Abstract
Release of lipopolysaccharide (LPS) (endotoxin) from bacteria into the bloodstream may cause serious unwanted stimulation of the host immune system. Some but not all antimicrobial peptides can neutralize LPS-stimulated proinflammatory responses. Salt resistance and serum stability of short antimicrobial peptides can be boosted by adding β-naphthylalanine to their termini. Herein, significant antiendotoxin effects were observed in vitro and in vivo with the β-naphthylalanine end-tagged variants of the short antimicrobial peptides S1 and KWWK.
TEXT
Lipopolysaccharide (LPS) (endotoxin) is the main constituent of the outer membrane of Gram-negative bacteria. The release of LPS from bacteria into the bloodstream during infection can cause serious unwanted stimulation of the host's immune system, leading to sepsis and septic shock in the patient (1, 2). Neutralizing LPS using anti-LPS or anti-tumor necrosis factor alpha (anti-TNF-α) antibodies has had only limited success (3).
Antimicrobial peptides (AMPs) have been found in all living things and deeply studied in prokaryotic (4) and eukaryotic cells (5, 6). AMPs were shown to play important roles in host innate defense (7, 8). Three models, the barrel-stave model, the toroidal pores model, and the carpet model, have been widely described for the mode of action of antimicrobial peptides (4, 7). AMPs can incorporate and disturb microbial membranes, and hence cause the death of them (9). Owing to this mechanism, AMPs may become promising therapeutics to overcome the problem of bacterial resistances (10). In addition, AMPs can act synergistically with current antibiotics to diminish bacterial resistance and reduce the amount of antibiotics (11, 12). Some AMPs have been shown to bind to LPS and neutralize LPS-stimulated proinflammatory responses (13–15). Related to this, different studies have shed light on AMPs with antiendotoxin properties (13, 14, 16–24). However, the rules governing the design of AMPs with antiendotoxin properties are still not very clear (13–15).
The potency of AMPs might be diminished under high-salt conditions (25–29). To overcome this drawback, we developed a strategy to increase the salt resistance of Trp- and His-rich AMPs by replacing their tryptophans or histidines with nonnatural bulky amino acid β-naphthylalanine (30). After which, we modified this strategy by adding β-naphthylalanine to termini of the short antimicrobial peptide S1 (Ac-KKWRKWLAKK-NH2) to boost its salt resistance and serum proteolytic stability (31). These studies allow us to hypothesize that β-naphthylalanine end-tagging may boost the antiendotoxin properties of short AMPs.
We designed and synthesized β-naphthylalanine end-tagged derivatives of S1 and KWWK. Antibacterial activities were determined by the standard broth microdilution method of the National Committee for Clinical Laboratory Standards. All MICs were measured in triplicate. All of the peptides studied demonstrated promising activities in the RPMI 1640 medium (LYM broth). However, the activities of S1 and KWWK-Nal were reduced or diminished under high-salt conditions. The MICs of S1-Nal-Nal, Nal-Nal-S1 (31), and KWWK-Nal-Nal were found to be effective (1.56 to ∼3.1 μg/ml) in LYM broth medium and high-salt conditions. Toxicities of the peptides were determined by measuring cell death using the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay against human fibroblast (HFW) cells (32). S1-Nal-Nal, Nal-Nal-S1, and KWWK-Nal-Nal all exhibit <10% or even no cell toxicity at 10 times their effective MICs (i.e., ∼30 μg/ml).
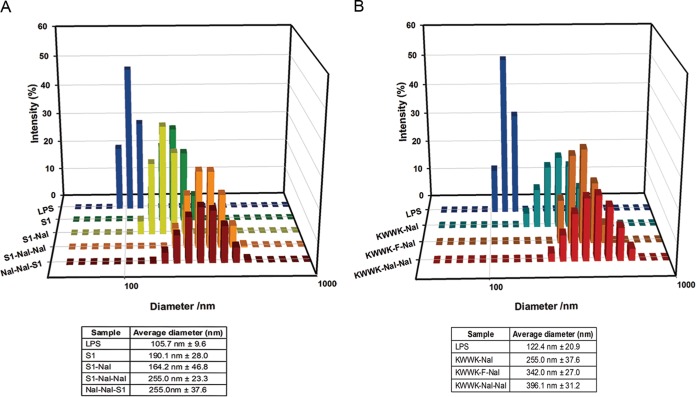
It has been reported that LPS aggregation promoted by polymyxin B and rBPI21 might inhibit the interaction of LPS with its cell receptors and, hence, block cytokine production (17, 33). Dynamic light scattering (DLS) was used to measure the size increase of LPS in the presence of S1 and its derivatives (17, 33). LPS and peptides were diluted to 25 μM in 20 mM sodium phosphate buffer with 150 mM NaCl at pH 7.4. The particle size of LPS in the absence or presence of peptides was detected on a Malvern Zetasizer Nano ZS (Malvern Instruments, United Kingdom). Figure 1A shows that S1 and its derivatives promoted LPS aggregation and increased its size (in the order S1-Nal-Nal = Nal-Nal-S1 > S1 > S1-Nal). Similarly, the KWWK derivatives can induce the aggregation of LPS (in the order KWWK-Nal-Nal > KWWK-F-Nal > KWWK-Nal) (Fig. 1B).
FIG 1.
Size distribution of LPS aggregates in the presence of different antimicrobial peptides. (A) S1 and its derivatives; (B) KWWK derivatives.
The ability of LPS neutralization in vitro was measured by a limulus amebocyte lysate (LAL) assay (Cape Cod Inc., USA) (19, 23). Peptides at concentrations of 12.5, 25, 50, 100, or 200 μg/ml were incubated with 5 endotoxin units (EU) of LPS in 96-well plates at 37°C. Fifty microliters of Pyrochrome reagent was added to each well, and the absorbance was measured at 405 nm by a microplate reader (PerkinElmer, USA). S1 had no LPS-neutralizing activity and S1-Nal had only a small effect. S1-Nal-Nal and Nal-Nal-S1 demonstrated dose-dependent LPS-neutralizing activities (Fig. 2). While KWWK-Nal-Nal demonstrated substantial LPS-neutralizing activity, KWWK-F-Nal and KWWK-Nal demonstrated very small activities (Fig. 2).
FIG 2.
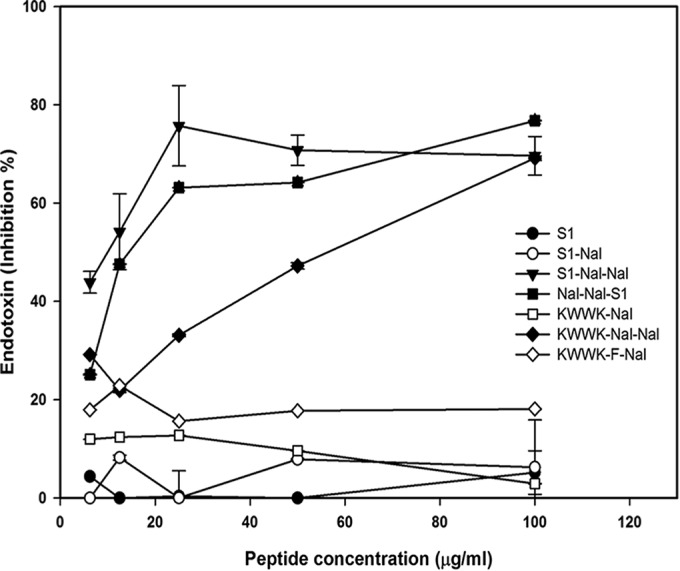
LPS-neutralizing activities determined by limulus amebocyte lysate (LAL) assay.
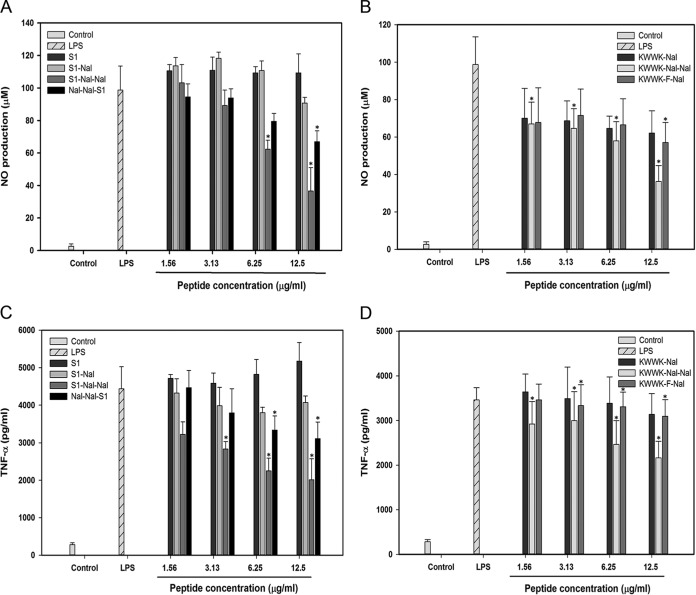
LPS can induce nitrite oxide production in macrophage cells (34). Nitrite oxide production can be determined by the Griess method to detect nitrite (NO2−) accumulation in a cultured medium (34). Murine macrophage J774A.1 cells were seeded at a density of 2 × 105 cells in 24-well plates. Cells were stimulated with 1 μg/ml LPS in the presence or absence of peptides for 24 h. After incubation, 50 μl supernatant was collected and mixed with an equal volume of Griess reagent and incubated at room temperature for 10 min. The level of nitrite oxide production was determined by measuring the absorbance at 540 nm on a microplate reader. S1 and its derivatives inhibited nitrite oxide production by LPS in a dose-dependent manner (in the order S1-Nal-Nal > Nal-Nal-S1 > S1-Nal > S1) (Fig. 3A). For the derivatives of KWWK, the inhibitory effect is KWWK-Nal-Nal > KWWK-F-Nal > KWWK-Nal (Fig. 3B).
FIG 3.
Inhibition of LPS-induced nitric oxide (NO) production by S1 and its derivatives (A) and KWWK derivatives (B) in murine macrophage J774A.1 cells. Inhibition of LPS-induced TNF-α production by S1 and its derivatives (C) and KWWK derivatives (D) in murine macrophage J774A.1 cells. Results are presented as means ± standard deviations (SD); n = 3. *, P < 0.05 versus LPS.
TNF-α plays an important role in septic shock and is generally used as an indicator for this disease. Similar to the LPS-induced nitrite oxide production in murine macrophage J774A.1 cells, the release of TNF-α in cultured medium was quantified using mouse enzyme-linked immunosorbent assay (eBioscience) (34). S1 and its derivatives inhibited TNF-α release (in the order S1-Nal-Nal > Nal-Nal-S1 > S1-Nal > S1) (Fig. 3C). For the derivatives of KWWK, the inhibitory effect is KWWK-Nal-Nal > KWWK-F-Nal > KWWK-Nal (Fig. 3D).
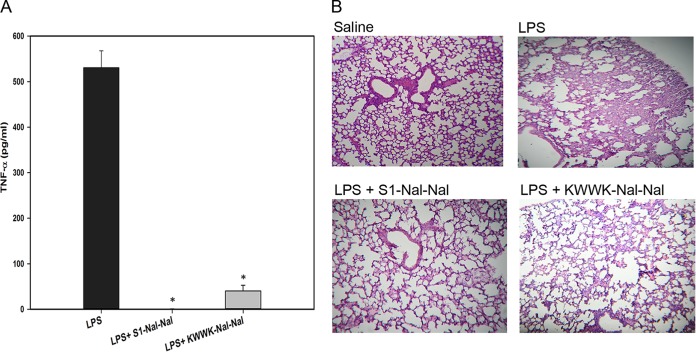
For the experimental mouse endotoxemia model, C57BL/6 mice were purchased from National Laboratory Animal Center (Taiwan). The weight of each mouse was approximately 20 g at the start of the experiments. Mice were divided into two groups (5 in each group with intraperitoneal [i.p.] injection of 18 mg/kg of body weight Escherichia coli O26:B6 LPS alone or 18 mg/kg LPS plus 10 mg/kg peptides). Blood was collected via tail vein 1.5 h after injection. Whole blood was centrifuged at 3,000 rpm at 4°C for 10 min, and supernatant was collected and measured by mouse TNF-α enzyme-linked immunosorbent assay (ELISA) kits (eBioscience). Serum TNF-α production increased dramatically in mice challenged with LPS (Fig. 4A). Significantly lower levels of TNF-α were observed in mice treated with S1-Nal-Nal or KWWK-Nal-Nal (Fig. 4A). Whereas LPS-challenged mice developed acute lung injury, pathological evaluation of the lung tissues confirmed the LPS-neutralizing effect on mice treated with S1-Nal-Nal and KWWK-Nal-Nal (Fig. 4B). The advantages of this endotoxemic mouse model are that LPS is a relatively pure compound that can be reliably measured, and its use can easily be standardized to demonstrate the efficacy of the experimental antiendotoxin agents (35). However, studies of some antisepsis agents that showed promise in the endotoxemia model failed in human clinical trials (36, 37). These failures have been attributed to the endotoxemia model alone being unable to accurately represent many important aspects of human sepsis. Thus, additional animal models such as the surgical implantation models, the cecal ligation and puncture (CLP) models, and the colon ascendens stent peritonitis (CASP) models may be needed to demonstrate the clinical relevance of sepsis (35).
FIG 4.
(A) Suppression of LPS-stimulated TNF-α release in endotoxemic mice (C57BL/6) by S1-Nal-Nal and KWWK-Nal-Nal. (B) Lung specimens, collected from sacrificed mice 24 h after injection, demonstrating the protective activities of S1-Nal-Nal and KWWK-Nal-Nal on LPS-stimulated endotoxemic mice. Results are presented as means ± standard deviations (SD); n = 3. *, P < 0.05 versus LPS.
Hydrophobicity has been known as a key factor in the development of effective LPS-neutralizing AMPs (15, 16, 38). Studies of NK-2 and N-acylated lactoferricin-derived LF11 indicated that neutralization of the LPS surface charges is a requirement but is not sufficient for LPS detoxification (15). A hydrophobic interaction would significantly increase LPS neutralization for AMPs (15). Here, we describe a strategy to boost the antiendotoxin activities of short antimicrobial peptides by adding the bulky, nonnatural, hydrophobic β-naphthylalanine residues to the termini of short antimicrobial peptides. Based on LPS aggregation, LPS neutralization in vitro, inhibition of LPS-induced nitrite oxide and TNF-α production in murine macrophage cells, and suppression of TNF-α release in an endotoxemic mouse model, it has been demonstrated that the β-naphthylalanine end-tagged S1-Nal-Nal, Nal-Nal-S1, and the ultrashort peptide KWWK-Nal-Nal all possess high antiendotoxin activities. Tests of these modified peptides in septic animal models are ongoing in our laboratory.
ACKNOWLEDGMENTS
This work is supported by grants from the Minister of Science and Technology, Taiwan. B.-S.Y. is supported by research grants from the National Taiwan University Hospital.
We thank Daniel Cheng for revising the manuscript.
REFERENCES
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. 2001. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Rietschel ET, Brade H, Holst O, Brade L, Muller-Loennies S, Mamat U, Zahringer U, Beckmann F, Seydel U, Brandenburg K, Ulmer AJ, Mattern T, Heine H, Schletter J, Loppnow H, Schonbeck U, Flad HD, Hauschildt S, Schade UF, Di Padova F, Kusumoto S, Schumann RR. 1996. Bacterial endotoxin: chemical constitution, biological recognition, host response, and immunological detoxification. Curr Top Microbiol Immunol 216:39–81. [DOI] [PubMed] [Google Scholar]
- 3.Fisher CJ Jr, Opal SM, Dhainaut JF, Stephens S, Zimmerman JL, Nightingale P, Harris SJ, Schein RM, Panacek EA, Vincent JL, Foulke GE, Warren EL, Garrard C, Park G, Bodmer MW, Cohen J, Van Der Linden C, Cross AS, Sadoff JC. 1993. Influence of an anti-tumor necrosis factor monoclonal antibody on cytokine levels in patients with sepsis. Crit Care Med 21:318–327. doi: 10.1097/00003246-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Drider D, Rebuffat S. 2011. Prokaryotic antimicrobial peptides. Springer, New York, NY. [Google Scholar]
- 5.Rishi P, Singh AP, Arora S, Garg N, Kaur IP. 2014. Revisiting eukaryotic anti-infective biotherapeutics. Crit Rev Microbiol 40:281–292. doi: 10.3109/1040841X.2012.749210. [DOI] [PubMed] [Google Scholar]
- 6.Silva PM, Goncalves S, Santos NC. 2014. Defensins: antifungal lessons from eukaryotes. Front Microbiol 5:97. doi: 10.3389/fmicb.2014.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andreu D, Rivas L. 1998. Animal antimicrobial peptides: an overview. Biopolymers 47:415–433. doi:. [DOI] [PubMed] [Google Scholar]
- 8.Tossi A, Sandri L, Giangaspero A. 2000. Amphipathic, alpha-helical antimicrobial peptides. Biopolymers 55:4–30. doi:. [DOI] [PubMed] [Google Scholar]
- 9.Zasloff M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 10.Hancock REW, Sahl HG. 2006. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol 24:1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- 11.Draper LA, Cotter PD, Hill C, Ross RP. 2013. The two peptide lantibiotic lacticin 3147 acts synergistically with polymyxin to inhibit Gram negative bacteria. BMC Microbiol 13:212. doi: 10.1186/1471-2180-13-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naghmouchi K, Baah J, Hober D, Jouy E, Rubrecht C, Sane F, Drider D. 2013. Synergistic effect between colistin and bacteriocins in controlling Gram-negative pathogens and their potential to reduce antibiotic toxicity in mammalian epithelial cells. Antimicrob Agents Chemother 57:2719–2725. doi: 10.1128/AAC.02328-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhattacharjya S. 2010. De novo designed lipopolysaccharide binding peptides: structure based development of antiendotoxic and antimicrobial drugs. Curr Med Chem 17:3080–3093. doi: 10.2174/092986710791959756. [DOI] [PubMed] [Google Scholar]
- 14.Pulido D, Nogues MV, Boix E, Torrent M. 2012. Lipopolysaccharide neutralization by antimicrobial peptides: a gambit in the innate host defense strategy. J Innate Immun 4:327–336. doi: 10.1159/000336713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenfeld Y, Shai Y. 2006. Lipopolysaccharide (endotoxin)-host defense antibacterial peptides interactions: role in bacterial resistance and prevention of sepsis. Biochim Biophys Acta 1758:1513–1522. doi: 10.1016/j.bbamem.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 16.Andra J, Lohner K, Blondelle S, Jerala R, Moriyon I, Koch MHJ, Garidel P, Brandenburg K. 2005. Enhancement of endotoxin neutralization by coupling of a C12-alkyl chain to a lactoferricin-derived peptide. Biochem J 385:135–143. doi: 10.1042/BJ20041270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Domingues MM, Castanho MA, Santos NC. 2009. rBPI(21) promotes lipopolysaccharide aggregation and exerts its antimicrobial effects by (hemi)fusion of PG-containing membranes. PLoS One 4:e8385. doi: 10.1371/journal.pone.0008385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohanram H, Bhattacharjya S. 2014. Resurrecting inactive antimicrobial peptides from the lipopolysaccharide trap. Antimicrob Agents Chemother 58:1987–1996. doi: 10.1128/AAC.02321-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ren J, Gao H, Tang M, Gu J, Xia P, Xiao G. 2010. Lipopolysaccharide (LPS) detoxification of analogue peptides derived from limulus anti-LPS factor. Peptides 31:1853–1859. doi: 10.1016/j.peptides.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Rosenfeld Y, Sahl HG, Shai Y. 2008. Parameters involved in antimicrobial and endotoxin detoxification activities of antimicrobial peptides. Biochemistry 47:6468–6478. doi: 10.1021/bi800450f. [DOI] [PubMed] [Google Scholar]
- 21.Singh S, Kasetty G, Schmidtchen A, Malmsten M. 2012. Membrane and lipopolysaccharide interactions of C-terminal peptides from S1 peptidases. Biochim Biophys Acta 1818:2244–2251. doi: 10.1016/j.bbamem.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 22.Singh S, Papareddy P, Kalle M, Schmidtchen A, Malmsten M. 2013. Importance of lipopolysaccharide aggregate disruption from the anti-endotoxic effects of heparin cofactor II peptides. Biochim Biophys Acta 1828:2709–2719. doi: 10.1016/j.bbamem.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 23.Srivastava S, Ghosh JK. 2013. Introduction of a lysine residue promotes aggregation of temporin L in lipopolysaccharides and augmentation of its antiendotoxin property. Antimicrob Agents Chemother 57:2457–2466. doi: 10.1128/AAC.00169-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao Y, Herrera AI, Bommineni YR, Soulages JL, Prakash O, Zhang G. 2009. The central kink region of fowlicidin-2, an alpha-helical host defense peptide, is critically involved in bacterial killing and endotoxin neutralization. J Innate Immun 1:268–280. doi: 10.1159/000174822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldman MJ, Anderson GM, Stolzenberg ED, Kari UP, Zasloff M, Wilson JM. 1997. Human b-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell 88:553–560. [DOI] [PubMed] [Google Scholar]
- 26.Lee IH, Cho Y, Lehrer RI. 1997. Effects of pH and salinity on the antimicrobial properties of clavanins. Infect Immun 65:2898–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothstein DM, Spacciapoli P, Tran LT, Xu T, Roberts FD, Dalla Serra M, Buxton DK, Oppenheim FG, Friden P. 2001. Anticandida activity is retained in P-113, a 12-amino-acid fragment of histatin 5. Antimicrob Agents Chemother 45:1367–1373. doi: 10.1128/AAC.45.5.1367-1373.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang CW, Yip BS, Cheng HT, Wang AH, Chen HL, Cheng JW, Lo HJ. 2009. Increased potency of a novel D-beta-naphthylalanine-substituted antimicrobial peptide against fluconazole-resistant fungal pathogens. FEMS Yeast Res 9:967–970. doi: 10.1111/j.1567-1364.2009.00531.x. [DOI] [PubMed] [Google Scholar]
- 29.Wu M, Maier E, Benz R, Hancock REW. 1999. Mechanism of interaction of different classes of cationic antimicrobial peptides with planar bilayers and with the cytoplasmic membrane of Escherichia coli. Biochem 38:7235–7242. doi: 10.1021/bi9826299. [DOI] [PubMed] [Google Scholar]
- 30.Yu HY, Tu CH, Yip BS, Chen HL, Cheng HT, Huang KC, Lo HJ, Cheng JW. 2011. Easy strategy to increase salt resistance of antimicrobial peptides. Antimicrob Agents Chemother 55:4918–4921. doi: 10.1128/AAC.00202-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chu HL, Yu HY, Yip BS, Chih YH, Liang CW, Cheng HT, Cheng JW. 2013. Boosting salt resistance of short antimicrobial peptides. Antimicrob Agents Chemother 57:4050–4052. doi: 10.1128/AAC.00252-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei SY, Wu JM, Kuo YY, Chen HL, Yip BS, Tzeng SR, Cheng JW. 2006. Solution structure of a novel tryptophan-rich peptide with bidirectional antimicrobial activity. J Bacteriol 188:328–334. doi: 10.1128/JB.188.1.328-334.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Domingues MM, Inacio RG, Raimundo JM, Martins M, Castanho MA, Santos NC. 2012. Biophysical characterization of polymyxin b interaction with LPS aggregates and membrane model systems. Biopolymers 98:338–344. doi: 10.1002/bip.22095. [DOI] [PubMed] [Google Scholar]
- 34.Kim JA, Ahn BN, Kong CS, Kim SK. 2011. Anti-inflammatory action of sulfated glucosamine on cytokine regulation in LPS-activated PMA-differentiated THP-1 macrophages. Inflamm Res 60:1131–1138. doi: 10.1007/s00011-011-0377-7. [DOI] [PubMed] [Google Scholar]
- 35.Nemzek JA, Hugunin KMS, Opp MR. 2008. Modeling sepsis in the laboratory: merging sound science with animal well-being. Comp Med 58:120–128. [PMC free article] [PubMed] [Google Scholar]
- 36.Buras JA, Holzmann B, Sitkovsky M. 2005. Animal models of sepsis: setting the stage. Nat Rev Drug Discov 4:854–865. doi: 10.1038/nrd1854. [DOI] [PubMed] [Google Scholar]
- 37.Deitch EA. 1998. Animal models of sepsis and shock: a review and lessons learned. Shock 9:1–11. [DOI] [PubMed] [Google Scholar]
- 38.Andra J, Koch MHJ, Bartels R, Brandenburg K. 2004. Biophysical characterization of endotoxin inactivation by NK-2, an antimicrobial peptide derived from mammalian NK-lysin. Antimicrob Agents Chemother 48:1593–1599. doi: 10.1128/AAC.48.5.1593-1599.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]