Abstract
Studies on amphotericin B (AmB) nephrotoxicity use diverse definitions of acute kidney injury (AKI). Here, we used the new Kidney Disease Improving Global Outcome (KDIGO) system to describe the incidence, predictors, and impact of AmB-induced AKI on hospital mortality in 162 patients treated with AmB (120 with deoxycholate preparation and 42 with liposomal preparation). KDIGO stage 1 requires an absolute increase of ≥0.3 mg/dl or ≥1.5× over baseline serum creatinine (SCr), while stage 2 requires ≥2×, and stage 3 requires ≥3×. A binary KDIGO definition (KDIGObin) corresponds to stage ≥1. For comparison, we included two definitions of AKI traditionally utilized in nephrotoxicity studies: ≥0.5 mg/dl (NT0.5) and ≥2× (NT2×) increase in baseline SCr. The overall incidence of AmB-induced AKI by KDIGObin was 58.6% (stage 1, 30.9%; stage 2, 18.5%; stage 3, 9.3%). Predictors of AKI by KDIGObin were older age and use of furosemide and angiotensin-converting enzyme inhibitor (ACE-I). Traditional criteria detected lower incidences of AKI, at 45.1% (NT0.5) and 27.8% (NT2×). Predictors of AKI by traditional criteria were older age and use of vancomycin (NT0.5) and use of vancomycin and vasopressors (NT2×). KDIGObin detected AKI 2 days earlier than the most sensitive traditional criterion. However, only traditional criteria were associated with intensive care unit (ICU) admission, mechanical ventilation, and mortality. In conclusion, the increase in sensitivity of KDIGObin is accompanied by a loss of specificity and ability to predict outcomes. Prospective studies are required to weigh the potential gain from early AKI detection against the potential loss from undue changes in management in patients with subtle elevations in SCr.
INTRODUCTION
Amphotericin B (AmB) is a powerful antifungal and antiparasitic agent that binds to the ergosterol component of microbial membranes, creating pores that result in cation leakage and cell death (1). AmB is the drug of choice for the treatment of severe forms of leishmaniasis and remains a lifesaving option for certain invasive fungal infections. Nevertheless, its use is limited by toxicity, including acute kidney injury (AKI).
AmB administration leads to direct renal vasoconstriction and causes a profound reduction in renal blood flow (2–4). In addition, AmB alters renal tubular cell membrane permeability (5, 6), allowing back diffusion of hydrogen ions and thereby impairing acid excretion. Recent data suggest that sodium entry through membrane pores activates mitogen-activated protein (MAP) kinases and increases intracellular calcium concentration, culminating in renal tubular cell injury (7). Therefore, AmB-induced AKI appears to result from a combination of ischemic and toxic insults (8). Phenotypically, AmB-induced AKI manifests itself through elevated serum creatinine (SCr) levels that may be accompanied by renal tubular acidosis, characterized by hyperchloremic metabolic acidosis, hypokalemia, and hypomagnesemia.
Mistro and coworkers (9) systematically reviewed the literature on AmB-induced AKI and assessed whether drug delivery in a locally prepared lipid emulsion or in liposomes reduced nephrotoxicity (9). In their meta-analysis, the authors summarized nine clinical trials comparing AmB in 5% dextrose with AmB in lipid emulsion and found an overall incidence of nephrotoxicity in 30.6% versus 12.2% of cases, respectively. They also summarized five clinical trials comparing AmB in 5% dextrose with liposomal AmB; the incidences of nephrotoxicity were 32.5% versus 14.5%, respectively. Nevertheless, a closer look at individual clinical trials included in the meta-analysis uncovers several issues. Most studies were small (only four had >100 patients), the populations were quite different, and the incidences of AmB-induced AKI varied widely, ranging from 1.3% (10) to 100% (11) for AmB in 5% dextrose, 1.2% (10) to 33.3% (12) for AmB in lipid emulsion, and 0% (13) and 18.7% for liposomal AmB (14). Most importantly, the definition of nephrotoxicity across trials was inconsistent. Several different nephrotoxicity criteria were used, but the most common were a ≥0.5-mg/dl increase in baseline SCr or ≥50% decrease in estimated glomerular filtration rate (NT0.5 criterion) (15) and a doubling of baseline SCr (NT2× criterion) (13, 14, 16, 17). Since the trials included in the meta-analysis by Mistro et al. (9) were published >13 years ago (all between 1992 and 2002), none of them used currently accepted consensus definitions of AKI (18–20).
The first consensus criterion for AKI was published in 2004 and called RIFLE (risk, injury, failure, loss, and end stage) (18). In 2007, the RIFLE criterion was modified to create the Acute Kidney Injury Network (AKIN) criteria (19). Finally, in 2012, characteristics of the RIFLE and AKIN criteria were merged to create the Kidney Disease Improving Global Outcome (KDIGO) criteria (20). Minejima et al. (21) demonstrated that the AKIN criteria facilitated the early detection of vancomycin-induced nephrotoxicity compared to that with the traditional NT0.5 criterion. To our knowledge, there are no studies comparing the performance of newer versus traditional diagnostic criteria on AmB-induced AKI.
The main objective of this study was to identify the incidence and predictors of AmB-induced AKI according to currently accepted staging systems. In addition, we aimed to describe the dynamics of AmB-induced AKI and assess its impact on hospital length of stay (LOS) and mortality.
MATERIALS AND METHODS
Site.
The study was conducted at Hospital Universitário Professor Edgard Santos, a tertiary care facility affiliated with the Medical School of the Federal University of Bahia, located in Salvador, Bahia, Brazil.
Design and population.
This was a retrospective cohort study of inpatients treated with AmB between 2006 and 2012. The year 2006 was chosen because it was the first year after the introduction of electronic medical records (EMRs) for laboratory data at our institution (August 2005); the year 2012 was the date of the initial draft of this project. According to our inpatient pharmacy, there were 734 treatments with AmB during that period of time. Exclusion criteria were retreatment (in these cases, only data from the first treatment were analyzed), use of fewer than three doses of AmB, age <18 years old, AKI or hemodialysis at the time of AmB initiation, intermittent use of AmB, such as weekly use in “day hospital” setting, use of nonintravenous forms of AmB, and missing critical data.
Variables collected.
We reviewed paper charts and electronic medical records for demographic variables, patient location (floor versus intensive care unit [ICU]), reason for AmB use, type of AmB used, AmB regimen, total AmB dose, renal protection strategy used, comorbidities (Charlson comorbidity index), concomitant nephrotoxic medications, use and dose of vasoactive drugs, serial laboratory data (blood urea nitrogen [BUN], SCr, potassium, magnesium, and bicarbonate levels), days on a ventilator (if applicable), days in the ICU (if applicable), days in the hospital, and in-hospital mortality.
Definitions.
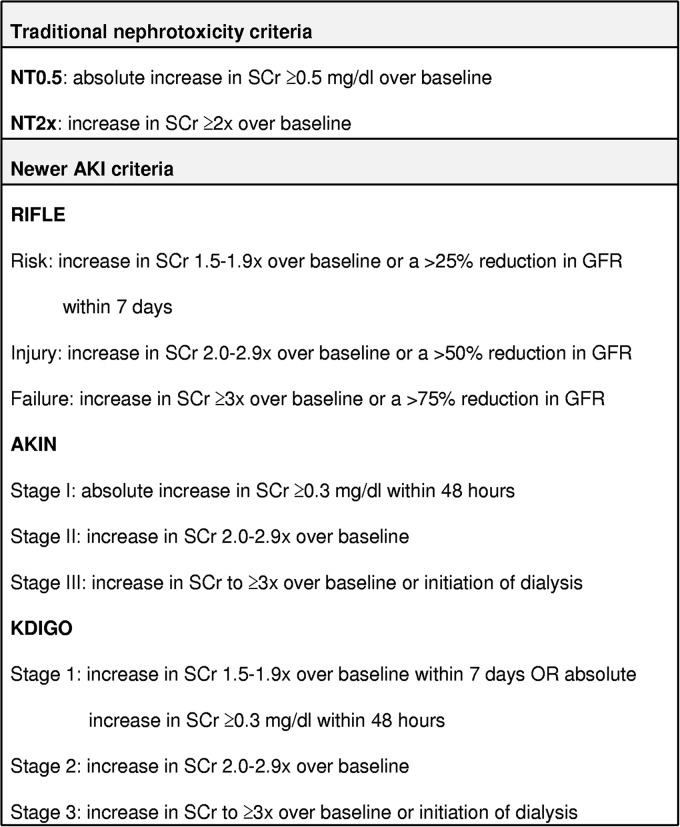
AKI was defined and staged according to RIFLE (18), AKIN (19), and KDIGO (20) criteria. For comparison, we also defined AKI using two traditional nephrotoxicity (NT) criteria (21). Since we did not have access to urinary output data, AKI diagnoses were based solely on the magnitude of the increases in SCr levels (Fig. 1).
FIG 1.
Summary of SCr-based AKI definitions. SCr, serum creatinine; AKI, acute kidney injury; NT, nephrotoxicity; RIFLE, risk, injury, failure, loss, and end stage; AKIN, Acute Kidney Injury Network; KDIGO, Kidney Diseases Improving Global Outcomes.
Due to the 48-h requirement, which practically entails having daily SCr measurements (or at least every other day), the AKIN criteria could not be applied to some patients. Since the newest KDIGO system combines characteristics of the RIFLE and AKIN criteria, we chose this system to stratify AKI in stages. In addition, we used binary definitions for RIFLE, AKIN, and KDIGO (RIFLEbin, AKINbin, and KDIGObin, respectively). In these binary definitions, any patient that fulfilled criteria for the initial stage (which in practice means a stage R or greater for RIFLE, stage I or greater for AKIN, or stage ≥1 for KDIGO) was classified as having AKI.
The baseline SCr level was defined as the value obtained on the morning of day zero, which was the day of the first AmB dose. If an SCr value was not available for that day, we used the value nearest to day zero or the admission SCr level.
Hypocalcemia and hypomagnesemia were diagnosed when at least one of three conditions was satisfied: (i) the presence of documented K+ or Mg2+ levels below the laboratory's limit of normal during AmB treatment, (ii) a history of replacement of these cations, or (iii) when these electrolyte disorders were listed as problems in the patient's chart.
Ethical approval.
The study was approved by the institutional review board of Hospital Universitário Professor Edgard Santos on 11 March 2013 (protocol no. 11123413.1.0000.0049). Given the retrospective nature of the study, we were granted a waiver of informed consent.
Statistical analyses.
The shape of the distribution of continuous data was analyzed using histograms and normality tests (Shapiro-Wilk). Normal-shaped data were summarized using means and standard deviations, and comparisons among groups were made with the Student t test; non-Gaussian data were summarized using median and interquartile range (IQR), and comparisons among groups were made with the Mann-Whitney U test. A comparison of hospital LOS across four groups (patients without AKI and AKI stages 1, 2, and 3 by KDIGO) was made using the Kruskal-Wallis test. Categorical data were summarized using absolute and relative frequencies, and comparisons among groups were made with the chi-square or Fisher's exact test, where appropriate. Time to AKI was analyzed in one of two different ways: (i) by summarizing the mean and median time to AKI only in those who developed AKI or (ii) by the Kaplan-Meier method. For those patients whose lengths of follow-up was >1 month, the survival time was censored at 30 days. The Kaplan-Meier data were summarized in one minus cumulative survival graphs and survival curves compared using the log rank test. Univariate logistic and Cox regression analyses were conducted to identify potential predictors of AKI. Variables with a P value of <0.20 on univariate analyses were included in multivariate, backward, logistic, and Cox regression models to identify independent predictors of AKI. A similar strategy was utilized to identify independent predictors of death. However, since AKI was our main independent variable in the time to mortality analyses, it was allocated to the second block of the regression and forced into the final model. A P value of <0.05 on final analyses was considered statistically significant. Multivariate logistic regression models were evaluated using the Hosmer-Lemeshow goodness-of-fit test, Nagelkerke's R2, and percent correct classification. The proportional hazards assumption was assessed by graphical methods (Kaplan-Meier curves and log-log plots). Statistical analyses were conducted using the software packages Stata version 12.1 and IBM SPSS Statistics version 20.0.
Sample size.
A priori sample size calculation was conducted using OpenEpi. Assuming an overall nephrotoxicity rate of 30% and a precision of 5%, a sample size of 122 would provide a confidence level of approximately 80%, a sample size of 186 patients would provide a confidence level of 90%, and a sample size of 245 patients would provide a confidence level of 95%.
RESULTS
Study population.
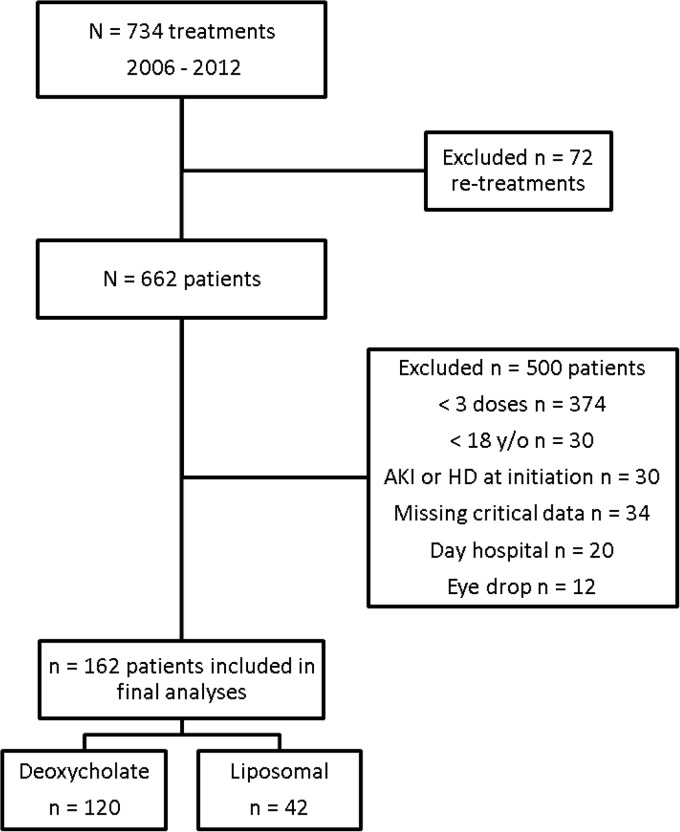
Out of 734 treatments with AmB that occurred at our institution between 2006 and 2012, 72 were retreatments and were excluded (Fig. 2). Out of the 662 patients that used AmB, 500 were excluded. Our final sample was composed of a cohort of 162 patients treated with AmB, with 120 with the deoxycholate preparation and 42 with the liposomal preparation. The median LOS in the hospital for the cohort was 32 days (IQR, 23 to 51 days).
FIG 2.
Flow chart demonstrating the process of selection of the sample of 162 patients treated with amphotericin B at our institution between 2006 and 2012. AKI, acute kidney injury; HD, hemodialysis.
Table 1 shows the demographic, clinical, and laboratory characteristics of the final sample. Patients were young, with a median age of 36 years, and there was a slight preponderance of males. The main indications for AmB treatment were suspected or confirmed fungal infection (40.8%), leishmaniasis (27.8%), and fever of unknown origin (22.2%). Only 23.5% had a Charlson comorbidity index of ≥4; approximately 1/3 needed to be treated at the intensive care unit at some point during the admission. The median baseline laboratory values were within normal limits.
TABLE 1.
Demographic, clinical, and laboratory characteristics of 162 hospitalized adults treated with intravenous AmB at a university hospitala
| Baseline variables | All (n = 162) | Type of AmB preparation |
P value | |
|---|---|---|---|---|
| Deoxycholate (n = 120) | Liposomal (n = 42) | |||
| Age (n = 161) (median [IQR]) (yr) | 35 (25–51) | 36 (24–51) | 33 (28–50) | 0.903 |
| Age quartiles (no. [%]) (yr) | ||||
| ≤25 | 43 (26.7) | 35 (29.4) | 8 (19.0) | 0.122 |
| 26–35 | 40 (24.8) | 24 (20.2) | 16 (38.1) | |
| 36–50 | 38 (23.6) | 30 (25.2) | 8 (19.0) | |
| >50 | 40 (24.8) | 30 (25.2) | 10 (23.8) | |
| Gender (no. [%]) | 0.187 | |||
| Male | 92 (56.8) | 64 (53.3) | 28 (66.7) | |
| Female | 70 (43.2) | 56 (46.7) | 14 (33.3) | |
| Residence (n = 160) (no. [%]) | 0.002 | |||
| Rural areas | 95 (59.4) | 61 (51.7) | 34 (81.0) | |
| Capital | 65 (40.6) | 57 (48.3) | 8 (19.0) | |
| AmB indication (no. [%]) | 0.000 | |||
| Teg. leishmaniasis | 33 (20.4) | 11 (9.2) | 22 (52.4) | |
| Visc. leishmaniasis | 12 (7.4) | 5 (4.2) | 7 (16.7) | |
| Suspected IFE | 26 (16.0) | 22 (18.3) | 4 (9.5) | |
| Confirmed IFE | 40 (24.8) | 34 (28.3) | 6 (14.3) | |
| FUO | 36 (22.2) | 34 (28.3) | 2 (4.8) | |
| Other | 15 (9.3) | 14 (11.7) | 1 (2.4) | |
| Charlson index (no. [%]) | 0.006 | |||
| 0–1 | 65 (40.1) | 40 (33.3) | 25 (59.5) | |
| 2–3 | 59 (36.4) | 51 (42.5) | 8 (19.0) | |
| ≥4 | 38 (23.5) | 29 (24.2) | 9 (21.4) | |
| Disease severity (no. [%])c | ||||
| Intensive care unit | 53 (32.7) | 48 (40.0) | 5 (11.9) | 0.002 |
| Vasopressor drugs | 35 (21.6) | 30 (25.0) | 5 (11.9) | 0.119 |
| Mechanical ventilation | 42 (26.1) | 38 (31.7) | 5 (11.9) | 0.022 |
| Serum lab values (median [IQR]) | ||||
| Cr (n = 162) (mg/dl) | 0.8 (0.7–1.1) | 0.8 (0.6–1.1) | 0.9 (0.8–1.1) | 0.350 |
| BUN (n = 103) (mg/dl) | 12.1 (7.9–18.2) | 12.6 (7.9–22.1) | 11.7 (10.3–14.5) | 0.088 |
| K+ (n = 98) (meq/liter) | 4.0 (3.6–4.4) | 3.9 (3.6–4.3) | 4.0 (3.6–4.5) | 0.906 |
| Mg2+ (n = 73) (mg/dl) | 1.8 (1.6–2.0) | 1.8 (1.6–2.0) | 1.8 (1.6–2.2) | 0.614 |
| HCO3− (n = 28) (meq/liter) | 21.6 (16.7–26.3) | 22.2 (17.7–26.2) | 18.7 (16.1–32.7) | 0.779 |
| Lactate (n = 23) (mmol/liter) | 1.6 (1.2–2.0) | 1.5 (1.2–2.0) | 2.0 (1.1–2.2) | 0.822 |
AmB amphotericin B.
Teg., tegumentary; Visc., visceral; IFE, invasive fungal infection; FUO, fever of unknown origin; BUN, blood urea nitrogen.
Intensive care unit, vasopressor drugs or mechanical ventilation reflect the need for these types of care at any point during the admission.
Compared to the deoxycholate group, patients in the liposomal group were more often from rural areas, had a higher frequency of leishmaniasis, a lower Charlson index, and needed less intensive care unit or mechanical ventilation (Table 1).
Treatment details.
The majority (83.3%) of patients initiated AmB at the ward. The deoxycholate preparation was used by 74.1% of patients; the median initial dose, maximum daily dose, and total cumulative dose were 50 mg/day (0.8 mg/kg of body weight/day), 50 mg/day (0.9 mg/kg/day), and 445 mg (7.7 mg/kg), respectively. The liposomal preparation was used by 25.9% of patients; the median initial dose, maximum daily dose, and total cumulative dose were 150 mg/day (2.2 mg/kg/day), 150 mg/day (2.6 mg/kg/day), and 1,450 mg (22.1 mg/kg), respectively. Overall, the median duration of AmB treatment was 10 days (10 versus 11 days for the deoxycholate and liposomal groups, respectively; P = 0.162). The median dose of normal saline used at the beginning of AmB treatment was 1,500 ml/day (1,500 versus 1,000 ml/day for the deoxycholate and liposomal groups, respectively; P = 0.968). The nephrotoxic drug that was most commonly used in combination with AmB was vancomycin (65/162 [40.1% of cases]); vancomycin use was much more common in the deoxycholate (59/120 [49.2%]) than in the liposomal group (6/42 [14.3%]) (P < 0.001).
Incidence of AmB-induced AKI.
The overall incidence of AmB-induced AKI by traditional criteria was 27.8% (45/162) for NT2× and 45.1% (73/162) for NT0.5 (Table 2). The RIFLE criteria diagnosed 10 additional cases of AKI, for an incidence of 51.2% (83/162). The KDIGO criterion was the most sensitive, detecting 22 cases that were missed by the NT0.5 criterion, for an AKI incidence of 58.6% (95/162).
TABLE 2.
Influence of the diagnostic criteria on the incidence of AKI during intravenous AmB use in 162 hospitalized adults treated at a university hospital, stratified by type of AmB preparation
| AKI criteria | No./total no. (%) (n = 162) | No./total no. (%) with AmB preparation type: |
P value | |
|---|---|---|---|---|
| Deoxycholate (n = 120) | Liposomal (n = 42) | |||
| Binarya | ||||
| NT2× | 45/162 (27.8) | 38/120 (31.7) | 7/42 (16.7) | 0.062 |
| NT0.5 | 73/162 (45.1) | 59/120 (49.2) | 14/42 (33.3) | 0.076 |
| RIFLEbin | 83/162 (51.2) | 65/120 (54.2) | 18/42 (42.9) | 0.207 |
| AKINbinb | 72/137 (52.6) | 55/102 (53.9) | 17/35 (48.6) | 0.584 |
| KDIGObin | 95/162 (58.6) | 72/120 (60.0) | 23/42 (54.8) | 0.553 |
| KDIGO stagec | ||||
| 1 | 50/162 (30.9) | 34/120 (28.3) | 16/42 (38.1) | 0.290 |
| 2 | 30/162 (18.5) | 25/120 (20.8) | 5/42 (11.9) | |
| 3 | 15/162 (9.3) | 13/120 (10.8) | 2/42 (4.8) | |
NT2×, traditional nephrotoxicity criterion of an SCr increase ≥2× over baseline; NT0.5, traditional nephrotoxicity criterion of an absolute SCr increase of ≥0.5 mg/dl over baseline; RIFLE, risk, injury, failure, loss, and end stage; RIFLEbin, SCr increase of ≥1.5× over baseline; AKIN, Acute Kidney Injury Network; AKINbin, absolute SCr increase of ≥0.3 mg/dl over baseline; KDIGO, Kidney Diseases Improving Global Outcomes; KDIGObin, absolute SCr increase of ≥0.3 mg/dl or ≥1.5× over baseline.
Unable to use AKIN criteria in 25 patients due to the 48-h requirement.
Numbers represent the maximum stage. Some patients with KDIGO stages 2 or 3 passed through stage 1, but they were classified as the maximum stage they reached.
The AKIN criteria could not be applied in 25 cases due to the absence of SCr values at critical time points, which hampered our ability to comply with the 48-h requirement. Since the KDIGO criteria combine characteristics of the RIFLE and AKIN criteria, this system was chosen to stratify AKI in stages. Most cases of AKI were mild, classified as stage 1 or 2. The incidence of severe KDIGO stage 3 AKI was <10% overall and <5% in patients using the liposomal preparation.
The incidence of AKI was numerically higher in the deoxycholate than in the liposomal group, but the differences were only marginally significant when using the traditional NT0.5 or NT2× criterion (Table 2). Patients on liposomal AmB also tended to have less severe cases of AKI, with a lower percentage of cases classified as stages 2 or 3.
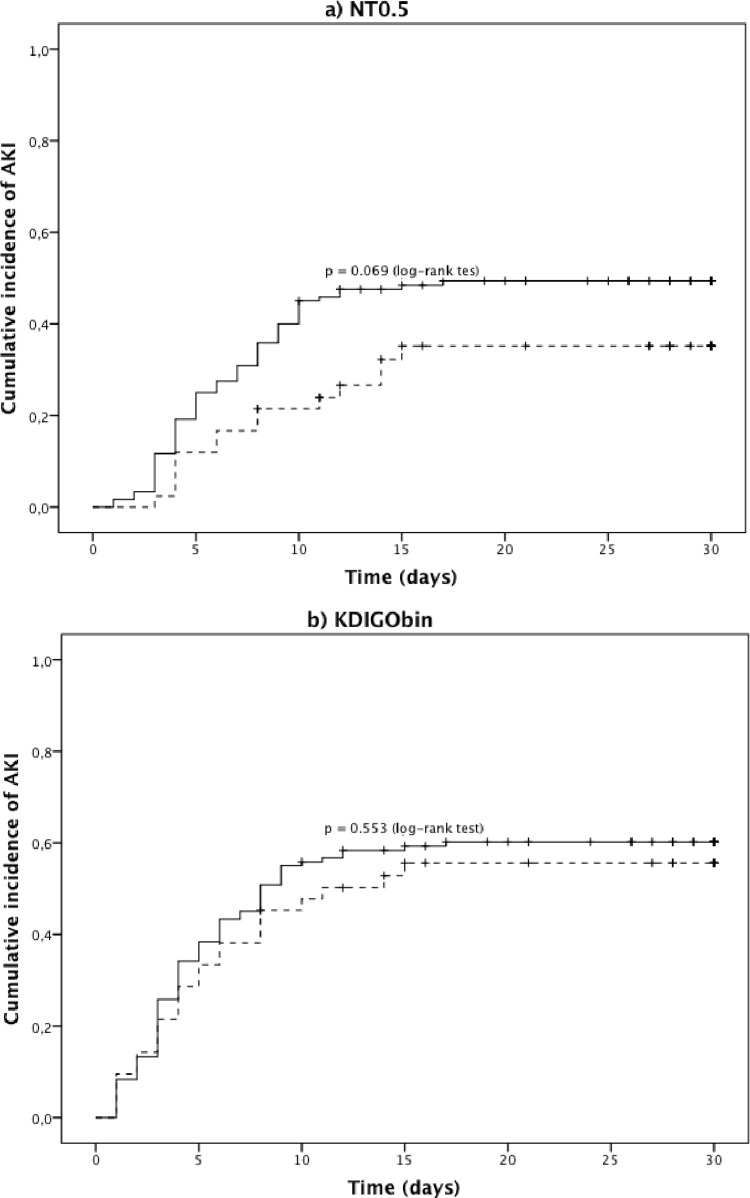
Regarding the time to diagnosis of AKI, the traditional NT0.5 criterion detected AmB-induced AKI at a median of 6.0 days (IQR, 4.0 to 9.0), whereas the newer KDIGO criteria detected it 2 days earlier, at a median of 4.0 days (IQR, 2.0 to 7.8) (Table 3). In a survival analysis using the Kaplan-Meier method, the KDIGObin also detected more cases of AKI, and at earlier time points, than the traditional NT0.5 criterion (Fig. 3). Nevertheless, this increased sensitivity of the KDIGObin definition occurred at the expense of a decreased ability to discriminate the differential nephrotoxic profiles of the two AmB preparations.
TABLE 3.
Influence of the diagnostic criteria on the time in days to diagnosis of AKI during intravenous AmB use in 162 hospitalized adults treated at a university hospital, stratified by type of AmB preparation
| AKI criteria (n)a | All (n = 162) (median [IQR]) | Type of AmB preparation (median [IQR]) |
|
|---|---|---|---|
| Deoxycholate (n = 120) | Liposomal (n = 42) | ||
| NT0.5 (73) | 6.0 (4.0 to 9.0) | 5.0 (4.0 to 9.0) | 7.0 (4.0 to 12.5) |
| NT2× (45) | 5.0 (3.0 to 8.0) | 5.0 (3.0 to 980) | 6.0 (4.0 to 8.0) |
| KDIGO stageb | |||
| 1 (76) | 4.0 (2.0 to 7.8) | 4.0 (2.5 to 7.5) | 4.0 (2.0 to 8.0) |
| 2 (40) | 5.5 (3.3 to 8.0) | 5.5 (3.0 to 8.3) | 5.5 (3.5 to 8.8) |
| 3 (15) | 7.0 (4.0 to 13.0) | 7.0 (3.5 to 13.0) | 8.0 (6.0 to 10.0) |
NT2×, traditional nephrotoxicity criterion of an SCr increase of ≥2× over baseline; NT0.5, traditional nephrotoxicity criterion of an absolute SCr increase of ≥0.5 mg/dl over baseline; KDIGO, Kidney Diseases Improving Global Outcomes.
The number of patients with AKI by the KDIGO criteria was 95. The sum of patients across the KDIGO stages add up to >95 because some patients progressed from stage 1 to stages 2 and 3 during treatment.
FIG 3.
Time to AKI stratified by AmB preparation according to NT0.5 and KDIGObin criteria. NT0.5, traditional nephrotoxicity criterion of an absolute SCr increase of ≥0.5 mg/dl over baseline; NT2×, traditional nephrotoxicity criterion of an SCr increase of ≥2× over baseline; KDIGO, Kidney Diseases Improving Global Outcomes; KDIGObin, absolute SCr increase of ≥0.3 mg/dl or ≥1.5× over baseline. Solid line, deoxycholate; dashed line, liposomal. (dashes and +) marks on the survival curves represent censored cases. The comparison among one minus cumulative survival curve for the two AmB preparations was 0.069 using the NT0.5 criterion, 0.081 using NT2× (not shown), and 0.553 using the KDIGObin criterion (log rank test).
Kinetics of AmB-induced AKI.
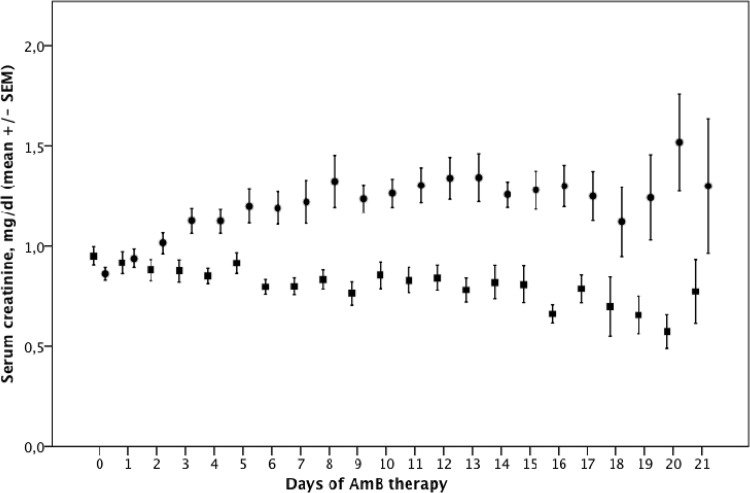
Figure 4 shows mean SCr values during AmB therapy, stratified by the presence of AKI by KDIGObin. In the AKI group, the mean SCr level started to rise at day two and peaked at day 8. Peak SCr in the AKI group was (mean ± standard deviation [SD]) 1.66 ± 0.67 mg/dl versus 1.04 ± 0.38 mg/dl in the no-AKI group (P = <0.001). The peak SCr level was also higher among AKI patients using the deoxycholate preparation than in those using the liposomal preparation (1.69 ± 0.71 mg/dl versus 1.58 ± 0.53 mg/dl, respectively), but the difference was not statistically significant (P = 0.488).
FIG 4.
SCr levels over time during AmB use, stratified by the occurrence of AKI according to the KDIGO criteria. SCr, serum creatinine; SEM, standard error of the mean. The circles represent the AKI group, and squares represent those without AKI by the Kidney Diseases Improving Global Outcomes (KDIGO) criteria. KDIGObin indicates an absolute SCr increase of ≥0.3 mg/dl or ≥1.5× over baseline.
Electrolyte imbalances.
Overall, 121/162 (74.7%) patients fulfilled our criteria for hypokalemia, and 107/162 (66.0%) patients required potassium replacement. Both hypokalemia (80.0% versus 59.5%, P = 0.015) and potassium replacement (71.7% versus 50.0%, P = 0.018) were significantly more common in patients using the deoxycholate versus the liposomal preparation, respectively.
Hypomagnesemia was detected in 101/162 (62.3%) patients, and magnesium replacement was required by 89/162 (54.9%) patients. Similarly, hypomagnesemia (68.3% versus 45.2%, P = 0.10) and magnesium replacement (60.8% versus 38.1%, P = 0.012) were more common in patients receiving deoxycholate versus liposomal AmB, respectively. There was no association, however, between these electrolyte imbalances and AKI, regardless of the AKI criteria used.
AKI management.
A change in management in response to AKI was documented in 59/95 (62.1%) patients classified by KDIGObin. Changes in management were more frequent in those in stage 3 (73%) than in stage 2 (66.7%) or stage 1 (56%) AKI. The most common changes were to discontinue AmB (n = 32), increase the amount of intravenous fluids (n = 19), decrease AmB dose (n = 17), switch from a deoxycholate to liposomal preparation (n = 8), switch the AmB regimen (e.g., from daily to every other day, n = 6), and discontinue concomitant nephrotoxins (n = 3).
Eleven patients (6.8%) required dialysis at a median of 9.0 (IQR, 5.0 to 15.0) days after the initiation of AmB. The median time on dialysis was 3.0 days (IQR, 0.0 to 8.0 days).
Predictors of AmB-induced AKI.
We then attempted to identify predictors of AKI. For these analyses, we used both logistic regression (treating AKI as the binary dependent variable) and Cox proportional hazards (using time to AKI as the dependent variable) analyses. In addition, we conducted these analyses using KDIGObin and traditional NT0.5 and NT2× criteria. The following predictor variables were analyzed: age (continuous), type of AmB (deoxycholate versus liposomal preparation), place of AmB initiation (ICU versus ward), diagnosis (leishmaniasis versus other), Charlson comorbidity index score (≥4 versus 1 through 3), and use of furosemide, ACE-I, polymyxin B, vancomycin, and vasopressors (all yes versus no). Since type of AmB was used as an independent variable, these analyses were performed for the entire group of 162 patients, without stratification into deoxycholate and liposomal subgroups.
Independent predictors of AKI in multivariate logistic regression models varied according to the criteria used to define AKI. When the KDIGObin criterion was used, age and use of furosemide were marginally significant. Age and use of vancomycin were predictors of AKI by NT0.5, whereas use of vancomycin and vasopressors predicted AKI by NT2× (Table 4). Cox proportional hazards models yielded similar findings (Table 5).
TABLE 4.
Univariate and multivariate logistic regression analyses to identify independent predictors of AKI by KDIGObin, NT0.5, and NT2×
| AKI criteria predictorsa | Univariate analysis (OR [95% CI]) | P valueb | Multivariate analysis (OR [95% CI]) | P valueb |
|---|---|---|---|---|
| KDIGObin | ||||
| Age (yr) | 1.02 (1.00–1.04) | 0.066 | 1.02 (1.00–1.04) | 0.096 |
| Deoxycholate preparation | 1.24 (0.61–2.52) | 0.553 | ||
| ICU initiation | 1.24 (0.53–2.91) | 0.618 | ||
| Leishmaniasis | 1.40 (0.69–2.85) | 0.353 | ||
| Charlson index ≥4 | 1.28 (0.61–2.71) | 0.519 | ||
| Furosemide | 2.26 (1.01–5.07) | 0.047 | 2.30 (0.98–5.39) | 0.056 |
| ACE-I | 2.29 (0.91–5.77) | 0.080 | ||
| Polymyxin B | 0.59 (0.22–1.63) | 0.309 | ||
| Vancomycin | 1.22 (0.64–2.32) | 0.540 | ||
| Vasopressors | 1.07 (0.50–2.30) | 0.854 | ||
| NT0.5 | ||||
| Age (yr) | 1.02 (1.00–1.04) | 0.064 | 1.02 (1.00–1.04) | 0.038 |
| Deoxycholate preparation | 1.93 (0.93–4.03) | 0.078 | ||
| ICU initiation | 1.99 (0.86–4.61) | 0.108 | ||
| Leishmaniasis | 0.85 (0.43–1.71) | 0.653 | ||
| Charlson index ≥4 | 1.13 (0.55–2.34) | 0.744 | ||
| Furosemide | 2.13 (1.01–4.49) | 0.048 | ||
| ACE-I | 1.66 (0.72–3.81) | 0.233 | ||
| Polymyxin B | 0.84 (0.30–2.32) | 0.734 | ||
| Vancomycin | 2.01 (1.06–3.81) | 0.032 | 2.24 (1.16–4.32) | 0.016 |
| Vasopressors | 1.61 (0.76–3.41) | 0.218 | ||
| NT2× | ||||
| Age (yr) | 1.00 (0.98–1.02) | 0.965 | ||
| Deoxycholate preparation | 2.32 (0.94–5.69) | 0.067 | ||
| ICU initiation | 2.47 (1.05–5.81) | 0.038 | ||
| Leishmaniasis | 0.47 (0.20–1.10) | 0.082 | ||
| Charlson index ≥4 | 1.27 (0.58–2.81) | 0.550 | ||
| Furosemide | 1.85 (0.85–4.02) | 0.080 | ||
| ACE-I | 1.68 (0.70–4.02) | 0.243 | ||
| Polymyxin B | 1.09 (0.36–3.30) | 0.874 | ||
| Vancomycin | 2.74 (1.35–5.54) | 0.005 | 2.27 (1.09–4.72) | 0.028 |
| Vasopressors | 3.34 (1.52–7.32) | 0.003 | 2.73 (1.21–6.16) | 0.015 |
Age was evaluated as a continuous variable; all other independent variables are categorical.
Variables with a P value of <0.20 in univariate analyses (in bold type) were included in a multivariate backward logistic regression model. Only the variables remaining at the final model are shown.
TABLE 5.
Univariate and multivariate Cox regression analyses to identify independent predictors of AKI by KDIGObin, NT0.5, and NT2×
| AKI criteria predictorsa | Univariate analysis (HR [95% CI]) | P valueb | Multivariate (HR [95% CI]) | P valueb |
|---|---|---|---|---|
| KDIGObin | ||||
| Age (yr) | 1.09 (1.00–1.02) | 0.143 | ||
| Deoxycholate preparation | 1.15 (0.72–1.83) | 0.569 | ||
| ICU initiation | 1.20 (0.71–2.03) | 0.497 | ||
| Leishmaniasis | 1.10 (0.71–1.70) | 0.679 | ||
| Charlson index ≥4 | 1.18 (0.37–3.77) | 0.776 | ||
| Furosemide | 1.42 (0.91–2.21) | 0.127 | ||
| ACE-I | 1.52 (0.93–2.50) | 0.095 | 1.53 (0.92–2.53) | 0.100 |
| Polymyxin B | 0.73 (0.35–1.50) | 0.384 | ||
| Vancomycin | 1.16 (0.77–1.76) | 0.466 | ||
| Vasopressors | 1.10 (0.68–1.80) | 0.693 | ||
| NT0.5 | ||||
| Age (yr) | 1.01 (1.00–1.02) | 0.104 | 1.01 (1.00–1.04) | 0.084 |
| Deoxycholate preparation | 1.68 (0.94–3.02) | 0.080 | ||
| ICU initiation | 1.59 (0.91–2.77) | 0.102 | ||
| Leishmaniasis | 0.83 (0.49–1.41) | 0.833 | ||
| Charlson index ≥4 | 1.07 (0.63–1.83) | 0.793 | ||
| Furosemide | 1.52 (0.92–2.51) | 0.101 | ||
| ACE-I | 1.29 (0.73–2.28) | 0.374 | ||
| Polymyxin B | 0.92 (0.42–2.00) | 0.826 | ||
| Vancomycin | 1.70 (1.07–2.69) | 0.024 | 1.76 (1.10–2.78) | 0.018 |
| Vasopressors | 1.45 (0.86–2.45) | 0.162 | ||
| NT2× | ||||
| Age (yr) | 1.00 (0.98–1.02) | 0.889 | ||
| Deoxycholate preparation | 2.00 (0.89–4.48) | 0.092 | ||
| ICU initiation | 2.07 (1.17–4.01) | 0.031 | ||
| Leishmaniasis | 0.51 (0.24–1.09) | 0.082 | ||
| Charlson index ≥4 | 1.21 (0.63–2.34) | 0.571 | ||
| Furosemide | 1.67 (0.89–3.14) | 0.112 | ||
| ACE-I | 1.50 (0.74–3.02) | 0.261 | ||
| Polymyxin B | 1.09 (0.43–2.77) | 0.853 | ||
| Vancomycin | 2.39 (1.32–4.33) | 0.004 | 2.00 (1.08–3.71) | 0.028 |
| Vasopressors | 2.58 (1.41–4.71) | 0.002 | 2.08 (1.11–3.91) | 0.022 |
Age was evaluated as a continuous variable; all other independent variables are categorical.
Variables with a P value of <0.20 in univariate analyses (in bold type) were included in a multivariate backward logistic regression model. Only the variables remaining at the final model are shown.
Impact of AmB-induced AKI on hospital LOS.
When considering all patients, median hospital LOS was not affected by the presence (or severity) of AKI (32 days in those without AKI; 30, 33, and 32 days in KDIGO stages 1, 2, and 3, respectively; P = 0.987). When analyzing only those patients who survived hospitalization, we found that median LOS was higher in patients with KDIGO stage 3 (62 days, n = 4 patients) than in those with earlier KDIGO stages (stage 2, 33 days, n = 21; stage 1, 30 days, n = 41) and those without AKI (33 days, n = 51), but the difference did not reach statistical significance (P = 0.205).
Median hospital LOS was significantly longer in patients using the deoxycholate AmB preparation than those using the liposomal AmB preparation when considering all patients (35 versus 27 days, respectively; P < 0.001) or only those who survived hospitalization (39 versus 27, respectively; P < 0.001).
Impact of AmB-induced AKI on hospital morbidity and mortality.
Using the KDIGObin definition, AmB-induced AKI was not associated with a need for ICU admission, mechanical ventilation, or mortality. When looking at the different KDIGO stages, the proportions of patients with KDIGO stage 1 who required ICU admission, mechanical ventilation, or died was actually lower than those of patients without AKI. The traditional NT criterion and KDIGO stage 2 or 3, however, were highly associated with all three outcomes (Table 6).
TABLE 6.
Impact of AKI on ICU admission, mechanical ventilation, and inpatient mortalitya
| AKI criteria by outcomeb | AKI by binary criteria |
AKI by KDIGO stage |
||||||
|---|---|---|---|---|---|---|---|---|
| No AKI | AKI | P value | No AKI | Stage 1 | Stage 2 | Stage 3 | P value | |
| ICU admission | 20/67 (29.9) | 8/50 (16.0) | 12/30 (40.0) | 13/15 (86.7) | <0.001 | |||
| NT2× | 28/117 (23.9) | 24/45 (55.6) | <0.001 | |||||
| NT0.5 | 22/89 (24.7) | 31/73 (42.5) | 0.017 | |||||
| KDIGObin | 20/67 (29.9) | 33/95 (34.7) | 0.514 | |||||
| Mechanical ventilation | 15/67 (22.4) | 7/50 (14.0) | 10/30 (33.3) | 11/15 (73.3) | <0.001 | |||
| NT2× | 22/117 (18.8) | 21/45 (46.7) | <0.001 | |||||
| NT0.5 | 16/89 (18.0) | 27/73 (37.0) | 0.006 | |||||
| KDIGObin | 15/67 (22.4) | 28/95 (29.5) | 0.314 | |||||
| Inpatient mortality | 16/67 (23.9) | 9/50 (18.0) | 9/30 (30.0) | 11/15 (73.3) | <0.001 | |||
| NT2× | 25/117 (21.4) | 20/45 (44.4) | 0.003 | |||||
| NT0.5 | 19/89 (21.3) | 26/73 (35.6) | 0.044 | |||||
| KDIGObin | 16/67 (23.9) | 29/95 (30.5) | 0.352 | |||||
Other than P values, data are presented as the no./total no. (%).
NT2×, traditional nephrotoxicity criterion of an SCr increase of ≥2× over baseline; NT0.5, traditional nephrotoxicity criterion of an absolute SCr increase of ≥0.5 mg/dl over baseline; KDIGO, Kidney Diseases Improving Global Outcomes; KDIGObin, absolute SCr increase of ≥0.3 mg/dl or ≥1.5× over baseline.
Overall, 45/162 patients (27.8%) died during hospital follow-up. The median time to death was 32 (IQR, 22.5 to 50.5) days. The impact of AKI on mortality was evaluated on univariate and multivariate analyses.
On univariate analyses, the NT0.5 criterion was mildly associated with mortality by logistic regression but not by Cox regression (Table 7). When AKI was classified using the KDIGO criterion as a binary variable, it became significantly associated with mortality only when stages 2 and 3 (KDIGO, ≥2) were combined. In fact, when looking at the KDIGO criteria as an ordinal variable, mortality associated with stage 1 AKI (9/52 [17.3%]) was even lower than in those without AKI (16/67 [23.9%]). Stage 3 AKI and need for dialysis were highly associated with mortality.
TABLE 7.
Impact of AKI criteria on the univariate association between AKI and mortality
| AKI criteria by regression methoda | No./total no. (%) | OR/HR (95% CI)b | P value |
|---|---|---|---|
| Univariate logistic regression | |||
| Binary | |||
| NT0.5 | 26/73 (35.6) | 2.04 (1.02–4.09) | 0.045 |
| NT2× | 20/45 (44.4) | 2.94 (1.41–6.14) | 0.004 |
| KDIGObin | 29/95 (30.5) | 1.40 (0.69–2.85) | 0.353 |
| KDIGO stage | |||
| 1 | 9/50 (18.0) | 0.70 (0.28–1.75) | 0.444 |
| 2 | 9/30 (30.0) | 1.37 (0.52–3.57) | 0.525 |
| 3 | 11/15 (73.3) | 8.77 (2.45–31.36) | 0.001 |
| Dialysis | 10/11 (90.9) | 33.14 (4.10–267.98) | <0.001 |
| Univariate Cox regression | |||
| Binary | |||
| NT0.5 | 26/73 (35.6) | 1.53 (0.84–2.81) | 0.168 |
| NT2× | 20/45 (44.4) | 2.23 (1.23–4.03) | 0.008 |
| KDIGObin | 29/95 (30.5) | 1.20 (0.65–2.24) | 0.559 |
| KDIGO stage | |||
| 1 | 9/50 (18.0) | 0.62 (0.26–1.46) | 0.270 |
| 2 | 9/30 (30.0) | 1.19 (0.52–2.72) | 0.682 |
| 3 | 11/15 (73.3) | 3.29 (1.52–7.13) | 0.002 |
| Dialysis | 10/11 (90.9) | 5.05 (2.47–10.34) | <0.001 |
NT2×, traditional nephrotoxicity criterion of an SCr increase of ≥2× over baseline; NT0.5, traditional nephrotoxicity criterion of an absolute SCr increase of ≥0.5 mg/dl over baseline; KDIGO, Kidney Diseases Improving Global Outcomes; KDIGObin, absolute SCr increase of ≥0.3 mg/dl or ≥1.5× over baseline.
Odds ratio (OR) was used for logistic regression data and hazard ratio (HR) for Cox regression data. 95% CI, 95% confidence interval.
In Table 8, we further explored the association between AKI by NT2× with mortality in multivariate models. AKI remained significantly associated with mortality even when adjusted for the Charlson comorbidity index and use of furosemide, vancomycin, and steroids (model 1). However, when the use of vasopressors and need for ICU and mechanical ventilation were included (model 2), AKI no longer predicted death.
TABLE 8.
Impact of AKI by NT2× on mortality, adjusted for potential confounders
| Variables by model typea | OR/HR (95% CI)b | P value |
|---|---|---|
| Logistic regression model | ||
| Unadjusted | ||
| NT2× | 2.94 (1.41–6.14) | 0.004 |
| Model 1 | ||
| NT2× | 2.279 (1.007–5.16) | 0.048 |
| Charlson index (≥4 vs 0–3) | 2.694 (1.104–6.11) | 0.033 |
| Steroids | 2.960 (1.225–7.15) | 0.019 |
| Vancomycin | 2.715 (1.193–6.18) | 0.016 |
| Furosemide | 2.597 (1.104–6.11) | 0.029 |
| Model 2 | ||
| NT2× | 1.06 (0.32–3.55) | 0.927 |
| Steroids | 6.06 (1.59–23.02) | 0.008 |
| Vasopressors | 3.60 (0.99–13.09) | 0.052 |
| Mechanical ventilation | 44.34 (12.28–160.02) | <0.001 |
| Cox regression model | ||
| Unadjusted | ||
| NT2× | 2.23 (1.23–4.03) | 0.008 |
| Model 1 | ||
| NT2× | 2.31 (1.27–4.19) | 0.006 |
| Charlson index (≥4 vs 0–3) | 2.04 (1.07–3.91) | 0.031 |
| Steroids | 2.12 (1.02–4.42) | 0.044 |
| Model 2 | ||
| NT2× | 1.27 (0.68–2.35) | 0.453 |
| Steroids | 2.14 (1.02–4.47) | 0.044 |
| Vasopressors | 2.20 (1.02–4.02) | 0.045 |
| Mechanical ventilation | 5.49 (2.40–12.56) | <0.001 |
NT2×, traditional nephrotoxicity criterion of an SCr increase of ≥2× over baseline. Variables included in model 1: first block (backward) Charlson's comorbidity index, use of furosemide, vancomycin, and steroids; second block (enter) AKI by NT2×. Variables included in model 2: first block (backward) all variables included in model 1 plus ICU, use of vasopressors, and mechanical ventilation; second block (enter) AKI by NT2×.
Odds ratio (OR) was used for logistic regression data and hazard ratio (HR) for Cox regression data. 95% CI, 95% confidence interval.
None of the 45 patients with a diagnosis of leishmaniasis in our data set ended up dying during hospital admission. Hence, this variable was not included as a predictor in multivariate mortality analyses due to the mathematical instability of the model. Therefore, we repeated all multivariate logistic and Cox regression analyses for predictors of mortality in the subset of 117 patients with a diagnosis other than leishmaniasis and found similar results (data not shown).
DISCUSSION
In 2001, Bellomo et al. (22) called attention to the fact that there were >30 different definitions of acute renal failure in use in the medical literature. Their call for a consensus definition of this syndrome was answered in 2004, with the publication of the RIFLE criteria (18). Subsequent evidence that minimal increases in SCr levels (as small as 0.3 mg/dl) were associated with worse outcomes (23) led to the incorporation of smaller increases in SCr into a modification of the RIFLE criteria called AKIN (19). More recently, the KDIGO guidelines attempted to harmonize earlier consensus definitions and staging criteria for AKI (20). Whether these criteria should be applied in routine clinical care is still a matter of debate (24).
Here, we showed that the overall incidence of AmB-induced AKI by the most sensitive traditional criterion (NT 0.5) was 45.4% and occurred at a median time of 6 days. Newer KDIGO criteria improved the recognition of AKI, raising the incidence to 58.6% and shortening the median time to detection to 4 days. The KDIGO criteria also allowed for staging of AKI severity. Of the 95 cases of AKI detected by the KDIGO criteria, 52 (54.7%) were stage 1, 28 (29.5%) were stage 2, and 15 (15.8%) were stage 3; 11 of the 15 stage 3 cases were dialyzed. AKI occurred more frequently, earlier, and reached higher stages in patients using the deoxycholate versus the liposomal preparation. To our knowledge, this is the first study to evaluate the performance of the KDIGO criteria in AmB-induced AKI.
Since the incidence and impact of AKI on outcomes are dependent upon the criteria used to define them (25), we sought to compare our findings with those of prior studies of AmB nephrotoxicity. In those studies, the most commonly used criterion to define renal toxicity was a doubling (or greater) of baseline SCr levels, which we termed NT2×. This definition is analogous to a KDIGO stage ≥2. Applying the NT2× definition to our data, the incidence of AKI secondary to deoxycholate AmB was 31.7%, which is comparable to that found by Nucci et al. (15), Walsh et al. (14), and Johnson et al. (17) (31.8%, 33.7%, and 37.5%, respectively); Moreau et al. (26) and Caillot et al. (12) found higher (56.3 and 66.7%, respectively) incidences, and Prentice et al. (16) encountered lower (23%) incidences. For the liposomal preparation, we detected AKI using the NT2× criterion in 16.7% of patients, which is in agreement with the findings of Walsh et al. (14) (18.7%) and slightly higher than the incidences detected by Leenders et al. (13), Prentice et al. (16), and Johnson et al. (17) (11.8%, 11.1%, and 9.4%, respectively).
Using the KDIGO criteria, however, we detected AmB-induced AKI in 60.0% of patients using the deoxycholate preparation and 54.8% of patients using the liposomal preparation. These incidences are significantly higher than those previously described, especially for the liposomal preparation. In fact, several AKI episodes must have gone clinically undetected, because no changes in management in response to AKI were made in approximately 1/3 of patients.
Minejima et al. (21) showed that the AKIN criteria were more sensitive than traditional criteria in detecting vancomycin nephrotoxicity. In their opinion, early detection of vancomycin nephrotoxicity has the potential to improve management because it may lead to changes in management that halt the process of renal injury. A similar rationale could be applied to AmB-induced AKI. Nevertheless, since our data are observational, prospective studies would be needed to confirm this hypothesis. A potential drawback of using a highly sensitive AKI criterion would be to discontinue or reduce AmB dose unnecessarily, potentially interfering with the management of the underlying infection. In the present study, KDIGO stage 1 AKI was not predictive of need for ICU admission, need for mechanical ventilation, or mortality. In fact, these outcomes were less common in patients with stage 1 AKI than in those without AKI. When AKI was defined by traditional criteria, however, there was a significant association with all of these outcomes.
In the present study, hospital LOS was associated with type of AmB preparation, being longer in recipients of deoxycholate AmB, but these patients also had more comorbidities and a greater need for transfer to the ICU and mechanical ventilation than recipients of liposomal AmB. We did not find a significant association between hospital LOS and AmB-induced AKI. In fact, when considering all patients, hospital LOS was almost identical in those with and without AKI. Since mortality can confound LOS data (since in those who die, what is measured is in fact time to death), we repeated the analysis considering only those patients who survived hospitalization and found a higher hospital LOS in patients with KDIGO stage 3 AKI than in those with earlier KDIGO stages and in those without AKI. This difference, however, did not reach statistical significance, perhaps due to a type II error, as we only had 4 patients with stage 3 AKI who survived hospitalization. Our findings are in disagreement with those of Ullmann et al. (27), who found that even small (50%) rises in SCr levels during AmB treatment were associated with significant increases in hospital LOS (average increase, 8.6 days; P = 0.006). This discrepancy might be due to the marked differences in population and design between our studies, as Ullmann et al. (27) evaluated hematology-oncology patients from multiple European centers, and we studied a heterogeneous group of patients with infectious diseases at a single center in Brazil. Nevertheless, other authors have also failed to demonstrate an association between AmB-induced AKI and increased hospital LOS (28, 29).
Independent predictors of AKI also varied according to the definition used. AKI by KDIGObin was predicted by older age and use of furosemide (logistic regression) and ACE-I (Cox regression). AKI by the NT0.5 criterion was predicted by older age and use of vancomycin, whereas the more severe episodes of AKI defined by the NT2× criterion were predicted by use of furosemide and vasopressors. Bates and coworkers (30) identified ICU admission and use of cyclosporine as independent predictors of AKI. In our study, ICU admission predicted AKI by traditional criteria on univariate analysis but was removed from the model after adjustment for other confounders. We did not have patients on cyclosporine for comparison.
Our study has several limitations. We applied newer diagnostic criteria for AKI to the retrospective data. Our ability to detect AKI may have been negatively influenced by the fact that we did not have daily SCr values for all patients and did not incorporate urine output data. Indeed, the AKIN criteria could not be applied to 25 patients due to missing SCr values at critical time points. Although we did not have formal data on fluid responsivity or renal imaging for all patients, we made an effort to exclude pre- or postrenal causes of AKI by thoroughly reviewing EMRs and paper charts. Since some patients were exposed to other renal insults, ischemic and/or toxic, we cannot state that use of AmB was the sole cause of AKI in all subjects. In this regard, an important limitation is the absence of data regarding exposure to iodinated contrast during hospitalization. Finally, our study population was somewhat unique compared to that in previous studies, as it was composed of mostly young patients and almost 1/3 had leishmaniasis. Therefore, the applicability of our findings to other patient groups is unclear.
In summary, we demonstrated that the newer AKI criteria are more sensitive than traditional criteria for detecting AmB-induced AKI. As expected, this improved sensitivity occurred at the expense of potential overdiagnosis of mild cases. Whether or not this increased sensitivity of newer AKI criteria will translate into earlier interventions and better renal and patient outcomes remains unproven. In our data set, mild cases of AKI were not predictive of worse outcomes. Potential drawbacks of overly sensitive AKI criteria also need to be considered, because undue AmB discontinuation or dose reduction might compromise treatment of the underlying disease.
ACKNOWLEDGMENTS
Paulo Novis Rocha conducted this project and wrote this paper while attending the Weill Cornell Graduate School of Medical Sciences Master of Science Program in Clinical Epidemiology and Health Services Research and was supported by the National Institutes of Health (NIH), Fogarty International Center, grant no. 5 U2R TW006885.
The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Carla Dinamerica Kobayashi was awarded a research scholarship from Programa Institucional de Bolsas de Iniciação Científica (PIBIC) funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ). Luna de Carvalho Almeida was awarded a research scholarship from Programa Institucional de Bolsas de Iniciação Científica (PIBIC) funded by Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB).
We acknowledge Gillian Leandro de Queiroga Lima and Lucia Noblat for their efforts in locating the paper charts at our medical records sector.
We thank Warren Johnson, Mary Charlson, and Carol Mancuso and the entire faculty and student body of the Weill Cornell Graduate School of Medical Sciences Master of Science Program in Clinical Epidemiology and Health Services Research for their inestimable feedback during all steps of this work.
REFERENCES
- 1.Baginski M, Sternal K, Czub J, Borowski E. 2005. Molecular modelling of membrane activity of amphotericin B, a polyene macrolide antifungal antibiotic. Acta Biochim Pol 52:655–658. [PubMed] [Google Scholar]
- 2.Heidemann HT, Gerkens JF, Jackson EK, Branch RA. 1983. Effect of aminophylline on renal vasoconstriction produced by amphotericin B in the rat. Naunyn Schmiedebergs Arch Pharmacol 324:148–152. doi: 10.1007/BF00497021. [DOI] [PubMed] [Google Scholar]
- 3.Sabra R, Branch RA. 1991. Mechanisms of amphotericin B-induced decrease in glomerular filtration rate in rats. Antimicrob Agents Chemother 35:2509–2514. doi: 10.1128/AAC.35.12.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sawaya BP, Weihprecht H, Campbell WR, Lorenz JN, Webb RC, Briggs JP, Schnermann J. 1991. Direct vasoconstriction as a possible cause for amphotericin B-induced nephrotoxicity in rats. J Clin Invest 87:2097–2107. doi: 10.1172/JCI115240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinmetz PR, Lawson LR. 1970. Defect in urinary acidification induced in vitro by amphotericin B. J Clin Invest 49:596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brezis M, Rosen S, Silva P, Spokes K, Epstein FH. 1984. Polyene toxicity in renal medulla: injury mediated by transport activity. Science 224:66–68. doi: 10.1126/science.6322305. [DOI] [PubMed] [Google Scholar]
- 7.Yano T, Itoh Y, Kawamura E, Maeda A, Egashira N, Nishida M, Kurose H, Oishi R. 2009. Amphotericin B-induced renal tubular cell injury is mediated by Na+ influx through ion-permeable pores and subsequent activation of mitogen-activated protein kinases and elevation of intracellular Ca2+ concentration. Antimicrob Agents Chemother 53:1420–1426. doi: 10.1128/AAC.01137-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sawaya B, Briggs J, Schnermann J. 1995. Amphotericin B nephrotoxicity: the adverse consequences of altered membrane properties. J Am Soc Nephrol 6:154–164. [DOI] [PubMed] [Google Scholar]
- 9.Mistro S, Maciel Ide M, de Menezes RG, Maia ZP, Schooley RT, Badaró R. 2012. Does lipid emulsion reduce amphotericin B nephrotoxicity? A systematic review and meta-analysis. Clin Infect Dis 54:1774–1777. doi: 10.1093/cid/cis290. [DOI] [PubMed] [Google Scholar]
- 10.Nath CE, Shaw PJ, Gunning R, McLachlan AJ, Earl JW. 1999. Amphotericin B in children with malignant disease: a comparison of the toxicities and pharmacokinetics of amphotericin B administered in dextrose versus lipid emulsion. Antimicrob Agents Chemother 43:1417–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barquist E, Fein E, Shadick D, Johnson J, Clark J, Shatz D. 1999. A randomized prospective trial of amphotericin B lipid emulsion versus dextrose colloidal solution in critically ill patients. J Trauma 47:336–340. doi: 10.1097/00005373-199908000-00020. [DOI] [PubMed] [Google Scholar]
- 12.Caillot D, Reny G, Solary E, Casasnovas O, Chavanet P, Bonnotte B, Perello L, Dumas M, Entezam F, Guy H. 1994. A controlled trial of the tolerance of amphotericin B infused in dextrose or in Intralipid in patients with haematological malignancies. J Antimicrob Chemother 33:603–613. doi: 10.1093/jac/33.3.603. [DOI] [PubMed] [Google Scholar]
- 13.Leenders AC, Daenen S, Jansen RL, Hop WC, Lowenberg B, Wijermans PW, Cornelissen J, Herbrecht R, van der Lelie H, Hoogsteden HC, Verbrugh HA, de Marie S. 1998. Liposomal amphotericin B compared with amphotericin B deoxycholate in the treatment of documented and suspected neutropenia-associated invasive fungal infections. Br J Haematol 103:205–212. doi: 10.1046/j.1365-2141.1998.00944.x. [DOI] [PubMed] [Google Scholar]
- 14.Walsh TJ, Finberg RW, Arndt C, Hiemenz J, Schwartz C, Bodensteiner D, Pappas P, Seibel N, Greenberg RN, Dummer S, Schuster M, Holcenberg JS. 1999. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. National Institute of Allergy and Infectious Diseases Mycoses Study Group. N Engl J Med 340:764–771. [DOI] [PubMed] [Google Scholar]
- 15.Nucci M, Loureiro M, Silveira F, Casali AR, Bouzas LF, Velasco E, Spector N, Pulcheri W. 1999. Comparison of the toxicity of amphotericin B in 5% dextrose with that of amphotericin B in fat emulsion in a randomized trial with cancer patients. Antimicrob Agents Chemother 43:1445–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prentice HG, Hann IM, Herbrecht R, Aoun M, Kvaloy S, Catovsky D, Pinkerton CR, Schey SA, Jacobs F, Oakhill A, Stevens RF, Darbyshire PJ, Gibson BE. 1997. A randomized comparison of liposomal versus conventional amphotericin B for the treatment of pyrexia of unknown origin in neutropenic patients. Br J Haematol 98:711–718. doi: 10.1046/j.1365-2141.1997.2473063.x. [DOI] [PubMed] [Google Scholar]
- 17.Johnson PC, Wheat LJ, Cloud GA, Goldman M, Lancaster D, Bamberger DM, Powderly WG, Hafner R, Kauffman CA, Dismukes WE, U.S. National Institute of Allergy and Infectious Diseases Mycoses Study Group . 2002. Safety and efficacy of liposomal amphotericin B compared with conventional amphotericin B for induction therapy of histoplasmosis in patients with AIDS. Ann Intern Med 137:105–109. doi: 10.7326/0003-4819-137-2-200207160-00008. [DOI] [PubMed] [Google Scholar]
- 18.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative Workgroup. 2004. Acute renal failure–definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8:R204-12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A, Acute Kidney Injury Network. 2007. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. 2012. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2:1–138. doi: 10.1038/kisup.2012.1. [DOI] [Google Scholar]
- 21.Minejima E, Choi J, Beringer P, Lou M, Tse E, Wong-Beringer A. 2011. Applying new diagnostic criteria for acute kidney injury to facilitate early identification of nephrotoxicity in vancomycin-treated patients. Antimicrob Agents Chemother 55:3278–3283. doi: 10.1128/AAC.00173-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellomo R, Kellum J, Ronco C. 2001. Acute renal failure: time for consensus. Intensive Care Med 27:1685–1688. doi: 10.1007/s00134-001-1120-6. [DOI] [PubMed] [Google Scholar]
- 23.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. 2005. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 24.Palevsky PM, Liu KD, Brophy PD, Chawla LS, Parikh CR, Thakar CV, Tolwani AJ, Waikar SS, Weisbord SD. 2013. KDOQI U.S. commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis 61:649–672. doi: 10.1053/j.ajkd.2013.02.349. [DOI] [PubMed] [Google Scholar]
- 25.Hoste EAJ, Schurgers M. 2008. Epidemiology of acute kidney injury: how big is the problem? Crit Care Med 36:S146–51. doi: 10.1097/CCM.0b013e318168c590. [DOI] [PubMed] [Google Scholar]
- 26.Moreau P, Milpied N, Fayette N, Ramée JF, Harousseau JL. 1992. Reduced renal toxicity and improved clinical tolerance of amphotericin B mixed with intralipid compared with conventional amphotericin B in neutropenic patients. J Antimicrob Chemother 30:535–541. doi: 10.1093/jac/30.4.535. [DOI] [PubMed] [Google Scholar]
- 27.Ullmann AJ, Sanz MA, Tramarin A, Barnes RA, Wu W, Gerlach BA, Krobot KJ, Gerth WC, Longitudinal Evaluation of Antifungal Drugs (LEAD I) Investigators. 2006. Prospective study of amphotericin B formulations in immunocompromised patients in 4 European countries. Clin Infect Dis 43:e29–38. doi: 10.1086/505969. [DOI] [PubMed] [Google Scholar]
- 28.Harbarth S, Burke JP, Lloyd JF, Evans RS, Pestotnik SL, Samore MH. 2002. Clinical and economic outcomes of conventional amphotericin B-associated nephrotoxicity. Clin Infect Dis 35:e120–7. doi: 10.1086/344468. [DOI] [PubMed] [Google Scholar]
- 29.Gubbins PO, Penzak SR, Polston S, McConnell SA, Anaissie E. 2002. Characterizing and predicting amphotericin B-associated nephrotoxicity in bone marrow or peripheral blood stem cell transplant recipients. Pharmacotherapy 22:961–971. doi: 10.1592/phco.22.12.961.33607. [DOI] [PubMed] [Google Scholar]
- 30.Bates DW, Su L, Yu DT, Chertow GM, Seger DL, Gomes DR, Platt R. 2001. Correlates of acute renal failure in patients receiving parenteral amphotericin B Kidney Int 60:1452–1459. [DOI] [PubMed] [Google Scholar]