Abstract
Extract from Gryllus bimaculatus crickets inhibits oxidation at the DNA level, with reduced production of 8-hydroxy-2'-deoxyguanosine (8-OHdG). Microarray analyses were performed with a rat 28K cDNA clone set array to identify the gene expression profiles of aged (10 months old) Wistar Kyoto rats treated for one month with 100 mg/kg G. bimaculatus ethanol extract to assess the effects. The extract produced a meaningful anti-edema effect, evident by the inhibition of creatinine phosphokinase activity. The weights of abdominal and ovarian adipose tissues were reduced and the proportion of unsaturated fatty acids in adipose tissues was increased in an extract dose-dependent manner. Compared with untreated control rats, rats treated with the extract displayed the upregulation of 1053 genes including Fas (tumor necrosis factor receptor superfamily, member 6), Amigo3 (adhesion molecule with an immunoglobulin-like domain), Reticulon 4, 3-hydroxy-3-methylglutaryl-coenzyme (Hmgcr; a reductase), related anti-fatigue (enzyme metabolism), and Rtn antioxidant, and the downregulation of 73 genes including Ugt2b (UDP glycosyltransferase 2 family), Early growth response 1, and Glycoprotein m6a. Data suggest that G. bimaculatus extract may have value in lessening the effects of aging, resulting in a differential gene expression pattern indicative of a marked stress response and lower expression of metabolic and biosynthetic genes.
Keywords: Anti-aging effect, G. bimaculatus, Wistar Kyoto rats, Microarray
INTRODUCTION
Water extracts of cricket (Gryllus bimaculatu, Gb) have been used in oriental medicine to treat fever and hypertension. Crickets are also reared in Korea as an amphibian food (1). The main components of Gb are protein; fat including essential fatty acids like oleic acid, linoleic acid and γ-linoleic acid; ash; and moisture (2). G. bimaculatus extract significantly decreases blood ethanol concentrations by enhancing liver mitochondrial alcohol metabolizing enzymes (3). Grasshoppers that are roasted can also be a food source, but these are not typically consumed in Korea. The observed adverse effect level (NOAEL) of Gb is reportedly higher than 5 g/kg body weight/day, as shown in a 13-week oral dose toxicity study in Sprague-Dawley rats (4).
Aging is a process of progressive decline in the physiological capacity of an organism, manifested by accumulated alteration and destabilization at the whole system level (5). Generally, oxidative stress is a cause of aging in lipid peroxidation, protein carbonyl content increase, and DNA damage. Aging is also associated with a differential gene expression pattern indicative of a marked or lower stress response of metabolic and biosynthetic genes (6). A recent report regarding protein and healthy aging showed that an evenly distributed protein diet provides a framework that allows older adults to benefit from the synergistic anabolic effect of protein and physical activity (7). Gb contains rich crude protein (52%), which can be one of these protein sources. Aging is not prevented with an antioxidant agent or fruits instead of a sufficient daily meal. Nevertheless, we compared Gb extract to blueberry as a positive control of anti-aging activity, which decreass D-galctose-induced oxidative stress and brain damage in rats (8), and its supplementation improved memory in middle-aged mice fed a high-fat diet (9).
In the present study, we demonstrate the potential value of Gb ethanol extract in lessening the deleterious aspects of aging in serum and the gene expression level of 10-month aged WKY rats, with treatment for one month.
MATERIALS AND METHODS
Materials. G. bimaculatus were reared on a cricket farm located in Jungsun, Kangwon-Do, South Korea. Crickets were freeze-dried in the Department of Agricultural Biology, National Academy of Agricultural Science, Korea.
Preparation of G. bimaculatus extract. To prepare the ethanol extract, 1kg dried G. bimaculatus was homogenized, soaked, and extracted three times with 70% ethanol by ultrasonification for 30 min. The samples were filtered through Whatman filter paper and concentrated by evaporation and freeze-drying. The dried powder was dissolved in saline prior to use. Blueberry (frozen, imported) was purchased in the commercial Mart, extracted with 70% ethanol by ultrasonification and freeze-dried in an identical manner.
Animals. Male, 10-month old Wistar Kyoto (WKY) rats weighing 469.9 ± 9.4 g, obtained from Japan SLC (Shizuoka, Japan), were divided into three groups of six rats. Control rats were purchased from Jung Ang Lab Animals (Seoul, Korea). All procedures were in accordance with the NIH Guidelines for Care and Use of Laboratory Animals. The rats were acclimated for one week under normal husbandry conditions (23 ± 2℃, 55 ± 10%, humidity and 12-hr light/dark cycle) and fed a standard diet (Rat and Mouse 18% 5L79; PMI Nutrition International, Brentwood, MO, USA) and water ad libitum. The rats were distributed into groups based on similar weights. The control group (I) was untreated. Group II was treated with 100 mg/kg G. bimaculatus ethanol extract in phosphate buffered saline. Group III was treated with 100 mg/kg blueberry ethanol extract. Each group was maintained for one month.
Organ weights. Absolute and relative (organ-to-body weight ratios) weights of the adrenal glands, kidneys, heart, liver, lung, spleen, stomach, pancreas, thymus, and ovaries were determined.
Measurement of blood pressure. Measurement of the direct model in vivo and the tail-cuff method (indirect) was performed on a weekly basis according to a previous report (10). Using a blood monitoring system (Japan Muromachi Co., MK-100), we monitored WKY rats that had received a single daily dose of Gb extract (100 mg/kg) orally (diluted in saline) over one month. Measurements were performed one week before, and four weeks after treatments.
Blood sampling and plasma assay. Following one month of treatment, blood (~3 mL) was collected from the posterior vena cava following light CO2 inhalation, and used for serum chemistry measurements of total protein, total bilirubin, glucose, glutamic pyruvic transaminase (GPT), glutamic oxaloacetic transaminase (GOT), γ-glutamyl transferase (GGT), alkaline phosphatase (ALP), creatinine phosphokinase (CK), lactic dehydrogenase (LDH), total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, blood urea nitrogen (BUN), creatinine, triglycerides, uric acid, sodium, potassium, and chloride. All were evaluated using a model 7060 automatic clinical analyzer (Hitachi, Tokyo, Japan).
Oxidative DNA (8-OHdG) damage quantitation. Among numerous types of oxidative DNA damage, the formation of 8-hydroxydeoxyguanosine (8-OHdG) was determined with an enzyme-linked immunoassay according to the manufacturer’s protocol (OxiselectTM oxidative DNA damage ELISA kit, Cell Biolabs, Inc., San Diego, CA, USA).
DNA microarray. Following histopathological analysis, microarray hybridization was performed on liver samples as described previously (11). Total RNA was isolated from the liver using a Qiagen RNeasy Midi Kit (Qiagen, Valencia, CA, USA). A regular microarray was carried out according to the manufacturer’s instructions for the FairPlayTM microarray labeling kit (Stratagene, La Jolla, CA, USA). Briefly, 20 μg total RNA was reverse-transcribed to single stranded cDNA. The cDNA was purified with ethanol precipitation and resuspended in 5 μl 2× coupling buffer, and coupled with 5 μL Cy3 or Cy5 dye for 1 hr in the dark. The labeled control liver cDNA and the treated liver cDNA were combined and purified. The labeled cDNA was mixed with 1.5 μL 10 μg/μL salmon DNA, 1.5 μL 8 μg/μL poly d(A), 1.5 μL 4 μg/μL yeast tRNA, 4.5 μL 20× SSC, and 0.75 μL 10% sodium dodecyl sulfate (SDS), heated at 99℃ for 2 min, and incubated at 45℃ for 15 min. The labeled DNA was loaded onto a microarray chip. A hybridization chamber was assembled with the microarray chip and submerged in a water bath overnight at 60℃. The microarray chip was washed in wash buffer I (2× SSC, 0.1% SDS) for 15 min, then in wash buffer II (1× SSC) for 5 min and wash buffer III (0.2× SSC) for 15 min. The slide was dried by centrifugation at 500 × g for 15 min, and scanned using a BMS Array Scanner and an applied precision Array WoRx eBiochip Reader (BioRad, Hercules, CA, USA) using the Cy3 and Cy5 channels (12).
Analysis of fatty acid composition of rat adipose tissue. For epididymidal and abdominal fat analysis, the concentration of 29 free fatty acids and fatty acid composition were analyzed in adipose tissues by gas chromatography-mass spectroscopy (GC-MS). Test tissue fatty acid of each removed adipose or epididymidal tissue (0.1 g) was prepared by chloroform: methanol (2:1) extraction over-night and the filtrated solution was removed under nitrogen gas. Subsequently, the lipids were saponified by alkaline hydrolysis of the phospholipid at 100℃ with 0.5 N methanolic sodium hydroxide, and methylated at 100℃ with 14% BF3 for 15 min. The top layer was transferred in petroleum ether and analyzed by GC/MS (Agilent 6890GC, Agilent 5973N mass detector, EI mode) with a HP-5 capillary column (Agilent Technologies, Palo Alto, CA, USA). The inlet temperature was 250℃ and the MS transfer line was kept constant at 230℃. The oven temperature was held at 180℃ for 20 min, then programmed at a 10℃/min increase to 230℃ and held 10 min. Quantification was achieved using a mixed 37 fatty acid standard: Sigma L9405, 10 rg/mL (Sigma-Aldrich Inc.), and linoleic acid (C18:2n6) as an internal standard.
Statistical analyses. The mean and standard error of all parameters studied were determined for each group using an ANOVA test. A Student’s t-test was carried out to determine significant differences between the control and treated groups. A p value < 0.05 was considered significant.
RESULTS
Clinical signs and food consumption. No deaths or adverse clinical signs were apparent due to the ingestion of the G. bimaculatus or blueberry extract. The level of food consumption was similar in all treated groups during the course of the study (data not shown).
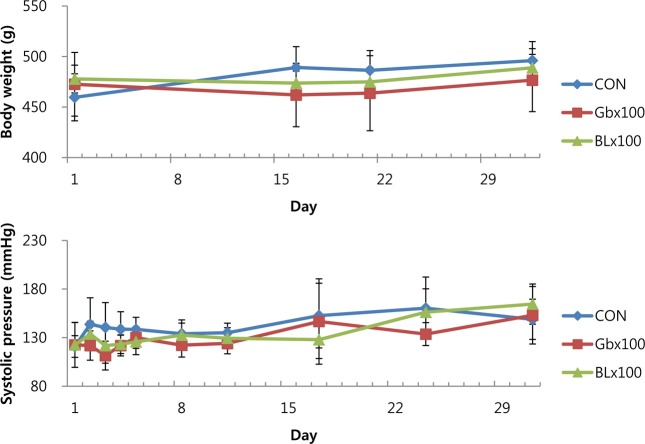
Body weight, blood pressure, and heart rate changes. There were no toxicologically significant differences in the mean body weight between the treatment groups (Fig. 1). During the 1-month administration period, the body weights of the male WKY rats in the treatment and control groups were comparable. The mean weekly body weight versus time data are presented in Fig. 1. No statistically significant differences in blood pressure (systolic blood pressure and heart rate) were observed between the 100 mg/kg G. bimaculatus extract-treated group and the control group.
Fig. 1. Effect of Gb extract on body weight and systolic blood pressure in 10-month old WKY rats treated orally with Gb ethanol extract for 1 month.
Hematology and blood chemistry. Some dose-dependent changes were observed between the treated and control groups with respect to the hematological parameters at the end of the experiment. An increase in activated partial thromboplastin time (PTT) was observed in the WKY rats in the treated groups (PTT: control, 37.2 ± 6.8 secs; G. bimaculatus extract 100 mg/kg, 44.2 ± 7.7 secs; blueberry extract 100 mg/kg, 32.2 ± 8.6 secs) but the differences were not significant. Minor changes were found in hematological parameters (eosinophils, neutrophils, lymphocytes, monocytes, and basophils) for some extract-treated (one side) male rat groups. However, the effects of the G. bimaculatus extract were not considered adverse because all changes in hematological data were within the normal physiological range (Table 1).
Table 1. Hematological findings of male WKY (10-month old) rats treated orally with Gb extract for 1 month.
| Item | Unit | CONa | Gb ex. 100 | BL ex.100 |
|---|---|---|---|---|
|
| ||||
| RBC | 103/mm3 | 9.14 ± 0.39 | 9.17 ± 0.24 | 9.07 ± 0.27 |
| WBC | 106/mm3 | 6.54 ± 2.26 | 7.72 ± 2.76 | 8.90 ± 2.08 |
| Hgb | g/dL | 16.93 ± 0.40 | 16.72 ± 0.33 | 16.50 ± 0.59 |
| Hct | % | 56.35 ± 2.56 | 56.10 ± 1.26 | 55.52 ± 2.45 |
| MCV | fL | 62.0 ± 0.5 | 61.2 ± 0.5 | 61.3 ± 1.5 |
| MCH | pg | 18.5 ± 0.5 | 18.3 ± 0.2 | 18.2 ± 0.1 |
| MCHC | g/dL | 30.0 ± 1.1 | 29.8 ± 0.2 | 29.8 ± 0.6 |
| PLT | 103/mm3 | 807.0 ± 73.3 | 775.6 ± 103.1 | 802.6 ± 16.8 |
| PTT | sec | 37.2 ± 6.8 | 44.2 ± 7.7 | 32.2 ± 8.6 |
| Thrombin time | sec | 37.2 ± 12.4 | 38.1 ± 5.34 | 44.0 ± 20.0 |
| Factor I | mg/dL | 333 ± 16.0 | 262 ± 57.4 | 275.3 ± 74.3 |
| PT | sec | 1.43 ± 0.04 | 1.44 ± 0.09 | 1.52 ± 0.05 |
| Neutrophil | % | 30.23 ± 6.88 | 28.08 ± 6.30 | 20.2 ± 2.67 |
| Lymphocyte | % | 67.1 ± 7.3 | 69.2 ± 6.5 | 76.5 ± 2.8 |
| Monocyte | % | 1.15 ± 0.31 | 1.38 ± 0.33 | 1.96 ± 0.27 |
| Eosinophil | % | 1.03 ± 0.39 | 0.88 ± 0.15 | 1.00 ± 0.188 |
| Basophil | % | 0.53 ± 0.17 | 0.46 ± 0.09 | 0.36 ± 0.09 |
Abbreviations:WBC, white blood cell; RBC, red blood cell; Hgb, hemoglobin; Hct, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; PLT, platelet. PTT, partial thromboplastin time; PT, prothrombin time.
aCON: PBS (as a vehicle) treated with murine normal diet.
Each value represents the mean ± S.E. * Statistically significant from the control (p < 0.05 vs. control).
Serum biochemistry. In the sera of the G. bimaculatus extract- and blueberry extract-treated WKY rat groups, creatinine phosphokinase (CK) levels were significantly lower than those in the control group after 1 month of treatment [control, 444.5 ± 297.9 IU/L; G. bimaculatus extract, 335.2 ± 179.1 IU/L (25% reduction); blueberry extract 309.0 ± 204.5 IU/L (30.5% reduction)]. Serum glucose levels were also lower versus the control (control, 218.6 ± 68.0 mg/dL; G. bimaculatus extract, 244.6 ± 50.3 mg/dL; blueberry extract, 273.6 ± 4.3 mg/dL), however the differences were not significant.
Table 2. Serological findings of male WKY (10-month old) rats treated orally with Gb extract for 1 month.
| Item | g/kg | CONa | GBex 100 | BL ex 100 |
|---|---|---|---|---|
|
| ||||
| Toal protein | g/dL | 7.76 ± 0.50 | 7.66 ± 0.31 | 7.18 ± 0.31 |
| CRP(HS) | 0.24 ± 0.11 | 0.24 ± 0.06 | 0.26 ± 0.06 | |
| ALP | IU/L | 117.8 ± 10.8 | 144 ± 6.88 | 12.97 ± 5.08 |
| AST | IU/L | 137.0 ± 37.8 | 133.2 ± 32.4 | 138.8 ± 20.6 |
| ALT | IU/L | 49.6 ± 10.4 | 49.6 ± 3.8 | 54.0 ± 13.8 |
| γGT | g/dL | 3 | 3 | 3 |
| CK | IU/L | 444.5 ± 297.9 | 335.2 ± 179.1 | 309.0 ± 204.5 |
| LDH | IU/L | 2452 ± 2029 | 1709 ± 1108 | 1372 ± 855 |
| Na | nmol/L | 136 ± 6.3 | 134 ± 4.4 | 134 ± 6.6 |
| K | nmol/L | 22.4 ± 8.4 | 25.4 ± 5.10 | 25.4 ± 8.05 |
| Cl | nmol/L | 95.2 ± 1.92 | 96.0 ± 2.0 | 96.0 ± 2.2 |
| BUN | mg/dL | 21.4 ± 2.19 | 17.5 ± 3.00 | 17.6 ± 1.01 |
| Uric acid | mg/dL | 5.74 ± 1.21 | 5.16 ± 0.46 | 4.82 ± 1.54 |
| T.Chol | mg/dL | 205.6 ± 26.2 | 203.4 ± 7.47 | 200.6 ± 17.01 |
| H.Chol | mg/dL | 177.4 ± 17.0 | 176.4 ± 2.8 | 175.0 ± 12.6 |
| TG | mg/dL | 73.8 ± 20.2 | 79.2 ± 7.0 | 81.2 ± 16.4 |
| Glucose | mg/dL | 218.6 ± 68.0 | 244.6 ± 50.3 | 273.6 ± 104.3 |
| Creatine | mg/dL | 0.64 ± 0.4 | 0.61 ± 0.02 | 0.68 ± 0.08 |
| Calcium | nmol/L | 11.86 ± 0.89 | 12.3 ± 0.18 | 11.96 ± 0.53 |
| IP | mg/dL | 166.7 ± 4.2 | 15.34 ± 3.31 | 16.88 ± 4.13 |
| Phoholipid | mg/dL | 216 ± 22.73 | 227 ± 8.01 | 222.8 ± 14.7 |
| Insulin | mU/mL | 0.2 | 0.2 | 0.2 |
| FFA | mEq/L | 749 ± 129.9 | 807.4 ± 189.0 | 642.6 ± 155.5 |
| Albumin | g/dL | 4.82 ± 0.24 | 4.92 ± 0.08 | 4.56 ± 0.21 |
| T. Bil | mg/dL | 0.16 ± 0.09 | 0.14 ± 0.06 | 0.14 ± 0.06 |
Abbreviations: ALP: alkaline phosphatase; AST(GOT), glutamate oxaloacetate transaminase; ALT(GPT), glutamate pyruvate transaminase; GGT, γ-glutamyl transferase; CK: creatinine phosphokinase; LDH, lactate dehydrogenase; Na, Sodium; K, potassium; Cl, chloride; BUN, blood urea nitrogen; T. Chol: total cholesterol; H. Chol: high cholesterol; l.Chol: low cholesterol; TG, triglyceride; Ca, calcium; IP, inorganic phosphorus.
aCON : PBS (vehicle) treated with murine normal diet.
Each value represents mean ± S.D. Statistically significant from control ( *p < 0.05).
DNA microarray. Microarray analysis using a mouse 28K cDNA clone set array was performed to identify the gene-expression profiles in the livers of G. bimaculatus extract-treated WKY rats, in order to clarify potential markers for aging. Compared with the control group, treated rats showed 1053 upregulated genes, including Fas (tumor necrosis factor receptor superfamily, member 6), Amigo3 (adhesion molecule with immunoglobulin-like domain), and Reticulon 4 (Rtn4) (Table 4). Rats treated with 100 mg/kg G. bimaculatus extract displayed the downregulation of 73 genes including UDP glycosyltransferase 2 (Ugt2b), Early growth response 1 (Egr1), and Glycoprotein m6a (Gpm6a) (Table 4). 3-Hydroxy-3-methylglutaryl-coenzyme (Hmgcr; a reductase), related anti-fatigue (enzyme metabolism), and Rtn antioxidant were also upregulated.
Table 4. Downregulated genes differentially expressed in the liver tissue of WKY 10-month aged rats treated with G. bimaculatus ethanol extract for 1 month.
| NO. | PairMean ratio* (test/control) | Description | Gene Symbol |
|---|---|---|---|
|
| |||
| 1 | 0.562 | UDP glycosyltransferase 2 family, polypeptide B | Ugt2b |
| 2 | 0.588 | RT1 class Ia, locus A2 /// RT1 class I, locus A3 /// RT1 class Ib, locus EC2 | RT1-A2 /// RT1-A3 /// RT1-EC2 |
| 3 | 0.636 | Ubiquitin D | Ubd |
| 4 | 0.668 | Glycoprotein m6a | Gpm6a |
| 5 | 0.690 | Early growth response 1 | Egr1 |
| 6 | 0.703 | sprouty homolog 2 (Drosophila) | Spry2 |
| 7 | 0.703 | Serine dehydratase | Sds |
| 8 | 0.711 | Insulin-like growth factor binding protein 1 | Igfbp1 |
| 9 | 0.725 | Elastin | Eln |
| 10 | 0.728 | Dual specificity phosphatase 1 | Dusp1 |
| 11 | 0.731 | Similar to interferon-inducible GTPase | RGD1309362 |
| 12 | 0.734 | Myelocytomatosis oncogene | Myc |
| 13 | 0.734 | EGF-like, fibronectin type III and laminin G domains | Egflam |
| 14 | 0.735 | MAX gene associated | Mga |
| 15 | 0.742 | Insulin receptor substrate 2 | Irs2 |
| 16 | 0.745 | HtrA serine peptidase 3 | Htra3 |
| 17 | 0.746 | Similar to interferon-inducible GTPase | MGC108823 |
| 18 | 0.750 | RT1 class Ib, locus EC2 | RT1-EC2 |
| 19 | 0.751 | Nuclear receptor subfamily 1, group D, member 2 | Nr1d2 |
| 20 | 0.752 | Hemoglobin, zeta | Hbz |
| 21 | 0.753 | Activating transcription factor 3 | Atf3 |
| 22 | 0.754 | Hypothetical protein LOC680687 | LOC680687 |
| 23 | 0.762 | Phosphorylase kinase, alpha 1 | Phka1 |
| 24 | 0.763 | AF4/FMR2 family, member 4 | Aff4 |
| 25 | 0.767 | TSPY-like 4 | Tspyl4 |
| 26 | 0.769 | UBX domain protein 4 | Ubxn4 |
| 27 | 0.769 | Similar to putative protein (5S487) | RGD1310819 |
| 28 | 0.770 | Tissue factor pathway inhibitor 2 | Tfpi2 |
| 29 | 0.772 | Interferon (alpha, beta and omega) receptor 1 | Ifnar1 |
| 30 | 0.772 | KRIT1, ankyrin repeat containing | Krit1 |
PairMean ratio* means pair Mean ratio (test/control).
Table 3. Upregulated genes differentially expressed in the liver tissue of WKY 10-month aged rats treated with G. bimaculatus ethanol extract for 1 month.
| NO. | PairMean ratio* (test/control) | Description | Gene symbol |
|---|---|---|---|
|
| |||
| 1 | 1.479 | Sema domain, immunoglobulin domain (Ig), short basic domain, (semaphorin) 3C | Sema3c |
| 2 | 1.361 | Similar to hypothetical protein MGC52110 | RGD1565095 |
| 3 | 1.349 | Calsequestrin 1 (fast-twitch, skeletal muscle) | Casq1 |
| 4 | 1.335 | 3-Hydroxy-3-methylglutaryl-Coenzyme A reductase | Hmgcr |
| 5 | 1.315 | ADP-ribosylation factor guanine nucleotide-exchange factor 1 | Arfgef1 |
| 6 | 1.311 | HAUS augmin-like complex, subunit 1 | Haus1 |
| 7 | 1.310 | Reticulon 4 | Rtn4 |
| 8 | 1.310 | Ligase III, DNA, ATP-dependent | Lig3 |
| 9 | 1.308 | WD repeat domain 89 | Wdr89 |
| 10 | 1.301 | Hypothetical protein LOC690785 | LOC690785 |
| 11 | 1.301 | Kelch-like 5 (Drosophila) | Klhl5 |
| 12 | 1.300 | Myeloid or mixed-lineage leukemia (trithorax homolog, Drosophila); translocated to, 3 | Mllt3 |
| 13 | 1.295 | Endothelin receptor type A | Ednra |
| 14 | 1.291 | URB2 ribosome biogenesis 2 homolog (S. cerevisiae) | Urb2 |
| 15 | 1.288 | Casein kinase 1, epsilon | Csnk1e |
| 16 | 1.287 | Mastermind like 2 (Drosophila) | Maml2 |
| 17 | 1.287 | Solute carrier family 2, (facilitated glucose transporter) member 8 | Slc2a8 |
| 18 | 1.286 | Fas (TNF receptor superfamily, member 6) | Fas |
| 19 | 1.283 | Claudin 1 | Cldn1 |
| 20 | 1.275 | Toll-like receptor 4 | Tlr4 |
| 21 | 1.270 | Adaptor-related protein complex 1, sigma 3 subunit | Ap1s3 |
| 22 | 1.270 | Chemokine (C-C motif) ligand 24 | Ccl24 |
| 23 | 1.270 | Adhesion molecule with Ig like domain 3 | Amigo3 |
| 24 | 1.269 | Eph receptor A2 | Epha2 |
| 25 | 1.268 | Vacuolar protein sorting 52 homolog (S. cerevisiae) | Vps52 |
| 26 | 1.268 | Similar to hypothetical protein MGC47256 | RGD1308694 |
| 27 | 1.268 | Secernin 1 | Scrn1 |
| 28 | 1.267 | tRNA methyltransferase 61 homolog A (S. cerevisiae) | Trmt61a |
| 29 | 1.256 | Growth differentiation factor 10 | Gdf10 |
| 30 | 1.254 | Spermatogenesis associated 5 | Spata5 |
*Corrected for background intensity using local background correction.
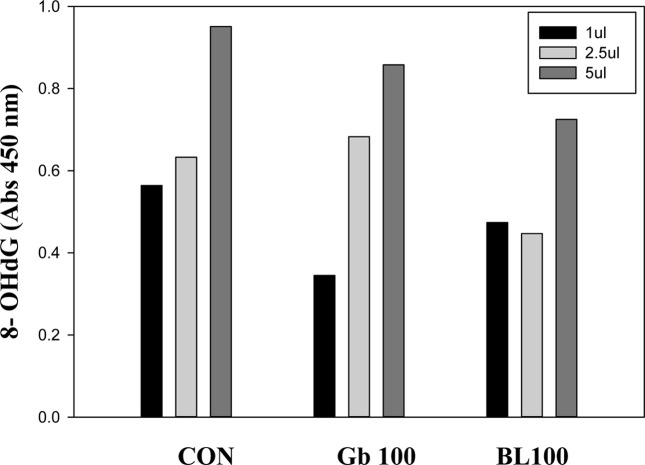
DNA oxidative damage (8-OHdG) quantitation. The formation of 8-hydroxydeoxyguanosine (8-OHdG) in serum was decreased in a treatment dose-dependent manner showing a decline in DNA oxidative damage (Fig. 2).
Fig. 2. 8-OHdG level in 10-month old WKY rats treated orally with Gb ethanol extract for 1 month. Gb100 or BL100 corresponds to the 100 mg/kg Gb ethanol or 100 mg/kg BL ethanol extract-treated group.
Fatty acid composition in rat adipose tissue. The fatty acid profile as indicated by GC-MS, showed a slight decrease in the arachidonic acid (C20: 4n6, AFA) concentration in epididymidal and abdominal fat of the male WKY rats in the Gb extract-fed group over a 1-month period compared with the control group (Table 5 and 6). There were also increases in the unsaturated fatty acid (FA) ratio especially single (mono) FA and decreases in saturated fatty acids in the Gb extract-fed groups.
Table 5. Fatty acid composition of abdominal fat of WKY 10-month aged rats treated with G. bimaculatus ethanol extract for 1 month.
| Fatty acid | Control | Gb extract | Blueberry extract |
|---|---|---|---|
|
| |||
| Myristoleic acid (C14:1) | 0.10 ± 0.01 | 0.22 ± 0.05* | 0.10 ± 0.03 |
| Myristic acid (C14:0) | 1.32 ± 0.23 | 1.48 ± 0.17 | 1.15 ± 0.17 |
| Pentadecanoic acid (C15:0) | 0.41 ± 0.06 | 0.35 ± 0.06 | 0.36 ± 0.05 |
| Palmitoleic acid (C16:1) | 4.76 ± 0.56 | 6.66 ± 3.41 | 5.84 ± 1.33 |
| Palmitic acid (C16:0) | 19.59 ± 0.37 | 19.67 ± 1.08 | 20.42 ± 1.62 |
| Heptadecanoic acid (C17:0) | 0.34 ± 0.03 | 0.30 ± 0.06 | 0.35 ± 0.01 |
| Linoleic acid (C18:2) | 42.90 ± 3.87 | 42.76 ± 1.92 | 44.81 ± 0.69 |
| Oleic acid (C18:1) | 24.07 ± 3.00 | 22.62 ± 3.19 | 21.13 ± 3.25 |
| Stearic acid (C18:0) | 4.07 ± 0.16 | 3.47 ± 1.13 | 3.32 ± 0.61 |
| Arachidonic acid (C20:4) | 0.83 ± 0.08 | 0.78 ± 0.07 | 0.86 ± 0.02 |
| Eicosapentaenoic acid (C20:5) | 0.13 ± 0.01 | 0.12 ± 0.03 | 0.14 ± 0.02 |
| Eicosatrienoic acid (C20:3) | 0.21 ± 0.01 | 0.22 ± 0.05 | 0.22 ± 0.01 |
| Eicosadienoic acid (C20:2) | 0.25 ± 0.02 | 0.25 ± 0.02 | 0.25 ± 0.01 |
| Eicosenoic acid (C20:1) | 0.40 ± 0.02 | 0.42 ± 0.05 | 0.37 ± 0.02 |
| Eicosanoic acid (C20:0) | 0.09 ± 0.01 | 0.10 ± 0.03 | 0.08 ± 0.01 |
| Docosahexaenoic acid (C22:6) | 0.53 ± 0.03 | 0.57 ± 0.22 | 0.60 ± 0.06 |
| Saturated fatty acid | 25.82 ± 0.25 | 25.38 ± 2.42 | 25.68 ± 1.34 |
| Unsaturated fatty acid (UFA) | 74.18 ± 0.25 | 74.62 ± 2.42 | 74.32 ± 1.34 |
| Single UFA | 29.33 ± 3.56 | 29.92 ± 0.56 | 27.43 ± 2.01 |
| Poly UFA | 44.85 ± 3.76 | 44.70 ± 2.11 | 46.89 ± 0.71 |
Each value represents mean ± S.D.
Asterisk (*) indicates significant difference compared with the control (p < 0.05).
Table 6. Fatty acid composition of epididymal fat of WKY 10-month aged rats treated with G. bimaculatus ethanol extract for 1 month.
| Control | GE extract | Blueberry extract | |
|---|---|---|---|
|
| |||
| Myristoleic acid (C14:1) | 0.15 ± 0.09 | 0.08 ± 0.02 | 0.11 ± 0.04 |
| Myristic acid (C14:0) | 1.49 ± 0.15 | 1.19 ± 0.28 | 1.51 ± 0.47 |
| Pentadecanoic acid (C15:0) | 0.42 ± 0.08 | 0.34 ± 0.04 | 0.43 ± 0.08 |
| Palmitoleic acid (C16:1) | 5.73 ± 2.11 | 4.03 ± 1.12 | 4.34 ± 0.34 |
| Palmitic acid (C16:0) | 21.15 ± 3.92 | 19.00 ± 5.21 | 22.47 ± 2.42 |
| Heptadecanoic acid (C17:0) | 0.31 ± 0.07 | 0.33 ± 0.08 | 0.41 ± 0.11 |
| Linoleic acid (C18:2) | 43.46 ± 5.90 | 44.23 ± 1.36 | 40.06 ± 5.58 |
| Oleic acid (C18:1) | 21.04 ± 3.84 | 24.07 ± 4.94 | 23.20 ± 3.04 |
| Stearic acid (C18:0) | 3.85 ± 1.32 | 4.55 ± 0.86 | 04.83 ± 1.30 |
| Arachidonic acid (C20:4) | 0.80 ± 0.26 | 0.73 ± 0.05 | 0.85 ± 0.18 |
| Eicosapentaenoic acid (C20:5) | 0.12 ± 0.04 | 0.11 ± 0.00 | 0.13 ± 0.03 |
| Eicosatrienoic acid (C20:3) | 0.21 ± 0.07 | 0.19 ± 0.03 | 0.22 ± 0.05 |
| Eicosadienoic acid (C20:2) | 0.28 ± 0.08 | 0.25 ± 0.06 | 0.30 ± 0.05 |
| Eicosenoic acid (C20:1) | 0.45 ± 0.03 | 0.46 ± 0.10 | 0.58 ± 0.11 |
| Eicosanoic acid (C20:0) | 0.12 ± 0.03 | 0.12 ± 0.03 | 0.15 ± 0.02 |
| Docosahexaenoic acid (C22:6) | 0.43 ± 0.26 | 0.35 ± 0.03 | 0.40 ± 0.11 |
| Saturated fatty acid | 27.33 ± 5.51 | 25.67 ± 4.93 | 29.89 ± 3.42 |
| Unsaturated fatty acid (UFA) | 72.67 ± 5.51 | 74.33 ± 4.93 | 70.11 ± 3.42 |
| Single UFA | 27.33 ± 2.31 | 28.33 ± 4.16 | 28.12 ± 2.79 |
| Poly UFA | 45.67 ± 6.66 | 45.67 ± 1.53 | 41.99 ± 6.02 |
DISCUSSION
Aging can be defined as a process of progressive decline in the physiological capacity of an organism, manifested by accumulated alteration and destabilization at the whole system level (5). In seeking to lessen the deleterious effects of aging, safer and more effective drugs developed from substances obtained from natural products including sericulture products and crude insect-based preparations, have been studied (13,14). Slowing aging using a range of approaches has included ingestion of anti-oxidants for repairing oxidative damage in DNA, protein, and lipid, as well as stem cell-mediated tissue regeneration, and gene therapy (15-17).
DNA microarray analysis is a powerful tool for examining the mechanisms of complex conditions including aging. The gene expression profile of the gastrocnemius muscle from 5- and 30-month old male C57BL/6 mice revealed that aging is associated with alterations at the mRNA level (6).
Gb extract contains many components such as amino acids, nucleic acids, sugar, lipid and some active substances (unpublished data). Thus, it can access multiple receptors and can prevent the causes of aging due to the fact that it contains amino acids as a protein source for healthy aging (7). For instance, Gb extract also contains trehalose (insect sugar), chitosan derivatives, essential fatty acids, nucleic acids and grypin (anti-hypertensive agent). Therefore, Gb extract showed a critical anti-aging effect at the DNA oxidative damage decrease level according to the reduction of 8-hydroxy-2'-deoxyguanosine. In addition, creatinine phosphokinase activity exhibited a 25% reduction with an antiedema effect on 10-month old male Wistar Kyoto rats in the present aging study. The glucose level of the treated groups was slightly increased, 11.9% with the Gb extract and 25% with the blueberry extract (25%), however this increase was not statistically significant compared with the control group. In the present study, with respect to the serum glucose level, Gb extract has a lesser effect than blueberry extract.
Furthermore, data generated from the DNA microarray supported the anti-aging effect of the extracted G. bimaculatus, with meaningful gene expression profiles in WKY rats being observed after a 1-month treatment. Fas (pro-apoptotic and matrix-degradation gene expression following induction of osteoarthritis in mature and aged rabbits (18), Amigo3 (19), and Rtn4 (whose expression protects SHSY5Y cells against multiple death insults (20) were upregulated (Table 4). Ugt2b, Egr1, and Gpm6a were downregulated (Table 5). Ugt2b and Egr1 play an important role in the regulation of cell proliferation, differentiation, and angiogenesis (21,22). Gpm6q, the sole member of the proteolipid protein family of tetraspan proteins to be expressed exclusively by neurons in the central nervous system (23,24), was down-regulated 0.67 fold.
In conclusion, G. bimaculatus extract has potential efficacy in the treatment of aging in WKY rats by significantly reducing CK in serum. Consequently, G. bimaculatus extract may be a protective nutraceutical for aging-related disorders including circulatory disorders. The gene expression profile of WKY rats treated with G. bimaculatus extract could be a valuable prognostic marker to identify potential therapeutic targets for aging.
Acknowledgments
This work was supported by the Rural Development Administration National Research project, PJ009827.
References
- 1.Ahn M.Y., Bae H.J., Kim I.S., You E.J., Kwack S.J., Kim H.S., Kim D.H., Ryu K.S., Lee H.S., Kim J.W., Kim I., Lee B.M. Genotoxic evaluation of the biocomponents of the cricket, Gryllus bimaculatus, using three mutagenicity tests. J. Toxicol. Environ. Health Part A. (2005);68:2111–2118. doi: 10.1080/15287390500182537. [DOI] [PubMed] [Google Scholar]
- 2.Ahn M.Y., Ryu K.S., Park B.Y., Kim D.W., Kim I., Kim S.H. Effects on cricket supplements on the chicken meats and its eggs. Korean J. Poult. Sci. (2000);27:197–202. [Google Scholar]
- 3.Lee Y.W., Lim S.S., Ryu K.S., Lee H.S., Kim I.S., Kim J.W., Ahn M.Y. Effect of water and methanol extracts of cricket (Gryllus bimaculatus) on alcohol metabolism. Korean J. Pharmacogn. (2004);35:175–178. [Google Scholar]
- 4.Ahn M.Y., Han J.W., Kim S.J., Hwang J.S., Yun E.Y. Thirteen-week oral dose toxicity study of G. bimaculatus in Sprague-Dawley rats. Toxicol. Res. (2011);27:231–240. doi: 10.5487/TR.2011.27.4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hou L., Huang J., Green C.D., Boyd-Kirkup J., Zhang W., Yu X., Gong W., Zhou B., Han J.D. Systems biology in aging: linking in old and the young. Curr. Genomics. (2012);13:558–565. doi: 10.2174/138920212803251418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weindruch R., Kayo T., Lee C.K., Prolla T.A. Microarray profiling of gene expression in aging and its alteration by caloric restriction in mice. J. Nutr. (2001);131:918S–923S. doi: 10.1093/jn/131.3.918S. [DOI] [PubMed] [Google Scholar]
- 7.Paddon-Jones D., Campbell W.W., Jacques P.F., Kritchevsky S.B., Moore L.L., Rodriguez N.R., van Loon L.J. Protein and healthy aging. Am. J. Clin. Nutr. (2015) doi: 10.3945/ajcn.114.084061. Pii: ajcn084061. [DOI] [PubMed] [Google Scholar]
- 8.Çoban J., Doğan-Ekici I., Aydın A.F., Betül-Kalaz E., Doğru-Abbasoğlu S., Uysal M. Blueberry treatment decreased D-galactose-induced oxidative stress and brain damage in rats. Metab. Brain Dis. (2015);30:793–802. doi: 10.1007/s11011-014-9643-z. [DOI] [PubMed] [Google Scholar]
- 9.Carey A.N., Gomes S.M., Shukitt-Hale B. Blueberry supplementation improves memory in middle-aged mice fed a high-fat diet. J. Agric. Food Chem. (2014);62:3972–3978. doi: 10.1021/jf404565s. [DOI] [PubMed] [Google Scholar]
- 10.Ahn M.Y., Jung Y.S., Jee S.D., Kim C.S., Lee S.H., Moon C.H., Cho S.I., Lee B.M., Ryu K.S. Anti-hypertensive effect of the Dongchunghacho, Isaria sinclairii, in the spontaneously hypertensive rats. Arch. Pharm. Res. (2007);30:493–501. doi: 10.1007/BF02980225. [DOI] [PubMed] [Google Scholar]
- 11.Ueno T., Fukuda N., Nagase H., Tsunemi A., Tahira K., Matsumoto T., Hiraoka-Yamamoto J., Ikeda K., Mitsumata M., Sato Y., Soma M., Matsumoto K., Yamori Y. Atherogenic dyslipidemia and altered hepatic gene expression in SHRSP.Z-Leprfa/IzmDmer rats. Int. J. Mol. Med. (2009);23:313–320. doi: 10.3892/ijmm_00000133. [DOI] [PubMed] [Google Scholar]
- 12.Song J., Liu H., Ressom H.W., Tiwari S., Ecelbarger C.M. Chronic Rosigiltazone therapy normalizes expression of ACE1, SCD1 and other genes in the kidney of obese Zucker rats as determined by microarray by microarray analysis. Exp. Clin. Endocrinol. Diabetes. (2008);116:315–325. doi: 10.1055/s-2008-1042429. [DOI] [PubMed] [Google Scholar]
- 13.Lin W.S., Chen J.Y., Wang J.C., Chen L.Y., Lin C.H., Hsieh T.R., Wang M.F., Fu T.F., Wang P.Y. The anti-aging effects of Ludwigia octovalvis on Drosophila melanogaster and SAMP8 mice. Age (Dordrecht) (2014);36:689–703. doi: 10.1007/s11357-013-9606-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jafari M., Felgner J.S., Bussel I.I., Hutchili T., Khodayari B., Rose M.R., Vince-Cruz C., Mueller L.D. Rhodiola: a promising anti-aging Chinese herb. Rejuvenation Res. (2007);10:587–602. doi: 10.1089/rej.2007.0560. [DOI] [PubMed] [Google Scholar]
- 15.Yan L.J. Positive oxidative stress in aging and aging related disease tolerance. Redox Biol. (2014);2:165–169. doi: 10.1016/j.redox.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan G.C. Role of heat shock proteins in stem cell behavior. Prog. Mol. Biol. Transl. Sci. (2012);111:305–322. doi: 10.1016/B978-0-12-398459-3.00014-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosaad Y.M. Hematopoietic stem cells, an overview. Tansfus. Apher Sci. (2014);51:68–82. doi: 10.1016/j.transci.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 18.Robertson C.M., Pennock A.T., Harwood F.L., Pomerleau A.C., Allen R.T., Amiel D. Characterization of pro-apoptotic and matrix degradative gene expression following induction of osteoarthritis in mature and aged rabbits. Osteoarthritis Cartilage. (2006);14:471–476. doi: 10.1016/j.joca.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Kuja-Panula J., Kiiltomäki M., Yamashiro T., Rouhiainen A., Rauvala H. AMIGO, a transmembrane protein implicated in axon tract development, defines a novel protein family with leucine-rich repeats. J. Cell Biol. (2003);160:963–973. doi: 10.1083/jcb.200209074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teng F.Y., Tang B.L. Nogo/RTN4 isoforms and RTN3 expression protect SH-SY5Y cells against multiple death insults. Mol. Cell. Biochem. (2013);384:7–19. doi: 10.1007/s11010-013-1776-6. [DOI] [PubMed] [Google Scholar]
- 21.Cho Y.E., Moon P.G., Lee J.E., Singh T.S., Kang W., Lee H.C., Lee M.H., Kim S.H., Baek M.C. Integrative analysis of proteomic and transcriptomic data for identification of pathways related to simvastatin-induced hepatotoxicity. Proteomics. (2013);13:1257–1275. doi: 10.1002/pmic.201200368. [DOI] [PubMed] [Google Scholar]
- 22.Guo B., Tian X.C., Li D.D., Yang Z.Q., Cao H., Zhang Q.L., Liu J.X., Yue Z.P. Expression, regulation and function of Egr1 during implantation and decidualization in mice. Cell Cycle. (2014);13:2626–2640. doi: 10.4161/15384101.2014.943581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper B., Fuchs E., Flügge G. Expression of the axonal membrane glycoprotein M6a is regulated by chronic stress. PLoS One. (2009);4:e3659. doi: 10.1371/journal.pone.0003659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alfonso J., Frick L.R., Silberman D.M., Palumbo M.L., Genaro A.M., Frasch A.C. Regulation of hippocampal gene expression is conserved in two species subjected to different stressors and antidepressant treatments. Biol. Psychiatry. (2006);59:244–251. doi: 10.1016/j.biopsych.2005.06.036. [DOI] [PubMed] [Google Scholar]