Abstract
Determinations of doxycycline 50% inhibitory concentrations (IC50) for 620 isolates from northwest Thailand were performed via the isotopic method, and the data were analyzed by the Bayesian method and distributed into two populations (mean IC50s of 13.15 μM and 31.60 μM). There was no significant difference between the group with low IC50s versus the group with high IC50s with regard to copy numbers of the Plasmodium falciparum tetQ (pftetQ) gene (P = 0.11) or pfmdt gene (P = 0.87) or the number of PfTetQ KYNNNN repeats (P = 0.72).
TEXT
The World Health Organization (WHO) recommends doxycycline in combination with quinine or artesunate as the second-line treatment for uncomplicated Plasmodium falciparum malaria (1). Doxycycline is currently one of the recommended chemoprophylactic regimens for travelers visiting areas of malaria endemicity, particularly in countries with multiple-drug resistance. The prophylactic failure of doxycycline against P. falciparum may be explained by drug resistance, but this has not yet been documented. Indeed, -cycline resistance in Plasmodium has been documented only as a consequence of drug pressure in a P. berghei murine malaria model (2).
Recent studies have suggested that P. falciparum mdt (pfmdt) and pftetQ copy numbers are potential molecular markers of decreased in vitro susceptibility to doxycycline in African P. falciparum isolates (3, 4). In addition, isolates with PfTetQ KYNNNN motif repeats have been associated with reduced in vitro susceptibility to doxycycline and with a significantly greater probability of a 50% inhibitory concentration (IC50) greater than the doxycycline resistance threshold of 35 μM (3, 5).
The objective of this study was to evaluate for the first time the distribution of doxycycline IC50s for P. falciparum isolates collected in Asian patients and to validate the use of the pftetQ and pfmdt genes as molecular markers of decreased in vitro susceptibility to doxycycline.
Clinical isolates were obtained from patients with acute P. falciparum malaria attending Shoklo Malaria Research Unit (SMRU) clinics between 2001 and 2010. The SMRU clinics are all located along the Thai-Myanmar border. Isolates were collected from primary infections with a parasite density of at least 5 parasites/1,000 red blood cells. Samples were kept at room temperature before being transported to the main laboratory, where they were immediately tested in vitro. The fresh parasite isolate samples were obtained as part of prospective clinical evaluations of antimalarial drug therapy. Written informed consent translated into the patient's own language was obtained from each participant, whose consent signature was witnessed. The studies were approved by the Ethics Committees of the Faculty of Tropical Medicine, Mahidol University, and Oxford University. All cases were microscopically confirmed to be falciparum malaria.
In vitro drug susceptibility was determined by the hypoxanthine uptake inhibition assay, which has been described previously (6). The reproducibility of the IC50 measurements was assessed regularly using cloned P. falciparum strain K1 (Table 1). There was a significant reduction in the doxycycline median IC50 for strain K1 in 2003 (P < 0.0001). The doxycycline IC50s for the 620 isolates ranged from 0.21 to 55.44 μM, with a mean of 14.0 μM ± 6.5 μM. The average parameter estimates for the IC50s and their distribution by year are given in Table 1 and in Fig. 1. There were significant differences in the doxycycline median IC50s for the sample isolates collected during the study period of 2001 to 2010. The reduction in the doxycycline median IC50 in sample isolates collected in 2003 can be explained only by a bias in methodology. Considering the reduction in the doxycycline median IC50 for strain K1 in 2003, only 7 isolates of 620 (1.1%) had a doxycycline IC50 greater than 35 μM, which was the threshold determined for reduced susceptibility to doxycycline (7), demonstrating that isolates with reduced susceptibility to doxycycline (according to the IC50 values) were rare even in Thailand, a geographic area known for multiple drug resistance. This cutoff value of 35 μM was determined for an exposure to doxycycline from 42 h to 48 h (7). A cutoff for in vitro resistance is defined for a specific methodology. For example, the in vitro effects and the IC50s for doxycycline are dependent on the duration of incubation (8–10), on gas conditions, i.e., O2 and CO2 levels (11, 12), and on methodology, i.e., isotopic test versus immunoenzymatic or SYBR green test (13, 14). The incubation time is one of the conditions that interferes significantly with the IC50s of antibiotics (10, 15). In the present study, the in vitro testing conditions (i.e., parasitemia, hematocrit, serum use, incubation time in the presence of doxycycline, isotopic test) were the same as those used in the previous works (3, 4, 7).
TABLE 1.
Statistical analysis of the 620 P. falciparum Thai isolates and K1 strain in vitro responses (IC50 in µM) to doxycycline by year
| Parameter | Value |
||||||
|---|---|---|---|---|---|---|---|
| 2001 | 2002 | 2003 | 2007 | 2008 | 2009 | 2010 | |
| Plasmodium falciparum clinical isolates | |||||||
| No. of isolates | 173 | 244 | 91 | 31 | 24 | 45 | 12 |
| Minimum IC50 | 2.37 | 0.83 | 0.21 | 7.89 | 9.04 | 7.08 | 14.42 |
| 25% percentile | 11.99 | 9.66 | 5.13 | 12.05 | 11.66 | 15.91 | 17.41 |
| Median | 14.56 | 12.45 | 8.93 | 14.77 | 14.56 | 17.79 | 19.86 |
| 75% percentile | 18.44 | 16.01 | 12.71 | 17.82 | 19.13 | 20.16 | 21.17 |
| Maximum IC50 | 29.28 | 55.44 | 30.48 | 21.5 | 27.33 | 33.36 | 29.26 |
| Mean | 15.16 | 13.44 | 9.61 | 15.08 | 15.89 | 18.37 | 19.93 |
| SD | 5.20 | 7.27 | 6.04 | 3.60 | 5.10 | 5.05 | 3.67 |
| SE | 0.40 | 0.47 | 0.63 | 0.64 | 1.042 | 0.75 | 1.06 |
| Lower 95% CI of mean | 14.38 | 12.52 | 8.36 | 13.76 | 13.73 | 16.86 | 17.6 |
| Upper 95% CI of mean | 15.94 | 14.36 | 10.87 | 16.4 | 19.89 | 16.86 | 22.26 |
| Plasmodium falciparum K1 clone | |||||||
| No. of isolates | 6 | 6 | 7 | 4 | 4 | 10 | 6 |
| 25% percentile | 15.82 | 13.81 | 8.59 | 11.79 | 8.54 | 15.89 | 17.52 |
| Median | 17.47 | 14.37 | 8.9 | 12.04 | 11.33 | 18.60 | 18.60 |
| 75% percentile | 18.85 | 15.06 | 9.54 | 12.28 | 13.76 | 21.93 | 19.31 |
FIG 1.
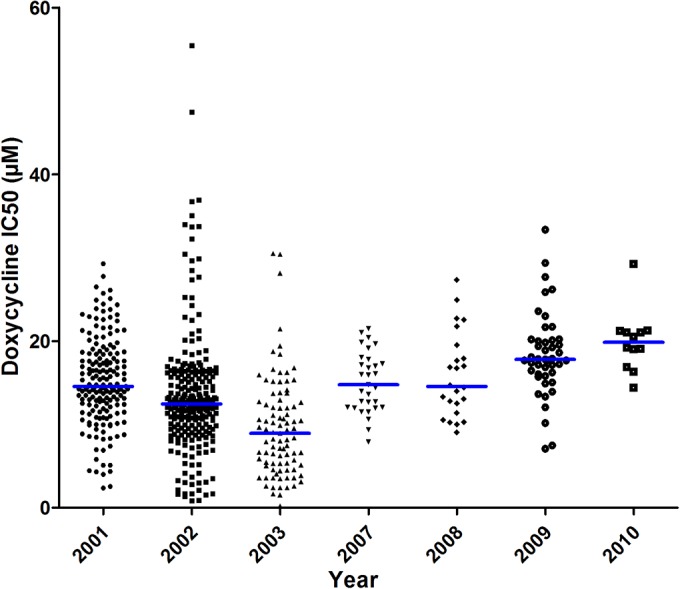
Doxycycline 50% inhibitory concentrations (IC50s) during the 2001-to-2010 period. The horizontal bars indicate the medians.
The distribution of doxycycline IC50s for 620 P. falciparum isolates was analyzed by the Bayesian method to identify the presence of subpopulations with different levels of doxycycline chemosusceptibility as previously described for doxycycline (4) and for pyronaridine and piperaquine (16). Two distributions were identified with, respectively, a mean value (± standard deviation) of 13.15 ± 5.25 μM for phenotypic group A, including 590 isolates (95.3%), and a mean value of 31.60 ± 9.39 μM for phenotypic group B, including 30 isolates (4.7%) (Fig. 2). This differs from the three doxycycline phenotypes observed in P. falciparum African isolates under the same laboratory conditions (3, 4).
FIG 2.
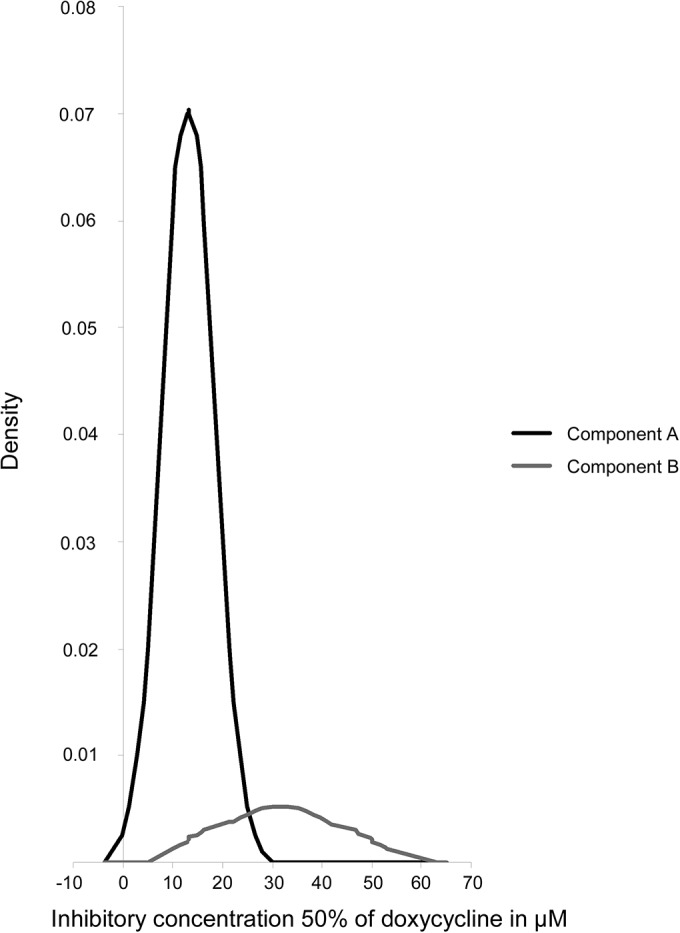
Distribution of the doxycycline IC50s of the 620 Plasmodium falciparum isolates from Thailand in the two-component mixture model (Bayesian mixture modeling approach).
Two recent studies have demonstrated that there was an association between the pfmdt and pftetQ copy numbers and the level of doxycycline susceptibility in Africa (3, 4). In addition, in Kenya, Achieng et al. showed that isolates with one copy of pfmdt had a median IC50 lower than those with two or more pfmdt copies (5). The quantification of pfmdt and pftetQ copy numbers of 59 P. falciparum Thai isolates randomly chosen from phenotypic group A with low or moderate doxycycline IC50s (mean, 9.41 μM [95% confidence interval [CI], 3.61 to 15.21 μM]) and all 30 isolates from group B with high doxycycline IC50s (mean, 29.16 μM [95% CI, 22.76 to 35.56 μM]) was performed by TaqMan real-time PCR under the same conditions as previously described for African isolates (3, 4, 17). Two isolates possessed two copies of pftetQ and one copy of pfmdt. Ten isolates had one copy of pftetQ and two copies of pfmdt. Only one isolate had two copies of both pftetQ and pfmdt. All isolates with two copies of pfmdt or pftetQ had one allelic family for each of the two genes (msp1 and msp2) as determined by the use of the nested PCR strategy previously described (18), confirming that these infections were clonal. Mixed-clone infections could influence TaqMan real-time PCR readouts, potentially leading to false-positive gene copy number data.
In Thai isolates, there was no association between pfmdt or pftetQ copy numbers and the level of susceptibility to doxycycline (Table 2 and Table 3). This result differs from data from previous studies in African isolates but is similar to data from a recent study from Senegal that found no significant association between doxycycline in vitro susceptibility and increased copy numbers of pftetQ (P = 0.08) or pfmdt (P = 0.07) (17).
TABLE 2.
Statistical analysis of pftetQ and pfmdt gene polymorphism in 89 Plasmodium falciparum isolates (Fisher's exact test)
| Gene copy no. and repeat frequency | No. (%) of isolates |
P value | |
|---|---|---|---|
| Component A | Component B | ||
| No. of pfmdt copies | |||
| >1 | 7 (12.1) | 4 (13.3) | |
| 1 | 51 (87.9) | 26 (86.7) | 1 |
| No. of pTetQ copies | |||
| >1 | 1 (1.7) | 2 (6.7) | |
| 1 | 58 (98.3) | 28 (93.3) | 0.26 |
| No. of PfTetQ KYNNNN repeats | |||
| <3 | 11 (18.6) | 7 (23.3) | |
| 3 | 48 (81.4) | 23 (76.7) | 0.59 |
TABLE 3.
Statistical analysis of the doxycycline IC50s (in µM) based on the pftetQ and pfmdt copy numbers and PfTetQ KYNNNN repeat numbers in 89 Plasmodium falciparum isolates
| Parameter | Value |
|||||
|---|---|---|---|---|---|---|
| No. of pftetQ copies |
No. of pfmdt copies |
No. of PfTetQ KYNNNN repeats |
||||
| 1 | >1 | 1 | >1 | <3 | 3 | |
| No. of isolates | 86 | 3 | 77 | 11 | 18 | 71 |
| Minimum IC50 | 2.17 | 15.20 | 2.17 | 2.58 | 3.03 | 2.17 |
| 25% percentile | 4.34 | 15.20 | 4.33 | 4.44 | 4.59 | 4.44 |
| Median IC50 | 14.62 | 25.88 | 14.68 | 14.9 | 14.64 | 14.68 |
| 75% percentile | 24.15 | 29.38 | 25.11 | 25.88 | 27.08 | 23.73 |
| Maximum IC50 | 55.44 | 29.38 | 55.44 | 36.92 | 36.92 | 55.44 |
| Mean | 15.66 | 23.49 | 15.85 | 16.49 | 16.99 | 15.65 |
| SD | 11.08 | 7.39 | 11.21 | 10.87 | 11.86 | 10.89 |
| SE | 1.20 | 4.27 | 1.28 | 3.28 | 2.79 | 1.29 |
| Lower 95% CI of mean | 13.28 | 5.14 | 13.30 | 9.19 | 13.92 | 14.87 |
| Upper 95% CI of mean | 18.03 | 41.84 | 18.39 | 23.79 | 20.71 | 16.43 |
| Mann-Whitney test P value | 0.11 | 0.87 | 0.72 | |||
In addition, reduced in vitro doxycycline susceptibility was found associated with PfTetQ KYNNNN sequence polymorphism: a total of <3 KYNNNN motif repeats is predictive of P. falciparum parasites with resistance in vitro, with IC50s of >35 μM (odds ratio of 15) (5). There was no association between doxycycline in vitro susceptibility and the number of PfTetQ KYNNNN repeats in Thai isolates (Table 2 and Table 3). This result differs from those obtained in Kenyan isolates (5). The difference in these data might be explained by the differences in the methods used for parasite IC50 evaluation (isotopic assay versus SYBR green I-based assay), data interpretation, sample size, parasite genetic background, population structure, and much more.
These results may also indicate that the molecular mechanisms of resistance to doxycycline are more complex than anticipated. The overexpression of pftetQ or pfmdt and the PfTetQ KYNNNN sequence polymorphism could confer reduced in vitro susceptibility to doxycycline in association with other contributing determinants which could modulate the in vitro response to doxycycline. Some genes which encoded apicoplast proteins such as apicoplast ribosomal protein S10 (arps10 gene; PF3D7_1460900.1) or ferrodoxin (fd gene; PF3D7_1318100), a key component of the apicoplast electron transport chain, might be involved in doxycycline resistance. These two genes might be also involved in P. falciparum artemisinin resistance (19). Thus, further studies are needed to better characterize the genetics of doxycycline resistance in P. falciparum.
ACKNOWLEDGMENTS
This study was supported by the Délégation Générale pour l'Armement (grant number 10CO405). The Shoklo Malaria Research Unit is part of the Mahidol Oxford University Research Unit, supported by The Wellcome Trust of Great Britain.
We thank Charlie Woodrow for his useful comments on the manuscript.
We declare that we have no competing interests.
REFERENCES
- 1.World Health Organization. 2011. Guidelines for the treatment of malaria, 2nd ed World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Jacobs RL, Koontz LC. 1976. Plasmodium berghei: development of resistance to clindamycin and minocycline in mice. Exp Parasitol 40:116–123. doi: 10.1016/0014-4894(76)90073-4. [DOI] [PubMed] [Google Scholar]
- 3.Briolant S, Wurtz N, Zettor A, Rogier C, Pradines B. 2010. Susceptibility of Plasmodium falciparum isolates to doxycycline is associated with pftetQ sequence polymorphisms and pftetQ and pfmdt copy numbers. J Infect Dis 201:153–159. doi: 10.1086/648594. [DOI] [PubMed] [Google Scholar]
- 4.Gaillard T, Briolant S, Houzé S, Baragatti M, Wurtz N, Hubert V, Lavina M, Pascual A, Travaillé C, Le Bras J, Pradines B. 2013. PftetQ and pfmdt copy numbers as predictive molecular markers of decreased ex vivo doxycycline susceptibility in imported Plasmodium falciparum malaria. Malar J 12:414. doi: 10.1186/1475-2875-12-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Achieng AO, Ingasia LA, Juma DW, Cheruiyot AC, Okudo CA, Yeda RA, Cheruiyot J, Akala HM, Johnson J, Andangalu B, Eyase F, Jura WG, Kamau E. 2014. Doxycycline reduced in vitro susceptibility in Plasmodium falciparum Kenyan field isolates is associated with PftetQ KYNNNN sequence polymorphism. Antimicrob Agents Chemother 58:5894–5899. doi: 10.1128/AAC.02788-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brockman A, Price RN, van Vugt M, Heppner DG, Walsh D, Sookto P, Wimonwattrawatee T, Looareesuwan S, White NJ, Nosten F. 2000. Plasmodium falciparum antimalarial drug susceptibility on the north-western border of Thailand during five years of extensive use of artesunate-mefloquine. Trans R Soc Trop Med Hyg 94:537–544. doi: 10.1016/S0035-9203(00)90080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briolant S, Baragatti M, Parola P, Simon F, Tall A, Sokhna C, Hovette P, Mamfoumbi MM, Koeck JL, Delmont J, Spiegel A, Castello J, Gardair JP, Trape JF, Kombila M, Minodier P, Fusai T, Rogier C, Pradines B. 2009. Multinormal in vitro distribution model suitable for the distribution of Plasmodium falciparum chemosusceptibility to doxycycline. Antimicrob Agents Chemother 53:688–695. doi: 10.1128/AAC.00546-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Draper MP, Bhatia B, Assefa H, Honeyman L, Garrity-Ryan LK, Verma AK, Gut J, Larson K, Donatelli J, Macone A, Klauner K, Leahy RG, Odinecs A, Ohemeng K, Rosenthal PJ, Nelson ML. 2013. In vitro and in vivo antimalarial efficacies of optimized tetracyclines. Antimicrob Agents Chemother 57:3131–3136. doi: 10.1128/AAC.00451-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pradines B, Spiegel A, Rogier C, Tall A, Mosnier J, Fusai T, Trape JF, Parzy D. 2000. Antibiotics for prophylaxis of Plasmodium falciparum infections: in vitro activity of doxycycline against Senegalese isolates. Am J Trop Med Hyg 62:82–85. [DOI] [PubMed] [Google Scholar]
- 10.Pradines B, Rogier C, Fusai T, Mosnier J, Daries W, Barret E, Parzy D. 2001. In vitro activities of antibiotics against Plasmodium falciparum are inhibited by iron. Antimicrob Agents Chemother 45:1746–1750. doi: 10.1128/AAC.45.6.1746-1750.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Divo AA, Geary TG, Jensen JB. 1985. Oxygen- and time-dependent effects of antibiotics and selected mitochondrial inhibitors on Plasmodium falciparum in culture. Antimicrob Agents Chemother 27:21–27. doi: 10.1128/AAC.27.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pascual A, Basco LK, Baret E, Amalvict R, Travers D, Rogier C, Pradines B. 2011. Use of the atmospheric generators for capnophilic bacteria Genbag-CO2 for the evaluation of in vitro Plasmodium falciparum susceptibility to standard anti-malarial drugs. Malar J 10:8. doi: 10.1186/1475-2875-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fall B, Diawara S, Sow K, Baret E, Diatta B, Fall KB, Mbaye PS, Fall F, Diémé Y, Rogier C, Wade B, Bercion R, Pradines B. 2011. Ex vivo susceptibility of Plasmodium falciparum isolates from Dakar, Senegal, to seven standard anti-malarial drugs. Malar J 10:310. doi: 10.1186/1475-2875-10-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wein S, Mynadier M, Tran Van Ba C, Cerdan R, Peyrottes S, Fraisse L, Vial H. 2010. Reliability of antimalarial sensitivity tests depends on drug mechanisms of action. J Clin Microbiol 48:1651–1660. doi: 10.1128/JCM.02250-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sidhu ABS, Sun Q, Nkrumah LJ, Dunne MW, Sacchettini JC, Fidock DA. 2007. In vitro efficacy, resistance selection, and structural modeling studies implicate the malarial parasite apicoplast as the target of azithromycin. J Biol Chem 282:2494–2504. doi: 10.1074/jbc.M608615200. [DOI] [PubMed] [Google Scholar]
- 16.Pascual A, Madamet M, Briolant S, Gaillard T, Amalvict R, Benoit N, Travers D, Pradines B. 2015. Multinormal in vitro distribution of Plasmodium falciparum susceptibility to piperaquine and pyronaridine. Malar J 14:49. doi: 10.1186/s12936-015-0586-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaillard T, Fall B, Tall A, Wurtz N, Diatta B, Lavina M, Fall KB, Sarr FD, Baret E, Diémé Y, Wade B, Bercion R, Briolant S, Pradines B. 2012. Absence of association between ex vivo susceptibility to doxycycline and pftetQ and pfmdt copy numbers in Plasmodium falciparum isolates from Dakar, Senegal. Clin Microbiol Infect 18:238–240. [DOI] [PubMed] [Google Scholar]
- 18.Henry M, Diallo I, Bordes J, Ka S, Pradines B, Diatta B, M'Baye PS, Sane M, Thiam M, Gueye PM, Wade B, Touze JE, Debonne JM, Rogier C, Fusai T. 2006. Urban malaria in Dakar, Senegal: chemosusceptibility and genetic diversity of Plasmodium falciparum isolates. Am J Trop Med Hyg 75:146–151. [PubMed] [Google Scholar]
- 19.Miotto O, Amato R, Ashley EA, MacInnis B, Almagro-Garcia J, Amaratunga C, Lim P, Mead D, Oyola SO, Dhorda M, Imwong M, Woodrow C, Manske M, Stalker J, Drury E, Campino S, Amenga-Etego L, Nguyen Thanh TN, Tran HT, Ringwald P, Bethell D, Nosten F, Phyo AP, Pukrittayakamee S, Chotivanich K, Chuor CM, Nguon C, Suon S, Sreng S, Newton PN, Mayxay M, Khanthavong M, Hongvanthong B, Htut Y, Han KT, Kyaw MP, Faiz MA, Fanello CI, Onamboko M, Mokuolu OA, Jacob CG, Takala-Harrison S, Plowe CV, Day NP, Dondorp AM, Spencer CCA, McVean G, Fairhurst RM, White NJ, Kwiatkowski DP. 2015. Genetic architecture of artemisinin-resistant Plasmodium falciparum. Nat Genet 47:226–234. doi: 10.1038/ng.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]


