Abstract
Cryptococcus gattii is the main etiological agent of cryptococcosis in immunocompetent individuals. The triazole drug itraconazole is one of the antifungals used to treat patients with cryptococcosis. Heteroresistance is an adaptive mechanism to counteract the stress of increasing drug concentrations, and it can enhance the ability of a microorganism to survive under antifungal pressure. In this study, we evaluated the ability of 11 C. gattii strains to develop itraconazole heteroresistance. Heteroresistant clones were analyzed for drug susceptibility, alterations in cell diameter, capsule properties, and virulence in a murine model. Heteroresistance to itraconazole was intrinsic in all of the strains analyzed, reduced both the capsule size and the cell diameter, induced molecular heterogeneity at the chromosomal level, changed the negatively charged cells, reduced ergosterol content, and improved the antioxidant system. A positive correlation between surface/volume ratio of original cells and the level of heteroresistance to itraconazole (LHI) was observed in addition to a negative correlation between capsule size of heteroresistant clones and LHI. Moreover, heteroresistance to itraconazole increased the engulfment of C. gattii by macrophages and augmented fungal proliferation inside these cells, which probably accounted for the reduced survival of the mice infected with the heteroresistant clones and the higher fungal burden in lungs and brain. Our results indicate that heteroresistance to itraconazole is intrinsic and increases the virulence of C. gattii. This phenomenon may represent an additional mechanism that contributes to relapses of cryptococcosis in patients during itraconazole therapy.
INTRODUCTION
Increased attention has been given to Cryptococcus gattii since an outbreak of devastating cryptococcosis in immunocompetent individuals (1). Azoles are antifungals widely used for both prophylactic therapy and the long-term management of cryptococcosis due to their efficacy and safety (2). Recent studies have described the emergence of heteroresistant clones of Cryptococcus species that are able to counteract the actions of azoles (3, 4). Heteroresistance is defined as resistance to antibiotics expressed by a subset of a microbial population that initially is considered susceptible to these antibiotics. These resistant subpopulations are able to adapt to increasing drug concentrations in a stepwise manner (5). Although it is known that heteroresistance can result in changes in morphology, growth patterns, and the virulence of Cryptococcus species, the clinical implications of this phenomenon remain unclear (3, 6).
Itraconazole is an alternative antifungal agent used in the treatment of cryptococcosis if fluconazole is unavailable or contraindicated (7). Although itraconazole cannot access the cerebrospinal fluid easily, some studies have demonstrated that it provides good results in the prophylaxis and treatment of cryptococcosis in patients with or without AIDS (8, 9).
In spite of the fact that itraconazole and fluconazole have the same principal mechanism of action (i.e., inhibition of sterol 14-α-demethylase), our group has previously shown that these drugs induced different levels of oxidative burst in vitro. Fluconazole treatment does not result in the formation of free radicals, while itraconazole induces oxidative stress at the beginning of treatment, which triggers the antioxidant enzymatic system (10).
Presently, no studies have focused on the characterization of the heteroresistance of C. gattii to itraconazole. In this study, we evaluated distinct parameters involved in the heteroresistance and their relationship to virulence in a murine model. Overall, our results demonstrate that the development of heteroresistance is correlated with an increase in the surface/volume (S/V) ratio in C. gattii cells and that heteroresistant clones are more virulent than the original strains from which they are derived.
MATERIALS AND METHODS
Ethics statement.
The protocol for the animal studies described here was approved by the Comissão de Ética no Uso de Animais (CEUA) of the Universidade Federal de Minas Gerais, Minas Gerais, Brazil (protocol 19/2013).
Cryptococcus gattii strains and study design.
Two strains from the ATCC, eight clinical strains (isolated from cerebrospinal fluid), and one strain from the environment of C. gattii, all from the culture collection of the Laboratório de Micologia/Universidade Federal de Minas Gerais, Minas Gerais, Brazil, were tested. The following assays were performed on all 11 of the strains: antifungal drug susceptibility testing, initial screening and stability of heteroresistance to itraconazole, time-kill curves, post antifungal effect, and capsule visualization and size measurements by India ink counterstaining.
Strains L135/03 and L27/01 were chosen for further experiments based on the cell size and level of heteroresistance to itraconazole.
Antifungal drug susceptibility testing.
The MIC for itraconazole (Sigma-Aldrich, St. Louis, MO) was determined by both the microdilution method (11) and spot tests on Sabouraud's dextrose agar (SDA) supplemented with different concentrations of itraconazole (3, 4, 12). For the spot tests, cell suspensions of different inocula (e.g., from 1 × 104 to 1 × 109 CFU/ml) of all the strains were plated onto SDA plates containing different concentrations of itraconazole (from 0.03 to 16.0 μg/ml). The growth pattern was read after 72 h of incubation at 37°C. All of the tests were performed in duplicate for each strain.
Initial screening and stability of heteroresistance to itraconazole.
Isolates were considered heteroresistant when a fraction of the inoculum was able to grow at a higher concentration than the MIC. This phenomenon occurred in the spot tests for all of the strains at 109 CFU/ml. Furthermore, cell suspensions of all of the strains (1 × 109 to 5 × 109 CFU/ml) were cultured onto SDA plates containing increasing concentrations of itraconazole (up to 16 μg/ml) in a stepwise manner until growth ceased (12). The culture plates were incubated at 37°C for 96 h. The clones resulting from this process were referred to as heteroresistant clones, while the cells that they were derived from were referred to as original cells.
In order to test the stability of the resistance to itraconazole, each strain was subcultured every 48 h on SDA plates without itraconazole. The MIC of each strain was determined after the 5th and 10th subculturing.
Time-kill curves and PAFE.
The evaluation of the time-kill kinetics (using concentrations equal to the MIC and 2×MIC) and the postantifungal effect (PAFE) (using concentrations equal to the MIC) of itraconazole against the original C. gattii strains and the heteroresistant clones were performed as previously described (10, 13).
Cell diameter, capsule size, and zeta potential measurements.
Original and heteroresistant yeast cells were visualized with an optical microscope (Axioplan; Carl Zeiss) following suspension in India ink. The capsule and diameter of at least 100 cells were measured using ImageJ 1.40 g software (http://rsb.info.nih.gov/ij/; National Institutes of Health, NIH, Bethesda, MD) (14). In addition, the surface-to-volume ratio (S/V) was calculated using the formula 3/r, where r is radius (15). The zeta potentials of the original and heteroresistant yeast cells were calculated using a zeta potential analyzer (Zetasizer NanoZS90; Malvern, United Kingdom) as described previously (16).
PFGE.
Pulsed-field gel electrophoresis (PFGE) was performed according to Santos et al. (17). Briefly, 108 cryptococcal cells and 10 mg/ml of lysing enzyme from Trichoderma harzianum (Sigma, St. Louis, MO, USA) were used for the preparation of spheroplast yeast cells. The running conditions for the PFGE were 100 to 200 s at 3.5 V for 16 h, followed by 200 to 300 s at 4.0 V for 32 h. The Saccharomyces cerevisiae chromosomal DNA PFGE marker (0.225 to 2.2 Mb) (Bio-Rad) was used as a size standard.
Ergosterol quantification.
The amount of ergosterol in the original and heteroresistant L135/03 and L27/01 C. gattii strains following a 1-h treatment with itraconazole was determined using n-heptane (Sigma-Aldrich). Spectrophotometric readings were taken at 282 nm as described previously (10, 17). A calibration curve using an ergosterol standard (Sigma-Aldrich) was constructed and used to calculate the amount of ergosterol in the yeast. The results are expressed as micrograms/milliliter of ergosterol and represent the means from 3 independent experiments.
Phagocytosis assay and IPR.
Murine peritoneal macrophages from C57BL/6 mice (2 × 105 cells/ml) were obtained 3 days after a thioglycolate (3%) injection and maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum. Macrophages were infected with the original or heteroresistant yeast cells from the L135/03 and L27/01 strains (6 × 104 cells/ml) opsonized with 10% murine serum and incubated for 3 or 27 h at 37°C under 5% CO2. The phagocytic index was calculated as the percentage of cells with internalized C. gattii after 27 h, while the intracellular proliferation rate (IPR) was calculated as the quotient of the intracellular yeast cell numbers at 27 h (the time point at which there was the maximum number of intracellular yeast) and 3 h (17). The results were confirmed by infecting J774 macrophages.
Measurement of ROS production, lipid peroxidation, and PER and SOD activities.
The original and heteroresistant yeast cells from the L135/03 and L27/01 strains were treated with itraconazole for 1 h prior to the tests. 2′,7′-Dichlorofluorescein diacetate (DCFH-DA; Invitrogen, Carlsbad, CA, USA) was used for reactive oxygen species (ROS) quantification, and fluorescence was measured with a fluorometer (Synergy 2 SL luminescence microplate reader; Biotek) (10, 17). The products of the lipid peroxidation were measured as thiobarbituric acid-reactive substances (TBARS) (10, 17). A cell extract of C. gattii cells was obtained for the measurement of superoxide dismutase (SOD) and peroxidase (PER) activities (10, 17).
All of the results are expressed as the ratio of the data from cells treated with itraconazole to the data from the growth control (medium without drug plus inocula) ± standard errors (SE).
Virulence of heteroresistant and original strains in C57BL/6 mice.
Six C57BL/6 male mice per group were anesthetized by intraperitoneal (i.p.) injection with ketamine hydrochloride (60 mg/kg of body weight) and xylazine (10 mg/kg) in sterile saline and then inoculated via the intratracheal (i.t.) or intravenous route with 30 μl of 1 × 106 CFU/ml of the original or heteroresistant yeast cells from the L135/03 and L27/01 strains of C. gattii. Phosphate-buffered saline (PBS) was used in control animals. The mice were monitored daily for survival.
Other groups of mice were i.t. infected with 106 cells of the original cells or heteroresistant clones of strain L135/03 to obtain lungs and brain at 10 days postinoculation. The organ homogenates were plated onto SDA to determine the fungal burden, expressed as CFU per gram (17).
MICs for itraconazole of colonies recovered from mice.
In order to test the stability of the heteroresistance to itraconazole, colonies recovered from mice infected with original or heteroresistant clones also were recovered on SDA plates supplemented with itraconazole. Briefly, cell suspensions (1 × 109 to 5 × 109 CFU/ml) were cultured onto SDA plates containing increasing concentrations of itraconazole. The culture plates were incubated at 37°C for 72 h. The MIC was the minimum concentration that inhibited 50% of growth compared to that of the control.
Histopathology.
C57BL/6 mice were inoculated by the intratracheal route with 106 cells of the original cell lines or heteroresistant clones of strain L135/03. Animals were euthanized at 10 days postinfection. Brain and lungs were fixed in formalin, embedded in paraffin, sectioned, and stained with hematoxylin-eosin (HE). Histopathological analysis was performed, and the score of yeast quantity was determined as 0 (absent), 1 (mild), 2 (moderate), and 3 (severe). The score of inflammation ranged from 0 to 6 (combined scores from 0 to 3 for two distinct lesions, i.e., peribrochial infiltrate and perivascular and alveolar thickening).
Analyses of 10th subculturing of cells from heteroresistant clones.
In order to better characterize the cells from the 10th subculturing from heteroresistant clones in medium without drug, we performed morphometric and survival curve experiments with L135/03 original and heteroresistant cells and from clones at the 10th subculturing. The methodologies used were the same as those described above for capsule and diameter sizes and survival curve.
Statistical analysis.
All statistical analyses were performed using GraphPad Prism, version 5.00, for Windows (GraphPad Software, San Diego, CA, USA). A one-way analysis of variance (ANOVA) using a Kruskal-Wallis nonparametric test was used to statistically compare the differences among groups, while an individual comparison between groups was done by the Bonferroni posttest. A 95% confidence interval was considered in all experiments. Statistically significant results were attained when the P value was less than the significance level (P < 0.05). The Pearson and Spearman correlation tests were used to determine the correlation between morphometric parameters and the level of heteroresistance to itraconazole. For the evaluation of the survival of mice, data were analyzed using the log-rank method. All tests were repeated at least twice.
RESULTS
Antifungal drug susceptibility testing and screening of heteroresistance.
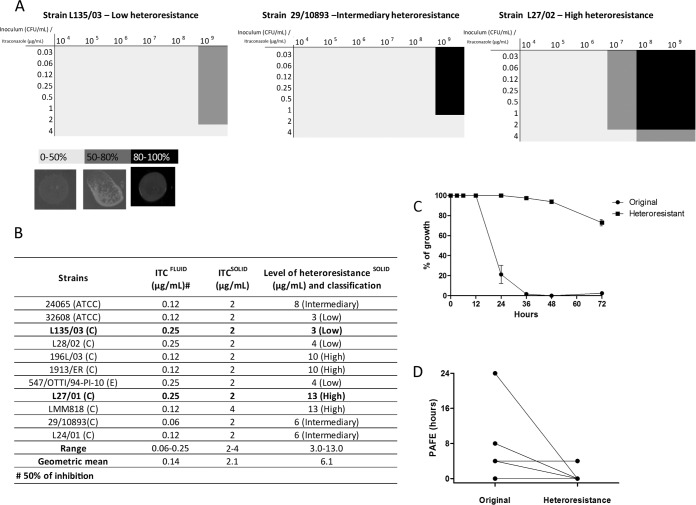
In the antifungal susceptibility testing, the geometric mean of the MIC values was 0.25 μg/ml (Fig. 1B). All strains were able to grow in the presence of itraconazole at 37°C only when the inoculum used was 109 CFU/ml (Fig. 1A). In the spot tests for this inoculum, the geometric mean of the MIC values was 2.2 μg/ml (Fig. 1B).
FIG 1.
Screening of original and itraconazole-heteroresistant C. gattii strains. (A) Checker board of the growth of the strains that showed low, medium, and high heteroresistance to increasing concentrations of itraconazole (ITC) on solid medium at 37°C and photos of spot test analyses representing growth of 0 to 50%, 50 to 80%, and 80 to 100% of a random C. gattii strain on SDA medium supplement with itraconazole. (B) Table showing the MIC of itraconazole against the strains in fluid and solid medium and the level of heteroresistance to itraconazole. (C) Time-kill curves. (D) Postantifungal effect (PAFE) in all of the C. gattii strains in which the downward lines represent the strains that showed a reduction in the PAFE and the horizontal lines represent the strains that maintained the same PAFE following induction of heteroresistance. The results of the time-kill curve are expressed as the percentage of growth compared with growth of the control. Results for the PAFE are expressed in hours. Data represent the means from 3 independent experiments consisting of duplicate assays. C, clinical; E, environmental.
All strains of C. gattii exhibited heteroresistance, but they did so at different levels that ranged from 3 μg/ml to 13 μg/ml. The median of the levels of heteroresistance to itraconazole (LHI) was used to calculate the cutoffs and separate the isolates into low (first quartile), intermediate (second quartile), and high (third quartile) heteroresistance (Fig. 1B). The patterns from the spot test analyses of serial dilutions are shown in Fig. 1A. Overall, the LHI varied from 1.5 to 6.5 times higher than the MIC values.
Following 10 sequential subcultures of highly resistant subclones derived from all of the strains in drug-free medium, all of the subclones reverted to the initial susceptibility to itraconazole.
Heteroresistance to itraconazole alters time-kill curves and postantifungal effect.
The kinetics of fungal death caused by itraconazole against the original strains and heteroresistant clones was evaluated. The original strains died faster, with a reduction of about 50% in metabolic activity after 24 h when using the MIC and 2×MIC (data not shown). In contrast, only a small reduction was observed for the heteroresistant strains after 72 h using the MIC (P < 0.001) and 2×MIC (Fig. 1C and data not shown, respectively).
The PAFE determined for itraconazole varied from 0 to 24 h (mean PAFE, 2.3 h) and from 0 to 4 h (mean PAFE, 0.6 h) for the original strains and heteroresistant clones, respectively. Four of the 11 strains analyzed showed a reduction in PAFE, while the other 7 strains maintained the same PAFE following the induction of heteroresistance (P = 0.11) (Fig. 1D).
Morphological alterations correlate with the level of heteroresistance in C. gattii cells.
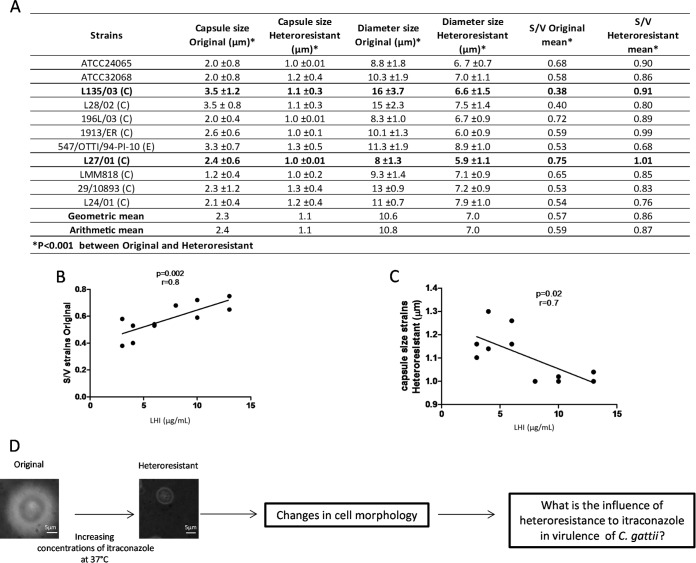
The arithmetic means of the capsule size and cell diameter of the strains were 2.4 and 10.8 μm, respectively, and their S/V ratios ranged from 0.38 to 0.7 (Fig. 2A). In contrast, the arithmetic means of the capsule size and cell diameter of heteroresistant clones were 1.1 and 7.0, respectively, and their S/V ratios ranged from 0.68 to 1.1. All of these parameters were significantly different between the original strains and the heteroresistant clones (P < 0.001 for all tests) (Fig. 2A). Interestingly, a positive correlation between the S/V ratio of the original cells and LHI was observed (P = 0.002, r = 0.8) (Fig. 2B). In addition, a negative correlation between the capsule size of heteroresistant clones and LHI was revealed (P = 0.02, r = 0.7) (Fig. 2C).
FIG 2.
Surface/volume (S/V) and capsule size are altered by the heteroresistance of clones to itraconazole (ITC). (A) Table listing capsule size, diameter sizes, and S/V of original and heteroresistant strains of C. gattii. (B) Correlation between S/V of original and heteroresistant strains (micrograms/milliliter). (C) Correlation between capsule size of heteroresistant cells and heteroresistance (micrograms/milliliter). (D) Schematic representation of the article's aim. C, clinical; E, environmental.
It is well established that smaller cells adapt faster to changes in environmental conditions than larger cells (17–19). In order to verify the influence of heteroresistance on ergosterol content and virulence of C. gattii, two strains were selected for further analysis, i.e., L135/03 (high S/V and low LHI) and L27/01 (low S/V and high LHI) (Fig. 2D).
PFGE banding patterns and ergosterol content are different in heteroresistant yeasts.
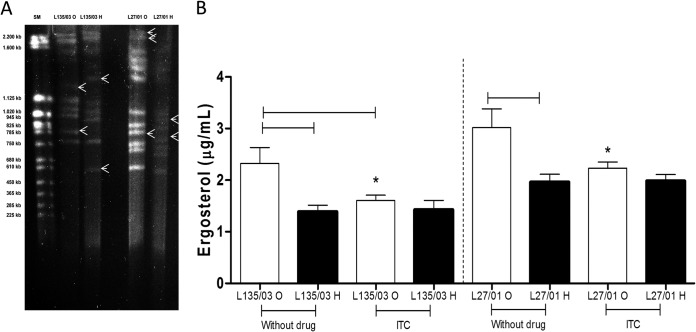
PFGE revealed that the original and heteroresistant yeast cells from strain L135/03 had the same number of chromosomes (nine) but different banding patterns, while the original and heteroresistant yeast cells from L27/01 had a different number of chromosomes (12 and 11, respectively) and different banding patterns, as shown in Fig. 3A.
FIG 3.
Heteroresistance to itraconazole promotes different banding patterns following PFGE and reduction of ergosterol content. (A) Banding patterns of original strains L135/03 and L27/01 and their heteroresistant clones following 1% PFGE. Lane 1, PFGE size marker (SM), 0.225 to 2.2 Mb S. cerevisiae chromosomal DNA. Arrows indicate the different bands observed between the original and heteroresistant cells. (B) Reduction in ergosterol content of original strains L135/03 and L27/01 and their heteroresistant clones following a 1-h treatment with itraconazole. Results are expressed in micrograms/milliliter. Statistically significant differences between original and heteroresistant cells and between cells treated with and without itraconazole are represented by the horizontal lines (P < 0.05). Statistically significant differences between original strains L135/03 and L127/01 subjected to the same treatment are represented by an asterisk (P < 0.05). Data represent the means ± SE from two independent experiments consisting of triplicate assays. ITC, itraconazole; O, original; H, heteroresistant.
Heteroresistant clones revealed a significantly lower ergosterol content than those of the original strains (L135/03, P < 0.01; L27/01, P = 0.01) (Fig. 3B). It is noteworthy that the L27/01 strain exhibited an LHI of 13 μg/ml and a higher content of ergosterol than L135/03 (P < 0.01), which had an LHI of 3 μg/ml and smaller amounts of ergosterol (Fig. 3B).
Heteroresistant cells are phagocytosed more but also are more resistant to macrophage killing than original cells.
The heteroresistant cells possessed lower cellular charges (L135/03, P = 0.02; L27/01, P < 0.01) (Fig. 4A) and were more susceptible to internalization than the original yeast cells (L135/03, P = 0.01; L27/01, P = 0.03) (Fig. 4B). However, the heteroresistant clones demonstrated a greater ability to proliferate inside macrophages (L135/03, P = 0.01; L27/01, P < 0.01) (Fig. 4C). It is important to note that the original (P < 0.01) and heteroresistant (P = 0.01) cells from strain L27/01 (high LHI) showed an intracellular proliferation rate significantly higher than that of L135/03 (low LHI).
FIG 4.
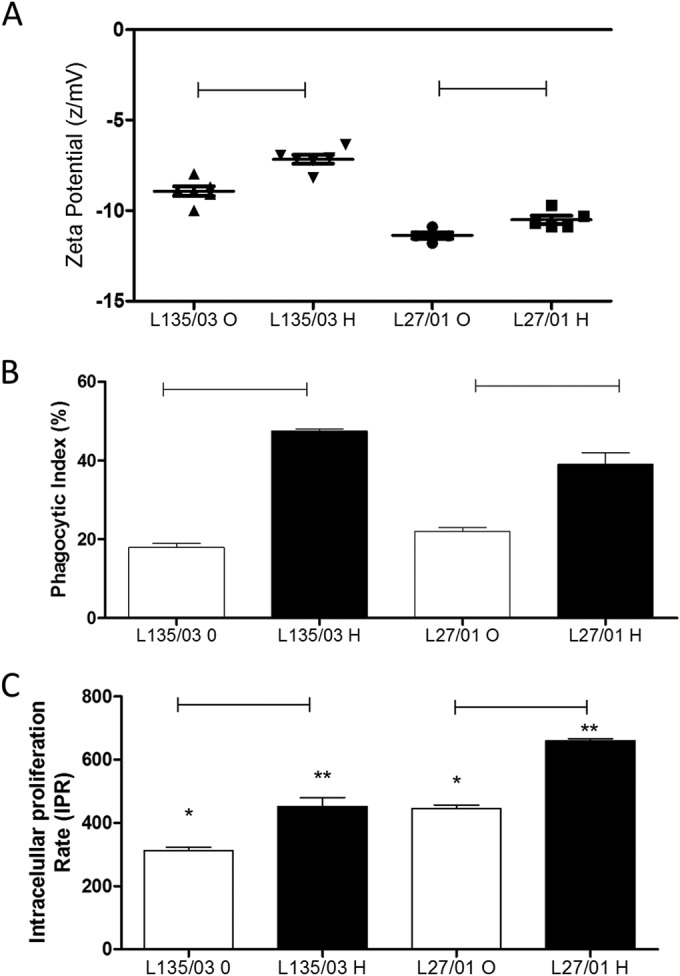
Heteroresistant cells are more readily internalized by macrophages but are more able to protect against oxidative burst. (A) The heteroresistant cells possessed lower cellular charges. (B) Phagocytic index. (C) Intracellular proliferation rate. Statistically significant differences between the original cells and their heteroresistant clones are represented by the horizontal lines (P < 0.05). Significant differences between the original L135/03 and L127/01 cells are represented by one asterisk (P < 0.05) and those between L135/03 and L127/01 heteroresistant clones by two asterisks (P < 0.05). Data represent the means ± SE from two independent experiments consisting of triplicate assays. O, original; H, heteroresistant.
Heteroresistant cells were more resistant to oxidative burst.
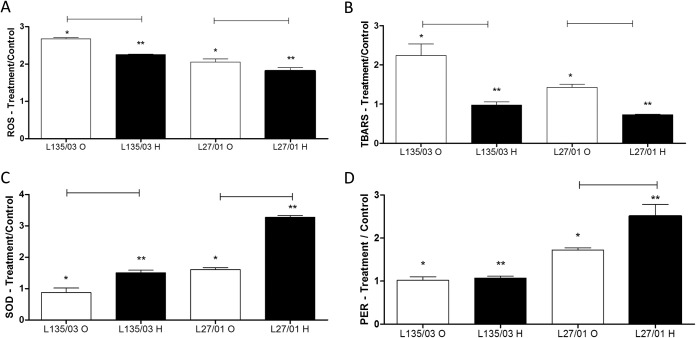
C. gattii cells were further tested to determine whether the induction of heteroresistance leads to a better adaptation to oxidative burst. The ROS measurement (L135/03, P < 0.01; L27/01, P = 0.03) (Fig. 5A) and TBARS assay (L135/03, P < 0.01; L27/01, P < 0.01) (Fig. 5B) showed that heteroresistant cells were less susceptible to oxidative stress caused by itraconazole. Results also demonstrated that ROS production (P < 0.05) (Fig. 5A) and lipid peroxidation (P < 0.05) (Fig. 5B) were higher for strain L135/03 (low LHI) than for strain L27/01 (high LHI).
FIG 5.
Heteroresistant cells are more resistant to oxidative burst. Shown are the amounts of reactive oxygen species (ROS) (A), lipid peroxidation (TBARs) (B), and total SOD (C) and PER (D) activities after 1 h of treatment with itraconazole. Results are expressed as the ratio of the treatment to the control. Statistical differences between the original cells and their heteroresistant clones are represented by the horizontal line (P < 0.05). Differences between L135/03 and L127/01 original cells are represented by one asterisk (P < 0.05) and those between L135/03 and L127/01 heteroresistant clones by two asterisks (P < 0.05). Data represent the means ± SE from two independent experiments consisting of triplicate assays. O, original; H, heteroresistant.
To explore the relationship between antioxidant enzymes and the heteroresistance to itraconazole, we measured the SOD and PER activities. The results showed that compared to the levels for the original cells, SOD activity (Fig. 6C) was higher in the heteroresistant cells originating from both of the strains exposed to itraconazole (L135/03, P < 0.01; L27/01, P < 0.01), but that the PER activity (Fig. 6D) was higher only in heteroresistant cells derived from strain L27/01 (high LHI) (P = 0.04). In line with the data from the ROS measurements and TBARS assay, cells from strain L135/03 (low LHI) treated with itraconazole showed less SOD and PER activities than those from strain L27/01 (high LHI).
FIG 6.
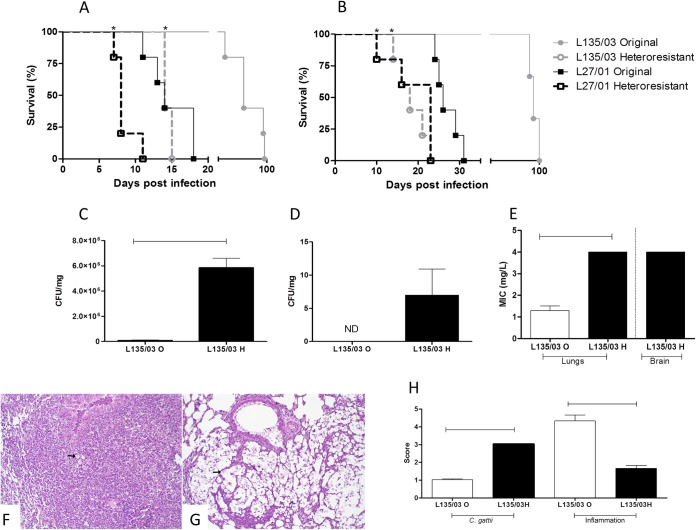
Heteroresistant clones were more virulent in C57/BL6 mice than in the original strains. Six mice per group were inoculated by the intratracheal (A) and intravenous (B) routes with 106 cells of the original cells or heteroresistant clones of strains L135/03 and L27/01. Animals were monitored daily. The test was repeated twice. C57BL/6 mice were inoculated by the intratracheal route with 106 cells of the original cell lines or heteroresistant clones of strain L135/03. Lungs (C) and brain (D) were removed, homogenized, diluted, and plated onto Sabouraud dextrose agar for measurement of CFU after 10 days postinfection. (E) Level of heteroresistance to itraconazole of C. gattii recovered from mice. (F) Histopathological analysis of lungs 10 days postinoculation with original L135/03 strains showed few yeasts (arrow) and intense, coalescent to diffuse inflammation. (G) Lungs of mice infected with the L135/03 heteroresistant clone showed numerous yeasts (arrow) and mild inflammation. HE was used as the stain, and the magnification is ×200. (H) Score of C. gattii amount and inflammatory lesions intensity in the lungs of C57BL/6 mice. Statistical differences between the original cells and their heteroresistant clones are represented by the horizontal line and asterisks (P < 0.05). O, original; H, heteroresistant; ND, nondetected.
Heteroresistance also enhances the ability of cells to survive under other stresses. For example, endogenous ROS play an important role in the antifungal activity of itraconazole (12).
Heteroresistance to itraconazole enhanced the virulence of C. gattii in C57BL/6 mice.
Mice infected by an intratracheal (P = 0.04) or an intravenous (P = 0.003) route with heteroresistant clones from C. gattii strain L135/03 (LHI, 3 μg/ml) succumbed significantly earlier than those infected with original cells from the same strain. The same results were obtained for mice infected with heteroresistant cells from strain L27/01 (LHI, 13 μg/ml) by an intratracheal (P = 0.002) or an intravenous (P = 0.005) route (Fig. 6A and B).
Interestingly, the original L27/01 strain (high LHI) also was more virulent than the original L135/03 strain (low LHI) by both routes (intratracheal, P = 0.04; intravenous, P = 0.002) (Fig. 6A and B). Altogether, the results raise the possibility that at least one mechanism behind the increased virulence of C. gattii-heteroresistant cells to itraconazole is their ability to escape host defenses through better internalization and survival in macrophages.
Further, strain L135/03 (original and heteroresistant) was used to infect animals for a better characterization of the disease caused by the different clones. After 10 days postinoculation, the fungal burden in lungs was significantly higher (P < 0.001) in mice infected with heteroresistant cells (Fig. 6C). It is important to note that the original L135/03 strain did not disseminate to the brain until 10 days postinoculation, while heteroresistant cells colonized the central nervous system (Fig. 6D).
Level of heteroresistance to itraconazole was maintained after infection of mice.
The ability of the heteroresistant clones recovered from the lungs (Fig. 6E) and brain to grow at higher concentrations of itraconazole was maintained, as verified in medium supplemented with this drug.
The heteroresistant clones induced lower inflammatory infiltrate in C57/BL6 mice than the original strains.
The heteroresistance to itraconazole influenced the inflammatory infiltrate in lungs (Fig. 6F, G, and H). Lungs of mice infected with original strain L135/03 showed few yeasts in pulmonary parenchyma, while lungs of mice infected with heteroresistant clones of strain L135/03 showed numerous yeasts. Intense, coalescent to diffuse inflammation characterized by moderated alveolar thickening and intense peribronchial and alveolar mononuclear cells (MN) and neutrophil infiltrate were observed in mice infected with original L135/03. On the other hand, mild inflammation characterized by mild peribronchial MN or mild alveolar neutrophil infiltrates were observed in mice infected with heteroresistant L135/03.
Cells from 10th subculturing of heteroresistant clones remain more virulent and smaller than original cells, although susceptibility to itraconazole returned to original levels.
The cell diameter (P < 0.001) and capsule size (P < 0.001) of cells from the 10th subculturing of L135/03 heteroresistant yeasts remained smaller than those of the original yeasts but were larger than those of heteroresistant cells (P < 0.001 for both parameters) (Fig. 7). Interestingly, yeasts from the 10th subculturing were equally as virulent as the heteroresistant L135/03 strain (P = 0.33) (data not shown). Indeed, the cells from the 10th subculture returned to the initial susceptibility to itraconazole.
FIG 7.
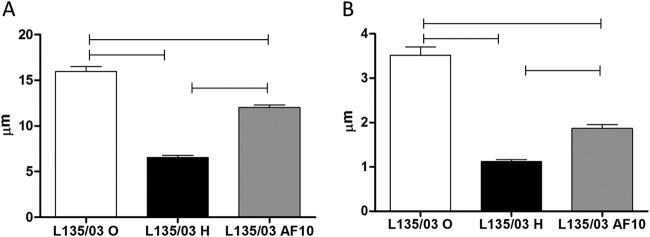
Cells from 10th subculturing of heteroresistant clones showed smaller cell diameter (A) and capsule size (B) than those of original yeasts, but they were larger than those of heteroresistant cells. Statistical differences are represented by the horizontal line (P < 0.05). O, original; H, heteroresistant; AF10, cells from 10th subculturing.
DISCUSSION
There are few data regarding the consequences of long-term exposure to itraconazole in C. gattii cells. In this study, we have provided the first evidence that heteroresistance to itraconazole in C. gattii is associated with a lower susceptibility to oxidative burst and enhanced virulence.
Heteroresistance to itraconazole was intrinsic to all of the 11 strains of C. gattii tested. It is important to note that the acquired itraconazole resistance of the clones was abolished in a stepwise manner upon repeated subculturing in drug-free medium. The selection of heteroresistant populations was evidenced only after the propagation of concentrated inocula (109 CFU/ml) at 37°C. Others have previously used the same procedure to screen for the presence of azole-resistant Candida species isolates (12). The temperature of 37°C was chosen to simulate the temperature of the mammalian body, since the ability to modify their physiology and grow well at this temperature is fundamental for invasive human fungal pathogens (20). In light of our findings, we cannot exclude the possibility of the selection of a heteroresistant population in patients undergoing long-term itraconazole therapy.
In our experiments, heteroresistant clones died slowly and exhibited short or no PAFEs of itraconazole. Based on the pharmacodynamic characteristics outlined above, we can surmise that heteroresistant cells would need longer and continuous exposure than the original cells to itraconazole in order to produce an efficient fungistatic effect.
It is important to highlight that the LHI differed among the strains tested and that 3 profiles were observed: low, intermediate, and high LHI. The heteroresistance was seen to alter the cell morphology and induce a reduction in the capsule size and cell diameter. In this way, a positive correlation between the S/V ratio of the original cells and the LHI was observed, while a negative correlation between the capsule size of heteroresistant clones and the LHI also was seen. Similar effects have been reported following the exposure of Cryptococcus neoformans to subinhibitory concentrations of fluconazole, voriconazole, amphotericin B, and terbinafine (16, 21). Indeed, the S/V ratio of a cell affects several aspects of its biology, since the rate of the cell's growth depends on, among other things, the rate of nutrient exchange.
According to the basics of microbiology, there are significant advantages of being small, because the higher S/V ratio of smaller cells supports a higher rate of nutrient exchange per cell volume compared with that of larger cells. This fact influences several aspects of the cell's evolution, since an amount of resources will allow a larger population of small cells than of large cells (15). Some authors have reported that capsule growth is associated with a slower yeast growth rate, implying a high-energy-consuming process (22). Although the capsule can provide resistance to some antifungals (e.g., amphotericin B), it does not have any effect on C. neoformans treated with itraconazole (23). In this way, modifications in S/V and capsule size by itraconazole increases the ability of cells to adapt more rapidly to abrupt changes in environmental conditions and to more easily exploit new habitats.
Certain stress conditions play a role in repressing the synthesis of capsule and ergosterol in yeast by altering cell integrity (24, 25). Researchers have demonstrated that C. neoformans overcomes the stress of contact with fluconazole through the formation of disomy in specific chromosomes, especially chromosome 1, which is closely associated with the ERG11 gene (6). Our results revealed different banding patterns between the original and heteroresistant cells by PFGE, suggesting an adaptive mechanism due to itraconazole pressure. Gene duplication and loss is a powerful mechanism in fungal evolution (26). Therefore, we presume that the genome reorganization, as seen in heteroresistant clones, is a consequence of the development of resistance to azoles.
Some authors also have reported that exposure of C. neoformans to subinhibitory concentrations of fluconazole alters the lipid profile of the yeast cells (27). Our data clearly demonstrated that heteroresistance to itraconazole reduced ergosterol levels in C. gattii cells. Given that capsule synthesis is associated with the accumulation of vesicles in the cell wall (27), it is possible that the inhibition of synthesis of ergosterol results in a defective membrane that interferes with the vesicular trafficking required for capsule synthesis.
In our study, heteroresistant cells from the strains with low (L135/03) and high LHI (L27/01) were significantly more phagocytosed than the original cryptococcal cells. These observations could be explained by the alterations in capsule (size and charge) observed in heteroresistant cells. Since cryptococcal cells are negatively charged, we hypothesized that the heteroresistance to itraconazole decreased the magnitude of the negative charge and reduced the electrostatic repulsion between the yeast cells and macrophages (16, 17).
Although capsule enlargement in C. neoformans confers resistance to oxidative stress, providing a mechanism for intracellular survival (23), our data demonstrated that heteroresistance significantly enhanced the intracellular proliferation rate of the yeast cells. To better understand these results, the susceptibility of heteroresistant clones to oxidative burst due to treatment with itraconazole was evaluated. Interestingly, heteroresistant clones were significantly more resistant to oxidative stress than the original cells following treatment with itraconazole (Fig. 5). Indeed, high-LHI (L27/01) strains showed lower levels of ROS and TBARS than low-LHI strains (L135/03). Furthermore, the greater resistance to oxidative burst in heteroresistant clones could be explained by the overexpression of the antioxidant enzymes PER and SOD. Our group previously showed that itraconazole led to ROS formation and lipid peroxidation in C. gattii cells, and this phenomenon strongly increased the activities of enzymes of the antioxidant system (10).
The antioxidant enzymes protect cryptococcal cells against oxidative stress and intracellular killing by macrophages. Previous studies demonstrated that melanin, SOD, glutathione peroxidases, and cytochrome C peroxidase (28) are components of the antioxidant system in cryptococci. Moreover, mitochondria depend on a manganese-containing superoxide dismutase (Sod2) that plays an essential role in the growth of C. neoformans at 37°C by regulating the steady-state concentration of ROS in mitochondria (29). With this in mind, we presume that the set of factors observed in heteroresistant clones, such as high S/V, small capsule size, and enhanced SOD and PER activities, will provide favorable conditions to these cells, allowing them to proliferate inside macrophages.
In order to determine if heteroresistance alters fungal virulence, we inoculated the original C. gattii and heteroresistant cells into C57BL/6 mice by intratracheal or intravenous routes. The heteroresistant clones were more virulent under both conditions (Fig. 6A and B). The growth of cryptococci within macrophages is one of the earliest observations reported during the cryptococcal host-to-pathogen interaction. The ability of cryptococci to grow within the phagosome relies on their ability to scavenge nutrients that allow them to proliferate (30). Our findings demonstrated that heteroresistant clones could survive better than the original cells following engulfment by macrophages, and that this phenomenon could explain the enhanced virulence observed in the murine model. Furthermore, the heteroresistant clones possessed capsules of reduced size, reinforcing data from previous studies that suggested that large polysaccharide capsules have difficulty in crossing the blood-brain barrier and in establishing infection in the central nervous system (31).
We observed that both original and heteroresistant cells with high LHI (L27/01) were more virulent than cells with low LHI (L135/03) in the intravenous model, which is unlike what was observed in the intratracheal model. These data could be attributed to the natural barriers that differ between the intravenous and intratracheal routes and to the severe pulmonary disease induced when C. gattii is inoculated intratracheally. For instance, within the lung, surfactant protein D (SP-D) acts as an opsonin of poorly encapsulated cryptococci. This process is beneficial to the pathogen, since opsonization with SP-D shows a large reduction in the destruction of acapsular mutants (32).
To better understand the influence of heteroresistance in virulence, we determined the CFU and histopathology for animals infected with strain L135/03. Higher fungal burden in lungs and brain (Fig. 6) of mice infected with heteroresistant clones induced less inflammation after 10 days of inoculation. Appropriate inflammatory balance is critical for the prognosis of cryptococcal infection, with Th1 and TH17 cytokines resulting in reduced intracellular proliferation (33). In addition, researchers observed that the anti-inflammatory cytokines interleukin-4 (IL-4) and IL-13 have a nonprotective effect, and the loss of the Th1 response is associated with development of cryptococcal meningoencephalitis (34). In this way, our results showed that in mice infected with heteroresistant clones, lesser inflammatory infiltrate and higher fungal burden were seen than in mice infected with the original strain, which resulted in poor prognosis (Fig. 6). We also observed that heteroresistant yeasts were more phagocytosed and proliferate more inside the macrophages (Fig. 4B and C). These results corroborate the observations found by researches that tried to correlate the Cryptococcus neoformans-macrophage interactions and the outcome of infection in the corresponding patients. They observed that patients with isolates that had both a high phagocytic index and high intracellular proliferation experienced a 5-fold-increased risk of death (35).
We demonstrated that the itraconazole heteroresistance levels of all C. gattii strains could be increased in a stepwise manner by exposing them to higher concentrations of the drug, and all reverted back to the original level of susceptibility by the 10th subculturing in drug-free medium. These results are similar to those found by others (3, 4). Interestingly, the cells recovered from lungs and brain are able to grow at higher concentrations of itraconazole (Fig. 6E). These differences, found in vivo and in vitro, may be explained by the fact that the strains still needed to adapt to free radicals from phagocytic cells in vivo, and there was no stress in vitro. Further, our group previously showed that the yeasts exposed to itraconazole increased the activities of enzymes of the antioxidant system for surviving oxidative burst and lipid peroxidation generated from itraconazole. In this way, we supposed that what occurred was a “cross adaptation,” and because of this phenomenon, the cells recovered from mice continued to be less susceptible to itraconazole. However, the clinical significance of this phenomenon should be studied more in the future.
In conclusion, heteroresistance to itraconazole was found to be intrinsic in all of the C. gattii strains tested and represents an adaptive mechanism for survival under azole stress. Our findings demonstrated that heteroresistance alters cell morphology (S/V and capsule size), the amounts of ergosterol in the cells, and antioxidant enzyme activities. These modifications may represent additional mechanisms resulting in the ineffectiveness of itraconazole treatment in patients with high fungal burden, since they lead to changes in pharmacodynamics parameters (e.g., time-kill curve and PAFE) and increased virulence of C. gattii.
ACKNOWLEDGMENTS
This study was supported by Fundação de Amparo a Pesquisa do Estado de Minas Gerais (grant PPM-00117-14) and Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq (grant 472438/2013-1). D.A.S. is a research fellow of the CNPq (grant 305154/2014-1).
We thank Carolina Leite, Kamyla Andrade, Laís Ramos, Sabrina Almeida, and Tawana Soares for technical support.
REFERENCES
- 1.McMullan BJ, Sorrell TC, Chen SC. 2013. Cryptococcus gattii infections: contemporary aspects of epidemiology, clinical manifestations and management of infection. Future Microbiol 8:1613–1631. doi: 10.2217/fmb.13.123. [DOI] [PubMed] [Google Scholar]
- 2.Zonios DI, Bennett JE. 2008. Update on azole antifungals. Semin Respir Crit Care Med 29:198–210. doi: 10.1055/s-2008-1063858. [DOI] [PubMed] [Google Scholar]
- 3.Sionov E, Chang YC, Garraffo HM, Kwon-Chung KJ. 2009. Heteroresistance to fluconazole in Cryptococcus neoformans is intrinsic and associated with virulence. Antimicrob Agents Chemother 53:2804–2815. doi: 10.1128/AAC.00295-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varma A, Kwon-Chung KJ. 2010. Heteroresistance of Cryptococcus gattii to fluconazole. Antimicrob Agents Chemother 54:2303–2311. doi: 10.1128/AAC.00153-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falagas ME, Makris GC, Dimopoulos G, Matthaiou DK. 2008. Heteroresistance: a concern of increasing clinical significance? Clin Microbiol Infect 14:101–104. doi: 10.1111/j.1469-0691.2007.01912.x. [DOI] [PubMed] [Google Scholar]
- 6.Sionov E, Lee H, Chang YC, Kwon-Chung KJ. 2010. Cryptococcus neoformans overcomes stress of azole drugs by formation of disomy in specific multiple chromosomes. PLoS Pathog 6:1–13. doi: 10.1371/journal.ppat.1000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, Harrison TS, Larsen RA, Lortholary O, Nguyen MH, Pappas PG, Powderly WG, Singh N, Sobel JD, Sorrell TC. 2010. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 50:291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denning DW, Tucker RM, Hanson LH, Hamilton JR, Stevens DA. 1989. Itraconazole therapy for cryptococcal meningitis and cryptococcosis. Arch Intern Med 10:2301–2308. [PubMed] [Google Scholar]
- 9.Batungwanayo J, Taelman H, Bogaerts J, Allen S, Lucas S, Kagame A, Clerinx J, Montane J, Saraux A, Muhlberger F. 1994. Pulmonary cryptococcosis associated with HIV-1 infection in Rwanda: a retrospective study of 37 cases. AIDS 8:1271–1276. doi: 10.1097/00002030-199409000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira GF, Baltazar LM, Santos JR, Monteiro AS, Fraga LAO, Resende-Stoianoff MA, Santos DA. 2013. The role of oxidative and nitrosative bursts caused by azoles and amphotericin B against the fungal pathogen Cryptococcus gattii. J Antimicrob Chemother 68:1801–1811. doi: 10.1093/jac/dkt114. [DOI] [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts: approved standard M27-A3. CLSI, Wayne, PA. [Google Scholar]
- 12.Claudino AL, Peixoto-Junior RF, Melhem MS, Szeszs MW, Lyon JP, Chavasco JK, Franco MC. 2009. Mutants with heteroresistance to amphotericin B and fluconazole in Candida. Braz J Microbiol 40:943–951. doi: 10.1590/S1517-83822009000400028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santos JR, Gouveia LF, Taylor EL, Resende-Stoianoff MA, Pianetti GA, Cesar IC, Santos DA. 2012. Dynamic interaction between fluconazole and amphotericin B against Cryptococcus gattii. Antimicrob Agents Chemother 56:2553–2558. doi: 10.1128/AAC.06098-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Araujo GS, Fonseca FL, Pontes B, Torres A, Cordero RJB, Zancopé-Oliveira RM, Casadevall A, Viana NB, Nimrichter L, Rodrigues ML, Garcia ES, Souza W, Frases S. 2012. Capsules from pathogenic and non-pathogenic Cryptococcus spp. manifest significant differences in structure and ability to protect against phagocytic cells. PLoS One 7:1–11. doi: 10.1371/journal.pone.0029561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madigan MT, Martinko JM, Stahl DA, Clarck DP. 2011. Brock biology of microorganisms, 11th ed Pearson/Benjamin Cummings, San Francisco, CA. [Google Scholar]
- 16.Nosanchuk JD, Cleare W, Franzot SP, Casadevall A. 1999. Amphotericin B and fluconazole affect cellular charge, macrophage phagocytosis, and cellular morphology of Cryptococcus neoformans at subinhibitory concentrations. Antimicrob Agents Chemother 43:233–239. doi: 10.1093/jac/43.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santos JR, Holanda RA, Frases S, Bravim M, Araujo Gde S, Santos PC, Costa MC, Ribeiro MJA, Ferreira GF, Baltazar LM, Miranda AS, Oliveira DB, Santos CMA, Fontes ACL, Gouveia LF, Resende-Stoianoff MA, Abrahão JS, Teixeira AL, Paixão TA, Souza DG, Santos DA. 2014. Fluconazole alters the polysaccharide capsule of Cryptococcus gattii and leads to distinct behaviors in murine cryptococcosis. PLoS One 13:e112669. doi: 10.1371/journal.pone.0112669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulz HN, Jorgensen BB. 2001. Big bacteria. Annu Rev Microbiol 55:105–137. doi: 10.1146/annurev.micro.55.1.105. [DOI] [PubMed] [Google Scholar]
- 19.Young KD. 2006. The selective value of bacterial shape. Microbiol Mol Biol Rev 70:660–703. doi: 10.1128/MMBR.00001-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perfect JR. 2006. Cryptococcus neoformans: the yeast that likes it hot. FEMS Yeast Res 6:463–468. doi: 10.1111/j.1567-1364.2006.00051.x. [DOI] [PubMed] [Google Scholar]
- 21.Guerra CR, Ishida K, Nucci M, Rozental S. 2012. Terbinafine inhibits Cryptococcus neoformans growth and modulates fungal morphology. Mem Inst Oswaldo Cruz 107:582–590. doi: 10.1590/S0074-02762012000500003. [DOI] [PubMed] [Google Scholar]
- 22.Maxson ME, Cook E, Casadevall A, Zaragoza O. 2007. The volume and hydration of the Cryptococcus neoformans polysaccharide capsule. Fungal Genet Biol 44:180–186. doi: 10.1016/j.fgb.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 23.Zaragoza O, Chrisman CJ, Castelli MV, Frases S, Cuenca-Estrella M, Rodríguez-Tudela JL, Casadevall A. 2008. Capsule enlargement in Cryptococcus neoformans confers resistance to oxidative stress suggesting a mechanism for intracellular survival. Cell Microbiol 10:2043–2057. doi: 10.1111/j.1462-5822.2008.01186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Meara TR, Alspaugh JA. 2012. The Cryptococcus neoformans capsule: a sword and a shield. Clin Microbiol Rev 25:387–408. doi: 10.1128/CMR.00001-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghannoum MA, Spellberg BJ, Ibrahim AS, Ritchie JA, Currie B, Spitzer ED, Edwards JE Jr, Casadevall A. 1994. Sterol composition of Cryptococcus neoformans in the presence and absence of fluconazole. Antimicrob Agents Chemother 38:2029–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wapinski I, Pfeffer A, Friedman N, Regev A. 2007. Natural history and evolutionary principles of gene duplication in fungi. Nature 449:54–61. doi: 10.1038/nature06107. [DOI] [PubMed] [Google Scholar]
- 27.Sakaguchi N, Baba T, Fukuzawa M, Ohno S. 1993. Ultrastructural study of Cryptococcus neoformans by quick-freezing and deep-etching method. Mycopathologia 121:133–141. doi: 10.1007/BF01104068. [DOI] [PubMed] [Google Scholar]
- 28.Olszewski MA, Zhang Y, Huffnagle GB. 2010. Mechanisms of cryptococcal virulence and persistence. Future Microbiol 5:1269–1288. doi: 10.2217/fmb.10.93. [DOI] [PubMed] [Google Scholar]
- 29.Giles SS, Batinic-Haberle I, Perfect J, Cox GM. 2005. Cryptococcus neoformans mitochondrial superoxide dismutase: an essential link between antioxidant function and high temperature growth. Eukaryot Cell 4:46–54. doi: 10.1128/EC.4.1.46-54.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnston SA, May RC. 2013. Cryptococcus interactions with macrophages: evasion and manipulation of the phagosome by a fungal pathogen. Cell Microbiol 15:403–411. doi: 10.1111/cmi.12067. [DOI] [PubMed] [Google Scholar]
- 31.Charlier C, Chretien F, Baudrimont M, Mordelet E, Lortholary O, Dromer F. 2005. Capsule structure changes associated with Cryptococcus neoformans crossing of the blood-brain barrier. Am J Pathol 166:421–432. doi: 10.1016/S0002-9440(10)62265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geunes-Boyer S, Oliver TN, Janbon G, Lodge JK, Heitman J, Perfect JR, Wright JR. 2009. Surfactant protein D increases phagocytosis of hypocapsular Cryptococcus neoformans by murine macrophages and enhances fungal survival. Infect Immun 77:2783–2794. doi: 10.1128/IAI.00088-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Voelz K, Lammas DA, May RC. 2009. Cytokine signaling regulates the outcome of intracellular macrophage parasitism by Cryptococcus neoformans. Infect Immun 77:3450–3457. doi: 10.1128/IAI.00297-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siddiqui AA, Brouwer AE, Wuthiekanun V, Jaffar S, Shattock R, Irving D, Sheldon J, Chierakul W, Peacock S, Day N, White NJ, Harrison TS. 2005. IFN-gamma at the site of infection determines rate of clearance of infection in cryptococcal meningitis. J Immunol 174:1746–1750. doi: 10.4049/jimmunol.174.3.1746. [DOI] [PubMed] [Google Scholar]
- 35.Alanio A, Desnos-Ollivier M, Dromer F. 2011. Dynamics of Cryptococcus neoformans-macrophage interactions reveal that fungal background influences outcome during cryptococcal meningoencephalitis in humans. mBio 2:e00158–11. doi: 10.1128/mBio.00158-11. [DOI] [PMC free article] [PubMed] [Google Scholar]