Abstract
The prognostic value of the primary lesion pretreatment apparent diffusion coefficient (ADC), which is obtained by diffusion-weighted magnetic resonance imaging (MR-DWI), remains unknown in nasopharyngeal carcinoma (NPC). Thus, to investigate whether the pretreatment ADC value as measured from the primary site on MR-DWI is an independent prognostic factor in NPC, we retrospectively reviewed a cohort of 541 patients with histologically-proven stage I-IVB NPC. All patients underwent MRI using a 3-Tesla system (Trio Tim; Siemens, Erlangen Germany). To calculate ADC, the primary lesion was designated on the ADC map at the level of the largest tumor diameter to cover most of the lesion, avoiding cystic or necrotic components. Median and mean (±SD) pretreatment ADC were 0.713 and 0.716 ± 0.079 × 10−3 mm2/s, respectively. Univariate and multivariate analysis confirmed high pretreatment ADC was a good prognostic factor for poor local relapse-free survival and disease-free survival. Furthermore, the area under the ROC curve for prediction of local failure significantly increased when pretreatment ADC was combined with T classification (P = 0.004). Thus, pretreatment ADC might provide useful information for predicting outcome and selecting high-risk patients appropriate for more aggressive therapy. Further studies are warranted to investigate the biological basis of this observation.
According to the International Agency for Research on Cancer, there were over 86,000 new cases of nasopharyngeal cancer (NPC) worldwide in 2012; 80% of which occurred in Asia and only 6% in Europe1. NPC differs from other head and neck cancers because of its distinctly skewed geographic and ethnic distribution, its aggressive natural behavior, and specific therapeutic considerations. Unlike most other head and neck cancers, which are mainly treated by surgery, radiotherapy is the mainstay treatment modality for NPC2. Although local control has greatly improved in recent years with the application of magnetic resonance imaging (MRI) and intensity-modulated radiotherapy (IMRT), the management of recurrent NPC remains a challenging clinical problem due to high rates of fatal complications and poor survival as well as a low quality of life after retreatment3. For instance, it has been reported that up to 50% of patients with recurrent disease die of treatment-induced injuries after re-irradiation4. Therefore, it is of vital importance to be able to identify patients at high risk of local failure in order that individualized treatment can be offered.
“In-field” recurrence (95% of the recurrent tumor volume within the 95% isodose distribution of the radical dose) is the major local recurrence pattern in NPC5. One possible explanation is that tumors that recur possess different biological characteristics to tumors that do not5. Although the TNM staging system has been the cornerstone of the assessment of prognosis in NPC, it is merely based on the anatomic extent of the tumor6 and lacks an assessment of biological information. Thus, the exploration of parameters that reflect the intrinsic biological features of the tumor is highly important, and the prognostic value of these parameters demands urgent investigation.
Apparent diffusion coefficient (ADC) is a parameter obtained by diffusion-weighted magnetic resonance imaging (MR-DWI), a component of functional MRI, and reflects the Brownian movement of water molecules. ADC indirectly reflects the microvascular circulation, cell density and membrane integrity of the tissue7. A high pretreatment ADC value has been shown to be associated with a poor treatment response and/or survival after chemotherapy or chemoradiotherapy in many malignancies, including breast cancer8, hepatic metastases in colorectal cancer9 and some other head and neck cancers10,11,12,13. However, the prognostic value of the pretreatment ADC in NPC is unknown. Thus, we retrospectively analyzed a cohort of patients to investigate whether the pretreatment ADC value as measured from the primary lesion on diffusion weighted MRI is an independent prognostic factor in NPC.
Patients and Methods
Patients
This retrospective study was approved by the institutional Committee for Clinical Studies and the requirement for informed consent was waived. Between November 2010 and May 2012, 640 consecutive patients with histologically-proven stage I-IVB NPC were enrolled. Of these, 99 (15.5%) patients were subsequently excluded, including 8 (1.3%) cases due to a lack of clinical information, 28 (4.4%) because of poor imaging quality and 63 (10.0%) whose lesion was too small to be distinguished from normal tissues on DW images (tumor volume range, 0.68-6.36 ml). The remaining 541 patients were included (tumor volume range, 6.65–43.2 ml). The mean age of the entire cohort was 45.3 years (range, 14–74 years), with a male-to-female ratio of 2.6:1. The majority (99.6%) of patients were diagnosed with non-keratinizing carcinoma, while only 0.4% (2/541) were diagnosed with keratinizing squamous cell carcinoma.
All patients underwent a pretreatment evaluation including a complete patient history, physical examination, hematology and biochemistry profiles, MRI of the neck and nasopharynx, chest radiography, abdominal sonography and a whole body bone scan using single photon emission computed tomography. Furthermore, positron emission tomography-computed tomography (PET-CT) was performed on 156/541 (28.8%) patients. Medical records and imaging studies were analyzed retrospectively, and all patients were staged according to the 7th edition of the International Union against Cancer/American Joint Committee on Cancer (UICC/AJCC) system6. The characteristics of the patients analyzed in this study are shown in Table 1.
Table 1. Clinical features of the 541 patients with NPC.
| Characteristic | No. of patients (%) |
|---|---|
| Age (years) | |
| Median | 45 |
| Range | 14–74 |
| Gender | |
| Male | 392 (72.4) |
| Female | 149 (27.6) |
| Pathologic typea | |
| keratinizing squamous cell carcinoma | 2 (0.4) |
| differentiated non-keratinizing carcinoma | 26 (4.8) |
| undifferentiated non-keratinizing carcinoma | 513 (94.8) |
| T categoryb | |
| T1 | 90 (16.6) |
| T2 | 93 (17.2) |
| T3 | 200 (36.9) |
| T4 | 158 (29.2) |
| N categoryb | |
| N0 | 83 (15.3) |
| N1 | 326 (60.3) |
| N2 | 87 (16.1) |
| N3 | 45 (8.0) |
| Stageb | |
| I | 24 (4.4) |
| II | 117 (21.6) |
| III | 206 (38.1) |
| IV A/B | 194 (35.9) |
| Chemotherapy | |
| Radiotherapy alone | 67 (12.4) |
| Chemoradiotherapy | 474 (87.6) |
Imaging
All patients underwent MRI using a 3-Tesla system (Trio Tim; Siemens, Erlangen Germany). The region from the suprasellar cistern to the inferior margin at the sternal end of the clavicle was examined with a head-and-neck coil. T1-weighted fast spin-echo images in the axial, coronal and sagittal planes (repetition time [TR]/echo time [TE], 650 ms/9 ms), T2-weighted fast spin-echo MR images in the axial plane (TR/TE, 2470 ms/90 ms) and a spin-echo echo-planar DWI sequence (matrix, 192 × 192; TR/TE, 5100 ms/96 ms; fov, 240; b-values, 0 and 1000 s/mm2; three signal averages) were obtained before contrast injection. After the intravenous administration of gadopentetate dimeglumine (0.1 mmol per kilogram of body weight; Magnevist, Schering, Berlin, Germany), axial and sagittal T1-weighted spin-echo sequences and coronal T1-weighted fat-suppressed spin-echo sequences were performed sequentially using the same parameters as applied before the injection of gadopentetate dimeglumine. Then, 5-mm thick sections were obtained with a 1-mm interslice gap for the axial plane, and 6-mm sections with a 1-mm interslice gap were obtained for the coronal and sagittal planes, resulting in a matrix size of 512 × 512.
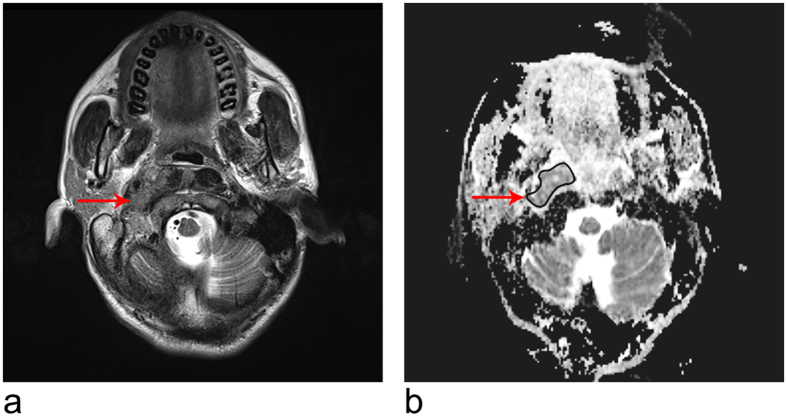
The ADC value was calculated as follows: the signal intensity (SI) of each pixel was fitted to the equation SI = SI0e-bD, where SI is the measured signal intensity, b is the b-value, D is the ADC, and SI0 is the SI at a b-value of 0. In this study, the ADC values were calculated using a b-value of 1,000 s/mm2. To calculate ADC, the primary lesion was designated as the region of interest (ROI) on the ADC map at the level of the largest tumor diameter to cover most of the lesion, avoiding cystic or necrotic components (with reference to T2-weighted and gadolinium-enhanced images). This procedure was carried out by two radiologists with more than 10 years of experience in MRI of head and neck cancer in consensus while blinded to the clinical outcomes. Representative ADC maps are shown in Fig. 1.
Figure 1. MR images of a 46-year-old woman.
(a) Axial T2-weighted fast spin-echo (2470/90) MR image showing the primary tumor (arrow) in the nasopharynx with parapharyngeal extension. The spin-echo echo-planar (5100/96) DW images were obtained with b values of 0 and 1000 s/mm2. (b) The lesion (arrow) shows intermediate signal intensity on the corresponding ADC map (ADC = 0.893 × 10−3 mm2/s). The patient experienced local failure 22.8 months after treatment.
Treatment
All patients were treated with IMRT; details of the techniques used at our center have previously been reported14. In total, 94.2% (377/400) of patients with stage III-IV A/B NPC received concurrent chemoradiotherapy ± neoadjuvant/adjuvant chemotherapy in conjunction with a platinum-based therapeutic clinical trial. When possible, salvage treatments such as brachytherapy, neck dissection and chemotherapy were provided in the event of documented relapse or persistent disease.
Patients were examined at least every 3 months during the first 2 years, and thereafter, a follow-up examination was performed every 5 months during years 3–5 or until death. Any residual disease found in the nasopharynx or cervical nodes within 6 months after completion of RT was regarded as local failure or regional failure, respectively. Distant failure was defined as the presence of metastases in locations beyond the regional nodes to which the cancer spread by vascular or lymphatic channels, such as the liver, bones or mediastinal lymph nodes15. Evaluations during follow-up included a complete patient history, physical examination, hematology and biochemistry profiles, MRI of the neck and nasopharynx, chest radiography, abdominal sonography and a whole body bone scan. All local recurrences were diagnosed by fibreoptic endoscopy and biopsy or MRI (or both) of the nasopharynx and the skull base showing progressive bone erosion and soft tissue swelling. Regional recurrences were diagnosed by clinical examination of the neck and, in doubtful cases, by fine needle aspiration or an MRI scan of the neck. Distant metastases were diagnosed by clinical symptoms, physical examinations, and imaging methods that included chest radiography, bone scan, MRI, CT and abdominal sonography.
Statistical analysis
Statistical analyses were performed using SPSS version 22.0 (IBM Corporation, Armonk, NY, USA). The independent samples t-test was used to examine the differences in continuous variables between groups. The primary outcome of interest was local relapse-free survival (LRFS). Secondary outcomes included distant metastasis-free survival (DMFS), overall survival (OS) and disease-free survival (DFS). LRFS, DMFS and OS were calculated from the first day of treatment to the first local relapse, distant metastasis or death, respectively. DFS was defined as the latency to the date of disease progression or death from any cause. Actuarial rates were calculated using the Kaplan–Meier method and compared using the log-rank test. Multivariate analysis using a Cox proportional hazards model was used to test the independent significance of different factors by backward elimination. When testing the association with local failure, host factors (age and gender), tumor factors (T and N classification) and treatment method (radiotherapy alone or chemoradiotherapy) were included as variables in all analyses. Area under the receiver-operating characteristic (ROC) curves were used to select the optimal cut-off point for ADC by maximizing the conditional Youden score and to assess the prognostic validity of adding pretreatment ADC to the current T classification, based on the method described by Hanley16 and Zweig17. The criterion for statistical significance was set at α = 0.05 and all P-values were based on two-sided tests.
Results
Pretreatment ADC level and tumor staging
Of the entire cohort of 541 patients with NPC, the median and mean ± SD pretreatment ADC levels were 0.713 and 0.716 ± 0.79 × 10−3 mm2/s, respectively, (range, 0.498 to 0.958 × 10−3 mm2/s). The pretreatment ADC levels of patients with T3/4 disease (mean, 0.722 ± 0.080 × 10−3 mm2/s; median, 0.716 × 10−3 mm2/s; interquartile range, 0.672–0.776 × 10−3 mm2/s) were significantly higher than those of patients with T1/2 disease (mean, 0.704 ± 0.075 × 10−3 mm2/s; median, 0.702 × 10−3 mm2/s; interquartile range, 0.654–0.752 × 10−3 mm2/s; P = 0.014).
Patterns of treatment failure
The median duration of follow-up for the entire cohort was 37.3 months (range, 4.5–52.0 months). A total of 23/541 (4.3%), 19/541 (3.5%), and 44/541 (7.8%) patients developed local failure, regional failure, and distant metastases, respectively; 25/541 (4.3%) patients died and 5/541 (0.7%) patients experienced both local-regional relapse and distant metastases. The 3-year LRFS, DMFS, OS and DFS rates were 95.7%, 92.5%, 95.3% and 86.0%, respectively.
Prognostic value of pretreatment ADC in NPC
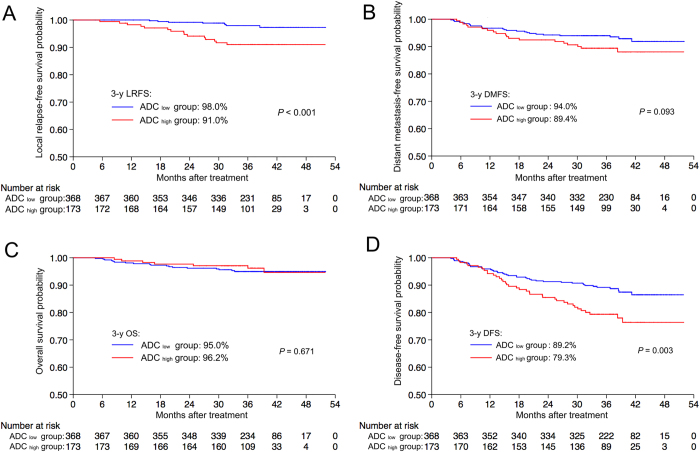
The optimal cut-off ADC value for local failure was 0.747 × 10−3 mm2/s (maximal conditional Youden score = 1.347; sensitivity, 65.2%; specificity, 69.5%; AUC [area under the ROC] = 0.68, P = 0.004). This value was selected to classify patients into ADC high (>0.747 × 10−3 mm2/s) and ADC low (<0.747 × 10−3 mm2/s) groups. The Kaplan-Meier survival curves for the two groups are shown in Fig. 2. The 3-year LRFS (91.0% vs. 98.0%, P < 0.001; Fig. 2A) and DFS (79.3% vs. 89.2%, P = 0.003; Fig. 2D) rates for the ADC high group were significantly lower than the corresponding rates for the ADC low group. There were no differences in the 3-year DMFS (89.4% vs. 94.0%, P = 0.093; Fig. 2B) and OS (95.0% vs. 96.2%, P = 0.671; Fig. 2C) rates between groups.
Figure 2. Kaplan-Meier curves of local relapse-free survival.
(A), distant metastasis-free survival (B), overall survival (C) and disease-free survival (D) for patients with NPC stratified as the ADC low and ADC high group. ADC low group = patients with a primary lesion pretreatment ADC value < 0.747 × 10−3 mm2/s; ADC high group = patients with a primary lesion pretreatment ADC value ≥ 0.747 × 10−3 mm2/s. Abbreviations: 3-y = 3-year; ADC = apparent diffusion coefficient; DFS = disease-free survival; DMFS = distant metastasis-free survival; LRFS = local relapse-free survival; OS = overall survival.
Multivariate analysis was performed to adjust for confounding factors (seen in Table 2). Pretreatment ADC was found to be an independent prognostic factor for LRFS; advanced T classification (T3-4 vs. T1-2) was also associated with an increased risk of local failure, though this effect did not reach statistical significance. Meanwhile, pretreatment ADC and N classification were independent prognostic factors for DFS.
Table 2. Univariate and multivariate analyses of prognostic factors in the 541 patients with NPC.
| Endpoint | Variable | Univariate analysis | Multivariate analysis |
|
|---|---|---|---|---|
| P-value | HR (95% CI) | P-valuea | ||
| Local relapse-free survival | ADC | <0.001 | 3.858 (1.634–9.111) | 0.002 |
| T classificationb | 0.031 | 3.153 (0.935–10.632) | 0.064 | |
| Distant metastasis-free survival | N classificationb | <0.001 | 3.139 (1.736–5.675) | <0.001 |
| Disease-free survival | ADC | 0.003 | 1.829 (1.177–2.843) | 0.007 |
| N classificationb | <0.001 | 2.386 (1.526–3.731) | <0.001 | |
Abbreviations: ADC = apparent diffusion coefficient; CI = confidence interval; HR = hazard ratio; NPC = nasopharyngeal carcinoma.
aP-values were calculated using an adjusted Cox proportional hazards model.
bAccording to the 7th UICC/AJCC staging system6.
In patients with T3-4 disease, a high pretreatment ADC was also found to be a good prognostic factor for poor 3-year LRFS (89.0% vs. 97.2%, P = 0.003) and DFS (78.7% vs. 88.1%, P = 0.013) in univariate analysis. Furthermore, multivariate analysis confirmed that pretreatment ADC was an independent prognostic factor for LRFS and DFS.
Prognostic validity of adding the pretreatment ADC of the primary lesion to the current T classification for local failure in NPC
Receiver-operating characteristic curves were used to evaluate the prognostic validity of adding the pretreatment ADC of the primary lesion to the T classification of the current TNM staging system for local failure in NPC. The AUC significantly increased when the pretreatment ADC (ADC ≥ 0.747 vs. ADC < 0.747 × 10−3 mm2/s) was added to T classification (0.699 vs. 0.653, P = 0.004; Fig. 3).
Figure 3. Receiver-operating characteristic curves for prediction of local failure after IMRT for the current T classification alone and the combination of the primary lesion pretreatment ADC value and T classification in patients with nasopharyngeal carcinoma (n = 541).
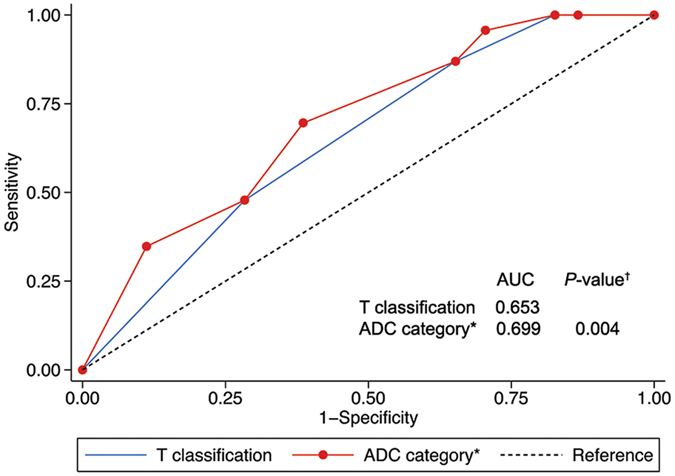
P-values are compared to T classification alone. *Pretreatment ADC of the primary lesion (ADC ≥ 0.747 vs. ADC < 0.747 × 10−3 mm2/s) combined with T classification. Abbreviations: ADC = apparent diffusion coefficient; AUC = area under the curve.
Discussion
To the best of our knowledge, this is the first study to determine the prognostic value of the pretreatment ADC of the primary lesion in NPC. A high pretreatment ADC was associated with advanced T classification, the pretreatment ADC of the primary lesion was an independent prognostic indicator of LRFS and DFS, and combining the pretreatment ADC with the current T classification resulted in superior prognostic value for local failure compared to T classification alone in patients with NPC.
The mechanism underlying the association between a high pretreatment ADC value and local failure may be correlated with the radiosensitivity of the tumor. A number of biological features, such as hypoxia, inflammation, cell density and cell membrane integrity, could affect the diffusion of water in tissues and hence the ADC value10; these factors may also influence the radiosensitivity of the tumor. For example, hypoxia in the tumor promotes inflammation18, which is correlated with a high ADC value as it increases the interstitial water content of the tissue19. Moreover, hypoxia is a well-known characteristic that is associated with low levels of radiosensitivity20,21. Accordingly, tumors with low oxygen tensions may exhibit high ADC values and have a low level of radiosensitivity. On the other hand, ADC values are inversely correlated with cell density in many malignancies22,23,24, and tumors with a high cell density are more likely to have a higher number of viable proliferative cells and may exhibit a better response to radiotherapy25. Therefore, it is expected that tumors with fewer viable proliferative cells would have high ADC values and lower radiosensitivity. However, these mechanisms need to be confirmed by future studies.
Currently, TNM staging is the cornerstone of the assessment of prognosis and the establishment of treatment strategies in NPC, and the T classification is supposed to reflect the risk of local failure26. However, the TNM staging system is only based on the anatomical extent of the tumor6 and lacks biological information. Additionally, recent reports have suggested that the prognostic validity of the T classifications may have become blurred in the IMRT era5,27. The pretreatment ADC of the primary lesion may reflect the intrinsic biological characteristics of the primary tumor and provide additional useful information regarding T classification. This study demonstrated that the pretreatment ADC was an independent prognostic indicator of local failure and combination of the pretreatment ADC with T classification had superior prognostic value for local failure compared to T classification alone, suggesting that the pretreatment ADC can enhance the prognostic ability of the current T classification system. Patients with a high pretreatment ADC value might benefit from more aggressive treatment strategies, such as the addition of molecular-targeted agents or radiosensitizer agents such as sodium glycididazole28,29,30. However, the value of these strategies needs to be addressed in future studies.
MR-DWI enables non-invasive in vivo assessment of the intrinsic biological characteristics of tissues, and has proven value for differential diagnosis31, staging32, monitoring the response to therapy7,33, prognostic evaluation10,34 and early detection of recurrence35 in many malignancies. Several studies have demonstrated high pretreatment ADC values correlate with unfavorable treatment responses or poor survival after radiotherapy and/or chemotherapy in various tumor types including breast cancer36, brain tumor37, bladder cancer38 and some head and neck cancers10. Interestingly, one study in renal cancer reported that patients with low ADC values had a high risk of metastasis after surgery39; it remains unclear whether this effect was due to intrinsic tumor characteristics or the treatment modalities and this issue awaits further research. Moreover, large changes in the ADC value after treatment could be a useful indicator of treatment efficacy at an early phase, prior to the shrinkage of tumor volume, such as in breast cancer40 and brain tumors41, which provides compelling evidence of the potential of ADC in treatment decision making.
Similarly, in NPC, Chen et al. found that a large increase in ADC was associated with good treatment response after the first cycle of neoadjuvant chemotherapy in a cohort study of 31 patients with stage III-IV disease, while there was no significant difference in pretreatment ADC between responders and non-responders42. This was possibly due to the limited sample size. Similar results were observed in another study by the same group: Hong et al. did not find significant differences in pretreatment ADC between patients with and without residual tumors detected by MRI or biopsy three months after radiotherapy7. However, in Hong’s study, the proportion of residual tumors diagnosed by imaging only or histology was not clear, and all of the “residual tumors” detected by imaging may not actually contain viable tumor cells as the residual rate (16%) was much higher than the histological residual rate at the same time point in previous study (6.8% in patients treated with 2D-CRT)43. Besides, though Razek et al. reported that a low pretreatment ADC correlated with a large tumor volume and lymph node metastasis44, all patients enrolled were from non-endemic regions and only 53.3% (16/30) had undifferentiated non-keratinizing carcinoma (which accounted for up to 94.8% of cases in our study). Furthermore, the relationship between ADC changes and clinical outcome was not explored. Thus the correlation between pretreatment ADC and long-term survival outcomes in NPC was unknown. Here, we firstly analyzed the correlation of pretreatment ADC and clinical outcome in a large cohort of patients with NPC from the endemic region, and found out that pretreatment ADC was an independent prognostic factor for local control and disease-free survival, which is in accordance with findings in breast cancer36, some head and neck cancers10 and other tumor types37,38.
Nevertheless, the application of pretreatment ADC values in the clinic may be subject to a number of limitations. Firstly, detection of small primary lesions on DWI is usually difficult, although patients with small primary tumors have a low risk of local failure27. Secondly, these results may not be easily generalized to other centers as a result of inter-institutional differences in DWI techniques, including the b-values used. Moreover, no standard method has been established to determine the ADC value. Other methods described in the literature have included drawing multiple small regions of interest on one or several sections, or calculating ADC from the entire lesion. As MRI has been accepted as the imaging modality of choice for patients with NPC45, the standardization of DWI techniques and calculation methods could allow DWI to be more widely used in clinical settings. Thirdly, this study only included patients from the endemic region in China, of whom only 0.4% had keratinizing squamous cell carcinoma, which accounts for up to 67% of cases of NPC in western countries46. Finally, this study has unavoidable biases due to its retrospective nature. Prospective multi-center studies are required to confirm the results of this analysis.
In conclusion, the pretreatment ADC of the primary lesion was an independent prognostic indicator of local failure in patients with NPC, thus pretreatment ADC may provide useful information for predicting outcome and selecting high-risk patients appropriate for more aggressive therapy. Further studies are warranted to investigate the biological foundation of this observation.
Additional Information
How to cite this article: Zhang, Y. et al. Prognostic value of the primary lesion apparent diffusion coefficient (ADC) in nasopharyngeal carcinoma: a retrospective study of 541 cases. Sci. Rep. 5, 12242; doi: 10.1038/srep12242 (2015).
Acknowledgments
This work was supported by grants from the Key Laboratory Construction Project of Guangzhou City, China (No. 121800085), the Health & Medical Collaborative Innovation Project of Guangzhou City, China (201400000001), the National Science & Technology Pillar Program during the Twelfth Five-year Plan Period (2014BAI09B10), the Sun Yat-sen University Clinical Research 5010 Program (No. 2012011), Science and Technology Project of Guangzhou City, China (No. 14570006), and the Planned Science and Technology Project of Guangdong Province (No. 2013B020400004).
Footnotes
Author Contributions Conception and design of the study: Yuan Z., X.L, Yun Z. and J.M. Acquisition of data: Yuan Z., X.L., Yun Z. and J.X.S. Analysis and interpretation of the data: Yuan Z., X.L. and Yun Z. Contributed reagents /materials/analysis tools: All authors. Writing and revision of the manuscript: All authors. All authors reviewed the manuscript.
References
- International Agency for Research on Cancer. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. (2012). Available at: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx (Accessed: 27nd March 2015).
- Lee A. W., Lin J. C. & Ng W. T. Current management of nasopharyngeal cancer. Seminars in radiation oncology 22, 233–244. 10.1016/j.semradonc.2012.03.008 (2012). [DOI] [PubMed] [Google Scholar]
- Xu T. et al. Recurrent nasopharyngeal carcinoma: a clinical dilemma and challenge. Current oncology 20, e406–419. 10.3747/co.20.1456 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y. M. et al. Prognostic model for survival of local recurrent nasopharyngeal carcinoma with intensity-modulated radiotherapy. British journal of cancer 110, 297–303. 10.1038/bjc.2013.715 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng W. T. et al. Clinical outcomes and patterns of failure after intensity-modulated radiotherapy for nasopharyngeal carcinoma. International journal of radiation oncology, biology, physics 79, 420–428. 10.1016/j.ijrobp.2009.11.024 (2011). [DOI] [PubMed] [Google Scholar]
- Edge S. B. & Compton C. C. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Annals of surgical oncology 17, 1471–1474. 10.1245/s10434-010-0985-4 (2010). [DOI] [PubMed] [Google Scholar]
- Hong J. et al. Value of magnetic resonance diffusion-weighted imaging for the prediction of radiosensitivity in nasopharyngeal carcinoma. Otolaryngology–head and neck surgery: official journal of American Academy of Otolaryngology-Head and Neck Surgery 149, 707–713. 10.1177/0194599813496537 (2013). [DOI] [PubMed] [Google Scholar]
- Hatakenaka M. et al. Apparent diffusion coefficients of breast tumors: clinical application. Magnetic resonance in medical sciences: MRMS: an official journal of Japan Society of Magnetic Resonance in Medicine 7, 23–29 (2008). [DOI] [PubMed] [Google Scholar]
- Koh D. M. et al. Predicting response of colorectal hepatic metastasis: value of pretreatment apparent diffusion coefficients. AJR. American journal of roentgenology 188, 1001–1008. 10.2214/AJR.06.0601 (2007). [DOI] [PubMed] [Google Scholar]
- Hatakenaka M. et al. Pretreatment apparent diffusion coefficient of the primary lesion correlates with local failure in head-and-neck cancer treated with chemoradiotherapy or radiotherapy. International journal of radiation oncology, biology, physics 81, 339–345. 10.1016/j.ijrobp.2010.05.051 (2011). [DOI] [PubMed] [Google Scholar]
- King A. D. et al. Head and neck squamous cell carcinoma: diagnostic performance of diffusion-weighted MR imaging for the prediction of treatment response. Radiology 266, 531–538. 10.1148/radiol.12120167 (2013). [DOI] [PubMed] [Google Scholar]
- Lambrecht M. et al. Integrating pretreatment diffusion weighted MRI into a multivariable prognostic model for head and neck squamous cell carcinoma. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology 110, 429–434. 10.1016/j.radonc.2014.01.004 (2014). [DOI] [PubMed] [Google Scholar]
- Ohnishi K. et al. Prediction of local failures with a combination of pretreatment tumor volume and apparent diffusion coefficient in patients treated with definitive radiotherapy for hypopharyngeal or oropharyngeal squamous cell carcinoma. Journal of radiation research 52, 522–530. 10.1269/jrr.10178 (2011). [DOI] [PubMed] [Google Scholar]
- Lai S. Z. et al. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? International journal of radiation oncology, biology, physics 80, 661–668. 10.1016/j.ijrobp.2010.03.024 (2011). [DOI] [PubMed] [Google Scholar]
- Edge S. B. et al. AJCC cancer staging manual (7th ed). New York, NY: Springer. 7 (2010). [Google Scholar]
- Hanley J. A. & McNeil B. J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143, 29–36. 10.1148/radiology.143.1.7063747 (1982). [DOI] [PubMed] [Google Scholar]
- Zweig M. H. & Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clinical chemistry 39, 561–577 (1993). [PubMed] [Google Scholar]
- Eltzschig H. K. & Carmeliet P. Hypoxia and inflammation. The New England journal of medicine 364, 656–665. 10.1056/NEJMra0910283 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami R. et al. Malignant supratentorial astrocytoma treated with postoperative radiation therapy: prognostic value of pretreatment quantitative diffusion-weighted MR imaging. Radiology 243, 493–499. 10.1148/radiol.2432060450 (2007). [DOI] [PubMed] [Google Scholar]
- Brizel D. M., Sibley G. S., Prosnitz L. R., Scher R. L. & Dewhirst M. W. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. International journal of radiation oncology, biology, physics 38, 285–289 (1997). [DOI] [PubMed] [Google Scholar]
- Nordsmark M. & Overgaard J. Tumor hypoxia is independent of hemoglobin and prognostic for loco-regional tumor control after primary radiotherapy in advanced head and neck cancer. Acta oncologica 43, 396–403 (2004). [DOI] [PubMed] [Google Scholar]
- Humphries P. D., Sebire N. J., Siegel M. J. & Olsen O. E. Tumors in pediatric patients at diffusion-weighted MR imaging: apparent diffusion coefficient and tumor cellularity. Radiology 245, 848–854. 10.1148/radiol.2452061535 (2007). [DOI] [PubMed] [Google Scholar]
- Manenti G. et al. Malignant renal neoplasms: correlation between ADC values and cellularity in diffusion weighted magnetic resonance imaging at 3 T. La Radiologia medica 113, 199–213. 10.1007/s11547-008-0246-9 (2008). [DOI] [PubMed] [Google Scholar]
- Squillaci E. et al. Correlation of diffusion-weighted MR imaging with cellularity of renal tumours. Anticancer research 24, 4175–4179 (2004). [PubMed] [Google Scholar]
- Hatakenaka M. et al. Apparent diffusion coefficient calculated with relatively high b-values correlates with local failure of head and neck squamous cell carcinoma treated with radiotherapy. AJNR. American journal of neuroradiology 32, 1904–1910. 10.3174/ajnr.A2610 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. et al. The seventh edition of the UICC/AJCC staging system for nasopharyngeal carcinoma is prognostically useful for patients treated with intensity-modulated radiotherapy from an endemic area in China. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology 104, 331–337, 10.1016/j.radonc.2011.10.009 (2012). [DOI] [PubMed] [Google Scholar]
- Guo R. et al. Is primary tumor volume still a prognostic factor in intensity modulated radiation therapy for nasopharyngeal carcinoma? Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology 104, 294–299. 10.1016/j.radonc.2012.09.001 (2012). [DOI] [PubMed] [Google Scholar]
- Chan A. T. et al. Multicenter, phase II study of cetuximab in combination with carboplatin in patients with recurrent or metastatic nasopharyngeal carcinoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 23, 3568–3576. 10.1200/JCO.2005.02.147 (2005). [DOI] [PubMed] [Google Scholar]
- Sung F. L. et al. Pharmacoproteomics study of cetuximab in nasopharyngeal carcinoma. Journal of proteome research 5, 3260–3267. 10.1021/pr050452g (2006). [DOI] [PubMed] [Google Scholar]
- Yang J. et al. [Phase II clinical trial of sodium glyci-didazole (CM-Na) combined with concurrent radiochemotherapy for advanced esophageal carcinoma]. Ai zheng=Aizheng=Chinese journal of cancer 27, 622–626 (2008). [PubMed] [Google Scholar]
- Wu L. M. et al. On the utility of quantitative diffusion-weighted MR imaging as a tool in differentiation between malignant and benign thyroid nodules. Academic radiology 21, 355–363. 10.1016/j.acra.2013.10.008 (2014). [DOI] [PubMed] [Google Scholar]
- Vandecaveye V. et al. Head and neck squamous cell carcinoma: value of diffusion-weighted MR imaging for nodal staging. Radiology 251, 134–146. 10.1148/radiol.2511080128 (2009). [DOI] [PubMed] [Google Scholar]
- Thoeny H. C., De Keyzer F. & King A. D. Diffusion-weighted MR imaging in the head and neck. Radiology 263, 19–32. 10.1148/radiol.11101821 (2012). [DOI] [PubMed] [Google Scholar]
- Nagamachi S. et al. Comparison of diagnostic and prognostic capabilities of (1)(8)F-FDG-PET/CT, (1)(3)(1)I-scintigraphy, and diffusion-weighted magnetic resonance imaging for postoperative thyroid cancer. Japanese journal of radiology 29, 413–422. 10.1007/s11604-011-0572-z (2011). [DOI] [PubMed] [Google Scholar]
- Xu J. F. et al. Value of diffusion-weighted magnetic resonance imaging on the follow-up of nasopharyngeal carcinoma after radiotherapy. Journal of X-ray science and technology 22, 605–612. 10.3233/XST-140448 (2014). [DOI] [PubMed] [Google Scholar]
- Park S. H. et al. Diffusion-weighted MR imaging: pretreatment prediction of response to neoadjuvant chemotherapy in patients with breast cancer. Radiology 257, 56–63. 10.1148/radiol.10092021 (2010). [DOI] [PubMed] [Google Scholar]
- Mardor Y. et al. Pretreatment prediction of brain tumors’ response to radiation therapy using high b-value diffusion-weighted MRI. Neoplasia 6, 136–142. 10.1593/neo.03349 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S. et al. Role of diffusion-weighted magnetic resonance imaging in predicting sensitivity to chemoradiotherapy in muscle-invasive bladder cancer. International journal of radiation oncology, biology, physics 83, e21–27. 10.1016/j.ijrobp.2011.11.065 (2012). [DOI] [PubMed] [Google Scholar]
- Yoshida S. et al. Apparent diffusion coefficient as a prognostic biomarker of upper urinary tract cancer: a preliminary report. European radiology 23, 2206–2214. 10.1007/s00330-013-2805-2 (2013). [DOI] [PubMed] [Google Scholar]
- Sharma U., Danishad K. K., Seenu V. & Jagannathan N. R. Longitudinal study of the assessment by MRI and diffusion-weighted imaging of tumor response in patients with locally advanced breast cancer undergoing neoadjuvant chemotherapy. NMR in biomedicine 22, 104–113, 10.1002/nbm.1245 (2009). [DOI] [PubMed] [Google Scholar]
- Mardor Y. et al. Early detection of response to radiation therapy in patients with brain malignancies using conventional and high b-value diffusion-weighted magnetic resonance imaging. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 21, 1094–1100 (2003). [DOI] [PubMed] [Google Scholar]
- Chen Y. et al. Diffusion-weighted magnetic resonance imaging for early response assessment of chemoradiotherapy in patients with nasopharyngeal carcinoma. Magnetic resonance imaging. 10.1016/j.mri.2014.02.009 (2014). [DOI] [PubMed] [Google Scholar]
- Kwong D. L. et al. The time course of histologic remission after treatment of patients with nasopharyngeal carcinoma. Cancer 85, 1446–1453 (1999). [DOI] [PubMed] [Google Scholar]
- Abdel Razek A. A. & Kamal E. Nasopharyngeal carcinoma: correlation of apparent diffusion coefficient value with prognostic parameters. La Radiologia medica 118, 534–539, 10.1007/s11547-012-0890-x (2013). [DOI] [PubMed] [Google Scholar]
- Lai V. & Khong P. L. Updates on MR imaging and (1)(8)F-FDG PET/CT imaging in nasopharyngeal carcinoma. Oral oncology 50, 539–548, 10.1016/j.oraloncology.2013.05.005 (2014). [DOI] [PubMed] [Google Scholar]
- Shedd D. P., Von Essen C. F. & Eisenberg H. Cancer of the nasopharynx in Connecticut, 1935 through 1959. Cancer 20, 508–511 (1967). [DOI] [PubMed] [Google Scholar]
- Thompson L. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Ear, nose, & throat journal 85, 74 (2006). [PubMed] [Google Scholar]