Abstract
To characterize the relationship between penicillin-binding protein 2 (PBP2/penA) and susceptibility to extended-spectrum cephalosporins (ESCs) and carbapenem antibiotics, we compared 17 PBP2 variants in Neisseria gonorrhoeae. Nonmosaic and mosaic variants of PBP2 caused decreased susceptibility to ESCs and, to a lesser extent, to carbapenems. An A501P substitution in mosaic XXXIV_A501P conferred decreased susceptibility to ESCs but restored carbapenem susceptibility to wild-type levels. These results could aid the molecular surveillance of antimicrobial resistance to these agents.
TEXT
The World Health Organization and the U.S. Centers for Disease Control and Prevention have named antimicrobial resistant gonococcus (AMR-GC) as a top concern to human health (1, 2). The recommended treatment for gonorrhea is an extended-spectrum cephalosporin (ESC), preferably ceftriaxone or, alternatively, cefixime, in combination with the macrolide azithromycin (3–5); however, treatment failures have been reported in many countries (6–10). Ertapenem has recently been explored as a potential treatment for gonorrhea (11–14). Resistance to β-lactam antibiotics, including ESCs and carbapenems, is primarily caused by changes in their cellular target, penicillin-binding protein 2 (PBP2), which is encoded by the penA gene in Neisseria gonorrhoeae. Nonmosaic variants of PBP2 contain an aspartic acid insertion after position 345 (termed D345a), while mosaic variants contain many segments from commensal Neisseria species that are less susceptible to ESCs (15, 16).
Surveillance of AMR-GC is important for predicting potential treatment failure due to empirical therapy; however, diagnosis of gonococcal infection is now done by nucleic acid amplification testing (NAAT) so fewer cultures are available for surveillance of resistance (17). In the absence of live cultures, a better understanding of the genetic markers of ESC resistance in N. gonorrhoeae might help to make molecular surveillance a viable alternative. To elucidate the link between susceptibility to ESC and carbapenem antibiotics and changes in their cellular target, we have conducted a systematic study of 17 variants of PBP2 in an isogenic background.
Characterization of PBP2 variants in clinical isolates.
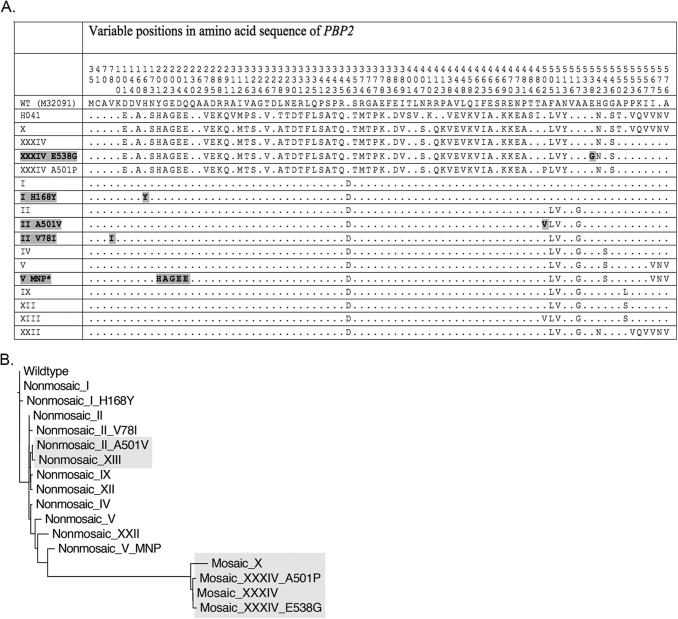
Seventeen alleles of penA were identified from whole-genome sequences of 169 clinical isolates collected across Canada between 1989 and 2013 (18) and from the F89 strain from France (10). Alleles were extracted from whole-genome sequence data by BLAST using the sequence of wild-type penA (GenBank accession no. M32091). Five alleles were novel, while 12 were known (19). GenBank accession nos. of the novel alleles are KP721215 (nonmosaic I H168Y), KP721216 (nonmosaic II A501V), KP721217 (nonmosaic II V78I), KP721218 (nonmosaic V MNP), and KP721219 (nonmosaic XXXIV E538G). Figure 1A highlights the amino acid positions that were altered in the PBP2 variants of this study and that of a high MIC H041 variant from Japan (19). Three allele pairs differed only by variation at A501: nonmosaic II and nonmosaic II_A501V; nonmosaic XII and nonmosaic XIII (which contains A501V); and mosaic XXXIV and mosaic XXXIV_A501P. In the dendrogram of PBP2 sequences, variants that were found here to be associated with elevated cephalosporin MICs are highlighted (Fig. 1B).
FIG 1.
Sequence variations and phylogenetic relationship between PBP2 variants of this study. (A) The altered positions in the amino sequences of wild-type PBP2 (M32091), H041 PBP2 (AB546858) (19), and the variants of this study are shown. The residue numbers of wild-type and mosaic variants were shifted by +1 to maintain alignment with the nonmosaic alleles, which contain an Asp insertion after position 345; thus A501P and A501V substitutions are shown at position 502. Dots indicate consensus with the wild-type sequence, while bold text indicates novel variants and substitutions. The last 12 alleles (I to XXII) are nonmosaic alleles. The variant named V MNP* was encoded by an allele containing multiple nucleotide polymorphisms (MNP) compared to variant V. (B) A dendrogram based on the amino acid sequences of the PBP2 variants of this study was created with ClustalX by the nearest neighbor-joining method. Highlighted branches contain either mosaic alleles or nonmosaic alleles with A501V substitutions.
Transformation of penA alleles into a susceptible recipient strain.
Wild-type penA was replaced by non-wild-type alleles in N. gonorrhoeae isolate NML 22890, which was chosen because it lacked the markers of ESC resistance (no mutations in penA, mtrR −35A deletion, MtrR A39T, MtrR G45D, PonA L421P, or PorB G120) except for PorB A121G. NML 22890 was highly susceptible to both cefixime (MIC of 0.001 mg/liter) and ceftriaxone (MIC of 0.00025 mg/liter). The penA gene (1,749 bp) along with 1,150 bp of the upstream sequence (containing a natural DNA uptake sequence) and 800 bp of the downstream sequence was amplified with primers 1150-F and 800-R (Table 1). Transformation was carried out by the method outlined by Dillard (20) by spotting 0.5 μg penA PCR product onto supplemented GC agar medium and selection on 0.0005 mg/liter ceftriaxone (2× MIC). Spontaneous resistance did not occur at a detectable frequency. The sequences of the penA alleles in the transformants were verified with primers P0-FS, P1-RCS, P2-RCS, P3-RCS, and either P4-RCS-nonmosaic or P4-RCS-mosaic (Table 1).
TABLE 1.
Primers used in this study
| Primera | Sequence (5′ to 3′) | Position (bp) |
|---|---|---|
| 1150-F | ACCCTGCGGTTTGATTTCCT | 1,150 upstream |
| 800-R | TGGTGAAGAGCGGTTTAGCC | 800 downstream |
| P0-FS | GGGTAATGGCGTTTTAATTC | 490 |
| P1-RCS | CGAGCTTGTCGATGTGCCGGT | 345 |
| P2-RCS | GCGGTCGAATACCATCAGGCA | 760 |
| P3-RCS | TTGGATGTGCGCGGCATTATG | 1,060 |
| P4-RCS-nonmosaic | TGATGGTTTCCGTAACCGA | 1,420 |
| P4-RCS-mosaic | TGATGGTTTCCGTTACTGA | 1,420 |
Primers were designed using the sequence of strain FA1090 (GenBank accession no. NC_002946.2).
Effects of PBP2 variants on cephalosporin susceptibility.
MICs were determined by agar dilution according to the standard method of the Clinical and Laboratory Standards Institute (CLSI) (21). Nonmosaic alleles I, I_H168Y, II, II_V78I, IV, V, V_MNP, IX, XII, and XXII caused 2-fold to 5-fold increases in cefixime MICs and 5-fold to 11-fold increases in ceftriaxone MICs with additional 2-fold to 3-fold increases contributed by the A501V substitutions in nonmosaic II A501V and nonmosaic XIII (Table 2). Variations in the C-terminal transpeptidase domain after residue 500 or in the N-terminal domain did not have a significant effect on ESC susceptibility in this study.
TABLE 2.
MICs of cephalosporin and carbapenem antibiotics against variants of PBP2 in isogenic strains of N. gonorrhoeae
| PBP2 variant | MIC (mg/liter)a |
|||
|---|---|---|---|---|
| Cefixime | Ceftriaxone | Ertapenem | Meropenem | |
| Wild type | 0.0023 ± 0.0015 | 0.0005 ± 0 | 0.016 ± 0 | 0.016 ± 0 |
| Nonmosaic I | 0.008 ± 0 | 0.004 ± 0 | 0.0267 ± 0.0092 | 0.016 ± 0 |
| Nonmosaic I H168Y | 0.0053 ± 0.0023 | 0.0033 ± 0.0012 | 0.0267 ± 0.0092 | 0.016 ± 0 |
| Nonmosaic II | 0.0067 ± 0.0023 | 0.0033 ± 0.0012 | 0.0267 ± 0.0092 | 0.016 ± 0 |
| Nonmosaic II A501V | 0.0213 ± 0.0092 | 0.008 ± 0 | 0.0267 ± 0.0092 | 0.016 ± 0 |
| Nonmosaic II V78I | 0.0067 ± 0.0023 | 0.004 ± 0 | 0.0267 ± 0.0092 | 0.016 ± 0 |
| Nonmosaic_IV | 0.008 ± 0 | 0.004 ± 0 | 0.0267 ± 0.0092 | 0.016 ± 0 |
| Nonmosaic_V | 0.008 ± 0 | 0.004 ± 0 | 0.032 ± 0 | 0.016 ± 0 |
| Nonmosaic V MNP* | 0.008 ± 0 | 0.004 ± 0 | 0.0213 ± 0.0092 | 0.016 ± 0 |
| Nonmosaic IX | 0.008 ± 0 | 0.004 ± 0 | 0.032 ± 0 | 0.0213 ± 0.0092 |
| Nonmosaic XII | 0.0107 ± 0.0046 | 0.0053 ± 0.0023 | 0.032 ± 0 | 0.0213 ± 0.0092 |
| Nonmosaic XIII | 0.032 ± 0 | 0.008 ± 0 | 0.032 ± 0 | 0.016 ± 0 |
| Nonmosaic XXII | 0.004 ± 0 | 0.0027 ± 0.0012 | 0.0213 ± 0.0092 | 0.016 ± 0 |
| Mosaic X | 0.125 ± 0 | 0.0107 ± 0.0046 | 0.125 ± 0 | 0.125 ± 0 |
| Mosaic XXXIV | 0.0637 ± 0.0006 | 0.008 ± 0 | 0.125 ± 0 | 0.0843 ± 0.0352 |
| Mosaic XXXIV E538G | 0.0637 ± 0.0006 | 0.008 ± 0 | 0.1047 ± 0.0352 | 0.0843 ± 0.0352 |
| Mosaic XXXIV A501P | 1 ± 0 | 0.25 ± 0 | 0.016 ± 0 | 0.016 ± 0 |
| ATCC 49226 | 0.0267 ± 0.0092 | 0.0133 ± 0.0046 | 0.125 ± 0 | 0.1047 ± 0.0352 |
| WHO F | 0.0033 ± 0.0012 | 0.001 ± 0 | 0.016 ± 0 | 0.0133 ± 0.0046 |
| F89 | ≥4 ± 0 | 2 ± 0 | 0.1047 ± 0.0352 | 0.1047 ± 0.0352 |
MICs were measured in triplicate by the agar dilution method and averages ± SD are shown.
Mosaic alleles X, XXXIV, and XXXIV_E538G conferred 27-fold to 53-fold increases in cefixime MICs and 16-fold to 21-fold increases in ceftriaxone MICs, with additional 16-fold to 30-fold increases conferred by the A501P substitution in mosaic XXXIV A501P. The larger effect of A501P than A501V may be due to the allelic context, or perhaps the proline causes significant chain bending in PBP2 (10). Mosaic XXXIV_A501P raised the MICs to 1 mg/liter for cefixime and 0.25 mg/liter for ceftriaxone, which were above the breakpoints for decreased susceptibility for both antibiotics according to WHO guidelines (2). The magnitudes of the changes conferred by these mosaic alleles were similar to those reported for similar alleles (10, 19, 22–25).
Effects of PBP2 variants on carbapenem susceptibility.
Ertapenem has been investigated as a potential future treatment for gonorrhea infections (11–14). Nonmosaic alleles, regardless of the presence of A501V, caused 2-fold decreased susceptibility to ertapenem, raising the MICs from 0.016 mg/liter to 0.032 mg/liter, but had no effect on meropenem MICs. Mosaic alleles caused 5-fold to 8-fold decreased susceptibility to both ertapenem and meropenem, increasing the MIC to ≥0.125 mg/liter. Resistance breakpoints for carbapenems against N. gonorrhoeae have not been established. Interestingly, susceptibilities to ertapenem and meropenem were restored by the A501P mutation in mosaic XXXIV_A501P, suggesting that this substitution might block the binding or inactivation of carbapenems by PBP2. Thus, carbapenem MICs were less affected than ESC MICs by PBP2 variants, and susceptibility to carbapenems was restored by the A501P substitution suggesting that carbapenem antibiotics may be an effective treatment for infections that are associated with mosaic XXXIV_A501P mutations.
Effects of PBP2 variants on susceptibility to other antibiotics.
All PBP2 variants had the same MICs as the recipient strain for gentamicin (MIC of 4 mg/liter), azithromycin (MIC of 0.063 mg/liter), spectinomycin (MIC of 16 mg/liter), erythromycin (MIC of 0.25 mg/liter), tetracycline (MIC of 1 mg/liter), and ciprofloxacin (MIC of 0.125 mg/liter). The average increases in penicillin MICs were 1.6-fold and 2.1-fold for the nonmosaic and mosaic alleles, respectively.
Elucidating the relationship between the genetic markers of resistance and antimicrobial susceptibility can help in interpretation of molecular surveillance data. In this comparative study of 17 PBP2 variants, we found that nonmosaic alleles caused small elevations in ESC MICs, mosaic alleles caused larger elevations in ESC MICs, and further contributions were made by substitutions at A501. Carbapenem antibiotics were less affected than ESCs, and the A501P substitution restored carbapenem susceptibility to wild-type levels, suggesting that carbapenem antibiotics may be effective against cases of ESC treatment failures that are associated with mosaic XXXIV_A501P mutations. The results of this study will strengthen the link between the genotype and phenotype for PBP2 variants and susceptibility to the last approved treatment for gonorrhea infections.
Nucleotide sequence accession numbers.
The novel alleles were deposited in GenBank under accession numbers of KP721215 (nonmosaic I H168Y), KP721216 (nonmosaic II A501V), KP721217 (nonmosaic II V78I), KP721218 (nonmosaic V MNP), and KP721219 (nonmosaic XXXIV E538G).
ACKNOWLEDGMENTS
We thank Gary Liu, Pam Sawatzky, Anton Kowalski, and Norman Barairo for technical assistance, Linda Hoang (British Columbia Centers for Disease Control, Vancouver, BC), Brigitte Lefebvre (Laboratoire de Santé Publique du Québec, Ste-Anne-de-Bellevue, QC), and Vanessa Allen (Public Health Ontario Laboratories, Toronto, ON) for providing clinical isolates, and Patrice Sednaoui (Institut Alfred Fournier, Centre National de Référence des Gonocoques, Paris, France) for providing N. gonorrhoeae strain F89.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2013. Antibiotic resistance threats in the United States, 2013. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 2.World Health Organization. 2012. Global action plan to control the spread and impact of antimicrobial resistance in Neisseria gonorrhoeae. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 3.Public Health Agency of Canada. 2013. Canadian guidelines on sexually transmitted infections: gonococcal infections. Public Health Agency of Canada, Ottawa, ON, Canada. [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2012. Update to CDC's sexually transmitted diseases treatment guidelines, 2010: oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR Morb Mortal Wkly Rep 61:590–594. [PubMed] [Google Scholar]
- 5.Bignell C, Unemo M, European STI Guidelines Editorial Board . 2013. 2012 European guideline on the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS 24:85–92. doi: 10.1177/0956462412472837. [DOI] [PubMed] [Google Scholar]
- 6.Unemo M, Golparian D, Hestner A. 2011. Ceftriaxone treatment failure of pharyngeal gonorrhoea verified by international recommendations, Sweden, July 2010. Euro Surveill 16(6):pii=19792 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19792. [PubMed] [Google Scholar]
- 7.Y Chen M, Stevens K, Tideman R, Zaia A, Tomita T, Fairley CK, Lahra M, Whiley D, Hogg G. 2013. Failure of 500 mg of ceftriaxone to eradicate pharyngeal gonorrhoea, Australia. J Antimicrob Chemother 68:1445–1447. doi: 10.1093/jac/dkt017. [DOI] [PubMed] [Google Scholar]
- 8.Allen VG, Mitterni L, Seah C, Rebbapragada A, Martin IE, Lee C, Siebert H, Towns L, Melano RG, Low DE. 2013. Neisseria gonorrhoeae treatment failure and susceptibility to cefixime in Toronto, Canada. JAMA 309:163–170. doi: 10.1001/jama.2012.176575. [DOI] [PubMed] [Google Scholar]
- 9.Unemo M, Golparian D, Potočnik M, Jeverica S. 2012. Treatment failure of pharyngeal gonorrhoea with internationally recommended first-line ceftriaxone verified in Slovenia, September 2011. Euro Surveill 17(25): pii=20200 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20200. [PubMed] [Google Scholar]
- 10.Unemo M, Golparian D, Nicholas R, Ohnishi M, Gallay A, Sednaoui P. 2012. High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob Agents Chemother 56:1273–1280. doi: 10.1128/AAC.05760-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quaye N, Cole MJ, Ison CA. 2014. Evaluation of the activity of ertapenem against gonococcal isolates exhibiting a range of susceptibilities to cefixime. J Antimicrob Chemother 69:1568–1571. doi: 10.1093/jac/dkt537. [DOI] [PubMed] [Google Scholar]
- 12.Unemo M, Golparian D, Limnios A, Whiley D, Ohnishi M, Lahra MM, Tapsall JW. 2012. In vitro activity of ertapenem versus ceftriaxone against Neisseria gonorrhoeae isolates with highly diverse ceftriaxone MIC values and effects of ceftriaxone resistance determinants: ertapenem for treatment of gonorrhea? Antimicrob Agents Chemother 56:3603–3609. doi: 10.1128/AAC.00326-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olsen B, Pham TL, Golparian D, Johansson E, Tran HK, Unemo M. 2013. Antimicrobial susceptibility and genetic characteristics of Neisseria gonorrhoeae isolates from Vietnam, 2011. BMC Infect Dis 13:40–47. doi: 10.1186/1471-2334-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen VG, Seah C, Martin I, Melano RG. 2014. Azithromycin resistance is coevolving with reduced susceptibility to cephalosporins in Neisseria gonorrhoeae in Ontario, Canada. Antimicrob Agents Chemother 58:2528–2534. doi: 10.1128/AAC.02608-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spratt BG, Bowler LD, Zhang QY, Zhou J, Smith JM. 1992. Role of interspecies transfer of chromosomal genes in the evolution of penicillin resistance in pathogenic and commensal Neisseria species. J Mol Evol 34:115–125. [DOI] [PubMed] [Google Scholar]
- 16.Ito M, Deguchi T, Mizutani K-S, Yasuda M, Yokoi S, Ito S-I, Takahashi Y, Ishihara S, Kawamura Y, Ezaki T. 2005. Emergence and spread of Neisseria gonorrhoeae clinical isolates harboring mosaic-like structure of penicillin-binding protein 2 in Central Japan. Antimicrob Agents Chemother 49:137–143. doi: 10.1128/AAC.49.1.137-143.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Unemo M. 2014. Challenges with antimicrobial susceptibility testing for Neisseria gonorrhoeae in the era of extensively drug-resistant gonorrhoea—molecular antimicrobial resistance testing crucial. Pathog Glob Health 108:214–215. doi: 10.1179/2047772414Z.000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demczuk W, Lynch T, Martin I, Van Domselaar G, Graham M, Bharat A, Allen V, Hoang L, Lefebvre B, Tyrrell G, Horsman G, Haldane D, Garceau R, Wylie J, Wong T, Mulvey MR. 2015. Whole-genome phylogenomic heterogeneity of Neisseria gonorrhoeae isolates with decreased cephalosporin susceptibility collected in Canada between 1989 and 2013. J Clin Microbiol 53:191–200. doi: 10.1128/JCM.02589-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohnishi M, Golparian D, Shimuta K, Saika T, Hoshina S, Iwasaku K, Nakayama S-I, Kitawaki J, Unemo M. 2011. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?: detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob Agents Chemother 55:3538–3545. doi: 10.1128/AAC.00325-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dillard JP. 2011. Genetic manipulation of Neisseria gonorrhoeae. Curr Protoc Microbiol Chapter 4:Unit4A.2. doi: 10.1002/9780471729259.mc04a02s23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clinical and Laboratory Standards Institute. 2014. Performance standards for antimicrobial susceptibility testing; 24th informational supplement. CLSI document M100-S24 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 22.Tomberg J, Unemo M, Ohnishi M, Davies C, Nicholas RA. 2013. Identification of amino acids conferring high-level resistance to expanded-spectrum cephalosporins in the penA gene from Neisseria gonorrhoeae strain H041. Antimicrob Agents Chemother 57:3029–3036. doi: 10.1128/AAC.00093-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takahata S, Senju N, Osaki Y, Yoshida T, Ida T. 2006. Amino acid substitutions in mosaic penicillin-binding protein 2 associated with reduced susceptibility to cefixime in clinical isolates of Neisseria gonorrhoeae. Antimicrob Agents Chemother 50:3638–3645. doi: 10.1128/AAC.00626-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao S, Duncan M, Tomberg J, Davies C, Unemo M, Nicholas RA. 2009. Genetics of chromosomally mediated intermediate resistance to ceftriaxone and cefixime in Neisseria gonorrhoeae. Antimicrob Agents Chemother 53:3744–3751. doi: 10.1128/AAC.00304-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohnishi M, Watanabe Y, Ono E, Takahashi C, Oya H, Kuroki T, Shimuta K, Okazaki N, Nakayama S-I, Watanabe H. 2010. Spread of a chromosomal cefixime-resistant penA gene among different Neisseria gonorrhoeae lineages. Antimicrob Agents Chemother 54:1060–1067. doi: 10.1128/AAC.01010-09. [DOI] [PMC free article] [PubMed] [Google Scholar]