Abstract
Respiratory syncytial virus (RSV) is the leading cause of bronchiolitis and pneumonia in infants. Effective treatment for RSV infection is a significant unmet medical need. While new RSV therapeutics are now in development, there are very few animal models that mimic the pathogenesis of human RSV, making it difficult to evaluate new disease interventions. Experimental infection of Holstein calves with bovine RSV (bRSV) causes a severe respiratory infection that is similar to human RSV infection, providing a relevant model for testing novel therapeutic agents. In this model, viral load is readily detected in nasal secretions by quantitative real-time PCR (qRT-PCR), and cumulative symptom scoring together with histopathology evaluations of infected tissue allow for the assessment of disease severity. The bovine RSV model was used to evaluate the antiviral activity of an RSV fusion inhibitor, GS1, which blocks virus entry by inhibiting the fusion of the viral envelope with the host cell membrane. The efficacy of GS1, a close structural analog of GS-5806 that is being developed to treat RSV infection in humans was evaluated in two randomized, blind, placebo-controlled studies in bRSV-infected calves. Intravenous administration of GS1 at 4 mg/kg of body weight/day for 7 days starting 24 h or 72 h postinoculation provided clear therapeutic benefit by reducing the viral load, disease symptom score, respiration rate, and lung pathology associated with bRSV infection. These data support the use of the bovine RSV model for evaluation of experimental therapeutics for treatment of RSV.
INTRODUCTION
Respiratory syncytial virus (RSV) is a member of the Paramyxoviridae family. RSV is an enveloped virus with a negative single-strand RNA genome that encodes 11 proteins, including 3 surface glycoproteins (F, G, and SH) and 4 proteins that comprise the viral RNA polymerase complex (N, P, L, and M2-1). The virus replicates in the ciliated respiratory epithelial cells that line the respiratory tract. Replication of the virus can induce syncytia (cell fusion) that can be readily observed in transformed cell lines and has also been detected in infant lung tissue from fatal cases of RSV infection (1). However, replication of RSV in primary human airway epithelial cells does not cause measurable cytopathic effects (2). While direct virus-induced cytopathic effects may not be the primary cause of clinical disease, virus replication activates the host immune responses which can lead to immune-mediated pathogenesis (3).
RSV circulates in the population as a single serotype with two major antigenic subgroups, A and B, that cause seasonal infections during fall and winter in the United States and other temperate regions of the world (4). Children younger than 2 years of age, immunocompromised individuals, and adults with underlying respiratory dysfunction such as asthma or chronic obstructive pulmonary disease (COPD) are at greater risk for developing complications due to RSV (5–10). While most infections resolve without medical intervention, RSV infection can cause acute bronchiolitis and pneumonia in infants and elderly adults (5, 11). Infection of the lower respiratory tract can cause severe pneumonia requiring hospitalization and resulting in mortality.
Despite extensive study of the clinical features and immunopathogenesis of RSV, there are no effective therapeutics or vaccines to treat RSV infection. A prophylactic monoclonal antibody Synagis (palivizumab) is recommended for preventing RSV infection among infants at high risk for lower respiratory tract RSV infection (12). Vaccine strategies using formalin-fixed RSV as the immunogen did not fully protect infants from RSV infection and in some cases enhanced disease, underscoring the challenges of developing effective infant vaccines for RSV (13). The only approved antiviral treatment for RSV is Virazole (ribavirin), which is delivered as an inhaled agent. Virazole has shown equivocal efficacy in bone marrow transplant patients and is not typically used to treat infants with RSV due to poor efficacy and tolerability, as well as the complexity of the specialized aerosol drug delivery system (14–16). A number of small-molecule inhibitors of RSV have been evaluated as potential therapeutics for treating RSV infections that target viral entry and replication (17–23). While several clinical candidates have been evaluated in human studies, only ribavirin has been approved for the treatment of RSV infection.
Preclinical development of new therapeutics requires evaluation of efficacy in animal models of RSV infection. The cotton rat model is commonly used to measure RSV replication and evaluate RSV therapeutics (24, 25). While cotton rats are permissive to RSV infection, virus replication does not produce quantifiable symptoms, and the extent of virus infection is measured by determining viral lung titers from infected animals following euthanasia. Thus, the cotton rat model is not suitable for measuring changes in disease pathogenesis resulting from antiviral therapy. Additional animal models have been developed using chimpanzees, African green monkeys, infant rhesus monkeys, and mice to study RSV infection (18, 26–29). As in the cotton rat model, virus replication does not induce significant disease, and symptoms tend to be mild or absent. Infection of neonatal lambs with RSV produces mild symptoms that include fever, tachypnea, and malaise as well as histologic lesions in lung tissue (27, 30). However, the natural history of RSV infection in this model has not been fully characterized, and there are limited data on quantitative viral load measurements in tissues over the course of infection or on the development of techniques to quantify symptoms to correlate virus replication with disease progression.
Bovine RSV (bRSV) is a natural pathogen of cattle that causes a disease in young animals similar to human RSV (hRSV) (31, 32). The virus replicates in the upper and lower respiratory tract to produce a spectrum of illness ranging from subclinical to severe bronchiolitis and pneumonia (33). The similarity in pathogenesis between bRSV and hRSV is underscored by studies that have demonstrated disease enhancement by a formalin-inactivated bRSV/alum vaccine made using the protocol that created the disease-enhancing RSV vaccine that sickened and killed several children in the 1960s (13, 34).
The goal of this study was to develop a preclinical animal model to evaluate efficacy of experimental therapeutics for treatment of human RSV infection. To validate the bRSV model for testing antiviral therapeutic interventions, we tested the therapeutic efficacy of a novel RSV fusion inhibitor GS1, a close structural analog of GS-5806 which has recently been shown to reduce RSV viral load and clinical symptoms in a human model of experimental RSV infection (Fig. 1) (20). The effects of GS1 on bRSV replication and disease symptoms were assessed in two randomized, placebo-controlled, blind efficacy studies in calves less than 8 weeks old to assess GS1 efficacy. The results demonstrate that the bRSV infection of young calves is a suitable model for quantifying the impact of antiviral intervention on the viral load and disease symptoms and can be used to evaluate the in vivo efficacy of RSV antiviral compounds.
FIG 1.
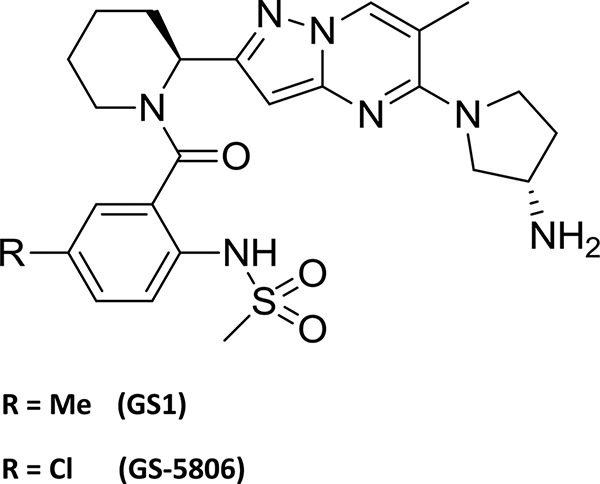
Structures of GS1 and GS-5806. Me, methyl.
MATERIALS AND METHODS
Compounds.
GS1 was synthesized by the Department of Medicinal Chemistry, Gilead Sciences (Foster City, CA) (Fig. 1).
Cells and viruses.
Primary bovine turbinate (BT) cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin (10 U/ml), and streptomycin (100 μg/ml) at 37°C with 5% CO2. Primary bovine alveolar type 2 (BAT2) cells were isolated from newborn calf lung as described previously (35). The cells were grown in DMEM-keratinocyte medium at a ratio of 1:1 (Invitrogen, Carlsbad, CA) supplemented with 2% FBS, 5 mM l-glutamine, 0.02% lactalbumin dehydrogenase, and penicillin (100 μg/ml)-streptomycin (100 μg/ml) (Invitrogen) at 37°C in a humidified incubator with 5% CO2.
Stocks of bRSV were prepared from a clinical isolate of a virulent bRSV strain (CA-1) as previously described (36, 37). Virus stocks were prepared by infecting a BT cell monolayer in a T75 culture flask with 3 × 105 PFU of virus. The infected cells were incubated until viral cytopathic effects (CPE) reached approximately 30 to 50% of the cell monolayer, usually by day 5 postinfection. The cultures were harvested and subjected to a single freeze-thaw cycle to release cell-associated virus. Aliquots of virus suspensions were placed on ice and administered to calves by aerosol within approximately 1 h. Additional aliquots were used to quantify viral titer by plaque assay, and any remaining virus was frozen at −80°C.
In vitro antiviral activity.
The antiviral activity of GS1 against bRSV was measured on BT and BAT2 cells using a cell viability assay that measures cellular ATP levels. Serial threefold dilutions of GS1 were prepared in dimethyl sulfoxide (DMSO) and added to cell culture media at 2× the final concentration. Cell monolayers were prepared in 96-well plates at 1 × 104 cells per well, and the next day, they were infected with bRSV at 0.05 PFU/cell (multiplicity of infection [MOI] of 0.05) in a final volume of 100 μl. A parallel set of plates was prepared and mock infected to measure compound cytotoxicity. The assay plates were incubated at 37°C in 5% CO2 for 4 days. The assay was developed by removal of 100 μl of media from each well followed by incubation at room temperature in a laminar flow hood to allow the plates to equilibrate. Following equilibration, 100 μl of Cell TiterGlo reagent (Promega) was added to each well, and luminescence was quantified on a Victor 96-well luminescence plate reader. The signal-to-background ratio was between 6 and 8 and was calculated by determining the ratio of luminescence signal from uninfected cells compared to signal from infected, untreated cells. The effective concentration of compound that inhibited 50% of the virus-induced cytopathic effects (EC50) was calculated by fitting the data to a logistic regression equation.
Pharmacokinetic studies of GS1 in calves.
The plasma pharmacokinetic parameters for GS1 were evaluated in male Holstein calves 12 weeks of age and weighing 180 to 230 lbs. The intravenous (i.v.) infusion was conducted via the jugular vein by slow i.v. bolus over a period of >2 min. Nasal swabs and blood samples were collected prior to GS1 administration and postdose at 0.08, 0.5, 1, 4, and 8 h on day 1 and day 4.
GS1 was formulated in sterile water with 5% dextrose (D5W) adjusted to pH 3 with 1 N HCl, sterile filtered through a 0.2-μm filter, and stored under refrigeration and protected from light. For a 2-mg/kg dose, GS1 was formulated at 10 mg/ml and administered at a dose volume of 0.2 ml/kg of body weight. For a 4-mg/kg dose, GS1 was formulated at 12 mg/ml and administered at a dose volume of 0.33 ml/kg.
Blood samples (approximately 1 ml each) were collected into labeled Vacutainer tubes containing EDTA-K3 as the anticoagulant and immediately placed on wet ice. Blood samples were centrifuged at 3,000 × g, the plasma fractions were transferred to separate tubes labeled with animal identification (ID) and sampling time and frozen immediately at −80°C.
Bronchoalveolar lavage fluid (BALF) was collected from each animal at 1 and 24 h after GS1 administration. Bronchoalveolar lavage was performed with phosphate-buffered saline (PBS) at room temperature in three aliquots with a total volume of 100 to 120 ml. The BALF samples were centrifuged at 300 × g for 10 min, and the supernatants and pellets were separated and frozen at −80°C until analysis.
Lung tissue was collected at necropsy at the end of the pharmacokinetic (PK) study. The samples were homogenized in 1 ml of PBS and stored at −80°C until analysis.
Liquid chromatography-mass spectrometry (LC-MS) analysis.
The high-performance liquid chromatography (HPLC) system consisted of a C18 column (50 × 3.0 mm) (Hypersil Gold column; Thermo Scientific, San Jose, CA) (5-μm particle size), Agilent 1200 HPLC pump, HTS PAL autosampler from LEAP Technologies (Carrboro, NC) with an API-4000 triple quadrupole mass spectrometer (Applied Biosystems, Foster City, CA) operating in multiple reaction monitoring mode (with positive electrospray ionization [ESI+], transition from 532.3 to 270.3, curtain gas of 25 liters/min, spray voltage of 5 kV, capillary temperature of 600°C, collision energy of 53 V). Mobile phase A contained water with 0.1% formic acid and 1% 2-propanol. Mobile phase B contained acetonitrile with 0.1% formic acid and 1% 2-propanol. The gradient was 5% to 95% mobile phase B in 3 min, followed by 95% mobile phase B for 1 min and equilibration time with 5% mobile phase B for 3 min at a flow rate of 0.5 ml/min. The average recovery of GS1 from plasma over a concentration range of 50 to 10,000 ng/ml was 68%. The detector response was found to be linear over 1 to 10,000 nM. The limit of detection was 2 nM in plasma and BALF and 6 nM in lung tissue. The intraday and interday accuracy of the quality control samples (150 to 7,500 ng/ml) ranged from 80 to 120%. The intraday and interday precision of the quality control samples (2 to 7,500 nM) ranged from 0.75% to 20%.
Because of the variable dilution of epithelial lining fluid (ELF) and nasal secretions during bronchoalveolar lavage and nasal swab wash, a dilution marker was used to determine the concentration of GS1 in the ELF and nasal secretion from the known BALF and nasal swab wash concentration. Endogenous urea was used as the dilution marker. For the analysis, 200-μl aliquots of 15N2-labeled urea (the internal standard) in methanol-acetonitrile-water (25%/25%/50%, vol/vol/vol; 200 μl) were added to BALF, nasal swab wash, and plasma samples (100 μl), and the samples were centrifuged, dried, and reconstituted in water for analysis. The analysis was performed with an HPLC system coupled to a Micromass Quattro Premier triple quadrupole mass spectrometer with an ESI+ source. An Aquasil C18 column (150 × 4.6 mm; 3 μm) was used with a shallow linear gradient of water-acetonitrile with 0.2% formic acid. The analytes were detected by monitoring the selected reaction monitoring (SRM) transitions (m/z 61 to 44 for urea and 63 to 45 for 15N2-labeled urea). The electrospray conditions were as follows: cone voltage of 25 V, source temperature of 135°C, and desolvation temperature of 435°C. The collision voltages were 12 and 11 V for urea and 15N2-labeled urea, respectively. The detector response for urea was found to be linear over 1 to 10,000 μM. The limit of detection was 2 μM in plasma BALF and nasal swab wash. The intraday and interday accuracy of the quality control samples (150 to 7,500 μΜ) ranged from 80 to 120%. The intraday and interday precision of the quality control samples (2 to 7,500 μM) ranged from 0.75% to 20%.
Pharmacokinetic analysis.
Pharmacokinetic parameters were calculated by model-independent methods using WinNonlin (version 5; SCI Software, Lexington, KY). Area under the drug concentration-time curve (AUC) and area under the first moment of the drug concentration-time curve (AUMC) were calculated by means of the trapezoidal rule and extrapolated to infinity (AUC from 0 h to infinity [AUC0–∞] and AUMC from 0 h to infinity [AUMC0–∞]). The elimination half-life at β phase (t1/2β) was calculated when possible from the slope of the terminal phase of the log plasma drug concentration-time points by linear regression. The volume of distribution at steady state (Vss) and systemic clearance (CL) were determined by the following formulas: Vss = (i.v. dose × AUMC0–∞)/AUC0–∞, and CL = i.v. dose/AUC0–∞.
Selection of cattle for antiviral efficacy studies.
Study protocols were approved by the University of California, Davis (UC Davis) Institutional Animal Care and Use Committee. Treatment of animals adhered to U.S. government principles for the utilization and care of vertebrate animals (38), the Guide for the Care and Use of Laboratory Animals (39), the Animal Welfare Act (40), and other applicable public laws and regulations. Male calves were obtained from local dairies and determined to be bRSV negative by qRT-PCR analysis of nasal swab samples. The calves were housed in individual runs at the UC Davis animal facility and allowed to acclimatize for at least 4 to 7 days. Four days prior to infection, all calves were treated for 3 consecutive days with a dose of the antibiotic cetiofur sodium Naxcel (1 to 2 ml/100 lb of body weight) by the intramuscular route. Calves were tested for gastrointestinal parasites and ectoparasites. Calves were also tested for salmonella if they arrived at the animal facility with symptoms of diarrhea. Only calves with body temperatures in the normal range that were bRSV negative and with lungs that auscultated within normal limits were selected for the study. Two catheters were inserted into the jugular vein in two calves in each group for dosing and PK sampling, and one jugular catheter was inserted into the remaining calves in each group for dosing only. Calves were housed individually to avoid contact that could disrupt catheter placement.
Randomization.
Calves were weighed, and an antibody titer test (indirect immunofluorescence assay [IFA] titer) was conducted on each calf (blood taken 2 or 3 days after arrival), and the mean bRSV antibody titer was calculated. Approximately 2 days prior to virus inoculation, the calves were randomized into groups such that the mean antibody titer and average body weight were similar in each group. The information on dosing for the groups was kept from the on-site study team until study completion (the study was performed in a blind manner).
Inoculation with bRSV.
Virus stocks amplified on BT cell cultures were used directly for inoculation. Approximately 5 ml of nebulized bRSV at 2 × 104 PFU/ml was administered to each calf over a 15- to 20-min period using a specially fitted face mask that covered the nose and mouth.
Experimental design. (i) Study 1.
Eight calves were randomized into two groups (groups 1 and 2 with four calves in each group) based upon weight and bRSV antibody titers (Fig. 2). On day 0, the animals were inoculated with bRSV as described above. On day 1 postinoculation, animals were administered GS1 at 2 mg/kg (group 1) or placebo (group 2) by i.v. injection twice daily (BID) for a total of 7 days. Nasal swabs and blood samples were collected daily for 10 days before each dose to measure viral load and drug levels, respectively. On day 7 postinoculation, BALF samples were collected to measure the viral load in the lung. Physical exams were conducted daily for the duration of the study to calculate the clinical symptom scores. The animals were euthanized at the end of the study, and relevant tissues were collected for histopathology evaluation.
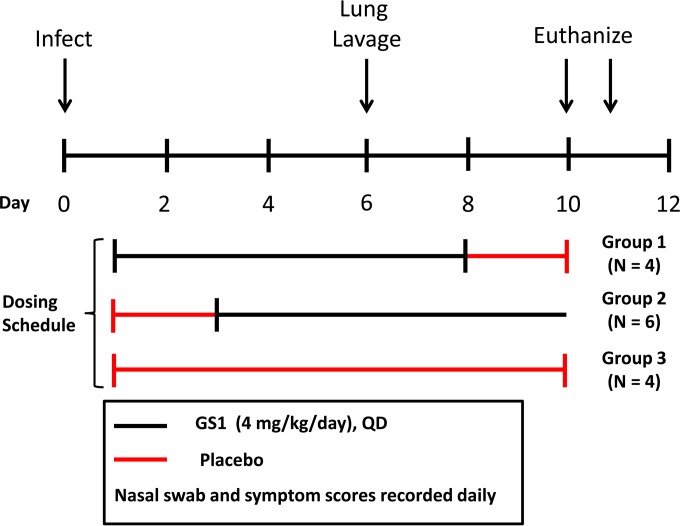
FIG 2.
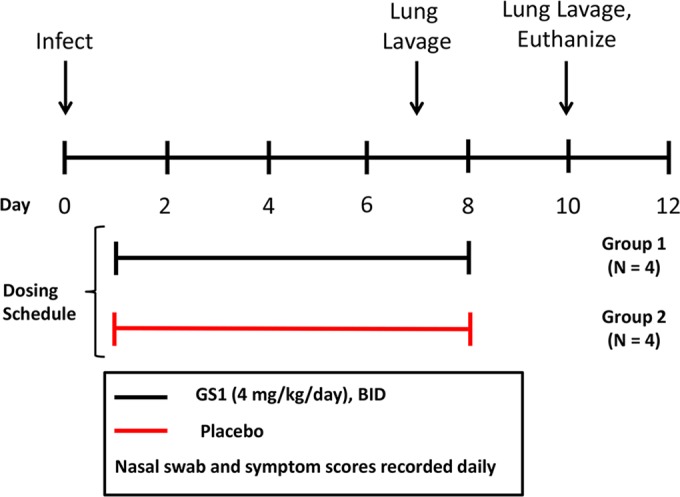
Experimental design for study 1. Calves were randomized into 2 groups based upon weight and bRSV antibody titers. On day 0, animals were inoculated with nebulized bRSV delivered to the respiratory tract through a face mask covering the nose and mouth. At 24 h postinoculation, each group of animals was administered GS1 at 2 mg/kg or placebo by intravenous injection BID for a total of 7 days. Nasal swabs and blood samples were collected daily for 10 days before each dose. On day 7 postinoculation, bronchiolar lavage was conducted on selected animals. Physical exams were conducted daily for the duration of the study to calculate the clinical symptom scores. The animals were euthanized on day 10 of the study, and tissues were collected for necropsy and histopathology.
(ii) Study 2.
Sixteen calves were enrolled and randomized into 3 groups (groups 1, 2, and 3) based upon weight and bRSV antibody titers (Fig. 3). Two calves in treatment group 3 (placebo) were excluded from the study prior to inoculation due to a possible blood clot and/or a bRSV-positive qRT-PCR test. The remaining animals (14 total) were inoculated with bRSV on day 0. One calf was included in the study but excluded from analyses due to a secondary infection. Group 1 (4 animals) was administered GS1 at 4 mg/kg/day once a day starting on day 1 and continuing for 7 days followed by administration of vehicle for 2 days. One animal in group 1 was treated but was excluded from analysis due to development of a secondary bacterial infection. Group 2 (6 animals) was treated with vehicle only for 2 days starting on day 1 followed by administration of GS1 at 4 mg/kg once a day for 7 days beginning on day 3 postinfection. Group 3 (4 animals) served as the placebo control group and received 9 consecutive vehicle treatments starting on day 1. Plasma samples were collected from 3 calves (2 from group 1 and 1 from group 3) on day 1 and 3 calves (2 from group 2 and 1 from group 3) on day 3 to quantify drug levels. Approximately 1 ml of blood was collected predose and at 0.3, 0.5, 1, 4, 8, and 24 h postdose. Nasal swabs and blood samples for the measurement of viral load and drug concentration were collected in the morning prior to the next dose for each animal. Physical exams were conducted daily for the duration of the study to calculate the clinical symptom scores. The animals were euthanized at the end of the study, and tissues were collected for histopathology evaluation.
FIG 3.
Experimental design for study 2. Fourteen calves were inoculated with nebulized bRSV on study day 0. Group 1 (4 animals) was administered GS1 at 4 mg/kg/day once per day starting on study day 1 and continuing for 7 days, followed by administration of vehicle for 2 days. Group 2 (6 animals) was treated with vehicle for 2 days starting on study day 1, followed by administration of GS1 at 4 mg/kg once per day for 7 days beginning on study day 3. Group 3 (4 animals) received 9 consecutive vehicle treatments starting on study day 1. Blood samples were taken for plasma isolation from 3 calves (2 from group 1 and 1 from group 3) on study day 1 and 3 calves from study day 3 (2 from group 2 and 1 from group 3) to quantify drug levels. Nasal swabs and a blood sample were taken in the morning prior to dosing of each animal to measure viral load and drug concentration, respectively. Physical exams were conducted daily for the duration of the study to calculate the clinical symptom scores. On study day 6, selected animals that appeared in reasonably good health underwent bronchial lavage. The animals were euthanized on study day 10 or 11, and tissues were collected for necropsy and histopathology.
Viral load measurements.
Nasal swabs were collected by inserting a sterile cotton swab in the posterior portion of the nasal cavity, withdrawing the swab, and immediately placing it with agitation in 1 ml of Hanks buffered saline solution in a sterile screw-cap tube on ice. Nasal epithelial cells were collected from these nasal swabs and isolated by centrifugation at 500 × g for 10 min. Lung alveolar macrophages were isolated from BALF by centrifugation at 500 × g for 10 min. Total RNA was isolated from nasal epithelia and alveolar macrophages using the RNeasy minikit (Qiagen). A total of 0.1 to 0.5 μg of RNA was reverse transcribed using a high-capacity reverse transcription kit (Applied Biosystems). qRT-PCR was performed using SYBR green master mix kit (Applied Biosystems) in a ViiA-7 real-time PCR (RT-PCR) system (Applied Biosystems). The amplification of the bRSV nucleocapsid (N) gene was used to monitor bRSV infection. The bovine glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was amplified as an internal control. A plasmid with an insert of full-length bRSV N gene and another plasmid with bovine GAPDH gene were used as standards to calculate viral copies and GAPDH copies in the analyzed RNA samples. Bovine RSV viral titer was reported as copies of N transcripts per 105 copies of GAPDH transcripts.
Symptom scoring.
Physical examinations were conducted prior to infection (baseline) and on each day of the study prior to dosing. Disease symptoms were recorded in a blind manner and used to calculate disease symptom scores. This method has previously been validated in numerous studies (32, 34, 36). The clinical symptom scores were based upon a compilation of observations and measurements that included temperature, respiratory rate, signs of anorexia, conjunctivitis, ocular discharge, nasal discharge, respiratory character, dyspnea, mouth breathing, cough, and auscultation. Respiratory rate was measured by a veterinarian (in a blind manner [unaware of the treatment group]) using a stethoscope to count inspirations for 1 min while timing with a second hand on a wrist watch.
Lung pathology.
Calves were euthanized humanely with an intravenous injection of barbiturate. The upper and lower respiratory tracts were removed and examined for gross lesions. Histopathology analysis was conducted in a blind manner by a board-certified veterinary pathologist.
Statistical analysis.
Data collected through day 10 postinfection were included in the analyses. Calf 908 (group 1) was excluded from analyses in study 2 due to a secondary bacterial infection. The area under the curve was computed for each calf for the log10 viral load, clinical symptom score, and respiration rate endpoints in each study. Before log10 transformation, a value of 1 was added to each measurement to prevent errors caused by log10 transformation of zero values. AUC of log10 viral load was assessed with a Wilcoxon rank sum test (two-sided test) (a P value of <0.05 was considered statistically significant) in study 1. All other endpoints were assessed with a rank analysis of covariance (ANCOVA) model (two-sided test) (a P value of <0.05 was considered statistically significant) with the baseline as a covariate to account for differing baseline values.
RESULTS
In vitro antiviral activity of GS1 against bRSV.
GS1 is a novel small-molecule RSV inhibitor that targets the RSV F protein and prevents virus entry into the host cell by inhibiting fusion of the viral envelope to the host cell membrane. The compound is a potent inhibitor of human RSV in vitro (mean EC50 = 0.3 ± 0.1 nM) but is inactive against other paramyxoviruses such as human metapneumovirus, parainfluenza virus, and henipaviruses (data not shown). The RSV F protein from bRSV is 82% identical at the amino acid level to the hRSV F protein, and amino acid residues known to confer drug resistance to fusion inhibitors in hRSV are 100% conserved. To determine whether GS1 was active against bRSV, the compound was evaluated for antiviral activity in bovine turbinate (BT) and bovine alveolar type 2 (BAT2) cells. The mean EC50s for GS1 in BT cells and BAT2 cells infected with bRSV were 0.36 ± 0.1 nM (n = 4) and 0.39 ± 0.3 nM (n = 4), respectively (Table 1), a potency consistent with that observed against hRSV. GS1 was not cytotoxic in the cell lines used for antiviral testing at the highest concentrations tested (25 μM).
TABLE 1.
Antiviral activity of GS1 for bRSVa
| Virus | Cell type | EC50 (nM) (SD) (n = 4) | CC50b (μM) (n = 4) |
|---|---|---|---|
| bRSV | BT | 0.36 (0.10) | >25 |
| BAT2 | 0.39 (0.30) | >25 | |
| hRSV | HEp-2 | 0.30 (0.10)c | >25 |
The mean values and standard deviations (SDs) are given.
CC50, concentration of 50% cellular cytotoxicity.
n = 14.
Multiple-dose pharmacokinetic profile of GS1 in calves.
The multiple-dose pharmacokinetic profiles for GS1 were measured in male Holstein calves to determine the safety, tolerability, and exposure of GS1 in plasma and in the respiratory tract (Table 2). These data were used to define the optimal dose level and dosing frequency for the subsequent efficacy studies. The stability of GS1 was similar in both bovine and human hepatic microsome assays in vitro. In addition, GS1 showed slightly higher plasma protein binding in bovine plasma than in human plasma (99.6% versus 97.1%, respectively). Twelve-week-old calves were administered GS1 as repeat i.v. injections at 4 mg/kg/day for 4 consecutive days. Blood samples were collected at selected time points, and plasma was isolated from the blood samples to measure compound concentrations. Nasal swab samples were collected concurrently with the blood samples, BALF samples were collected at 1 and 24 h after administration of the compound, and lung tissue was collected 24 h after the last dose at necropsy to measure compound concentrations.
TABLE 2.
Pharmacokinetic properties of GS1 in calves following 4-day once-a-day i.v. bolus administration (4 mg/kg)
| PK parametera (units) | Mean (range)b on: |
|
|---|---|---|
| Day 1 | Day 4 | |
| AUC0–last (nM · h) | 14,649 (9,723–19,575) | 23,251 (12,433–34,069) |
| CL (liter/h/kg) | 0.58 (0.37–0.78) | 0.39 (0.17–0.61) |
| Vss (liter/kg) | 3.7 (3.1–4.3) | 3.1 (3.2–3.0) |
| MRTINF (h) | 6.8 (5.5–8.2) | 11.3 (5.2–17.5) |
| t1/2β (h) | 6.0 (4.8–7.3) | 9.0 (4.9–13.2) |
| Cmax (nM) | 3,180 (2,270–4,090) | 2,880 (2,180–3,580) |
| C8 (nM) | 606 (291–921) | 1,154 (358–1,950) |
| C24 (nM) | 123 (44–201) | 340 (55–624) |
The pharmacokinetic (PK) parameters are as follows: AUC0–last, area under the concentration-time curve over 24-h dosing interval; CL, plasma clearance; Vss, volume of distribution at steady state; MRTINF, mean residence time; t1/2β, elimination half-life at β phase calculated from the slope of the terminal phase; Cmax, maximal concentration; C8, concentration at the 8-h time point; C24, concentration at the 24-h time point.
The mean (range) values for two animals are given.
After repeated 4-mg/kg doses of GS1, the plasma half-lives of GS1 were 6 h and 9 h on day 1 and 4, respectively. The area under the concentration-time curve over the 24-h dosing interval (AUC0–last) was 15,494 nM · h on day 1 (range, 9,723 to 19,575 nM · h) and 23,251 nM · h (range, 12,433 to 34,069 nM · h) on day 4. The data suggested an increase in systemic exposure of GS1 following multiple doses at 4 mg/kg/day. However, it should be noted that only a small number of animals (n = 2 per time point) was included in the pharmacokinetic analysis; therefore, the differences could not be assessed statistically. Notably, GS1 appeared to distribute into lung tissue. In a separate single-dose i.v. study, the GS1 concentration in lung tissue was 145-fold higher than the concentration in plasma (Table 3). Moreover, the concentration of GS1 was notably higher in BALF (15-fold) and nasal swab (4-fold) samples than in plasma samples (Table 3). These data indicate preferential distribution of GS1 into therapeutically relevant compartments and tissues.
TABLE 3.
Drug concentrations in different compartments at 24 h following administration of GS1 (4 mg/kg i.v. bolus) to healthy calves
| Compartment or sample | GS1 concn (μM) at 24 ha | Ratio to plasmab |
|---|---|---|
| Plasma | 5.8 | 1.0 |
| Nasal swab | 78c | 4.0 |
| BALF | 88 | 15 |
| Lung tissue | 1,140 | 195 |
Mean values for two animals.
Ratio of the GS1 concentration in the compartment or sample compared to the GS1 concentration in plasma.
At the 12-h time point.
Based upon the pharmacokinetic profile, the dose of 4 mg/kg/day was selected for subsequent efficacy evaluations to ensure adequate compound exposure for antiviral efficacy.
Therapeutic efficacy of GS1 administered twice daily (BID).
The efficacy of GS1 was assessed in randomized, placebo-controlled, blind studies to eliminate potential bias associated with symptom scoring and efficacy measurements (Fig. 2). The first study (study 1) evaluated BID administration of GS1 at 2 mg/kg/dose initiated 24 h after the bRSV inoculation. The study was conducted with 8 calves that were randomized into GS1-treated (group 1 [n = 4]) or placebo-treated (group 2 [n = 4]) groups. A physical exam was conducted daily on each animal, and symptoms of bRSV infection were recorded. Nasal wash samples were collected daily to measure viral load and drug levels. On day 6 postinfection, lung lavage samples were obtained for analysis of viral load in lung lavage fluid. On day 11, the animals were euthanized, and tissues were collected for histopathology.
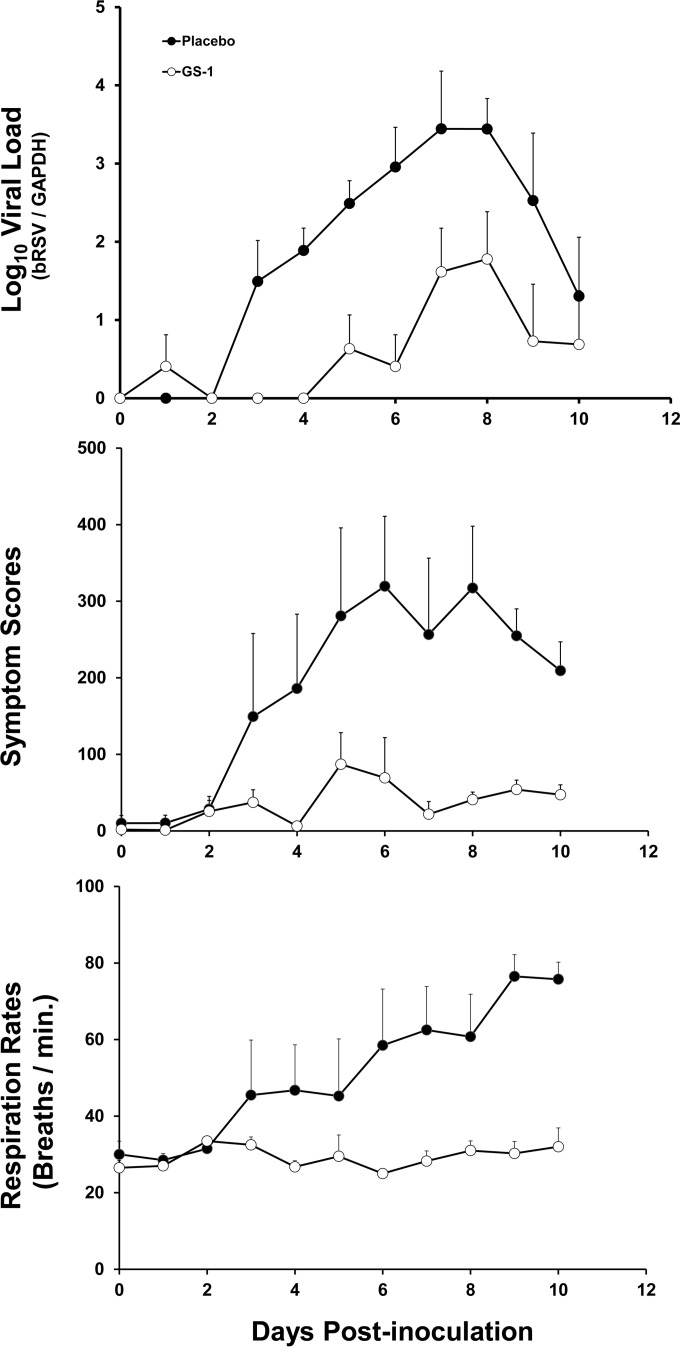
All inoculated calves became infected with bRSV as determined by positive qRT-PCR values from nasal wash samples. Most animals showed clinical signs of infection by day 2 postinoculation based upon symptom scores. In placebo-treated calves, the viral loads and symptom scores increased over the course of infection and reached a peak between day 6 and 8 postinoculation followed by a decline until the end of the study (Fig. 4). Respiratory rates increased over the course of the study even though viral loads and symptom scores were in decline.
FIG 4.
Mean viral loads, symptom scores, and respiration rates for calves in study 1. Viral loads were quantified from nasal swabs by qRT-PCR, and bRSV RNA values were normalized to GADPH cellular mRNA values. Cumulative symptom scores and respiration rates were calculated based upon daily physical exams for each animal. The mean values of viral load and each score (n = 4 per group) are plotted as a function of time postinoculation, and the positive error bars represent the standard errors of the means.
Calves treated twice daily with GS1 had reduced viral loads in nasal wash samples, lower symptom scores, and lower respiration rates compared to placebo-treated animals (Fig. 4 and Table 4; see Table S1 in the supplemental material). Mean AUC values of the viral load curve for GS1-treated and placebo-treated animals were 5.9 and 18.9 log10 bRSV/GAPDH equivalents · day/ml (P = 0.061), respectively. The mean AUC values of symptom scores in GS1-treated and placebo-treated animals were 366.3 and 1,912.4 score · day (P = 0.018), respectively. A decrease in respiration rates in treated animals compared to placebo-treated animals was also noted (293.0 versus 508.6 breaths · day/min (P = 0.027).
TABLE 4.
Viral loads, symptom scores, and respiration rates in bRSV-infected calves treated with GS1 or placebo BID (study 1)
| Parameter (units) | Meana (SD) for group treated with: |
P value | |
|---|---|---|---|
| GS1 (n = 4) | Vehicle (n = 4) | ||
| AUC of log10 viral load (log10 genome equivalents · day/ml) | 5.9 (3.3) | 18.9 (7.2) | 0.061b |
| AUC of symptom score (score · day) | 366.3 (278.1) | 1,912.4 (1,172.5) | 0.018c |
| AUC of respiration rate (no. of breaths · day/min) | 293.0 (35.1) | 508.6 (144.7) | 0.027c |
The median values were similar to the mean values.
Wilcoxon rank sum test.
Rank ANCOVA.
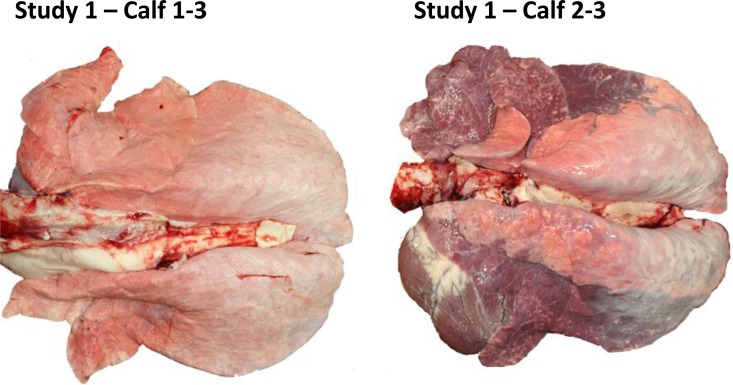
Gross and histological lung pathologies were evaluated. There were clear differences observed between the placebo-treated group and the GS1 treatment group with regard to the percentage of lung consolidation and presence of typical bRSV lesions (Fig. 5; see Table S3 in the supplemental material). The mean percent lung consolidation for placebo-treated calves was 33.8% compared to 0% for GS1-treated calves. The lung consolidation was estimated by the pathologist by calculating the percent consolidation for each lobe and the overall consolidation for the lung. Bronchointerstitial pneumonia was a common finding in the infected placebo-treated calves, but it was not observed in GS1-treated animals. Placebo-treated calves also had evidence of bronchiolitis obliterans and type II epithelial cell hyperplasia, and several calves had syncytial cells present in lung sections. Three of four placebo-treated calves had bRSV detected in lung sections by immunohistochemistry, while in contrast, none of the GS1-treated calves had bRSV antigen detected in lung sections. Together, these results demonstrate that BID dosing of GS1 initiated at 24 h postinoculation reduced virus replication and clinical symptoms as well as disease pathology associated with bRSV infection.
FIG 5.
Lung tissue from calves in study 1 treated with GS1 or placebo. Calf 1-3 (calf 3 from group 1) (treated with GS1) showed no gross lesions with 0% lung consolidation, with the exception of a focal fibrous adhesion on the thoracic wall. In addition, there was mild neutrophilic bronchial exudate with occasional lymphoid aggregates in bronchial and bronchiolar epithelium, and there was no active bronchopneumonia or exudation in lung. No evidence of bRSV was detected by immune staining of lung tissue. In contrast, calf 2-3 (calf 3 from group 2) (treated with placebo) showed 60% lung consolidation and widespread bronchointerstitial pneumonia with neutrophilic exudation in which bronchioles had hyperplastic epithelium with focal necrosis and surrounding lymphoplasmocytic infiltrate. Multifocal epithelial syncytia and syncytia in alveolar spaces was observed. Immune staining showed bRSV-positive signal in the lung tissue. A description of the histopathology results for individual animals is shown in Table S3 in the supplemental material.
Therapeutic efficacy of GS1 administered QD.
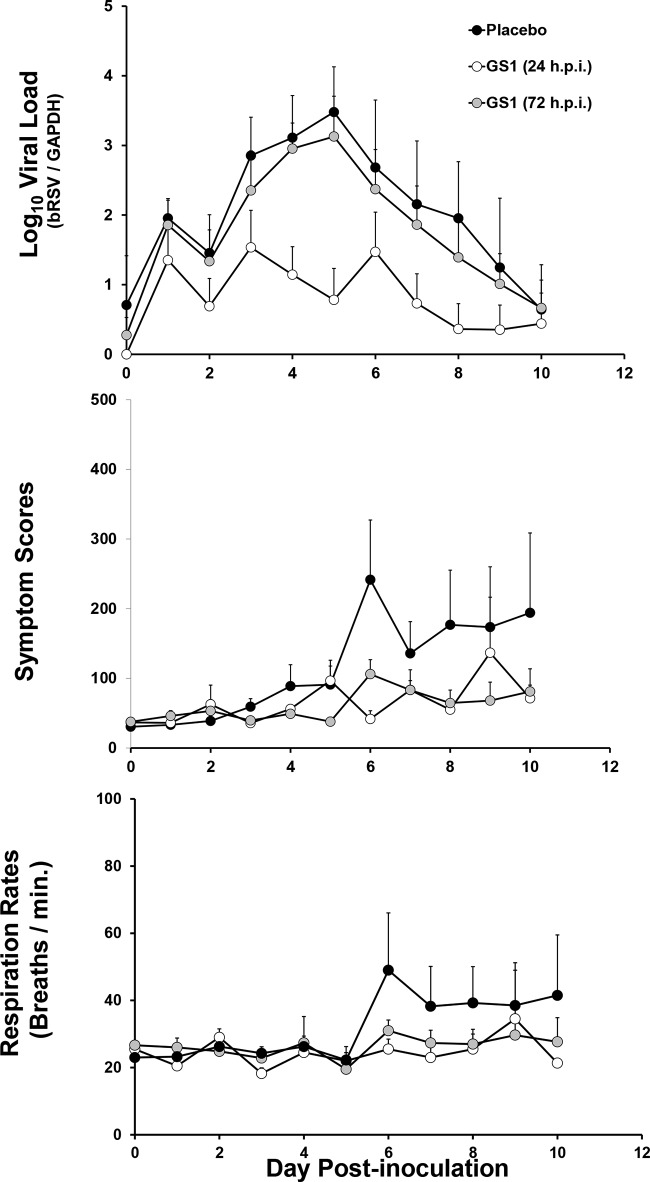
Following demonstration of the efficacy of BID dosing of GS1, a second randomized placebo-controlled study was conducted to assess the efficacy of the compound dosed once daily (QD). In addition, this study was also designed to compare the efficacy of early versus delayed initiation of treatment with GS1. Fourteen male calves 4 to 6 weeks of age were randomized into three groups and infected with bRSV. The calves were treated with GS1 at 4 mg/kg once daily initiated at 24 h (group 1) or 72 h (group 2) after bRSV inoculation or treated with placebo (group 3; Fig. 3). Treatment was administered for a total of 7 days, and the viral load was measured from nasal swabs taken daily and BALF samples taken at day 6 postinoculation and at the end of the study. Clinical symptom scores were assessed daily as described for study 1.
Initiation of treatment at day 1 and day 3 with QD administration of 4 mg/kg GS1 to bRSV-infected calves reduced viral loads as well as clinical symptom scores and respiration rates compared to placebo treatment (Fig. 6 and Table 5). However, the magnitude of the antiviral effects was greater in animals that received the early treatment at day 1 postinoculation. The treatment with GS1 initiated at day 1 and day 3 reduced the mean viral load AUC by 94% and 50%, respectively, compared to the values for treatment with placebo (AUCs of 8.6, 18.8, and 21.6 log10 bRSV/GAPDH equivalents · day/ml for the day 1 treatment initiation, day 3 treatment initiation, and placebo treatment, respectively) (Table 5; see Table S2 in the supplemental material). Similar trends were observed for the reduction in AUCs of clinical symptom scores and respiration rates in the GS1-treated groups versus placebo-treated group (Fig. 6; individual animal data in Table S2 in the supplemental material).
FIG 6.
Mean viral loads, symptom scores, and respiration rates for calves in study 2. Viral loads were quantified from nasal swabs by qRT-PCR, and bRSV RNA values were normalized to GADPH cellular mRNA. Cumulative symptom scores and respiration rates were calculated based upon daily physical exams of each animal. The mean values for placebo-treated calves (n = 3) and GS1-treated calves on day 1 of treatment (n = 4) (white circles) or day 3 of treatment (n = 6) (gray circles) are plotted as a function of time postinfection (p.i.), and the positive error bars represent the standard errors of the means.
TABLE 5.
Viral loads, symptom scores, and respiration rates in bRSV-infected cattle treated with QD GS1 or placebo (study 2)
| Parameter (units) | Mean (SD) for group treated witha: |
P valuec | ||
|---|---|---|---|---|
| GS1 |
Vehicle (n = 4) | |||
| Early (n = 3)b | Delayed (n = 6) | |||
| AUC of log10 viral load (log10 genome equivalents · day/ml) | 8.6 (8.0) | 18.8 (8.1) | 21.6 (12.3) | 0.33 |
| AUC of symptom score (score · day) | 657.7 (182.6) | 606.4 (297.3) | 1,150.8 (643.7) | 0.18 |
| AUC of respiration rate (no. of breaths · day/min) | 221.0 (17.1) | 262.7 (60.6) | 319.5 (110.4) | 0.22 |
The GS1 groups were treated early (24 h postinoculation [h.p.i.]) or later (delayed) (72 h.p.i.) The median values were similar to the mean values.
One animal (group 1, calf 1-3 [calf 3 from group 1]) was removed from analysis due to secondary infection.
Rank ANCOVA testing the null hypothesis of no difference between any treatment.
Differences in lung pathology were identified between GS1- and placebo-treated animals. The mean percent lung consolidation values were 7.5 and 9.4 in animals from early and delayed treatment groups, respectively, compared to 22.4 consolidation observed in placebo-treated group animals. While the evaluation of the lung tissue lesions was somewhat complicated by the presence of a common bovine lung pathogen Mycoplasma bovis, the bronchiolitis and bronchointerstitial pneumonia associated with bRSV infection was observed primarily in the placebo-treated calves (Fig. 7; see Table S4 in the supplemental material).
FIG 7.
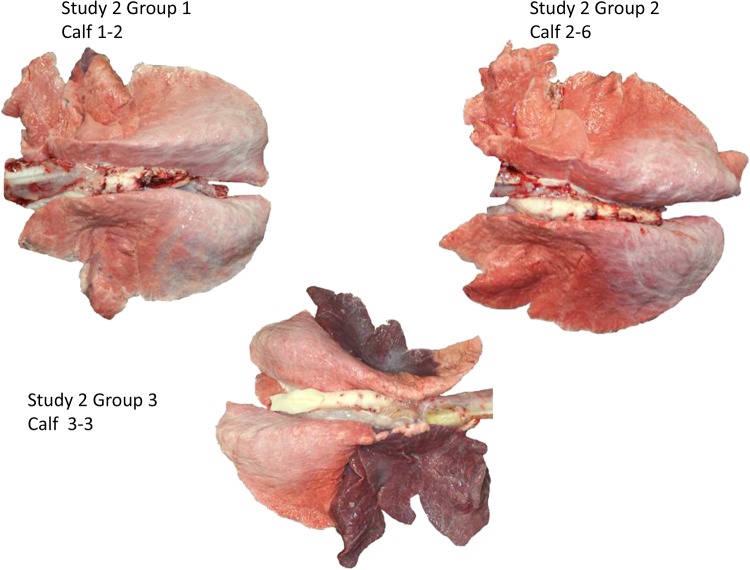
Lung tissue from calves in study 2 treated with GS1 or placebo. Lung tissue from calf 1-2 (calf 2 from group 1) from study group 1 (GS1 treatment at 24 h postinfection) showed minimal multifocal multilobular atelectasis (<1% of lung mass) and mild bilateral neutrophilic histiocytic bronchopneumonia. Mycoplasma bovis was isolated from the tissue, but bRSV antigen was not detected by immunostaining. Lung tissue from calf 2-6 (calf 6 from group 2) from study group 2 (GS1 treatment at 72 h postinfection) showed minimal focal multilobular consolidation in the right middle lobe (<1% of lung mass) with regional focal multilobular atelectasis with minimal histiocytic neutrophilic bronchopneumonia. Mycoplasma bovis was isolated from lung tissue but was negative for bRSV by immune staining. Lung tissue from calf 3-3 (calf 3 from group 3) from study group 3 (placebo-treated animal) showed severe bilateral anteroventral pulmonary consolidation (45 to 50% of the lung mass). The affected lung lobes showed extensive multilobular areas of neutrophilic bronchopneumonia with multifocal necrosuppurative bronchiolitis and focal necrosis. Arcanobacterium pyogenes and Mycoplasma bovis were isolated from lung tissue, but bRSV was not detected by immune staining. A description of the histopathology results for individual animals is shown in Table S4 in the supplemental material.
DISCUSSION
RSV infection can lead to serious respiratory illness among high-risk pediatric, elderly, and immunocompromised patients. Currently, there are no effective antiviral agents available for the treatment of RSV infection in these high-risk patient populations. One of the limitations of developing new clinically effective antiviral therapies is the lack of animal models of RSV infection with validated clinical endpoints that mirror the pathogenesis of human infection. Experimental infection of Holstein calves with bRSV causes respiratory disease with similar viral dynamics, pathology, and clinical symptoms relative to pediatric human RSV infection, making this a candidate model system to study efficacy of antiviral therapeutics.
During the course of bRSV infection, viral load quantification can be conducted by qRT-PCR using an internal host gene (GADPH) as a standard to normalize for total RNA input, thereby reducing the variability related to sample collection. The optimized nasal swab procedure maximizes the recovery of nasal epithelial cells which harbor significant amounts of virus relative to virus found in the mucous layer. Quantification of viral load in nasal secretions and in lung lavage fluids of the infected animals over the course of the infection detected peak viral titers between 6 and 8 days postinoculation, with a subsequent decline in viral load.
Calves inoculated with bRSV develop respiratory symptoms within 3 days postinoculation, reaching a peak between 6 to 9 days postinoculation, followed by decline in symptoms observed until the infection resolves (32). Symptoms include mucous hypersecretion, spontaneous cough, occasional wheeze, dyspnea, and increased respiratory rates with high viral loads detected in nasal secretions and in BALF samples. Composite symptom scoring is an effective method to quantify observations conducted during physical exams (41, 42). The composite scoring system used in this study was designed to quantify observed physical symptoms that relate to disease severity. To reduce the potential for experimental bias introduced by subjective scoring, all examinations of clinical symptoms were conducted in a blind manner by an independent assessor. While there is a general correlation between viral load in the nasal secretions and the observed severity of symptoms, the symptom scoring could have been complicated by the presence of a secondary lung infection with other bovine pathogens such as Mycoplasma bovis. In most animals, this can be successfully managed by conditioning animals with antibiotics prior to their enrollment in efficacy studies. Histopathology evaluation of lung tissue at necropsy mirrors symptom scores with evidence of lung consolidation, virus-induced syncytia, bronchiolitis, and interstitial pneumonia in untreated animals infected with bRSV (see Tables S3 and S4 in the supplemental material).
Overall, the ability to objectively quantify viral load, symptom scores, respiratory parameters, and lung pathology indicate that bRSV-infected calves represent a robust model to test the in vivo efficacy of novel RSV therapeutics. This is in contrast to other RSV models that are typically reported for the evaluation of RSV therapies that do not adequately model the pathogenesis of the infection.
The utility of the bRSV infection model for evaluation of antiviral therapeutics was validated using GS1, a novel small-molecule inhibitor with potent in vitro activity against both bRSV and hRSV. GS1 is a close structural analog of GS-5806, which is currently in clinical development for treatment of RSV infection (43). GS-5806 has been tested in healthy adult human subjects experimentally infected with a clinical strain of RSV (20). Oral GS-5806 administration was initiated following the confirmation of RSV infection by PCR analysis of nasal wash samples and continued for 5 days. Various GS-5806 treatment regimens, including single-dose administration reduced the viral load in nasal secretions, mucous weight, and clinical symptom scores.
Consistent with pharmacokinetic parameters predictive of antiviral efficacy, the administration of GS1 once or twice a day for 7 days to bRSV-infected animals reduced viral loads and disease symptom scores relative to placebo-treated animals. In addition, GS1-treated animals showed improved respiratory function with respiration rates approaching baseline levels. While BID treatment initiated at 24 h postinoculation had the most profound impact on viral load, symptom scores, and lung pathology, delayed QD administration initiated at 72 h postinoculation also showed evidence of antiviral efficacy. Examination of lung tissue at necropsy demonstrated clear differences in histopathology between GS1- and placebo-treated animals, even in animals that received drug treatment at 72 h postinoculation (see Table S4 in the supplemental material). Drug-treated animals had reduced lung consolidation and minimal evidence of bronchiolitis and pneumonia. These results indicate that virus replication in the respiratory tracts of infected calves drives the disease and that inhibition of virus replication with GS1 provides clinical benefit. Importantly, all dosing regimens of GS1 were found to be safe and well tolerated in treated Holstein calves.
The present model of bRSV infection relies on aerosolized nasal delivery of virus which ensures that both the upper and lower respiratory tract are infected in all of the inoculated animals. While most human RSV infections begin in the upper respiratory tract and cause mild disease with cold-like symptoms that resolve without the need for medical intervention, in some high-risk individuals, including a relatively large proportion of the pediatric population, the infection will spread into the lower respiratory tract. Lower respiratory tract infections typically result in more severe disease and are the most common cause of RSV-associated bronchiolitis and pneumonia that often require hospitalization. Consistent with human disease, lung pathology in bRSV-infected calves showed evidence of bronchiolitis and pneumonia prominent in placebo-treated animals, indicating that bRSV replication in the lower respiratory tracts of young calves causes a significant respiratory disease.
Treatment of upper respiratory tract infections with antivirals can prevent progression of RSV infection to the lower respiratory tract and potentially reduce the need for medical intervention (15). While the current bovine RSV model provides a means for quantifying bRSV disease endpoints, it does not model the kinetics of progression from upper to lower respiratory tract infection observed in humans. Administration of virus by intranasal inoculation instead of aerosolized delivery might provide a more natural route of infection that could mirror the progression of infection in humans, but it would require substantial additional optimization. The mode of transmission of naturally occurring bRSV infection is thought to be identical to the mode of transmission in humans (44). Aerosolized virus enters the upper respiratory tract, and the resulting infection causes upper respiratory tract symptoms that resolve without medical intervention. Other factors, such as environmental stress or reduced host defense mechanisms that restrict virus replication in the lung, increase the risk of developing pneumonia and bronchiolitis.
One of the challenges of the current model is the natural variability in the endpoints measured primarily due to differences in the permissiveness to experimental RSV infection among individual animals. While consistent trends were observed in the current study with respect to antiviral efficacy, the small sample sizes and variability in endpoint measurements reduced the statistical power for some of the measured therapeutic effects observed in treated animals. Although the study outcome could be improved by increasing the number of animals enrolled in the study, this would bring additional technical challenges associated with effectively handling increased numbers of large animals during the therapeutic study.
An additional challenge is the variable homology of bRSV to hRSV requiring that compounds developed to treat hRSV be evaluated for antiviral activity in vitro against bRSV to confirm cross-reactivity before considering in vivo evaluation in the bovine efficacy model. A survey of published RSV antiviral compounds identified several compounds that target both bRSV and hRSV (see Table S5 in the supplemental material). Polymerase inhibitors ribavirin and BI-D were found to be active against both bRSV and hRSV, while the fusion inhibitor TMC-353121, but not BMS-433771, was active against both viruses. The RSV nucleocapsid inhibitor RSV-604 and the RSV polymerase inhibitor YM-53403 were active only against hRSV. The differential responses to these inhibitor classes against bovine RSV underscore the need to evaluate inhibitors in vitro before testing in the bovine RSV model.
While GS1 provided measurable antiviral efficacy resulting in reductions in viral loads, the emergence of compound-resistant variants was not evaluated. In these studies, we did not isolate replication-competent virus from nasal secretions to measure virus susceptibility to GS1 after treatment.
In conclusion, GS1 treatment of Holstein calves experimentally infected with bRSV provided validation of this animal model for assessing the efficacy of experimental therapeutics for treatment of RSV infection. The pathophysiology of disease in this model is similar to human RSV infection, and the availability of quantitative clinical endpoints provides a robust system for evaluating the efficacy of RSV therapeutics. GS1 treatment initiated up to 3 days postinoculation provided measurable antiviral and clinical efficacy, demonstrating that RSV fusion is an effective target for therapeutic antiviral intervention. These data are consistent with a recent study demonstrating clinical efficacy of GS-5806, an RSV fusion inhibitor and close analog of GS1, in humans experimentally infected with RSV (20). Importantly, since experimental RSV infection in humans is restricted primarily to the upper respiratory tract, the outcome of GS1 testing in the bRSV infection model suggests that this novel class of RSV fusion inhibitors can effectively suppress the RSV infection both in the upper and lower respiratory tracts. In summary, the results of the present studies indicate that bovine RSV infection in calves represents a relevant animal model for the assessment of in vivo efficacy for RSV therapeutics and should help advance the preclinical testing and development of novel interventions for the treatment of RSV infection.
Supplementary Material
ACKNOWLEDGMENT
This work was funded by Gilead Sciences, Inc.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00487-15.
REFERENCES
- 1.Neilson KA, Yunis EJ. 1990. Demonstration of respiratory syncytial virus in an autopsy series. Pediatr Pathol 10:491–502. doi: 10.3109/15513819009067138. [DOI] [PubMed] [Google Scholar]
- 2.Zhang L, Peeples ME, Boucher RC, Collins PL, Pickles RJ. 2002. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J Virol 76:5654–5666. doi: 10.1128/JVI.76.11.5654-5666.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ioannidis I, McNally B, Willette M, Peeples ME, Chaussabel D, Durbin JE, Ramilo O, Mejias A, Flano E. 2012. Plasticity and virus specificity of the airway epithelial cell immune response during respiratory virus infection. J Virol 86:5422–5436. doi: 10.1128/JVI.06757-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borchers AT, Chang C, Gershwin ME, Gershwin LJ. 2013. Respiratory syncytial virus-a comprehensive review. Clin Rev Allergy Immunol 45:331–379. doi: 10.1007/s12016-013-8368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, Auinger P, Griffin MR, Poehling KA, Erdman D, Grijalva CG, Zhu Y, Szilagyi P. 2009. The burden of respiratory syncytial virus infection in young children. N Engl J Med 360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falsey AR. 2005. Respiratory syncytial virus infection in elderly and high-risk adults. Exp Lung Res 31(Suppl 1):77. [PubMed] [Google Scholar]
- 7.Waghmare A, Campbell AP, Xie H, Seo S, Kuypers J, Leisenring W, Jerome KR, Englund JA, Boeckh M. 2013. Respiratory syncytial virus lower respiratory disease in hematopoietic cell transplant recipients: viral RNA detection in blood, antiviral treatment, and clinical outcomes. Clin Infect Dis 57:1731–1741. doi: 10.1093/cid/cit639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson DJ, Lemanske RF Jr. 2010. The role of respiratory virus infections in childhood asthma inception. Immunol Allergy Clin North Am 30:513–522. doi: 10.1016/j.iac.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Busse WW, Lemanske RF Jr, Gern JE. 2010. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet 376:826–834. doi: 10.1016/S0140-6736(10)61380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wedzicha JA. 2004. Role of viruses in exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc 1:115–120. doi: 10.1513/pats.2306030. [DOI] [PubMed] [Google Scholar]
- 11.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. 2005. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 12.Romero JR. 2003. Palivizumab prophylaxis of respiratory syncytial virus disease from 1998 to 2002: results from four years of palivizumab usage. Pediatr Infect Dis J 22(Suppl 2):S46–S54. doi: 10.1097/00006454-200302001-00007. [DOI] [PubMed] [Google Scholar]
- 13.Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH. 1969. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 89:422–434. [DOI] [PubMed] [Google Scholar]
- 14.American Academy of Pediatrics Committee on Infectious Diseases. 1993. Use of ribavirin in the treatment of respiratory syncytial virus infection. Pediatrics 92:501–504. [PubMed] [Google Scholar]
- 15.Shah DP, Ghantoji SS, Shah JN, El Taoum KK, Jiang Y, Popat U, Hosing C, Rondon G, Tarrand JJ, Champlin RE, Chemaly RF. 2013. Impact of aerosolized ribavirin on mortality in 280 allogeneic haematopoietic stem cell transplant recipients with respiratory syncytial virus infections. J Antimicrob Chemother 68:1872–1880. doi: 10.1093/jac/dkt111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seo S, Campbell AP, Xie H, Chien JW, Leisenring WM, Englund JA, Boeckh M. 2013. Outcome of respiratory syncytial virus lower respiratory tract disease in hematopoietic cell transplant recipients receiving aerosolized ribavirin: significance of stem cell source and oxygen requirement. Biol Blood Marrow Transplant 19:589–596. doi: 10.1016/j.bbmt.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olszewska W, Ispas G, Schnoeller C, Sawant D, Van de Casteele T, Nauwelaers D, Van Kerckhove B, Roymans D, De Meulder M, Rouan MC, Van Remoortere P, Bonfanti JF, Van Velsen F, Koul A, Vanstockem M, Andries K, Sowinski P, Wang B, Openshaw P, Verloes R. 2011. Antiviral and lung protective activity of a novel respiratory syncytial virus fusion inhibitor in a mouse model. Eur Respir J 38:401–408. doi: 10.1183/09031936.00005610. [DOI] [PubMed] [Google Scholar]
- 18.Cianci C, Genovesi EV, Lamb L, Medina I, Yang Z, Zadjura L, Yang H, D'Arienzo C, Sin N, Yu KL, Combrink K, Li Z, Colonno R, Meanwell N, Clark J, Krystal M. 2004. Oral efficacy of a respiratory syncytial virus inhibitor in rodent models of infection. Antimicrob Agents Chemother 48:2448–2454. doi: 10.1128/AAC.48.7.2448-2454.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wyde PR, Laquerre S, Chetty SN, Gilbert BE, Nitz TJ, Pevear DC. 2005. Antiviral efficacy of VP14637 against respiratory syncytial virus in vitro and in cotton rats following delivery by small droplet aerosol. Antiviral Res 68:18–26. doi: 10.1016/j.antiviral.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 20.DeVincenzo J, Whitley RJ, Scaglioni C, Harrison L, Farrell E, McBride S, Mackman RL, Jordan R, Xin Y, Ramanathan S, O'Riordan T, Lewis SA, Li X, Toback SL, Lin SL, Chien JW. 2014. Oral GS-5806 reduces viral load and clinical symptoms in a respiratory syncytial virus challenge study. N Engl J Med 371:711–722. doi: 10.1056/NEJMoa1401184. [DOI] [PubMed] [Google Scholar]
- 21.Henderson EA, Alber DG, Baxter RC, Bithell SK, Budworth J, Carter MC, Chubb A, Cockerill GS, Dowdell VC, Fraser IJ, Harris RA, Keegan SJ, Kelsey RD, Lumley JA, Stables JN, Weerasekera N, Wilson LJ, Powell KL. 2007. 1,4-Benzodiazepines as inhibitors of respiratory syncytial virus. The identification of a clinical candidate. J Med Chem 50:1685–1692. [DOI] [PubMed] [Google Scholar]
- 22.Sudo K, Miyazaki Y, Kojima N, Kobayashi M, Suzuki H, Shintani M, Shimizu Y. 2005. YM-53403, a unique anti-respiratory syncytial virus agent with a novel mechanism of action. Antiviral Res 65:125–131. doi: 10.1016/j.antiviral.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Liuzzi M, Mason SW, Cartier M, Lawetz C, McCollum RS, Dansereau N, Bolger G, Lapeyre N, Gaudette Y, Lagace L, Massariol MJ, Do F, Whitehead P, Lamarre L, Scouten E, Bordeleau J, Landry S, Rancourt J, Fazal G, Simoneau B. 2005. Inhibitors of respiratory syncytial virus replication target cotranscriptional mRNA guanylylation by viral RNA-dependent RNA polymerase. J Virol 79:13105–13115. doi: 10.1128/JVI.79.20.13105-13115.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prince GA, Jenson AB, Horswood RL, Camargo E, Chanock RM. 1978. The pathogenesis of respiratory syncytial virus infection in cotton rats. Am J Pathol 93:771–791. [PMC free article] [PubMed] [Google Scholar]
- 25.Boukhvalova MS, Prince GA, Blanco JC. 2009. The cotton rat model of respiratory viral infections. Biologicals 37:152–159. doi: 10.1016/j.biologicals.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bem RA, Domachowske JB, Rosenberg HF. 2011. Animal models of human respiratory syncytial virus disease. Am J Physiol Lung Cell Mol Physiol 301:L148–L156. doi: 10.1152/ajplung.00065.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Derscheid RJ, Ackermann MR. 2012. Perinatal lamb model of respiratory syncytial virus (RSV) infection. Viruses 4:2359–2378. doi: 10.3390/v4102359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eyles JE, Johnson JE, Megati S, Roopchand V, Cockle PJ, Weeratna R, Makinen S, Brown TP, Lang S, Witko SE, Kotash CS, Li J, West K, Maldonado O, Falconer DJ, Lees C, Smith GJ, White P, Wright P, Loudon PT, Merson JR, Jansen KU, Sidhu MK. 2013. Nonreplicating vaccines can protect African green monkeys from the Memphis 37 strain of respiratory syncytial virus. J Infect Dis 208:319–329. doi: 10.1093/infdis/jit169. [DOI] [PubMed] [Google Scholar]
- 29.Vaughan K, Rhodes GH, Gershwin LJ. 2005. DNA immunization against respiratory syncytial virus (RSV) in infant rhesus monkeys. Vaccine 23:2928–2942. doi: 10.1016/j.vaccine.2004.10.046. [DOI] [PubMed] [Google Scholar]
- 30.Derscheid RJ, van Geelen A, Gallup JM, Kienzle T, Shelly DA, Cihlar T, King RR, Ackermann MR. 2014. Human respiratory syncytial virus Memphis 37 causes acute respiratory disease in perinatal lamb lung. Biores Open Access 3:60–69. doi: 10.1089/biores.2013.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elazhary MA, Galina M, Roy RS, Fontaine M, Lamothe P. 1980. Experimental infection of calves with bovine respiratory syncytial virus (Quebec strain). Can J Comp Med 44:390–395. [PMC free article] [PubMed] [Google Scholar]
- 32.Gershwin LJ, Anderson ML, Wang C, Berghaus LJ, Kenny TP, Gunther RA. 2011. Assessment of IgE response and cytokine gene expression in pulmonary efferent lymph collected after ovalbumin inhalation during experimental infection of calves with bovine respiratory syncytial virus. Am J Vet Res 72:134–145. doi: 10.2460/ajvr.72.1.134. [DOI] [PubMed] [Google Scholar]
- 33.Valarcher JF, Taylor G. 2007. Bovine respiratory syncytial virus infection. Vet Res 38:153–180. doi: 10.1051/vetres:2006053. [DOI] [PubMed] [Google Scholar]
- 34.Gershwin LJ, Schelegle ES, Gunther RA, Anderson ML, Woolums AR, Larochelle DR, Boyle GA, Friebertshauser KE, Singer RS. 1998. A bovine model of vaccine enhanced respiratory syncytial virus pathophysiology. Vaccine 16:1225–1236. doi: 10.1016/S0264-410X(98)80123-0. [DOI] [PubMed] [Google Scholar]
- 35.Zekarias B, Mattoo S, Worby C, Lehmann J, Rosenbusch RF, Corbeil LB. 2010. Histophilus somni IbpA DR2/Fic in virulence and immunoprotection at the natural host alveolar epithelial barrier. Infect Immun 78:1850–1858. doi: 10.1128/IAI.01277-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gershwin LJ, Berghaus LJ, Arnold K, Anderson ML, Corbeil LB. 2005. Immune mechanisms of pathogenetic synergy in concurrent bovine pulmonary infection with Haemophilus somnus and bovine respiratory syncytial virus. Vet Immunol Immunopathol 107:119–130. doi: 10.1016/j.vetimm.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Berghaus LJ, Corbeil LB, Berghaus RD, Kalina WV, Kimball RA, Gershwin LJ. 2006. Effects of dual vaccination for bovine respiratory syncytial virus and Haemophilus somnus on immune responses. Vaccine 24:6018–6027. doi: 10.1016/j.vaccine.2006.03.077. [DOI] [PubMed] [Google Scholar]
- 38.US Interagency Research Animal Committee. 1991. Principles for the utilization and care of vertebrate animals used in testing, research, and training. National Academies Press, Washington, DC. [Google Scholar]
- 39.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 40.US Congress. 1966. Public Law 89-544 Act of August 24, 1966. United States Code, Title 7, Sections 2131-2156. [Google Scholar]
- 41.Walter MJ, Castro M, Kunselman SJ, Chinchilli VM, Reno M, Ramkumar TP, Avila PC, Boushey HA, Ameredes BT, Bleecker ER, Calhoun WJ, Cherniack RM, Craig TJ, Denlinger LC, Israel E, Fahy JV, Jarjour NN, Kraft M, Lazarus SC, Lemanske RF Jr, Martin RJ, Peters SP, Ramsdell JW, Sorkness CA, Sutherland ER, Szefler SJ, Wasserman SI, Wechsler ME. 2008. Predicting worsening asthma control following the common cold. Eur Respir J 32:1548–1554. doi: 10.1183/09031936.00026808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ricart S, Marcos MA, Sarda M, Anton A, Munoz-Almagro C, Pumarola T, Pons M, Garcia-Garcia JJ. 2013. Clinical risk factors are more relevant than respiratory viruses in predicting bronchiolitis severity. Pediatr Pulmonol 48:456–463. doi: 10.1002/ppul.22633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mackman RL, Sangi M, Sperandio D, Parrish JP, Eisenberg E, Perron M, Hui H, Zhang L, Siegel D, Yang H, Saunders O, Boojamra C, Lee G, Samuel D, Babaoglu K, Carey A, Gilbert BE, Piedra PA, Strickley R, Iwata Q, Hayes J, Stray K, Kinkade A, Theodore D, Jordan R, Desai MC, Cihlar T. 2015. Discovery of an oral respiratory syncytial virus (RSV) fusion inhibitor (GS-5806) and clinical proof of concept in a human RSV challenge study. J Med Chem 58:1630–1643. doi: 10.1021/jm5017768. [DOI] [PubMed] [Google Scholar]
- 44.Sacco RE, McGill JL, Pillatzki AE, Palmer MV, Ackermann MR. 2014. Respiratory syncytial virus infection in cattle. Vet Pathol 51:427–436. doi: 10.1177/0300985813501341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.