Abstract
Solithromycin, a new macrolide and the first fluoroketolide, is in late-stage clinical development and, like older macrolides, is primarily metabolized and excreted through liver-dependent mechanisms. This study evaluated the safety and pharmacokinetics of solithromycin in patients with chronic liver disease. This open-label, multiple-dose study in subjects with hepatic impairment and in healthy control subjects (matched for age, weight, and sex) enrolled 8 Child-Pugh class A (mild), 8 class B (moderate), and 8 class C (severe) patients and 9 healthy controls. Subjects (n = 33) received one 800-mg dose on day 1 followed by once-daily doses of 400 mg on days 2 through 5. The most commonly reported adverse events were mild diarrhea and mild headache, and no significant differences were noted between hepatically impaired subjects and healthy controls. The pharmacokinetics of plasma solithromycin in subjects with mild and moderate impairment was similar to that in control subjects. In subjects with severe impairment, total exposure to solithromycin at steady state (area under the plasma concentration-time curve [AUC0–tau]) was decreased compared to that in control subjects, which may have been related to the higher body mass index of individuals in this group. No greater accumulation was noted in any hepatically impaired cohort on day 5 compared to that in control subjects. No decrease in dosage is therefore needed when administering solithromycin to patients with mild, moderate, or severe hepatic impairment. Solithromycin was well tolerated in this patient population, and no significant differences in safety, compared to healthy controls, were noted.
INTRODUCTION
Solithromycin (CEM-101) is a new macrolide antibiotic, the first fluoroketolide, being developed in both oral and intravenous formulations for the treatment of bacterial infections. Solithromycin has in vitro activity against both respiratory (1–4) and urogenital (4–7) pathogens. In a phase 2 study in patients with community-acquired bacterial pneumonia (CABP), treatment with a once-daily 5-day regimen of solithromycin showed efficacy comparable to, and was better tolerated than, levofloxacin (8). A phase 2 single-dose study in patients with uncomplicated urogenital gonorrhea showed a microbiological success rate of 100% at the 2 doses evaluated (9). Solithromycin has been evaluated in one phase 3 oral CABP study (ClinicalTrials registration no. NCT01756339), in which noninferiority to moxifloxacin was demonstrated (10), and is currently being evaluated in a phase 3 intravenous to oral CABP study (ClinicalTrials registration no. NCT01968733) and in a phase 3 gonorrhea study (ClinicalTrials registration no. NCT02210325).
Solithromycin exhibits nonlinear pharmacokinetics (PK) and accumulates moderately over multiple dosing regimens due to autoinhibition. Therefore, a loading dose regimen with lower daily maintenance doses was chosen for clinical development. In healthy subjects, the loading dose of 800 mg solithromycin on day 1 achieves peak plasma concentrations of ∼1.0 μg/ml; similar peak concentrations are achieved on day 5 after once-daily dosing of 400 mg for 4 days (11). Food does not appear to have an effect on the bioavailability of solithromycin (11).
Solithromycin may be used to treat infections in patients with hepatic impairment. CYP3A4 metabolism and hepatic elimination are likely its major metabolic and clearance pathways (data not shown). Solithromycin is metabolized by CYP3A4, and, as a mechanism-based inhibitor of CYP3A isozymes, it inhibits its own metabolism. It does not induce CYP3A isozymes. Biliary excretion in animal models is extensive; in humans, the majority of radioactivity was excreted in the feces after administration of [14C]solithromycin (data not shown).
This hepatic impairment study was designed based on the current FDA guidance (23), which recommends using the Child-Pugh classification system to determine the degree of liver impairment and a sample size of at least 8 subjects in the moderate-impairment arm and the healthy control arm. This document also acknowledges that statistical significance of differences between groups (e.g., 90% confidence intervals between 80% and 125% or 50% and 200%) may not be achievable given the small sample sizes. A review of published literature on PK studies of marketed macrolide antibiotics in hepatically impaired subjects revealed that clarithromycin was evaluated in 7 (moderate and severe) subjects (12), azithromycin was evaluated in 16 (mild and moderate) subjects (13), and telithromycin was evaluated in 12 (mild, moderate, and severe) subjects (14).
We evaluated the PK, protein binding, and safety of solithromycin in 24 subjects with mild, moderate, and severe hepatic impairment compared to 9 healthy subjects with normal hepatic function.
MATERIALS AND METHODS
This was an open-label, nonrandomized, parallel-group study conducted at 2 centers in the United States between March and August 2013. The study was conducted in accordance with good clinical practice guidelines and conformed to the ethical principles of the Declaration of Helsinki. The protocol and informed consent documents were approved by two institutional review boards (IRB) (Aspire IRB and University of Miami, Human Subjects Research Office). Written informed consent was obtained from all subjects prior to enrollment. Liver disease severity was assessed by Child-Pugh scores.
Male and female subjects (ages 18 to 75 years) with mild (Child-Pugh score of 5 or 6), moderate (Child-Pugh score of 7 to 9), and severe (Child-Pugh score of ≥10) hepatic impairment and with a total body mass index (BMI) of ≥18 and ≤40 kg/m2 were enrolled. Healthy control subjects with normal hepatic function, matched for age (±10 years), weight (±20%), and sex (approximately the same ratio of men to women), were also enrolled. All subjects had to provide written informed consent, to adhere to the lifestyle guideline restrictions (abstaining from smoking, alcohol, and xanthine-containing beverages), and be confined to the clinical research unit as required by the protocol. Subjects were required to have a QT interval with Fridericia correction (QTcF) of ≤470 ms and to have aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels of less than or equal to the upper limit of normal (ULN) for healthy control subjects and <6 × ULN for hepatically impaired subjects. Subjects with hepatorenal syndrome or a surgical portocaval shunt were excluded; however, those who had undergone a transjugular intrahepatic portosystemic shunt (TIPS) procedure were enrolled. Concomitant use of medications that are moderate to strong substrates, inhibitors, or inducers of CYP3A4 was prohibited.
Subjects with mild impairment and matched controls received one dose of 800 mg (4 200-mg capsules) on day 1 followed by once-daily 400-mg doses (2 200-mg capsules) on days 2 through 5. In all cases, the study medication was administered under the direct supervision of study staff. The first and last doses (days 1 and 5) were administered in the fasted state, and subjects drank ∼240 ml of water with each dose. Subjects were confined in the study center during the 5-day dosing period and for at least 72 h after the last dose for PK sampling. All subjects returned for a follow-up visit 14 (±2) days after the last dose. Preliminary PK and safety data were reviewed from the previous groups prior to dosing the next group (moderate and severe hepatic impairment groups), and no dosage modifications were required; all groups received the same dosing regimen.
Safety was evaluated by clinical laboratory tests (on days 2, 4, 6, 8, and 19), physical examination, vital signs, 12-lead electrocardiograms (ECGs), and adverse events (AEs). All reported AEs were coded to a standard set of terms, using the Medical Dictionary for Regulatory Activities (MedDRA) version 15.1. The number of subjects experiencing treatment-emergent adverse events (TEAEs) and the number of TEAEs were summarized by the MedDRA-preferred term for each cohort.
Blood samples for the measurement of plasma concentrations of solithromycin and its active side chain metabolites, N-acetyl-CEM-101 and CEM-214, were collected on days 1 and 5 at 0 (predose), 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, and 16 h postdose. On days 2 to 4, samples were collected at predose only, and on days 6 to 8, they were collected at 24, 36, 48, and 72 h postdose. Plasma samples were stored frozen at −70°C and analyzed in batches using a validated liquid chromatography-mass spectrometry (LC-MS) method at a central laboratory. The range of quantification was 10 to 10,000 ng/ml for CEM-101 (1 to 1,000 ng/ml for N-acetyl-CEM-101 and CEM-214); deuterated CEM-101 was added as an internal standard. The precision ranged from 4.4% to 5.6%, and the accuracy ranged from −5.4% to −0.7%.
Additional blood samples were obtained (at predicted maximum observed concentration [Cmax]) 4 h postdose on days 1 and 5 to measure unbound solithromycin concentrations in plasma for evaluation of protein binding. These samples were stored frozen at −70°C after collection and incubated in batches in an equilibrium dialysis chamber, and samples from each side of the membrane were analyzed using a validated LC-MS method at a central laboratory.
Urine was collected from all subjects over 24 h postdose on days 1 and 5 for PK analysis of solithromycin, N-acetyl-CEM-101, and CEM-214; stored frozen at −70°C after collection; and analyzed using a nonvalidated LC-MS method at a central laboratory. The range of quantification was 50 to 50,000 ng/ml for CEM-101 (5 to 5,000 ng/ml for N-acetyl-CEM-101 and CEM-214); deuterated CEM-101 was added as an internal standard. The precision ranged from 4.7% to 9.2%, and the accuracy ranged from −10.0% to 0%.
PK parameters were calculated from the plasma and urine solithromycin samples, N-acetyl-CEM-101, and CEM-214 concentration-time data following dosing on days 1 and 5. The following PK parameters were calculated using noncompartmental analyses as appropriate: areas under the plasma concentration-time curves (AUC0–t, AUC0–inf, AUC0–tau), percentage of the area extrapolated for calculation of AUC0–inf (AUC%ext), Cmax, time of occurrence of Cmax (tmax), the apparent oral clearance (CL/F), volume of distribution at steady state (Vss/F), percentage of bound plasma protein, amount of drug excreted in urine, cumulative amount excreted in urine, percentage of dose excreted in urine, and renal clearance (CLR). Descriptive statistics, including mean, standard deviation (SD), and coefficient of variation (CV), were calculated for the plasma concentrations and PK parameters of solithromycin and its 2 side chain metabolites and for the fractional and cumulative amounts excreted in the urine.
An analysis of variance (ANOVA) was performed on the ln-transformed PK parameters AUC0–t, AUC0–inf, AUC0–tau, and Cmax for solithromycin, as applicable, between each impaired group and the control group, using PROC MIXED of SAS version 9.3 for day 1 and day 5 separately. The ANOVA model included group as a fixed effect. Each ANOVA included calculation of least-square means (LSM), the difference between the LSM of the impairment group (group A [mild], B [moderate], or C [severe]) and the healthy matched control group (group D), and the standard error and 90% confidence interval (CI) associated with this difference. These were transformed back to the original concentration or ratio scale, and the geometric mean values were reported. Ratios of means and their 90% CIs were expressed as percentages of the impaired group over the matched control group. The comparisons of interest were group A versus group D, group B versus group D, and group C versus group D.
RESULTS
Thirty-three subjects were enrolled in the study and assigned to treatment. Eight subjects with mild hepatic impairment (Child-Pugh class A, mean Child-Pugh score of 5.625), 8 with moderate hepatic impairment (Child-Pugh class B, mean Child-Pugh score of 7.375), 8 with severe hepatic impairment (Child-Pugh class C, mean Child-Pugh score of 10.625), and 9 healthy matched controls were enrolled. The demographic characteristics of the subjects are shown in Table 1; the groups were well matched for age, weight, and sex.
TABLE 1.
Demographic data of enrolled subjects
| Trait | Result by group |
||||
|---|---|---|---|---|---|
| Child-Pugh class Aa (n = 8) | Child-Pugh class Ba (n = 8) | Child-Pugh class Ca (n = 8) | Healthy control (n = 9) | Hepatically impaired total (n = 24) | |
| Sex (n [%]) | |||||
| Female | 3 (38) | 0 (0) | 3 (38) | 3 (33) | 6 (25) |
| Male | 5 (63) | 8 (100) | 5 (63) | 6 (67) | 18 (75) |
| Race (n [%]) | |||||
| Asian | 2 (25) | 0 (0) | 0 (0) | 0 (0) | 2 (8) |
| White | 6 (75) | 8 (100) | 8 (100) | 9 (100) | 22 (92) |
| Ethnicity (n [%]) | |||||
| Hispanic or Latino | 2 (25) | 5 (63) | 5 (63) | 5 (56) | 12 (50) |
| Not Hispanic or Latino | 6 (75) | 3 (38) | 3 (38) | 4 (44) | 12 (50) |
| Age (yr) | |||||
| Mean | 56.9 | 57.1 | 55.6 | 57.9 | 56.5 |
| Minimum | 49 | 49 | 42 | 51 | 42 |
| Maximum | 67 | 68 | 65 | 66 | 68 |
| Wt (kg) | |||||
| Mean | 77.1 | 89.2 | 85.0 | 77.4 | 83.8 |
| Minimum | 68.0 | 71.0 | 65.2 | 66.0 | 65.2 |
| Maximum | 106.9 | 115.3 | 110.5 | 89.6 | 115.3 |
| BMI, mean (kg/m2) | 27.1 | 29.5 | 31.2 | 27.3 | 29.3 |
Child-Pugh classes: A, mild hepatic impairment, mean score of 5.625; B, moderate impairment, mean score of 7.375; C, severe impairment, mean score of 10.625.
Among the three hepatically impaired groups, the most frequent cause of hepatic disease was hepatitis C virus infection, followed by alcohol abuse and/or alcoholic cirrhosis. The majority of the moderately and severely hepatically impaired subjects had one or more medical conditions commonly associated with hepatic disease, such as hepatic encephalopathy, ascites, or portal hypertension. Many hepatically impaired subjects were on concomitant medications, most frequently diuretics, insulin, oral hypoglycemic medications, antihypertensive drugs, and antianxiety drugs.
Thirty-two subjects completed the study in accordance with the protocol; 1 subject (a healthy control) was discontinued by the investigator after dosing on day 2 due to a mild rash AE, which resolved after treatment with diphenhydramine. These 32 subjects were included in the PK analyses, and all 33 subjects who received the study drug were included in the safety analyses.
Solithromycin was well tolerated by both healthy subjects and hepatically impaired subjects. No deaths or serious AEs were reported in this study. Overall, 16 TEAEs were reported by 13 subjects, with 9 of 24 (38%) hepatically impaired subjects and 4 of 9 (44%) healthy matched controls reporting AEs (Table 2). Of these AEs, 12 were considered mild and 4 were considered moderate in severity. The investigator considered 11 AEs to be related to the study drug and 5 AEs to be unrelated. All events resolved by the end of the study.
TABLE 2.
Treatment-emergent adverse events
| Adverse eventa | No. (%) of subjects |
||||
|---|---|---|---|---|---|
| Child-Pugh class |
Healthy controls (n = 9) | Hepatically impaired total (n = 24) | |||
| A (n = 8) | B (n = 8) | C (n = 8) | |||
| All subjects with adverse events | 1 (13) | 4 (50) | 4 (50) | 4 (44) | 9 (38) |
| Gastrointestinal disorders | 1 (13) | 4 (50) | 2 (25) | 1 (11) | 7 (29) |
| Diarrhea | 1 (13) | 4 (50) | 2 (25) | 0 (0) | 7 (29) |
| Gastritis | 0 (0) | 0 (0) | 0 (0) | 1 (11) | 0 (0) |
| General disorders and administration site conditions | 0 (0) | 0 (0) | 1 (13) | 0 (0) | 1 (4) |
| Noncardiac chest pain | 0 (0) | 0 (0) | 1 (13) | 0 (0) | 1 (4) |
| Investigations | 0 (0) | 0 (0) | 1 (13) | 1 (11) | 1 (4) |
| Blood creatine phosphokinase increased | 0 (0) | 0 (0) | 1 (13) | 0 (0) | 1 (4) |
| Hepatic enzyme increased | 0 (0) | 0 (0) | 0 (0) | 1 (11) | 0 (0) |
| Nervous system disorders | 0 (0) | 0 (0) | 1 (13) | 2 (22) | 1 (4) |
| Headache | 0 (0) | 0 (0) | 1 (13) | 2 (22) | 1 (4) |
| Skin and subcutaneous tissue disorders | 0 (0) | 0 (0) | 0 (0) | 1 (11) | 0 (0) |
| Rash | 0 (0) | 0 (0) | 0 (0) | 1 (11) | 0 (0) |
Adverse events are classified according to MedDRA version 15.1.
The most common AE was mild diarrhea, reported a total of 7 times by 7 subjects (21%), including 1 mildly hepatically impaired subject, 4 moderately hepatically impaired subjects, and 2 severely hepatically impaired subjects. All 7 of these events were considered related to the study drug.
All remaining AEs were experienced by ≤3 subjects (≤9%). Of these, 3 AEs (headache, increased hepatic enzyme, and rash), all experienced by healthy control subjects, were considered treatment related.
There were no clinically significant shifts in chemistry, hematology, or coagulation parameters in this study. The mild AE of increased ALT on day 4 (70 IU/liter; 1.3 × ULN) was reported in a healthy 60-year-old female control subject.
Many hepatically impaired subjects had liver function tests above the ULN at baseline and throughout the study. Mean AST was above the ULN (41 IU/liter) at all pre- and postdose time points for all subjects in the moderately and severely hepatically impaired groups. Mean direct bilirubin was above the ULN (0.5 mg/dl) at all pre- and postdose time points for the severely hepatically impaired group. For this reason, mean changes from baseline results on day 6 (after 5 days of study drug administration) and day 8 (upon discharge) for ALT, AST, and direct and total bilirubin are presented in Table 3. None of the mean changes from baseline were considered clinically important in any group.
TABLE 3.
Liver-related chemistry changes from baseline, days 6 and 8
| Laboratory test | Day | Change from baseline (mean ± SD) in: |
|||
|---|---|---|---|---|---|
| Child-Pugh class A | Child-Pugh class B | Child-Pugh class C | Healthy control | ||
| ALT (IU/liter) | 6 | 6.4 ± 10.2 | 6.9 ± 8.9 | 2.5 ± 9.8 | 4.6 ± 6.2 |
| 8 | 4.0 ± 8.0 | 7.8 ± 6.9 | 6.3 ± 14.6 | 2.6 ± 4.5 | |
| AST (IU/liter) | 6 | 3.4 ± 7.9 | −1.5 ± 7.7 | 0.0 ± 26.5 | −0.4 ± 2.5 |
| 8 | 0.4 ± 5.9 | 0.1 ± 10.6 | 5.8 ± 22.4 | −0.6 ± 2.9 | |
| Direct bilirubin (mg/dl) | 6 | 0.06 ± 0.15 | 0.03 ± 0.05 | 0.11 ± 0.25 | 0.04 ± 0.09 |
| 8 | 0.00 ± 0.08 | 0.03 ± 0.05 | 0.04 ± 0.21 | 0.00 ± 0.05 | |
| Total bilirubin (mg/dl) | 6 | 0.00 ± 0.24 | 0.04 ± 0.29 | 0.14 ± 0.57 | 0.03 ± 0.20 |
| 8 | −0.13 ± 0.13 | −0.01 ± 0.25 | 0.00 ± 0.55 | −0.03 ± 0.17 | |
ECGs were obtained in triplicate, on days 1, 3, and 5, prior to dosing and at 4 h postdose. No clinically important ECG shifts from normal at baseline to abnormal postdose were observed. There were no significant changes in QTcF postdose in any group. There were no ECG AEs in this study, and all individual subject ECG abnormalities were considered not clinically significant. Mean change from baseline in heart rate, across all groups, ranged from 1 to 11 beats per minute (bpm) at postdosing time points and did not differ significantly between study subjects with and without liver disease. Tachycardia (defined as a heart rate of >100 bpm) was not observed at any time point in any study subject.
Plasma concentrations of solithromycin on days 1 and 5 are presented in Fig. 1 and 2. Select plasma PK parameters of solithromycin on days 1 and 5 and the results of inferential statistical analyses comparing the Cmax and AUC in subjects with hepatic impairment and those with normal hepatic function are presented in Table 4. Each group (mild, moderate, severe, control) consisted of 8 subjects in this analysis.
FIG 1.
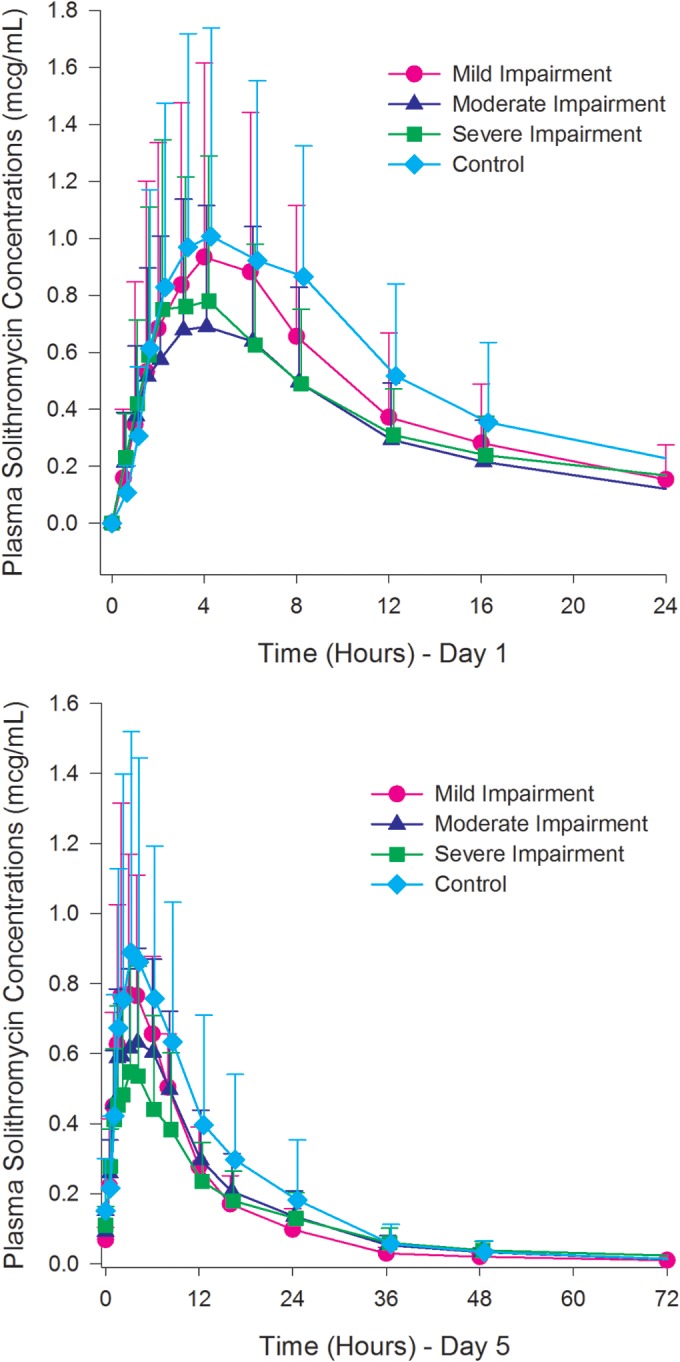
Mean (+SD) plasma concentrations of solithromycin on day 1 and day 5.
FIG 2.
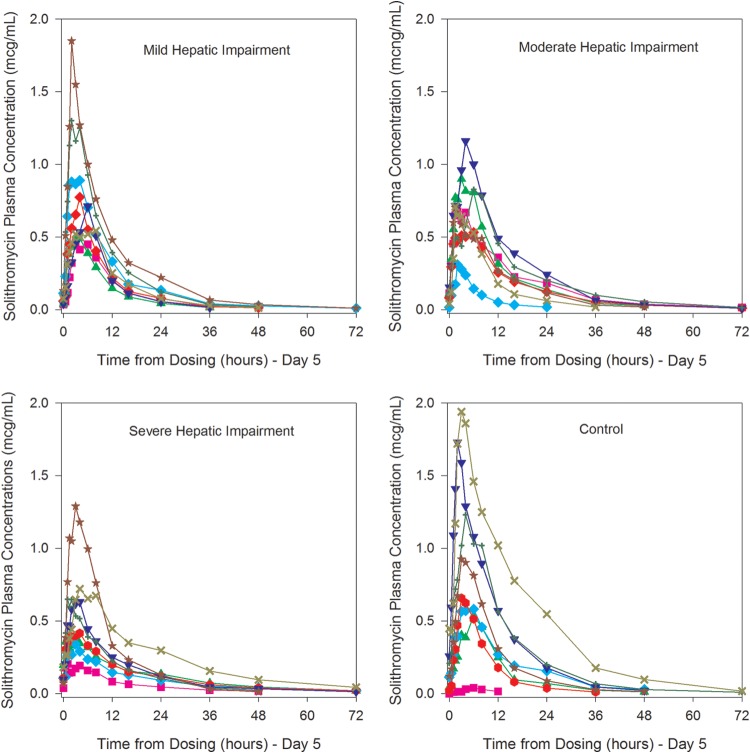
Individual plasma concentrations of solithromycin on day 5 in subjects with Child-Pugh class A (mild), Child-Pugh class B (moderate), and Child-Pugh class C (severe) impairment and in healthy control subjects. Colors/symbols are used to represent individual subjects.
TABLE 4.
Statistical comparison of select plasma solithromycin pharmacokinetic parameters, days 1 and 5
| Compared groups and parametera | Geometric LSMb |
Geometric mean ratio (test/reference)c (%) | 90% CI | CV (%) | |
|---|---|---|---|---|---|
| Test group | Reference group | ||||
| Day 1 | |||||
| Mildly impaired vs control | |||||
| Cmax (μg/ml) | 0.782 | 0.709 | 110.34 | 46.56–261.51 | 134.12 |
| AUC0–inf (μg · h/ml) | 8.039 | 12.376 | 64.96 | 30.02–140.59 | 100.49 |
| Moderately impaired vs control | |||||
| Cmax (μg/ml) | 0.558 | 0.709 | 78.75 | 33.23–186.63 | 134.12 |
| AUC0–inf (μg · h/ml) | 9.491 | 12.376 | 76.69 | 34.62–169.88 | 100.49 |
| Severely impaired vs control | |||||
| Cmax (μg/ml) | 0.712 | 0.709 | 100.37 | 42.35–237.88 | 134.12 |
| AUC0–inf (μg · h/ml) | 9.112 | 12.376 | 73.63 | 33.24–163.09 | 100.49 |
| Day 5 | |||||
| Mildly impaired vs control | |||||
| Cmax (μg/ml) | 0.786 | 0.649 | 121.08 | 64.38–227.71 | 85.78 |
| AUC0–tau (μg · h/ml) | 7.900 | 10.492 | 75.30 | 47.89–118.39 | 54.92 |
| Moderately impaired vs control | |||||
| Cmax (μg/ml) | 0.684 | 0.649 | 105.36 | 56.02–198.14 | 85.78 |
| AUC0–tau (μg · h/ml) | 7.509 | 10.492 | 71.58 | 45.52–112.55 | 54.92 |
| Severely impaired vs control | |||||
| Cmax (μg/ml) | 0.504 | 0.649 | 77.69 | 41.31–146.11 | 85.78 |
| AUC0–tau (μg · h/ml) | 6.176 | 10.492 | 58.86 | 37.44–92.55 | 54.92 |
Parameters were ln transformed prior to analysis. n = 8/group.
Geometric least-squares means were calculated by exponentiating the LSMs from the ANOVA.
Percent geometric mean ratio = 100 × (test/reference).
The observed variability of the PK parameters of solithromycin was higher in this study than in previous studies, with the intrasubject CV between 54.92% and 134.12% across both AUC and Cmax values. The high variability and the small sample size of the study (n = 8 per cohort) preclude making statistical conclusions based on the 90% CIs of the ratios of LSM for Cmax and AUC. The results from the statistical analyses are therefore also discussed based on the ratios of LSMs and the overall tendency, if any, related to the degree of hepatic impairment.
All 90% CIs were outside the 50% to 200% range and, in most cases, expanded outside this range on both the lower and upper limits. Based on the ratios of LSMs, there were no apparent trends in Cmax and AUC with regard to the degree of hepatic impairment or compared to the control group.
Although the variability of the PK parameters at steady state was lower than after a single dose, it remained high for all parameters (CV, >50%). Based on the ratios of LSMs, AUC0–tau appeared to decrease with increasing degree of hepatic impairment. For subjects with severe hepatic impairment, the ratio of LSM was 58.9%, and the 90% CI for AUC0–tau was entirely below 100%. An effect of hepatic impairment on the Cmax and AUC0–t parameters was not as apparent, with a 12% lower Cmax for the severe impairment group than for the control group. This observed difference may be somewhat underestimated because one subject in the control group had a low Cmax and AUC0–t.
The volume of distribution at steady state (Vss/F) also increased with increasing severity of liver impairment. On day 5, control subjects had a mean Vss/F of 542 liters, and mild, moderate, and severe subjects had mean Vss/F values of 778, 832, and 1,749 liters, respectively. The median CL/F values ranged from 75 to 97 liters/h and were relatively comparable for all groups.
Plasma concentrations of the active side chain metabolites were low relative to the parent concentrations, with exposure (AUCtau) to CEM-214 at <5% of parent exposure while exposure to N-acetyl-CEM-101 ranged from ∼5% to 21% of parent exposure (see Tables S1 and S2 in the supplemental material). For N-acetyl-CEM-101, based on the ratios of LSMs, Cmax and AUC values seemed to increase with increasing degree of hepatic impairment (see Table S1 in the supplemental material). For CEM-214, based on the ratios of LSMs, moderate and severe hepatic impairments appeared to decrease the Cmax and AUC values by at least 40% and 30%, respectively (see Table S2 in the supplemental material).
The protein binding of solithromycin was evaluated 4 h postdose on days 1 and 5 and was slightly lower for subjects with moderate and severe hepatic impairment than for control subjects. Hepatic impairment had a minimal effect on the protein binding of solithromycin following multiple once-daily doses for 5 days, with a decrease in the mean percentage of protein bound of ∼9%, from 71% to 62%, between subjects with severe hepatic impairment relative to the control group with normal hepatic function. A slight increase in the free fraction with increased degree of hepatic impairment was observed.
Mean urinary excretion parameters for solithromycin following a single oral 800-mg dose (day 1) and following multiple 400-mg doses (day 5) are presented in Table 5.
TABLE 5.
Mean pharmacokinetic parameters for urine solithromycin, days 1 and 5
| Pharmacokinetic parametera | Result (mean ± SD) by group |
|||
|---|---|---|---|---|
| Child-Pugh class A (n = 8) | Child-Pugh class B (n = 8) | Child-Pugh class C (n = 8) | Healthy control (n = 8) | |
| Day 1 | ||||
| CumAe total (mg) | 38.4 ± 19.1 | 51.5 ± 31.2 | 44.2 ± 29.3 | 38.1 ± 19.2 |
| Total dose (%) | 4.8 ± 2.4 | 6.4 ± 3.9 | 5.5 ± 3.7 | 4.8 ± 2.4 |
| CLR (liter/h) | 4.9 ± 2.2 | 6.5 ± 1.8 | 5.7 ± 3.4 | 4.9 ± 3.5 |
| Day 5 | ||||
| CumAe total (mg) | 35.3 ± 8.8 | 45.6 ± 20.6 | 47.3 ± 18.9 | 41.1 ± 19.4 |
| Total dose (%) | 8.8 ± 2.2 | 11.4 ± 5.2 | 11.8 ± 4.7 | 10.3 ± 4.9 |
| CLR (liter/h) | 4.4 ± 0.8 | 5.6 ± 1.6 | 7.6 ± 3.1 | 4.6 ± 1.8 |
CumAe, cumulative amount of drug excreted in urine. CLR was calculated using matching intervals between plasma and urine data.
The urinary excretion of solithromycin over 24 h following a single oral 800-mg dose (day 1) and following multiple 400-mg doses (day 5) appeared to be similar across groups. There were no apparent trends with the degree of hepatic impairment. Approximately 8.8% to 11.8% of the dose was recovered in urine in the 24 h after the 400-mg dose on day 5. In addition, no apparent time dependency was observed in the PK of solithromycin in urine, as CLR was generally similar on days 1 and 5 within each cohort.
Only very small amounts of the active side chain metabolites were recovered in the urine. After a single 800-mg dose, <1% was recovered as N-acetyl-CEM-101 or CEM-214 over 24 h. On day 5 over 24 h, <1.3% was recovered as N-acetyl-CEM-101 and <2% as CEM-214. Hepatic impairment did not affect the renal elimination of solithromycin and its N-acetyl-CEM-101 and CEM-214 metabolites.
DISCUSSION
While the overall prevalence of cirrhosis in the United States is difficult to ascertain, the incidence of mortality from chronic liver disease in 2008 in the United States was reported to be 25.7 deaths/100,000 people, and chronic liver disease ranked twelfth among the leading causes of death (15). In 2004, >2.4 million ambulatory care visits in the United States were attributed to chronic liver disease (16). Chronic liver disease is also a significant contributor to morbidity and mortality in the European Union and Asia (17). The importance of knowledge guiding drug dosing in the setting of liver disease is thus of great importance. While dosage adjustments have been recommended for erythromycin (18), newer macrolides, such as azithromycin, clarithromycin, and the ketolide telithromycin, have not required dosage adjustments due to hepatic impairment (12–14).
Solithromycin, given orally as an 800-mg loading dose on day 1 followed by 400 mg once daily on days 2 to 5, was safe and well tolerated by the hepatically impaired and healthy matched subjects in this study. The AE profiles of hepatically impaired subjects did not differ significantly from those of the age-, weight-, and sex-matched control subjects. Mean changes from baseline in liver function tests were also similar between the hepatically impaired and healthy subjects.
The PK of plasma solithromycin in subjects with mild and moderate hepatic impairment were similar to that in subjects with normal liver function. Total exposure to solithromycin at steady state (AUC0–tau) decreased by ∼41% for subjects with severe hepatic impairment compared to that in subjects with normal hepatic function. With an LSM AUC of 6.176 μg · h/ml in the severely hepatically impaired group after day 5 and an MIC90 to Streptococcus pneumoniae of 0.25 (19), an AUC/MIC ratio of ∼24 would be achieved, which should still produce a >2-log kill based on previous studies (20). This decrease in plasma exposure may reflect the >3-fold larger mean Vss/F observed in severely impaired subjects receiving solithromycin; a similar increase was observed with clarithromycin in severely impaired subjects (13), but azithromycin was not evaluated in severely impaired subjects (14). The severely impaired cohort had the highest mean BMI (31.2 kg/m2), which may have contributed to the larger volume of distribution observed. The CL/F values were relatively comparable for all groups. There was no apparent relationship between CL/F and the degree of hepatic impairment. There was no evidence of greater accumulation in hepatically impaired subjects than in healthy subjects on day 5. These data suggest that no dosage decrease is needed for solithromycin administration in patients with chronic liver disease, regardless of the degree of hepatic impairment.
As observed with other macrolides, plasma exposure of solithromycin exhibited a high degree of variability in small cohorts. One healthy subject had low plasma levels, as seen in Fig. 2. It is known that the plasma concentrations of macrolide antibiotics are variable and that they concentrate intracellularly and in the lung (12, 21). In the recently completed phase 3 oral CABP study, solithromycin was demonstrated to be noninferior to moxifloxacin in 860 moderate to moderately severe pneumonia patients (10).
Within each group, the mean AUC0–tau following the 400-mg once-daily solithromycin doses was roughly 20% to 30% lower than the AUC0–inf following a single 800-mg dose. These results suggest time-dependent PK and autoinhibition of CYP3A4, consistent with prior evaluations.
The systemic exposure of N-acetyl-CEM-101 increased by 2- to 3-fold while the systemic exposure of CEM-214 decreased by 50% to 60% for subjects with severe hepatic impairment compared to those in the control group. These results may suggest a potential change in the relative contribution of the different metabolic pathways of solithromycin with hepatic impairment. N-Acetylation is primarily an extrahepatic metabolic pathway and appears to increase with increasing severity of liver dysfunction. As both of these metabolites are much less active than the parent (22) and present at much lower concentrations than the parent, these changes would not be expected to be clinically relevant.
Hepatic impairment had a minimal effect on the protein binding of solithromycin following multiple once-daily dosing for 5 days, with a difference of 9% between the severely hepatically impaired group and the control group with normal hepatic function. Solithromycin was not highly bound to plasma proteins, with mean values of <75% in this study across all groups. Therefore, small variations in the free fraction of solithromycin are not expected to have any significant impact on the disposition of solithromycin.
Hepatic impairment did not affect the renal elimination of solithromycin or its N-acetyl-CEM-101 and CEM-214 metabolites. Approximately 5% of the dose was excreted as solithromycin in the urine over 24 h after a single dose and ∼10% was excreted over 24 h after 5 once-daily doses. As the first dose was twice as large as subsequent doses (800 versus 400 mg), the actual amounts excreted in the urine over 24 h postdose were similar on days 1 and 5.
In conclusion, patients with mild, moderate, or severe chronic liver disease should not require a decrease in dosage when solithromycin is administered once daily for 5 days.
Supplementary Material
ACKNOWLEDGMENT
This clinical trial was supported by Cempra, Inc.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.04652-14.
REFERENCES
- 1.Farrell DJ, Sader HS, Castanheira M, Biedenbach DJ, Rhomberg PR, Jones RN. 2010. Antimicrobial characterisation of CEM-101 activity against respiratory tract pathogens, including multidrug-resistant pneumococcal serogroup 19A isolates. Int J Antimicrob Agents 35:537–543. doi: 10.1016/j.ijantimicag.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 2.Jones RN, Sader HS, Biedenbach DJ. 2009. Antimicrobial characterization of CEM-101: activity against staphylococci, beta-haemolytic and viridans group streptococci, abstr P-1100 Abstr 19th Eur Congr Clin Microbiol Infect Dis. [Google Scholar]
- 3.McGhee P, Clark C, Kosowska-Shick KM, Nagai K, Dewasse B, Beachel L, Abbelbaum PC. 2010. In vitro activity of CEM-101 against Streptococcus pneumoniae and Streptococcus pyogenes with defined macrolide resistance mechanisms. Antimicrob Agents Chemother 54:230–238. doi: 10.1128/AAC.01123-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waites KB, Crabb DM, Duffy LB. 2009. Comparative in vitro susceptibilities of human mycoplasmas and ureaplasmas to a new investigational ketolide, CEM-101. Antimicrob Agents Chemother 53:2139–2141. doi: 10.1128/AAC.00090-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Golparian D, Fernandes P, Ohnishi M, Jensen JS, Unemo M. 2012. In vitro activity of the new fluoroketolide solithromycin (CEM-101) against a large collection of clinical Neisseria gonorrhoeae isolates and international reference strains including those with various high-level antimicrobial resistance: potential treatment option for gonorrhea? Antimicrob Agents Chemother 56:2739–2742. doi: 10.1128/AAC.00036-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mallegol J, Fernandes P, Seah C, Guyard C, Melano RG. 2013. Determination of in vitro activity of solithromycin at different pHs and its intracellular activity tested against clinical isolates of Neisseria gonorrhoeae from a laboratory collection. Antimicrob Agents Chemother 57:4322–4328. doi: 10.1128/AAC.00564-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roblin PM, Kohlhoff SA, Parker C, Hammerschlag MR. 2010. In vitro activity of CEM-101, a new fluoroketolide antibiotic, against Chlamydia trachomatis and Chlamydia (Chlamydophila) pneumoniae. Antimicrob Agents Chemother 54:1358–1359. doi: 10.1128/AAC.01343-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oldach D, Clark K, Schranz J, Das A, Craft JC, Scott D, Jamieson BD, Fernandes P. 2013. Randomized, double-blind, multicenter phase 2 study comparing the efficacy and safety of oral solithromycin (CEM-101) to those of oral levofloxacin in the treatment of patients with community-acquired bacterial pneumonia. Antimicrob Agents Chemother 57:2526–2534. doi: 10.1128/AAC.00197-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hook EW, Jamieson B, Oldach D, Harbison H, Whittington A, Fernandes P. 2013. A phase II dose ranging study to evaluate the efficacy and safety of single-dose oral solithromycin (CEM-101) for treatment of patients with uncomplicated urogenital gonorrhea, abstr O02.5 STI & AIDS World Congress, Vienna, Austria. [Google Scholar]
- 10.Oldach DW, Barrera CM, Metev H, Dvoretskiy LI, Mykietuk A, Mitha I, DeSalvo MC, Tanaseanu CM, Szabo P, Clark K, Jamieson B, Das A, Keedy K, Fernandes P. 2015. Oral solithromycin versus oral moxifloxacin for treatment of adult community-acquired bacterial pneumonia (CABP): results of the global phase-3 trial SOLITAIRE-ORAL, abstr 69069 Abstr Am Thorac Soc Int Conf. [Google Scholar]
- 11.Still JG, Schranz J, Degenhardt TP, Scott D, Fernandes P, Guiterrez MJ, Clark K. 2011. Pharmacokinetics of solithromycin (CEM-101) after single or multiple oral doses and effects of food on single-dose bioavailability in healthy adult subjects. Antimicrob Agents Chemother 55:1997–2003. doi: 10.1128/AAC.01429-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu SY, Granneman GR, Pichotta PJ, Decourt JP, Girault J, Fourtillan JB. 1993. Effect of moderate or severe hepatic impairment on clarithromycin pharmacokinetics. J Clin Pharmacol 33:480–485. doi: 10.1002/j.1552-4604.1993.tb04692.x. [DOI] [PubMed] [Google Scholar]
- 13.Mazzei T, Surrenti C, Novelli A, Crispo A, Fallani S, Carla V, Surrenti E, Periti P. 1993. Pharmacokinetics of azithromycin in patients with impaired hepatic function. J Antimicrob Chemother 31(Suppl E):57–63. doi: 10.1093/jac/31.suppl_E.57. [DOI] [PubMed] [Google Scholar]
- 14.Cantalloube C, Bhargava V, Sultan E, Vacheron F, Batista I, Montay G. 2003. Pharmacokinetics of the ketolide telithromycin after single and repeated doses in patients with hepatic impairment. Int J Antimicrob Agents 22:112–121. doi: 10.1016/S0924-8579(03)00128-6. [DOI] [PubMed] [Google Scholar]
- 15.Asrani SK, Larson JJ, Yawn B, Therneau TM, Kim WR. 2013. Underestimation of liver-related mortality in the United States. Gastroenterology 145:375–382. doi: 10.1053/j.gastro.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Everhart JE, Ruhl CE. 2009. Burden of digestive diseases in the United States, part III: liver, biliary tract, and pancreas. Gastroenterology 136:1134–1144. doi: 10.1053/j.gastro.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 17.Blachier M, Lelue H, Peck-Radosavljevic M, Valla D, Roudot-Thoraval F. 2013. The burden of liver disease in Europe: a review of available epidemiologic data. J Hepatol 58:593–608. doi: 10.1016/j.jhep.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Kucers A, Bennett N. 1987. Erythromycin, p 851–882. In William Heinemann. (ed), The use of antibiotics: a comprehensive review with clinical emphasis, 4th ed William Heinemann Medical Books Ltd., London, United Kingdom. [Google Scholar]
- 19.Farrell DJ, Mendes RE, Jones RN. 2015. Antimicrobial activity of solithromycin against serotyped macrolide-resistant Streptococcus pneumoniae isolates collected from U.S. medical centers in 2012. Antimicrob Agents Chemother 59:2432–2434. doi: 10.1128/AAC.04568-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andes DR, Okusanya OO, Forrest A, Bhavnani SM, Fernandes P, Ambrose PG. 2010. Pharmacokinetic-pharmacodynamic analysis of solithromycin (CEM-101) against Streptococcus pneumoniae using data from a murine-lung infection model, abstr A1-688 Abstr 50th Intersci Conf Antimicrob Agents Chemother. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodvold KA, Gotfried MH, Still JG, Clark K, Fernandes P. 2012. Comparison of plasma, epithelial lining fluid, and alveolar macrophage concentrations of solithromycin (CEM-101) in healthy adult subjects. Antimicrob Agents Chemother 56:5076–5081. doi: 10.1128/AAC.00766-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pereira DE, Degenhardt T, Fernandes P. 2010. Comparison of CEM-101 metabolism in mice, rats, monkeys and humans, abstr A1-687 Abstr 50th Intersci Conf Antimicrob Agents Chemother. [Google Scholar]
- 23.Department of Health and Human Services. 2003. Pharmacokinetics in patients with impaired hepatic function—study design, data analysis, and impact on dosing and labeling. HHS, Washington, DC: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm072123.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.