reach therapeutically effective levels in alveolar lining fluid without increasing the risk for vancomycin-mediated nephrotoxicity (26, 27). Although linezolid has better lung bioavailability, emerging resistance in both S. aureus and coagulase-negative staphylococci has been reported (26, 28). Additionally, the 23S rRNA methyltransferase gene (cfr), which is linked to linezolid resistance, has been reported to be plasmid encoded in S. aureus and coagulase-negative staphylococci, which could allow resistance to spread (29, 30). Consequently, a treatment approach that complements antibiotic therapy, such as MAb prophylaxis or treatment, holds promise for combating difficult-to-treat bacterial infections with susceptible or resistant bacteria.
One limitation of studying S. aureus infections in mice is that murine immune cells exhibit reduced susceptibility to some S. aureus toxins (e.g., Panton-Valentine leukocidin or LukGH [LukAB]), relative to their human counterparts (31). These toxins may contribute more significantly to disease in humans than in mice, and the effects of therapeutic agents targeting AT may be overestimated in mice. Therefore, it is important to test anti-S. aureus therapeutic strategies in more than one animal species, to increase confidence in their potential for humans.
One key factor in successfully treating bacterial infections, particularly pneumonia, is a rapid time to initiation of antibiotic therapy (27). Not surprisingly, Kollef and Ward found that delays in the initiation of appropriate antibiotic therapy in bacterial pneumonia were associated with significant increases in mortality rates (20). We found the effective treatment windows for vancomycin and linezolid to be <10 h in the immunocompromised S. aureus pneumonia model. When antibiotic therapy was initiated 10 h postinfection, with an antibiotic dose found to be effective when treatment was initiated 1 h postinfection, neither antibiotic could protect the animals from succumbing to the infection. When a single dose of MEDI4893* was administered along with the antibiotic course, most animals survived, indicating that neutralization of the pathogenic effects of AT in combination with antibiotic therapy increased the effective treatment window for both linezolid and vancomycin. Also, mice that had been prophylactically treated with MEDI4893* were more effectively treated with antibiotics than were those that had received an isotype-matched control MAb in a model designed to mimic a breakthrough infection following MEDI4893* prophylaxis. These preclinical data suggest that adjunctive therapy with an AT-neutralizing MAb could complement and provide benefits over antibiotic monotherapy for critically ill pneumonia patients, as well as breakthrough infections following immunoprophylaxis with MEDI4893. Taken together, the data presented herein provide further support for targeting AT with a neutralizing MAb for the prevention of S. aureus pneumonia even in the context of a compromised immune system, and they support the development of MEDI4893 for the prevention of S. aureus pneumonia in patients at high risk for infection.
Supplementary Material
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00510-15.
REFERENCES
- 1.Quartin AA, Scerpella EG, Puttagunta S, Kett DH. 2013. A comparison of microbiology and demographics among patients with healthcare-associated, hospital-acquired, and ventilator-associated pneumonia: a retrospective analysis of 1184 patients from a large, international study. BMC Infect Dis 13:561. doi: 10.1186/1471-2334-13-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK. 2008. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol 29:996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 3.Grgurich PE, Hudcova J, Lei Y, Sarwar A, Craven DE. 2012. Management and prevention of ventilator-associated pneumonia caused by multidrug-resistant pathogens. Expert Rev Respir Med 6:533–555. doi: 10.1586/ers.12.45. [DOI] [PubMed] [Google Scholar]
- 4.Hua L, Hilliard JJ, Shi Y, Tkaczyk C, Cheng LI, Yu X, Datta V, Ren S, Feng H, Zinsou R, Keller A, O'Day T, Du Q, Cheng L, Damschroder M, Robbie G, Suzich J, Stover CK, Sellman BR. 2014. Assessment of an anti-alpha-toxin monoclonal antibody for prevention and treatment of Staphylococcus aureus-induced pneumonia. Antimicrob Agents Chemother 58:1108–1117. doi: 10.1128/AAC.02190-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ragle BE, Bubeck Wardenburg J. 2009. Anti-alpha-hemolysin monoclonal antibodies mediate protection against Staphylococcus aureus pneumonia. Infect Immun 77:2712–2718. doi: 10.1128/IAI.00115-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foletti D, Strop P, Shaughnessy L, Hasa-Moreno A, Casas MG, Russell M, Bee C, Wu S, Pham A, Zeng Z, Pons J, Rajpal A, Shelton D. 2013. Mechanism of action and in vivo efficacy of a human-derived antibody against Staphylococcus aureus α-hemolysin. J Mol Biol 425:1641–1654. doi: 10.1016/j.jmb.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 7.DiGiandomenico A, Keller AE, Gao C, Rainey GJ, Warrener P, Camara MM, Bonnell J, Fleming R, Bezabeh B, Dimasi N, Sellman BR, Hilliard J, Guenther CM, Datta V, Zhao W, Gao C, Yu XQ, Suzich JA, Stover CK. 2014. A multifunctional bispecific antibody protects against Pseudomonas aeruginosa. Sci Transl Med 6:262ra155. doi: 10.1126/scitranslmed.3009655. [DOI] [PubMed] [Google Scholar]
- 8.Berube BJ, Bubeck Wardenburg J. 2013. Staphylococcus aureus alpha-toxin: nearly a century of intrigue. Toxins (Basel) 5:1140–1166. doi: 10.3390/toxins5061140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inoshima I, Inoshima N, Wilke GA, Powers ME, Frank KM, Wang Y, Bubeck Wardenburg J. 2011. A Staphylococcus aureus pore-forming toxin subverts the activity of ADAM10 to cause lethal infection in mice. Nat Med 17:1310–1314. doi: 10.1038/nm.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craven RR, Gao X, Allen IC, Gris D, Bubeck Wardenburg J, McElvania-Tekippe E, Ting JP, Duncan JA. 2009. Staphylococcus aureus α-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PLoS One 4:e7446. doi: 10.1371/journal.pone.0007446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kebaier C, Chamberland RR, Allen IC, Gao X, Broglie PM, Hall JD, Jania C, Doerschuk CM, Tilley SL, Duncan JA. 2012. Staphylococcus aureus α-hemolysin mediates virulence in a murine model of severe pneumonia through activation of the NLRP3 inflammasome. J Infect Dis 205:807–817. doi: 10.1093/infdis/jir846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powers ME, Kim HK, Wang Y, Bubeck Wardenburg J. 2012. ADAM10 mediates vascular injury induced by Staphylococcus aureus α-hemolysin. J Infect Dis 206:352–356. doi: 10.1093/infdis/jis192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inoshima N, Wang Y, Bubeck Wardenburg J. 2012. Genetic requirement for ADAM10 in severe Staphylococcus aureus skin infection. J Invest Dermatol 132:1513–1516. doi: 10.1038/jid.2011.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oganesyan V, Peng L, Damschroder MM, Cheng L, Sadowska A, Tkaczyk C, Sellman BR, Wu H, Dall'Acqua WF. 2014. Mechanisms of neutralization of a human anti-α-toxin antibody. J Biol Chem 289:29874–29880. doi: 10.1074/jbc.M114.601328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dall'Acqua WF, Kiener PA, Wu H. 2006. Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn). J Biol Chem 281:23514–23524. doi: 10.1074/jbc.M604292200. [DOI] [PubMed] [Google Scholar]
- 16.Hilliard JJ, Datta V, Tkaczyk C, Hamilton M, Sadowska A, Jones-Nelson O, O'Day T, Weiss WJ, Szarka S, Nguyen V, Prokai L, Suzich J, Stover CK, Sellman BR. 2015. Anti-alpha toxin monoclonal antibody and antibiotic combination therapy improves disease outcome and accelerates healing in a Staphylococcus aureus dermonecrosis model. Antimicrob Agents Chemother 59:299–309. doi: 10.1128/AAC.03918-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robbie GJ, Criste R, Dall'acqua WF, Jensen K, Patel NK, Losonsky GA, Griffin MP. 2013. A novel investigational Fc-modified humanized monoclonal antibody, motavizumab-YTE, has an extended half-life in healthy adults. Antimicrob Agents Chemother 57:6147–6153. doi: 10.1128/AAC.01285-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dall'Acqua WF, Woods RM, Ward ES, Palaszynski SR, Patel NK, Brewah YA, Wu H, Kiener PA, Langermann S. 2002. Increasing the affinity of a human IgG1 for the neonatal Fc receptor: biological consequences. J Immunol 169:5171–5180. doi: 10.4049/jimmunol.169.9.5171. [DOI] [PubMed] [Google Scholar]
- 19.Bubeck Wardenburg J, Bae T, Otto M, Deleo FR, Schneewind O. 2007. Poring over pores: α-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat Med 13:1405–1406. doi: 10.1038/nm1207-1405. [DOI] [PubMed] [Google Scholar]
- 20.Kollef MH, Ward S. 1998. The influence of mini-BAL cultures on patient outcomes: implications for the antibiotic management of ventilator-associated pneumonia. Chest 113:412–420. doi: 10.1378/chest.113.2.412. [DOI] [PubMed] [Google Scholar]
- 21.Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. 2005. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest 128:3854–3862. doi: 10.1378/chest.128.6.3854. [DOI] [PubMed] [Google Scholar]
- 22.Song Y, Baer M, Srinivasan R, Lima J, Yarranton G, Bebbington C, Lynch SV. 2012. PcrV antibody-antibiotic combination improves survival in Pseudomonas aeruginosa-infected mice. Eur J Clin Microbiol Infect Dis 31:1837–1845. doi: 10.1007/s10096-011-1509-2. [DOI] [PubMed] [Google Scholar]
- 23.Parker D, Prince A. 2011. Innate immunity in the respiratory epithelium. Am J Respir Cell Mol Biol 45:189–201. doi: 10.1165/rcmb.2011-0011RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maretzky T, Reiss K, Ludwig A, Buchholz J, Scholz F, Proksch E, de Strooper B, Hartmann D, Saftig P. 2005. ADAM10 mediates E-cadherin shedding and regulates epithelial cell-cell adhesion, migration, and β-catenin translocation. Proc Natl Acad Sci U S A 102:9182–9187. doi: 10.1073/pnas.0500918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zusman O, Avni T, Leibovici L, Adler A, Friberg L, Stergiopoulou T, Carmeli Y, Paul M. 2013. Systematic review and meta-analysis of in vitro synergy of polymyxins and carbapenems. Antimicrob Agents Chemother 57:5104–5111. doi: 10.1128/AAC.01230-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scheetz MH, Wunderink RG, Postelnick MJ, Noskin GA. 2006. Potential impact of vancomycin pulmonary distribution on treatment outcomes in patients with methicillin-resistant Staphylococcus aureus pneumonia. Pharmacotherapy 26:539–550. doi: 10.1592/phco.26.4.539. [DOI] [PubMed] [Google Scholar]
- 27.Woods C, Colice G. 2014. Methicillin-resistant Staphylococcus aureus pneumonia in adults. Expert Rev Respir Med 8:641–651. doi: 10.1586/17476348.2014.940323. [DOI] [PubMed] [Google Scholar]
- 28.Mendes RE, Hogan PA, Streit JM, Jones RN, Flamm RK. 2014. ZyvoxR Annual Appraisal of Potency and Spectrum (ZAAPS) program: report of linezolid activity over 9 years (2004–12). J Antimicrob Chemother 69:1582–1588. doi: 10.1093/jac/dkt541. [DOI] [PubMed] [Google Scholar]
- 29.Tewhey R, Gu B, Kelesidis T, Charlton C, Bobenchik A, Hindler J, Schork NJ, Humphries RM. 2014. Mechanisms of linezolid resistance among coagulase-negative staphylococci determined by whole-genome sequencing. mBio 5(3):e00894-14. doi: 10.1128/mBio.00894-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mendes RE, Deshpande LM, Bonilla HF, Schwarz S, Huband MD, Jones RN, Quinn JP. 2013. Dissemination of a pSCFS3-like cfr-carrying plasmid in Staphylococcus aureus and Staphylococcus epidermidis clinical isolates recovered from hospitals in Ohio. Antimicrob Agents Chemother 57:2923–2928. doi: 10.1128/AAC.00071-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malachowa N, Kobayashi SD, Braughton KR, Whitney AR, Parnell MJ, Gardner DJ, Deleo FR. 2012. Staphylococcus aureus leukotoxin GH promotes inflammation. J Infect Dis 206:1185–1193. doi: 10.1093/infdis/jis495. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.