Abstract
Olanexidine gluconate [1-(3,4-dichlorobenzyl)-5-octylbiguanide gluconate] (development code OPB-2045G) is a new monobiguanide compound with bactericidal activity. In this study, we assessed its spectrum of bactericidal activity and mechanism of action. The minimal bactericidal concentrations of the compound for 30-, 60-, and 180-s exposures were determined with the microdilution method using a neutralizer against 320 bacterial strains from culture collections and clinical isolates. Based on the results, the estimated bactericidal olanexidine concentrations with 180-s exposures were 869 μg/ml for Gram-positive cocci (155 strains), 109 μg/ml for Gram-positive bacilli (29 strains), and 434 μg/ml for Gram-negative bacteria (136 strains). Olanexidine was active against a wide range of bacteria, especially Gram-positive cocci, including methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci, and had a spectrum of bactericidal activity comparable to that of commercial antiseptics, such as chlorhexidine and povidone-iodine. In vitro experiments exploring its mechanism of action indicated that olanexidine (i) interacts with the bacterial surface molecules, such as lipopolysaccharide and lipoteichoic acid, (ii) disrupts the cell membranes of liposomes, which are artificial bacterial membrane models, (iii) enhances the membrane permeability of Escherichia coli, (iv) disrupts the membrane integrity of S. aureus, and (v) denatures proteins at relatively high concentrations (≥160 μg/ml). These results indicate that olanexidine probably binds to the cell membrane, disrupts membrane integrity, and its bacteriostatic and bactericidal effects are caused by irreversible leakage of intracellular components. At relatively high concentrations, olanexidine aggregates cells by denaturing proteins. This mechanism differs slightly from that of a similar biguanide compound, chlorhexidine.
INTRODUCTION
Antiseptics have several important uses in infection control in clinical settings, including hand hygiene and disinfection of surgical and catheter insertion sites. Antiseptics prevent infection by decreasing the number of microorganisms, thereby decreasing the transmission of pathogens. Currently, health care-associated infections caused by multidrug-resistant organisms, including methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, and certain Gram-negative bacilli, are substantial problems.
Chlorhexidine digluconate is one of the most effective antiseptics, because it has broad-spectrum antibacterial activity, persistent efficacy, and residual activity. Moreover, it is compatible with most materials and is used safely in various preparations for humans. However, it may not have sufficient activity to eradicate some pathogens, such as methicillin-resistant S. aureus (1) and vancomycin-resistant enterococci (2).
Povidone-iodine (PVP-I) is a broad-spectrum microbicide that can inactivate not only bacteria but also viruses (3). However, PVP-I may not function well in the presence of organic materials, such as blood or pus, which can rapidly neutralize its bactericidal activity (4). Additionally, PVP-I must not be administered to pregnant or lactating women, because it can induce transient hypothyroidism in the fetus or newborn (5).
New antiseptics are critical for preventing incurable infections, but only a few new agents have been launched in the past 50 years.
Olanexidine [1-(3,4-dichlorobenzyl)-5-octylbiguanide] (formerly OPB-2045), an antimicrobial agent with a biguanide group, was synthesized in 1997 (Fig. 1) (6). To optimize its use as a topical antiseptic, olanexidine was converted to the gluconate salt, and a solubilizing agent, polyoxyethylene (20) polyoxypropylene (20) glycol (POEPOPG), was added to make the olanexidine gluconate formulation. The resulting formulation (OPB) had more potent bactericidal activity against methicillin-resistant S. aureus and vancomycin-resistant enterococci in both in vitro and in vivo animal models than chlorhexidine and PVP-I (7). Clinical trials for OPB were completed in Japan by a similar method to the one described in the U.S. Food and Drug Administration Tentative Final Monograph (FDA-TFM) for patient preoperative skin preparation drug products (8). OPB was shown to be effective as an antiseptic, and the new drug application of this compound was submitted in Japan in 2014 (the clinical data will be reported separately).
FIG 1.
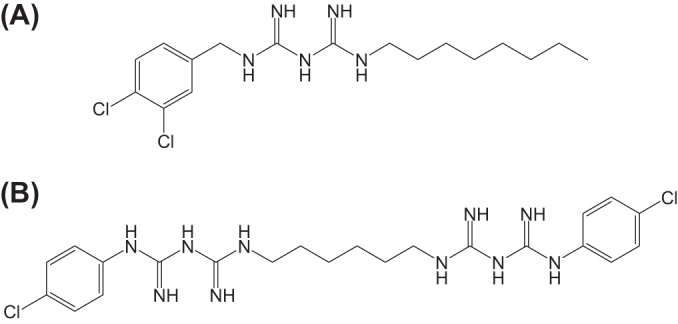
Chemical structures of olanexidine (A) and chlorhexidine (B).
In this study, we sought to determine the spectrum of bactericidal action and mechanism of action of OPB.
MATERIALS AND METHODS
Materials.
The olanexidine gluconate formulation (OPB), which contains POEPOPG as a solubilizing agent in addition to the active ingredient, olanexidine, was prepared by Otsuka Pharmaceutical Factory, Inc. (Tokushima, Japan). Hibitane gluconate solution (Sumitomo Dainippon Pharma Co., Ltd., Osaka, Japan) was used as chlorhexidine digluconate. Isodine solution 10% (Meiji Seika Pharma Co., Ltd., Tokyo, Japan) was used as povidone-iodine. These test antiseptics were diluted with distilled water to the desired concentration.
Bacterial strains were purchased from the ATCC (Manassas, VA), the Biological Resource Center, National Institute of Technology and Evaluation (NBRC, Kisarazu, Japan), or Microbiologics, Inc. (St. Cloud, MN). Bacterial clinical isolates were collected and identified from specimens from patients in Japanese medical facilities at Mitsubishi Chemical Medience Co. (Tokyo, Japan) from May through June 2012. Bacterial media were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
Bodipy TR cadaverine [5-(((4-(4,4-difluoro-5-(2-thienyl)-4-bora-3a,4a-diaza-s-indacene-3-yl)phenoxy)acetyl)amino)–pentylamine, hydrochloride] (BC), lipid A from Escherichia coli strain K-12, and nitrocefin were purchased from Thermo Fisher Scientific, Inc. (Waltham, MA), List Biological Laboratories, Inc. (Campbell, CA), and Merck KGaA (Darmstadt, Germany), respectively. Lipopolysaccharides from E. coli O111:B4, lipoteichoic acid from S. aureus (LTA), and 3,3-dipropylthiadicarbocyanine iodide [DiSC3(5)] were purchased from Sigma-Aldrich Co. LLC (St. Louis, MO).
The phospholipids were purchased from NOF Co. (Tokyo, Japan), Sigma-Aldrich Co. LLC, or Avanti Polar Lipids, Inc. (Alabaster, AL). All other chemicals were purchased from Wako Pure Chemical Industries, Ltd. and were of special grade.
In vitro bactericidal activity.
The minimal bactericidal concentration (MBC) is the lowest concentration of an antiseptic that eradicates the bacteria in the indicated exposure time. We determined the MBCs for each test antiseptic against each of the various bacterial strains obtained from culture collections and clinical isolates (a total of 320 strains). MBCs were measured with a microdilution technique, a method employed after modifying a previously reported method (9, 10), which is different from the method used in the Clinical and Laboratory Standards Institute guideline (11). Briefly, 11 concentrations for each of the three antiseptics were prepared using distilled water with the 2-fold serial dilution method. Test bacterial strains were grown in an appropriate broth, washed once in sterile saline, and diluted in distilled water. A 50-μl aliquot of each challenge strain containing ≥2 × 105 CFU/ml (intended to evaluate a ≥3-log10 reduction) was exposed to 50 μl of each concentration of each antiseptic for 30, 60, and 180 s at 23°C ± 3°C. The actual inoculum density (in log10CFU per milliliter) of 320 strains was 6.9 ± 0.6 (mean ± standard deviation [SD]), ranging from 5.1 to 8.1. After exposure, 10 μl of the reaction mixture was transferred to 200 μl of soybean-casein digest broth containing 0.1% lecithin and 0.7% polysorbate 80 as neutralizing agents (SCDLP broth) and incubated at 35°C for >20 h until the growth of test bacteria was observed in the well in which distilled water instead of test antiseptics was used. After incubation, bacterial growth was evaluated visually based on the turbidity of the SCDLP broth. The minimum final concentration with no turbidity was designated the MBC. Concurrently, to confirm that the bactericidal activity of the test antiseptics was neutralized with the SCDLP broth, 205 μl of SCDLP broth containing test bacteria with the same inoculum density was added to 5 μl of 2-fold serial dilutions of the test antiseptics and incubated. A turbid medium indicated positive bacterial growth, and bactericidal activity was considered to be neutralized. That concentration was designated the neutralizing concentration. When the MBC was lower than the neutralizing concentration, the MBC was recorded. When the MBC was greater than the neutralizing concentration, it was reported as greater than the upper limit of the neutralizing concentration.
For bacterial strains that did not grow in the SCDLP broth, the neutralization mixture was transferred to 2 ml of an appropriate broth and incubated. After incubation, the MBC of each antiseptic at each exposure time was similarly determined.
Fluorescent probe displacement assay for LPS, lipid A, or LTA binding to olanexidine.
Lipopolysaccharide (LPS), lipid A, or LTA binding was assessed using the fluorescent probe BC, as previously described (12–14). Briefly, the displacement assay was performed by adding a 5-μl aliquot of the test antiseptic dilutions to 95 μl of LPS (9.5 μg/ml), lipid A (9.4 μg/ml), or LTA (9.5 μg/ml) in 20 mM HEPES buffer (pH 7.2) containing BC (9.5 μM). Five minutes later, fluorescence was measured on a PowerScan MX multiplex plate reader (BioTek Instruments, Inc.), with 580 and 620 nm as the BC excitation and BC emission wavelengths, respectively. Each measurement was conducted in triplicate, and the 50% effective dose (ED50) and its 95% confidence interval were calculated from the dose-response curve using a sigmoidal (four-parameter) curve fitting, as described previously (12). These analyses were performed using the SAS 9.2 software (SAS Institute Japan, Tokyo, Japan) and EXSUS 7.7 software (CAC Exicare Corporation, Tokyo, Japan).
Liposome leakage assay.
The calcein-encapsulated liposome leakage assay was performed as described previously (15, 16). Briefly, a phospholipid solution in chloroform-methanol containing various types of phospholipids (ca. 20 mg) was placed in a round-bottom flask. After evaporating the solvent with a rotating evaporator and vacuum pump, the residual film was hydrated with the 70 mM calcein solution (1.5 ml). The suspension was freeze-thawed for five cycles and then successively extruded through polycarbonate filters (twice through a 0.6-μm-pore filter, five times through a 0.1-μm-pore filter, and five times through two stacked 0.1-μm-pore filters). Calcein-entrapped large unilamellar vesicles (LUVs) were separated from free calcein on a Sephadex G-50 column (1.5 cm by 30 cm; buffer, 10 mM HEPES-150 mM NaCl-1 mM EDTA [pH 7.2]).
Phospholipid concentrations were measured by phosphorus analysis and were considered to represent the concentrations of LUV (17). The LUV leakage assay was performed by adding a 10-μl aliquot of the antiseptic dilutions to 190 μl of LUV. The release of calcein from LUV was monitored by fluorescence at an excitation wavelength of 490 nm and an emission wavelength of 520 nm. The maximum fluorescence intensity corresponding to 100% leakage was determined by adding Triton X-100 to LUV for a final concentration of 1%. The final value (percent leakage) was the mean ± SD of triplicate measurements.
The molar ratio of the prepared LUVs was as follows: phosphatidylglycerol (PG)/phosphatidylcholine (PC) (1:1) vesicle from 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol (POPG) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), PG/phosphatidylethanolamine (PE) (1:1) vesicle from POPG and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE), PG/PE (3:7) vesicle from POPG and POPE, PG/cardiolipin (CL) (16:1) vesicle from l-α-phosphatidyl-dl-glycerol (from egg yolk lecithin) and cardiolipin (from bovine heart), and PG/CL/lysyl-phosphatidylglycerol (L-PG) (13:1:3) vesicle from l-α-phosphatidyl-dl-glycerol (from egg yolk lecithin), cardiolipin (from bovine heart), and 1,2-dioleoyl-sn-glycero-3-(phospho-rac-(3-lysyl(1-glycerol))).
Outer and inner membrane permeabilization assay with Gram-negative strains.
E. coli strain ML35 (ATCC 43827), a lactose permease-deficient strain with constitutive cytoplasmic β-galactosidase activity, was transformed using the plasmid vector pBR322 (TaKaRa Bio, Inc., Tokyo, Japan), which carries tetracycline and ampicillin resistance genes by the calcium chloride method. The transformed strain, E. coli ML-35p, selected by ampicillin resistance, constitutively expressed cytoplasmic β-galactosidase and periplasmic β-lactamase and was lactose permease deficient. The organism was used for the outer and inner membrane permeabilization assay, as described below (18–20).
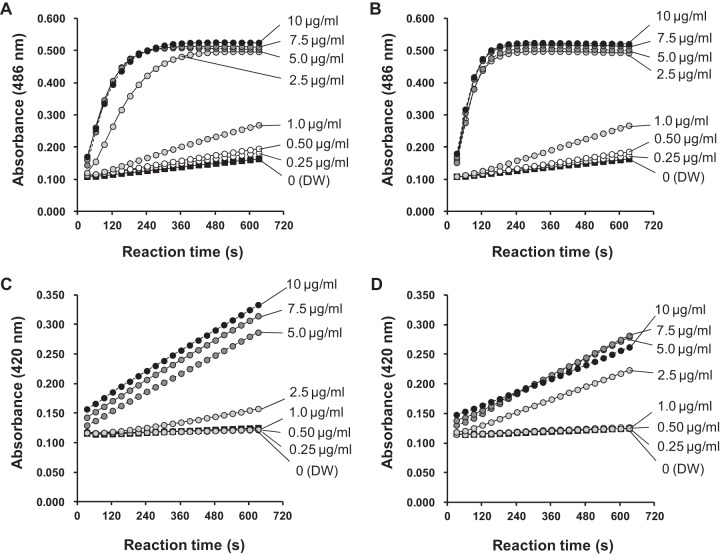
Nitrocefin, a chromogenic cephalosporin, cannot cross the outer membrane and is excluded from the periplasmic space. However, permeabilization of the outer membrane allows nitrocefin to enter the periplasm, where it is cleaved by a β-lactamase and produces a color change that can be monitored spectrophotometrically. Briefly, the organisms were cultivated at 35°C during log-phase growth, harvested by centrifuge, washed with 5 mM HEPES buffer (pH 7.2), and then diluted to about 1 × 108 CFU/ml in 5 mM HEPES buffer containing 20 μg/ml nitrocefin and 5 μM carbonyl cyanide 3-chlorophenylhydrazone. A 5-μl aliquot of the test antiseptic dilution was added to a 195-μl aliquot of the above-mentioned cell suspension, and the absorbance at 486 nm was measured at 30-s intervals for 10 min at room temperature.
Because this strain of E. coli has no lactose permease, o-nitrophenyl β-d-galactopyranoside (ONPG) cannot traverse the inner membrane to be cleaved by cytoplasmic β-galactosidase to o-nitrophenol unless the inner membrane becomes permeable. ONPG cleavage produces a color change that can be measured spectrophotometrically. Briefly, the organism pellet, prepared by a method similar to that used in the outer membrane permeabilization assay, was diluted to about 1 × 108 CFU/ml in 5 mM HEPES buffer containing 0.75 mg/ml ONPG. A 5-μl aliquot of the antiseptic dilutions was added to a 195-μl aliquot of the above-mentioned cell suspension, and the absorbance at 420 nm was measured at 30-s intervals for 10 min at room temperature. Mean values of triplicate measurements were used in the analysis.
Membrane depolarization assay with Gram-positive strains.
Cytoplasmic membrane integrity was determined using the membrane potential-sensitive cyanine probe DiSC3(5). This probe is known to distribute between bacterial cells and the surrounding medium, depending on the membrane potential gradient. Once inside the membrane, the probe aggregates and self-quenches. With the addition of a membrane-disrupting agent, such as antiseptics, the probe is released, and an increase in fluorescence can be monitored over time (12, 21–23). In this experiment, S. aureus (strain ATCC 29213) cultivated at 35°C during log-phase growth was harvested by centrifuge, washed with 5 mM HEPES-KOH buffer (pH 7.2) containing 20 mM glucose, and then diluted to about 2 × 107 CFU/ml in 5 mM HEPES-KOH buffer containing 20 mM glucose and 100 mM KCl. The probe DiSC3(5) was added to make a final concentration of 0.4 μM, and the probe was incorporated for 4 min at room temperature. The resulting solution (cell suspension) was allowed to stand for 4 min, which gave a stable baseline. Fluorescence (using 620 and 670 nm as excitation and emission wavelengths, respectively) was measured at 20-s intervals for 5 min after adding a 5-μl aliquot of antiseptic dilution to a 195-μl aliquot of the above-mentioned cell suspension. The mean of triplicate measurements was used in the analysis.
Protein denaturation assay.
The protein-denaturing property of OPB was estimated with a hemoglobin denaturation assay, as previously described (24, 25). Briefly, test antiseptic dilutions prepared with distilled water (12 concentrations from 0 to 20,000 μg/ml prepared by the serial 2-fold dilution method) were placed on a 96-well microplate. An equal amount of 20 mM HEPES buffer (pH 7.2) with or without 0.05% hemoglobin was added, and the wells were incubated for 5 min at room temperature. The absorbance at 418 nm was measured, and the hemoglobin denaturation ratio (HDR%) was calculated as HDR% = 100 − ([A(TH) − A(TB)]/[A(WH) − A(WB)]) × 100, where A(TH) is the absorbance of the test antiseptic dilution mixed with hemoglobin solution, A(TB) is the absorbance of the test antiseptic dilution mixed with buffer solution, A(WH) is the absorbance of distilled water mixed with the hemoglobin solution, and A(WB) is the absorbance of distilled water mixed with the buffer solution. The measurement was replicated four times.
RESULTS
In vitro bactericidal effects of olanexidine.
The well-known MBC assay is a method to determine the MBCs by subcultivating a culture broth after MICs are determined (11), and it has a long exposure time (contact time) with antiseptic agents (e.g., about 24 h). However, we determined the MBCs for 30-, 60-, and 180-s exposures using a microdilution method with a neutralizer, because the short-term efficacy of antiseptics is of great importance. The bactericidal effects of OPB were evaluated by MBCs and compared to those of chlorhexidine and PVP-I (summary of results in Table 1; see also Table S1 in the supplemental material for full details). OPB exhibited broad-spectrum bactericidal activity against the following bacteria: Gram-positive cocci, such as Staphylococcus spp., including methicillin-resistant S. aureus, and Enterococcus spp., including vancomycin-resistant enterococci; Gram-positive bacilli, such as Corynebacterium spp.; and Gram-negative bacteria, such as Pseudomonas aeruginosa and Serratia marcescens. OPB had a spectrum of bactericidal activity comparable to that of commercial antiseptics, such as chlorhexidine gluconate (CHG) and PVP-I.
TABLE 1.
Summary of the bactericidal effects of olanexidine against various bacterial strains by exposure time and MBCa
| Strain tested (no. of strains) | Exposure time (s) | Minimal bactericidal concn (range) (μg/ml) for: |
||
|---|---|---|---|---|
| Olanexidine gluconate | Chlorhexidine digluconate | Povidone-iodine | ||
| Gram-positive cocci except Enterococcus spp. (21) | 30 | 13.6 to 1,740 | 39.1 to >2,500 | ≤48.8 to 781 |
| 60 | ≤6.8 to 869 | 9.8 to >2,500 | ≤48.8 to 781 | |
| 180 | ≤6.8 to 434 | 9.8 to >1,250 | ≤48.8 to 391 | |
| Enterococcus spp. (34) | 30 | 13.6 to 434 | 5,000 to >5,000 | 195 to >50,000 |
| 60 | ≤6.8 to 217 | 78.1 to >5,000 | 97.7 to >50,000 | |
| 180 | ≤6.8 to 54.3 | 9.8 to >5,000 | ≤48.8 to 1,560 | |
| Gram-positive bacilli (9) | 30 | ≤6.8 to 1,740 | 19.5 to >2,500 | 97.7 to 781 |
| 60 | ≤6.8 to 1,740 | 19.5 to >2,500 | ≤48.8 to 781 | |
| 180 | ≤6.8 to 109 | 9.8 to >2,500 | 97.7 to 781 | |
| Gram-negative strains except Burkholderia cepacia (34) | 30 | ≤6.8 to 869 | 39.1 to >5,000 | ≤48.8 to 3,130 |
| 60 | ≤6.8 to 434 | 19.5 to >5,000 | ≤48.8 to 781 | |
| 180 | ≤6.8 to 54.3 | ≤4.9 to 2,500 | ≤48.8 to 781 | |
| B. cepacia (2) | 30 | >6,950 | >5,000 | 391 |
| 60 | 1,740 to >6,950 | >5,000 | 391 | |
| 180 | 434 | 625 | 195 | |
The full data set is given in Table S1 in the supplemental material.
OPB also showed bactericidal activity against clinical isolates of methicillin-resistant S. aureus, methicillin-susceptible S. aureus, coagulase-negative Staphylococcus, Enterococcus faecalis, Corynebacterium spp., E. coli, P. aeruginosa, Klebsiella pneumoniae, Acinetobacter baumannii, and S. marcescens (Table 2).
TABLE 2.
MBCs of olanexidine against clinical isolates
| Strain (no. of strains) | Exposure time (s) | Minimal bactericidal concn (range) (μg/ml) for: |
||
|---|---|---|---|---|
| Olanexidine gluconate | Chlorhexidine digluconate | Povidone-iodine | ||
| Methicillin-resistant S. aureus (30)a | 30 | 217 to >3,480 | 2,500 to >5,000 | 781 to 1,560 |
| 60 | 217 to 869 | 2,500 to >5,000 | 781 to 1,560 | |
| 180 | 54.3 to 217 | 2,500 to >5,000 | 195 to 781 | |
| Methicillin-susceptible S. aureus (20) | 30 | 217 to >3,480 | 156 to >2,500 | 391 to 1,560 |
| 60 | 217 to 1,740 | 78.1 to >2,500 | 195 to 781 | |
| 180 | ≤6.8 to 869 | ≤4.9 to 156 | ≤48.8 to 781 | |
| Coagulase-negative Staphylococcus (20) | 30 | 217 to >869 | 156 to >1,250 | 195 to 1,560 |
| 60 | 109 to 869 | 39.1 to >1,250 | 97.7 to 1,560 | |
| 180 | 13.6 to 109 | 19.5 to >625 | ≤48.8 to 781 | |
| E. faecalis (30)a | 30 | 54.3 to 434 | 625 to >5,000 | 781 to 50,000 |
| 60 | 27.1 to 217 | 313 to >5,000 | 391 to 3,130 | |
| 180 | 13.6 to 109 | 156 to 5,000 | 195 to 1,560 | |
| Corynebacterium spp. (20) | 30 | ≤6.8 to 54.3 | ≤4.9 to >78.1 | ≤48.8 to 781 |
| 60 | ≤6.8 to 27.1 | ≤4.9 to >78.1 | ≤48.8 to 391 | |
| 180 | ≤6.8 to 13.6 | ≤4.9 to 78.1 | ≤48.8 to 391 | |
| E. coli (20) | 30 | 54.3 to 217 | 19.5 to 625 | 195 to 781 |
| 60 | 54.3 to 217 | 9.8 to 313 | 97.7 to 781 | |
| 180 | 27.1 to 109 | ≤4.9 to 78.1 | ≤48.8 to 391 | |
| P. aeruginosa (20) | 30 | 27.1 to 869 | 39.1 to >5,000 | 195 to 781 |
| 60 | 13.6 to 217 | 39.1 to >5,000 | 195 to 781 | |
| 180 | ≤6.8 to 54.3 | 19.5 to 313 | 195 to 781 | |
| K. pneumoniae (20) | 30 | 13.6 to 54.3 | 19.5 to 78.1 | 195 to 781 |
| 60 | ≤6.8 to 27.1 | 19.5 to 78.1 | 97.7 to 391 | |
| 180 | ≤6.8 to 27.1 | 9.8 to 39.1 | ≤48.8 to 391 | |
| A. baumannii (20) | 30 | 13.6 to 109 | 39.1 to 156 | ≤48.8 to 391 |
| 60 | 13.6 to 54.3 | 19.5 to 156 | ≤48.8 to 391 | |
| 180 | 13.6 to 27.1 | 9.8 to 78.1 | ≤48.8 to 391 | |
| S. marcescens (20) | 30 | 27.1 to 3,480 | 78.1 to >5,000 | 97.7 to 391 |
| 60 | 13.6 to 434 | 39.1 to >5,000 | 97.7 to 391 | |
| 180 | 13.6 to 217 | 19.5 to 78.1 | 97.7 to 391 | |
These data were collected in the current study but are included in reference 7, which was published first.
Binding affinity of olanexidine to LPS, lipid A, and LTA.
To clarify the mechanism of action, we examined the binding affinity of OPB to LPS, lipid A, and LTA with the BC displacement assay. LPS, of which the toxic moiety is lipid A, and LTA are bacterial surface molecules of Gram-negative and Gram-positive bacteria, respectively, and both are widely conserved. The assay monitors the competitive displacement of BC bound to LPS, lipid A, or LTA by other putative LPS-, lipid A-, or LTA-binding molecules as a change in fluorescence intensity. When OPB was added, it displaced the BC probe bound to LPS, lipid A, or LTA in a dose-dependent manner, which increased the fluorescence of BC by decreasing in binding occupancy of BC to LPS, lipid A, and LTA (see Fig. S1 in the supplemental material). OPB had binding affinity to LPS, lipid A, and LTA, and the ED50 values for OPB binding were calculated (Table 3). The ED50 values of OPB to LPS, lipid A, and LTA were 20, 15, and 43 μmol/liter (11, 8.4, and 25 μg/ml, as olanexidine gluconate), respectively, being almost comparable. Chlorhexidine also had binding affinity to LPS, lipid A, and LTA, and those ED50s were 210, 30, and 680 μmol/liter (190, 27, and 610 μg/ml, as chlorhexidine digluconate), respectively.
TABLE 3.
Binding affinity of olanexidine to bacterial surface molecules, as determined by the Bodipy TR cadaverine displacement assay
| Bacterial surface molecule | Median effective dose (95% confidence interval) (μmol/liter) for: |
|||
|---|---|---|---|---|
| Olanexidine gluconate | Chlorhexidine digluconate | Polymyxin B sulfate salt (positive control) | Alexidine dihydrochloride (positive control) | |
| LPS-bound BC | 20 (18 to 22) | 210 (98 to 440) | 3.8 (2.6 to 5.6) | Not tested |
| Lipid A-bound BC | 15 (14 to 15) | 30 (27 to 33) | 3.1 (2.8 to 3.3) | Not tested |
| LTA-bound BC | 43 (37 to 50) | 680 (610 to 760) | Not tested | 18 (14 to 22) |
Membrane barrier-disrupting effects of olanexidine.
The effects of OPB on membrane barrier disruption were examined by the dye release assay, which uses the calcein-entrapped liposomes consisting of various phospholipids. In this assay, the membrane barrier function is determined by monitoring fluorescence, because the calcein entrapped in the liposome is self-quenched but fluoresces if it leaks from the liposome.
Generally, the lipid compositions of bacterial membranes are anionic lipid PG and CL (26–28). The typical lipid compositions of Gram-negative bacteria are those containing a high concentration of zwitterionic lipid PE. On the other hand, Gram-positive bacteria contain L-PG (26–28). We prepared PG/PE vesicles (molar ratios, 1:1 and 3:7) and PG/CL/L-PG (13:1:3) vesicle as model membranes of Gram-negative and Gram-positive bacteria, respectively. We also prepared PG/PC and PG/CL vesicles to evaluate the effects of phospholipid type on the results.
OPB disrupted the artificial membrane, which depended on the phospholipid composition of the membrane (Fig. 2). Calcein was released by adding OPB to the LUVs containing PE, but it was not released when OPB was added into the LUV containing PC; that is, OPB disrupted the membrane barrier of the LUVs containing PE but had no effect on the LUV containing PC (Fig. 2). The effect of OPB was dose dependent for the PG/PE (1:1) vesicle but not for the PG/PE (3:7) vesicle. The effect of OPB was small against the PG/CL vesicle, but it became stronger against the vesicle containing L-PG (Fig. 2). These effects were apparent within 1 min after adding OPB and continued for ≥30 min (data not shown). On the other hand, chlorhexidine had small membrane barrier-disrupting effects on all types of LUV. As a result, the monobiguanide compound, OPB, and the bisbiguanide compound, chlorhexidine, produced different results.
FIG 2.
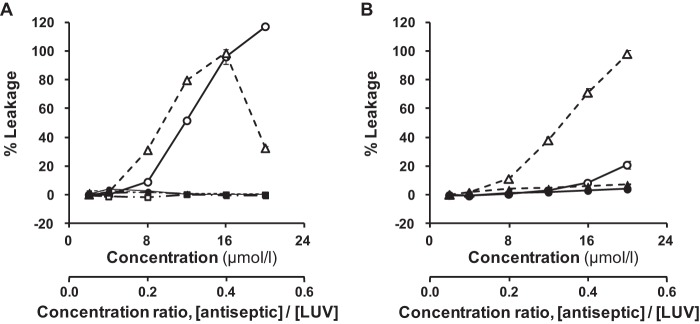
Effects of olanexidine and chlorhexidine on calcein release from calcein-encapsulated LUVs with different lipid compositions after 11 min of exposure. (A) PG/PC (1:1) vesicle (square), PG/PE (1:1) vesicle (circle), and PG/PE (3:7) vesicle (triangle). (B) PG/CL (16:1) vesicle (circle) and PG/CL/L-PG (13:1:3) vesicle (triangle). Empty symbols, olanexidine gluconate; filled symbols, chlorhexidine gluconate. The concentration ratio of an antiseptic to the LUV is also shown below the x axis of each graph, where [LUV] was 40 μmol/liter. Each point and vertical bar represents the mean ± SD (n = 3).
Effects on outer and inner membrane permeabilization in Gram-negative strains.
Because OPB interacted with LPS/lipid A and the bacterial membrane as described above, we tested the effect on membrane permeabilization using E. coli as a representative Gram-negative bacterium. At concentrations of ≥2.5 μg/ml, both OPB and chlorhexidine rapidly increased the permeability of the outer membrane of E. coli ML-35p, as shown by increased nitrocefin permeation (Fig. 3). However, neither antiseptic clearly affected outer membrane permeability at concentrations of ≤1.0 μg/ml. OPB enhanced the inner membrane permeability of the strain in a time-dependent manner at concentrations of ≥5.0 g/ml, as indicated by an increase in ONPG permeation (Fig. 3). The OPB concentration at which the apparent effect on permeabilization was observed differed slightly between the outer and inner membranes. Chlorhexidine also increased the inner membrane permeability of the strain at concentrations of ≥2.5 μg/ml. The MICs of OPB and chlorhexidine against E. coli ML-35p were 4.0 and 1.3 μg/ml, respectively. The MBCs of OPB and chlorhexidine against E. coli after 3 min of exposure were 13.6 to 109 μg/ml and ≤4.9 to 78.1 μg/ml, respectively (Table 2; see also Table S1 in the supplemental material). Accordingly, the MIC seems to be related to the concentration that causes inner membrane permeabilization.
FIG 3.
Changes in outer and inner membrane permeabilization caused by olanexidine and chlorhexidine in E. coli ML-35p cells incubated in 5 mM HEPES buffer containing 20 μg/ml nitrocefin and 5 μM carbonyl cyanide 3-chlorophenylhydrazone with olanexidine gluconate (A) or chlorhexidine digluconate (B). The bacteria were incubated in 5 mM HEPES buffer containing 0.75 mg/ml o-nitrophenyl β-d-galactopyranoside with olanexidine gluconate (C) or chlorhexidine digluconate (D). The concentrations of olanexidine and chlorhexidine are shown on the right side of each graph. Distilled water (DW) was used as a negative control.
Effect on depolarization of bacterial cytoplasmic membrane in Gram-positive bacterial strains.
Given that OPB interacted with LTA and bacterial membrane, as described above, we tested cytoplasmic membrane integrity using S. aureus as a representative Gram-positive bacterium. Changes in membrane integrity were detected by membrane depolarization using a fluorescence probe, DiSC3(5), in S. aureus ATCC 29213 (Fig. 4). Increased fluorescence intensity indicated that OPB depolarized the membrane at concentrations between 0.25 and 1.0 μg/ml, and the maximum effect was reached within about 3 min. The time to reach a peak of fluorescence intensity became shorter as concentrations increased. In the range of 2.5 to 10 μg/ml, OPB immediately depolarized the membrane of S. aureus.
FIG 4.
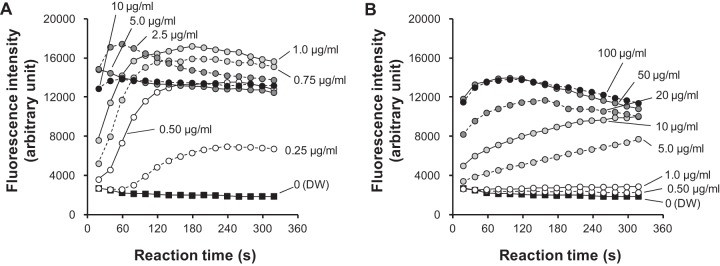
Effects of olanexidine and chlorhexidine on the cytoplasmic membrane of S. aureus. The effects were monitored by changes in the membrane potential using a fluorescence probe, DiSC3(5). (A) Olanexidine gluconate. (B) Chlorhexidine digluconate. Distilled water (DW) was used as a negative control.
Chlorhexidine depolarized the membrane at concentrations between 5.0 and 50 μg/ml, and the time to peak fluorescence intensity also became shorter with increased concentrations. At a concentration of ≤1.0 μg/ml, chlorhexidine did not increase fluorescence intensity. In addition, fluorescence intensity tended to decrease after peaking. At a concentration of ≥50 μg/ml, the effect of chlorhexidine on membrane depolarization seemed to be constant.
The MICs of OPB and chlorhexidine against S. aureus ATCC 29213 were 0.63 and 1.3 μg/ml, respectively, and the MBCs of OPB and chlorhexidine at 3 min of exposure against the strain were 109 and >1,250 μg/ml, respectively (see Table S1 in the supplemental material). Thus, the MIC of OPB seems to be related to the concentration that causes depolarization, which was not true for chlorhexidine.
Protein-denaturing effects of olanexidine.
Using hemoglobin as a representative protein and as an easily detectable protein when denatured, we examined the protein-denaturing effect of OPB. OPB showed a clear hemoglobin-denaturing effect at concentrations of ≥160 μg/ml (Table 4), but chlorhexidine showed only a slight hemoglobin-degenerating effect at a concentration of 10,000 μg/ml by this assay method. The hemoglobin-degenerating effect of the positive control, hexadecylpyridinium chloride monohydrate, was the same as that reported elsewhere (25).
TABLE 4.
Protein-denaturing effects of olanexidine on hemoglobin by concentrationa
| Test substance | HDR% for concn (μg/ml) of: |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 9.8 | 19.5 | 39.1 | 78.1 | 156 | 313 | 625 | 1,250 | 2,500 | 5,000 | 10,000 | |
| Olanexidine gluconate | −1.5 ± 3.3 | −2.6 ± 2.9 | 0.7 ± 2.7 | 0.8 ± 2.5 | 15.0 ± 3.1 | 36.1 ± 1.9 | 45.3 ± 1.6 | 46.2 ± 1.3 | 47.8 ± 1.9 | 48.4 ± 1.6 | 47.7 ± 1.5 |
| Chlorhexidine digluconate | −0.1 ± 1.8 | 0.2 ± 1.1 | −1.8 ± 0.5 | −1.7 ± 1.9 | −1.2 ± 2.4 | −3.1 ± 2.3 | −2.4 ± 1.6 | −1.6 ± 1.8 | −0.9 ± 1.0 | −1.8 ± 2.3 | 4.4 ± 0.4 |
| Hexadecyl-pyridinium chloride monohydrate (positive control) (25) | 0.8 ± 1.2 | 5.9 ± 0.7 | 7.0 ± 0.8 | 20.8 ± 0.9 | 38.9 ± 0.8 | 38.5 ± 0.8 | 37.9 ± 1.0 | 37.4 ± 0.7 | 38.1 ± 0.7 | 38.5 ± 1.3 | 39.8 ± 1.1 |
Values represent the mean ± SD (n = 4).
DISCUSSION
One of the most important characteristics of an antiseptic is its bactericidal activity rather than bacteriostatic activity, because an antiseptic needs to kill bacteria quickly. The MBC determination method using a neutralizer, which we report in this article, is better than other methods in that it can evaluate MBCs with a short exposure time and thus the spectrum of bactericidal activity of an antiseptic. The method used in the study is a semiquantitative method for measuring bactericidal activity, for which the results are not precise, as MICs are not and should be interpreted accordingly. However, the values obtained from our method appear to be reasonable considering the results of the qualitative suspension test (time-kill study) for CHG and PVP-I that were previously reported (29–31).
In this study, we used an olanexidine gluconate formulation (OPB) as a test antiseptic instead of an olanexidine aqueous solution. We confirmed that the base of the formulation, which is the solution containing the same amounts of POEPOPG and gluconic acid with same pH, has no biological activity (data not shown). Therefore, we believe that the results of this study using the olanexidine gluconate formulation (OPB) are from olanexidine itself.
Based on the results of MBCs, olanexidine had antimicrobial activity against a wide range of Gram-positive and Gram-negative bacteria, including clinical isolates. The activity against Gram-positive bacteria, especially Enterococcus spp. and Staphylococcus spp., was pronounced.
An important step in the bacterium-killing mechanism of an antiseptic is the first encounter with the bacterial surface. Therefore, we examined the binding affinity of olanexidine against LPS/lipid A and LTA, which are widely conserved molecules on the bacterial surface. Olanexidine seems to bind to LPS, lipid A, and LTA with better affinity than that of chlorhexidine, based on the 95% confidence intervals of those ED50s (Table 3).
Biguanides strongly interact with the negatively charged phospholipid PG (32, 33). To clarify the effects of olanexidine on membrane disruption, we tested it against an artificial membrane, calcein-entrapped LUV, which consists of various phospholipids. Olanexidine clearly disrupted the membrane of the LUVs containing phospholipids with an ammonio group (-N+H3), such as PE and L-PG. However, it had no such activity against other types of LUVs (Fig. 2). For all types of LUVs containing acidic PG, olanexidine seemed to interact with the phospholipid membranes only through surface adsorption, but it did not deform the membranes. The biguanide group in olanexidine is a strong base; therefore, it is completely monoprotonated at physiological pH. Electrostatic attraction between the positive charge on the biguanide group and the negative charge on the PG molecule is the most probable driving force for the complex formation between olanexidine and the PG molecule. The hydrophobic octamethylene group, which is bound to the biguanide group in the olanexidine molecule, may also be important in expanding the PG bilayer. This group seems to act as a wedge that is inserted into the more hydrophobic sites in the bilayer. On the other hand, the bisbiguanide compound chlorhexidine may not insert so easily into these hydrophobic sites. Such an insertion would produce a larger distance between each phospholipid molecule and cause electrostatic repulsion if the phospholipids with an ammonio group were contained in the liposomal bilayer, which destabilizes the LUV and causes leakage of calcein. Because olanexidine had no effect on the LUVs containing PC, the barrier-disrupting activity could not be explained by the electronic charge alone. The shape of the PC might differ from that of PE because of the relatively large headgroup (-N+(CH3)3) of the PC. The shape of phospholipids is related to the size of the polar headgroup and apolar tails of the molecule, which may be involved in the stability of liposome as a molecular assembly. Therefore, liposomes with differently shaped phospholipids are considered to exert different activities. The PC-containing LUV that was bound to olanexidine may be a stable molecular assembly, because it was not disrupted. The lack of a dose-related effect of olanexidine against the PG/PE (3:7) vesicle may also be related to the stability of the LUV bound to a large amount of olanexidine. In addition, this assay may be affected by the fatty acid composition of phospholipids. We confirmed that egg yolk PC with different fatty acid compositions slightly increased the membrane-disrupting activities of olanexidine and chlorhexidine (data not shown).
Biological membranes include not only phospholipids but also proteins and glycolipids. Binding to membrane proteins or glycolipids may exert synergistic effects on membrane disruption. In our in vitro tests, effects on phospholipid membranes containing several components and the effective concentration against actual microorganisms were unknown, so we studied such issues using bacteria.
In the test detecting the movement of substrates across the membranes using E. coli ML-35p, olanexidine enhanced outer membrane and inner membrane permeability (Fig. 3). It also disrupted the membranes of the LUVs containing PE, a high-level component of the E. coli membrane, which might be related to its effect on membrane permeabilization in bacteria. These results indicate that olanexidine binds to the cell membrane of E. coli, which disrupts membrane integrity and exhibits its bacteriostatic and bactericidal effects through the irreversible leakage of intracellular components.
This hypothesis is supported by observations with electron microscopy of P. aeruginosa and methicillin-resistant S. aureus treated by olanexidine hydrochloride. Sakagami et al. (34, 35) reported that olanexidine hydrochloride released intracellular components from such bacteria at the level of the MIC. On the other hand, chlorhexidine did not disrupt the membrane of the LUVs but did disrupt that of E. coli. Because chlorhexidine has binding affinity to LPS, it might disrupt the membrane of the LUV containing LPS.
In the test using S. aureus, we detected the loss of membrane potential caused by the disruption of membrane integrity. Olanexidine changed the membrane potential of S. aureus, and the effective concentration was related to the MIC. Olanexidine also disrupted the membrane of L-PG containing LUV, which mimicked the S. aureus membrane, but chlorhexidine did not. This difference might be related to the bactericidal effect against S. aureus. The results above, combined with the electron microscope observations by Sakagami et al. (35), suggest that olanexidine binds to the surface of S. aureus, where it disrupts the membrane, causing cytoplasmic components to leak from the cell, thereby killing it.
In addition, a hemoglobin denaturation assay revealed that olanexidine denatured protein at relatively high concentrations (≥ 160 μg/ml). Observations with electron microscopy revealed that olanexidine hydrochloride also affects the agglutination of P. aeruginosa at nearly the same concentration (34). This effect might be explained by a protein-denaturing effect.
Olanexidine gluconate exhibited antimicrobial activity against a wide range of bacteria, especially Gram-positive bacteria. It interacted with the surface molecules and phospholipids that are widely conserved in Gram-negative and -positive bacteria and disrupted the membranes of liposomes, E. coli, and S. aureus. It also denatured protein at relatively high concentrations. From these results, the mechanism of action was considered to be follows: olanexidine binds to the cell membrane, disrupts membrane integrity, and exerts its bacteriostatic and bactericidal activities by causing the irreversible leakage of intracellular components. At relatively high concentrations, olanexidine aggregates the cells through a protein-denaturing effect. The mechanism of action differs between the monobiguanide compound olanexidine and the bisbiguanide compound chlorhexidine. Therefore, olanexidine will be a new choice for preventing health care-associated infections and may provide better protection against infections caused by pathogens resistant to chlorhexidine.
Supplementary Material
ACKNOWLEDGMENTS
This study was funded by Otsuka Pharmaceutical Factory, Inc, and was led and mainly conducted by the authors at Otsuka Pharmaceutical Factory, Inc. Part of the experiments was outsourced and conducted at Tanabe R&D Service Co., Ltd. and Mitsubishi Chemical Medience Co.
We thank Motoya Kikuchi and Kazumasa Hashimoto (Otsuka Pharmaceutical Factory, Inc.), Kinue Ohguro (Otsuka Pharmaceutical Co., Ltd.), Masato Onozawa (Tanabe R&D Service Co., Ltd.), and Takako Iyoda, Makoto Suzuki, and Fumiaki Ikeda (Mitsubishi Chemical Medience Co.) for technical support. We thank Hiroko Inoue and Hideki Uchimi (Otsuka Pharmaceutical Factory, Inc.) for their critical review of the manuscript and many helpful suggestions.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.05048-14.
REFERENCES
- 1.Kobayashi H, Tsuzuki M, Hosobuchi K. 1989. Bactericidal effects of antiseptics and disinfectants against methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol 10:562–564. doi: 10.1086/645952. [DOI] [PubMed] [Google Scholar]
- 2.Kampf G, Höfer M, Wendt C. 1999. Efficacy of hand disinfectants against vancomycin-resistant enterococci in vitro. J Hosp Infect 42:143–150. doi: 10.1053/jhin.1998.0559. [DOI] [PubMed] [Google Scholar]
- 3.Sauerbrei A, Wutzler P. 2010. Virucidal efficacy of povidone-iodine-containing disinfectants. Lett Appl Microbiol 51:158–163. doi: 10.1111/j.1472-765X.2010.02871.x. [DOI] [PubMed] [Google Scholar]
- 4.Zamora JL, Price MF, Chuang P, Gentry LO. 1985. Inhibition of povidone-iodine's bactericidal activity by common organic substances: an experimental study. Surgery 98:25–29. [PubMed] [Google Scholar]
- 5.Ito S. 2000. Drug therapy for breast-feeding women. N Engl J Med 343:118–126. doi: 10.1056/NEJM200007133430208. [DOI] [PubMed] [Google Scholar]
- 6.Tsubouchi H, Ohguro K, Yasumura K, Ishikawa H, Kikuchi M. 1997. Synthesis and structure-activity relationship of novel antiseptics. Bioorganic Med Chem Let 7:1721–1724. doi: 10.1016/S0960-894X(97)00297-7. [DOI] [Google Scholar]
- 7.Inoue Y, Hagi A, Nii T, Tsubotani Y, Nakata H, Iwata K. 2015. Novel antiseptic compound OPB-2045G shows potent bactericidal activity against methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus both in vitro and in vivo: a pilot study in animals. J Med Microbiol 64:32–36. doi: 10.1099/jmm.0.080861-0. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Food and Drug Administration. 1994. Topical antimicrobial drug products for over-the-counter human use; tentative final monograph (TFM) for health-care antiseptic drug products. Federal Register 59:31402–31452. [Google Scholar]
- 9.Jono K, Takayama T, Kuno M, Higashide E. 1986. Effect of alkyl chain length of benzalkonium chloride on the bactericidal activity and binding to organic materials. Chem Pharm Bull (Tokyo) 34:4215–4224. doi: 10.1248/cpb.34.4215. [DOI] [PubMed] [Google Scholar]
- 10.Sasatsu M, Shimizu K, Noguchi N, Kono M. 1994. Evaluation of antiseptics by the modified phenol coefficient method: sensitivity of methicillin-resistant Staphylococcus aureus. Biol Pharm Bull 17:136–138. doi: 10.1248/bpb.17.136. [DOI] [PubMed] [Google Scholar]
- 11.Jabes D, Brunati C, Candiani G, Riva S, Romanó G, Maffioli S, Rossi R, Simone M, Gaspari E, Donadio S. 2014. Pharmacological properties of NAI-603, a well-tolerated semisynthetic derivative of ramoplanin. Antimicrob Agents Chemother 58:1922–1929. doi: 10.1128/AAC.01620-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torrent M, Navarro S, Moussaoui M, Nogués MV, Boix E. 2008. Eosinophil cationic protein high-affinity binding to bacteria-wall lipopolysaccharides and peptidoglycans. Biochemistry 47:3544–3555. doi: 10.1021/bi702065b. [DOI] [PubMed] [Google Scholar]
- 13.Zorko M, Jerala R. 2008. Alexidine and chlorhexidine bind to lipopolysaccharide and lipoteichoic acid and prevent cell activation by antibiotics. J Antimicrob Chemother 62:730–737. doi: 10.1093/jac/dkn270. [DOI] [PubMed] [Google Scholar]
- 14.Wood SJ, Miller KA, David SA. 2004. Anti-endotoxin agents. 1. Development of a fluorescent probe displacement method optimized for the rapid identification of lipopolysaccharide-binding agents. Comb Chem High Throughput Screen 7:239–249. doi: 10.2174/1386207043328832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Machida S, Niimi S, Shi X, Ando Y, Yu Y. 2000. Design of a novel membrane-destabilizing peptide selectively acting on acidic liposomes. Biosci Biotechnol Biochem 64:985–994. doi: 10.1271/bbb.64.985. [DOI] [PubMed] [Google Scholar]
- 16.Matsuzaki K, Fukui M, Fujii N, Miyajima K. 1991. Interactions of an antimicrobial peptide, tachyplesin I, with lipid membranes. Biochim Biophys Acta 1070:259–264. doi: 10.1016/0005-2736(91)90173-6. [DOI] [PubMed] [Google Scholar]
- 17.Ames BN. 1966. Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol 8:115–118. [Google Scholar]
- 18.Balakrishna R, Wood SJ, Nguyen TB, Miller KA, Suresh Kumar EV, Datta A, David SA. 2006. Structural correlates of antibacterial and membrane-permeabilizing activities in acylpolyamines. Antimicrob Agents Chemother 50:852–861. doi: 10.1128/AAC.50.3.852-861.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eriksson M, Nielsen PE, Good L. 2002. Cell permeabilization and uptake of antisense peptide-peptide nucleic acid (PNA) into Escherichia coli. J Biol Chem 277:7144–7147. doi: 10.1074/jbc.M106624200. [DOI] [PubMed] [Google Scholar]
- 20.Silvestro L, Weiser JN, Axelsen PH. 2000. Antibacterial and antimembrane activities of cecropin A in Escherichia coli. Antimicrob Agents Chemother 44:602–607. doi: 10.1128/AAC.44.3.602-607.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nüsslein K, Arnt L, Rennie J, Owens C, Tew GN. 2006. Broad-spectrum antibacterial activity by a novel abiogenic peptide mimic. Microbiology 152:1913–1918. doi: 10.1099/mic.0.28812-0. [DOI] [PubMed] [Google Scholar]
- 22.Wu M, Hancock RE. 1999. Interaction of the cyclic antimicrobial cationic peptide bactenecin with the outer and cytoplasmic membrane. J Biol Chem 274:29–35. doi: 10.1074/jbc.274.1.29. [DOI] [PubMed] [Google Scholar]
- 23.Maruyama M, Yamauchi S, Akiyama K, Sugahara T, Kishida T, Koba Y. 2007. Antibacterial activity of a virgatusin-related compound. Biosci Biotechnol Biochem 71:677–680. doi: 10.1271/bbb.60429. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi T, Itagaki H, Fukuda T, Tamura U, Sato Y, Suzuki Y. 1995. Hemoglobin denaturation caused by surfactants. Biol Pharm Bull 18:540–543. doi: 10.1248/bpb.18.540. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi T, Itagaki H, Fukuda T, Tamura U, Kato S. 1993. Quantitative evaluation for the prediction of eye irritation using hemoglobin. AATEX 2:25–31. [Google Scholar]
- 26.Epand RF, Pollard JE, Wright JO, Savage PB, Epand RM. 2010. Depolarization, bacterial membrane composition, and the antimicrobial action of ceragenins. Antimicrob Agents Chemother 54:3708–3713. doi: 10.1128/AAC.00380-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kilelee E, Pokorny A, Yeaman MR, Bayer AS. 2010. Lysyl-phosphatidylglycerol attenuates membrane perturbation rather than surface association of the cationic antimicrobial peptide 6W-RP-1 in a model membrane system: implications for daptomycin resistance. Antimicrob Agents Chemother 54:4476–4479. doi: 10.1128/AAC.00191-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mishra NN, Bayer AS, Tran TT, Shamoo Y, Mileykovskaya E, Dowhan W, Guan Z, Arias CA. 2012. Daptomycin resistance in enterococci is associated with distinct alterations of cell membrane phospholipid content. PLoS One 7:e43958. doi: 10.1371/journal.pone.0043958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koburger T, Hübner O, Braun M, Siebert J, Kramer A. 2010. Standardized comparison of antiseptic efficacy of triclosan, PVP-iodine, octenidine dihydrochloride, polyhexanide and chlorhexidine digluconate. J Antimicrob Chemother 65:1712–1719. doi: 10.1093/jac/dkq212. [DOI] [PubMed] [Google Scholar]
- 30.Haley CE, Marling-Cason M, Smith JW, Luby JP, Mackowiak PA. 1985. Bactericidal activity of antiseptics against methicillin-resistant Staphylococcus aureus. J Clin Microbiol 21:991–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawamura-Sato K, Wachino J, Kondo T, Ito H, Arakawa Y. 2008. Reduction of disinfectant bactericidal activities in clinically isolated Acinetobacter species in the presence of organic material. J Antimicrob Chemother 61:568–576. doi: 10.1093/jac/dkm498. [DOI] [PubMed] [Google Scholar]
- 32.Ikeda T, Ledwith A, Bamford CH, Hann RA. 1984. Interaction of a polymeric biguanide with phospholipid membranes. Biochim Biophys Acta 769:57–66. doi: 10.1016/0005-2736(84)90009-9. [DOI] [PubMed] [Google Scholar]
- 33.Ikeda T, Tazuke S, Watanabe M. 1983. Interaction of biologically active molecules with phospholipid membranes. I. Fluorescence depolarization studies on the effect of polymeric biocide bearing biguanide groups in the main chain. Biochim Biophys Acta 735:380–386. doi: 10.1016/0005-2736(83)90152-9. [DOI] [PubMed] [Google Scholar]
- 34.Sakagami Y, Mimura M, Kajimura K, Yokoyama H, Nishimura H. 1999. Electron-microscopic study of the bactericidal effect of OPB-2045, a new mono-biguanide disinfectant produced from biguanide group compounds, against Pseudomonas aeruginosa. J Pharm Pharmacol 51:201–206. doi: 10.1211/0022357991772141. [DOI] [PubMed] [Google Scholar]
- 35.Sakagami Y, Kajimura K, Nishimura H. 2000. Electron-microscopic study of the bactericidal effect of OPB-2045, a new disinfectant produced from biguanide group compounds, against methicillin-resistant Staphylococcus aureus. J Pharm Pharmacol 52:1547–1552. doi: 10.1211/0022357001777603. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.