Abstract
Mycobacterium tuberculosis is wrapped in complex waxes, impermeable to most antibiotics. Comparing Mycobacterium bovis BCG and M. tuberculosis mutants that lack phthiocerol dimycocerosates (PDIM) and/or phenolic glycolipids with wild-type strains, we observed that glycopeptides strongly inhibited PDIM-deprived mycobacteria. Vancomycin together with a drug targeting lipid synthesis inhibited multidrug-resistant (MDR) and extensively drug-resistant (XDR) clinical isolates. Our study puts glycopeptides in the pipeline of potential antituberculosis (TB) agents and might provide a new antimycobacterial drug-screening strategy.
TEXT
Mycobacterium tuberculosis remains a leading cause of morbidity, tuberculosis (TB), and mortality in the world. M. tuberculosis is intrinsically resistant to most classical antibiotics, partly because of its impermeable cell wall (1–6). Due to selective mutations in M. tuberculosis, almost one-third of new TB patients are now infected with first-line drug-resistant strains, monoresistant strains, or multidrug-resistant (MDR) strains. Consequently, second-line therapies are often implemented, leading to the appearance of extensively drug-resistant (XDR) strains (7, 8). There is therefore a growing urgency in the need for new antimycobacterial therapies.
The mycobacterial cell wall is composed of peptidoglycan covalently attached to arabinogalactan, which are in turn esterified by very-long-chain mycolic acids. Various noncovalently attached lipids are embedded at the outer surface and necessary for capsule formation. Among these lipids, two related waxes, phthiocerol dimycocerosates (PDIM) and phenolic glycolipids (PGL), are involved in virulence (9–11). PDIM and PGL are only or mostly, respectively, found in pathogenic mycobacteria, but their roles in antibiotic resistance remain unclear (12–16). In Mycobacterium marinum, a mild (2- to 10-fold) increase in antibiotic susceptibility was observed in PDIM- and PGL-deficient strains (14, 15). In contrast, in PDIM- and PGL-deficient M. tuberculosis, no change was detected (13).
The present study aimed to understand how mycobacteria can become susceptible to glycopeptides. Using PDIM-negative and/or PGL-negative strains of Mycobacterium bovis BCG and M. tuberculosis, we investigated the correlation between the absence of PDIM and the glycopeptide susceptibility. Subsequently, we investigated whether vancomycin could synergistically inhibit MDR and XDR strains with a mycobacterial lipids synthesis inhibitor.
We recently reported that the chaperonin Cpn60.1/GroEL-1/Hsp60-1 of M. bovis BCG was necessary for the integrity of the cell wall as the Δcpn60.1 strain showed an abnormal mycobacterial cell wall with a lack of PDIM and mycolates with two more carbon atoms (17). We investigated the susceptibility of the wild-type (WT), Δcpn60.1, and complemented Δcpn60.1 M. bovis BCG (GL2 strain) strains to several antituberculosis drugs. We used the NCCLS agar proportion method (18) to determine the MIC scale range of each antibiotic. We inoculated equal quantities of several dilutions of a 3 McFarland standard inoculum on 7H11 agar supplemented with oleic acid-albumin-dextrose (Difco Laboratories) with or without drug (10-fold dilution assays). The BacT/Alert MP (mycobacteria process) system was used to determine the MIC more accurately. BacT/Alert MP bottles (11 ml) supplemented with 0.5 ml restoring fluid were inoculated with 0.1 ml water or drug solution and 0.4 ml of mycobacterial suspension (0.5 McFarland standard in 7H9 medium, 0.05% Tween 80, 10% albumin-dextrose). A 100-fold diluted bacterial inoculum was injected in a drug-free vial, as a 1/100 proportional growth control. The concentration of the antibiotic in a bottle flagged positive in the same amount of time as the 1/100 control bottle was considered the MIC (19).
The WT M. bovis BCG strain and the Δcpn60.1 mutant were susceptible to all antituberculosis drugs (Table 1), but the Δcpn60.1 mutant showed a MIC 5 times lower for rifampin. This increase in susceptibility was totally abolished by reintroducing expression of Cpn60.1. We unexpectedly also observed that the Δcpn60.1 mutant showed 100-fold higher susceptibility to glycopeptides (teicoplanin and vancomycin), usually not used in the treatment of tuberculosis or Gram-negative bacterial infection because of their outer membranes. This gain in susceptibility was totally abolished in the complemented strain, suggesting that loss of Cpn60.1 conferred an unusual and very high susceptibility to this class of antibiotic.
TABLE 1.
Antibiotic susceptibility for the three M. bovis BCG strains and H37Rv M. tuberculosis
| Antibiotic | MIC (μg/ml)a |
|||
|---|---|---|---|---|
| WT BCG | Δcpn60.1 | Δcpn60.1Comp | H37Rv | |
| Isoniazid | 0.1 | 0.1 | 0.1 | 0.1 |
| Rifampin | 0.05 | 0.01 | 0.05 | 1 |
| Ethambutol | 1 | 1 | 1 | 5 |
| Streptomycin | 0.2 | 0.2 | 0.2 | 1 |
| Ethionamide | 4 | 4 | 4 | 5 |
| Ciprofloxacin | 0.25 | 0.25 | 0.25 | 1 |
| Moxifloxacin | 0.05 | 0.05 | 0.05 | 0.25 |
| Teicoplanin | >1,000 | 17.5 | >1,000 | 100 |
| Vancomycin | >500 | 5 | >500 | 65 |
| Cerulenin | 0.75 | 0.75 | 0.75 | 2.5 |
The BacT/Alert MP system was used to determine the MIC more accurately. Δcpn60.1 Comp, the complemented Δcpn60.1 strain.
To assess if the glycopeptide susceptibility of the Δcpn60.1 M. bovis BCG mutant was linked to a PGL deficiency in addition to the PDIM deficiency, as previously reported (17), we analyzed its lipid composition in more detail (see the supplemental material). The mass spectra of the lipid extract of the Δcpn60.1, complemented, and WT strains confirmed the longer chain lengths in α-mycolates (2 extra carbons) and the absence of intact PDIM (see Fig. S1A to C in the supplemental material) and showed the absence of intact PGL in the Δcpn60.1 mutant (see Fig. S1A, B, D, and E).
To assess the impact of either PDIM or PGL deficiency on vancomycin susceptibility, we determined the vancomycin susceptibility of the wild type (BCG Pasteur 1173P2), the PMM50 mutant (BCG ΔppsE, PDIM− and PGL−), and the PMM137 mutant (BCG ΔfadD26, PDIM− and PGL+) (20, 21). The wild-type BCG Pasteur 1173P2 was resistant to vancomycin, like the related GL2 strain, with a MIC of approximatively 100 μg/ml (data not shown). All M. bovis BCG mutants defective in the PDIM component, regardless of the presence or absence of PGL, presented a MIC of around 5 μg/ml to vancomycin (Fig. 1A and B) as observed for the mutant Δcpn60.1 (Table 1). To assess the impact of PDIM in M. tuberculosis, the vancomycin susceptibilities of two M. tuberculosis strains, wild-type H37Rv (naturally deficient for PGL) or PMM56 (H37Rv ΔppsE, PDIM−), were compared (22). The wild-type M. tuberculosis H37Rv was resistant to vancomycin, with a MIC of approximatively 65 μg/ml (Table 1). The M. tuberculosis PMM56 mutant (ΔppsE) showed a MIC of between 0.5 and 1 μg/ml to vancomycin (Fig. 1C), allowing us to make the same association between PDIM deficiency and the increase in vancomycin susceptibility in M. tuberculosis.
FIG 1.
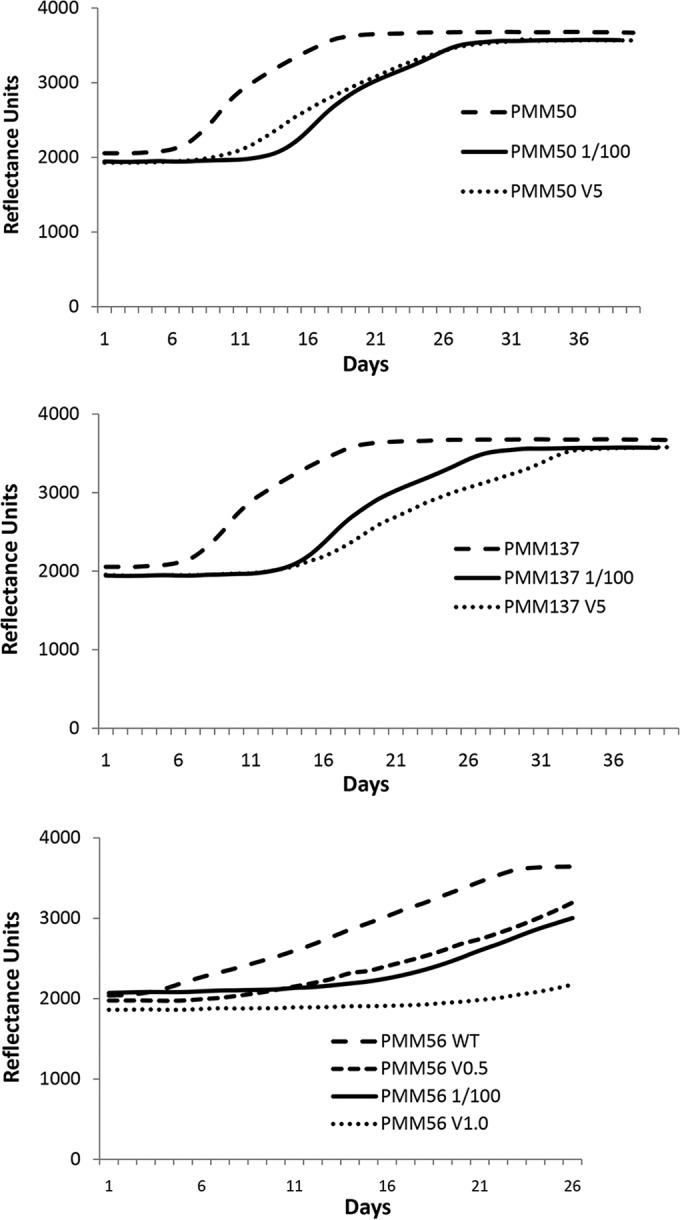
The lack of PDIM in mycobacteria is associated with glycopeptide susceptibility. Typical fluorometric reflectance results showing mycobacterial cell growth in the absence and presence of 5 (V5), 1 (V1.0), and 0.5 (V0.5) μg/ml vancomycin. (A) Representative growth curves of PMM50 M. bovis BCG (PDIM and PGL deficient) diluted (1/100) or not diluted. The MIC corresponds to a concentration of 5 μg/ml vancomycin. (B) Representative growth curves of PMM137 M. bovis BCG (PDIM deficient, PGL positive) diluted (1/100) or not diluted. The MIC corresponds to a concentration of 5 μg/ml vancomycin. (C) Representative growth curves of PMM56 M. tuberculosis (PDIM deficient) diluted (1/100) or not diluted. The MIC is between 0.5 and 1.0 μg/ml vancomycin.
The potential clinical use of vancomycin in combination with a cell wall-targeting drug was investigated as a proof of concept. The combination of vancomycin and cerulenin, a potent long-chain lipid synthesis inhibitor (23, 24), was used at a sub-MIC on M. tuberculosis H37Rv and on MDR and XDR M. tuberculosis clinical isolates. Thirteen clinically unrelated isolates were selected from a M. tuberculosis collection (Tuberculosis Center, Public Health Research Institute [PHRI], NJ) (25). A synergistic effect was evaluated in the BacT/Alert MP system by the x/y methodology (26–29). A Δx/Δy quotient of <0.5 indicates enhanced drug action, with x being the growth index (GI) value obtained for the vial with the combination of drugs and y being the lowest GI value obtained with any of the single drugs used within the combination tested. A combination of vancomycin (5 μg/ml) and cerulenin (0.5 μg/ml) inhibited 99% of the H37Rv M. tuberculosis growth (data not shown). Interestingly, the combination of vancomycin (6 μg/ml) with cerulenin (1 μg/ml) was synergistically effective, inhibiting the growth of 6 MDR and 3 XDR out of 10 MDR and 3 XDR M. tuberculosis clinical isolates (Table 2).
TABLE 2.
Synergic effect of vancomycin and cerulenin in M. tuberculosis multidrug-resistant clinical isolates
| Isolatea | FPb | TN no.c | Resistance profiled |
Synergic effecte | MICf to vancomycin (μg/ml) | |
|---|---|---|---|---|---|---|
| First-line drugs | Second-line drugs | |||||
| 1 | OO1 | 16054 | INH, RIF, EMB, STR | RMC, PAS | + (0.30) | Between 50 and 75 |
| 2 | BE | 17182 | INH, RIF | ETH, PAS | ++ (0.10) (V5C0.5)f | Between 50 and 75 |
| 3 | W283 | 14178 | INH, RIF, EMB, PZA, STR | KAN, CAP, RFB, PAS | + (0.43) (V5C0.5)f | >200 |
| 4 | OO1 | 18048 | INH, RIF, PZA, STR | RFB, RMC, PAS | + (0.32) | >200 |
| 5 | P | 16442 | INH, RIF, PZA, STR | RMC, PAS | + (0.20) | Between 75 and 100 |
| 6 | P23 | 16906 | INH, RIF, EMB, PZA, STR | ETH, RMC, PAS | + (0.25) | 20 |
| 7 | BE | 18460 | INH, RIF, EMB, STR | ETH, CYC, CIP, KAN, CAP, RFB, RMC, PAS | +++ (0.05) | >200 |
| 8 | W | 2550 | INH, RIF, EMB, PZA, STR | ETH, OFX, KAN, CYC, PAS | + (0.28) | >200 |
| 9 | HD15 | 18985 | INH, RIF, EMB, PZA, STR | CYC, CIP, OFX, KAN, AMI, CAP, RFB, RMC, PAS | + (0.13) | 200 |
| 10 | W | 14614 | INH, RIF, EMB, STR | ETH, AMI, KAN, RFB, RIP | >0.5 | 200 |
| 11 | W12 | 15183 | INH, RIF, EMB, STR | ETH, AMI, KAN | >0.5 | >200 |
| 12 | W | 14003 | INH, RIF, EMB, STR | ETH, RFB, RMC, PAS | >0.5 | >200 |
| 13 | W148 | 13438 | INH, RIF, EMB, PZA, STR | KAN, AMI, RFB, RIP | >0.5 | >200 |
Isolates 7 to 9 are XDR strains.
FP, fingerprint name based on IS6110 typing and PHRI nomenclature.
TN no., tracking number, a PHRI unique identifier for each isolate.
Abbreviations: AMI, amikacin; CAP, capreomycin; CIP, ciprofloxacin; CYC, cycloserin; EMB, ethambutol; ETH, ethionamide; INH, isoniazid; KAN, kanamycin; OFX, ofloxacin; PAS, para-aminosalicylic acid; PZA, pyrazinamide; RFB, rifabutin; RIF, rifampin; RIP, rifapentine; RMC, rifamycin; STR, streptomycin.
Synergy between vancomycin (6 μg/ml) and cerulenin (1 μg/ml). Values in parentheses show Δx/Δy quotients. A Δx/Δy quotient of <0.5 in the case of a two-drug combination indicates a synergic effect of the drug action. The respective Δx/Δy quotients are illustrated as follows: +, <0.5; ++, <0.1; +++, <0.05.
These values were obtained from tests with 5 μg/ml vancomycin (V5) and 0.5 μg/ml cerulenin (C0.5). The BacT/Alert MP system was used to determine the MIC more accurately.
Vancomycin is a large (molecular weight [MW] of 1,449) hydrophilic molecule able to form a hydrogen bond with the terminal d-alanyl-d-alanine moieties during peptidoglycan biosynthesis, thereby preventing bacterial cell wall backbone synthesis. The target of vancomycin, ubiquitous in bacteria, is thus only easily reachable on bacteria with thin cell walls or without an outer lipid membrane (6) or without PDIM, as suggested by our results either using various M. bovis BCG and M. tuberculosis mutants, or cotreated by a drug targeting long-chain lipid synthesis, such as cerulenin.
Interestingly, Arain et al. had already reported in 1994 that some M. tuberculosis strains were potentially inhibited in vitro by the coadministration of teicoplanin with ethambutol (30). Despite the fact that the route of administration of glycopeptides restricts their use in ambulatory care, our results suggest that it might be interesting to investigate if these antibiotics might be useful for treating multidrug-resistant (MDR) and extensively drug-resistant (XDR) infections, an important issue as these strains are emerging all over the world (8).
Supplementary Material
ACKNOWLEDGMENTS
We thank Christophe Guilhot for supplying the M. bovis BCG ΔppsE PMM50 strain, the M. bovis BCG Δfad26 PMM137 strain, and the M. tuberculosis ΔppsE PMM56 strain. We thank Barry Kreiswirth for supplying the M. tuberculosis MDR and XDR strains. We also thank Jean-Paul Dehaye for critically reading the manuscript.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.04856-14.
REFERENCES
- 1.Koul A, Arnoult E, Lounis N, Guillemont J, Andries K. 2011. The challenge of new drug discovery for tuberculosis. Nature 469:483–490. doi: 10.1038/nature09657. [DOI] [PubMed] [Google Scholar]
- 2.Barry CE III, Mdluli K. 1996. Drug sensitivity and environmental adaptation of mycobacterial cell wall components. Trends Microbiol 4:275–281. doi: 10.1016/0966-842X(96)10031-7. [DOI] [PubMed] [Google Scholar]
- 3.Gao LY, Laval F, Lawson EH, Groger RK, Woodruff A, Morisaki JH, Cox JS, Daffe M, Brown EJ. 2003. Requirement for kasB in Mycobacterium mycolic acid biosynthesis, cell wall impermeability and intracellular survival: implications for therapy. Mol Microbiol 49:1547–1563. doi: 10.1046/j.1365-2958.2003.03667.x. [DOI] [PubMed] [Google Scholar]
- 4.Barkan D, Liu Z, Sacchettini JC, Glickman MS. 2009. Mycolic acid cyclopropanation is essential for viability, drug resistance, and cell wall integrity of Mycobacterium tuberculosis. Chem Biol 16:499–509. doi: 10.1016/j.chembiol.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sambandan D, Dao DN, Weinrick BC, Vilchèze C, Gurcha SS, Ojha A, Kremer L, Besra GS, Hatfull GF, Jacobs WR Jr. 2013. Keto-mycolic acid-dependent pellicle formation confers tolerance to drug-sensitive Mycobacterium tuberculosis. mBio 4:e00222. doi: 10.1128/mBio.00222-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambert PA. 2002. Cellular impermeability and uptake of biocides and antibiotics in Gram-positive bacteria and mycobacteria. J Appl Microbiol 92:46S–54S. doi: 10.1046/j.1365-2672.92.5s1.7.x. [DOI] [PubMed] [Google Scholar]
- 7.Raviglione M, Marais B, Floyd K, Lonnroth K, Getahun H, Migliori GB, Harries AD, Nunn P, Lienhardt C, Graham S, Chakaya J, Weyer K, Cole S, Kaufmann SH, Zumla A. 2012. Scaling up interventions to achieve global tuberculosis control: progress and new developments. Lancet 379:1902–1913. doi: 10.1016/S0140-6736(12)60727-2. [DOI] [PubMed] [Google Scholar]
- 8.Dalton T, Cegielski P, Akksilp S, Asencios L, Campos Caoili J, Cho SN, Erokhin VV, Ershova J, Gler MT, Kazennyy BY, Kim HJ, Kliiman K, Kurbatova E, Kvasnovsky C, Leimane V, van der Walt M, Via LE, Volchenkov GV, Yagui MA, Kang H, Global PETTS Investigators, Akksilp R, Sitti W, Wattanaamornkiet W, Andreevskaya SN, Chernousova LN, Demikhova OV, Larionova EE, Smirnova TG, Vasilieva IA, Vorobyeva AV, Barry CE III, Cai Y, Shamputa IC, Bayona J, Contreras C, Bonilla C, Jave O, Brand J, Lancaster J, Odendaal R, Chen MP, Diem L, Metchock B, Tan K, Taylor A, Wolfgang M, Cho E, Eum SY, Kwak HK, Lee J, Lee J, Min S, Degtyareva I, Nemtsova ES, Khorosheva T, Kyryanova EV, Egos G, Perez MT, Tupasi T, Hwang SH, Kim CK, Kim SY, Lee HJ, Kuksa L, Norvaisha I, Skenders G, Sture I, Kummik T, Kuznetsova T, Somova T, Levina K, Pariona G, Yale G, Suarez C, Valencia E, Viiklepp P. 2012. Prevalence of and risk factors for resistance to second-line drugs in people with multidrug-resistant tuberculosis in eight countries: a prospective cohort study. Lancet 380:1406–1417. doi: 10.1016/S0140-6736(12)60734-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daffé M, Laneelle MA. 1988. Distribution of phthiocerol diester, phenolic mycosides and related compounds in mycobacteria. J Gen Microbiol 134:2049–2055. [DOI] [PubMed] [Google Scholar]
- 10.Jackson M, Stadthagen G, Gicquel B. 2007. Long-chain multiple methyl-branched fatty acid-containing lipids of Mycobacterium tuberculosis: biosynthesis, transport, regulation and biological activities. Tuberculosis 87:78–86. doi: 10.1016/j.tube.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Onwueme KC, Vos CJ, Zurita J, Ferreras JA, Quadri LE. 2005. The dimycocerosate ester polyketide virulence factors of mycobacteria. Prog Lipid Res 44:259–302. doi: 10.1016/j.plipres.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Camacho LR, Ensergueix D, Perez E, Gicquel B, Guilhot C. 1999. Identification of a virulence gene cluster of Mycobacterium tuberculosis by signature-tagged transposon mutagenesis. Mol Microbiol 34:257–267. doi: 10.1046/j.1365-2958.1999.01593.x. [DOI] [PubMed] [Google Scholar]
- 13.Camacho LR, Constant P, Raynaud C, Laneelle MA, Triccas JA, Gicquel B, Daffe M, Guilhot C. 2001. Analysis of the phthiocerol dimycocerosate locus of Mycobacterium tuberculosis. Evidence that this lipid is involved in the cell wall permeability barrier. J Biol Chem 276:19845–19854. [DOI] [PubMed] [Google Scholar]
- 14.Chavadi SS, Edupuganti UR, Vergnolle O, Fatima I, Singh SM, Soll CE, Quadri LE. 2011. Inactivation of tesA reduces cell wall lipid production and increases drug susceptibility in mycobacteria. J Biol Chem 286:24616–24625. doi: 10.1074/jbc.M111.247601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu J, Tran V, Li M, Huang X, Niu C, Wang D, Zhu J, Wang J, Gao Q, Liu J. 2012. Both phthiocerol dimycocerosates and phenolic glycolipids are required for virulence of Mycobacterium marinum. Infect Immun 80:1381–1389. doi: 10.1128/IAI.06370-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azad AK, Sirakova TD, Fernandes ND, Kolattukudy PE. 1997. Gene knockout reveals a novel gene cluster for the synthesis of a class of cell wall lipids unique to pathogenic mycobacteria. J Biol Chem 272:16741–16745. doi: 10.1074/jbc.272.27.16741. [DOI] [PubMed] [Google Scholar]
- 17.Wang XM, Lu C, Soetaert K, S'Heeren C, Peirs P, Laneelle MA, Lefevre P, Bifani P, Content J, Daffe M, Huygen K, De Bruyn J, Wattiez R. 2011. Biochemical and immunological characterization of a cpn60.1 knockout mutant of Mycobacterium bovis BCG. Microbiology 157:1205–1219. doi: 10.1099/mic.0.045120-0. [DOI] [PubMed] [Google Scholar]
- 18.National Committee for Clinical Laboratory Standards. 2003. Susceptibility testing of mycobacteria, nocardia, and other aerobic actinomycetes. Approved standard M24-A. National Committee for Clinical Laboratory Standards, Wayne, PA. [PubMed] [Google Scholar]
- 19.Mor N, Vanderkolk J, Heifets L. 1994. Inhibitory and bactericidal activities of levofloxacin against Mycobacterium tuberculosis in vitro and in human macrophages. Antimicrob Agents Chemother 38:1161–1164. doi: 10.1128/AAC.38.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siméone R, Leger M, Constant P, Malaga W, Marrakchi H, Daffe M, Guilhot C, Chalut C. 2010. Delineation of the roles of FadD22, FadD26 and FadD29 in the biosynthesis of phthiocerol dimycocerosates and related compounds in Mycobacterium tuberculosis. FEBS J 277:2715–2725. doi: 10.1111/j.1742-4658.2010.07688.x. [DOI] [PubMed] [Google Scholar]
- 21.Astarie-Dequeker C, Le Guyader L, Malaga W, Seaphanh FK, Chalut C, Lopez A, Guilhot C. 2009. Phthiocerol dimycocerosates of M. tuberculosis participate in macrophage invasion by inducing changes in the organization of plasma membrane lipids. PLoS Pathog 5:e1000289. doi: 10.1371/journal.ppat.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siméone R, Constant P, Malaga W, Guilhot C, Daffe M, Chalut C. 2007. Molecular dissection of the biosynthetic relationship between phthiocerol and phthiodiolone dimycocerosates and their critical role in the virulence and permeability of Mycobacterium tuberculosis. FEBS J 274:1957–1969. doi: 10.1111/j.1742-4658.2007.05740.x. [DOI] [PubMed] [Google Scholar]
- 23.Rastogi N, Goh KS, Horgen L, Barrow WW. 1998. Synergistic activities of antituberculous drugs with cerulenin and trans-cinnamic acid against Mycobacterium tuberculosis. FEMS Immunol Med Microbiol 21:149–157. doi: 10.1111/j.1574-695X.1998.tb01161.x. [DOI] [PubMed] [Google Scholar]
- 24.Parrish NM, Kuhajda FP, Heine HS, Bishai WR, Dick JD. 1999. Antimycobacterial activity of cerulenin and its effects on lipid biosynthesis. J Antimicrob Chemother 43:219–226. doi: 10.1093/jac/43.2.219. [DOI] [PubMed] [Google Scholar]
- 25.Mathema B, Kurepina NE, Bifani PJ, Kreiswirth BN. 2006. Molecular epidemiology of tuberculosis: current insights. Clin Microbiol Rev 19:658–685. doi: 10.1128/CMR.00061-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathys V, Wintjens R, Lefevre P, Bertout J, Singhal A, Kiass M, Kurepina N, Wang XM, Mathema B, Baulard A, Kreiswirth BN, Bifani P. 2009. Molecular genetics of para-aminosalicylic acid resistance in clinical isolates and spontaneous mutants of Mycobacterium tuberculosis. Antimicrob Agents Chemother 53:2100–2109. doi: 10.1128/AAC.01197-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Werngren J, Klintz L, Hoffner SE. 2006. Evaluation of a novel kit for use with the BacT/ALERT 3D system for drug susceptibility testing of Mycobacterium tuberculosis. J Clin Microbiol 44:2130–2132. doi: 10.1128/JCM.02218-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.David S. 2001. Synergic activity of d-cycloserine and β-chloro-d-alanine against Mycobacterium tuberculosis. J Antimicrob Chemother 47:203–206. doi: 10.1093/jac/47.2.203. [DOI] [PubMed] [Google Scholar]
- 29.Singh P, Wesley C, Jadaun GP, Malonia SK, Das R, Upadhyay P, Faujdar J, Sharma P, Gupta P, Mishra AK, Singh K, Chauhan DS, Sharma VD, Gupta UD, Venkatesan K, Katoch VM. 2007. Comparative evaluation of Löwenstein-Jensen proportion method, BacT/ALERT 3D system, and enzymatic pyrazinamidase assay for pyrazinamide susceptibility testing of Mycobacterium tuberculosis. J Clin Microbiol 45:76–80. doi: 10.1128/JCM.00951-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arain TM, Goldstein BP, Scotti R, Resconi A. 1994. Synergic activity of teicoplanin and ethambutol against Mycobacterium tuberculosis. J Antimicrob Chemother 33:359–360. doi: 10.1093/jac/33.2.359. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


