Abstract
The skin exposure to solar irradiation and photoreactive xenobiotics may produce abnormal skin reaction, phototoxicity. Phototoxicity is an acute light-induced response, which occurs when photoreacive chemicals are activated by solar lights and transformed into products cytotoxic against the skin cells. Multifarious symptoms of phototoxicity are identified, skin irritation, erythema, pruritis, and edema that are similar to those of the exaggerated sunburn. Diverse organic chemicals, especially drugs, are known to induce phototoxicity, which is probably from the common possession of UV-absorbing benzene or heterocyclic rings in their molecular structures. Both UVB (290~320 nm) and UVA (320~400 nm) are responsible for the manifestation of phototoxicity. Absorption of photons and absorbed energy (hv) by photoactive chemicals results in molecular changes or generates reactive oxygen species and depending on the way how endogenous molecules are affected by phototoxicants, mechanisms of phototoxcity is categorized into two modes of action: Direct when unstable species from excited state directly react with the endogenous molecules, and indirect when endogeneous molecules react with secondary photoproducts. In order to identify phototoxic potential of a chemical, various test methods have been introduced. Focus is given to animal alternative test methods, i.e., in vitro, and in chemico assays as well as in vivo. 3T3 neutral red uptake assay, erythrocyte photohemolysis test, and phototoxicity test using human 3-dimensional (3D) epidermis model are examples of in vitro assays. In chemico methods evaluate the generation of reactive oxygen species or DNA strand break activity employing plasmid for chemicals, or drugs with phototoxic potential.
Keywords: Phototoxicity, UV, Animal alternative test method, Sunlight, In vitro toxicology
DEFINITION OF PHOTOTOXICITY
Terrestrial animals that include human, are constantly exposed to the irradiation of sunlight. Accordingly, the skin, which is located on the front-line that delineates the interior from exterior of the body,is the most affected by sunlight. Indeed, sunlight-induced skin burn, rash (erythema), hyperpigmentation, photoaging and skin cancers are the most commonly observed skin-associated problems (1). Incidentally, skin is the organ with the largest surface area in the body (1.5~2.0 m2) and frequently exposed to exogenous xenobiotics. Phototoxicity develops when photoactive or photoreactive chemicals come in contacts with, are absorbed into the skin and activated by sunlight, of which products have cytotoxic potential against the skin tissue (Fig. 1).
Fig. 1. Phototoxicity is initiated when photoactive or photoreactive chemicals are activated by UV lights. Photoreactivity is exemplified by photo-addition, production of reactive oxygen species (ROS). In final consequence, the reactions which derived by solar UV irradiation cause cytotoxicity against skin cells and, ultimately, skin irritation occurs.

Phototoxicity can be also manifested by systemically absorbed chemicals that find their ways to the skin and activated by incident sunlight as can be evidently exemplified by systemically administered flouroquinolone antibiotics (2). Accordingly, to cover this phototoxicant category, phototoxicity is defined in OECD TG 432 (Test No. 432: In vitro 3T3 NRU Phototoxicity Test) as “a toxic response from a substance applied to the body which is either elicited or increased (apparent at lower dose levels) after subsequent exposure to light, or that is induced by skin irradiation after systemic administration of a substance” (3). Phototoxicity is generally an acute light-induced responses of the skin to photoreactive chemicals. Therefore, the most common clinical manifestations of phototoxicity coincide with the symptoms of skin irritation or exaggerated sunburn, i.e., erythema, pruritis and edema (4). In distinction from phototoxicity, photosensitization or photoallergenicity is also referred along with photo-irritation as subcategories of phototoxicity (5). Difference of photosensitization from photoirritation is the presence of immune responses through the recognition of photo-haptenized products by antigen-presenting cells or T cells, and elicitation of allergic reaction upon repeated exposure. Generally, chemicals with photoirritation potential may induce both photo-irritation and photosensitization. In the current paper, focus is given mainly to phototoxicity as a broader term.
Phototoxic chemicals. A wide spectrum of organic chemicals, especially drugs possess a concern of phototoxicity probably because of the common existence of UV-absorbing benzene or heterocyclic rings as chromophores in their structures (6,7). Examples can be readily found in various classes of drugs (8); antibiotics (quinolones (9), tetracyclines and sulfonamides), antihistamines, anti-malarial drugs (quinine, chloroquine), anti-cancer agents (5-fluorouracil, vinblastine, vemurafenib (10) and dacarbazine), cardiac drugs (amiodarone, nifedipine, quinidine and diltiazem), diuretics (furosemide, thiazides and hydrochlorothiazide), anti-diabetics (sulfonylurea and glyburide), non-steroidal anti-inflammatory drugs (naproxen, piroxicam, diclofenac, celecoxib (11) and ketoprofen), anti-fibrotic drug (piferindone (12,13)) and psychiatric drugs (phenothiazines, tricyclic antidepressants and imipramine). Dermatological drugs also have a concern of phototoxicity but a certain class of drugs capitalize phototoxic responses as their mode of pharmacological action as can be exemplified by photodynamic therapy like 5-aminolevulinic acid and 8-methoxy psoralen (7). In addition to drugs, endogenous chemical (protoporphyrin), sunscreen (4-aminobenzoic acid and its esters in sunscreens) or environmental agents (anthracene in coal tar) have phototoxic potential (14). Indeed, UV irradiation or artificial light sources can result in photodecomposition of many drugs and photostability poses a major hurdle to ensure appropriate shelf-life of drugs. During photodecomposition, photoexcited drug molecules may transform into toxic compounds or produce reactive oxygen species that can ultimately inflict toxicity towards the skin cells (6).
MECHANISM OF PHOTOTOXICITY
UV-Visible light. Manifestation of phototoxicity is a mixed contribution of sunlight and photoreactive chemicals. Sun light that arrives on the earthen surface is a composite of a spectrum of lights from visible (400~700 nm wavelength) to ultraviolet regions (290~400 nm) along with energy waves in the infra-red region (700~2,400 nm). Actual solar light is not composed of a consistent but variable intensity of energy waves (6~7% ultraviolet light; around 42% visible light and 51% near infra-red, total solar irradiance is 1 kilowatt/m2) as can be shown below (Fig. 2).
Fig. 2. Solar spectrum and irradiance on a clear day. (from Newport website, http://www.newport.com).
Energy and extent of penetration also varies depending on the wavelength of incoming light. Shorter the wavelength is, stronger becomes the energy of the incident light. Conversely, longer the wavelength is, the deeper the light penetrates into the skin since the light with short wavelength readily imparts energy to the adjacent tissues on its trajectory. Accordingly, UVA and UVB, lights of shortest wavelength in incident sunlight are mainly accountable for the manifestation of phototoxicity. Of these, UVB (290~320 nm), the light with the shortest wavelength that reaches the earthen surface is approximately 1,000 fold stronger than UVA (320~400 nm) in inflicting burn or DNA damages but fortunately, UVB only goes as deep as epidermal layer while UVA penetrates as far as to subcutaneous layer. Furthermore, UVB loses its energy during traveling the atmosphere and the level at the sea level is 100 fold less than that of UVA (7).
Modes of phototoxicity. Mechanisms of molecular phototoxicity can be classified into direct and indirect mode of actions (Fig. 3). Direct mode is distinguished by the existence of unstable species which is derived from excited state and reacts directly with the endogenous molecules, whereas secondary photoproducts of the phototoxic compound react with the biological targets in the indirect mode (15).
Fig. 3. Two types of modes of phototoxicity: Direct and Indirect modes. Direct mode is when unstable phototoxic compound reacts with endogenous molecules directly. On the other hand, indirect mode is initiated when endogenous molecules bind to photoproducts of the phototoxic compound. Both modes result in the alteration of biological substrate, in which induces biological responses.
Photochemical reaction. Since UV/VIS absorption is prerequisite for photochemical reaction and thus for the manifestation of phototoxicity, chemicals that do not absorb UV/VIS within the range of natural incident sunlight (290~700 nm) do not cause phototoxicity (16,17). Photoactive chemicals absorb photons and the absorbed energy (hv) results in molecular changes of the chemical itself or generates reactive oxygen species that can cause toxicity to the target tissues (Fig. 4A). Intermediates of photochemical reactions include radicals and radical ions that can directly inflict cytotoxicity. Photo-excited molecule can also transfer energy to adjacent oxygen that results in the formation of highly cytotoxic singlet oxygen as in indirect modes (14) (Fig. 4B). Superoxide anion and hydrogen peroxide can be also generated by photoexcitation and transfer of energy to neighboring water molecules in indirect modes (18).
Fig. 4. Photochemical reaction of representative phototoxicants: (A) Chlorpromazine, (adopted from Irene K.E. Kochevar (15)), (B) Avobenzone (From Sayre et al. (19)).
TEST METHODS FOR PHOTOTOXICITY AND ANIMAL ALTERNATIVES
It is essential to ensure the photosafety of chemicals when there are chances of human exposure as can be clearly exemplified by pharmaceuticals (20) or cosmetics (21). To evaluate the potential of phototoxicity of a chemical, various test methods that range from in silico (22), in chemico (23), in vitro to in vivo assay, have been introduced. In chemico assays like ROS generation (24), in vitro test that include 3T3 NRU assay and 3D epidermis model, and in vivo studies employing guinea pig, mouse or pigmented rats have been developed and routinely used (25).
Light source for phototoxicity. Light source for phototoxicity is extremely important since wavelengths absorbed by the test chemical (absorption spectrum) and the dose of light (achievable in a reasonable exposure time) should be sufficient to induce phototoxicity (26). Solar simulators that simulate natural sunlight are considered the ideal artificial light source (Fig. 5).
Fig. 5. Commercial solar simulators: Newport, Suntest CPS+ or CPS (Atlas), SXL-2500V2 (Seric).
The irradiation power distribution of the filtered solar simulator should be close to that of outdoor daylight. Solar simulators are equipped with xenon arcs or (doped) mercury-metal halide arcs. They also should be suitably filtered to attenuate the highly cytotoxic UVB wavelengths. The spectrum recorded below these filters should not deviate from standardized outdoor daylight (Specification: FDA CFR Part 201.327, ISO 24444:2010(e), CIE-85-1989).
Nevertheless, other UVA light source like UVA lamp can be also used with a proper UV dosimeter to check the intensity and wavelength. The intensity of light (irradiance) varies depending on the sources, and it should be regularly checked before each phototoxicity test using a suitable broadband UV-meter. The UV-meter must have been calibrated before each measurement. Accordingly, irradiation time depends on the intensity of light source (e.g., for 1.7 mW/cm2 light source, 50 min exposure time is necessary to achieve 5 J/cm2). Irradiation time also varies depending on the test methods. A dose of 5 J/cm2 (as measured in the UVA range) was determined to be non-cytotoxic but sufficiently potent to excite chemicals to elicit phototoxic reactions in 3T3 Neutral red uptake assay.
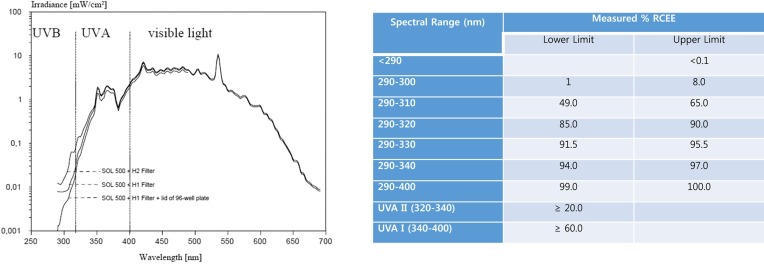
Fig. 6. Phototoxicity and its evaluations: Spectral power distribution of a filtered solar simulator (adopted from OECD TG432 (3), %RCEE, Relative Cumulative Erythemal Effectiveness (27)).
3T3 Neutral red uptake assay. 3T3 NRU assay has been officially approved by OECD and endorsed as OECD TG432 in 13th April 2004 (3). This test evaluates photocytotoxicity by determining the relative reduction in cell viability after the exposure to the test article in the presence versus absence of UV/VIS irradiation. Decision to conduct 3T3 NRU phototoxicity test is being made for the chemicals that show absorption spectra in UV/VIS region when dissolved in appropriate solvent (17). It has been suggested that if the molar extinction/absorption coefficient is less than 10 litre x mol−1 x cm−1 the chemical is unlikely to be photoreactive (e.g., In UV cuvette with 1 cm long light path, OD of 0.05 M solution shall be less than 0.5 to be considered as non-photoreactive based on the equation “absorbance = extinction coefficient x path length x concentration”) (26). 3T3 NRU test exhibits highly sensitive but low specific predictive capacity (a sensitivity of 93% and a specificity of 84%). 3T3 NRU test has many limitations. It cannot predict adverse effects other than photo(cyto)toxicity that may arise from combined action of a chemical and light such as photogenotoxicity, photoallergy(photosensitization) or photocarcinogenicity. 3T3 NRU test is only being employed for hazard identification while its utility for the assessment of phototoxic potency is not warranted.In particular, this assay system lacks metabolic activity which is critical in the manifestation of systemically exposed chemicals. Therefore, for systemically exposed chemicals that require metabolic activation like monocrotaline, riddelliine, and heliotrine (pyrrolizidine alkaloids) (28), in vivo animal studies are recommended (5,29).
Fundamental test principle of 3T3 NRU is the comparison of cell viability in the presence or absence of UV/Vis irradiation as determined with vital dye, neutral red, which is a weak cationic dye that readily penetrates cell membranes and accumulating intracellularly in lysosomes of viable cells. Base cell-line is Balb/c 3T3 cell, which is mouse fibroblast developed from mouse embryos by G.T. Todaro in 1968. 3T3 designation stands for “3-day transfer, inoculum 3 × 105 cells” in 20 cm2 dish, and this cell is relatively stable, readily available and easy to handle (30). Dermal fibroblast is one of target cells of phototoxicity providing a solid rationale for the employment of 3T3 cells.
To decide whether the test article is phototoxic or not in 3T3 NRU assay, the concentration-response shall be obtained in the presence and in the absence of irradiation. Photo-Irritation-Factor (PIF) or Mean Photo Effect (MPE) shall be calculated (31). PIF is the ratio of IC50 (concentration that decreases cell viability by 50%) of non-irradiated over irradiated as shown in Fig. 7.
Fig. 7. Prediction model of Photocytotoxicity by PIF (Photo-irritation factor).

When IC50 cannot be obtained, MPE is calculated as following equation

PIF < 2 or an MPE < 0.1 predicts: “no phototoxicity”. A PIF > 2 and < 5 or an MPE > 0.1 and < 0.15 predicts: “probable phototoxicity” and PIF > 5 or an MPE > 0.15 predicts: “phototoxicity” (Fig. 8).
Fig. 8. Photo effect calculation: The photo effect (PEC) at an arbitrary concentration C is defined as the product of the response effect (REC) and the dose effect (DEC), i.e. PEC = REC × DEC. The definition is illustrated as adopted from (31). Calculation of the photo effect at the concentration 0.4 follows the equations given in the text gives: response effect RE0.4 = (66% − 11%)/100% = 0.55, dose effect DE0.4 = (0.4/0.16 − 1)/(0.4/0.16 + 1) = 0.43, and photo effect PE0.4 = 0.24. The mean photo effect is obtained by averaging over the values for the photo effect at various concentrations (31).

Erythrocyte hemolysis test. Cellular membranes are vulnerable to photochemically generated ROS and radicals. UVA-induced damages of erythrocyte and resultant hemolysis (photohemolysis)is capitalized to assess phototoxic potential of test articles (32). Sheep red blood cells (SRBC) are incubated with chemicals and irradiated with UVA at 20 J/cm2. Following irradiation, SRBCs were incubated in the dark for 2 hr at room temperature and then for a further 1 hr at 37℃, after which hemolysis were measured with Drabkin’s reagent and the measurement of UV absorbance at 540 nm. Extent of phototoxicity was assessed by the release of hemoglobin from SRBC, i.e., photohemolytic activityas following equation (33).

ADE: optical density of exposed drug solution with erythrocytes
AD: optical density of exposed drug solution without erythrocytes
C: optical density of 100% hemolytic control solution
Phototoxicants like ciprofloxacin, norfloxacin or enoxacin significantly increase photohemolytic activity beyond 20% at 100 μg/mL. The sensitivity, specificity, and accuracy of this test were 67%, 73%, and 73%, respectively for 24 chemicals (8 fragrances, 5 UV absorbers, 4 drugs, 4 antimicrobials and 3 dyes) as compared with in vivo guinea pig test (34). Low sensitivity may be problematic and its performance is much inferior to that of 3T3 NRU test, which may explain the decreased use of this test recently.
In vitro human 3D epidermis model. To overcome the limitations of in vitro cell-based methods, 3D reconstructed epidermis model is being investigated for the application to phototoxicity tests (35,36). Basically the test principle is similar to 3T3 NRU test, namely the assessment of the difference of tissue viability between in the presence and absence of UV/VIS irradiation. Similar prediction model employing PIF and MPE may be utilized (37). In 3D epidermis model, however, water-insoluble materials can be tested and a certain extent of metabolic capacity is preserved in the primary keratinocytes in the epidermal layer that may be applied to the toxicants requiring metabolic activation (38). Moreover, measurement of cytokine production like IL-1β (Interleukin-1β) (39), comet assay (40) and histological examination are possible that can be considered into the further evaluation of photoallergenicity and photocarcinogenecity.
In vivo methods employing guinea pig, mouse or pigmented rats. Laboratory animals such as mice and guinea pigs are being employed to simulate real-life scenario of human phototoxicity. Animals are exposed to chemicals topically or systemically and irradiated with appropriate dose of UVA (generally 10 J/cm2 for the guinea pig test, 20 J/cm2 for mouse test (41)). Scoring of erythema and edema from 0 to 4 is summed up and maximum score during 72 hrs of observation is averaged per animal to generate irritation index. Phototoxicity index is being obtained by the equation “Irritation index of UVA-irradiated - Irritation index of non-irradiated site” (42). Phototoxicity index over 0.6 indicates the potential of phototoxicity. Alternatively, ear thickness may be measured to estimate edema in mouse tests. These in vivo tests well reflect the pathophysiological process of phototoxicity in human but sacrifices of animals, expenses and time required to conduct the test poses many problems especially in the era of wide-spread awareness of animal welfare and ethics. Non-animal based phototoxicity tests are gaining popularity these days to overcome these problems (43).
In chemico methods for phototoxicity evaluation. Cell-free test-tube methods, namely in chemico have been explored to assess phototoxicity. Information on light absorbance and photostability of test article has been analyzed to predict phototoxicity (44). Employing the generation of reactive oxygen species during photoexcitation and subsequent photoreaction, phototoxic potential of a chemical can be evaluated in chemico (12). Singlet oxygen is detected by p-nitrosodimethylaniline (RNO) bleaching while Nitro Blue-Tetrazolium Test (NBT-formazan reaction) is employed to determine peroxide generation as depicted below (24),
Singlet oxygen + imidazole
→ [peroxide intermediate] → oxidized imidazole
[peroxide intermediate] + RNO
→ RNO bleaching + products
ROS generation assay exhibited the sensitivity and specificity of 90% and 76.9% for cosmetics and 100% and 75% for non-cosmetic chemicals. DNA strand breaking activity is another way to evaluate UV induced phototoxicity of different kinds of chemicals, or drugs in chemico through quantifying open or closed circular DNA. This assay also does not require live cells or tissues, but plasmid. Plasmid is dissolved in buffer and mixed with test articles. After the mixture has been irradiated with UV, samples are subjected to electrophoresis. Quantity of breakage DNA is analyzed by fluorescent-based technology. UV induced phototoxic compound results opening DNA strands and it is dependent on drug concentration and UV irradiation dose (33). These tests do not require live cells or tissues that can add to the variability of test results. However, these methods have limitations that include lack of metabolic activation capacity, inapplicability of water-insoluble materials (oils, solids, gels, formulated products), and inability to predict photogenotoxicity, photoallergy(photosensitization) or photocarcinogenicity. This test is limited to hazard identification, not for an assessment of phototoxic potency.
CONCLUSION
Sunlight-associated health problems such as skin cancers, photoaging and hyper-pigmentation are in the trend of increasing frequency and severity. As the awareness of the safety increases, combined effects of sunlight and chemicals as well as its intrinsic toxicity draw attention from general public, which supports the initiative to study the mechanism of phototoxicity and the novel method to evaluate it. Current in vitro test methods still leave many points to be improved, especially, inability to provide the information on phototoxic potential and lack of metabolic capacity. In this regards, further attention and efforts from toxicologist and photochemists is necessary which will provide a better solution in near future.
Acknowledgments
This work is supported by Ministry of Food and Drug Safety of Korea, Grant No. 13172MFDS987.
References
- 1.Freeman A.K., Gordon M. Dermatologic diseases and problems. Geriatric Medicine. (4th edition) Springer; New York: (2006). pp. 869–881. [Google Scholar]
- 2.Boudon S.M., Morandi G., Prideaux B., Staab D., Junker U., Odermatt A., Stoeckli M., Bauer D. Evaluation of Sparfloxacin Distribution by Mass Spectrometry Imaging in a Phototoxicity Model. J. Am. Soc. Mass Spectrom. (2014);25:1803–1809. doi: 10.1007/s13361-014-0947-3. [DOI] [PubMed] [Google Scholar]
- 3.OECD. Test No. 432: In vitro 3T3 NRU Phototoxicity Test. OECD; Paris: (2004). OECD Publishing. No. 432. [Google Scholar]
- 4.Lugovic L., Situm M., Ozanic-Bulic S., SjerobabskiMasnec I. Phototoxic and photoallergic skin reactions. Coll. Antropol. (2007);31 Suppl 1:63–67. [PubMed] [Google Scholar]
- 5.FDA U. Guidance for industry - Photosafety Testing. FDA Publishing; US: (2003). pp. 1–22. [Google Scholar]
- 6.Tønnesen H.H. Formulation and stability testing of photolabile drugs. Int. J. Pharm. (2001);225:1–14. doi: 10.1016/S0378-5173(01)00746-3. [DOI] [PubMed] [Google Scholar]
- 7.Gonçalo M. Phototoxic and photoallergic reactions. Contact Dermatitis, Springer; (2011). pp. 361–376. [Google Scholar]
- 8.Ferguson J. Photosensitivity due to drugs. Photodermatol. Photoimmunol. photomed. (2002);18:262–269. doi: 10.1034/j.1600-0781.2002.02778.x. [DOI] [PubMed] [Google Scholar]
- 9.Adachi T., Satou Y., Satou H., Shibata H., Miwa S., Iwase Y., Yamamoto T., Nishida A., Masutomi N. Assessment of 8-methosypsoralen, lomefloxacin, sparfloxacin, and Pirfenidone phototoxicity in Long-Evans rats. Int. J. Toxicol. (2015);34:16–23. doi: 10.1177/1091581814559397. [DOI] [PubMed] [Google Scholar]
- 10.Boudon S.M., Plappert-Helbig U., Odermatt A., Bauer D. Characterization of vemurafenib phototoxicity in a mouse model. Toxicol. Sci. (2014);137:259–267. doi: 10.1093/toxsci/kft237. [DOI] [PubMed] [Google Scholar]
- 11.Yazici A.C., Baz K., Ikizoglu G., Kokturk A., Uzumlu H., Tataroglu C. Celecoxib?induced photoallergic drug eruption. Int. J. Dermatol. (2004);43:459–461. doi: 10.1111/j.1365-4632.2004.02149.x. [DOI] [PubMed] [Google Scholar]
- 12.Onoue S., Seto Y., Kato M., Aoki Y., Kojo Y., Yamada S. Inhalable powder formulation of pirfenidone with reduced phototoxic risk for treatment of pulmonary fibrosis. Pharm. Res. (2013);30:1586–1596. doi: 10.1007/s11095-013-0997-4. [DOI] [PubMed] [Google Scholar]
- 13.Seto Y., Inoue R., Kato M., Yamada S., Onoue S. Photosafety assessments on pirfenidone: photochemical, photobiological, and pharmacokinetic characterization. J. Photochem. Photobiol. B. (2013);120:44–51. doi: 10.1016/j.jphotobiol.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Chignell C.F., Motten A.G., Buettner G.R. Photoinduced free radicals from chiorpromazine and related phenothiazines: relationship to phenothiazine-induced photosensitization. Environ. Health Perspect. (1985);64:103–110. doi: 10.1289/ehp.8564103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kochevar K.E. Phototoxicity mechanisms: chlorpromazine photosensitized damage to DNA and cell membranes. J. Invest. Dermatol. (1981);77:59–64. doi: 10.1111/1523-1747.ep12479244. [DOI] [PubMed] [Google Scholar]
- 16.ICH. ICH guideline S10 on photosafety evaluation of pharmaceuticals. FDA; US: (2014). [Google Scholar]
- 17.OECD. TG101: UV-VIS Absorption Spectra. OECD; Paris: (1981). pp. 1–6. [Google Scholar]
- 18.Garg S., Rose A.L., Waite T.D. Photochemical production of superoxide and hydrogen peroxide from natural organic matter. Geochim. Cosmochim. Acta. (2011);75:4310–4320. doi: 10.1016/j.gca.2011.05.014. [DOI] [Google Scholar]
- 19.Sayre R.M., Dowdy J.C., Gerwig A.J., Shlelds W.J., Lioyd R.V. Unexpected photolysis of the sunscreen octinoxate in the presence of the sunscreen avobenzone. Photochem. Photobiol. (2005);81:452–456. doi: 10.1562/2004-02-12-RA-083.1. [DOI] [PubMed] [Google Scholar]
- 20.ICH Photosafety Evaluation of Pharmaceuticals S10. FDA; US: (2013). pp. 1–15. [Google Scholar]
- 21.Vinardell M.P. The use of non-animal alternatives in the safety evaluations of cosmetics ingredients by the Scientific Committee on Consumer Safety (SCCS). Regul. Toxicol. Pharmacol. (2015);71:198–204. doi: 10.1016/j.yrtph.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 22.Haranosono Y., Kurata M., Sakaki H. Establishment of an in silico phototoxicity prediction method by combining descriptors related to photo-absorption and photoreaction. J. Toxicol. Sci. (2014);39:655–664. doi: 10.2131/jts.39.655. [DOI] [PubMed] [Google Scholar]
- 23.Onoue S., Tsuda Y. Analytical studies on the prediction of photosensitive/phototoxic potential of pharmaceutical substances. Pharm. Res. (2006);23:156–164. doi: 10.1007/s11095-005-8497-9. [DOI] [PubMed] [Google Scholar]
- 24.Onoue S., Kawamura K., Igarashi N., Zhou Y., Fujikawa M., Yamada H., Tsuda Y., Seto Y., Yamada S. Reactive oxygen species assay-based risk assessment of druginduced phototoxicity: classification criteria and application to drug candidates. J. Pharm. Biomed. Anal. (2008);47:967–972. doi: 10.1016/j.jpba.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 25.Guideline IHT. ICH guideline M3 (R2) on nonclinical safety studies for the conduct of human clinical trials and marketing authorization for pharmaceuticals. ICH, European Medicines Agency; (2009). pp. 1–26. [Google Scholar]
- 26.Bauer D., Averett L.A., De Smedt A., Kleinman M.H., Muster W., Pettersen B.A., Robles C. Standardized UV-vis spectra as the foundation for a threshold-based, integrated photosafety evaluation. Regul. Toxicol. Pharmacol. (2014);68:70–75. doi: 10.1016/j.yrtph.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Diffey B.L. Sources and measurement of ultraviolet radiation. Methods. (2002);28:4–13. doi: 10.1016/S1046-2023(02)00204-9. [DOI] [PubMed] [Google Scholar]
- 28.Wang C.C., Xia Q., Li M., Wang S., Zhao Y., Tolleson W.H., Yin J.J., Fu P.P. Metabolic activation of pyrrolizidine alkaloids leading to phototoxicity and photogenotoxicity in human HaCaT keratinocytes. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. (2014);32:362–384. doi: 10.1080/10590501.2014.969980. [DOI] [PubMed] [Google Scholar]
- 29.Schümann J., Boudon S., Ulrich P., Loll N., Garcia D., Schaffner R., Streich J., Kittel B., Bauer D. Integrated preclinical photosafety testing strategy for systemically applied pharmaceuticals. Toxicol. Sci. (2014);139:245–256. doi: 10.1093/toxsci/kfu026. [DOI] [PubMed] [Google Scholar]
- 30.Spielmann H., Balls M., Brand M., Döring B., Holzhütter H.G., Kalweit S., Klecak G., Eplattenier H.L., Liebsch M., Lovell W.W., Maurer T., Moldenhauer F., Moore L., Pape W.J., Pfanenbecker U., Potthast J., De Silva O., Steiling W., Willshaw A. EEC/COLIPA project on in vitro phototoxicity testing: First results obtained with a Balb/c 3T3 cell phototoxicity assay. Toxicol. In Vitro. (1994);8:793–796. doi: 10.1016/0887-2333(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 31.Peters B., Holzhütter H.G. In vitro phototoxicity testing: development and validation of a new concentration response analysis software and biostatistical analyses related to the use of various prediction models. Altern. Lab. Anim. (2002);30:415–432. doi: 10.1177/026119290203000405. [DOI] [PubMed] [Google Scholar]
- 32.Pape W.J., Maurer T., Pfannenbecker U., Steiling W. The red blood cell phototoxicity test (photohaemolysis and haemoglobin oxidation): EU/COLIPA validation programme on phototoxicity (phase II). Altern. Lab. Anim. (2001);29:145–162. doi: 10.1177/026119290102900208. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto T., Tsurumaki Y., Takei M., Hosaka M., Oomori Y. In vitro method for prediction of the phototoxic potentials of fluoroquinolones. Toxicol. In Vitro. (2001);15:721–727. doi: 10.1016/S0887-2333(01)00089-3. [DOI] [PubMed] [Google Scholar]
- 34.Sugiyama M., Itagaki H., Hariya T., Murakami N., Kato S. In vitro assays to predict phototoxicity of chemicals:(I) Red blood cell hemolysis assay. AATEX. (1994);2:183–191. [Google Scholar]
- 35.Netzaff F., Lehr C.M., Wertz P.W., Schaefer U.F. The human epidermis models EpiSkin (R), SkinEthic (R) and EpiDerm (R): An evaluation of morphology and their suitability for testing phototoxicity, irritancy, corrosivity, and substance transport. Eur. J. Pharm. Biopharm. (2005);60:167–178. doi: 10.1016/j.ejpb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 36.Portes P., Pygmalion M.J., Popovic E., Cottin M., Mariani M. Use of human reconstituted epidermis Episkin® for assessment of weak phototoxic potential of chemical compounds. Photodermatol. Photoimmunol. Photomed. (2002);18:96–102. doi: 10.1034/j.1600-0781.2002.180207.x. [DOI] [PubMed] [Google Scholar]
- 37.Bernard F.X., Barrault C., Deguercy A., De Wever B., Rosdy M. Development of a highly sensitive in vitro phototoxicity assay using the SkinEthicTM reconstructed human epidermis. Cell Biol. Toxicol. (2000);16:391–400. doi: 10.1023/A:1007604612003. [DOI] [PubMed] [Google Scholar]
- 38.Aardema M.J., Barnett B.B., Mun G.C., Dahl E.L., Curren R.D., Hewitt N.J., Pfuhler S. Evaluation of chemicals requiring metabolic activation in the EpiDerm 3D human reconstructed skin micronucleus (RSMN) assay. Mutat. Res. (2013);750:40–49. doi: 10.1016/j.mrgentox.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 39.Lelièvre D., Justine P., Christiaens F., Bonaventure N., Coutet J., Marrot L., Cotovio J. The EpiSkin phototoxicity assay (EPA): development of an in vitro tiered strategy using 17 reference chemicals to predict phototoxic potency. Toxicol. In Vitro. (2007);21:977–995. doi: 10.1016/j.tiv.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 40.Reus A.A., Reisinger K., Downs T.R., Carr G.J., Zeller A., Corvi R., Krul C.A., Pfuhler S. Comet assay in reconstructed 3D human epidermal skin models--investigation of intra- and inter-laboratory reproducibility with coded chemicals. Mutagenesis. (2013);28:709–720. doi: 10.1093/mutage/get051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsumoto N., Akimoto A., Kawashima H., Kim S. Comparative study of skin phototoxicity with three drugs by an in vivo mouse model. J. Toxicol. Sci. (2010);35:97–100. doi: 10.2131/jts.35.97. [DOI] [PubMed] [Google Scholar]
- 42.Wagai N., Tawara K. Possible reasons for differences in phototoxic potential of a 5 quinolone antibacterial agents: generation of toxic oxygen. Free Radical Res. Commun. (1992);17:387–398. doi: 10.3109/10715769209083143. [DOI] [PubMed] [Google Scholar]
- 43.Clewell H.J., 3rd. Coupling of computer modeling with in vitro methodologies to reduce animal usage in toxicity testing. Toxicol. Lett. (1993);68:101–117. doi: 10.1016/0378-4274(93)90123-F. [DOI] [PubMed] [Google Scholar]
- 44.Henry B., Foti C., Alsante K. Can light absorption and photostability data be used to assess the photosafety risks in patients for a new drug molecule? J. Photochem. Photobiol. B. (2009);96:57–62. doi: 10.1016/j.jphotobiol.2009.04.005. [DOI] [PubMed] [Google Scholar]