Abstract
Bacterial biofilms are difficult to treat using available antimicrobial agents, so new antibiofilm strategies are needed. We previously showed that 20, 200, and 2,000 μA of electrical current reduced bacterial biofilms of Staphylococcus aureus, Staphylococcus epidermidis, and Pseudomonas aeruginosa. Here, we tested continuous direct current at lower amperages, intermittent direct current, and combinations of surface materials (Teflon or titanium) and electrode compositions (stainless steel, graphite, titanium, or platinum) against S. aureus, S. epidermidis, and P. aeruginosa biofilms. In addition, we tested 200 or 2,000 μA for 1 and 4 days against biofilms of 33 strains representing 13 species of microorganisms. The logarithmic reduction factor was used to measure treatment effects. Using continuous current delivery, the lowest active amperage was 2 μA for 1, 4, or 7 days against P. aeruginosa and 5 μA for 7 days against S. epidermidis and S. aureus biofilms. Delivery of 200 μA for 4 h a day over 4 days reduced P. aeruginosa, S. aureus, and S. epidermidis biofilms on Teflon or titanium discs. A reduction of P. aeruginosa, S. aureus, and S. epidermidis biofilms was measured for 23 of 24 combinations of surface materials and electrode compositions tested. Four days of direct current delivery reduced biofilms of 25 of 33 strains studied. In conclusion, low-amperage current or 4 h a day of intermittent current delivered using a variety of electrode compositions reduced P. aeruginosa, S. aureus, and S. epidermidis biofilms on a variety of surface materials. The electricidal effect was observed against a majority of bacterial species studied.
INTRODUCTION
Chronic infections associated with medical devices such as joint replacements and other types of orthopedic instrumentation, prosthetic heart valves, pacemakers, implantable defibrillators, urinary catheters and stents, peritoneal dialysis catheters, intravascular catheters, cerebrospinal fluid shunts, breast implants, and vascular grafts and stents are common in today's medical practice. When these devices become infected, they must often be removed to successfully cure the associated infection. Device removal is associated with significant morbidity, cost, and, in some cases, mortality. Prosthetic joint removal, for example, may mean that a patient is left without a functional joint for months, along with the requirement for two surgeries (i.e., infected implant removal and eventual replacement) (1). Intrathoracic device infections may require major surgical procedures, involving repeat sternotomy. The removal of some devices, such as vascular graft bypasses, may be impossible, rendering associated infection incurable with current approaches.
The pathogenesis of device-associated infections relates to the presence of microorganisms in biofilms. Existence within a biofilm represents a survival strategy for microorganisms, protecting them from environmental influences, the host immune system, and, unfortunately, therapeutic levels of conventional antimicrobial agents. Biofilms exhibit dramatically reduced susceptibility to killing by antimicrobial agents compared to their planktonic counterparts. In some cases, antibiotics can paradoxically increase densities of bacterial biofilms (2). Several mechanisms underlie biofilm-associated antimicrobial resistance, including an altered growth rate with the presence of so-called “persister” cells, the failure of the compound to penetrate the biofilm matrix, etc., of which the first is most important.
We previously described a new antibiofilm strategy, the electricidal effect. In our original studies, biofilms of Staphylococcus aureus Xen 30, Staphylococcus epidermidis Xen 43, or Pseudomonas aeruginosa Xen 5 on Teflon discs were exposed to direct electric current (20, 200 or 2,000 μA) for up to 7 days, delivered by stainless steel or graphite electrodes placed on either side of the discs in a flow chamber (3). We observed dose- and time-dependent antibiofilm effects of direct electrical current, as measured by decreases in cell numbers on the discs (3). Sandvik et al. recently reported an electricidal effect against S. epidermidis RP62A and P. aeruginosa ERC-1 (4). In addition, our group delivered 200 μA of direct current for 21 days to S. epidermidis Xen 43-infected stainless steel implants placed in the medullary cavity of the tibiae of rabbits and demonstrated a reduction in bacterial quantity compared to untreated animals (5).
Whether or not the electricidal effect applies to other species and strains of bacteria or to fungi is unknown, as is the effect of smaller amounts of direct current than originally studied and that of intermittent current delivery and different surface materials and electrode compositions. These parameters, which can be likened, in part, to defining the pharmacodynamic properties of an antibiotic, were assessed in the studies described herein.
(Presented in part at the 51st ICAAC in Chicago, IL, 17 to 20 September 2011, and at the 113th ASM General Meeting in Denver, CO, 18 to 21 May 2013.)
MATERIALS AND METHODS
Microorganisms.
Three strains each of S. aureus (USA 300, IDRL-4284, and IDRL-6169), S. epidermidis (Xen 43, RP62A, and IDRL-6461), P. aeruginosa (Xen 5, PA14, and IDRL-7262), Candida albicans (GDH2346, IDRL-7033, and IDRL-7034), Candida glabrata (IDRL-3828, IDRL-5067, and IDRL-8404), Escherichia coli (IDRL-6199, IDRL-7029, and IDRL-8110), Enterococcus faecalis (ATCC 29212, IDRL-7107, and IDRL-8618), Propionibacterium acnes (IDRL-7844, IDRL-7751, and IDRL-7676), and Streptococcus mutans group (IDRL-6249, IDRL-7131, and IDRL-7448), and six strains of Corynebacterium species (C. jeikeium IDRL-6016 and IDRL-9345, C. amycolatum IDRL-6281 and IDRL-7372, C. aurimucosum IDRL-8271, and C. striatum IDRL-7652) were studied. The Xen strains were generous gifts of Perkin-Elmer Caliper Life Sciences (formerly Xenogen Corp.), Waltham, MA; PA14 was from Daniel Hassett (University of Cincinnati, Cincinnati, OH); GDH2346 was from Jyotsna Chandra and Mahmoud Ghannoum (University Hospitals of Cleveland and Case Western Reserve University, Cleveland, OH) (6, 7); USA300 was from Henry F. Chambers (University of California San Francisco); RP62A (ATCC 35984) was from the American Type Culture Collection, Manassas, VA; and the IDRL isolates were clinical isolates collected at Mayo Clinic, Rochester, MN.
Biofilms.
Bacterial and fungal biofilms were grown on Teflon or titanium (materials present in implanted materials) discs (12.5 by 1 mm) in 2 ml of Trypticase soy broth (TSB) containing 106 CFU in 24-well microtiter plates for 24 h on an orbital shaker at 37°C, with the exception of C. albicans and C. glabrata, which were grown at 30°C for 48 h with 1 and 2% glucose, respectively; S. mutans group, which was grown anaerobically; P. acnes, which was grown anaerobically in brain heart infusion broth (BHIB) for 64 h; and the Corynebacterium species, which were grown in TSB supplemented with 0.1% Tween 80.
Flow buffer.
Phosphate flow buffer (1×) was made with 426 mg of Na2HPO4, 205 mg of KH2PO4, 640 mg of glucose, and 1 liter of distilled water, filter sterilized, and stored at 4°C. The stock flow buffer was diluted to 3% in sterile water for each experiment. We added 50% BHIB to the flow buffer for P. acnes.
Electrical treatment device.
Previously designed treatment devices that deliver direct current to polycarbonate test chambers were used (3, 8). Specifically, an eight-channel computer controlled current generator designed by the Mayo Clinic Division of Engineering (Rochester, MN) was programmed to deliver 20 to 2,000 μA of direct current, as well as to deliver intermittent direct current (3, 8). Alternatively, a Keithley 2400 SourceMeter (Cleveland, OH) was used. For each experiment, discs with preformed biofilms, as described above, were rinsed by dipping them in sterile saline to remove planktonic bacteria. The discs were placed in a groove in an upright position in the polycarbonate test chambers containing 10 ml of 3% phosphate flow buffer; electrodes were inserted 3 mm from each side of the discs (Fig. 1). Next, 3% phosphate flow buffer was continuously pumped through each test chamber at 3 ml/h (L/S precision variable-speed console drive; Masterflex, Vernon Hills, IL) in room air at ambient temperature, with the exception of S. mutans group and P. acnes, which were tested anaerobically, the latter at 37°C. All testing was performed in triplicate.
FIG 1.
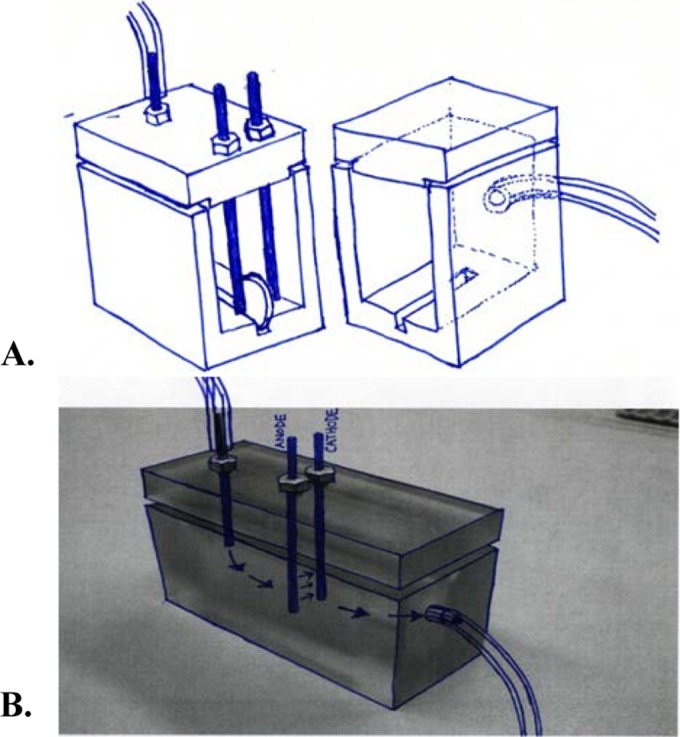
(A) Polycarbonate chamber and disc placement 3 mm from electrodes. (B) Polycarbonate chamber showing inflow and outflow of phosphate buffer.
Biofilm density and reduction.
Biofilm density was determined by quantitative culture. Briefly, discs were aseptically removed from test chambers, dipped into sterile saline, and placed into culture tubes containing 1 ml of sterile saline. Biofilms were removed from the discs by vortexing and sonication (9), quantitatively cultured on BBL Trypticase soy agar with 5% sheep blood (Becton Dickinson, Franklin Lakes, NJ), and the CFU were counted. Biofilm reduction was expressed using the logarithmic reduction factor (LRF), i.e., the log[(mean CFU/cm2 of nonexposed discs)/(mean CFU/cm2 of exposed discs)] (3, 8).
Continuous direct current studies.
Direct current (2, 5, and 10 μA) was continuously delivered from the anode to the cathode via stainless steel electrodes in test chambers containing S. aureus IDRL-4284, S. epidermidis Xen 43, or P. aeruginosa Xen 5 biofilm-laden Teflon discs. After 1, 4, and 7 days, the bacterial density was assessed as described above, and the LRF was calculated.
Intermittent direct current studies.
In these experiments, 200 μA was intermittently delivered to test chambers containing S. aureus IDRL-4284, S. epidermidis Xen 43, or P. aeruginosa Xen 5 biofilm-laden Teflon or titanium discs via stainless steel electrodes for 4 days. The computer controlled current source was programmed to deliver current for 4 days of 200 μA for 24, 12, 8, 6, 4, or 2 h/day. After treatment, the discs were quantitatively cultured, and the LRF was calculated.
Disc and electrode type combination studies.
To test the effect using various surface materials and electrode compositions, experiments were performed for 4 days using 200 μA delivered via stainless steel, graphite, titanium, or platinum electrodes to S. aureus IDRL-4284, S. epidermidis Xen 43, or P. aeruginosa Xen 5 biofilms on titanium or Teflon discs. After treatment, the discs were quantitatively cultured, and the LRF was calculated.
Testing of other bacterial and fungal species.
Tests with other species (C. albicans, Corynebacterium species, E. coli, E. faecalis, P. aeruginosa, P. acnes, S. aureus, S. epidermidis, and S. mutans group) were performed using 200 μA delivered for 1 and 4 days via stainless steel electrodes. C. albicans, C. glabrata, Corynebacterium species, and E. faecalis were also tested using 2,000 μA for 1 and 4 days.
Statistical methods.
Statistical analyses were performed using SAS software (SAS Institute, Inc., Cary, NC). A one-way analysis of variance was performed with each current delivery strategy and no current delivery to determine whether electrical current reduced biofilms. Using the Wilcoxon rank-sum test, we compared the LRFs of continuous and all intermittent strategies and the LRFs of all the disc/electrode combinations. All tests were two sided; P values of <0.05 were considered statistically significant.
RESULTS
Continuous direct current studies.
Since we have previously demonstrated an electricidal effect against S. aureus Xen 30, S. epidermidis Xen 43, and P. aeruginosa Xen 5 using 20, 200, and 2,000 μA of direct current (3), we postulated that smaller amounts of current would also reduce biofilms. There was a significant reduction in P. aeruginosa Xen 5 biofilms with all amperages and time points studied. S. epidermidis Xen 43 biofilm was significantly reduced using 5 μA for 7 days and 10 μA for 1 day. S. aureus IDRL-4284 showed a significant reduction in biofilm quantity using 10 μA for 1, 4, or 7 days and 5 μA for 7 days (Table 1).
TABLE 1.
LRFs after 1, 4, and 7 days of 2, 5, and 10 μA for P. aeruginosa Xen 5, S. aureus IDRL-4284, and S. epidermidis Xen 43
| Strain and treatment (μA) | LRFa |
||
|---|---|---|---|
| 1 day | 4 days | 7 days | |
| P. aeruginosa Xen 5 | |||
| 2 | 2.58* | 4.56* | 3.78* |
| 5 | 0.48* | 4.12* | 4.81* |
| 10 | 1.49* | 2.19* | 2.65* |
| S. aureus IDRL-4284 | |||
| 2 | 0.14 | -0.75 | 0.20 |
| 5 | –0.04 | 0.36 | 0.90* |
| 10 | 0.86* | 2.58* | 0.82* |
| S. epidermidis Xen 43 | |||
| 2 | 0.36 | 1.46 | 1.22 |
| 5 | 0.74 | 0.17 | 2.47* |
| 10 | 0.78* | 1.30 | 1.11* |
LRF, logarithmic reduction factor. *, P < 0.05.
Intermittent direct current studies.
Because in earlier studies we had observed a reduction in biofilms with continuous current, we hypothesized that intermittent current delivery would also reduce biofilms. A significant reduction of biofilms was detected at all intermittent current time points studied, except for S. aureus grown on Teflon discs and S. epidermidis grown on Teflon and titanium discs treated with 2 h/day of 200 μA current (Fig. 2). Compared to continuous current (24 h/day), intermittent current delivery had no significant differences in effect with the exception of S. epidermidis treated for 2 h/day (P = 0.0463 and P = 0.0495 for Teflon and titanium, respectively) and S. aureus grown on titanium discs treated for 4 and 2 h/day (P = 0.0463). Decreasing the duration of daily current delivery yielded higher viable bacterial counts and thus a time-dependent decline in LRFs. No significant differences in LRFs were detected between biofilms grown on Teflon or titanium discs, except for S. epidermidis, which demonstrated a higher reduction when treated for 8 h/day on titanium versus Teflon discs (P = 0.0369).
FIG 2.
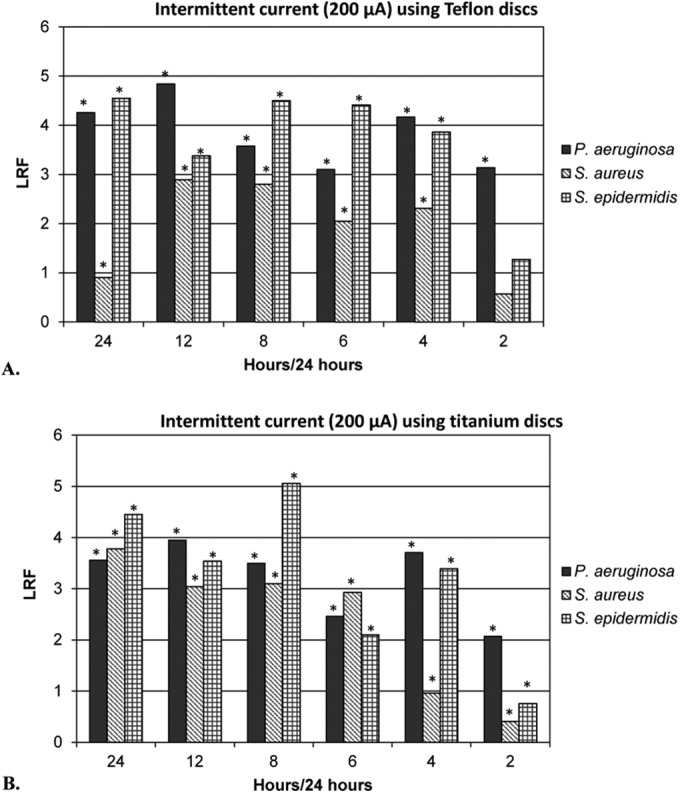
LRF after 4 days of intermittent current using stainless steel electrodes. Pseudomonas aeruginosa Xen 5, Staphylococcus aureus IDRL-4284, and Staphylococcus epidermidis Xen 43 on Teflon (A) and titanium (B) discs. *, LRFs with P value of <0.05 when comparing 0 with 200 μA for each time point.
Disc and electrode type combination studies.
In the continuous and intermittent current studies, stainless steel electrodes were used to deliver current to Teflon or titanium discs; corrosion of stainless steel electrodes was observed. Therefore, we tested various surface materials and electrode compositions. None of the non-stainless-steel electrodes exhibited corrosion. For the three species tested using 4 days of 200 μA, a significant electricidal effect was observed for 23 of 24 disc and electrode combinations, the exception being S. epidermidis grown on Teflon discs using titanium electrodes (Table 2). The data recorded using 200 μA delivered for 4 days using stainless steel electrodes to biofilms on Teflon discs is shown in Table 3.
TABLE 2.
LRFs for disc and electrode combinations using 200 μA for 4 days for P. aeruginosa Xen 5, S. aureus IDRL-4284, and S. epidermidis Xen 43
| Strain and disc type | LRF with indicated electrode typea |
|||
|---|---|---|---|---|
| Stainless steel | Graphite | Titanium | Platinum | |
| P. aeruginosa Xen 5 | ||||
| Teflon | 4.26* | 2.23* | 4.99* | 2.02* |
| Titanium | 3.56* | 2.51* | 1.85* | 1.87* |
| S. aureus IDRL-4284 | ||||
| Teflon | 1.66* | 4.78* | 5.00* | 4.38* |
| Titanium | 3.78* | >3.01* | 3.89* | 3.43* |
| S. epidermidis Xen 43 | ||||
| Teflon | 4.55* | 3.06* | 0.33 | 1.73* |
| Titanium | 4.45* | 5.51* | 1.93* | 2.11* |
LRF, logarithmic reduction factor. *, P < 0.05.
TABLE 3.
LRFs for different species of bacteria with 200 and 2,000 μA delivered for 1 and 4 days
| Species and strain | LRFa |
|||
|---|---|---|---|---|
| 200 μA |
2,000 μA |
|||
| 1 day | 4 days | 1 day | 4 days | |
| Pseudomonas aeruginosa | ||||
| PA14 | 1.93* | >4.12* | ||
| IDRL-7262 | 2.67* | 2.46* | ||
| Xen 5 | 4.11* | 4.26* | ||
| Staphylococcus aureus | ||||
| USA300 | 0.31 | 1.20* | ||
| IDRL-6169 | 1.64* | 3.83* | ||
| IDRL-4284 | 0.01 | 1.66* | ||
| Staphylococcus epidermidis | ||||
| RP62A | 0.74* | 1.62* | ||
| IDRL-6461 | 1.82* | >2.92* | ||
| Xen 43 | 2.30* | 4.55* | ||
| Escherichia coli | ||||
| IDRL-7029 | 0.42* | 2.85* | ||
| IDRL-6199 | 1.47* | 4.17* | ||
| IDRL-8110 | 1.27* | 2.30* | ||
| Enterococcus faecalis | ||||
| ATCC 29212 | 1.38* | 1.52* | 1.20* | 2.40* |
| IDRL-8618 | 1.71* | 1.20* | 0.97 | 1.16* |
| IDRL-7107 | 1.71* | –0.27 | 0.32 | 1.55* |
| Propionibacterium acnes | ||||
| IDRL-7676 | 0.75 | 0.93* | ||
| IDRL-7751 | 0.33 | 1.74* | ||
| IDRL-7844 | 0.58* | 3.17* | ||
| Streptococcus mutans groupb | ||||
| IDRL-7131 | 3.19* | 3.82* | ||
| IDRL-6249 | 3.41* | 2.99* | ||
| IDRL-7448 | 2.83* | 1.52* | ||
| Candida albicans | ||||
| IDRL-7033 | 0.48 | 0.81 | –0.18 | 0.52 |
| IDRL-7034 | 0.67* | 0.85 | –0.24 | –0.14 |
| GDH2346 | 0.10 | 0.38 | –0.05 | 0.80* |
| Candida glabrata | 0.05 | 0.56* | ||
| IDRL-3828 | –0.10 | 0.45* | ||
| IDRL-5067 | 0.14 | –0.77 | ||
| IDRL-8404 | ||||
| Corynebacterium jeikeium | ||||
| IDRL-6016 | –0.55 | –0.20 | 0.59 | 0.61 |
| IDRL-9345 | 0.47 | 0.99 | –0.91 | –0.75 |
| Corynebacterium amycolatum | ||||
| IDRL-6281 | 1.10* | 0.42 | –0.03 | 0.32 |
| IDRL-7372 | 0.21 | 0.21 | 0.52 | 1.60* |
| Corynebacterium aurimucosum | ||||
| IDRL-8271 | –0.94 | –0.15 | 0.78* | –0.24 |
| Corynebacterium striatum | ||||
| IDRL-7652 | –0.67 | 0.10 | –2.02 | –0.26 |
LRF, logarithmic reduction factor. *, P < 0.05.
S. mutans group isolates were studied in an anaerobic environment.
Testing of other bacterial and fungal species.
Based on our having observed a reduction in S. aureus IDRL-4284, S. epidermidis Xen 43, and P. aeruginosa Xen 5 biofilms, we hypothesized that direct electrical current would also reduce biofilms of other bacterial as well as fungal species. All three strains of E. coli studied showed significant reductions in biofilm amounts using 200 μA delivered for 1 and 4 days (P ≤ 0.0495). All three strains of E. faecalis studied showed significant reductions in biofilm amounts using 2,000 μA delivered for 4 days (P = 0.0495). The two additional strains of P. aeruginosa studied showed significant reductions in biofilms using 200 μA delivered for 1 and 4 days (P ≤ 0.0495). All three strains of P. acnes showed significant reductions in biofilms using 200 μA for 4 days (P = 0.0495). Two of three strains of S. aureus showed significant biofilm reductions using 200 μA for 4 days (P ≤ 0.0495). The two additional strains of S. epidermidis showed significant biofilm reductions using 200 μA for 1 and 4 days (P ≤ 0.0495). All three strains of S. mutans showed significant reductions in biofilms using 200 μA for 1 and 4 days (P ≤ 0.0495) (Table 3). Only one strain of Corynebacterium species, C. amycolatum IDRL-7372, demonstrated a reduction in biofilm quantity and that occurred when applying 2000 μA for 4 days (P = 0.0495). For C. albicans strains, a significant reduction was measured for only one of three study isolates, C. albicans GDH2346 using 2000 μA delivered for 4 days (P = 0.0495). For C. glabrata, a significant biofilm reduction was observed for two of three strains tested, IDRL-3828 and IDRL-5067, using 2,000 μA delivered for 4 days (P = 0.0495). Overall, with 4 days of either 200 or 2,000 μA, 25 of the 33 strains tested showed significant biofilm reduction.
DISCUSSION
In this study, we have shown an electricidal effect, a reduction in viable biofilm bacteria with exposure to direct electrical current, with most experimental parameters studied.
Intermittent current application demonstrated an effect with just 4 h a day of treatment over a period of 4 days. There was a time-dependent effect observed with intermittent treatment, with increasing hours of current delivery yielding greater effects (Fig. 2). The dynamics of time-dependent LRF decline differed for each microorganism. Intermittent current delivery may result in interruption of cell division and cell stress as electrical current is variously applied (10, 11). The different disc and electrode combinations studied exhibited a significant electricidal effect for all combinations studied, except for S. epidermidis grown on Teflon discs with titanium electrodes, the last possibly due to the small sample size tested.
P. aeruginosa, S. epidermidis, and S. aureus biofilms were reduced with as little as 5 μA, delivered for 7 days. Biofilms of all six Gram-negative bacteria tested exposed to 200 μA for just 1 day showed significant reductions, which contrasted with the Gram-positive bacteria tested, some of which required more than a day of treatment with the same amount of current to show significant reductions (Table 3). It is possible that differences in cell wall structure account for these observations (12).
Among the multiple species of bacteria tested, the electricidal effect was observed using 200 μA with E. coli, P. aeruginosa, P. acnes, S. aureus, S. epidermidis, and S. mutans group. For strains with no clear effect using 200 μA, amperage was increased to 2,000 μA. Among the species exposed to 2,000 μA for 4 days, activity was observed with two strains of C. glabrata (IDRL-3828 and IDRL-5067) and all three stains of E. faecalis studied. Only one strain of C. albicans (GDH2346) showed a reduction in biofilm using 2,000 μA for 4 days, possibly due to the presence of yeast and hyphal forms and/or rapid adaptation to changes in environmental pH, stress responses, and/or nutrient acquisition systems, and/or metabolic plasticity (13). There was no effect against Corynebacterium biofilms using 200 or 2,000 μA, with the exception of one strain of C. amycolatum (IDRL-7372), which showed an effect when using 2,000 μA for 4 days; this may, hypothetically, be due to the mycolic acid- and other lipid-containing cell envelope of Corynebacterium species (14).
Oxidative stress may play a role in the electricidal effect (15–19). It is also possible that direct electrical current enhances repulsive forces between bacteria and surface materials (20–24). Additional factors that could affect biofilm integrity include changes in the pH (4, 25), temperature (25), and/or concentration of chlorine, nitrogen, and/or hydrogen ions (4, 25, 26), when applying the low amperage direct current. Sandvik et al. recently showed that electrolysis creates hypochlorous acid from chloride in solution, which aides direct electrical current in reducing S. epidermidis and P. aeruginosa biofilms (4). Notably there was no chlorine added to our study media. Sandvik et al. also noted a pH gradient between electrodes (4); we similarly found a pH gradient between electrodes, with a lower pH at the anode and a higher pH at the cathode (data not shown). Stevenson et al. found that there was an increased temperature (a ∼20°C elevation, 21 to 37°C) and dramatic changes in pH when using platinum electrodes with prolonged application of direct electrical current (25). Although we did not test the temperature of our electrodes or biofilm surfaces directly, we did not find an increase in temperature in our flow buffer (data not shown). Because we used flow cells and performed our experiments at ambient temperature, effects of temperature differentials, if present, may have been minimized. Studies investigating the mechanism(s) of the electricidal effect are ongoing in our laboratory.
Others have used electricity to kill bacteria or detach them from various surfaces, with an overall consensus that electricity is effective in reducing the amount of bacteria present (21, 24, 27). Liu et al. found that S. aureus and S. epidermidis biofilms can be removed from intravascular catheters with low-amperage (10 μA) direct electrical current (21). Drees et al. showed that direct electrical current of 25 to 350 mA applied to planktonic suspensions of P. aeruginosa or E. coli resulted in a significant reduction in viable cells (28). In addition, Merriman et al. tested four different electrical stimulation types, continuous microamperage direct current, high-voltage pulsed current, low-voltage monophasic milliamperage pulsed current, and low-voltage biphasic milliamperage pulsed current on culture plates containing S. aureus (29). These researchers found that microamperage direct current and high-voltage pulsed current had significant inhibitory effects, while the other two electrical stimulation types did not (29).
There are several potential uses of the electricidal effect, including applications to infections associated with orthopedic hardware, as assessed in our animal study (5), and intravascular catheter infection. Before considering such approaches, however, potential toxicity will need to be addressed (4, 5), and a strategy that results in biofilm eradication and not just reduction adopted.
Corrosion of the stainless steel electrodes using 200 and 2,000 μA was observed. It is possible the associated biochemical reaction may have contributed to reduction in biofilm mass (30). However, a similar reduction in bacterial biofilm was observed with other electrode types, including graphite, titanium, and platinum, that did not corrode (Table 2). A limitation to our study is that we have only tested direct, and not alternating, current. Also, the experiments were performed at ambient temperature. Typically medical biofilms would be found in the human body at 37°C. However, testing with P. aeruginosa Xen 5 and S. aureus Xen 30 at both ambient temperature and 37°C using 2,000 μA delivered for 48 h showed a similar reduction in biofilms (data not shown). Another potential limitation to our study is that only 24 h, relatively young biofilms were studied; in separate experiments, we observed a significant reduction in more mature (72-h-old), S. aureus, S. epidermidis, and P. aeruginosa biofilms when treated with 1 and 4 days of 200 μA direct current (data not shown).
In conclusion, P. aeruginosa S. epidermidis, and S. aureus biofilms were reduced with as little as 5 μA, delivered for 7 days. Further, low amperage current, delivered for as few as 4 h a day for 4 days, reduced the biofilms of the same species. The electricidal effect was observed using a variety of surface materials and electrode compositions and against a number of bacterial species beyond P. aeruginosa S. epidermidis, and S. aureus, including E. coli, P. acnes, S. mutans group, and E. faecalis.
ACKNOWLEDGMENTS
Research reported here was supported by the National Institute of Allergy and Infectious Diseases under award R01 AI091594 and the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award R01 AR056647.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We thank Ashley Piper, Megan Lindsey, and Alex Fondaw for their contributions to this study.
REFERENCES
- 1.Tande AJ, Patel R. 2014. Prosthetic joint infection. Clin Microbiol Rev 27:302–345. doi: 10.1128/CMR.00111-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim CK, Karau MJ, Greenwood-Quaintance KE, Tilahun AY, David CS, Mandrekar JN, Patel R, Rajagopalan G. 2014. Superantigens in Staphylococcus aureus isolated from prosthetic joint infection. Diagn Microbiol Infect Dis 81:201–207. doi: 10.1016/j.diagmicrobio.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.del Pozo JL, Rouse MS, Mandrekar JN, Steckelberg JM, Patel R. 2009. The electricidal effect: reduction of Staphylococcus and Pseudomonas biofilms by prolonged exposure to low-intensity electrical current. Antimicrob Agents Chemother 53:41–45. doi: 10.1128/AAC.00680-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandvik EL, McLeod BR, Parker AE, Stewart PS. 2013. Direct electric current treatment under physiologic saline conditions kills Staphylococcus epidermidis biofilms via electrolytic generation of hypochlorous acid. PLoS One 8:e55118. doi: 10.1371/journal.pone.0055118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Del Pozo JL, Rouse MS, Euba G, Kang C-I, Mandrekar JN, Steckelberg JM, Patel R. 2009. The electricidal effect is active in an experimental model of Staphylococcus epidermidis chronic foreign body osteomyelitis. Antimicrob Agents Chemother 53:4064–4068. doi: 10.1128/AAC.00432-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. 2001. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol 183:5385–5394. doi: 10.1128/JB.183.18.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shuford JA, Rouse MS, Piper KE, Steckelberg JM, Patel R. 2006. Evaluation of caspofungin and amphotericin B deoxycholate against Candida albicans biofilms in an experimental intravascular catheter infection model. J Infect Dis 194:710–713. doi: 10.1086/506452. [DOI] [PubMed] [Google Scholar]
- 8.del Pozo JL, Rouse MS, Mandrekar JN, Sampedro MF, Steckelberg JM, Patel R. 2009. Effect of electrical current on the activities of antimicrobial agents against Pseudomonas aeruginosa, Staphylococcus aureus, and Staphylococcus epidermidis biofilms. Antimicrob Agents Chemother 53:35–40. doi: 10.1128/AAC.00237-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK, Osmon DR, Mandrekar JN, Cockerill FR, Steckelberg JM, Greenleaf JF, Patel R. 2007. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med 357:654–663. doi: 10.1056/NEJMoa061588. [DOI] [PubMed] [Google Scholar]
- 10.Giladi M, Porat Y, Blatt A, Wasserman Y, Kirson ED, Dekel E, Palti Y. 2008. Microbial growth inhibition by alternating electric fields. Antimicrob Agents Chemother 52:3517–3522. doi: 10.1128/AAC.00673-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Racyte J, Bernard S, Paulitsch-Fuchs AH, Yntema DR, Bruning H, Rijnaarts HH. 2013. Alternating electric fields combined with activated carbon for disinfection of Gram-negative and Gram-positive bacteria in fluidized bed electrode system. Water Res 47:6395–6405. doi: 10.1016/j.watres.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Garcia D, Gomez N, Manas P, Raso J, Pagan R. 2007. Pulsed electric fields cause bacterial envelopes permeabilization depending on the treatment intensity, the treatment medium pH and the microorganism investigated. Int J Food Microbiol 113:219–227. doi: 10.1016/j.ijfoodmicro.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Mayer FL, Wilson D, Hube B. 2013. Candida albicans pathogenicity mechanisms. Virulence 4:119–128. doi: 10.4161/viru.22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gebhardt H, Meniche X, Tropis M, Kramer R, Daffe M, Morbach S. 2007. The key role of the mycolic acid content in the functionality of the cell wall permeability barrier in Corynebacterineae. Microbiology 153:1424–1434. doi: 10.1099/mic.0.2006/003541-0. [DOI] [PubMed] [Google Scholar]
- 15.Boles BR, Singh PK. 2008. Endogenous oxidative stress produces diversity and adaptability in biofilm communities. Proc Natl Acad Sci U S A 105:12503–12508. doi: 10.1073/pnas.0801499105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boor KJ. 2006. Bacterial stress responses: what doesn't kill them can make them stronger. PLoS Biol 4:18–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cabiscol E, Tamarit J, Ros J. 2000. Oxidative stress in bacteria and protein damage by reactive oxygen species. Int Microbiol 3:3–8. [PubMed] [Google Scholar]
- 18.Marles-Wright J, Lewis RJ. 2007. Stress responses of bacteria. Curr Opin Struct Biol 17:755–760. doi: 10.1016/j.sbi.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Miller RA, Britigan BE. 1997. Role of oxidants in microbial pathophysiology. Clin Microbiol Rev 10:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jucker BA, Harms H, Zehnder AJ. 1996. Adhesion of the positively charged bacterium Stenotrophomonas (Xanthomonas) maltophilia 70401 to glass and Teflon. J Bacteriol 178:5472–5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu WK, Brown MR, Elliott TS. 1997. Mechanisms of the bactericidal activity of low amperage electric current (DC). J Antimicrob Chemother 39:687–695. doi: 10.1093/jac/39.6.687. [DOI] [PubMed] [Google Scholar]
- 22.Pareilleux A, Sicard N. 1970. Lethal effects of electric current on Escherichia coli. Appl Microbiol 19:421–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ueshima M, Tanaka S, Nakamura S, Yamashita K. 2002. Manipulation of bacterial adhesion and proliferation by surface charges of electrically polarized hydroxyapatite. J Biomed Mater Res 60:578–584. doi: 10.1002/jbm.10113. [DOI] [PubMed] [Google Scholar]
- 24.van der Borden AJ, van der Mei HC, Busscher HJ. 2005. Electric block current induced detachment from surgical stainless steel and decreased viability of Staphylococcus epidermidis. Biomaterials 26:6731–6735. doi: 10.1016/j.biomaterials.2004.04.052. [DOI] [PubMed] [Google Scholar]
- 25.Stevenson M, Baylor K, Netherton BL, Stecker MM. 2010. Electrical stimulation and electrode properties. 2. Pure metal electrodes. Am J Electroneurodiagnostic Technol 50:263–296. [PubMed] [Google Scholar]
- 26.Rossmeisl J, Qu Z-W, Zhu H, Kroes G-J, Nørskov JK. 2007. Electrolysis of water on oxide surfaces. J Electroanal Chem 207:83–89. [Google Scholar]
- 27.Rosenberg B, Vancamp L, Krigas T. 1965. Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature 205:698–699. doi: 10.1038/205698a0. [DOI] [PubMed] [Google Scholar]
- 28.Drees KP, Abbaszadegan M, Maier RM. 2003. Comparative electrochemical inactivation of bacteria and bacteriophage. Water Res 37:2291–2300. doi: 10.1016/S0043-1354(03)00009-5. [DOI] [PubMed] [Google Scholar]
- 29.Merriman HL, Hegyi CA, Albright-Overton CR, Carlos J Jr, Putnam RW, Mulcare JA. 2004. A comparison of four electrical stimulation types on Staphylococcus aureus growth in vitro. J Rehabil Res Dev 41:139–146. doi: 10.1682/JRRD.2004.02.0139. [DOI] [PubMed] [Google Scholar]
- 30.Davis CP, Wagle N, Anderson MD, Warren MM. 1991. Bacterial and fungal killing by iontophoresis with long-lived electrodes. Antimicrob Agents Chemother 35:2131–2134. doi: 10.1128/AAC.35.10.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]


