Abstract
We compared six colistin susceptibility testing (ST) methods on 61 carbapenem-nonsusceptible Klebsiella pneumoniae (n = 41) and Acinetobacter baumannii (n = 20) clinical isolates with provisionally elevated colistin MICs by routine ST. Colistin MICs were determined by broth microdilution (BMD), BMD with 0.002% polysorbate 80 (P80) (BMD-P80), agar dilution (AD), Etest, Vitek2, and MIC test strip (MTS). BMD was used as the reference method for comparison. The EUCAST-recommended susceptible and resistant breakpoints of ≤2 and >2 μg/ml, respectively, were applied for both K. pneumoniae and A. baumannii. The proportions of colistin-resistant strains were 95.1, 77, 96.7, 57.4, 65.6, and 98.4% by BMD, BMD-P80, AD, Etest, MTS, and Vitek2, respectively. The Etest and MTS methods produced excessive rates of very major errors (VMEs) (39.3 and 31.1%, respectively), while BMD-P80 produced 18% VMEs, AD produced 3.3% VMEs, and Vitek2 produced no VMEs. Major errors (MEs) were rather limited by all tested methods. These data show that gradient diffusion methods may lead to inappropriate colistin therapy. Clinical laboratories should consider the use of automated systems, such as Vitek2, or dilution methods for colistin ST.
INTRODUCTION
The increasing occurrence of infections due to multidrug-resistant (MDR) Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacteriaceae led to the revival of old and neglected antibiotics that may remain active, such as polymyxins (polymyxin B and colistin) (1, 2). Colistin is increasingly being used as a last-resort treatment option for infections caused by MDR organisms (2, 3), particularly carbapenem-resistant (CR) Gram-negative bacteria (4). However, during the last years, increasing colistin resistance emerged worldwide, especially among Klebsiella pneumoniae and A. baumannii isolates, further limiting treatment options (5–7). In Europe, the evolving colistin resistance is more pronounced in southern countries (notably Greece, Romania, and Italy) (8–10).
Rapid and reliable colistin susceptibility testing (ST) is needed in routine clinical laboratories to allow appropriate therapeutic decision-making. Thus far, few studies have assessed the performance of colistin ST methods, displaying controversial results, and thus, the most accurate one is still challenging (11).
Disk diffusion, commonly used in many clinical laboratories, yielded high error rates compared to MIC-based methods and is considered unreliable for the detection of colistin resistance (12–14). Among commercial methods, gradient diffusion strips are convenient tests for determining colistin MICs, but their performance is not well established. Some studies demonstrated very good correlations between the results of Etest (bioMérieux, Marcy l'Etoile, France) and broth microdilution (BMD) or agar dilution (AD) methods for colistin ST (13–17), while other reports questioned the reliability of Etest (18, 19). Another gradient diffusion test, MIC test strip (MTS) (Liofilchem SRL, Italy), has not been evaluated for colistin ST to the best of our knowledge. Colistin ST using automated methods has been tested in limited studies that tested mainly the performance of the Vitek2 system (bioMérieux) (13, 16, 19).
AD and BMD are widely considered standard methods for MIC ST (20), which, however, are not convenient for routine clinical laboratories. Additionally, for BMD, technical issues, such as the type or surface pretreatment of microtiter trays, have influenced colistin MICs significantly (3, 21). In particular, colistin displays various levels of adherence to different surfaces used for MIC trays, such as polystyrene, resulting in reduced antibiotic concentrations actually being present under experimental conditions (22). The addition of the surfactant polysorbate 80 (P80) to BMD (BMD-P80) minimized colistin adhesion to BMD panels and thus significantly reduced colistin MICs, affecting mainly bacteria with relatively low MICs (≤2 μg/ml) (18, 21, 23). Nevertheless, the CLSI does not recommend the use of P80 for colistin ST by BMD (24). In addition, according to recent observations, P80 exhibited a synergistic effect with colistin, perhaps enhancing its interaction with the bacterial cell membrane (J. D. Turnidge, presented at the ESCMID Conference on Reviving Old Antibiotics, Vienna, Austria, 22 to 24 October 2014). Hence, further studies on the accuracy of BMD-P80 in determining the colistin susceptibility of Gram-negative bacteria are needed.
The breakpoints for colistin developed by various organizations differ (24, 25), further complicating the interpretation of antimicrobial ST results. Additionally, most studies investigating the accuracy of colistin ST methods involved predominantly colistin-susceptible Gram-negative isolates, while they have scarcely been tested on colistin-resistant isolates. In this study, we evaluated various colistin MIC testing methods, such as BMD, BMD-P80, AD, Etest, MTS, and Vitek2, using a collection of carbapenem-nonsusceptible K. pneumoniae and A. baumannii isolates with provisionally elevated colistin MICs according to routine ST.
(Part of this work was presented at the 54th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy [ICAAC], Washington, DC, 5 to 9 September 2014 [30].)
MATERIALS AND METHODS
Bacterial isolates.
A total of 61 carbapenem-nonsusceptible clinical isolates collected from four tertiary Greek hospitals during 2008 to 2013 were studied. In particular, this collection contained nonduplicate previously characterized KPC, OXA-48, or VIM carbapenemase-producing K. pneumoniae (n = 41) and OXA-58 or OXA-23 carbapenemase-producing A. baumannii (n = 20) isolates. The isolates were recovered from patients with bloodstream (42.6%), respiratory tract (16.4%), urinary tract (16.4%), skin and soft tissue (11.5%), and other (13.1%) infections. Colistin was deemed necessary for the treatment of the respective infections; however, the isolates were identified as being colistin nonsusceptible (MIC > 2 μg/ml) by routine laboratory ST, based mainly on the use of automated systems. Due to the existing controversies in colistin ST, to further evaluate the susceptibility results and verify that colistin could not be used for infections caused by these bacteria, the isolates were submitted to the Department of Microbiology at the Medical School of the University of Athens. Prior to use in this study, the isolates had been stored in glycerol stocks at −70°C and subcultured twice before testing.
Susceptibility testing.
Colistin MICs were determined by BMD, BMD-P80, AD, Etest, MTS, and Vitek2 methods. Dilution methods were performed according to CLSI procedures (20, 24), using colistin sulfate powder (batch number SLBK0713V; Sigma-Aldrich, St. Louis, MO). Stock solutions of colistin were reconstituted in sterile distilled water, in accordance with the manufacturer's instructions, immediately prior to use. BMD and BMD-P80 MIC determinations (concentration range, 0.125 to 128 μg/ml) were conducted by using tissue culture-treated round-bottom polystyrene 96-well trays (Costar 3799; Corning, NY, USA), using a bacterial inoculum of 5 × 105 CFU/ml in cation-adjusted Mueller-Hinton broth (Sigma-Aldrich) without or with the addition of 0.002% P80 (Sigma-Aldrich). For AD, colistin was incorporated into Mueller-Hinton II agar (MHA; Sigma-Aldrich) plates at concentrations of 0.125 to 128 μg/ml and with a final inoculum of 104 CFU/spot. Etest and MTS methods, which included the same colistin concentration gradient range (0.016 to 256 μg/ml), were performed with MHA plates, according to the manufacturer's instructions. Etest and MTS MICs between 2-fold dilutions were rounded up to the next 2-fold dilution. The Vitek2 AST-EXN8 susceptibility card (bioMérieux), reporting colistin MICs of ≤0.5 to ≥16 μg/ml, was employed according to the manufacturer's recommendations. For data analysis, when needed, MICs of ≥16 μg/ml determined by Vitek2 were considered 16 μg/ml. All methods were performed simultaneously with a single inoculum for each strain, with incubation at 35°C ± 2°C for 18 to 20 h, and results were reviewed by two independent observers. Escherichia coli ATCC 25922 and P. aeruginosa ATCC 27853 were used for quality control (24).
Interpretation of results and data analysis.
The CLSI provides susceptibility breakpoints for colistin against A. baumannii (susceptible, MIC of ≤2 μg/ml; resistant, MIC of ≥4 μg/ml) but not Enterobacteriaceae (24). For consistency, EUCAST-recommended breakpoints, which are available for both organisms (susceptible, MIC of ≤2 μg/ml; resistant, MIC of >2 μg/ml) (25) were applied.
Data were analyzed by comparing the results produced by the BMD-P80, AD, Etest, MTS, and Vitek2 methods with those produced by standard BMD. Essential agreement (EA) was defined as the percentage of MICs within ±1 log2 dilution of the MIC determined by BMD. Categorical agreement (CA) was defined as the percentage of isolates classified in the same susceptibility category by BMD and the method under evaluation. Very major errors (VMEs) denoted a false-susceptible result, and major errors (MEs) denoted a false-resistant result (26). It should be noted that because our collection included mainly colistin-resistant isolates and to avoid overestimation of MEs, for the estimation of errors, the total number of tested isolates was used as the denominator.
Acceptable performance was evaluated according to criteria established by the International Organization for Standardization: ≥90% for essential or category agreement and ≤3% for VMEs or MEs (27). Statistical analysis was performed by using Student's t test, and differences were considered statistically significant at a P value of <0.05.
RESULTS
Susceptibility to colistin, EA, CA, and errors.
The susceptibilities of the study isolates to colistin, MIC50s/MIC90s, EAs, CAs, and errors by each method are presented in Table 1. The colistin MICs per method are shown in Table 2.
TABLE 1.
Colistin susceptibilities of isolates and MIC50s/MIC90s determined by the ST methods and EA, CA, and types of errors produced by BMD-P80, AD, Etest, MTS, and Vitek2 compared to BMD
| Method and isolate group | No. (%) of isolates |
MIC (μg/ml) |
No. (%) of isolates with: |
|||||
|---|---|---|---|---|---|---|---|---|
| Susceptible | Resistant | 50% | 90% | EA | CA | VME | ME | |
| BMD | ||||||||
| All isolates | 3 (4.9) | 58 (95.1) | 8 | 32 | ||||
| K. pneumoniae | 1 (2.4) | 40 (97.6) | 8 | 32 | ||||
| A. baumannii | 2 (10) | 18 (90) | 4 | 32 | ||||
| BMD-P80 | ||||||||
| All isolates | 14 (23) | 47 (77) | 4 | 16 | 58 (95.1) | 50 (82) | 11 (18) | 0 (0) |
| K. pneumoniae | 6 (14.6) | 35 (85.4) | 8 | 32 | 39 (95.1) | 36 (87.8) | 5 (12.2) | 0 (0) |
| A. baumannii | 8 (40) | 12 (60) | 4 | 16 | 19 (95) | 14 (70) | 6 (30) | 0 (0) |
| AD | ||||||||
| All isolates | 2 (3.3) | 59 (96.7) | 16 | 64 | 34 (55.7) | 56 (91.8) | 2 (3.3) | 3 (4.9) |
| K. pneumoniae | 0 (0) | 41 (100) | 16 | 64 | 23 (56.1) | 40 (97.6) | 0 (0) | 1 (2.4) |
| A. baumannii | 2 (10) | 18 (90) | 8 | 128 | 11 (55) | 16 (80) | 2 (10) | 2 (10) |
| Etest | ||||||||
| All isolates | 26 (42.6) | 35 (57.4) | 4 | 8 | 31 (50.8) | 36 (59) | 24 (39.3) | 1 (1.6) |
| K. pneumoniae | 17 (41.5) | 24 (58.5) | 4 | 8 | 20 (48.8) | 23 (56.1) | 17 (41.5) | 1 (2.4) |
| A. baumannii | 9 (45) | 11 (55) | 4 | 8 | 11 (55) | 13 (65) | 7 (35) | 0 (0) |
| MTS | ||||||||
| All isolates | 21 (34.4) | 40 (65.6) | 4 | 4 | 40 (65.6) | 41 (67.2) | 19 (31.1) | 1 (1.6) |
| K. pneumoniae | 14 (34.1) | 27 (65.9) | 4 | 4 | 22 (53.7) | 26 (63.4) | 14 (34.1) | 1 (2.4) |
| A. baumannii | 7 (35) | 13 (65) | 4 | 8 | 18 (90) | 15 (75) | 5 (25) | 0 (0) |
| Vitek2 | ||||||||
| All isolates | 1 (1.6) | 60 (98.4) | 8 | ≥16 | 48 (78.6) | 59 (96.7) | 0 (0) | 2 (3.3) |
| K. pneumoniae | 1 (2.4) | 40 (97.6) | ≥16 | ≥16 | 31 (75.6) | 41 (100) | 0 (0) | 0 (0) |
| A. baumannii | 0 (0) | 20 (100) | 4 | ≥16 | 17 (85) | 18 (90) | 0 (0) | 2 (10) |
TABLE 2.
Colistin MICs for isolates
| Organism (total no. of isolates) | Method | No. of isolates with MIC (μg/ml) of: |
Geometric mean MIC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | >128 | |||
| K. pneumoniae (41) | BMD | 0 | 0 | 1 | 12 | 16 | 6 | 4 | 1 | 0 | 1 | 9.2 |
| BMD-P80 | 0 | 0 | 6 | 14 | 12 | 4 | 3 | 1 | 0 | 1 | 7.0 | |
| AD | 0 | 0 | 0 | 5 | 7 | 9 | 15 | 5 | 0 | 0 | 18.3 | |
| Etest | 3 | 6 | 8 | 17 | 4 | 3 | 0 | 0 | 0 | 0 | 2.8 | |
| MTS | 1 | 1 | 12 | 25 | 0 | 2 | 0 | 0 | 0 | 0 | 3.2 | |
| Vitek2 | 0 | 0 | 1 | 4 | 10 | 26 | 0 | 0 | 0 | 0 | 11.2 | |
| A. baumannii (20) | BMD | 0 | 0 | 2 | 8 | 6 | 1 | 1 | 1 | 1 | 0 | 7.5 |
| BMD-P80 | 0 | 1 | 7 | 5 | 4 | 2 | 0 | 0 | 1 | 0 | 4.6 | |
| AD | 0 | 1 | 1 | 4 | 3 | 2 | 6 | 0 | 2 | 1 | 14.9 | |
| Etest | 6 | 0 | 3 | 6 | 4 | 1 | 0 | 0 | 0 | 0 | 2.1 | |
| MTS | 0 | 0 | 7 | 9 | 2 | 1 | 1 | 0 | 0 | 0 | 4.0 | |
| Vitek2 | 0 | 0 | 0 | 10 | 5 | 5 | 0 | 0 | 0 | 0 | 6.7 | |
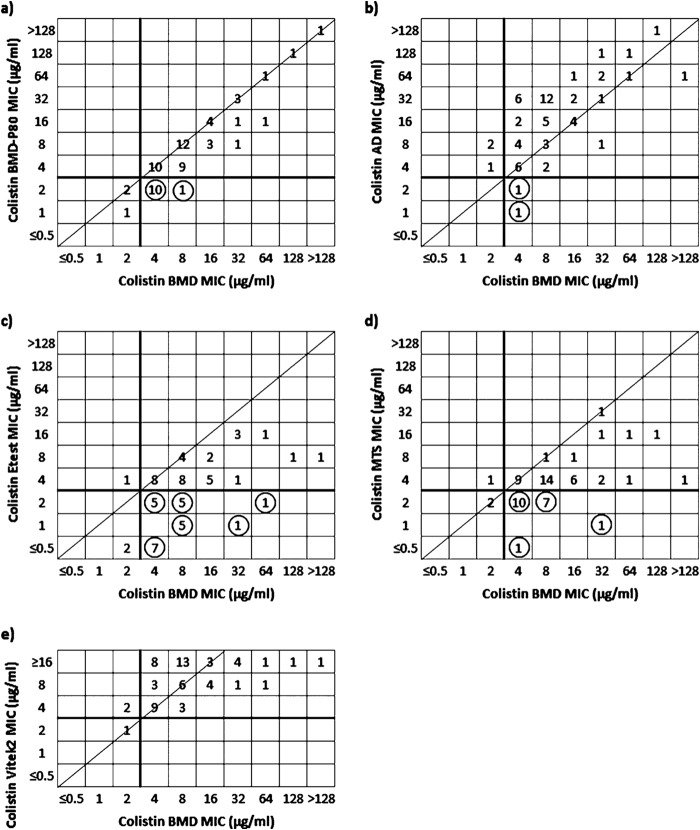
In particular, by BMD, 58 isolates (95.1%) were colistin resistant. A trend toward lower colistin MICs was noted for BMD-P80; considerably fewer isolates were colistin resistant (47 isolates; 77%), and the CA with BMD was 82%. AD produced susceptibility results similar to those of BMD, and high rates of CA (91.8%) were observed for both pathogens. Discordant susceptibility rates with serious interpretative errors and unacceptable CA were observed for Etest and MTS (59 and 67.2%, respectively). The in vitro activity of colistin was found to be importantly higher by Etest, with only 57.4% of the isolates being classified as resistant; 65.6% of the isolates were classified as resistant by MTS. Vitek2 categorized 98.4% of isolates as colistin resistant, showing excellent CA with BMD (96.7%).
The EA was highest for BMD-P80 (95.1% overall), exceeding the acceptable performance threshold for antimicrobial ST methods. By BMD-P80, 55.7% of the isolates exhibited MICs identical to those determined by BMD, and 39.3% of isolates displayed MICs that were 1 log2 dilution lower. AD generated rather low EA rates (55.7% overall) and trends toward higher colistin MICs; it produced MICs that were 1, 2, and 3 log2 dilutions higher than those determined by BMD for 26.2%, 29.5%, and 9.8% of the isolates, respectively. In contrast, Etest produced MICs that were 1, 2, 3, and >3 log2 dilutions lower than those determined by BMD for 29.5%, 18%, 19.7%, and 11.5% of the isolates, respectively, resulting in the lowest rate of EA (50.8% overall; 48.8 and 55% for K. pneumoniae and A. baumannii, respectively). Similarly, MICs obtained by MTS were 1, 2, 3, and >3 log2 dilutions lower for 42.6%, 23%, 6.6%, and 4.9% of the isolates, respectively. MTS generated an overall EA rate of 65.6%, with a low EA rate for K. pneumoniae isolates (53.7%) but an appropriate EA for A. baumannii (EA of 90%). For Vitek2, the EA rate was 78.6% in total; 31.1% of the isolates had MICs equal to those determined by BMD, while 47.5% of the isolates had MICs that were 1 log2 dilution higher or lower (Table 3).
TABLE 3.
Differences in log2 dilutions of MICs obtained by BMD-P80, AD, Etest, MTS, and Vitek2 compared to those obtained by BMD
| Method and isolate group | No. (%) of isolates showing MIC difference (log2 dilution) of: |
|||||||
|---|---|---|---|---|---|---|---|---|
| >−3 | −3 | −2 | −1 | 0 | 1 | 2 | 3 | |
| BMD-P80 | ||||||||
| All isolates | 3 (4.9) | 24 (39.3) | 34 (55.7) | |||||
| K. pneumoniae | 2 (4.9) | 12 (29.3) | 27 (65.9) | |||||
| A. baumannii | 1 (5) | 12 (60) | 7 (35) | |||||
| AD | ||||||||
| All isolates | 3 (4.9) | 3 (4.9) | 15 (24.6) | 16 (26.2) | 18 (29.5) | 6 (9.8) | ||
| K. pneumoniae | 2 (4.9) | 2 (4.9) | 11 (26.8) | 10 (24.4) | 11 (26.8) | 5 (12.2) | ||
| A. baumannii | 1 (5) | 1 (5) | 4 (20) | 6 (30) | 7 (35) | 1 (5) | ||
| Etest | ||||||||
| All isolates | 7 (11.5) | 12 (19.7) | 11 (18) | 18 (29.5) | 12 (19.7) | 1 (1.6) | ||
| K. pneumoniae | 4 (9.8) | 7 (17.1) | 10 (24.4) | 12 (29.3) | 7 (17.1) | 1 (2.4) | ||
| A. baumannii | 3 (15) | 5 (25) | 1 (5) | 6 (30) | 5 (25) | |||
| MTS | ||||||||
| All isolates | 3 (4.9) | 4 (6.6) | 14 (23) | 26 (42.6) | 13 (21.3) | 1 (1.6) | ||
| K. pneumoniae | 2 (4.9) | 3 (7.3) | 14 (34.1) | 15 (36.6) | 6 (14.6) | 1 (2.4) | ||
| A. baumannii | 1 (5) | 1 (5) | 11 (55) | 7 (35) | ||||
| Vitek2 | ||||||||
| All isolates | 1 (1.6) | 2 (3.3) | 2 (3.3) | 11 (18) | 19 (31.1) | 18 (29.5) | 8 (13.1) | |
| K. pneumoniae | 1 (2.4) | 2 (4.9) | 7 (17.1) | 11 (26.8) | 13 (31.7) | 7 (17.1) | ||
| A. baumannii | 2 (10) | 4 (20) | 8 (40) | 5 (25) | 1 (5) | |||
In terms of errors (Table 1 and Fig. 1), high rates of VMEs (18%) and no MEs were detected for BMD-P80, while AD exhibited 3.3% VMEs and 4.9% MEs. The Etest yielded the highest rates of VMEs overall (39.3%) and limited MEs (1.6%); MTS produced high rates of VMEs (31.1%) and also limited MEs (1.6%). No VMEs were observed for Vitek2; it yielded 3.3% MEs.
FIG 1.
Scattergrams showing numbers of isolates (n = 61) with colistin MICs determined by BMD-P80 (a), AD (b), Etest (c), MTS (d), and Vitek2 (e) versus BMD as a reference. Solid lines represent the 2015 EUCAST breakpoint for susceptibility (≤2 μg/ml). The diagonal line indicates absolute agreement. VMEs are indicated by circles.
DISCUSSION
Colistin therapy is commonly necessary for the treatment of serious infections caused by CR Gram-negative bacteria (4). Trends toward elevated colistin MICs have been noted worldwide (5, 8), underlining the importance of accurate colistin susceptibility results. Also, suggestions for the optimal methods to be used for colistin ST have not been formulated by the CLSI. In the present study, we evaluated the performances of six colistin ST methods against carbapenem-nonsusceptible K. pneumoniae and A. baumannii clinical isolates with provisionally elevated colistin MICs.
Much debate on the need to add the surfactant P80 to test systems for polymyxins has recently arisen. Overall, the available evidence for the performance of BMD-P80 in testing of isolates with elevated colistin MICs is currently limited. We observed that BMD-P80 showed the highest EA and a relatively high CA with BMD but produced significantly low MICs (P < 0.001) (data not shown); differences in susceptibility rates were modest for K. pneumoniae and more pronounced for A. baumannii. The most important differences occurred among isolates with BMD MICs of 4 to 8 μg/ml, resulting in 18% VMEs. Slighter, but still significant, decreases in colistin MICs were also shown in a recent study comparing BMD with and BMD without P80 (21). However, in another study comparing BMD with and BMD without P80 using a different type of MIC tray, P80 did not alter MIC values for isolates with colistin MICs of ≥2 μg/ml (28).
As far as AD, previous reports have shown results concordant with those of BMD (12, 13). Similarly, in our study, the CA between AD and BMD was acceptable, and errors were relatively limited. However, the EA was low, because AD overall tended to produce MICs that were 1 to 2 log2 dilutions higher than those determined by BMD, as was also observed previously (12).
Among commercial methods, Etest is convenient and widely applied in clinical laboratories, but concerns have been expressed regarding its suitability for colistin ST (11, 18, 19). Supporting its limitations, in our study, the Etest method generally produced lower MICs than did BMD, resulting in false sensitivity, with rates of VMEs being severalfold beyond the acceptable range for both species; EA and CA rates were also low. Considering that many clinical laboratories currently rely on Etest to determine colistin susceptibility of MDR Gram-negative bacteria, such VMEs could result in inappropriate colistin administration. Previously reported studies, which tested mainly isolates with low MICs, reported higher EA levels and much lower VME rates than those in our study. However, a study including higher numbers of K. pneumoniae and A. baumannii isolates with increased MICs pointed out considerably lower MICs by Etest compared with BMD-P80 and high VME rates (18), confirming our observations. It should be noted that Etest was recently shown to detect sufficiently colistin-heteroresistant K. pneumoniae isolates with PhoPQ alterations; however, our collection did not include isolates with evident heteroresistance (29).
The second gradient diffusion method tested, MTS, also produced lower MICs than those determined by BMD for most isolates, resulting in a shift toward susceptibility. Interestingly, a substantial number of isolates with MTS MICs at the susceptible breakpoint (2 μg/ml) exhibited just 1 or 2 dilutions higher MICs by BMD, thus being interpreted as resistant. Overall, MTS performed better than Etest in terms of EA, CA, and VMEs.
In many clinical laboratories, the main routine antimicrobial ST methods in use are automated systems, such as Vitek2. Previous studies reported that Vitek colistin ST for A. baumannii exhibited appropriate performance, using BMD or AD as a reference (13, 16, 19). However, those studies included mainly colistin-susceptible A. baumannii isolates. As far as the Enterobacteriaceae, controversial findings have been reported about the reliability of Vitek2 colistin ST, implying that it was either unreliable compared with AD (19) or comparable in terms of agreement with BMD (13). Our data suggest that Vitek2 appears to be a useful method for rapid detection of colistin resistance, as it exhibited excellent CA with BMD. It was able to identify all colistin-resistant isolates, with MEs being observed for only two A. baumannii isolates.
In conclusion, the findings of the present study showed substantial discordance between the tested methods, with none of the methods under comparison meeting the criteria for acceptable antimicrobial susceptibility test performance. Important shortcomings of gradient diffusion testing methods, which may lead to inappropriate selection of colistin therapy, were probably the most notable observation of this study. Therefore, it is critical for clinical laboratories to be aware of these discrepancies and consider applying a reference method for colistin ST, especially when colistin administration is deemed necessary. In routine clinical practice in most regions worldwide, where a reference method can hardly be implemented, the interpretation of colistin susceptibility should preferably be based on results of automated systems such as Vitek2.
ACKNOWLEDGMENT
This study was supported by internal funding.
REFERENCES
- 1.Landman D, Georgescu C, Martin DA, Quale J. 2008. Polymyxins revisited. Clin Microbiol Rev 21:449–465. doi: 10.1128/CMR.00006-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, Paterson DL. 2006. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis 6:589–601. doi: 10.1016/S1473-3099(06)70580-1. [DOI] [PubMed] [Google Scholar]
- 3.Nation RL, Li J, Cars O, Couet W, Dudley MN, Kaye KS, Mouton JW, Paterson DL, Tam VH, Theuretzbacher U, Tsuji BT, Turnidge JD. 2015. Framework for optimisation of the clinical use of colistin and polymyxin B: the Prato polymyxin consensus. Lancet Infect Dis 15:225–234. doi: 10.1016/S1473-3099(14)70850-3. [DOI] [PubMed] [Google Scholar]
- 4.Karaiskos I, Giamarellou H. 2014. Multidrug-resistant and extensively drug-resistant Gram-negative pathogens: current and emerging therapeutic approaches. Expert Opin Pharmacother 15:1351–1370. doi: 10.1517/14656566.2014.914172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sader HS, Farrell DJ, Flamm RK, Jones RN. 2014. Antimicrobial susceptibility of Gram-negative organisms isolated from patients hospitalized in intensive care units in United States and European hospitals (2009-2011). Diagn Microbiol Infect Dis 78:443–448. doi: 10.1016/j.diagmicrobio.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 6.Ah YM, Kim AJ, Lee JY. 2014. Colistin resistance in Klebsiella pneumoniae. Int J Antimicrob Agents 44:8–15. doi: 10.1016/j.ijantimicag.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Cai Y, Chai D, Wang R, Liang B, Bai N. 2012. Colistin resistance of Acinetobacter baumannii: clinical reports, mechanisms and antimicrobial strategies. J Antimicrob Chemother 67:1607–1615. doi: 10.1093/jac/dks084. [DOI] [PubMed] [Google Scholar]
- 8.European Centre for Disease Prevention and Control. 2013. Antimicrobial resistance surveillance in Europe. Annual report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). European Centre for Disease Prevention and Control, Stockholm, Sweden: http://www.ecdc.europa.eu/en/publications/Publications/antimicrobial-resistance-surveillance-europe-2013.pdf. [Google Scholar]
- 9.Zarkotou O, Pournaras S, Voulgari E, Chrysos G, Prekates A, Voutsinas D, Themeli-Digalaki K, Tsakris A. 2010. Risk factors and outcomes associated with acquisition of colistin-resistant KPC-producing Klebsiella pneumoniae: a matched case-control study. J Clin Microbiol 48:2271–2274. doi: 10.1128/JCM.02301-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agodi A, Voulgari E, Barchitta M, Quattrocchi A, Bellocchi P, Poulou A, Santangelo C, Castiglione G, Giaquinta L, Romeo MA, Vrioni G, Tsakris A. 2014. Spread of a carbapenem- and colistin-resistant Acinetobacter baumannii ST2 clonal strain causing outbreaks in two Sicilian hospitals. J Hosp Infect 86:260–266. doi: 10.1016/j.jhin.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Humphries RM. 2015. Susceptibility testing of the polymyxins: where are we now? Pharmacotherapy 35:22–27. doi: 10.1002/phar.1505. [DOI] [PubMed] [Google Scholar]
- 12.Gales AC, Reis AO, Jones RN. 2001. Contemporary assessment of antimicrobial susceptibility testing methods for polymyxin B and colistin: review of available interpretative criteria and quality control guidelines. J Clin Microbiol 39:183–190. doi: 10.1128/JCM.39.1.183-190.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lo-Ten-Foe JR, de Smet AM, Diederen BM, Kluytmans JA, van Keulen PH. 2007. Comparative evaluation of the VITEK 2, disk diffusion, Etest, broth microdilution, and agar dilution susceptibility testing methods for colistin in clinical isolates, including heteroresistant Enterobacter cloacae and Acinetobacter baumannii strains. Antimicrob Agents Chemother 51:3726–3730. doi: 10.1128/AAC.01406-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maalej SM, Meziou MR, Rhimi FM, Hammami A. 2011. Comparison of disc diffusion, Etest and agar dilution for susceptibility testing of colistin against Enterobacteriaceae. Lett Appl Microbiol 53:546–551. doi: 10.1111/j.1472-765X.2011.03145.x. [DOI] [PubMed] [Google Scholar]
- 15.Arroyo LA, García-Curiel A, Pachón-Ibañez ME, Llanos AC, Ruiz M, Pachón J, Aznar J. 2005. Reliability of the E-test method for detection of colistin resistance in clinical isolates of Acinetobacter baumannii. J Clin Microbiol 43:903–905. doi: 10.1128/JCM.43.2.903-905.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SY, Shin JH, Lee K, Joo MY, Park KH, Shin MG, Suh SP, Ryang DW, Kim SH. 2013. Comparison of the Vitek2, MicroScan, and Etest methods with the agar dilution method in assessing colistin susceptibility of bloodstream isolates of Acinetobacter species from a Korean university hospital. J Clin Microbiol 51:1924–1926. doi: 10.1128/JCM.00427-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldstein FW, Ly A, Kitzis MD. 2007. Comparison of Etest with agar dilution for testing the susceptibility of Pseudomonas aeruginosa and other multidrug-resistant bacteria to colistin. J Antimicrob Chemother 59:1039–1040. doi: 10.1093/jac/dkm046. [DOI] [PubMed] [Google Scholar]
- 18.Hindler JA, Humphries RM. 2013. Colistin MIC variability by method for contemporary clinical isolates of multidrug-resistant Gram-negative bacilli. J Clin Microbiol 51:1678–1684. doi: 10.1128/JCM.03385-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan TY, Ng SY. 2007. Comparison of Etest, Vitek and agar dilution for susceptibility testing of colistin. Clin Microbiol Infect 13:541–544. doi: 10.1111/j.1469-0691.2007.01708.x. [DOI] [PubMed] [Google Scholar]
- 20.Clinical and Laboratory Standards Institute. 2015. Methods for dilution of antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 10th ed CLSI document M07-A10. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 21.Albur M, Noel A, Bowker K, Macgowan A. 2014. Colistin susceptibility testing: time for a review. J Antimicrob Chemother 69:1432–1434. doi: 10.1093/jac/dkt503. [DOI] [PubMed] [Google Scholar]
- 22.Karvanen M, Malmberg C, Mohamed A, Lagerback P, Friberg LE, Cars O. 2011. Colistin is extensively lost during normal experimental conditions, abstr D-690 Abstr 51st Intersci Conf Antimicrob Agents Chemother, Chicago, IL, 17 to 20 September 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sader HS, Rhomberg PR, Flamm RK, Jones RN. 2012. Use of a surfactant (polysorbate 80) to improve MIC susceptibility testing results for polymyxin B and colistin. Diagn Microbiol Infect Dis 74:412–414. doi: 10.1016/j.diagmicrobio.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 24.Clinical and Laboratory Standards Institute. 2015. Performance standards for antimicrobial susceptibility testing; 25th informational supplement. CLSI document M100-S25. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 25.EUCAST. 1 January 2015. Breakpoint tables for interpretation of MICs and zone diameters, version 5.0. EUCAST Laboratory for Antimicrobial Susceptibility Testing, Växjö, Sweden: http://www.eucast.org/clinical_breakpoints/. [Google Scholar]
- 26.Clinical and Laboratory Standards Institute. 2008. Development of in vitro susceptibility testing criteria and quality control parameters; approved guideline, 3rd ed CLSI document M23-A3. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 27.International Organization for Standardization. 2007. ISO 20776-2:2007(E). Clinical laboratory testing and in vitro diagnostic test systems. Susceptibility testing of infectious agents and evaluation of performance of antimicrobial susceptibility test devices. Part 2: evaluation of performance of antimicrobial susceptibility test devices. International Organization for Standardization, Geneva, Switzerland. [Google Scholar]
- 28.Sutherland CA, Nicolau DP. 2014. To add or not to add polysorbate 80: impact on colistin MICs for clinical strains of Enterobacteriaceae and Pseudomonas aeruginosa and quality controls. J Clin Microbiol 52:3810. doi: 10.1128/JCM.01454-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jayol A, Nordmann P, Brink A, Poirel L. 2015. Heteroresistance to colistin in Klebsiella pneumoniae associated with alterations in the PhoPQ regulatory system. Antimicrob Agents Chemother 59:2780–2784. doi: 10.1128/AAC.05055-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pournaras S, Dafopoulou K, Zarkotou O, Poulou A, Dimitroulia E, Tsakris A. 2014. Comparative evaluation of colistin susceptibility testing methods among multidrug-resistant Klebsiella pneumoniae and Acinetobacter baumannii clinical isolates with elevated colistin MICs, abstr D-852. Abstr 54th Intersci Conf Antimicrob Agents Chemother. American Society for Microbiology, Washington, DC. [Google Scholar]