Abstract
Cornus officinalis is widely distributed in Korea, and its fruit has been used to make as herbal drug for traditional medicine in Korea, Japan, and China because of its tonic, analgesic, and diuretic properties. However, the effects of C. officinalis methanol extract (COME) on melanogenesis remain poorly understood. We evaluated the melanogenic capability of COME in melan-a cells, which are immortalized mouse melanocytes. COME increased melanin synthesis in a dose-dependent manner. Treatment with 12.5 μg/mL of COME significantly increased melanin content by 36.1% (p < 0.001) to a level even higher than that (31.6%) of 3-isobutyl-1-methyl-xanthine, a well-known pigmentation agent. COME also upregulated tyrosinase activity and its messenger RNA and protein expression. In addition, COME upregulated the expression of tyrosinase-related proteins 1 and 2 and microphthalmia-associated transcription factor-M messenger RNA expression. These results imply that COME may be appropriate for development as a natural product to treat hair graying.
Keywords: Cornus officinalis, Hair graying, Melan-a cells, Melanogenesis, Tyrosinase activity
INTRODUCTION
Hair color has important socioeconomic implications and propels a multimillion-dollar hair product industry. Premature graying (canities) is a common and intriguing phenomenon frequently discussed in the context of environmental or endogenous processes that accelerate aging such as pollution, ultraviolet light exposure, inflammation, or even psychoemotional stress (1). Despite these pressing interests, the phenomenon has not yet been satisfactorily explained, and a scientifically sound therapeutic strategy remains to be developed.
Melanin production is restricted to the melanocytes of the skin, hair follicles, and pigment epithelium in the retina. Skin and hair follicle melanins are the products of melanogenesis, a complex, phylogenetically ancient biochemical pathway. They are formed in cytoplasmic organelles called melanosomes produced by neural-crest-derived pigment cells called melanocytes. In human hair follicles, melanocytes located in the nascent bulb undergo mitosis and begin organizing the dermal papilla during the onset of anagen (2). The melanocytes responsible for hair pigmentation are located in the bulb of the follicle, in which they transfer melanins to the cortical keratinocytes of the hair shaft, thus defining the hair pigmentation unit (2). The large variety in hair color derives from different relative amounts of brown/black eumelanins and yellow/red phaeomelanins. Eumelanins and phaeomelanins differ not only in color but also in the size, shape, and packaging of their granules (3). Both types of melanin are derived from a common tyrosinasedependent pathway with the same precursor, tyrosine. Moreover, several enzymes must be activated in melanocytes to increase melanin synthesis.
Melanocyte-specific tyrosinase, tyrosinase-related protein-1 (TRP-1) and TRP-2 are involved in melanogenesis, in which tyrosine is converted into melanins (4). In particular, tyrosinase plays a pivotal role in the modulation of melanin production by catalyzing the hydroxylation of tyrosine to 3,4-dihydroxy-phenylalanine (DOPA) and the subsequent oxidation of DOPA, which results in the formation of dopaquinone (5). Tyrosinase, TRP-1, and TRP-2 share 70~80% nucleotide sequence homology and 30~40% amino acid identity. 3-Isobutyl-1-methylxanthine (IBMX) plays a key role in the regulation of melanogenesis via the cellular cyclic adenosine 3',5'-monophosphate (cAMP) pathway (6). The cAMP-dependent pathway upregulates microphthalmia-associated transcription factor (MITF), which stimulates the synthesis of key melanogenic proteins such as tyrosinase, TRP-1, and TRP-2 (7). Thus, IBMX is generally used as a positive control in pigmenting research. MITF-M transactivates tyrosinase, TRP-1, and TRP-2 melanogenic genes in vitro via binding to an M-box motif present in the promoters of those genes (8).
Despite recent evidence that hair graying is caused at least in part by a decline in the number of melanocyte stem cells, the factors that cause this decline are unknown. All studies to date have confirmed that pigment loss is due to a marked reduction in melanogenically active melanocytes in the hair bulb of gray anagen hair follicles (9). In the absence of a natural treatment for graying hair, colorants are the mainstay for recovering lost hair color. However, studies have shown that a small number of long-term users of permanent hair dyes (particularly black dyes) may develop irritating and allergic contact reactions (commonly due to p-phenylenediamine) that can cause dermatitis and even hair loss (10).
Cornus officinalis Sieb. et Zucc. (C. officinalis) is widely distributed in Korea, and its fruit has been used to make an herbal drug for traditional medicine in Korea, Japan, and China because of its tonic, analgesic, and diuretic properties (11). The major chemical constituents of C. officinalis fruit are saponins, phenolic acid (gallic acid, tannic acid), and loganin. Saponins and phenolic acid have known antioxidant activities (12). C. officinalis is also a medicinal plant with potent free radical scavenging activity against not only reactive oxygen species (e.g., hydrogen peroxide, superoxide anion, and hydroxyl radical) but also many other free radicals (e.g., peroxynitrate, peroxy radical) (13). Recent studies have reported that hot water and ethanol extracts of C. officinalis block oxidative reactions in melanogenesis, thereby inhibiting melanogenesis in B16 melanoma cells and that loganin and cornuside have inhibitory effects on melanogenesis (14). However, the effects of methanol extracts of C. officinalis on melanogenesis have not been thoroughly studied.
In the present study, we investigated the effects of C. officinalis methanol extract (COME) on melanogenesis by measuring melanin synthetic capability, tyrosinase activity, and melanogenic gene and protein expression in melan-a cells.
MATERIALS AND METHODS
Materials. Dimethyl sulfoxide (DMSO), 2,6-di-tertbutylate hydroxytoluene (BHT), 3,4-dihydroxy-L-phenylalanine (L-DOPA), β-actin, 1,1-diphenyl-2-picryl hydrazyl (DPPH), tannic acid, L-tyrosine, ascorbic acid, Folin-Ciocalteu’s phenol reagent, 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT), IBMX, MITF-M, and diethylene glycol reagent were obtained from Sigma Chemical Company (St. Louis, MO, USA). TRP-1 and TRP-2 were obtained from Amersham Company (Bucks, UK). Propylene glycol was purchased from Chemical Innovation Company (Seoul, Korea). Methanol extract from the fruit of C. officinalis (serial number: 014-046) was obtained from the Korea Plant Extract Bank (Daejeon, Korea). This specimen was dissolved in DMSO before use.
Antioxidant activity analysis. The total polyphenol content of COME was determined with the Folin-Denis assay (15). One milliliter of test agent dissolved in DMSO was placed into test tube followed by the addition of 1 mL of Folin-Ciocalteu’s phenol reagent. The tubes were allowed to stand for 3 min. One milliliter of 10% Na2CO3 was added, and the mixture was shaken vigorously. After the tubes stood for 60 min, absorbance at 760 nm was measured. A standard curve was prepared with tannic acid.
Total flavonoid content of COME was determined using the modified method of Davies et al. (16). One milliliter of test agent was placed into test tubes followed by the addition of 10 mL diethylene glycol reagent and 1 mL 1 N NaOH. The mixture was shaken vigorously and reacted in hot water at 37℃ for 60 min before absorbance at 420 nm was measured. A standard curve was prepared with rutin.
DPPH radical scavenging effects were evaluated according to the method of Blois (17). COME was dissolved in DMSO to final concentrations of 100, 500, and 1,000 μg/mL. One milliliter of the test agents were placed into each test tube followed by the addition of 4 mL of 4 × 10−4 M DPPH. The mixture was shaken vigorously and kept for 10 sec in hot water at 60℃ before absorbance at 525 nm was measured. BHT was used as the positive control. The free-radical-scavenging activity of each solution was then calculated as a percent of inhibition.
Cell culture. The melan-a cells used in this study were obtained from Dr. Dorothy Bennett (St. George’s Hospital, UK). These highly pigmented and immortalized cells were derived from C57BL/6 mice. The cells were grown in Roswell Park Memorial Institute medium (RPMI-1640) supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin, and 200 nM 12-O-tetradecanoylphorbol-13-acetate at 37℃ in an incubator with 10% CO2 for 72 hr.
MTT assay. The melan-a cells were divided in a 96-well plate (0.5 × 104 cells/well) and grown in the incubator at 37℃ with 10% CO2 for 24 hr. Then, 200 μL of COME diluted with RPMI-1640 medium to various concentrations (3.125, 6.25, 12.5, and 25.0 μg/mL) was placed in the wells, and the cells were grown in the incubator at 37℃ with 10% CO2 for 48 hr. Then, the cells were placed in medium containing 0.5 μg/mL MTT and grown in the incubator at 37℃ with 10% CO2 for 3 hr. After centrifuging the plate at 180 ×g for 10 min, the cells settled. The medium was removed, 200 μL of DMSO was added, and the cells were dissolved for 15 min on a plate-shaker. Absorbance was measured at 540 nm with an enzyme-linked immunosorbent assay (ELISA) reader.
Melanin assay. The melan-a cells were divided in a 96-well plate (2 × 104 cells/well) and grown in an incubator at 37℃ with 10% CO2 for 24 hr. Then, 200 μL of COME diluted with RPMI-1640 medium to concentrations of 1.563, 3.125, 6.25, and 12.5 μg/mL was put in the wells, and the cells were grown in the incubator with 10% CO2 at 37℃ for 72 hr. After the cells washed, the treatment was repeated. Next, the cells were dissolved in 1 N NaOH, and optical density was measured at 490 nm (OD 490) with an ELISA reader. Melanin content was estimated as the OD 490 value/μg of protein and expressed as a percentage relative to the untreated control value (100%).
Tyrosinase activity assay. For intracellular tyrosinase activity assay, melan-a cells were seeded in 60-mm cell culture dishes (4 × 105 cells/well) for 24 hr and then treated with 5 mL COME (0~12.5 μg/mL) for 48 hr. The cells were washed with phosphate buffer solution, detached with 200 μL 1% Triton X-100, transferred to Eppendorf tubes, extracted on ice with agitation, and centrifuged at 18,000 ×g for 20 min at 4℃. Thereafter, 100 μL L -DOPA was added, the mixture was incubated at 37℃ under 10% CO2 for 1 hr, and OD 490 was measured with an ELISA reader. Tyrosinase activity was estimated as the OD 490/μg protein/min and expressed as a percentage of the untreated control value (100%). For cell-extracted tyrosinase activity assay, after centrifugation of cultured melan-a cells, 50 μL supernatant was mixed with 49 μL 0.1 M phosphate buffer solution (pH 6.8) and 1 μL COME (0~12.5 μg/mL). L-DOPA (0.2%, 100 μL) was added, the absorbance measured, and the percentage activation was calculated.
Reverse transcription-polymerase chain reaction (RT-PCR). Total RNA was isolated with the Trizol-reagent (Life Technologies, CA, USA) according to the manufacturer’s instructions. Five micrograms of total RNA were reverse transcribed with 8 μL Moloney murine leukemia virus reverse transcriptase (M-MLV RT) 5 × buffer, 3 μL 10 mM deoxyribonucleotide triphosphates (dNTPs), 0.45 μL 40 U/μL RNase inhibitor, 0.3 μL 200 U/μL M-MLV RT (Promega, Madison, USA), and 1.5 μL 50 μM oligo dT (Bioneer, Cheongju, Korea) in a 40-μL volume. Single-stranded complementary DNA was then amplified via PCR with 4 μL 5 × green Go Taq Flexi buffer, 0.4 μL 10 mM dNTPs, 0.1 μL 5 U/μL Taq polymerase, 1.2 μL 25 mM MgCl2 (Promega), and 0.4 μL 20 μM each specific sense and anti-sense primers of tyrosinase, TRP-1, TRP-2, MITF-M, or β-Actin.
The primer sequences used for PCR were as follows: 5'-CAT TTT TGA TTT GAG TGT CT-3' (forward), 5'-TGT GGT AGT CGT CTT TGT CC-3' (reverse) for tyrosinase; 5'-GCT GCA GGA GCC TTC TTT CTC-3' (forward), 5'-AAG ACG CTG CAC TGC TGG TCT-3' (reverse) for TRP-1; 5'-GGA TGA CCG TGA GCA ATG GCC-3' (forward), 5'-CGG TTG TGA CCA ATG GGT GCC-3' (reverse) for TRR-2; 5'-TAC AGA AAG TAG AGG GAG GAG GAC TAA G-3' (forward), 5'-CAC AGT TGG AGT TAA GAG TGA GCA TAG CC-3' (reverse) for MITF-M; 5'-ACC GTG AAA AGA TGA CCC AG-3' (forward), 5'-TAC GGA TGT CAA CGT CAC AC-3' (reverse) for βActin. The expected sizes of the PCR product for tyrosinase, TRP-1, TRP-2, MITF-M, and β-Actin, respectively, were 1192, 268, 1044, 326, and 528 base pairs.
The following PCR conditions were applied: tyrosinase and TRP-1, 28 cycles of denaturation at 94℃ for 60 sec, annealing at 56℃ for 60 sec, and extension at 72℃ for 60 sec; TRP-2, 28 cycles of denaturation at 94℃ for 60 sec, annealing at 64℃ for 60 sec, and extension at 72℃ for 60 sec; MITF-M, 30 cycles of denaturation at 94℃ for 30 sec, annealing at 54℃ for 30 sec, and extension at 72℃ for 30 sec; β-Actin, 30 cycles of denaturation at 94℃ for 30 sec, annealing at 51℃ for 30 sec, and extension at 72℃ for 60 sec. The PCR products were analyzed on 1.2% agarose gel. β-Actin was used as an internal control to evaluate the relative expression of tyrosinase, TRP-1, TRP-2, and MITF-M.
Western blot analysis. Cell lysates were prepared by sonicating melan-a cells in 0.1 M Tris-HCl (pH 7.2) buffer containing 1% Nonidet P-40, 0.01% sodium dodecyl sulfate, and a protease inhibitor cocktail (Roche, Mannheim, Germany). The protein concentration of cell lysates was measured using a Pierce Protein Assay Kit (Pierce Biotechnology, Inc., Rockford, IL, USA) with bovine serum albumin as the standard. Equal amounts of protein (10 μg) were loaded onto each lane and separated with electrophoresis on a 10% polyacrylamide gel. After being transblotted onto nitrocellulose, the membranes were incubated with antibodies against tyrosinase/prolyl endoprotease-7 (PEP-7, 1:10,000 dilution), TRP-1/PEP-1 (1:10,000), and TRP-2/PEP-8 (1:10,000), which were kindly provided by Dr. Vincent J. Hearing (National Institutes of Health, USA). Next, the membranes were incubated with horseradish-peroxidase-conjugated anti-rabbit immunoglobulin G (1:1,000 dilution; Amersham Biosciences, Buckinghamshire, UK). Immunoreactive bands were detected with chemiluminescence using electrochemical luminescence reagents (Amersham Biosciences). β-Actin was used as an internal control for immunoblotting.
Statistical analysis. Differences in values between the groups were evaluated statistically using one-way analysis of variance followed by Duncan’s multiple range test for a post hoc comparison by using SPSS 21.0 for windows (IBM, Armonk, NY, USA). Statistical significance was defined as p < 0.05.
RESULTS
Antioxidant capability of COME. Polyphenol and flavonoid compounds in COME totaled 54.8 and 59.4 mg/g, respectively (Fig. 1). The electron-donating capabilities of BHT (the positive control) at 100, 500 and 1,000 μg/mL were 16.3%, 44.1%, and 68.4%, respectively (Fig. 2). Whereas those of COME were 64.9%, 95.1% and 95.7%, respectively. The electron-donating capability of COME was higher than that of BHT.
Fig. 1. Total polyphenol and flavonoid content of Cornus officinalis methanol extract (COME). Values are means ± standard deviation (SD) of three measurements.

Fig. 2. Electron-donating capabilities of 2,6-di-tert-butylate hydroxytoluene (BHT) and Cornus officinalis methanol extract (COME). Values are means ± SD of three measurements.
Cytotoxicity of COME in melan-a cells. Greater than 80% cell viability was observed at COME concentrations between 3.125 and 12.5 μg/mL, but cell viability was reduced to 75.5% at 25 μg/mL (Fig. 3). Therefore, the maximum permissible level for COME application to melan-a cells was 12.5 μg/mL. The maximum permissible level for IBMX application was also 12.5 μg/mL.
Fig. 3. Cell viability of melan-a cells treated with 3-isobutyl-1-methylxanthine (IBMX) and Cornus officinalis methanol extract (COME). Values are means ± SD of three measurements.

Effect of COME on melanin synthesis. Compared with the control cells, cells treated with COME at concentrations of 3.125, 6.25, and 12.5 μg/mL had melanin content that increased significantly by 16.3%, 28.0%, and 36.1%, respectively (p < 0.001), in a dose-dependent manner (Fig. 4). Treatment with IBMX at 12.5 μg/mL also increased melanin content significantly by 31.6% compared with melanin content in the control (p < 0.00l).
Fig. 4. Effect of Cornus officinalis methanol extract (COME) on the synthesis of melanin in melan-a cells. Values are means ± SD of three measurements. C: control, IBMX: 3-isobutyl-1-methylxanthine. Values with different superscripts are significantly different (p < 0.001) with analysis of variance and Duncan’s multiple range test. *p < 0.05 compared with the control group.

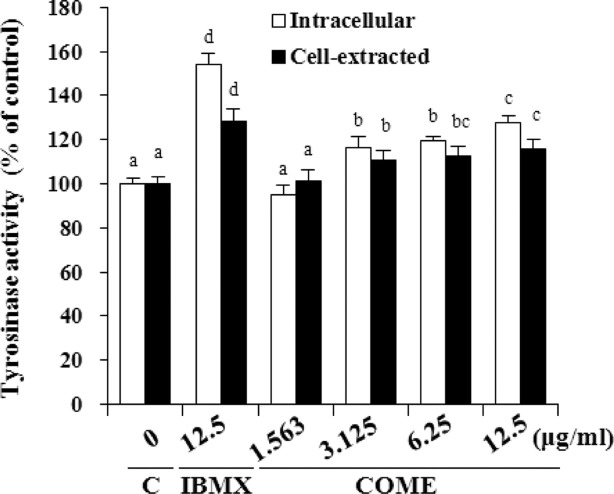
Effect of COME on tyrosinase activity. COME treatment at concentrations of 3.125, 6.25, and 12.5 μg/mL significantly increased intracellular tyrosinase activity by 16.4%, 19.8%, and 27.8%, respectively (p < 0.00l) (Fig. 5). Compared with that in the control group, intracellular tyrosinase activity in cells treated with 12.5 μg/mL IBMX also increased significantly by 54.2% (p < 0.00l). Cell-extracted tyrosinase activity in the cells treated with 3.125, 6.25, and 12.5 μg/mL COME increased significantly by 10.8%, 12.8%, and 15.7%, respectively (p < 0.001). Compared with that in the control, cell-extracted tyrosinase activity in the cells treated with 12.5 μg/mL IBMX increased significantly by 28.4% (p < 0.00l).
Fig. 5. Effect of Cornus officinalis methanol extract (COME) on tyrosinase activity in melan-a cells. COME was added to melan-a cells, and intracellular tyrosinase activity was measured after 60 min. COME was added to the cell extract and cell-extracted tyrosinase activity was measured after 60 min. Values are means ± SD of three measurements. C: control, IBMX: 3-isobutyl-1-methylxanthine. Values with different superscripts are significantly different (p < 0.001) with analysis of variance and Duncan’s multiple range test.
Effect of COME on messenger RNA (mRNA) and protein expression of tyrosinase, TRP-1, TRP-2, and MITF-M. IBMX and COME remarkably induced tyrosinase and MITFM mRNA expression (Fig. 6) but did not affect TRP-1 and TRP-2 at transcriptional levels. IBMX and COME affected tyrosinase, TRP-1, and TRP-2 at translational levels (Fig. 7).
Fig. 6. Effect of Cornus officinalis methanol extract (COME) on melanogenic gene expression in melan-a cells. COME upregulated tyrosinase (A) and microphthalmia-associated transcription factor-M (MITF-M; D) gene expression but did not affect tyrosinase-related protein 1 (TRP-1; B) and TRP-2 (C) gene expression. Values are means ± SD of three measurements. C: control, IBMX: 3-isobutyl-1-methylxanthine. Values with different superscripts are significantly different (p < 0.001) with analysis of variance and Duncan’s multiple range test.
Fig. 7. Effects of Cornus officinalis methanol extract (COME) on melanogenic protein expression in melan-a cells. COME upregulated tyrosinase (A), tyrosinase-related protein 1 (TRP-1; B), and TRP-2 (C) protein expression. Values are means ± SD of three measurements. C: control, IBMX: 3-isobutyl-1-methylxanthine. Values with different superscripts are significantly different (p < 0.001) with analysis of variance and Duncan’s multiple range test.
DISCUSSION
Continuous melanin synthesis during hair growth (anagen) generates hydrogen peroxideand other free radicals, thereby placing hair follicle melanocytes under high oxidative stress conditions that induce apoptosis of melanocytes in the pigmentary unit (18). Circumstantial evidence has shown that oxidative stress may be a pivotal mechanism contributing to hair graying and hair loss (10). Oxidative stress can be generated in or outside of hair follicle melanocytes by ultraviolet light and psychoemotional (19) and inflammatory (20) stress. Such stress may overwhelm hair follicle melanocyte antioxidant capacity and speed up the accumulation of terminal damage in aging hair follicles.
Phenolic acids are plant metabolites present throughout the plant kingdom. As polyphenol compounds, flavonoids have antioxidant, anti-inflammatory, and antiviral effects (21). DPPH radicals react with suitable reducing agents, electrons become paired off, and the solution loses color stoichiometrically with the number of electrons taken up (17). The DPPH radical has been widely used as a model system for investigations of the scavenging activities of natural compounds including phenolic compounds, flavonoids, or crude mixtures such as ethanol or water extracts of plants (22).
In the current study, the total polyphenol compound content in COME was 54.8 mg/g, which is similar to the 49.7 mg/g in C. officinalis hot water extract reported by Kim and Kim (23). The total flavonoid content in COME was 59.4 mg/g, which is much higher than the 5.67 mg/g in ethanol extract reported by Jeon et al. (24). The electron-donating capability of COME at 1,000 μg/mL was 95.7%, which is higher than that of BHT (68.4%) and that in C. officinalis hot water extract at the same concentration (42.7%) reported by Kim and Kim (23). These results indicate that COME is a potent antioxidant.
Melan-a cells derived from C57BL/6 mice are highly pigmented and immortalized cells with an abundance of tyrosinase enzyme and substantial melanin synthetic capability. These cells can be cultured for long periods (25). The dendrites of melanocytes stretch the melanocyte membrane to connect with keratinocytes, which allows the transfer of melanosomes into keratinocytes. The dendrite length can be more than twice the length of the melanocyte body due to overexpansion of the melanocyte itself. In the microscopic examination in the current study, COME effectively stimulated melanin synthesis and dendrite production. In the quantitative analysis, COME significantly increased melanogenesis (p < 0.001) as well as tyrosinase activity (p < 0.001) at concentrations above 3.125 μg/mL in a dosedependent manner. Cell-extracted tyrosinase activity was relatively weaker than intracellular tyrosinase activity. In general, a pigmenting effect can be promoted by increasing tyrosinase activity and melanin synthesis. Melanin synthesis is initiated by tyrosinase, which catalyzes the oxidation of tyrosine to DOPAchrome via a two-step reaction (5). Therefore, increases in tyrosinase activity in melanin synthesis account for increase in melanin levels (26).
At the concentrations above 3.125 μg/mL, COME significantly stimulated tyrosinase and TRP-2 protein expression (p < 0.001) but did not affect TRP-1 protein expression at the lower concentrations of 3.125 and 6.25 μg/mL (p > 0.05), although a significant increase in translation occurred at 12.5 μg/mL (p < 0.001). At concentrations above 3.125 μg/mL, COME significantly stimulated tyrosinase and MITF-M transcription (p < 0.001), but no significant induction was observed in TRP-1 and TRP-2 gene transcription. MITF is involved in the differentiation, growth, and survival of pigment cells via a number of signaling pathways. The increase in MITF-M expression induces the upregulation of the tyrosinase gene family, which leads to increased melanin synthesis (27). Choi et al. (28) reported that of particular interest, MITF-M is highly expressed in black hair but is not detected in white hair. The cAMP-mediated activation of protein kinase A induces the expression of MITF, a master transcriptional regulator for melanogenic enzymes and tyrosinase family proteins (29).
The results of the current study suggest that COME might act as a pigmenting agent through the upregulation of MITF-M in the cAMP-dependent melanogenic pathway. However, an explanation for the lack of significant TRP-1 and TRP-2 mRNA induction by COME and IBMX despite the upregulation of MITF-M transcription observed in this study remains to be elucidated through further detailed studies.
In conclusion, COME treatment of melan-a cells stimulated melanogenesis by increasing tyrosinase activity on the transcriptional and translational levels. COME also induced TRP-1 and TRP-2 translation with upregulation of MITF-M transcription. Therefore, COME might be useful as a natural black pigmentation agent for the prevention of hair graying. In addition, which biologically active components contained in COME are responsible for stimulating melanogenesis is need to be investigated further in the future.
References
- 1.Melov S. Animal models of oxidative stress, aging, and therapeutic antioxidant interventions. Int. J. Biochem. Cell Biol. (2002);34:1395–1400. doi: 10.1016/S1357-2725(02)00086-9. [DOI] [PubMed] [Google Scholar]
- 2.Commo S., Gaillard O., Bernard B.A. Human hair graying is linked to a specific depletion of hair follicle melanocytes affecting both the bulb and the outer root sheath. Br. J. Dermatol. (2004);150:435–443. doi: 10.1046/j.1365-2133.2004.05787.x. [DOI] [PubMed] [Google Scholar]
- 3.Slominski A., Tobin D.J., Shibahara S., Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. (2004);84:1152–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi T., Urabe K., Winder A., Jiménez-Cervantes C., Imokawa G., Brewington T., Solano F., García-Borrón J.C., Hearing V.J. Tyrosinase related protein 1 (TRP1) functions as a DHICA oxidase in melanin biosynthesis. EMBO J. (1994);13:5818–5825. doi: 10.1002/j.1460-2075.1994.tb06925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hearing V.J., Tsukamoto K. Enzymatic control of pigmentation in mammals. FASEB J. (1991);5:2902–2909. [PubMed] [Google Scholar]
- 6.Hoh J.F., Rossmanith G.H., Hamilton A.M. Effects of dibutyryl cyclic AMP, ouabain, and xanthine derivatives on cross bridge kinetics in rat cardiac muscle. Circ. Res. (1991);68:702–713. doi: 10.1161/01.RES.68.3.702. [DOI] [PubMed] [Google Scholar]
- 7.Park H.Y., Wu C., Yonemoto L., Murphy-Smith M., Wu H., Stachur C.M., Gilchrest B.A. MITF mediates cAMP-induced protein kinase C-β expression in human melanocytes. Biochem. J. (2006);359:571–578. doi: 10.1042/BJ20051388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertolotto C., Abbe P., Hemesath T.J., Bille K., Fisher D.E., Ortonne J.P., Ballotti R. Microphthalmia gene product as a signal transducer in cAMP-induced differentiation of melanocytes. J. Cell Biol. (1998);142:827–835. doi: 10.1083/jcb.142.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thibaut S., Collin C., Langbein L., Schweizer J., Gautier B., Bernard B.A. Hair keratin pattern in human hair follicles grown in vitro. Exp. Dermatol. (2003);12:160–164. doi: 10.1034/j.1600-0625.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- 10.Trüeb R.M. Oxidative stress in ageing of hair. Int. J. Trichology. (2009);1:6–14. doi: 10.4103/0974-7753.51923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim D.K., Kwak J.H. A furan derivative from Cornus officinalis. Arch. Pharm. Res. (1998);21:787–789. doi: 10.1007/BF02976779. [DOI] [PubMed] [Google Scholar]
- 12.Johns T., Mahunnah R.L., Sanaya P., Chapman L., Ticktin T. Saponins and phenolic content in plant dietary additives of a traditional subsistence community, the Batemi of Ngorongoro District, Tanzania. J. Ethnopharmacol. (1999);66:1–10. doi: 10.1016/S0378-8741(98)00179-2. [DOI] [PubMed] [Google Scholar]
- 13.Peng Q., Wei Z., Lau B.H. Fructus corni attenuates oxidative stress in macrophages and endothelial cells. Am. J. Chin. Med. (1998);26:291–300. doi: 10.1142/S0192415X98000336. [DOI] [PubMed] [Google Scholar]
- 14.Nawa Y., Endo J., Ohta T. The inhibitory effect of the components of Cornus officinalis on melanogenesis. J. Cosmet. Sci. (2007);58:505–517. [PubMed] [Google Scholar]
- 15.Folin O., Denis W. On phosphotungstic-phosphomolybdic compounds as color reagents. J. Biol. Chem. (1912);12:239–243. [Google Scholar]
- 16.Davies R., Massey R.C., Mcweeny D.J. The catalysis of the N-nitrosation of secondary amines by nitrosophenols. Food Chem. (1980);6:115–122. doi: 10.1016/0308-8146(80)90027-8. [DOI] [Google Scholar]
- 17.Blois M.S. Antioxidant determinations by the use of a stable free radical. Nature. (1958);181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- 18.Arck P.C., Overall R., Spatz K., Liezman C., Handjiski B., Klapp B.F., Birch-Machin M.A., Peters E.M. Towards a “free radical theory of graying”: melanocyte apoptosis in the aging human hair follicle is an indicator of oxidative stress induced tissue damage. FASEB J. (2006);20:1567–1569. doi: 10.1096/fj.05-4039fje. [DOI] [PubMed] [Google Scholar]
- 19.Irie M., Asami S., Nagato S., Ikeda M., Miyata M., Kasai H. Psychosocial factors as a potential trigger of oxidative DNA damage in human leukocytes. Jap. J. Cancer Res. (2001);92:367–376. doi: 10.1111/j.1349-7006.2001.tb01104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akar A., Arca E., Erbil H., Akay C., Sayal A., Gür A.R. Antioxidant enzymes and lipid peroxidation in the scalp of patients with alopecia areata. J. Dermatol. Sci. (2002);29:85–90. doi: 10.1016/S0923-1811(02)00015-4. [DOI] [PubMed] [Google Scholar]
- 21.Seyoum A., Asres K., El-Fiky F.K. Structure radical scavenging activity relationships of flavonoids. Phytochemistry. (2006);67:2058–2070. doi: 10.1016/j.phytochem.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Chang H.C., Huang G.J., Agrawal D.C., Kuo C.L., Wu C.R., Tsay H.S. Antioxidant activities and polyphenol contents of six folk medicinal ferns used as “Gusuibu”. Bot. Stud. (2007);48:397–406. [Google Scholar]
- 23.Kim H.J., Kim Y.C. Antidiabetic and renoprotective effects of Corni Fructus extract in db/db mice. Mol. Cell. Toxicol. (2010);6:135–142. doi: 10.1007/s13273-010-0020-7. [DOI] [Google Scholar]
- 24.Jeon Y.H., Kim M.H., Kim M.R. Antioxidative, antimutagenic, and cytotoxic activities of ethanol extracts from Cornus officinalis. J. Korean Soc. Food Sci. Nutr. (2008);37:1–7. doi: 10.3746/jkfn.2008.37.1.1. [DOI] [Google Scholar]
- 25.Sheffield M.V., Yee H., Dorvault C.C., Weilbaecher K.N., Eltoum I.A., Siegal G.P., Fisher D.E., Chhieng D.C. Comparison of five antibodies as markers in the diagnosis of melanoma in cytologic preparations. Am. J. Clin. Pathol. (2002);118:930–936. doi: 10.1309/EWK9-LUPR-6BC5-1GXV. [DOI] [PubMed] [Google Scholar]
- 26.Yokoyama K., Yasumoto K., Suzuki H., Shibahara S. Cloning of the human DOPAchrome tautomerase/tyrosinase-related protein 2 gene and identification of two regulatory regions required for its pigment cell-specific expression. J. Biol. Chem. (1994);269:27080–27087. [PubMed] [Google Scholar]
- 27.Levy C., Khaled M., Fisher D.E. MITF: master regulator of melanocyte development and melanoma oncogene. Trends Mol. Med. (2006);12:406–414. doi: 10.1016/j.molmed.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Choi Y.J., Yoon T.J., Lee Y.H. Changing expression of the genes related to human hair graying. Eur. J. Dermatol. (2008);18:397–399. doi: 10.1684/ejd.2008.0434. [DOI] [PubMed] [Google Scholar]
- 29.Buscà R., Ballotti R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigm. Cell Res. (2000);13:60–69. doi: 10.1034/j.1600-0749.2000.130203.x. [DOI] [PubMed] [Google Scholar]