Abstract
Borrelia burgdorferi is the causative agent of Lyme disease, which affects an estimated 300,000 people annually in the United States. When treated early, the disease usually resolves, but when left untreated, it can result in symptoms such as arthritis and encephalopathy. Treatment of the late-stage disease may require multiple courses of antibiotic therapy. Given that antibiotic resistance has not been observed for B. burgdorferi, the reason for the recalcitrance of late-stage disease to antibiotics is unclear. In other chronic infections, the presence of drug-tolerant persisters has been linked to recalcitrance of the disease. In this study, we examined the ability of B. burgdorferi to form persisters. Killing growing cultures of B. burgdorferi with antibiotics used to treat the disease was distinctly biphasic, with a small subpopulation of surviving cells. Upon regrowth, these cells formed a new subpopulation of antibiotic-tolerant cells, indicating that these are persisters rather than resistant mutants. The level of persisters increased sharply as the culture transitioned from the exponential to stationary phase. Combinations of antibiotics did not improve killing. Daptomycin, a membrane-active bactericidal antibiotic, killed stationary-phase cells but not persisters. Mitomycin C, an anticancer agent that forms adducts with DNA, killed persisters and eradicated growing and stationary cultures of B. burgdorferi. Finally, we examined the ability of pulse dosing an antibiotic to eliminate persisters. After addition of ceftriaxone, the antibiotic was washed away, surviving persisters were allowed to resuscitate, and the antibiotic was added again. Four pulse doses of ceftriaxone killed persisters, eradicating all live bacteria in the culture.
INTRODUCTION
All pathogens studied to date form persisters, dormant variants of regular cells which are tolerant to killing by antibiotics. The ability to produce persisters explains the puzzling recalcitrance of chronic infections to antibiotics that are effective against the same pathogen in vitro. Indeed, many chronic infections are caused by drug-susceptible pathogens (1, 2). The immune system can effectively remove sessile cells from the blood and many of the tissues, and this accounts for the efficacy of antibiotics, including bacteriostatic compounds, in treating uncomplicated infections. When the immune response is limited, the result is often a chronic infection (2). Biofilms are a well-studied example of immune evasion and serve as a paradigm for understanding chronic infections. In biofilms, cells are protected from the large components of the immune system by a surface exopolymer (3–5). Antibiotics kill the regular cells, but dormant persisters survive, and when the concentration of antibiotic drops, they resuscitate and repopulate the biofilm (2). This scenario is supported by our finding of high-persister (hip) Pseudomonas aeruginosa isolates selected in the course of prolonged antibiotic treatment (6). Isolated from patients with late-stage cystic fibrosis, hip mutants of P. aeruginosa can produce 1,000 times more persisters than the parent strain; this indicates that selection for increased tolerance (rather than resistance) provided the pathogen with a survival advantage. Similarly, hip mutants are selected during treatment of oral thrush caused by Candida albicans (7). In Salmonella enterica serovar Typhimurium, entrance of pathogens into human cells where they are protected from the immune system is accompanied by a sharp increase in persister formation and tolerance to killing by antibiotics (8). In tuberculosis, dormant cells are likely responsible for the need of a lengthy treatment of the acute stage and for the latent form of the disease. Mycobacterium tuberculosis hides from the immune system in macrophages or in granulomas (9).
Borrelia burgdorferi causes Lyme disease, with 300,000 estimated cases annually in the United States alone (10). When treated early with antibiotics, the disease usually resolves (11, 12). If treatment is delayed, the pathogen spreads throughout the body and can cause meningitis, arthritis, and carditis. Meningitis and carditis are mostly self-limited, but Lyme arthritis can persist for years (13, 14). A substantial proportion of patients receiving their first course of antibiotics for Lyme arthritis do not respond fully to a 28-day course of treatment. In such cases, retreatment with additional courses of antibiotics is recommended (13, 15, 16). B. burgdorferi avoids immune attack by antigenic variation of surface components and by decreasing exposure of antigens (17–19). In this regard, Lyme disease resembles other chronic infections where the pathogen is protected from the immune system, and persister cells may enable it to survive treatment with antibiotics. In Escherichia coli, the model organism for the study of persisters, dormant cells are formed primarily through expression of toxin-antitoxin (TA) modules. Toxins confer dormancy by either inhibiting protein synthesis or by decreasing the energy level of cells (20–22). TA modules are widely spread among bacteria and are copiously present in some pathogens. E. coli has more than 30 TA modules and M. tuberculosis has more than 75 (23, 24). Interestingly, there are apparently no TA modules in the genome of B. burgdorferi (25). Virtually nothing is known about persisters in this species. In this study, we report formation of drug-tolerant persisters in B. burgdorferi and describe possible approaches to their elimination.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Borrelia burgdorferi strain B31 5A19 that had been passaged five times in vitro was kindly provided by Monica Embers (26). B. burgdorferi was grown in Barbour-Stoenner-Kelly-II (BSK-II) liquid medium in a microaerophilic chamber (34°C, 3% O2, 5% CO2). Cultures were started by thawing −80°C glycerol stocks of B. burgdorferi (titer, approximately 107 CFU/ml) and diluting 1:20 into fresh BSK-II medium.
BSK-II liquid medium was prepared according to protocol received from Monica Embers' lab by adding the following ingredients to 400 ml of deionized water and mixing thoroughly: 20 g bovine serum albumin (Sigma), 2 g neopeptone (Fluka), 0.8 g yeastolate (BD), 4 g HEPES sodium salt (Sigma), 2.4 g 10× CMRL (US Biologicals), 0.28 g sodium citrate (Fisher), 0.32 g sodium pyruvate (Sigma), 2 g glucose (Fisher), 0.16 g N-acetylglucosamine (Sigma), and 0.88 g sodium pyruvate (Sigma). The pH of the medium was adjusted to 7.6, and 24 ml of rabbit serum (Sigma) was added. The medium was then filtered through a 0.22-μm filter.
Semisolid plating was used to obtain CFU counts (27). First, BSK 1.5× medium for semisolid plating was prepared as described by Samuels (27). The following ingredients were added to 1 liter of deionized water (LabChem, Inc.) and mixed thoroughly: 8.33 g neopeptone (Fluka), 4.22 g yeastolate (BD), 9.99 g HEPES acid (Fisher), 8.33 g glucose (Fisher), 1.22 g sodium citrate (Fisher), 1.33 g sodium pyruvate (Sigma), 0.670 g N-acetylglucosamine (Sigma), and 7.66 g sodium bicarbonate (Sigma). The pH of the medium was adjusted to 7.5, and then 83.25 g of bovine serum albumin (Sigma) was added. The medium was stirred for 1 h and then filtered using a 0.22-μm filter. After preparation, 1.5× BSK-II was stored at 4°C and used within 7 days. On the day of plating, 125 ml of 1.5× BSK was mixed with 6 ml rabbit serum and 19 ml 1× CMRL (97.89 mg/ml) and equilibrated to 55°C; 1.7% agarose (Lonza) was melted and equilibrated to 55°C. When all ingredients had equilibrated to 55°C, 1.7% agarose was added to 1.5× BSK at a ratio of 2:1 (BSK/agarose) to create BSK agarose. Eight milliliters of BSK agarose was dispensed into 60-mm petri dishes as bottom agar and allowed to solidify. For the top agar, 100 μl of the given dilution of B. burgdorferi was mixed with 5 ml of 55°C BSK agarose and poured onto the bottom agar plates and allowed to solidify. The plates were incubated in zip-top bags in a microaerophilic chamber (34°C, 3% O2, 5% CO2) for at least 21 days to obtain visible colonies.
Antimicrobial agents.
Amoxicillin (Sigma), doxycycline hydrochloride (MP Biomedicals), ceftriaxone disodium salt hemi (heptahydrate) (Sigma), and vancomycin hydrochloride (Sigma) were dissolved in water. Mitomycin C (Sigma), gemifloxacin mesylate (Tecoland Corporation), and spectinomycin dihydrochloride pentahydrate (RPI) were dissolved in dimethyl sulfoxide (DMSO). Daptomycin cyclic lipopeptide (Sigma) was dissolved in a 5 μg/ml solution of calcium chloride. Stock solutions of antibiotics were aliquoted and stored at −20°C until use. Antibiotics did not undergo freeze-thaw cycles.
Killing experiments.
B. burgdorferi was cultured in liquid BSK-II medium for 3 days to late exponential growth phase or for 5 days to stationary phase. Antibiotics were then added to the culture. The cultures were incubated in the microaerophilic chamber (34°C, 3% O2, 5% CO2). At a given time point, an aliquot of the culture was washed twice by centrifuging the culture at 13,200 rpm for 5 min and resuspending the pellet in an equal volume of fresh BSK-II medium. The cultures were then serially diluted in fresh BSK-II medium. One hundred microliters of the appropriate dilution was mixed with 5 ml of BSK agarose and poured as top agar. Plates were incubated in the microaerophilic chamber until visible colonies appeared (at least 21 days).
Growth-persister experiments.
Cultures of B. burgdorferi were started as described above. At each time point, an aliquot of a growing culture was removed, diluted, and plated for CFU counts to generate the growth curve. A second aliquot (1 ml or 3 ml) was removed at the same time and challenged for 5 days with the indicated antibiotic. After 5 days, an aliquot of challenged culture was removed, washed twice, diluted, and plated for CFU counts to generate the persister curve.
MIC testing.
A slightly modified version of the broth microdilution method (28) was used. B. burgdorferi was grown in liquid culture for 3 days to reach exponential phase and then back diluted 1:10 into fresh BSK-II medium to make the inoculum. All antibiotics were prepared as stock solutions in solvent (water or DMSO) based on the concentration to be tested and diluted in 2-fold increments in a 96-well stock plate. Two microliters per well of the antibiotic stock solution was transferred to the 96-well MIC plate to which 198 μl of the B. burgdorferi inoculum was added (final concentration of approximately 106 cells/well). Media, growth, and vehicle controls were included on each plate. The MIC plate was covered with Breath-Easy film (Diversified Biotech) and incubated in the microaerophilic chamber (34°C, 3% O2, 5% CO2) for 72 h. The lowest concentration of antibiotics that inhibited growth was interpreted as the MIC. All MIC assays were repeated at least twice in triplicate.
RESULTS
Characterization of B. burgdorferi persisters.
The presence of persisters is indicated by a biphasic pattern in a time-dependent killing experiment. The bulk of the population is rapidly killed, followed by a lower rate of death in a subpopulation of tolerant cells (29, 30). In order to determine whether B. burgdorferi forms persisters, time-dependent killing experiments were performed with antibiotics commonly prescribed to patients with Lyme disease. Doxycycline is a bacteriostatic protein synthesis inhibitor; amoxicillin and ceftriaxone inhibit bacterial cell wall synthesis and are bactericidal for many bacteria. MICs of doxycycline, amoxicillin, and ceftriaxone were determined (Table 1). Levels of antibiotics close to what is achievable with standard clinically prescribed treatment dosing were chosen to evaluate persister formation in B. burgdorferi, and we used colony forming unit (CFU) counts to determine viability.
TABLE 1.
Selected antibiotics tested against B. burgdorferi
| Drug | Class | MIC (μg/ml)a | Maximum serum concn (μg/ml) | Reference |
|---|---|---|---|---|
| Amoxicillin | β-Lactam | 0.06 | 7.6 | 57 |
| Ceftriaxone | Cephalosporin | 0.01 | 256.9 | 58 |
| Doxycycline | Tetracycline | 0.25 | 2.6–5.9 | 59 |
| Gemifloxacin | Fluoroquinolone | 0.125 | 2.33 | 38 |
| Spectinomycin | Aminoglycoside | 2 | 140–160 | 41 |
| Daptomycin | Lipopeptide | 12.5–25 | 55–133 | 60 |
| Vancomycin | Glycopeptide | 0.25 | 40 | 61 |
| Mitomycin C | Antitumor antibiotic | 0.2 | 3.2 | 62 |
The MIC was determined by the broth microdilution method.
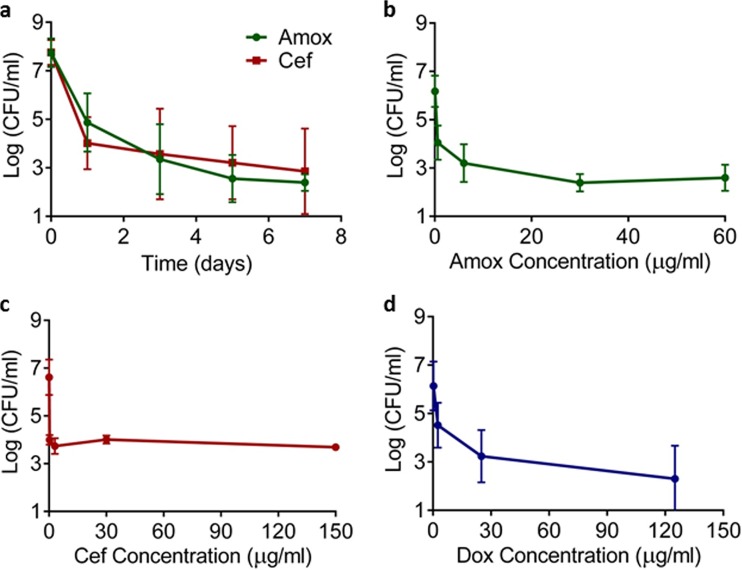
Amoxicillin (6 μg/ml, 100× MIC) and ceftriaxone (3 μg/ml, 300× MIC) at clinically achievable levels killed the majority of cells in the first day, after which a slow phase of death followed for the next 6 days (Fig. 1a). This characteristic biphasic pattern of killing is consistent with the presence of drug-tolerant persister cells.
FIG 1.
Killing of B. burgdorferi by antibiotics. (a) Time-dependent killing. Antibiotics were added to an exponentially growing culture; samples were taken over time, washed, diluted, and plated in semisolid BSK-II medium for CFU counts. The culture was treated with amoxicillin (Amox) (6 μg/ml) or ceftriaxone (Cef) (3 μg/ml) (n = 9). (b to d) Dose-dependent killing. A late exponential culture of B. burgdorferi culture was exposed to antibiotics for 5 days, and surviving cells were determined by CFU count. The culture was treated with amoxicillin (b), ceftriaxone (c), or doxycycline (Dox) (d) (n = 6). Error bars represent standard errors.
Previous studies have shown that the persister fraction in other bacteria remains relatively unchanged even as the antibiotic level increases. We sought to determine if B. burgdorferi persisters behaved similarly in a dose-dependent killing experiment. As the concentration of amoxicillin and ceftriaxone increased, the fraction of surviving cells remained largely unchanged (Fig. 1b and c). Doxycycline is a bacteriostatic antibiotic but at higher concentrations appeared to effectively kill B. burgdorferi (Fig. 1d). Again, the fraction of surviving cells did not change significantly with increasing levels of the compound. Thus, B. burgdorferi forms persisters capable of surviving very high concentrations of antibiotics, which exceed what is clinically achievable.
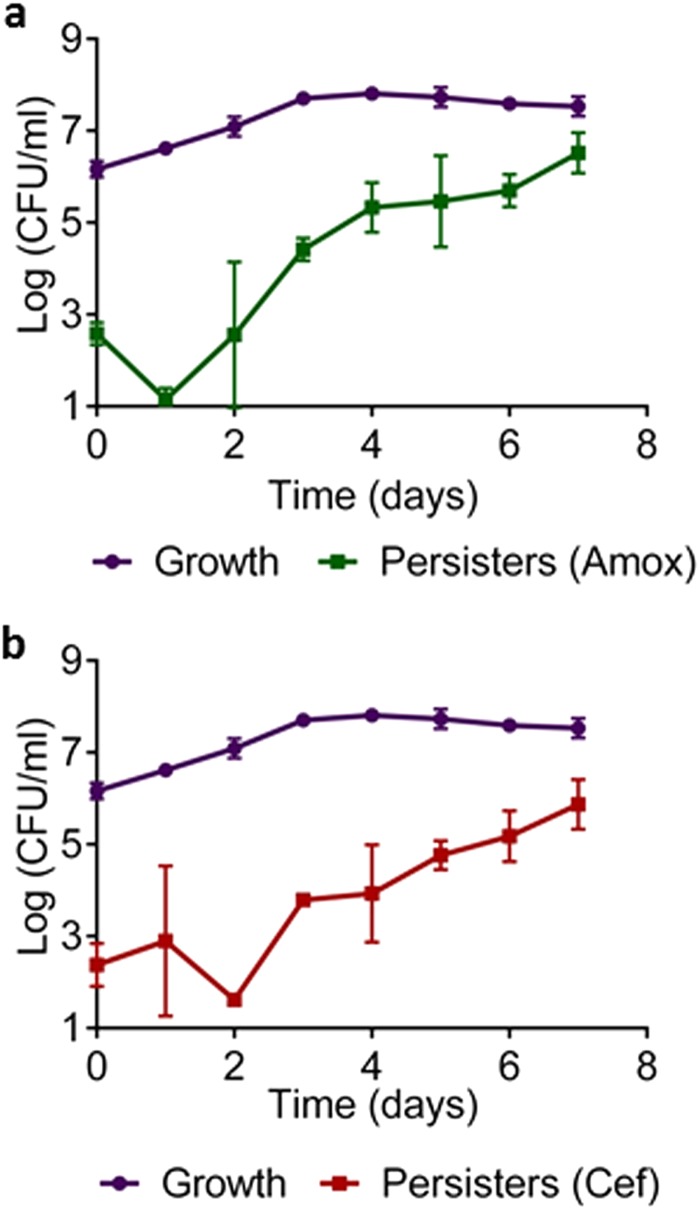
Density-dependent formation is a common feature of persisters reported for all pathogens examined so far, including E. coli, Staphylococcus aureus, P. aeruginosa, and M. tuberculosis (6, 31–33). In order to test this property in B. burgdorferi, samples from a growing culture were removed over time, exposed to a lethal dose of antibiotic for 5 days, and then plated for CFU. There was a characteristic dip in persister levels in the early log phase, which is probably due to the resuscitation of dormant cells carried over from the inoculum (Fig. 2). At the midlog phase, there is a sharp increase in persister levels, which continues as the density of the culture rises. In E. coli, once the culture reaches stationary state, complete tolerance is achieved for β-lactams that only kill growing cells (34). In B. burgdorferi, we observe a very different picture; amoxicillin and ceftriaxone kill stationary cells fairly well, yet the fraction of persisters continues to increase. One possibility is that this “stationary” culture actually represents a steady state where some cells die and others grow.
FIG 2.
Growth-dependent persister formation in B. burgdorferi. Growth in BSK-II medium was determined by CFU count. Persister levels were determined by taking samples from the growing culture, exposing to antibiotic for 5 days, and counting CFU. (a) Amoxicillin (Amox) (6 μg/ml) (n = 6); (b) ceftriaxone (Cef) (3 μg/ml) (n = 6). Error bars represent standard errors.
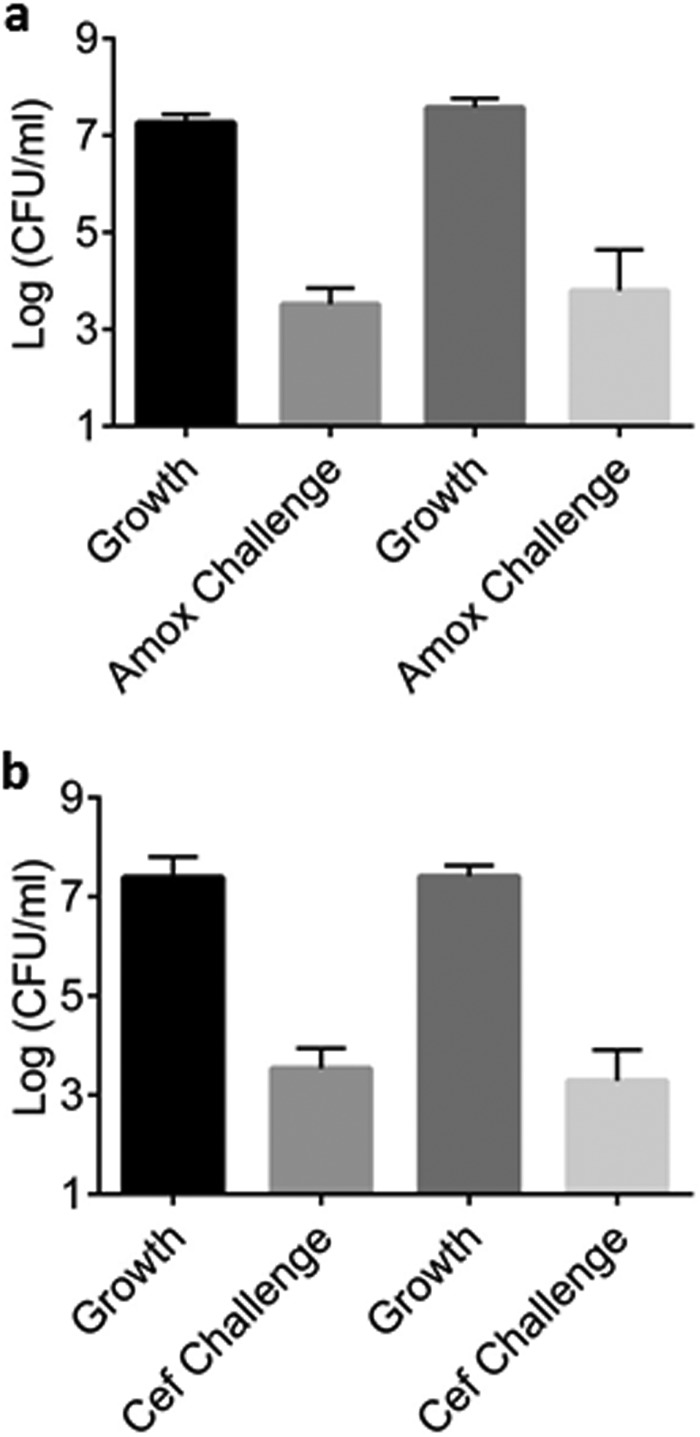
Next, we tested whether the B. burgdorferi cells surviving antibiotic treatment are drug-tolerant persisters or resistant mutants. For this, colonies produced by the surviving cells were regrown and tested for MIC. The amoxicillin and ceftriaxone MIC remained unchanged, showing that surviving cells had not acquired or developed a genetic mechanism for antibiotic resistance. The population grown from the surviving cells produced the same level of persisters as the original population (Fig. 3). These experiments show that B. burgdorferi forms typical persister cells.
FIG 3.
Persister formation is not heritable. Colonies recovered from a persister experiment before and after antibiotic treatment were used to inoculate fresh BSK-II medium. The colonies were allowed to grow for 3 days and treated with the same antibiotic used in the original persister experiment for 5 days (Amox, 6 µg/ml; Cef, 3 µg/ml). Persister levels of the colonies recovered after antibiotic treatment (right two bars) were not significantly different than the colonies recovered before antibiotic treatment (left two bars) (n = 5). Error bars represent standard errors. Amox, amoxicillin; Cef, ceftriaxone.
Eradication of B. burgdorferi persisters. (i) Drug combinations.
Some antibiotics act synergistically, such as sulfonamide and trimethoprim, polymyxin and gentamicin, and aminoglycosides and β-lactams (35), and we wanted to see if a combination of compounds known to be active against B. burgdorferi would increase the efficiency of killing regular and persister cells.
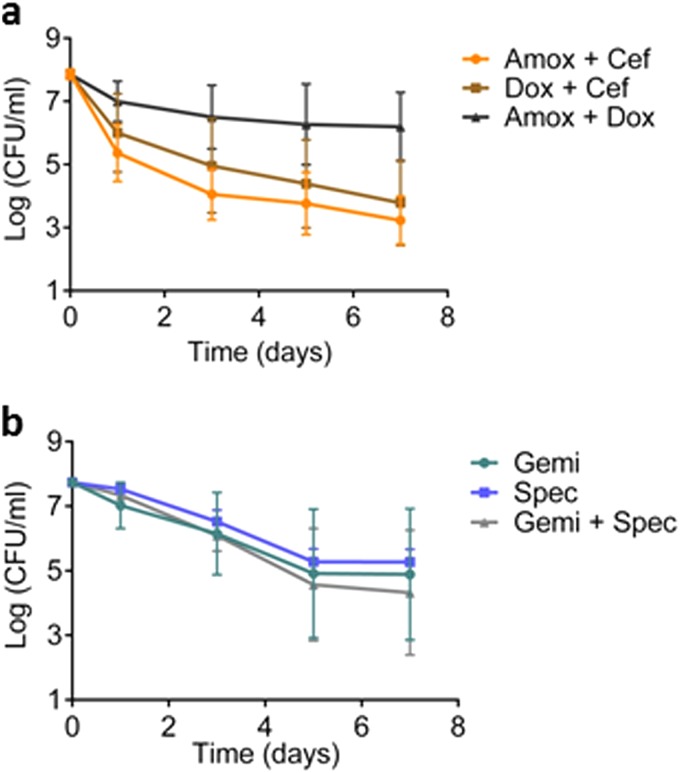
All possible two-drug combinations of amoxicillin, ceftriaxone, and doxycycline were tested with a late-exponential-phase culture in a time-dependent killing experiment and found to be no more effective than the drugs used individually for killing B. burgdorferi (Fig. 4a). Doxycycline actually inhibited the action of amoxicillin. We have shown previously that fluoroquinolones and aminoglycosides can kill nongrowing cells (36, 37), and we next tested these compounds against B. burgdorferi. The pathogen is generally poorly susceptible to compounds from these classes. However, the B. burgdorferi MICs for gemifloxacin (fluoroquinolone) and spectinomycin (aminoglycoside) are within achievable human dosing levels, so we chose to test them (38–41) (Table 1). Gemifloxacin and spectinomycin were ineffective in killing B. burgdorferi at tested concentrations (Fig. 4b). Combining these compounds also did not improve killing (Fig. 4b).
FIG 4.
Killing of B. burgdorferi with drug combinations. (a) Time-dependent killing of late exponential B. burgdorferi cultures exposed to the indicated antibiotics in combination. Amoxicillin (Amox) (6 μg/ml), ceftriaxone (Cef) (3 μg/ml), and doxycycline (Dox) (2.5 μg/ml) (n = 6). (b) Killing of late exponential B. burgdorferi exposed to gemifloxacin (Gemi) (1.5 μg/ml) and/or spectinomycin (Spec) (160 μg/ml) singly or in combination (n = 6). An aliquot was taken at indicated time points, washed, diluted, and plated on semisolid BSK-II medium for CFU counts. Error bars represent standard errors.
(ii) Experimental compounds.
Having shown that combinations of clinically prescribed antibiotics for Lyme disease are unable to effectively kill persister bacteria, we sought to examine some novel potential antimicrobial agents. We recently showed that acyldepsipeptide (ADEP4), an activator of the ClpP protease, effectively kills persisters in S. aureus (42). In the presence of ADEP4, the protease cleaves mature proteins, forcing the cell to self-digest. However, ADEP4 did not have significant activity against B. burgdorferi (not shown), which may be due to poor penetration.
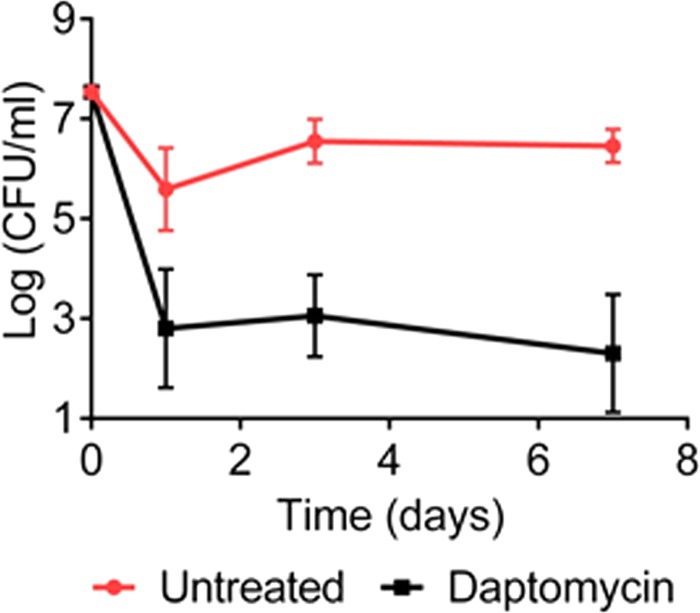
We then considered whether knowledge of B. burgdorferi biology might be exploited to predict vulnerability to existing approved compounds. B. burgdorferi lives under microaerophilic conditions, where the capacity for energy generation is limited by comparison to aerobic organisms. Daptomycin is the only approved membrane-acting antibiotic that disrupts the proton motive force. The B. burgdorferi MIC to daptomycin was fairly high, 12 to 25 μg/ml (Table 1), in accordance with published data (43). Daptomycin was highly bactericidal against B. burgdorferi, but a remaining subpopulation of persisters survived (Fig. 5), suggesting that B. burgdorferi persisters can tolerate a drop in the energy level. Next, we tested vancomycin. This large glycopeptide antibiotic binds to lipid II, a precursor of peptidoglycan, on the outside of the cytoplasmic membrane. Vancomycin is highly effective against Gram-positive bacteria, but does not penetrate across the outer membrane of Gram-negative species. Surprisingly, the vancomycin MIC with B. burgdorferi is low, 0.25 μg/ml, which is similar to Gram-positive species. B. burgdorferi cells have an outer membrane; the basis for this anomaly is unclear. Vancomycin effectively killed growing cells of B. burgdorferi, but not persisters, and was comparable to ceftriaxone (not shown). We also tested teixobactin, a compound we recently discovered, which also binds lipid II (44). At 1.2 kDa, teixobactin is considerably smaller than vancomycin (1.8 kDa), but it did not exhibit good activity in killing B. burgdorferi (data not shown).
FIG 5.
Killing of B. burgdorferi by daptomycin. Time-dependent killing of stationary-phase B. burgdorferi exposed to daptomycin (81 μg/ml) (n = 3). Error bars represent standard errors.
(iii) Prodrugs.
Growth under microaerophilic conditions suggests vulnerability to compounds whose action depends specifically on a low-oxygen environment. Nitroaromatic compounds such as metronidazole are prodrugs that are converted into reactive drugs by bacterial nitroreductases. These enzymes are expressed under anaerobic or microaerophilic conditions and target pathogens living in these environments (i.e., Helicobacter pylori, Clostridium difficile, and E. coli). We found that some nitroaromatic compounds, like nitrofurantoin, are effective in killing E. coli persisters (45). However, we did not detect homologs of nitroreductases in the genome of B. burgdorferi. The MIC for nitroaromatic compounds (nitrofurantoin, nitrofurazone, and metronidazole) was too high to make them useful agents for killing B. burgdorferi persisters (data not shown).
Another compound that depends on a reductive environment for action is the prodrug mitomycin C. Upon entering the cell, mitomycin C is reduced into an active drug which then forms covalent adducts with DNA (46). Originally discovered in a screen for antibiotics, mitomycin C is now used as an anticancer agent. Cancers often create a microaerophilic environment, which together with rapid cell division, accounts for the relatively selective action of mitomycin C against them. Functional RecBC and RecFOR pathways are required to repair DNA damaged by mitomycin C in E. coli (46). Interestingly, according to genomic data, B. burgdorferi lacks the genes of the RecFOR pathway (47), further suggesting vulnerability to this compound.
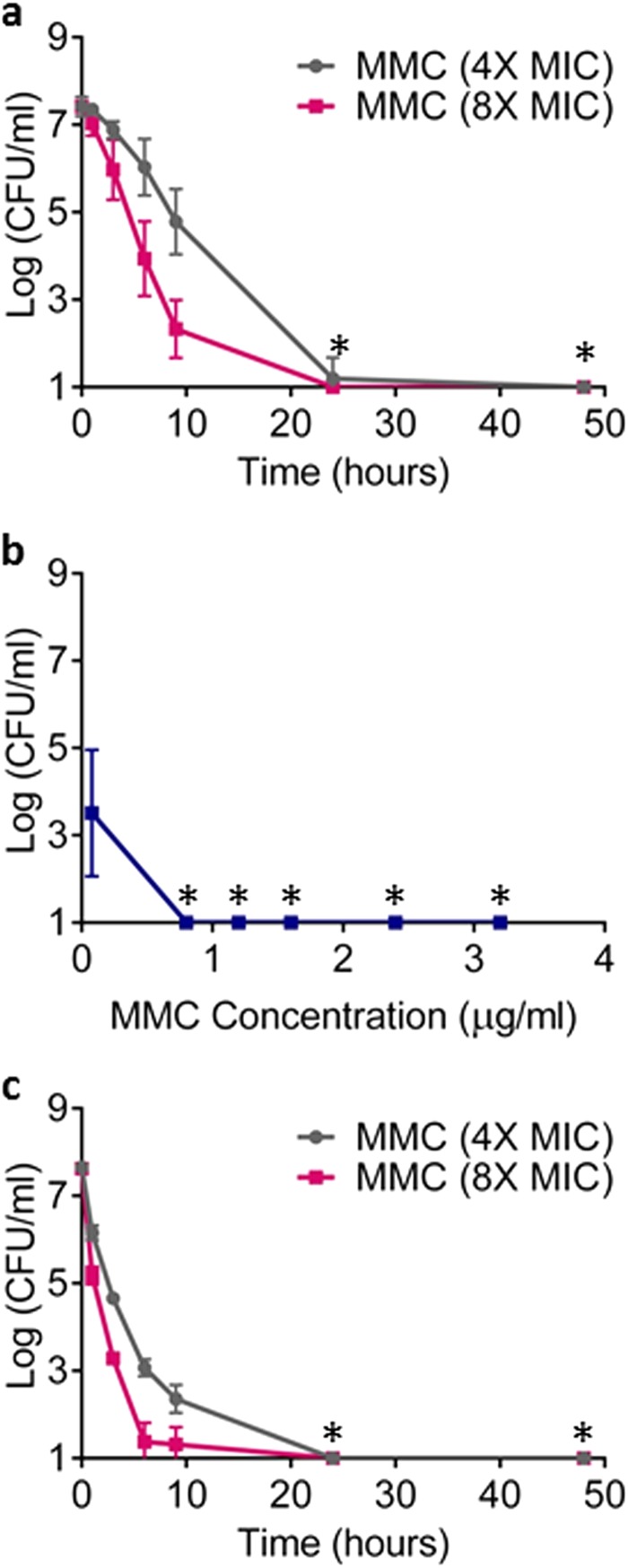
Mitomycin C eradicated a late exponential culture of B. burgdorferi within 24 h, with no detectable persisters remaining (Fig. 6a). This was observed with a low, clinically achievable dose of the compound, 1.6 μg/ml or 8× MIC. In a dose-dependent experiment, eradication of a late exponential culture was achieved within 5 days with a 0.8 μg/ml (4× MIC) dose of the compounds (Fig. 6b). Finally, mitomycin C was tested against a stationary culture of B. burgdorferi. Surprisingly, eradication was achieved with a low dose of 4× MIC within 24 h (Fig. 6c). It appears that a stationary population is more susceptible to this compound than an exponentially growing one.
FIG 6.
Killing of B. burgdorferi by mitomycin C (MMC). (a, c) Time-dependent killing of B. burgdorferi. Three independent cultures of B. burgdorferi either at late exponential phase (a) or stationary phase (c) of growth were treated with MMC, 0.8 μg/ml (4× MIC) or 1.6 μg/ml (8× MIC). (b) Dose-dependent killing of late exponential cultures of B. burgdorferi after 5-day exposure to increasing concentrations of MMC. An aliquot was taken at indicated time points, washed, diluted, and plated on semisolid BSK-II medium for CFU counts (n = 6). The x axis is the limit of detection. Asterisks represent eradication to the limit of detection.
(iv) Pulse dosing.
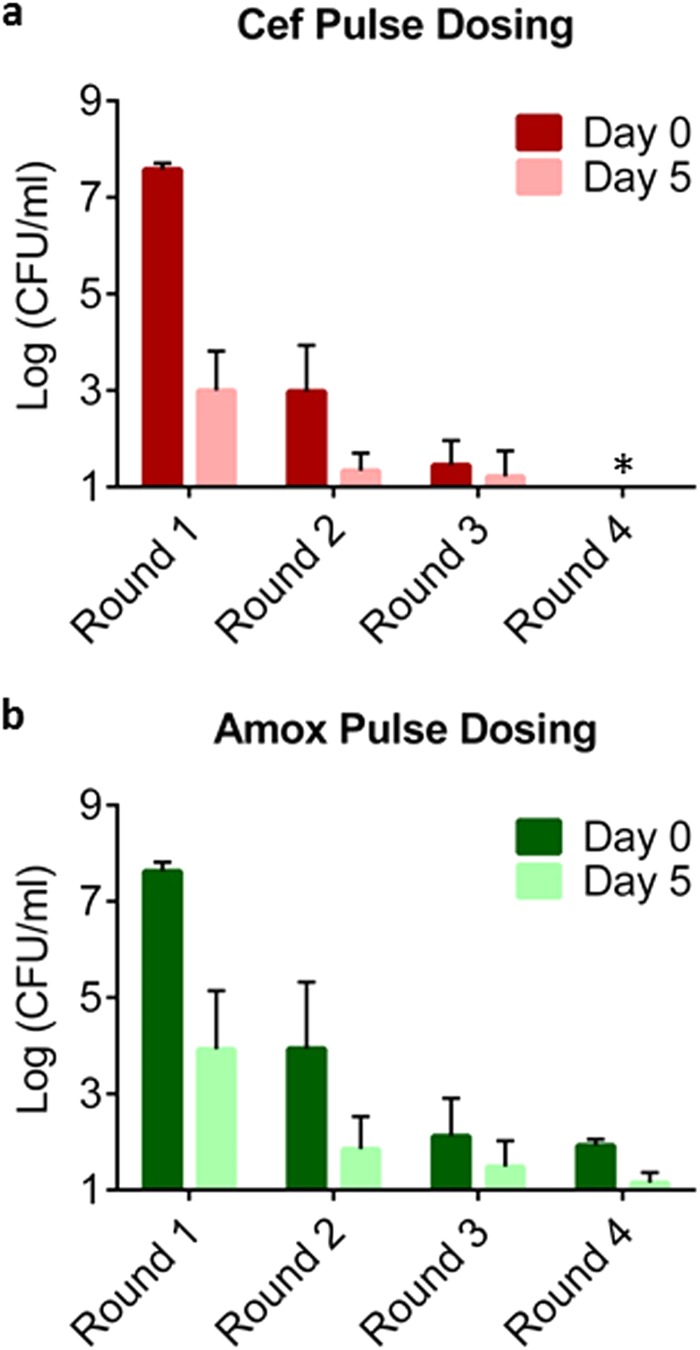
Apart from identifying compounds capable of killing persisters, it may also be possible to eliminate them with conventional bactericidal antibiotics using pulse dosing. Based on our results, the level of persisters is lowest during early exponential growth (Fig. 2). We reasoned that allowing growth to resume and then retreating them as they enter the exponential phase may kill persisters surviving an antibiotic challenge. Eradication of the culture can then be achieved after several rounds of killing and regrowth. To test this, a culture of B. burgdorferi was exposed to amoxicillin or ceftriaxone. The surviving persisters were allowed to resuscitate for a short period of time in fresh medium, and then exposed to the antibiotic again for a second round of killing. Persisters were substantially diminished after four rounds of killing with amoxicillin, and were eradicated below the limit of detection after four rounds of killing with ceftriaxone (Fig. 7). Additionally, we found that a ceftriaxone solution stored under experimental conditions (in BSK-II medium at 34°C, 3% O2, and 5% CO2) does not lose activity, as measured by MICs against B. burgdorferi, for up to 20 days. The activity of amoxicillin measured similarly, however, dropped 20-fold over 20 days, which suggests degradation over time. The resulting MIC was still lower than the concentration used in killing experiments. This pulse-dosing experiment shows that a population of the pathogen can be eradicated with conventional antibiotics commonly used to treat the disease.
FIG 7.
Pulse dosing results in effective killing of B. burgdorferi persisters. Late exponential cultures of B. burgdorferi were treated with ceftriaxone (Cef) (3 μg/ml) (a) or amoxicillin (Amox) (6 μg/ml) (b) for 5 days. This represents the first round of killing. The cultures were then washed and allowed to recover in fresh BSK-II medium for 24 h. They were then treated again with amoxicillin (6 μg/ml) or ceftriaxone (3 μg/ml) for a further 5 days to give the second round of killing. This was repeated for a total of four rounds of killing with a 24-h period of growth in fresh medium between each round. Bars represent the averages of at least three independent cultures (n = 3 to 6), and error bars represent standard errors. The x axis is the limit of detection. Asterisks represent eradication to the limit of detection.
DISCUSSION
The presence of drug-tolerant persisters can explain the recalcitrance of chronic infections to antimicrobial therapy, especially in cases when the disease is caused by a susceptible pathogen. While some chronic infections are ancient (leprosy, syphilis, tuberculosis), many cases in developed countries are consequences of otherwise successful medical intervention. Various indwelling devices (catheters, prostheses, heart valves) provide a substratum for biofilms that protect persisters from the immune system (2). Even in bacterial infections that are routinely successfully treated with antibiotics, there is dependence upon the host immune system to control persisting bacteria that are not eradicated by antibiotics. The role of the immune system becomes evident when these same infections involve immunocompromised hosts and antibiotic eradication of the infection becomes much more difficult.
B. burgdorferi is a pathogen that can affect immunocompetent hosts. It establishes long-term infections lasting years to lifelong in its natural (i.e., mice) and incidental (i.e., humans) hosts in the absence of antibiotic therapy (14, 48). Treatment in the early stages of disease results in good outcomes. Delays in diagnosis and treatment lead to sequelae that may require additional treatment. For example, patients who develop arthritis, which typically begins after 1 month of untreated infection, often do not respond fully to a first course of 28 days of antibiotics (49). The majority of these patients have evidence of B. burgdorferi DNA in their synovial fluid and will respond to additional 1- or 2-month courses of antibiotics (13, 16). A smaller minority of patients referred to as “antibiotic-resistant Lyme arthritis” patients will continue to have arthritis with synovial fluid that is PCR negative for B. burgdorferi DNA. These patients typically respond to anti-inflammatory agents such as methotrexate or tumor necrosis factor (TNF) inhibitors. These two groups of patients should be distinguished from patients with “chronic Lyme disease” that exhibit fatigue, myalgias, and arthralgias without clear evidence for the presence of the pathogen. For the first group of Lyme arthritis patients responsive to antibiotics, given that there is no reported resistance to clinically used tetracyclines, β-lactams, and cephalosporins in the pathogen, the need for lengthy courses of therapy is unclear. The presence of persister cells is one possible explanation, and this is a pattern that is seen in other infections where persister cells are thought to be relevant for disease in vivo.
We found that similar to other pathogens, the pattern of killing of B. burgdorferi by bactericidal antibiotics is biphasic, with a small subpopulation of surviving persisters. These surviving clones are not resistant mutants; upon regrowth, they form a new persister subpopulation. Also similar to E. coli, S. aureus, and other pathogens, the density of persisters increases as the culture deviates from strictly exponential growth, reaching a maximum at stationary state. This is probably due to a deterioration of growth conditions resulting in increasing numbers of dormant cells. However, it is notable that the stationary state in B. burgdorferi is atypical, as amoxicillin and ceftriaxone continue to kill the majority of cells despite an increase in the level of persisters in the population. Cell wall-acting antibiotics do not normally kill nongrowing cells; one possibility is that stationary state B. burgdorferi cultures represent a steady state of growing and dying cells. The ability of β-lactams to kill nongrowing cells was also observed in M. tuberculosis where a combination of meropenem and a β-lactamase inhibitor was able to kill viable but nonreplicative cells (50). The authors speculate that peptidoglycan remodeling continues in these nonreplicating cells allowing for the activity of the β-lactam. This is another possible explanation of the killing of stationary-phase B. burgdorferi that we observed with amoxicillin and ceftriaxone.
In a recently published study, Iyer et al. (51) treated two different strains of B. burgdorferi with ceftriaxone and were unable to detect live B. burgdorferi by subculture in liquid medium. However, the cell density in that study was 107 cells/ml, and according to our data, persister levels in this early exponential culture are low. In some of the biological replicates treated with ceftriaxone, we have not been able to recover live cells. At higher cell densities, the presence of persisters is unambiguous.
One common strategy for improving elimination of infective agents is to combine existing compounds. For example, β-lactams and aminoglycosides are known to synergize with each other to achieve effective killing of Enterococci (52). We tested combinations of standard antibiotics used in treatment of Lyme disease as well as a combination of a fluoroquinolone and an aminoglycoside, compounds that often synergize and are capable of killing nongrowing cells. However, there was no synergy in killing B. burgdorferi with any of the tested combinations.
We recently described the efficient killing of persisters in S. aureus (42) and in E. coli (45) and tested these compounds against B. burgdorferi. ADEP4, an activator of the Clp protease, causes massive protein degradation in S. aureus, killing regular cells and persisters. However, ADEP4 was not active against B. burgdorferi. We also reported that nitrofuran prodrugs are effective in killing E. coli persisters. Nitrofurans are reduced by bacterial nitroreductases into generally reactive compounds, explaining their activity against persisters. Nitroreductases are expressed under anaerobic or microaerophilic conditions. B. burgdorferi is a microaerophilic organism but does not have obvious homologs of a nitroreductase, and nitrofurans we tested were fairly inactive.
We also tested daptomycin, a lipopeptide that acts by increasing the K+ permeability of the membrane. Being in a low-energy (microaerophilic) environment, the pathogen may be vulnerable to membrane-acting compounds. Daptomycin killed the majority of cells in a stationary culture, but the level of surviving persisters was comparable to that of a stationary culture treated with ceftriaxone. In a recent publication, daptomycin was reported to kill B. burgdorferi persisters more effectively than regular cells (43). This conclusion was based on equating stationary cells with persisters. As follows from our experiments, a stationary culture harbors a small subpopulation of persisters. The actual level of stationary cells apparently surviving treatment by daptomycin in that study was very high, 28%, as determined by LIVE/DEAD staining. Under similar conditions, we detect about 103 (0.002%) surviving persisters by CFU count. It appears that LIVE/DEAD staining may be over-reporting the level of live B. burgdorferi cells.
Another weakness of the pathogen is its apparently limited ability for DNA repair. Based on the genome, B. burgdorferi lacks recFOR. In E. coli, RecBC and RecFOR are required for repair of DNA damage caused by mitomycin C, an anticancer drug. Mitomycin C at a low, clinically achievable dose (8× MIC) eradicated B. burgdorferi persisters in exponential and stationary cultures within 24 h. A highly reduced environment activates mitomycin C, and this contributes to its selective action in microaerophilic tumors. While the killing of persisters by mitomycin C is impressive, given the toxicity of this drug, this is more of a proof-of-principle for a compound exploiting the weaknesses of this pathogen rather than a clinically useful agent. Treatment with mitomycin C can result in serious negative side effects, and it should not be used for treatment of Lyme disease. This agent will be useful to examine the possible contribution of persisters to the disease in an animal model of infection.
Another peculiar feature of B. burgdorferi and a weakness of the pathogen is the lack of development of resistance to any antibiotic used to treat Lyme disease. Even attempts to raise mutants resistant to amoxicillin and ceftriaxone in vitro have been unsuccessful. Joseph Bigger proposed an interesting strategy for elimination of persisters in 1944 in the first publication describing these cells (53). The rationale is to add an antibiotic to kill off regular cells, wash it away, and allow the culture to start regrowing, at which point persisters will resuscitate. Reintroducing antibiotics will kill the regrowing bacteria. The argument against pulse dosing is that this protocol invites resistance development. Given that this is not a concern for B. burgdorferi, pulse dosing may be an effective strategy, and we performed pulse dosing with amoxicillin and ceftriaxone. Persisters were eradicated with ceftriaxone in four pulses. These experiments form the basis for testing pulse dosing in an animal model and, if successful, in humans.
While we have identified the presence of B. burgdorferi persisters in cultures of the organism, the mechanisms by which they are able to survive remain unknown. There are multiple pathways of persister formation in other bacteria. The study of persisters so far identified redundant TA modules as a main component responsible for persister formation in E. coli and S. Typhimurium (8, 20, 22). TA modules are widely spread among bacteria but are surprisingly absent from the genome of B. burgdorferi. Other components leading to persister formation in E. coli have been detected as well, including the stringent response (54), various metabolic processes (55, 56), global regulators, and protein stabilizing chaperones (56). Future work will determine if these or other processes are involved in persister formation in B. burgdorferi and if persisters play a role in the pathogenesis of Lyme disease in humans.
ACKNOWLEDGMENTS
This work was supported by grants from Lyme Research Alliance, a T-R01AI085585 grant from the NIH to Kim Lewis, and grants R21AI082436 and U01AI109656 from the NIH to Linden T. Hu.
We thank Monica E. Embers from Tulane University for providing us with the B. burgdorferi B31 5A19 strain and Yi-Pin Lin from John Leong's lab at Tufts University for his help with the semisolid plating method.
REFERENCES
- 1.Burns JL, Van Dalfsen JM, Shawar RM, Otto KL, Garber RL, Quan JM, Montgomery AB, Albers GM, Ramsey BW, Smith AL. 1999. Effect of chronic intermittent administration of inhaled tobramycin on respiratory microbial flora in patients with cystic fibrosis. J Infect Dis 179:1190–1196. doi: 10.1086/314727. [DOI] [PubMed] [Google Scholar]
- 2.Lewis K. 2010. Persister cells. Annu Rev Microbiol 64:357–372. doi: 10.1146/annurev.micro.112408.134306. [DOI] [PubMed] [Google Scholar]
- 3.Jesaitis AJ, Franklin MJ, Berglund D, Sasaki M, Lord CI, Bleazard JB, Duffy JE, Beyenal H, Lewandowski Z. 2003. Compromised host defense on Pseudomonas aeruginosa biofilms: characterization of neutrophil and biofilm interactions. J Immunol 171:4329–4339. doi: 10.4049/jimmunol.171.8.4329. [DOI] [PubMed] [Google Scholar]
- 4.Leid JG, Shirtliff ME, Costerton JW, Stoodley AP. 2002. Human leukocytes adhere to, penetrate, and respond to Staphylococcus aureus biofilms. Infect Immun 70:6339–6345. doi: 10.1128/IAI.70.11.6339-6345.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vuong C, Voyich JM, Fischer ER, Braughton KR, Whitney AR, DeLeo FR, Otto M. 2004. Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cell Microbiol 6:269–275. doi: 10.1046/j.1462-5822.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- 6.Mulcahy LR, Burns JL, Lory S, Lewis K. 2010. Emergence of Pseudomonas aeruginosa strains producing high levels of persister cells in patients with cystic fibrosis. J Bacteriol 192:6191–6199. doi: 10.1128/JB.01651-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lafleur MD, Qi Q, Lewis K. 2010. Patients with long-term oral carriage harbor high-persister mutants of Candida albicans. Antimicrob Agents Chemother 54:39–44. doi: 10.1128/AAC.00860-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helaine S, Cheverton AM, Watson KG, Faure LM, Matthews SA, Holden DW. 2014. Internalization of Salmonella by macrophages induces formation of nonreplicating persisters. Science 343:204–208. doi: 10.1126/science.1244705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barry CE III, Boshoff HI, Dartois V, Dick T, Ehrt S, Flynn J, Schnappinger D, Wilkinson RJ, Young D. 2009. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol 7:845–855. doi: 10.1038/nrmicro2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hinckley AF, Connally NP, Meek JI, Johnson BJ, Kemperman MM, Feldman KA, White JL, Mead PS. 2014. Lyme disease testing by large commercial laboratories in the United States. Clin Infect Dis 59:676–681. doi: 10.1093/cid/ciu397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steere AC. 2001. Lyme disease. N Engl J Med 345:115–125. doi: 10.1056/NEJM200107123450207. [DOI] [PubMed] [Google Scholar]
- 12.Hu LT. 2012. In the clinic. Lyme disease. Ann Intern Med 157:ITC2-2–ITC2-16. doi: 10.7326/0003-4819-157-3-201208070-01002. [DOI] [PubMed] [Google Scholar]
- 13.Puius YA, Kalish RA. 2008. Lyme arthritis: pathogenesis, clinical presentation, and management. Infect Dis Clin North Am 22:289–300. doi: 10.1016/j.idc.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Steere AC, Schoen RT, Taylor E. 1987. The clinical evolution of Lyme arthritis. Ann Intern Med 107:725–731. doi: 10.7326/0003-4819-107-5-725. [DOI] [PubMed] [Google Scholar]
- 15.Steere AC, Angelis SM. 2006. Therapy for Lyme arthritis: strategies for the treatment of antibiotic-refractory arthritis. Arthritis Rheum 54:3079–3086. doi: 10.1002/art.22131. [DOI] [PubMed] [Google Scholar]
- 16.Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, Krause PJ, Bakken JS, Strle F, Stanek G, Bockenstedt L, Fish D, Dumler JS, Nadelman RB. 2006. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 43:1089–1134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 17.Liang FT, Yan J, Mbow ML, Sviat SL, Gilmore RD, Mamula M, Fikrig E. 2004. Borrelia burgdorferi changes its surface antigenic expression in response to host immune responses. Infect Immun 72:5759–5767. doi: 10.1128/IAI.72.10.5759-5767.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coutte L, Botkin DJ, Gao L, Norris SJ. 2009. Detailed analysis of sequence changes occurring during vlsE antigenic variation in the mouse model of Borrelia burgdorferi infection. PLoS Pathog 5:e1000293. doi: 10.1371/journal.ppat.1000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radolf JD, Caimano MJ, Stevenson B, Hu LT. 2012. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol 10:87–99. doi: 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dorr T, Vulic M, Lewis K. 2010. Ciprofloxacin causes persister formation by inducing the TisB toxin in Escherichia coli. PLoS Biol 8:e1000317. doi: 10.1371/journal.pbio.1000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Germain E, Castro-Roa D, Zenkin N, Gerdes K. 2013. Molecular mechanism of bacterial persistence by HipA. Mol Cell 52:248–254. doi: 10.1016/j.molcel.2013.08.045. [DOI] [PubMed] [Google Scholar]
- 22.Maisonneuve E, Shakespeare LJ, Jorgensen MG, Gerdes K. 2011. Bacterial persistence by RNA endonucleases. Proc Natl Acad Sci U S A 108:13206–13211. doi: 10.1073/pnas.1100186108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Yamaguchi Y, Inouye M. 2011. Regulation of growth and death in Escherichia coli by toxin-antitoxin systems. Nat Rev Microbiol 9:779–790. doi: 10.1038/nrmicro2651. [DOI] [PubMed] [Google Scholar]
- 24.Sala A, Bordes P, Genevaux P. 2014. Multiple toxin-antitoxin systems in Mycobacterium tuberculosis. Toxins (Basel) 6:1002–1020. doi: 10.3390/toxins6031002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pandey DP, Gerdes K. 2005. Toxin-antitoxin loci are highly abundant in free-living but lost from host-associated prokaryotes. Nucleic Acids Res 33:966–976. doi: 10.1093/nar/gki201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Purser JE, Norris SJ. 2000. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc Natl Acad Sci U S A 97:13865–13870. doi: 10.1073/pnas.97.25.13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samuels DS. 1995. Electrotransformation of the spirochete Borrelia burgdorferi. Methods Mol Biol 47:253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dever LL, Jorgensen JH, Barbour AG. 1992. In vitro antimicrobial susceptibility testing of Borrelia burgdorferi: a microdilution MIC method and time-kill studies. J Clin Microbiol 30:2692–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis K. 2007. Persister cells, dormancy and infectious disease. Nat Rev Microbiol 5:48–56. doi: 10.1038/nrmicro1557. [DOI] [PubMed] [Google Scholar]
- 30.Maisonneuve E, Gerdes K. 2014. Molecular mechanisms underlying bacterial persisters. Cell 157:539–548. doi: 10.1016/j.cell.2014.02.050. [DOI] [PubMed] [Google Scholar]
- 31.Conlon BP. 2014. Staphylococcus aureus chronic and relapsing infections: evidence of a role for persister cells: an investigation of persister cells, their formation and their role in S. aureus disease. Bioessays 36:991–996. doi: 10.1002/bies.201400080. [DOI] [PubMed] [Google Scholar]
- 32.Keren I, Minami S, Rubin E, Lewis K. 2011. Characterization and transcriptome analysis of Mycobacterium tuberculosis persisters. mBio 2:e00100-11. doi: 10.1128/mBio.00100-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norton JP, Mulvey MA. 2012. Toxin-antitoxin systems are important for niche-specific colonization and stress resistance of uropathogenic Escherichia coli. PLoS Pathog 8:e1002954. doi: 10.1371/journal.ppat.1002954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tuomanen E, Cozens R, Tosch W, Zak O, Tomasz A. 1986. The rate of killing of Escherichia coli by beta-lactam antibiotics is strictly proportional to the rate of bacterial growth. J Gen Microbiol 132:1297–1304. [DOI] [PubMed] [Google Scholar]
- 35.Levin S, Harris AA. 1975. Principles of combination therapy. Bull N Y Acad Med 51:1020–1038. [PMC free article] [PubMed] [Google Scholar]
- 36.Spoering AL, Lewis K. 2001. Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. J Bacteriol 183:6746–6751. doi: 10.1128/JB.183.23.6746-6751.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keren I, Kaldalu N, Spoering A, Wang Y, Lewis K. 2004. Persister cells and tolerance to antimicrobials. FEMS Microbiol Lett 230:13–18. doi: 10.1016/S0378-1097(03)00856-5. [DOI] [PubMed] [Google Scholar]
- 38.Gee T, Andrews JM, Ashby JP, Marshall G, Wise R. 2001. Pharmacokinetics and tissue penetration of gemifloxacin following a single oral dose. J Antimicrob Chemother 47:431–434. doi: 10.1093/jac/47.4.431. [DOI] [PubMed] [Google Scholar]
- 39.Hunfeld KP, Kraiczy P, Wichelhaus TA, Schafer V, Brade V. 2000. New colorimetric microdilution method for in vitro susceptibility testing of Borrelia burgdorferi against antimicrobial substances. Eur J Clin Microbiol Infect Dis 19:27–32. doi: 10.1007/s100960050005. [DOI] [PubMed] [Google Scholar]
- 40.Kraiczy P, Weigand J, Wichelhaus TA, Heisig P, Backes H, Schafer V, Acker G, Brade V, Hunfeld KP. 2001. In vitro activities of fluoroquinolones against the spirochete Borrelia burgdorferi. Antimicrob Agents Chemother 45:2486–2494. doi: 10.1128/AAC.45.9.2486-2494.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wagner JG, Novak E, Leslie LG, Metzler CM. 1968. Absorption, distribution, and elimination of spectinomycin dihydrochloride in man. Int Z Klin Pharmakol Ther Toxikol 1:261–285. [PubMed] [Google Scholar]
- 42.Conlon BP, Nakayasu ES, Fleck LE, LaFleur MD, Isabella VM, Coleman K, Leonard SN, Smith RD, Adkins JN, Lewis K. 2013. Activated ClpP kills persisters and eradicates a chronic biofilm infection. Nature 503:365–370. doi: 10.1038/nature12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng J, Wang T, Shi W, Zhang S, Sullivan D, Auwaerter PG, Zhang Y. 2014. Identification of novel activity against Borrelia burgdorferi persisters using an FDA approved drug library. Emerg Microbes Infect 3:e49. doi: 10.1038/emi.2014.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ling LL, Schneider T, Peoples AJ, Spoering AL, Engels I, Conlon BP, Hughes DE, Epstein S, Jones M, Lazarides L, Steadman V, Cohen DR, Felix CR, Fetterman KA, Millet BP, Nitti AG, Zullo AM, Chen C, Lewis K. 2015. A new antibiotic kills pathogens without detectable resistance. Nature 517:455–459. doi: 10.1038/nature14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fleck LE, North EJ, Lee RE, Mulcahy LR, Casadei G, Lewis K. 2014. A screen for and validation of prodrug antimicrobials. Antimicrob Agents Chemother 58:1410–1419. doi: 10.1128/AAC.02136-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keller KL, Overbeck-Carrick TL, Beck DJ. 2001. Survival and induction of SOS in Escherichia coli treated with cisplatin, UV-irradiation, or mitomycin C are dependent on the function of the RecBC and RecFOR pathways of homologous recombination. Mutat Res 486:21–29. doi: 10.1016/S0921-8777(01)00077-5. [DOI] [PubMed] [Google Scholar]
- 47.Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, White O, Ketchum KA, Dodson R, Hickey EK, Gwinn M, Dougherty B, Tomb JF, Fleischmann RD, Richardson D, Peterson J, Kerlavage AR, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams MD, Gocayne J, Weidman J, Utterback T, Watthey L, McDonald L, Artiach P, Bowman C, Garland S, Fuji C, Cotton MD, Horst K, Roberts K, Hatch B, Smith HO, Venter JC. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 48.Barthold SW, de Souza MS, Janotka JL, Smith AL, Persing DH. 1993. Chronic Lyme borreliosis in the laboratory mouse. Am J Pathol 143:959–971. [PMC free article] [PubMed] [Google Scholar]
- 49.Marques A. 2008. Chronic Lyme disease: a review. Infect Dis Clin North Am 22:341–360. doi: 10.1016/j.idc.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hugonnet JE, Tremblay LW, Boshoff HI, Barry CE III, Blanchard JS. 2009. Meropenem-clavulanate is effective against extensively drug-resistant Mycobacterium tuberculosis. Science 323:1215–1218. doi: 10.1126/science.1167498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iyer R, Mukherjee P, Wang K, Simons J, Wormser GP, Schwartz I. 2013. Detection of Borrelia burgdorferi nucleic acids after antibiotic treatment does not confirm viability. J Clin Microbiol 51:857–862. doi: 10.1128/JCM.02785-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Graham JC, Gould FK. 2002. Role of aminoglycosides in the treatment of bacterial endocarditis. J Antimicrob Chemother 49:437–444. doi: 10.1093/jac/49.3.437. [DOI] [PubMed] [Google Scholar]
- 53.Bigger JW. 1944. Treatment of staphylococcal infections with penicillin. Lancet 244:497–500. [Google Scholar]
- 54.Maisonneuve E, Castro-Camargo M, Gerdes K. 2013. (p)ppGpp controls bacterial persistence by stochastic induction of toxin-antitoxin activity. Cell 154:1140–1150. doi: 10.1016/j.cell.2013.07.048. [DOI] [PubMed] [Google Scholar]
- 55.Spoering AL, Vulic M, Lewis K. 2006. GlpD and PlsB participate in persister cell formation in Escherichia coli. J Bacteriol 188:5136–5144. doi: 10.1128/JB.00369-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hansen S, Lewis K, Vulic M. 2008. Role of global regulators and nucleotide metabolism in antibiotic tolerance in Escherichia coli. Antimicrob Agents Chemother 52:2718–2726. doi: 10.1128/AAC.00144-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gordon C, Regamey C, Kirby WM. 1972. Comparative clinical pharmacology of amoxicillin and ampicillin administered orally. Antimicrob Agents Chemother 1:504–507. doi: 10.1128/AAC.1.6.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patel IH, Chen S, Parsonnet M, Hackman MR, Brooks MA, Konikoff J, Kaplan SA. 1981. Pharmacokinetics of ceftriaxone in humans. Antimicrob Agents Chemother 20:634–641. doi: 10.1128/AAC.20.5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Agwuh KN, MacGowan A. 2006. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J Antimicrob Chemother 58:256–265. doi: 10.1093/jac/dkl224. [DOI] [PubMed] [Google Scholar]
- 60.Dvorchik BH, Brazier D, DeBruin MF, Arbeit RD. 2003. Daptomycin pharmacokinetics and safety following administration of escalating doses once daily to healthy subjects. Antimicrob Agents Chemother 47:1318–1323. doi: 10.1128/AAC.47.4.1318-1323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rybak MJ. 2006. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis 42(Suppl):S35–S39. doi: 10.1086/491712. [DOI] [PubMed] [Google Scholar]
- 62.den Hartigh J, McVie JG, van Oort WJ, Pinedo HM. 1983. Pharmacokinetics of mitomycin C in humans. Cancer Res 43:5017–5021. [PubMed] [Google Scholar]