Abstract
Ceftaroline-avibactam is a new combination of the antibiotic ceftaroline with a novel non-β-lactam β-lactamase inhibitor, avibactam. The purpose of the present study was to investigate the effect of ceftaroline-avibactam on the human intestinal microbiota. Fourteen healthy volunteers received ceftaroline-avibactam (600 mg ceftaroline fosamil and 600 mg avibactam) intravenously over 2 h every 8 h on days 1 to 6 and as a single dose on day 7. Fecal samples were collected on day −1 (within 24 h of the first infusion on day 1) and on days 2, 5, 7, 9, 14, and 21. Escherichia coli numbers decreased during the study and normalized on day 21. An increased number of Klebsiella bacteria appeared on day 14 and normalized on day 21. The number of other enterobacteria decreased during the study, and the number of enterococci decreased from days 2 to 7 and normalized on day 9. Candida numbers increased from days 5 to 9 and normalized after day 14. The number of lactobacilli decreased during the study and recovered on day 14. The number of bifidobacteria decreased on day 2 and normalized on day 21. The number of Bacteroides bacteria was unchanged. Clostridium difficile numbers decreased on days 7 and 9 and increased on days 14 and 21. A toxigenic C. difficile strain was detected in one volunteer on day 21 with no reported adverse events. Plasma samples were collected on days −1, 2, 5, and 7. Ceftaroline and avibactam concentrations were 0 to 34.5 mg/liter and 0 to 61.6 mg/liter, respectively, in plasma and 0 to 35.4 mg/kg and 0 to 98.5 mg/kg, respectively, in feces. (This study is registered in the European Clinical Trials Database [https://eudract.ema.europa.eu/] under number EudraCT 2012 004921-25.)
INTRODUCTION
Bacteria producing extended-spectrum β-lactamases (ESBLs) are considered resistant to most cephalosporins, penicillins, and monobactams (1, 2). Avibactam is a novel non-β-lactam β-lactamase inhibitor with a spectrum of activity encompassing class A, class C, and some class D β-lactamases. Avibactam, when combined with ceftaroline, has been shown to be active against strains that express a combination of β-lactamase types and strains that are concomitantly resistant to other antibacterial agents, such as fluoroquinolones (1–3). Currently, the options for the treatment of Gram-negative infections, especially with multidrug-resistant strains, including ESBL producers, are very limited. Until recently, there have been no new investigational agents under early or late development specifically targeted to combat these bacteria (1, 2, 4). Hence, the availability and development of new agents to treat these infections are welcome additions to the existing treatments.
Knowledge about the interaction between antimicrobial agents and the normal microbiota gives the clinician the possibility to choose agents associated with lesser degrees of ecological disturbances (5, 6). Consequently, the risk of the development of resistant strains and the transfer of resistance elements between microorganisms is reduced (5, 6). Consideration of the ecological consequences is also an important step to prevent distribution of resistant strains between persons (5, 6). The extent to which disturbances occur depends on the spectrum of the agent, the dose, the route of administration, pharmacokinetic (PK) properties, pharmacodynamic (PD) properties, and in vivo inactivation of the agent (7).
The primary objective was to investigate the effect of the administration of ceftaroline-avibactam on the intestinal microbiota of healthy volunteers. The secondary objectives were to investigate the safety, tolerability, and PK of ceftaroline-avibactam in healthy volunteers, to measure ceftaroline-avibactam plasma and fecal concentrations using bioactivity techniques, and to describe the in vitro susceptibility of new colonizing bacteria in the intestinal microbiota to ceftaroline-avibactam during and after ceftaroline-avibactam administration.
MATERIALS AND METHODS
Study design.
An open-label multiple-dose study (EudraCT 2012 004921-25) in healthy volunteers was designed to investigate the effect on the intestinal microbiota of ceftaroline-avibactam in 15 healthy volunteers (9 females and 6 males) during multiple administrations over 7 days. However, one female volunteer was withdrawn from the study on day 2 after four doses due to an adverse event (disseminated maculopapular rash) and was not evaluated. Fourteen volunteers completed the study.
The volunteers received ceftaroline-avibactam (600 mg ceftaroline fosamil and 600 mg avibactam) by intravenous (i.v.) infusion given over 2 h, every 8 h on days 1 to 6 (inclusive) and as a single dose on day 7.
The duration of the study for each healthy volunteer was 6 weeks. The study comprised five visits. Visit 1, for screening evaluation, occurred within 21 days prior to visit 2. Visit 2 was the start of the treatment period; eligible healthy volunteers were admitted to the study center on day −1 (within 24 h of the first infusion on day 1) for baseline assessments. The healthy volunteers remained resident at the study center until the completion of assessments on day 8. Intravenous infusions of ceftaroline-avibactam every 8 h on days 1 to 6 and a single dose on day 7 were administered to the healthy volunteers. Visit 3 (day 10) was an outpatient visit; visit 4, the poststudy follow-up, took place on day 14. Visit 5, the end-of-study assessment, took place on day 21.
Volunteers.
Fifteen healthy volunteers were included in the study. Volunteers were recruited through information about the study on the Clinical Pharmacology Trial Unit website of the Karolinska University Hospital (Stockholm, Sweden). A physical examination was carried out on each volunteer at the screening visit and included measurements of blood pressure, heart rate, and clinical laboratory tests as well as an interview on medical and surgical history. Inclusion criteria for men and women were age 18 to 45 years and normal findings in the medical history and physical examination. Each volunteer had a body mass index (BMI) between 19 and 30 kg/m2. The female subjects were of nonchildbearing potential or willing to take adequate contraceptive measures during the entire study period and for 3 months after completion of the study. The volunteers had to adhere to the visit schedule and concomitant therapy prohibitions and had to be compliant with the treatment (assessed at the screening visit). Volunteers understood and signed an informed consent form at the screening visit prior to any investigational procedure.
The exclusion criteria were as follows: underlying known disease, a surgical or medical condition with a history of predisposition to candidiasis overgrowth, known or suspected achlorhydria or surgery that bypasses or excludes the duodenum, myasthenia gravis, or hepatic impairment. People with known allergies or sensitivities to the active substance or to any of the excipients or components of the formulation being tested were excluded, as were patients who were currently enrolled in another investigational drug or device study or who participated in such a study in the past 3 months prior to the screening visit. People who had not undergone at least a 4-month washout period following a treatment with any systemic or topical antibiotics were excluded, as were people who used prohibited medications (such as antibiotics, barbiturates, carbamazepine, diphenylhydantoin, primidone, phenytoin, quinapril, activated charcoal, cholestyramine, bismuth chelates, sucralfate, isotretinoin, methoxyflurane, sulfonylurea, oral antidiabetic agents, anticoagulants of the dicoumarol type, and bivalent or trivalent ions, i.e., aluminum, zinc, calcium, magnesium, or iron preparations) prior to the study and who were unwilling to refrain from use during the study. Additional exclusions were clinically significant laboratory abnormalities at the screening laboratory evaluation; seropositivity for hepatitis B surface antigen (HBsAg), hepatitis C virus (HCV) antibody, and/or HIV; a history of chronic alcoholism (>7 units of alcohol per week, in which 1 unit corresponds to 360 ml of beer, 150 ml of wine, or 45 ml of spirits); a history of drug abuse or a positive test result for any drug abuse; excessive intake (>5 cups per day or approximately 500 mg of caffeine per day) of tea, coffee, beverages containing caffeine, or chocolate; or categorization as vulnerable, as defined in the International Conference on Harmonisation Guideline for Good Clinical Practice.
Approvals.
The study protocol submitted to the Regional Ethics Committee in Stockholm (Stockholm, Sweden) and to the Medical Products Agency (Uppsala, Sweden) was approved before starting the trial.
Collection of plasma samples.
Blood samples for the determination of avibactam and ceftaroline were collected in the opposite arm from which the investigational product was being infused at day −1, day 2 at 1 h after the start of administration, day 5 at 1 h after the start of administration, and day 7 at 1, 3, and 12 h after the start of administration. Blood samples were collected into blood collection tubes containing lithium-heparin as an anticoagulant and were labeled appropriately. After collection, blood samples were immediately put on ice and were centrifuged within 30 min at 1,500 × g for 10 min in order to obtain plasma. Plasma samples were stored at −70°C within 30 min after centrifugation.
Collection of fecal samples.
Fecal samples were collected on day −1 (predose), during administration on days 2, 5, and 7, and postdose on days 9, 14, and 21 according to the study design. In case no specimen was obtained from feces passed on a specified collection day or no specimen was passed on that day, the first subsequent fecal specimen passed after that day was collected. If more than one specimen was passed on a specified collection day, only the first specimen of that day was collected. Fecal samples (10 g) were collected in sterile plastic containers and were kept at 4°C until transportation to the microbiological laboratory, where they were frozen at −70°C and processed within 14 days.
Determination of ceftaroline and avibactam in plasma and feces.
Ceftaroline and avibactam concentrations in plasma and feces were analyzed microbiologically by using the agar well diffusion method. For the determination of the ceftaroline concentration, Micrococcus luteus ATCC 9341 was used as an indicator strain; for the determination of the avibactam concentration, Escherichia coli CCUG 55971 was used as an indicator strain. All samples were analyzed within a time frame for which the stability of ceftaroline and avibactam in the samples had been validated and shown to be acceptable.
Plasma samples were centrifuged and supernatants were analyzed without further processing. The plasma supernatants were diluted according to the need. The fecal samples were first diluted 1:3 (weight/volume) in Milli-Q water, homogenized thoroughly, and then centrifuged at 5,000 rpm for 12 min. The supernatants were used for the analysis. The fecal supernatants were diluted according to a scheme. The lower limit of detection for ceftaroline was 0.032 mg/kg of feces and 0.032 mg/liter of the plasma samples. The standard curves were linear within a concentration range of 0.032 to 32 mg/liter for feces and a range of 0.032 to 32 mg/liter for plasma samples. The correlation coefficient of the standard curves was 0.99. All samples were analyzed in duplicate.
The lower limit of detection for avibactam was 0.5 mg/kg of feces and 0.5 mg/liter of plasma. The standard curves were linear within a concentration range of 0.5 to 16 mg/liter for feces and a range of 0.5 to 16 mg/liter for plasma samples. The correlation coefficient of the standard curves was 0.99. All samples were analyzed in duplicate.
Processing of specimens for microbiological analyses.
Fecal samples were diluted 10-fold to 10−7 and inoculated on nonselective and selective agars, as described previously (8, 9). The aerobic agar plates were incubated for 24 h at 37°C and the anaerobic plates were incubated for 48 h at 37°C in anaerobic jars (GasPak; BBL, Baltimore, MD, USA). After incubation, different colony types were counted, isolated in pure culture, and identified to the genus level. The lower limit of detection for quantitative cultures was 100 CFU, i.e., log10 = 2. All isolates were analyzed according to Gram reaction and colony morphology, followed by biochemical tests or matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) technology. Anaerobic microorganisms were identified by MALDI-TOF technology (10). All the fecal samples, including the predose samples, were cultured for Clostridium difficile. C. difficile strains were further characterized by the cytotoxic neutralization assay and PCR ribotyping, as described recently (11).
Antibiotic susceptibility tests.
The MICs of ceftaroline-avibactam were determined for new colonizing bacterial strains isolated from the fecal samples by the agar dilution method (12, 13). The final inoculum for aerobic bacteria was 104 CFU per spot, and the final inoculum for anaerobic bacteria was 105 CFU per spot. Inoculated plates were incubated for 24 h (aerobic bacteria) and 48 h (anaerobic bacteria) at 37°C. Reference strains were Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 29213, Enterococcus faecalis ATCC 29212, Bacteroides fragilis ATCC 25285, and Clostridium difficile ATCC 700057. The strains were considered resistant according to breakpoints defined by the Clinical and Laboratory Standards Institute (CLSI) (12, 13). The tentative breakpoint for ceftaroline-avibactam was set at 1 mg/liter ceftaroline and 4 mg/liter avibactam, and this concentration of ceftaroline-avibactam was used in the agar plates in order to isolate primary resistant strains. The MIC was defined as the lowest concentration of the drug that inhibited growth completely. For anaerobic strains, brucella agar supplemented with hemin (5 μg/ml), vitamin K1 (1 μg/ml), and leaked sheep blood (5% vol/vol) was used. For aerobic strains, Mueller-Hinton agar was used.
Safety.
The following safety assessments were performed: physical examination, vital signs, electrocardiogram, adverse events monitoring, and monitoring of clinical laboratory parameters. The investigator was responsible for necessary acute medical treatment of any adverse event during the trial and ensured that appropriate medical care was maintained thereafter, if necessary. All findings were reported on an adverse events page in the case report form and in the volunteer's medical record.
Statistical methods.
Descriptive statistics and individual case analyses were used. For quantitation of viable counts, the numbers of CFU were determined as the log10 CFU per gram of feces. A CFU of <2 log10 was considered below the limit of detection. For calculations, a CFU number below the detection limit was set to zero. Genus/species-specific changes in viable counts within the entire study population were analyzed to evaluate the ecological impact on the microbiota.
RESULTS
The age range of the subjects was 22 to 34 years (mean age, 27 years). The mean height was 178 cm (range, 158 to 195 cm), the mean weight was 71.4 kg (range, 53.3 to 88.5 kg), and the mean body mass index (BMI) was 22.5 kg/m2 (range, 19.9 to 25.1 kg/m2).
Effect of ceftaroline-avibactam on the aerobic intestinal microbiota.
Fig. 1 shows the effect of ceftaroline-avibactam on the aerobic intestinal microbiota. The number of E. coli bacteria decreased during administration of ceftaroline-avibactam and normalized on day 21. An increased number of Klebsiella bacteria appeared on day 14 and normalized on day 21. The numbers of other enterobacteria decreased during the administration of ceftaroline-avibactam; the number of enterococci decreased from day 2 until day 7 and normalized on day 9. Candida numbers increased from day 5 to day 9 and normalized after day 14.
FIG 1.
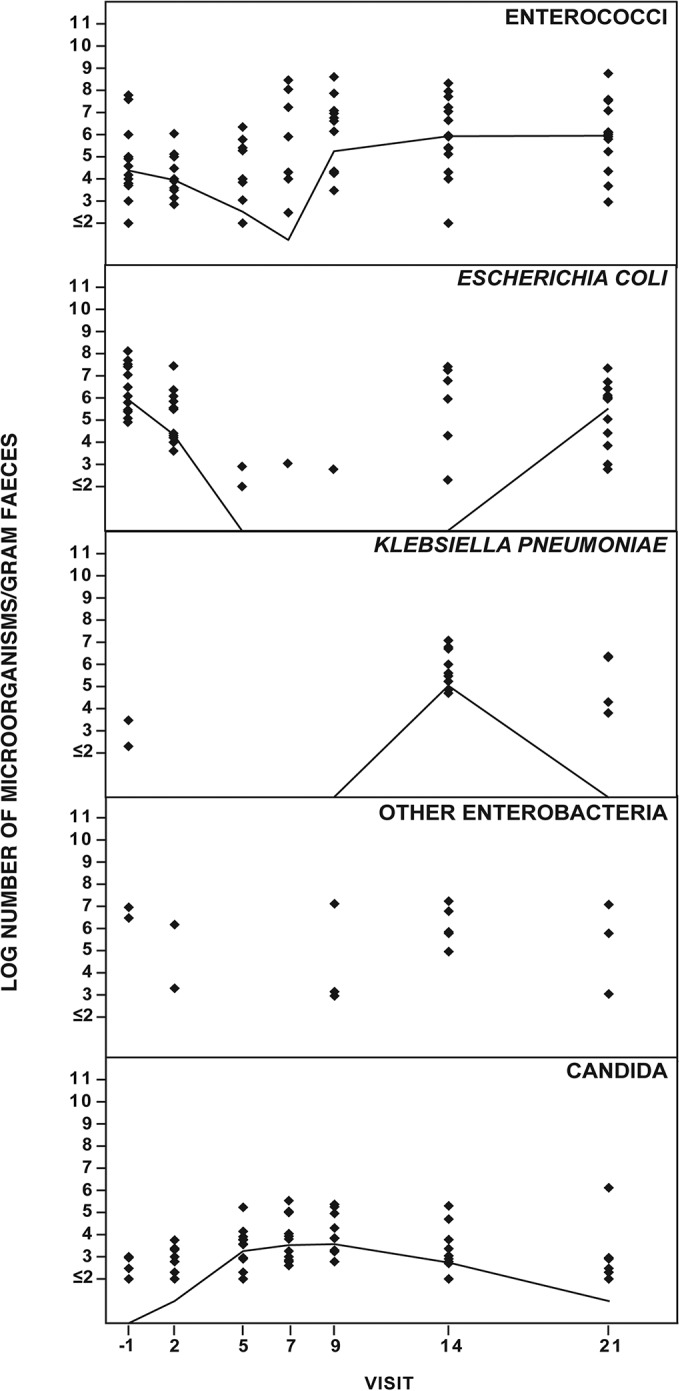
Effect of ceftaroline-avibactam (600 mg ceftaroline fosamil and 600 mg avibactam) i.v. over 1 h every 8 h on days 1 to 6 and a single dose on day 7 on intestinal aerobic microbiota of 14 healthy volunteers.
Effect of ceftaroline-avibactam on the anaerobic intestinal microbiota.
Fig. 2 shows the effect of ceftaroline-avibactam on the anaerobic intestinal microbiota. The number of lactobacilli decreased during administration of ceftaroline-avibactam and normalized on day 14. The number of bifidobacteria decreased on day 2 and was not normalized until day 21. The numbers of Bacteroides bacteria were unchanged throughout the study. C. difficile numbers decreased on days 7 and 9 and increased on days 14 and 21; the numbers of CFU were higher than those at baseline. A toxigenic C. difficile strain (4.5 × 103) was isolated from feces in one of the volunteers (volunteer 14), but only on day 21. The isolate was the SE21 PCR ribotype. All fecal samples from volunteer 14 were analyzed for the presence of the C. difficile toxin B gene. Only the sample from day 21 was positive. No adverse events were reported by the volunteer.
FIG 2.
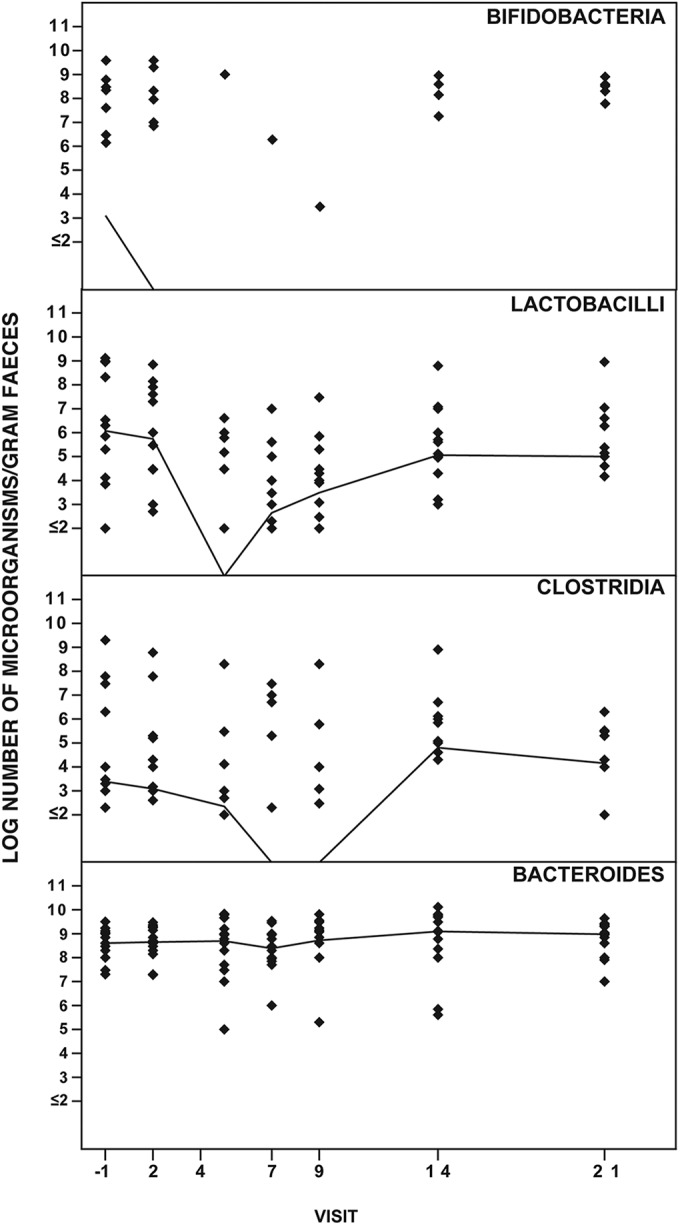
Effect of ceftaroline-avibactam (600 mg ceftaroline fosamil and 600 mg avibactam) i.v. over 1 h every 8 h on days 1 to 6 and a single dose on day 7 on intestinal anaerobic microbiota of 14 healthy volunteers.
Plasma and fecal concentrations of ceftaroline and avibactam.
The plasma concentrations of ceftaroline as determined by bioassay are shown in Table 1. None of the volunteers had any detectable ceftaroline at day −1. On day 2, the mean ceftaroline plasma concentration was 16.8 mg/liter and the median was 14.5 mg/liter (range, 8.4 to 31.4 mg/liter); on day 5, the mean plasma concentration of ceftaroline was 15.6 mg/liter, and the median was 13.9 mg/liter (range, 6.4 to 30.1 mg/liter). On day 7, the volunteers received only 1 dose of ceftaroline, and 3 samples were collected at 1, 3, and 12 h after the start of the administration. The mean plasma concentrations of ceftaroline at 1, 3, and 12 h were 16.7 mg/liter (range, 8.9 to 34.5 mg/liter), 4.9 mg/liter (range, 2.1 to 12.3 mg/liter), and 0.1 mg/liter (range, 0 to 0.3 mg/liter), respectively. The corresponding median values were 13.0 mg/liter, 2.7 mg/liter, and 0.1 mg/liter.
TABLE 1.
Plasma concentration of ceftaroline and avibactam in volunteers receiving ceftaroline-avibactama
| Drug and visit day | Sampling time after start of administration (h) | Plasma concn (mg/liter) |
||||
|---|---|---|---|---|---|---|
| Mean | Median | Minimum | Maximum | SD | ||
| Ceftaroline | ||||||
| −1 | 0 | 0 | 0 | 0 | 0 | |
| 2 | 1 | 16.8 | 14.5 | 8.4 | 31.4 | 7.4 |
| 5 | 1 | 15.6 | 13.9 | 6.4 | 30.1 | 7.1 |
| 7 | 1 | 16.7 | 13.0 | 8.9 | 34.5 | 8.2 |
| 7 | 3 | 4.9 | 2.7 | 2.1 | 12.3 | 4.0 |
| 7 | 12 | 0.1 | 0.1 | 0 | 0.3 | 0.1 |
| Avibactam | ||||||
| −1 | 0 | 0 | 0 | 0 | 0 | |
| 2 | 1 | 21.3 | 14.2 | 5.9 | 61.6 | 16.8 |
| 5 | 1 | 20.8 | 11.2 | 5.5 | 55.8 | 18.6 |
| 7 | 1 | 20.3 | 10.6 | 0 | 59.2 | 21.0 |
| 7 | 3 | 2.3 | 1.0 | 0 | 8.2 | 2.7 |
| 7 | 12 | 0 | 0 | 0 | 0 | 0 |
Ceftaroline-avibactam administered as 600 mg ceftaroline fosamil and 600 mg avibactam i.v. over 1 h every 8 h on days 1 to 6 and a single dose on day 7.
The plasma concentrations of avibactam are shown in Table 1. On day 2, the mean avibactam plasma concentration was 21.3 mg/liter, and the median was 14.2 mg/liter (range, 5.9 to 61.6 mg/liter); on day 5, the mean plasma concentration of avibactam was 20.8 mg/liter, and the median was 11.2 mg/liter (range, 5.5 to 55.8 mg/liter). On day 7, the volunteers received only 1 dose of ceftaroline-avibactam, and 3 samples were collected at 1, 3, and 12 h after the start of the administration. The mean plasma concentrations of avibactam at 1, 3, and 12 h were 20.3 mg/liter (range, 0 to 59.2 mg/liter), 2.3 mg/liter (range, 0 to 8.2 mg/liter), and 0 mg/liter (range, 0 to 0 mg/liter), respectively. The corresponding median values were 10.6 mg/liter, 1.0 mg/liter, and 0 mg/liter.
The fecal concentrations of ceftaroline and avibactam as determined by bioassay are shown in Table 2. None of the volunteers had any detectable concentration of ceftaroline or avibactam at days −1, 9, 14, and 21. Ceftaroline and/or avibactam was detected in the fecal samples of few volunteers: on day 2, ceftaroline was detected in 7 volunteers and avibactam in 1 volunteer; on day 5, ceftaroline was detected in 3 volunteers and avibactam in 2 volunteers; and on day 7, ceftaroline was detected in 5 volunteers and avibactam in 1 volunteer. The fecal concentration of ceftaroline on day 2 was 0 to 18.5 mg/kg, on day 5 was 0 to 35.4 mg/kg, and on day 7 was 0 to 1.4 mg/kg. The fecal concentration of avibactam on day 2 was 0 to 3.7 mg/kg, on day 5 was 0 to 98.5 mg/kg, and on day 7 was 0 to 1.6 mg/kg.
TABLE 2.
Fecal concentration of ceftaroline and avibactam in volunteers receiving ceftaroline-avibactama
| Drug and visit day | Fecal concn (mg/kg) |
||||
|---|---|---|---|---|---|
| Mean | Median | Minimum | Maximum | SD | |
| Ceftaroline | |||||
| −1 | 0 | 0 | 0 | 0 | 0 |
| 2 | 1.7 | 0 | 0 | 18.4 | 4.9 |
| 5 | 2.9 | 0 | 0 | 35.4 | 9.5 |
| 7 | 0.3 | 0 | 0 | 1.4 | 0.5 |
| 9 | 0 | 0 | 0 | 0 | 0 |
| 14 | 0 | 0 | 0 | 0 | 0 |
| 21 | 0 | 0 | 0 | 0 | 0 |
| Avibactam | |||||
| −1 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0.3 | 0 | 0 | 3.7 | 1.0 |
| 5 | 7.1 | 0 | 0 | 98.5 | 26.3 |
| 7 | 0.1 | 0 | 0 | 1.6 | 0.4 |
| 9 | 0 | 0 | 0 | 0 | 0 |
| 14 | 0 | 0 | 0 | 0 | 0 |
| 21 | 0 | 0 | 0 | 0 | 0 |
Ceftaroline-avibactam administered as 600 mg ceftaroline fosamil and 600 mg avibactam i.v. over 1 h every 8 h on days 1 to 6 and a single dose on day 7.
New colonizing ceftaroline-avibactam-resistant bacteria in the intestinal microbiota.
All aerobic and anaerobic bacterial isolates collected from ceftaroline-avibactam (1 mg/liter ceftaroline and 4 mg/liter avibactam)-containing agar plates were identified, and the MICs were determined. New colonizing isolates are shown in Table 3. During the observation period, a total of 289 Bacteroides spp. were isolated, and all of them were resistant to ceftaroline-avibactam (MIC, ≥1 to >256 mg/liter).
TABLE 3.
MICs of ceftaroline-avibactam for the new colonizing bacteria in volunteers receiving ceftaroline-avibactama
| Bacteria | No. of volunteers | MIC (mg/liter) |
|---|---|---|
| Enterococcus faecium | 5 | 0.5–8 |
| Enterococcus faecalis | 4 | 1–8 |
| Enterococcus durans | 1 | 1 |
| Alpha-hemolytic streptococci | 2 | 4 |
| Streptococcus salivarius | 2 | 1–4 |
| Enterobacter cloacae | 1 | 8 |
| Bacillus spp. | 1 | 4 |
| Acinetobacter baumannii | 1 | 4 |
| Stenotrophomonas maltophilia | 1 | 64 |
| Micrococcus spp. | 1 | 4 |
| Pseudomonas aeruginosa | 1 | 1 |
| Clostridium spp. | 7 | 1–128 |
| Lactobacillus spp. | 8 | 1–128 |
| Anaerobic Gram-positive rods | 6 | 1–16 |
Ceftaroline-avibactam administered as 600 mg ceftaroline fosamil and 600 mg avibactam i.v. over 1 h every 8 h on days 1 to 6 and a single dose on day 7.
Safety and tolerability.
All the volunteers in the study reported at least one adverse event. No serious adverse events were reported. One subject discontinued the study due to an adverse event, a disseminated maculopapular rash. The most commonly reported adverse events belonged to the system organ class (SOC) of skin and subcutaneous tissue disorders (80% [12 of 15] of the volunteers) and gastrointestinal disorders (66.7% [10 of 15] of the volunteers). Maculopapular rash (n = 8; 53.3%), pruritus and rash (each n = 6; 40%), and papular rash (n = 2; 13.3%) were reported by the 15 volunteers. One volunteer each reported the adverse events of papules, erythematous rash, or skin fissures (6.7% each). Flatulence was reported by 5 volunteers (33.3%), and loose stools was reported by 3 (20.0%) of the 15 volunteers.
Overall, 14 (93.3%) of the 15 volunteers reported at least one adverse event considered to be causally related to the investigational product by the investigator. All the adverse events reported in the study were considered to be of mild intensity except for 5 adverse events of moderate intensity reported by 4 volunteers. A total of 9 mild adverse events and 2 moderate adverse events reported by 7 volunteers remained unresolved at the end of the study.
The plasma albumin and platelet counts decreased slightly from day −1 through day 8 and returned to prestudy values by the end of the study. The mean creatinine values showed a moderate increase during dosing and normalized by the end of the study. The mean creatinine on day −1 was 72.6 μmol/liter (range, 54 to 90 μmol/liter), on day 5, 83 μmol/liter (range, 65 to 106 μmol/liter), on day 8, 83.4 μmol/liter (range, 66 to 114 μmol/liter), and on day 21, 75 μmol/liter (range, 56 to 109 μmol/liter). One volunteer tested positive on a pregnancy test at visit 4 (day 14). The pregnancy was electively terminated.
DISCUSSION
The spectra of cephalosporins are wide, and major disturbances in the normal microbiota might be expected, in particular with the agents that are excreted through bile, like ceftriaxone (5, 7, 14–17). There is an increased risk of developing C. difficile infection and colonization with yeasts in connection with cephalosporin administration (5, 15). Usually, β-lactamase inhibitors have no antibacterial activity of their own (18). The ecological impact of ceftaroline was studied by Panagiotidis et al. (19), and no fecal concentration of ceftaroline was found in the feces in healthy volunteers. There was a minor impact on the numbers of E. coli microorganisms, while the numbers of enterococci and Candida albicans microorganisms were not affected. There were moderate decreases in the numbers of bifidobacteria and lactobacilli during the first 7 days, while the numbers of clostridia increased during the same period. No impact on the numbers of Bacteroides microorganisms was noticed. Similar results were obtained in the present study. The aerobic and anaerobic fecal microbiota was suppressed during the i.v. administration of ceftaroline-avibactam, and the microbiota was normalized at day 21. In the ceftaroline-avibactam study, a fecal concentration of ceftaroline was found in 7 volunteers on day 2 and in 4 volunteers on day 5. A fecal concentration of avibactam was found in 1 volunteer on day 2 and in 2 volunteers on day 5. There was no correlation among the fecal concentrations and a moderate increase of plasma creatinine levels. Ceftaroline and avibactam are mainly excreted through urine (20). Avibactam was also studied in combination with ceftazidime in 12 healthy volunteers (21). A fecal concentration of avibactam, an impact on the microbiota, and toxigenic C. difficile colonization were found in healthy volunteers (21). Toxigenic C. difficile strains were found in 5 of the 12 volunteers; in 4 of these volunteers, loose stools were reported as adverse events (21). In the previous ceftaroline study, six toxigenic C. difficile strains were isolated from 2 subjects, and the MICs were 4 to 8 mg/liter (19). No clinical symptoms were observed in the 2 subjects (19).
In the current study, a toxigenic C. difficile strain was detected in 1 volunteer only on day 21, with no reported adverse events. Therefore, these findings are likely to have no clinical relevance. The MIC value for the C. difficile strain was 1 mg/liter. In the previous study, no new colonizing aerobic and anaerobic bacteria resistant to ceftaroline (MIC, ≥4 mg/liter) were found (19). In the current study, new colonizing aerobic and anaerobic bacteria resistant to ceftaroline-avibactam (MIC, ≥1 mg/liter) were found. All the Bacteroides spp. were resistant to ceftaroline-avibactam (MIC, ≥1 to >256 mg/liter). In the previous study, all B. fragilis group strains were β-lactamase producers and resistant to ceftaroline (MIC, ≥64 mg/liter) (19).
Colonization resistance is maintained by obligate anaerobes, but the knowledge is limited about the specific members of the microbiota that inhibit colonization by pathogens or opportunistic pathogens. The effects of antibiotics on the microbiota and its potential alterations have impacts on pathogen colonization and nosocomial infections. Antibiotic use may facilitate resistance gene transfer across the intestinal tract (6, 22). Further clinical studies with the avibactam combination are needed to know the pharmacokinetics and its clinical significance.
ACKNOWLEDGMENTS
This study was funded by AstraZeneca, Paddington, London, United Kingdom.
We declare no competing interests.
REFERENCES
- 1.Curello J, MacDougall C. 2014. Beyond susceptible and resistant, part II: treatment of infections due to Gram-negative organisms producing extended-spectrum beta-lactamases. J Pediatr Pharmacol Ther 19:156–164. doi: 10.5863/1551-6776-19.3.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toussaint KA, Gallagher JC. 2015. Beta-lactam/beta-lactamase inhibitor combinations: from then to now. Ann Pharmacother 49:86–98. doi: 10.1177/1060028014556652. [DOI] [PubMed] [Google Scholar]
- 3.Stachyra T, Levasseur P, Pechereau MC, Girard AM, Claudon M, Miossec C, Black MT. 2009. In vitro activity of the β-lactamase inhibitor NXL104 against KPC-2 carbapenemase and Enterobacteriaceae expressing KPC carbapenemases. J Antimicrob Chemother 64:326–329. doi: 10.1093/jac/dkp197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Agata EM. 2004. Rapidly rising prevalence of nosocomial multidrug-resistant, Gram-negative bacilli: a 9-year surveillance study. Infect Control Hosp Epidemiol 25:842–846. doi: 10.1086/502306. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan A, Edlund C, Nord CE. 2001. Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect Dis 1:101–114. doi: 10.1016/S1473-3099(01)00066-4. [DOI] [PubMed] [Google Scholar]
- 6.Rashid MU, Weintraub A, Nord CE. 2012. Effect of new antimicrobial agents on the ecological balance of human microflora. Anaerobe 18:249–253. doi: 10.1016/j.anaerobe.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 7.European Medicines Agency. 2000. Points to consider on pharmacokinetics and pharmacodynamics in the development of antibacterial medicinal products. CPMP/EWP/2655/99. European Agency for the Evaluation of Medicinal Products, London, United Kingdom. [Google Scholar]
- 8.Rashid MU, Dalhoff A, Backstrom T, Bjorkhem-Bergman L, Panagiotidis G, Weintraub A, Nord CE. 2014. Ecological impact of MCB3837 on the normal human microbiota. Int J Antimicrob Agents 44:125–130. doi: 10.1016/j.ijantimicag.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 9.Rashid MU, Zaura E, Buijs MJ, Keijser BJ, Crielaard W, Nord CE, Weintraub A. 2015. Determining the long-term effect of antibiotic administration on the human normal intestinal microbiota using culture and pyrosequencing methods. Clin Infect Dis 60:S77–S84. doi: 10.1093/cid/civ137. [DOI] [PubMed] [Google Scholar]
- 10.Versalovic J, Carroll KC, Jorgensen JH, Funke G, Landry ML, Warnock DW (ed). 2011. Manual of clinical microbiology, 10th ed ASM Press, Washington, DC. [Google Scholar]
- 11.Rashid MU, Lozano HM, Weintraub A, Nord CE. 2013. In vitro activity of cadazolid against Clostridium difficile strains isolated from primary and recurrent infections in Stockholm, Sweden. Anaerobe 20:32–35. doi: 10.1016/j.anaerobe.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 12.CLSI. 2012. Methods for antimicrobial susceptibility testing of anaerobic bacteria; approved standard—8th ed CLSI M11-A8. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 13.CLSI. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—9th ed. CLSI M7-A9. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 14.Nord CE, Heimdahl A, Lundberg C, Marklund G. 1987. Impact of cefaclor on the normal human oropharyngeal and intestinal microflora. Scand J Infect Dis 19:681–685. doi: 10.3109/00365548709117204. [DOI] [PubMed] [Google Scholar]
- 15.Takesue Y, Yokoyama T, Akagi S, Ohge H, Murakami Y, Sakashita Y, Sasaki M, Itaha H, Matsuura Y. 1999. Changes in intestinal flora after administration of panipenem/betamipron or sulbactam/cefoperazone for treatment of postoperative infections in gastrectomy patients. J Infect Chemother 5:52–57. doi: 10.1007/s101560050009. [DOI] [PubMed] [Google Scholar]
- 16.Kager L, Brismar B, Malmborg AS, Nord CE. 1986. Impact of cefbuperazone on the colonic microflora in patients undergoing colorectal surgery. Drugs Exp Clin Res 12:983–986. [PubMed] [Google Scholar]
- 17.Arvidsson A, Alvan G, Angelin B, Borga O, Nord CE. 1982. Ceftriaxone: renal and biliary excretion and effect on the colon microflora. J Antimicrob Chemother 10:207–215. doi: 10.1093/jac/10.3.207. [DOI] [PubMed] [Google Scholar]
- 18.Wilson SE, Nord CE. 1995. Clinical trials of extended spectrum penicillin/beta-lactamase inhibitors in the treatment of intraabdominal infections. European and North American experience. Am J Surg 169(5A Suppl):21S–26S. [PubMed] [Google Scholar]
- 19.Panagiotidis G, Backstrom T, Asker-Hagelberg C, Jandourek A, Weintraub A, Nord CE. 2010. Effect of ceftaroline on normal human intestinal microflora. Antimicrob Agents Chemother 54:1811–1814. doi: 10.1128/AAC.01716-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riccobene TA, Su SF, Rank D. 2013. Single- and multiple-dose study to determine the safety, tolerability, and pharmacokinetics of ceftaroline fosamil in combination with avibactam in healthy subjects. Antimicrob Agents Chemother 57:1496–1504. doi: 10.1128/AAC.02134-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rashid M-U, Rosenborg S, Panagiotidis G, Söderberg Löfdal K, Weintraub A, Nord CE. 2015. Ecological effect of ceftazidime/avibactam on the normal human intestinal microbiota. Int J Antimicrob Agents 46:60–65. doi: 10.1016/j.ijantimicag.2015.02.027. [DOI] [PubMed] [Google Scholar]
- 22.Donskey CJ. 2004. The role of the intestinal tract as a reservoir and source for transmission of nosocomial pathogens. Clin Infect Dis 39:219–226. doi: 10.1086/422002. [DOI] [PubMed] [Google Scholar]


