Background: Human and animal studies have shown associations between insulin growth factor 2 (IGF2) and diabetes.
Results: Overexpression of insulin growth factor 2 in β-cells leads to β-cell dysfunction and makes islets more vulnerable to β-cell damage and immune attack.
Conclusion: IGF2 may play an important role in the predisposition and development of diabetes.
Significance: This study unravels an unprecedented role of IGF2 on physiology of β-cells.
Keywords: beta cell (B-cell), diabetes, endoplasmic reticulum stress (ER stress), insulin-like growth factor (IGF), islet
Abstract
The human insulin-like growth factor 2 (IGF2) and insulin genes are located within the same genomic region. Although human genomic studies have demonstrated associations between diabetes and the insulin/IGF2 locus or the IGF2 mRNA-binding protein 2 (IGF2BP2), the role of IGF2 in diabetes pathogenesis is not fully understood. We previously described that transgenic mice overexpressing IGF2 specifically in β-cells (Tg-IGF2) develop a pre-diabetic state. Here, we characterized the effects of IGF2 on β-cell functionality. Overexpression of IGF2 led to β-cell dedifferentiation and endoplasmic reticulum stress causing islet dysfunction in vivo. Both adenovirus-mediated overexpression of IGF2 and treatment of adult wild-type islets with recombinant IGF2 in vitro further confirmed the direct implication of IGF2 on β-cell dysfunction. Treatment of Tg-IGF2 mice with subdiabetogenic doses of streptozotocin or crossing these mice with a transgenic model of islet lymphocytic infiltration promoted the development of overt diabetes, suggesting that IGF2 makes islets more susceptible to β-cell damage and immune attack. These results indicate that increased local levels of IGF2 in pancreatic islets may predispose to the onset of diabetes. This study unravels an unprecedented role of IGF2 on β-cells function.
Introduction
β-Cell dysfunction and reductions in β-cell mass are pathological alterations central to the development of both type 1 (T1D)6 and type 2 diabetes (T2D) (1). In particular, T2D may begin with insulin resistance, but overt T2D only develops when β-cells fail to compensate for the increased insulin demand. Thus, the alteration in the function of β-cells would be a primary defect in the pathogenesis of diabetes (2).
The concept of loss of β-cell differentiation was first introduced by Jonas et al. (3) in 1999 to describe a change in the phenotype of β-cells by which functions of the fully differentiated cells necessary for optimal functioning, such as insulin secretion, are lost (4). The progressive changes in β-cell phenotypes observed during dedifferentiation include the down-regulation of many genes that are highly expressed in β-cells, e.g. insulin (INS), solute carrier family member 2 (SLC2A2 or GLUT2), glucokinase (GCK), and pancreatic and duodenal homeobox 1 (PDX1) (5). The term dedifferentiation has been used, for example, to describe the phenotype observed in knock-out mice for the transcription factor Foxo1 specifically in β-cells. In this animal model that develops overt hyperglycemia, β-cells were described to have dedifferentiated to a state of immaturity characterized by positivity for markers of multipotency and plasticity and for markers of endocrine progenitor cells and by lack the of insulin production (6). Other authors have also used the term dedifferentiation to describe the loss of β-cell identity due to the inactivation of transcription factors that define β-cell fate (7, 8). Importantly, recent evidence attributes to dedifferentiation of β-cells a causative role in β-cell failure in T2D (6, 9, 10).
In parallel to β-cell dedifferentiation, many animal models of T1D also show endoplasmic reticulum (ER) stress. Given that β-cells need to produce insulin in large quantities, they probably represent a cell type vulnerable to ER stress (11). In fact, ER stress has been proposed as a mechanism responsible for β-cell dysfunction and death in type 2 diabetes (12), and it has been reported to precede the onset of T1D in the nonobese diabetic mouse model (13). The major indicators of the ER stress response, such as X-box binding protein 1 (Xbp1) and DNA-damage inducible transcript 3 (Ddit3/Chop), are up-regulated in islets from type 1 and type 2 diabetic models (12, 14, 15).
Insulin-like growth factor 2 (IGF2) is a growth-promoting polypeptide that shares a high degree of structural homology with insulin. IGF2 is synthesized primarily by the liver, but it is also produced locally by many tissues, where it acts in an autocrine/paracrine manner (16). IGF2 is expressed at high levels during embryonic development, but its expression is progressively shut down in most tissues after birth. In humans, the IGF2 gene is located on chromosome 11p15.5, in close linkage with the INS and tyrosine hydroxylase (TH) genes. Because the IGF2 and INS genes share their promoter, the human pancreas can express INS-IGF2 hybrid transcripts (17). Indeed, in human β-cells insulin is the most abundant transcript, followed by INS-IGF2 hybrids and IGF2 transcripts (18). Genome-wide association studies performed in humans from Caucasian and Asian populations have demonstrated associations between the IDDM2 locus containing the INS and IGF2 genes and diabetes (19). In particular, variations in the size and sequence of a regulatory element of the insulin promoter that includes a variable number of tandem repeats (VNTR) have been found to confer susceptibility to T1D (the shortest version of VNTR or class I) or to T2D (the longest version of VNTR or class III) (20, 21). Pugliese et al. (20) suggested that although there is clear evidence supporting the concept that the INS VNTR element is the main factor conferring susceptibility to diabetes to the IDDM2 locus, there is also the possibility that, at least in some instances, both the INS and IGF2 locus modulate IDDM2-conferred susceptibility. In contrast, the IGF2BP2 (IMP2) gene, involved in the regulation of IGF2 expression by binding to the 5′-UTR of the IGF2 mRNA to promote its translation (22), has also been associated with genome-wide association studies with an increased risk of T2D and gestational diabetes in Caucasian, Asian, and Arabic populations (23–25). IGF2BP2 is expressed in β-cells and in insulin-sensitive tissues in postnatal life (22), and an altered expression or regulation of the IGF2BP2 gene may end up impacting the levels of IGF2 in these cells. Furthermore, old Goto-Kakizaki rats, a nonobese model of mild T2D, show increased IGF2 mRNA in their islets (26).
We previously showed that transgenic mice overexpressing IGF2 specifically in β-cells (Tg-IGF2) display several primary defects characteristic of T2D, such as hyperinsulinemia, mild hyperglycemia, and altered glucose and insulin tolerance tests (27). These observations together with the evidence from human genome-wide association studies suggested that altered levels of IGF2 in β-cells could confer susceptibility to diabetes. Here, we studied the mechanisms by which IGF2 exerted its action on β-cells of Tg-IGF2 to help unravel the role that this growth factor plays in the development of overt diabetes in humans. To this end, we studied in detail the phenotype of transgenic β-cells at the initial stages of the diabetic process. Local IGF2 overexpression led to β-cell dysfunction as well as down-regulation of typical markers of β-cell differentiation. Up-regulation of markers of ER stress and immune response-related genes was also documented in islets of Tg-IGF2 mice, and this agreed with the observed increased susceptibility to β-cell death and diabetes development. Thus, in this study we provide evidence that local pancreatic overexpression of IGF2, besides causing islet hyperplasia and hyperinsulinemia (27), directly affects β-cells leading to cell dysfunction and susceptibility to damage and as a consequence predisposes to the onset of diabetes.
Experimental Procedures
Animals
Heterozygous male transgenic mice expressing either mouse Igf2 or human IFNβ under the control of the rat insulin promoter-I (RIP-I) were used (27, 28). Tg-IGF2 mice were in C57Bl6/SJL background, and Tg-IFNβ mice, a model of islet lymphocytic infiltration with increased susceptibility to autoimmune diabetes (29), were in CD1 background, and double Tg-IFNβ/IGF2 mice were obtained by crossing the two lines (C57Bl6/SJL+CD1). Diabetes was induced as described previously (29). All mice were fed ad libitum with a standard chow diet (2018S Teklad Global, Harlan) and maintained under conditions of controlled temperature and light (12 h light/dark cycles). When stated, mice were fasted for 16 h. Animal care and experimental procedures were approved by the Ethics Committee in Animal and Human Experimentation of the Universitat Autònoma de Barcelona.
Immunohistochemistry and Histopathology
For immunohistochemical detection of INS, GLUT2, PDX1, MAFa, and p62, pancreata were fixed for 12–24 h in formalin, embedded in paraffin, and sectioned. Sections were then incubated overnight at 4 °C with the following antibodies: guinea pig anti-porcine insulin (Sigma); rabbit anti-human glucagon (Signet Labs, Dedham, MA); rabbit polyclonal anti-GLUT2 (AB1342, Chemicon International Inc., EEUU); rabbit anti-human IGF2 ((PAC1) GroPep Ltd., Adelaide, Australia); rabbit polyclonal anti-MAFa (Santa Cruz Biotechnology, sc-66958); rabbit polyclonal anti-PDX1 (Millipore, AB3243); rat anti-mouse MAC-2 (Cedarlane CL8942AP); and mouse anti-human p62 (BD Biosciences 610833) as a autophagy marker. As secondary antibodies, peroxidase-conjugated rabbit anti-guinea pig IgG (Dako, Denmark), biotinylated goat anti-rabbit (Pierce), TRITC-conjugated goat anti-guinea pig (Molecular Probes Leiden, The Netherlands), biotinylated goat anti-rabbit (Molecular Probes), biotinylated rabbit anti-rat (Dako, Glostrup, Denmark), and biotinylated horse anti-mouse (Vector Laboratories, Burlingame, CA) antibodies were used. Streptavidin-conjugated Alexa 488 (Molecular Probes) or streptavidin-conjugated Alexa 568 (Molecular Probes) were used as fluorochromes. Images were obtained with a Nikon Eclipse E800 microscope (Nikon, Tokyo, Japan). For laser-scanning confocal analysis, a TCS SP2 microscope (Leica Microsystems, Heidelberg, Germany) was used.
Morphometric Analysis
β-Cell mass determination was performed as described previously (29). Macrophage infiltration was measured as the percentage of MAC-2-positive area per islet area in islets from three different sections per mouse, with four animals per group.
Islets Isolation and Culture
Pancreatic islets were isolated as described previously (29) and then cultured in RPMI 1640 medium (11 mm glucose), supplemented with 1% BSA, 2 mm glutamine, penicillin/streptomycin at 37 °C in an atmosphere of 95% humidified air, 5% CO2. To study the effects of recombinant IGF2 on β-cells, after overnight culture, pools of islets were treated with 13.5 nm recombinant IGF2 (792-MG, R&D Systems) and then cultured for 48 h. To express Igf2 in islets, after isolation and overnight culture, pools of islets were infected with Igf2-expressing adenoviruses (Ad5/CMV-IGF2) or with noncoding adenoviruses as control (Ad5/CMV-Null) and incubated for 48 h. After treatments islets were hand-picked and processed. For qPCR analysis, islets were cultured overnight to recuperate from isolation stress and then hand-picked and processed to obtain RNA.
Cell Culture
INS-1 cells were cultured in RPMI 1640 medium (11 mm glucose), 2 mm glutamine, 10 mm Hepes, 1 mm sodium pyruvate, 50 μm 2-mercaptoethanol, and 10% FBS at 37 °C in an atmosphere of 95% humidified air, 5% CO2. For cell culture studies, medium was modified, and 10% FBS was substituted for 1% BSA, and cells were treated with 200 nm wortmannin (W1628, Sigma) followed an hour later by 100 ng/ml recombinant IGF2, or with either alone, and then cultured for 48 h.
Gene Expression Analysis
For qPCR analysis, total RNA was extracted from isolated islets using Tripure Isolation Reagent (Roche Applied Science) and RNeasy mini kit (Qiagen, Hilden, Germany). Total RNA (1 μg) was retrotranscribed using the Transcriptor First Strand cDNA synthesis kit (Roche Applied Science). RT-PCR was performed using LightCycler 480 SYBR Green I MasterMix (Roche Applied Science). The primers used for murine islets and INS-1 cells (rat) are listed in the Table 1. Results were analyzed using the mathematical model of Pfaffl (30). All samples were processed in triplicate, and a mean Ct was calculated. The Ct for each transcript of interest was normalized by the Ct obtained for the reference gene RplpO. Then, the relative expression of each gene in each sample was calculated by dividing the value obtained for each sample by the mean of wild-type/control values.
TABLE 1.
Primers used for murine islets and INS-1 cells (rat)
| Gene | Forward primer (5′ to 3′) | Reverse primer (5′ to 3′) |
|---|---|---|
| Mouse | ||
| Foxa2 | 5′GAG-CAG-CAA-CAT-CAC-CAC-G3′ | 5′CGT-AGG-CCT-TGA-GGT-CCA-T3′ |
| Pdx1 | 5′GAA-ATC-CAC-CAA-AGC-TAC-G3′ | 5′CGG-GTT-CCG-CTG-TGT-AAG3′ |
| RplpO | 5′TCC-CAC-CTT-GTC-TCC-AGT-CT3′ | 5′ACT-GGT-CTA-GGA-CCC-GAG-AAG3′ |
| Ins | 5′GCG-ATT-GTG-GAT-CAG-TGC-T3′ | 5′AGG-TGG-GCC-TTA-GTT-GCA-C3′ |
| Gck | 5′ATG-ACA-GAG-CCA-GGA-TGG-AG | 5′CGG-CTC-ATC-ACC-TTC-TTC-AG3′ |
| Glut2 | 5′CTG-GAG-CCC-TCT-TGA-CGG-GA3′ | 5′CCA-GTC-CTG-AAA-TTA-GCC-CAC-A3′ |
| Serca2 | 5′GTC-CTA-ACT-GTG-GTG-TTT-TCC-TC3′ | 5′GTT-TAG-GAA-GCC-GTT-ACT-CCA-G3′ |
| Chop | 5′GCG-ACA-GAG-CCA-GAA-TAA-CA3′ | 5′GAT-GCA-CTT-CCT-TCT-GGA-ACA3′ |
| B2-microglobulin | 5′ACT-CCA-AGA-CCC-AGA-AAC-TGT-C3′ | 5′ACT-GGT-AGG-AGT-AGG-GAT-GCA-C3′ |
| H2-Aa | 5′CTC-TGA-TTC-TGG-GGG-TCC-T 3′ | 5′ACC-ATA-GGT-GCC-TAC-GTG-GT3′ |
| β2/NeuroD1 | 5′CTC-TGA-TTC-TGG-GGG-TCC-T3′ | 5′ACC-ATA-GGT-GCC-TAC-GTG-GT3′ |
| Hnf4a | 5′TGC-CAA-CCT-CAA-TTC-ATC-CA3 | 5′GCT-CGA-GGC-TCC-GTA-GTG-TT3′ |
| Mafa | 5′AGG-AGG-AGG-TCA-TCC-GAC-TG3′ | 5′CTT-CTC-GCT-CTC-CAG-AAT-GTG3′ |
| Xbp1s | 5′GCT-GAG-TCC-GCA-GCA-GGT3′ | 5′ACA-GGG-TCC-AAC-TTG-TCC-AG3′ |
| XBPt | 5′TCA-GTT-TCC-TCC-GCA-GCG-CTT-T3 | 5′GCT-GCC-GCT-CAT-GGT-ACC-CG3′ |
| Igf2r | 5′TCT-GTG-TTG-GCT-CGT-CAC-TC3′ | 5′CCG-GTG-ACA-GAC-GTT-GAT-GA3′ |
| Insr-B | 5′GCC-AGT-GAG-TGC-TGC-TCA-T3′ | 5′TAC-TGT-CCT-CGG-CAC-CAT-T3′ |
| Insr-A | 5′CCC-ACC-CTT-TGA-GTC-TGA-TG3′ | 5′GCT-TTC-GGG-ATG-GCC-T3′ |
| Gcg | 5′GGC-ACA-TTC-ACC-AGC-GAC-TA3′ | 5′GTC-CCT-TCA-GCA-TGC-CTC-TC3′ |
| Rat | ||
| Foxa2 | 5′CCA-GAC-AAC-GCG-AGT-CCT3′ | 5′ACG-GCT-CCC-AGC-ATA-CTT-T3′ |
| Pdx1 | 5′CTG-TCG-TGC-CAT-GTG-AAC-C3′ | 5′TTC-TCT-AAA-TTG-GTC-CCA-GGA-A3′ |
| RplpO | 5′GAT-GCC-CAG-GGA-AGA-CAG3 | 5′CAC-AAT-GAA-GCA-TTT-TGG-GTA-G3′ |
| INS | 5′ GCT-CTG-TAC-CTG-GTG-TGT-GG 3′ | 5′ CCA-AGG-TCT-GAA-GAT-CCC-CG 3 |
| Gck | 5′GCC-CAG-TTG-TTG-ACT-CTG-GT | 5′CAT-CAC-CTT-CTT-CAG-GTC-TTC 3′ |
| GLUT2 | 5′TGA-AGG-ATC-TGC-TCA-CAT-AGT-CA 3′ | 5′CCA-ACA-TGG-CTT-TGA-TCC-TT 3′ |
| Serca | 5′TGT-TAA-TCA-AGA-CAA-AAA-GAA-CAT-GC3′ | 5′GGA-TCT-TGC-CGA-TCT-CAG-TAT-T3′ |
Hormone and Metabolite Assays
Blood glucose levels and serum insulin concentrations were measured as described previously (29). The concentration of serum glucagon was determined by radioimmunoassay with the Millipore glucagon radioimmunoassay kit (EMD Millipore Corp., Billerica, MA). Glucose tolerance was evaluated as described previously (29). For insulin release determination, glucose (3 g/kg body weight) was injected intraperitoneally, and venous blood was collected from tail vein at 0, 2, 5, 15, and 30 min in pre-chilled tubes (Microvette® CB 300, SARSTEDT) and immediately centrifuged to separate plasma, which was stored at −20 °C. Insulin levels were measured by ELISA (Crystal Chemical, Chicago).
Microarray Analysis
RNA samples were obtained following Affymetrix recommendations (Expression Analysis Technical Manual). Microarray analysis was performed by Progenika (Bilbao, Spain). The final gene list contained only those probe sets with a p < 0.05. For the interpretation, cross-checking and visualization of the data, FatiGO term enrichment (release date 11/20/2010), Database for Annotation, Visualization and Integrated Discovery (DAVID; david.abcc.ncifcrf.gov), Kyoto Encyclopedia of Genes and Genomes, and GeneCodis were used. Array data have been submitted to MIAMExpress (E-MEXP-3786).
Statistical Analysis
All values are expressed as the mean ± S.E. Differences between groups were compared by Student's t test. A p value less than 0.05 was considered statistically significant.
Results
Local IGF2 Overexpression Impairs Islet Function
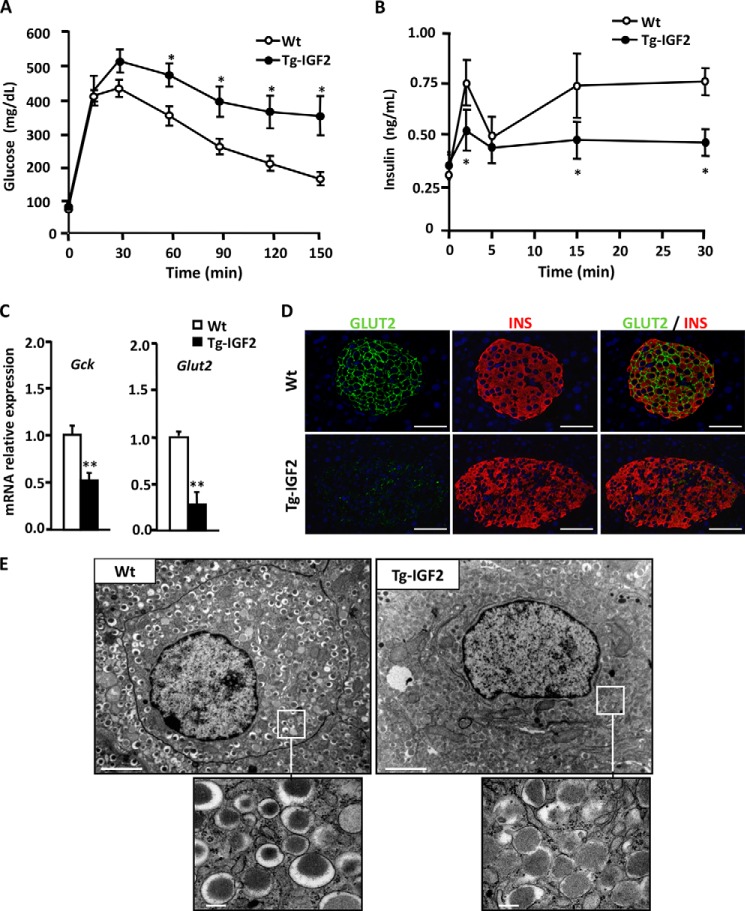
Transgenic mice overexpressing IGF2 specifically in β-cells (Tg-IGF2) have disrupted islet structure and islet hyperplasia and develop a pre-diabetic state (27). To study the role of IGF2 in β-cell function at the initial stages of the diabetic process, we performed a glucose tolerance test and in vivo insulin release (IR) test on 4-month-old mice. Tg-IGF2 mice showed glucose intolerance (Fig. 1A) (27) and were accompanied by reduced insulin release (Fig. 1B). In particular, the first phase of insulin secretion in response to glucose was greatly diminished in these mice (Fig. 1B), suggesting an impaired secretory response by β-cells. Tg-IGF2 mice were, however, hyperinsulinemic (WT, 1.2 ± 0.16 ng/ml; Tg-IGF2, 2.2 ± 0.19 ng/ml, p < 0.05 (27)), which reflected the fact that these animals have a β-cell mass 3-fold higher than WT littermates (27). In contrast, Tg-IGF2 mice presented a normal α-cell mass (27), and no differences were observed in the levels of islet glucagon mRNA (1 ± 0.35 versus 0.81 ± 0.19 relative units for wild type and Tg-IGF2, respectively, p ≤ 0.31) or serum glucagon (WT = 100.9 ± 8.2 versus Tg-IGF2 = 131.2 ± 26.5 pg/ml, p ≤ 0.21). In agreement with the abnormal insulin release shown by Tg-IGF2 upon glucose stimulation, the β-cell glucose sensors, solute carrier family member 2 (Slc2a2 or Glut2) and glucokinase (Gck), were down-regulated in transgenic islets (Fig. 1C). Immunohistochemical analysis further confirmed the dramatic reduction in SLC2A2 protein in insulin-positive cells in these animals (Fig. 1D). Moreover, the ultrastructural analysis of β-cells by electron microscopy revealed an increased number of immature insulin secretory granules with low or intermediate electron density occupying most of the cytoplasm of Tg-IGF2 β-cells (Fig. 1E). Altogether, these results provide evidence of β-cell dysfunction and inadequate secretory response in Tg-IGF2 mice.
FIGURE 1.
Local overexpression of IGF2 alters islet functionality. A, Tg-IGF2 mice showed altered glucose tolerance test at 4 months of age. Three-month-old WT (white circle) and Tg-IGF2 (black circle) mice were given an intraperitoneal injection of 2 mg of glucose/g bw, and blood glucose was measured at the indicated times as described under “Experimental Procedures.” Results are expressed as mean ± S.E. of 10 animals/group. *, p < 0.05. B, in vivo insulin release test after intraperitoneal glucose injection (3 g/kg bw) to 4-month-old WT mice (white circles) and Tg-IGF2 mice (black circles), n = 10 animals/group (*, p < 0.05). C, analysis of the expression of β-cell glucose sensor genes, Gck and Slc2a2/Glut2, by qPCR on WT (white bars) and Tg-IGF2 (black bars) islets from 4-month-old mice. Data are mean ± S.E. of at least three pools of islets from eight animals/group. **, p < 0.01. D, immunohistochemical detection of INS (red) and GLUT2 (green) in pancreatic sections of 3-month-old WT and Tg-IGF2 mice. Original magnification ×400 (scale bar, 50 μm). E, electron microscopy of β-cells in pancreatic islets isolated from 4-month-old WT and Tg-IGF2 mice. Tg-IGF2 β-cells presented altered ultrastructure (×10,000) (scale bar, 2 μm). Inset images show secretory granules in more detail (×80,000) (scale bar, 0.2 μm).
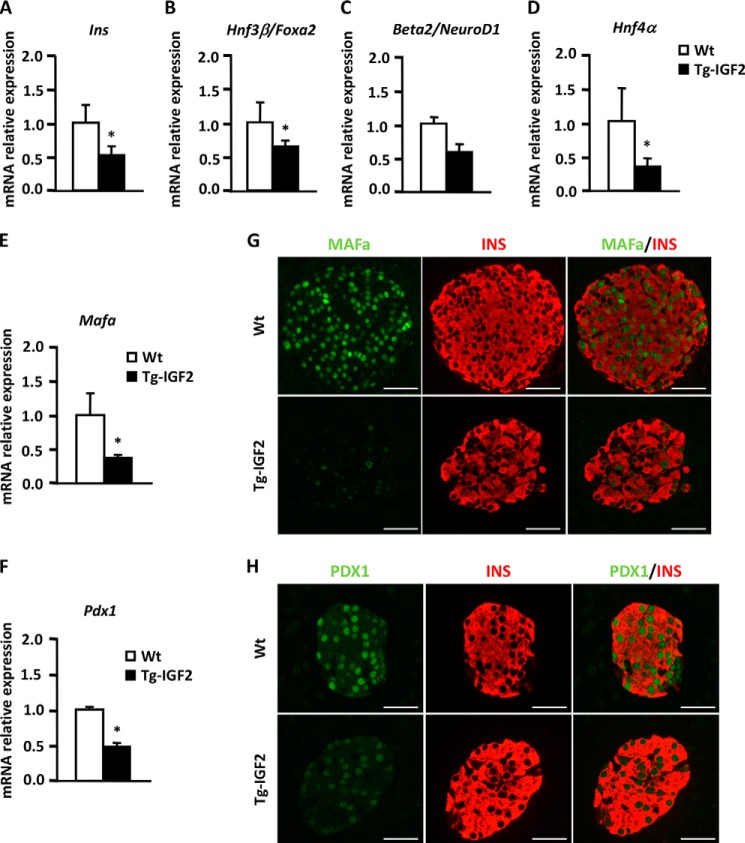
To gain insight into the IGF2-mediated molecular mechanisms that could account for β-cell dysfunction, we determined the expression of key genes involved in β-cell functionality. By qPCR, we observed that Insulin I mRNA levels were decreased in Tg-IGF2 compared with WT islets (Fig. 2A), and this agreed with the loss of the second phase of insulin release (Fig. 1A). The expression of critical transcription factors for insulin production such as forehead box A2 (Foxa2), neurogenic differentiation 1 (Neurod1), hepatic nuclear factor 4α (Hnf4α), v-maf musculoaponeurotic fibrosarcoma oncogene family (Mafa), and pancreatic and duodenal homeobox 1 (Pdx1) was also down-regulated (Fig. 2, B–F). Decreased production of MAFa and PDX1 was further confirmed by immunohistochemical analysis (Fig. 2, G and H). In addition, we performed microarray analysis on islets isolated from Tg-IGF2 and WT mice. After normalization and statistical filtering of the data, more than 300 genes with a 2-fold up- or down-regulation were identified, with the majority of them down-regulated and involved, according to gene ontology classification, in insulin secretion, development, cellular stress, immunity, transport, electron transport chain, and replication (Table 2). Noticeably, there was down-regulation of several genes characteristic of fully differentiated and functional β-cells, such as proprotein convertase subtilisin/kexin type 1 (Pcsk1/PC1/3), forkhead box A2 (Foxa2), and lipin 1 (lpin1) involved in insulin secretion, insulin promoter factor 1, homeodomain transcription factor (Ipf1/Pdx1), and solute carrier family 2, member 2 (Slc2a2). Altogether, these results support the notion that β-cells undergo a process of dedifferentiation resulting in functional alterations due to the overexpression of IGF2.
FIGURE 2.
IGF2 overexpression leads to changes in β-cell phenotype. A–F, confirmation of the differential gene expression of β-cell markers in WT (white bars) and Tg-IGF2 (black bars) islets from 4-month-old mice by qPCR. Data are expressed as mean ± S.E. of at least three pools of islets from eight animals/group. *, p < 0.05. G, immunohistochemical detection of INS (red) and MAFa (green) in pancreatic sections from 3-month-old mice. Original magnification ×400 (scale bar, 50 μm). H, immunohistochemical detection of INS (red) and PDX1 (green) in pancreatic sections from 3-month-old mice. Original magnification ×400 (scale bar, 50 μm).
TABLE 2.
Differentially expressed genes
Microarray expression analysis in islets from WT and Tg-IGF2 2-month-old mice. Classification of up- and down-regulated genes according to their biological function reported in the literature. RNA was isolated from three pools of islets from nine animals per group.
| Gene description | Gene symbol | Fold change | p value | RefSeq |
|---|---|---|---|---|
| Development | ||||
| Myeloid ecotropic viral integration site-related gene 1 | Meis2 | 0.67 | 0.03160 | NM 010825 |
| ISL1 transcription factor, LIM/homeodomain (islet 1) | Isl1 | 0.42 | 0.04980 | NM_021459 |
| Insulin promoter factor 1, homeodomain transcription factor | Pdx1 | 0.58 | 0.03740 | NM_ 008814 |
| Notch gene homolog 3 (Drosophila) | Nocth3 | 2.11 | 0.00484 | NM 00876.2 |
| Insulin secretion | ||||
| Activating transcription factor 2 | Atf2 | 0.45 | 0.02090 | NM_009715 |
| Calcyphosphine 2 | Caps2 | 0.35 | 0.03340 | NM_178278 |
| Dipeptidylpeptidase 4 | Dpp4 | 0.60 | 0.01890 | NM 010074 |
| ERO1-like (Saccharomyces cerevisiae) /// hypothetical gene supported by AK009667; NM_01577 | Ero1l /// LOC434 | 0.44 | 0.00139 | NM_015774 |
| Forkhead box A2 | Foxa 2 | 0.58 | 0.04580 | NM_010446 |
| Gap junction membrane channel protein α9 | Gja9/Cx36 | 0.38 | 0.03470 | NM 010290 |
| Potassium channel, subfamily K, member 16 | Kcnk16 | 0.52 | 0.00696 | NM_029006 |
| Lipin 1 | Lpin1 | 0.59 | 0.03970 | NM 172950 |
| Proprotein convertase subtilisin/kexin type 1 | Pcsk1/PC1/3 | 0.46 | 0.01790 | NM 13628 |
| N-Ethylmaleimide-sensitive fusion protein | Nsf | 0.70 | 0.03610 | NM_008740 |
| Rap guanine nucleotide exchange factor (GEF) 3 | Rapgef3 | 2.15 | 0.02210 | NM_144850 |
| Synaptotagmin-like 4 | Sytl4 | 0.33 | 0.04260 | NM_013757 |
| Transthyretin | Ttr | 0.40 | 0.02690 | NM_013697 |
| Urocortin 3 | Ucn3 | 0.23 | 0.00894 | NM_031250 |
| Metal-response element-binding transcription factor 1 | Mtf1 | 0.52 | 0.03510 | NM_008636 |
| Stress | ||||
| Autocrine motility factor receptor | Amfr | 0.62 | 0.02980 | NM_011787 |
| Activating transcription factor 2 | Atf2 | 0.45 | 0.02090 | NM_009715 |
| ATPase, Cu2+ transporting, α-polypeptide | Atp7a | 0.73 | 0.03470 | NM_009726 |
| Der1-like domain family, member 2 | Derl2 | 0.53 | 0.01260 | NM_033562 |
| ERO1-like (S. cerevisiae) /// hypothetical gene supported by AK009667; NM_01577 | Ero1l | 0.44 | 0.00139 | NM_015774 |
| F-box and leucine-rich repeat protein 5 | Fbxl5 | 0.65 | 0.03600 | NM_178729 |
| Forkhead box M1 | Foxm1 | 1.33 | 0.03840 | NM_027697 |
| Heat shock protein 1 | Hspb1 | 1.91 | 0.02100 | NM_013560 |
| Methyl CpG-binding protein 2 | Mecp2 | 0.63 | 0.02480 | NM_010788 |
| Metal-response element-binding transcription factor 1 | Mtf1 | 0.52 | 0.03510 | NM_008636 |
| Polo-like kinase 3 (Drosophila) | Plk3 | 0.42 | 0.00505 | NM_013807 |
| Autophagy 10-like (S. cerevisiae) | Atg10 | 0.64 | 0.04880 | NM_025770 |
| Immune response | ||||
| Alanyl-tRNA synthetase | Aars | 0.68 | 0.04430 | NM 146217 |
| Glutamic acid decarboxylase 1 | Gad1 | 0.21 | 0.01030 | NM 008077 |
| Cathepsin H | Ctsh | 2.42 | 0.02940 | NM_007801 |
| Immunoglobulin heavy chain (J558 family) | Igh-VJ558 | 8.33 | 0.00888 | NM_134051 |
| Endothelial differentiation, sphingolipid G-protein-coupled receptor, 3 | Edg3 | 2.96 | 0.03100 | NM_010101 |
| Histocompatibility 2, class II antigen A, α | H2-Aa | 2.70 | 0.01940 | NM_010378 |
| Transport | ||||
| Solute carrier family 9 (sodium/hydrogen exchanger), isoform 3 regulator 2 | Slc9a3r2 | 1.50 | 0.03590 | NM_023055 |
| Solute carrier family 35, member 3 | Slc35a3 | 0.66 | 0.04800 | NM_144902 |
| Solute carrier family 2 (facilitated glucose transporter), member 2 | Slc2a2 | 0.24 | 0.04170 | NM_031197 |
| solute carrier family 20, member 2 | Slc20a2 | 0.60 | 0.04070 | NM_011394 |
| Solute carrier family 12, member 7 | Scl12a7 | 0.57 | 0.03840 | NM_011390 |
| Solute carrier family 4, sodium bicarbonate cotransporter, member 7 | Slc4a7 | 0.47 | 0.01890 | NM_001033270 |
| Solute carrier family 2 (facilitated glucose transporter), member 5 | Slc2a5 | 0.61 | 0.04950 | NM_019741 |
| Potassium large conductance calcium-activated channel, β member 2 | Kcnmb2 | 0.50 | 0.00456 | NM_028231 |
| Activating transcription factor 2 | Atf2 | 0.45 | 0.02090 | NM_009715 |
| Calcium channel, voltage-dependent, β2 subunit | Cacnb2 | 0.68 | 0.02080 | NM_023116 |
| Transport chain | ||||
| ATPase, H+-transporting, V1 subunit B, isoform 2 | Atp6v1b2 | 0.77 | 0.04870 | NM_007509 |
| ATPase, H+-transporting, V0 subunit C | Atp6v0c | 0.61 | 0.04770 | NM_009729 |
| ATPase, aminophospholipid transporter (APLT), class I, type 8A, member 1 | Atp8a1 | 0.62 | 0.04630 | NM_009727 |
| ATPase, Cu2+-transporting, α-polypeptide | Atp7a | 0.64 | 0.04200 | NM_009726 |
| ATPase, Ca2+-sequestering | Atp2c1 | 0.55 | 0.03890 | NM_175025 |
| ATPase, Ca2+-transporting, cardiac muscle, slow twitch 2 | Atp2a2 | 0.49 | 0.03040 | NM_009722 |
| Cytochrome P450, family 4, subfamily v, polypeptide 3 | Cyp4v3 | 0.53 | 0.03790 | NM_133969 |
| NADH dehydrogenase (ubiquinone) 1, subcomplex unknown, 2 | Ndufc2 | 0.60 | 0.03220 | NM_024220 |
| Replication | ||||
| Cyclin D2 | Ccnd2 | 0.49 | 0.04340 | NM_009829 |
| Prolactin receptor | Prlr | 0.45 | 0.02650 | NM_011169 |
| B-cell translocation gene 1, anti-proliferative | Btg1 | 0.68 | 0.03340 | NM_007569 |
IGF2 Overexpression Induces ER Stress in β-Cells
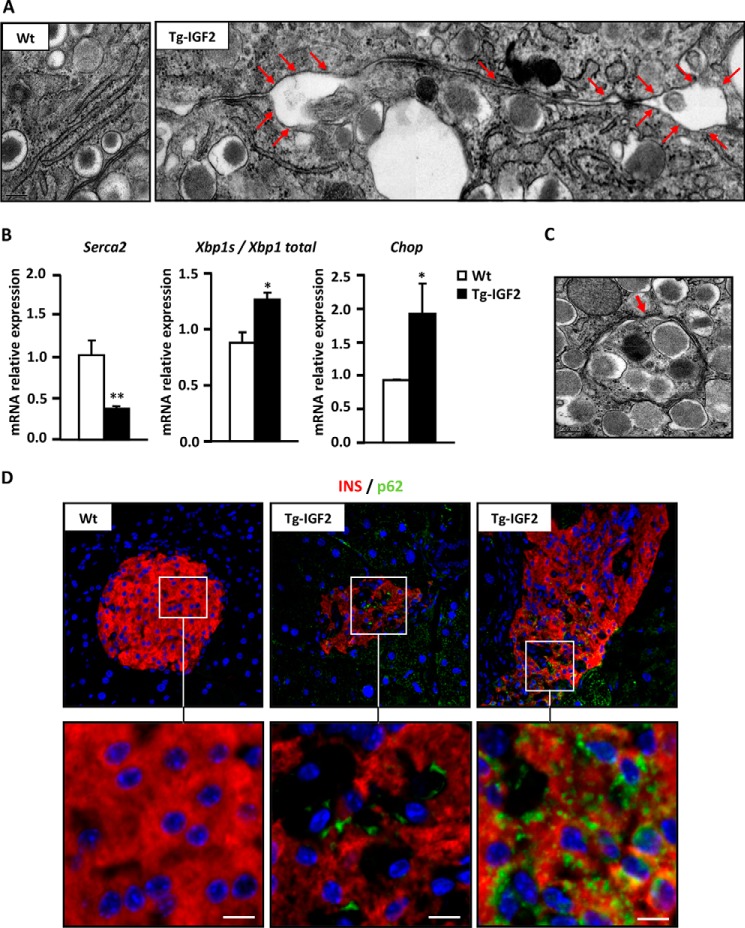
It has been reported that endoplasmic reticulum (ER) stress is involved in β-cell failure (31). Electron microscopy observation of Tg-IGF2 β-cells showed an expanded ER network (Fig. 3A, arrows), which is indicative of ER stress induced by the unfolded protein response (32). Accordingly, microarray analysis (Table 2) and qPCR (Fig. 3B) revealed down-regulation of the ATPase, Ca2+-transporting cardiac muscle, slow twitch 2 (Atp2a2/Serca2) gene in Tg-IGF2 islets. The SERCA protein regulates Ca2+ flux in and out of the ER and, as a consequence, controls proper protein folding (33). Moreover, markers of ER stress such as spliced x-box-binding protein 1 (Xbp1s) and DNA damage-inducible transcript 3 (Ddit3/Chop) were also up-regulated in Tg-IGF2 (Fig. 3B).
FIGURE 3.
ER stress and autophagy in Tg-IGF2 islets. A, electron microscopy of β-cells in islets of 4-month-old WT and Tg-IGF2 mice. Rough endoplasmic reticulum showing a normal lamellar arrangement is observed in WT, whereas Tg-IGF2 presented altered ER lamellar arrangement and dilatations as indicated by red arrows (composite image). Original magnification ×100,000. B, validation of differential gene expression of ER stress markers in WT (white bars) and Tg-IGF2 (black bars) islets from 4-month-old mice by qPCR. Data are expressed as mean ± S.E. of at least three pools of islets from eight animals/group. *, p < 0.05; **, p < 0.01. C, electron microscopy of β-cells in islets of 4-month-old Tg-IGF2 mice. An autophagic body, a cytoplasmic inclusion surrounded by a double membrane containing part of the cytoplasm, is indicated by red arrows in an image corresponding to Tg-IGF2 β-cells (×80,000). D, immunohistochemical detection of insulin (red) and LGAL3/p62 (green) in pancreatic sections from 3-month-old mice. Inset images show in detail the positive p62 signal observed in β-cells of Tg-IGF2 islets. Scale bar, 8 μm.
Autophagy has also been associated with ER stress and plays an important role in diabetes pathogenesis (34). Electron microscopy analysis revealed the presence in Tg-IGF2 β-cells of autophagic bodies or autophagosomes, identified as portions of the cytoplasm surrounded by a double membrane (Fig. 3C). An increase in the immunohistochemistry signal intensity of SQSTM1 (p62), a ubiquitin-binding protein also known as sequestosome 1 that co-localizes to autophagic bodies (35), was detected in most of the unstructured islets of Tg-IGF2 mice (Fig. 3D), confirming an increase in autophagy in Tg-IGF2 islets.
IGF2 Is Sufficient to Trigger β-Cell Dysfunction
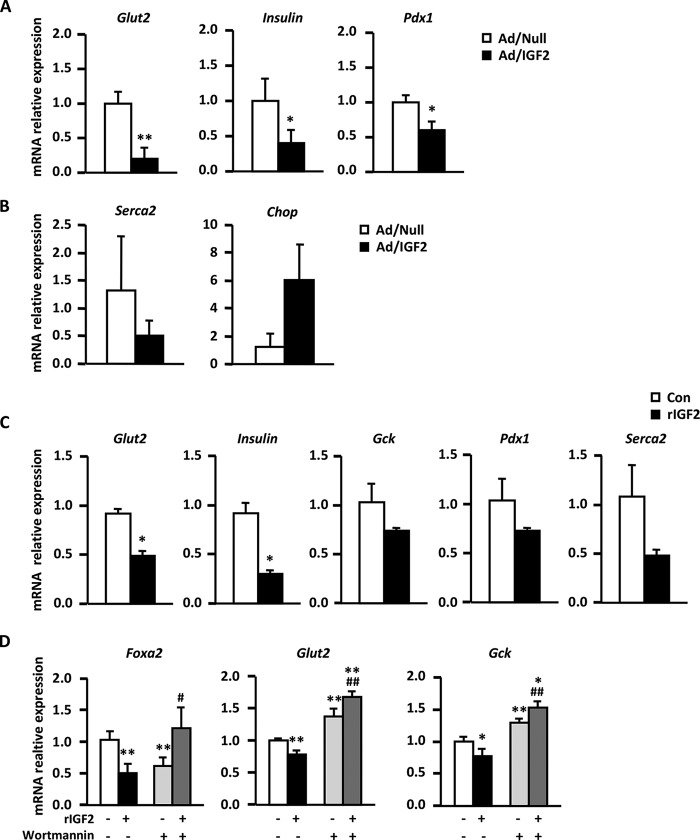
To discard the possibility that the β-cell dysfunction observed in Tg-IGF2 mice was due to IGF2 overexpression from early embryonic development, islets isolated from adult WT animals were transduced in vitro with adenoviral vectors encoding murine Igf2 (Ad/IGF2) or with null vectors (Ad/Null) that served as controls. Islets transduced with Ad/IGF2 vectors presented a similar phenotype to that observed in Tg-IGF2 islets, e.g. decreased Slc2a2, Pdx1, insulin I, and Atpa2/Serca2 expression and increased levels of Ddit3/Chop, although changes in Serca2 and Ddit3/Chop did not reach statistical significance (Fig. 4, A and B). Thus, IGF2 overexpression in adult islets is sufficient to alter β-cell functionality by diminishing insulin production and inducing ER stress.
FIGURE 4.
IGF2 effects on adult WT islets. A, expression of β-cell markers by qPCR analysis in WT islets transduced with null control vector (Ad/Null, white bars) or adenovirus expressing IGF2 (Ad/IGF2, black bars). B, expression of ER stress genes by qPCR in the same adenovirus-transduced islets. Three pools of islets from eight animals/group were used. C, gene expression analysis of β-cell markers by qPCR in WT islets incubated for 48 h with either vehicle (white bars) or 100 ng/ml recombinant IGF2 protein (black bars). Three pools of islets from eight animals/group were used. D, analysis by qPCR of Foxa2, Slc2a2/Glut2, and Gck genes in INS-1 cells treated for 48 h with vehicle (white bars), 100 ng/ml recombinant IGF2 protein (black bars), 200 nm of the PI3K inhibitor wortmannin (light gray bars), or both (dark gray bars). Results shown represent the data obtained for at least six wells/condition and from three independent experiments. Data are expressed as mean ± S.E. *, p < 0.05; **, p < 0.01 versus vehicle-treated cells. #, p < 0.05, and ##, p <0.01 versus recombinant IGF2-treated cells.
To study whether the phenotype of Tg-IGF2- and Ad/IGF2-transduced islets was due to the forced overexpression of IGF2 rather than a direct effect of IGF2 on β-cells, islets isolated from adult WT mice were also incubated for 48 h with recombinant IGF2 protein (rIGF2) to mimic the chronic exposure to IGF2 of Tg-IGF2 islets. Similarly to Tg-IGF2 and Ad/IGF2-transduced islets, rIGF2-treated islets presented a significant decrease in Slc2a2 and insulin I expression and a nonstatistically significant down-regulation of Gck, Pdx1, and Atp2a2/Serca2 (Fig. 4C). Similar results were obtained in rIGF2-treated INS-1 cells (data not shown).
Given that IGF2 binds with high affinity to the insulin (INSR), insulin-like growth factor 1 (IGF1R), and IGF2 receptors, we quantified the expression of these receptors in Tg-IGF2 islets. No differences were observed in the expression of IGF2R, IGF1R, or INSR-B (data not shown). However, a moderate (about 40%) statistically significant decrease in INSR-A expression was documented in Tg-IGF2 islets (WT 1.03 ± 0.11 versus Tg-IGF2 0.67 ± 0.05, p = 0.03). Next, we analyzed the effects of IGF2 on gene expression by inhibiting both INSR and IGF1R downstream signaling pathways using wortmannin, a PI3K inhibitor (36). INS-1 cells cultured in the presence of rIGF2 showed decreased expression of markers of β-cell identity (Fig. 4D), which recapitulated the observations made in Tg-IGF2 islets (Figs. 1B and 2). The addition of wortmannin per se altered the expression of Foxa2, Slc2a2, and Gck (Fig. 4D). Despite this unexplained observation, we did document a significant inhibition of the effects of rIGF2 by wortmannin treatment (Fig. 4D), indicating that at least partially the IGF2 effects on β-cell expression were mediated by PI3K activation.
IGF2 Transgenic Islets Are More Susceptible to β-Cell Damage
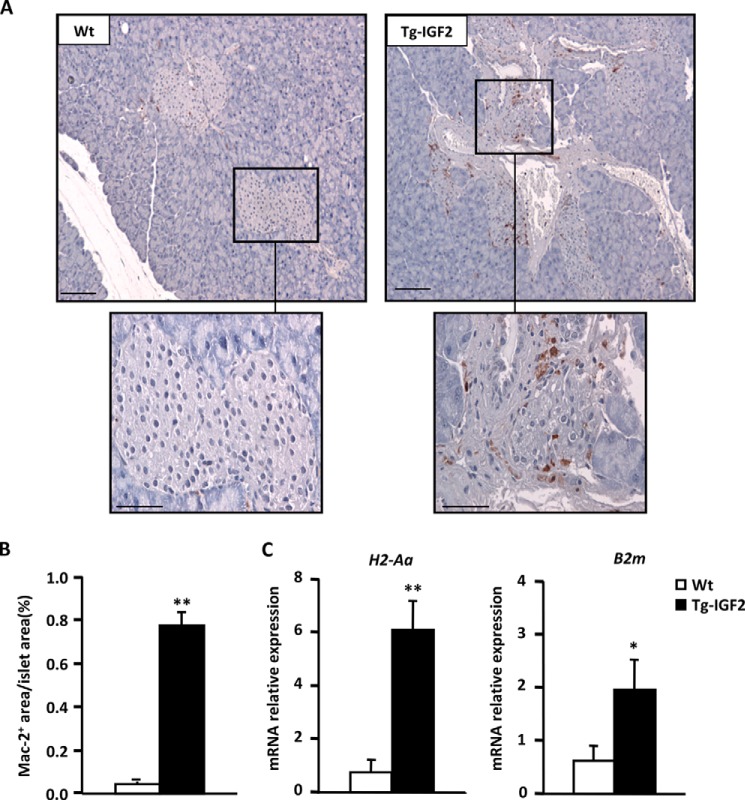
Microarray analysis of Tg-IGF2 islets showed altered expression of several genes involved in the immune response, such as cathepsin H (Ctsh), immunoglobulin heavy chain (J558 family, Igh-VJ558), endothelial differentiation, sphingosine 1-phosphate receptor 3 (Edg3), and histocompatibility 2, class II antigen A, α (H2-Aa) (data not shown). Although CD4- and CD8- positive lymphocytes could not be detected in pancreata from Tg-IGF2 mice (Table 2), immunostaining against LGAL3/MAC-2 revealed the presence of macrophages infiltrating Tg-IGF2 islets (Fig. 5, A and B). qPCR analysis confirmed the increased expression of H2-Aa and β2-microglobulin (B2m) (Fig. 5C), indicating an up-regulation of major histocompatibility complex (MHC) class I and II antigens in Tg-IGF2 islets, likely due, at least in part, to macrophage infiltration. These results suggested that Tg-IGF2 β-cells could be more susceptible to immune cell-mediated damage.
FIGURE 5.
IGF2 overexpression leads to islet macrophage infiltration. A, macrophage infiltration was determined by LGAL3/MAC-2 immunostaining on islet sections from 3-month-old mice. Original magnification ×10 (scale bar, 100 μm) and insets ×40 (scale bar, 50 μm). B, quantification of LGAL3/MAC-2-positive area. Data are expressed as mean ± S.E. of percentage of LGAL3/MAC-2-positive islet areas/total islet areas. n = 5 animals/group. WT (white bars) and Tg-IGF2 (black bars). C, analysis of gene expression by qPCR of histocompatibility 2, class II antigen Aα (H2-Aa), β-2 microglobulin (B2-m) genes in WT (white bars), and Tg-IGF2 (black bars) islets from 4-month-old mice. Results are expressed as mean ± S.E. of at least three pools of islets from eight animals/group. *, p < 0.05; **, p < 0.01.
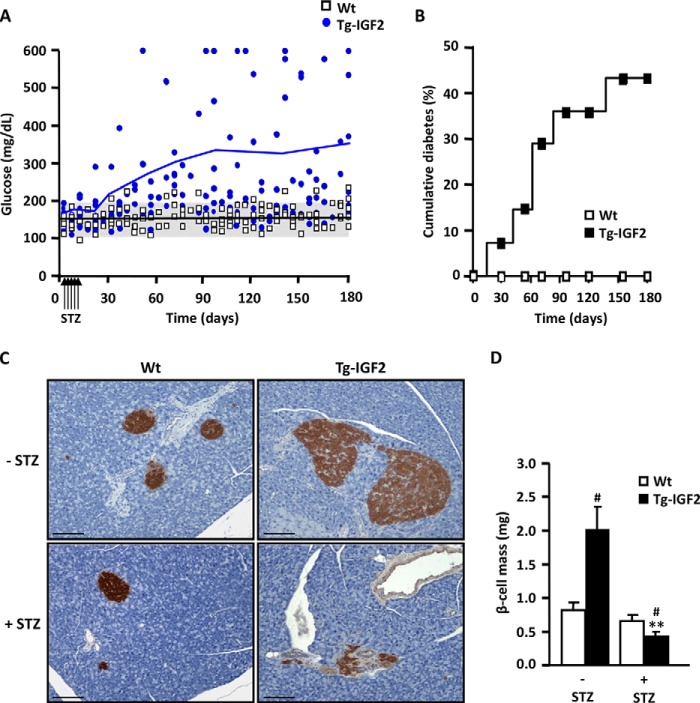
To verify this hypothesis and to determine the degree of sensitivity to damage of their β-cells, Tg-IGF2 mice received five daily consecutive injections of very low doses of streptozotocin (STZ), 20 mg/kg bw, a dose too low to affect WT mice (29). In contrast to WT mice, which remained normoglycemic, Tg-IGF2 mice showed a progressive increase in blood glucose levels (Fig. 6A) with ∼45% of transgenics becoming diabetic 6 months post-STZ administration (Fig. 6B). This increased incidence of diabetes was due to a drastic reduction in β-cell mass in STZ-treated Tg-IGF2 mice (Fig. 6, C and D) even though these mice have a pre-STZ β-cell mass 3-fold higher than that of WT mice (27). Thus, these results confirmed that local overexpression of IGF2 in β-cells increased susceptibility to injury caused by an external toxin, such as STZ.
FIGURE 6.
Transgenic IGF2 mice are more sensitive to streptozotocin treatment. A, blood glucose levels were monitored in fed mice for 6 months following treatment with very low doses of STZ for 5 consecutive days (20 mg/kg bw). WT mice (white squares) and transgenic IGF2 mice (blue circles) (n = 15 animals/group). B, cumulative incidence of diabetes before and after STZ treatment. Mice were considered diabetic when two consecutive glucose measurements were >250 mg/dl. WT mice (white squares) and transgenic IGF2 mice (black squares) (n = 15 animals/group). C, insulin immunostaining of pancreatic sections 6 months after STZ treatment. Transgenic IGF2 mice clearly presented fewer insulin-positive cells. Representative images, original magnification ×10 (scale bar, 100 μm). D, β-cell mass was measured 6 months after STZ treatment in WT (white bars) and Tg-IGF2 (black bars) mice. Nine pancreatic sections from each individual mouse (four mice/group) were analyzed as described under “Experimental Procedures.” Results are expressed as mean ± S.E. #, p < 0.05 versus WT; **, p < 0.01 versus Tg-IGF2-STZ.
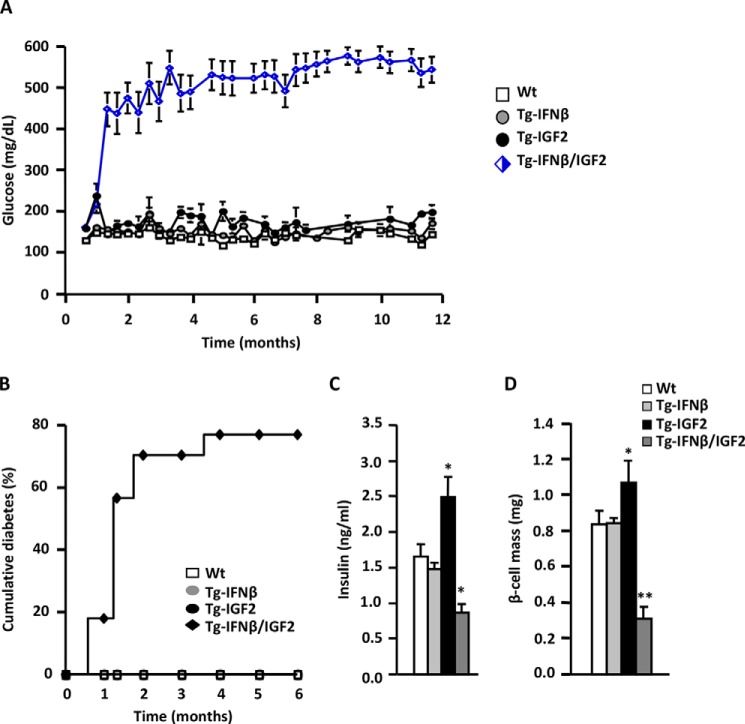
To further study the increased susceptibility of Tg-IGF2 β-cells to damage, Tg-IGF2 mice (C57Bl6/SJL background) were crossed with transgenic mice expressing human interferon β specifically in β-cells (Tg-IFNβ, CD1 background). Tg-IFNβ mice have islet lymphocytic infiltration and increased susceptibility to develop autoimmune diabetes with similar characteristics to humans type 1 diabetes (29). Although WT, Tg-IGF2, and Tg-IFNβ mice (all in 50% C57Bl6/SJL-50% CD1 background) remained normoglycemic during the 12-month follow-up, double transgenic mice Tg-IFNβ/IGF2 (50% C57Bl6/SJL-50% CD1 background) were highly hyperglycemic as early as 1 month of age (Fig. 7A), with 75% of them developing spontaneous diabetes during the first 2 months of life (Fig. 7B). At 3 month of age, WT and Tg-IFNβ mice were normoinsulinemic and had normal β-cell mass, whereas Tg-IGF2 mice showed hyperinsulinemia and β-cell hyperplasia (Fig. 7, C and D). β-Cell hyperplasia in Tg-IGF2 mice was lower that what we previously described (Fig. 6D) (27). This was likely due to a variation in the genetic background from 100% C57Bl6/SJL in the original study, a background more prone to islet hyperplasia (37, 38), to 50% C57Bl6/SJL-50%CD1 in the Tg-IGF2 that were littermates of Tg-IFNβ/IGF2. In agreement with the high incidence of spontaneous diabetes, double Tg-IFNβ/IGF2 mice had lower circulating levels of insulin and decreased β-cell mass (Fig. 7, C and D).
FIGURE 7.
Double IFNβ/IGF2 transgenic mice develop diabetes spontaneously. A, blood glucose levels were monitored in fed WT (white squares), transgenic IFNβ (gray circles), transgenic IGF2 (black circles), and double transgenic IFNβ/IGF2 mice (blue-white rhombus) mice from 0.5 to 12 months of age, n = 40 animals/group. B, cumulative incidence of diabetes in WT (white squares), transgenic IFNβ (gray circles), transgenic IGF2 (black circles), and double transgenic IFNβ/IGF2 (black rhombus) mice, n = 40 animals/group. Mice were considered diabetic when two consecutive glucose measurements were >250 mg/dl. C, serum insulin levels at 3 months of age in WT (white bars), Tg-IFNβ (light gray bars), Tg-IGF2 (black bars), and double Tg-IGF2/IFNβ (dark gray bars) mice. Results are expressed as mean ± S.E. of 15 animals/group. *, p < 0.05 versus WT. D, β-cell mass was measured at 3 months of age in WT (white bars), Tg-IFNβ (light gray bars), Tg-IGF2 (black bars), and double Tg-IFNβ/IGF2 (dark gray bars) mice. Nine pancreatic sections from each individual mouse (four mice/group) were analyzed as described under “Experimental Procedures.” Results are means ±S.E. of six animals/group. *, p < 0.05; **, p < 0.01 versus WT.
Discussion
Our group previously reported that local overexpression of IGF2 in β-cells predisposes to the development of T2D (27). Mice overexpressing IGF2 are mildly hyperglycemic, hyperinsulinemic, and insulin-resistant, and when subjected to a high-fat diet they develop overt diabetes (27). The increased β-cell mass and the high degree of islet disorganization present in Tg-IGF2 mice could account for the islet functional failure that characterizes these animals. The molecular bases underlying the observed phenotype, however, were not fully understood nor to what extent the overexpression of IGF2 during embryonic development contributed to the process. In this study, we demonstrate that β-cell-restricted IGF2 overexpression triggers β-cell dedifferentiation and dysfunction and increases their susceptibility to damage, highlighting the important role that this growth factor may have in the progression of T2D.
In our previous work, we reported that insulin expression in total pancreata and in vitro glucose-stimulated insulin secretion by isolated islets were greater in Tg-IGF2 than in WT mice, which agreed with the hyperinsulinemia described in transgenic animals (27). For the in vitro experiments, an equal number of WT and Tg-IGF2 islets were used, but because transgenic islets are considerably larger, this study was not illustrative of the amount of insulin produced by each β-cell. In this work, we observed that Tg-IGF2 mice secreted less insulin in response to a glucose overload than WT mice. Other animal models with islet hyperplasia also display impaired insulin secretion, such as the IR/IRS-1−/− and IRS1−/− knock-out mice (39, 40), and this has been attributed to altered β-cell functionality. Noticeably, β-cell dysfunction and alterations in insulin secretion have been described to precede insulin resistance in human patients at risk of developing T2D (41, 42). Similarly, Tg-IGF2 mice show glucose intolerance from an early age (2 months) and develop insulin resistance when they are older than 4 months (27). Thus, β-cell failure would be a primary defect and not just a simple consequence of diabetes progression in this model. A clear reduction in the expression of the main glucose sensors of the β-cell, the glucose transporter GLUT2 and the glucose-phosphorylating enzyme glucokinase, was documented in islets from Tg-IGF2 mice or in wild-type islets treated with recombinant IGF2 or with adenoviral vectors that mediate overexpression of IGF2 in β-cells. In addition, microarray profiling analysis of Tg-IGF2 islets revealed alterations in the expression of several genes involved in insulin secretion and cell transport, as well as of several transcription factors important for β-cell differentiation and functionality, such as Pdx1, Isl1, MafA, and Beta2/Neurod1. These changes could partially explain the clear β-cell dysfunction observed in our transgenic mice, mainly the reduced insulin synthesis and secretion, and the dedifferentiated phenotype of Tg-IGF2 β-cells. Other type 2 diabetic animal models, such as db/db mice and Zucker and Goto-Kakizaki rats, which have similar characteristics to Tg-IGF2 mice, i.e. islet hyperplasia, hyperinsulinemia, insulin resistance, and altered insulin secretion and response to glucose, also show a partial loss of β-cell phenotype (12, 43). Moreover, mice lacking FoxO1 specifically in β-cells have high blood glucose concentration associated with reduced β-cell mass that was attributed to β-cell dedifferentiation rather than to β-cell death (6). A recent study proposes that β-cell dedifferentiation, rather than apoptosis, is the main mechanism of loss of insulin-positive cells in a mouse model of T2D and that intensive insulin therapy, through the reversal of hyperglycemia, leads to re-differentiation of β-cells to a mature phenotype (9). These observations suggest that β-cell dedifferentiation is a common process in T2D (6) and that at least some forms of diabetes mellitus may result from loss of β-cell identity (7, 8). Our study supports this notion by demonstrating that IGF2 alone is capable of inducing changes in the β-cell phenotype, increasing the susceptibility to develop diabetes. Of note, when Tg-IGF2 mice were treated with low nondiabetogenic STZ doses, ∼45% of transgenic mice developed overt diabetes despite the fact that GLUT2, the transporter by which STZ enters the β-cell, was down-regulated.
Tg-IGF2 cells also presented other important cellular alterations. ER stress has been proposed as a mechanism to explain β-cell dysfunction and death in T2D (12, 44). Tg-IGF2 islets showed signs of ER stress. By electron microscopy, we observed the characteristic dilatations of the ER that accompany ER stress (31). In addition, the expression of markers of ER stress such as Xbp1s and Ddit3/Chop was increased, and the expression of Serca2, the main transporter of Ca2+ to the ER (45), was reduced. Some of these modifications in the expression of ER stress markers were also observed in wild-type islets treated with adenoviral vectors encoding Igf2 or in wild-type islets treated with recombinant protein, indicating that IGF2 per se was sufficient to induce ER stress in β-cells.
On the other hand, Tg-IGF2 islets showed increased expression of genes involved in the immune response, such as MHC class I and II, and a considerable number of macrophages infiltrated transgenic islets. Similarly, islets from Goto-Kakizaki rats, a model of T2D, have macrophage infiltration and an increased number of MHC-II-positive cells (46, 47). Macrophage infiltration has also been observed in islets from human T2D subjects (41). The immunological alterations observed at the islet level could probably predispose IGF2 islets to be more susceptible to immune cell-mediated attack and β-cell damage. Indeed, double transgenic Tg-IFNβ/IGF2 spontaneously developed overt diabetes at a very early age, without the need for administering STZ. These results indicate that islets overexpressing Igf2 have increased susceptibility to β-cell death. In addition, increased macrophage infiltration and expression of MHC-II molecules could contribute to the increased sensitivity to STZ of Tg-IGF2 β-cells.
The reproducibility of the observations made in Tg-IGF2 islets or in wild-type islets treated with IGF2 recombinant protein or with Igf2-encoding adenoviral vectors supports the idea that most of the observations made were direct effects of IGF2. Therefore, these observations were not the result of the overexpression of the growth factor during fetal pancreatic development.
The binding of IGF2 to IGF-II/Man-6-P receptor, its own receptor, has no other biological function than clearing IGF2 from serum and targeting it for degradation in lysosomes (48), thus balancing IGF2 activity by controlling the extracellular levels of IGF2 (49). Our in vitro studies demonstrated that IGF2 effects on β-cells were at least partially mediated through IGF1R or INSR signaling, because inhibition of the common downstream effector PI3K reversed the down-regulation of Glut2, Foxa2, and Gck expression. IGF2 can bind to INSR. In particular, isoform A (INSR-A) binds IGF2 with high affinity, and its activation by IGF2 elicits a unique signaling pathway different from that of insulin (50). Indeed, we observed a down-regulation of the INSR-A receptor, similar to what had previously been documented after hyperstimulation with insulin in various target tissues (51). Moreover, IGF2 can act through IGF1R and may even act through hybrid INSR-A/IGF1R receptors (52). Although we have observed detrimental effects of IGF2 on β-cell functionality, which resulted in increased sensitivity to β-cell damage, others have demonstrated that the interaction of IGF2 with the IGF1R is required for GLP-1-induced protection against β-cell apoptosis (53).
In summary, although there is still no direct evidence of the increase of IGF2 in T2D in humans, this study demonstrates that local overexpression of IGF2, specifically in β-cells, causes profound changes in β-cells that lead to their dedifferentiation, ER stress, and β-cell dysfunction. As a consequence of exposure to increased levels of IGF2, islets are predisposed to be more sensitive to immune- and nonimmune-mediated β-cell damage, triggering the onset of diabetes.
Acknowledgments
We thank Ivet Elias (Universitat Autònoma de Barcelona) for helpful discussions and Marta Moya (Universitat Autònoma de Barcelona) for technical support.
This work was supported in part by Ministerio de Economía y Competitividad, Plan Nacional I+D+I, Grants SAF2008-00962 and SAF2011-24698, and Generalitat de Catalunya Grants 2009 SGR-224 and ICREA Academia, Spain. The authors declare that they have no conflicts of interest with the contents of this article.
- T1D
- type 1 diabetes
- T2D
- type 2 diabetes
- INS
- insulin
- ER
- endoplasmic reticulum
- qPCR
- quantitative real time PCR
- STZ
- streptozotocin
- TRITC
- tetramethylrhodamine isothiocyanate
- bw
- body weight
- VNTR
- variable number of tandem repeats
- IR
- insulin release
- rIGF2
- recombinant IGF2.
References
- 1. Butler P. C., Meier J. J., Butler A. E., Bhushan A. (2007) The replication of beta cells in normal physiology, in disease, and for therapy. Nat. Clin. Pract. Endocrinol. Metab. 3, 758–768 [DOI] [PubMed] [Google Scholar]
- 2. Chang-Chen K. J., Mullur R., Bernal-Mizrachi E. (2008) β-Cell failure as a complication of diabetes. Rev. Endocr. Metab. Disord. 9, 329–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jonas J.-C., Sharma A., Hasenkamp W., Ilkova H., Patanè G., Laybutt R., Bonner-Weir S., Weir G. C. (1999) Chronic hyperglycemia triggers loss of pancreatic β cell differentiation in an animal model of diabetes. J. Biol. Chem. 274, 14112–14121 [DOI] [PubMed] [Google Scholar]
- 4. Weir G. C., Aguayo-Mazzucato C., Bonner-Weir S. (2013) β-Cell dedifferentiation in diabetes is important, but what is it? Islets 5, 233–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weir G. C., Marselli L., Marchetti P., Katsuta H., Jung M. H., Bonner-Weir S. (2009) Towards a better understanding of the contributions of overwork and glucotoxicity to the beta-cell inadequacy of type 2 diabetes. Diabetes Obes. Metab. 11, 82–90 [DOI] [PubMed] [Google Scholar]
- 6. Talchai C., Xuan S., Lin H. V., Sussel L., Accili D. (2012) Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell 150, 1223–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Puri S., Akiyama H., Hebrok M. (2013) VHL-mediated disruption of Sox9 activity compromises β-cell identity and results in diabetes mellitus. Genes Dev. 27, 2563–2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guo S., Dai C., Guo M., Taylor B., Harmon J. S., Sander M., Robertson R. P., Powers A. C., Stein R. (2013) Inactivation of specific β cell transcription factors in type 2 diabetes. J. Clin. Invest. 123, 3305–3316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang Z., York N. W., Nichols C. G., Remedi M. S. (2014) Pancreatic β cell dedifferentiation in diabetes and redifferentiation following insulin therapy. Cell Metab. 19, 872–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kitamura T. (2013) The role of FOXO1 in β-cell failure and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 9, 615–623 [DOI] [PubMed] [Google Scholar]
- 11. Iwawaki T., Oikawa D. (2013) The role of the unfolded protein response in diabetes mellitus. Semin. Immunopathol. 35, 333–350 [DOI] [PubMed] [Google Scholar]
- 12. Chan J. Y., Luzuriaga J., Bensellam M., Biden T. J., Laybutt D. R. (2013) Failure of the adaptive unfolded protein response in islets of obese mice is linked with abnormalities in β-cell gene expression and progression to diabetes. Diabetes 62, 1557–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tersey S. A., Nishiki Y., Templin A. T., Cabrera S. M., Stull N. D., Colvin S. C., Evans-Molina C., Rickus J. L., Maier B., Mirmira R. G. (2012) Islet β-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the nonobese diabetic mouse model. Diabetes 61, 818–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Engin F., Yermalovich A., Nguyen T., Ngyuen T., Hummasti S., Fu W., Eizirik D. L., Mathis D., Hotamisligil G. S. (2013) Restoration of the unfolded protein response in pancreatic β cells protects mice against type 1 diabetes. Sci. Transl. Med. 5, 211ra156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Allagnat F., Christulia F., Ortis F., Pirot P., Lortz S., Lenzen S., Eizirik D. L., Cardozo A. K. (2010) Sustained production of spliced X-box binding protein 1 (XBP1) induces pancreatic beta cell dysfunction and apoptosis. Diabetologia 53, 1120–1130 [DOI] [PubMed] [Google Scholar]
- 16. LeRoith D., Roberts C. T., Jr. (2003) The insulin-like growth factor system and cancer. Cancer Lett. 195, 127–137 [DOI] [PubMed] [Google Scholar]
- 17. Monk D., Sanches R., Arnaud P., Apostolidou S., Hills F. A., Abu-Amero S., Murrell A., Friess H., Reik W., Stanier P., Constância M., Moore G. E. (2006) Imprinting of IGF2 P0 transcript and novel alternatively spliced INS-IGF2 isoforms show differences between mouse and human. Hum. Mol. Genet. 15, 1259–1269 [DOI] [PubMed] [Google Scholar]
- 18. Nica A. C., Ongen H., Irminger J.-C., Bosco D., Berney T., Antonarakis S. E., Halban P. A., Dermitzakis E. T. (2013) Cell-type, allelic, and genetic signatures in the human pancreatic beta cell transcriptome. Genome Res. 23, 1554–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Awata T., Kawasaki E., Ikegami H., Kobayashi T., Maruyama T., Nakanishi K., Shimada A., Iizuka H., Kurihara S., Osaki M., Uga M., Kawabata Y., Tanaka S., Kanazawa Y., Katayama S. (2007) Insulin gene/IDDM2 locus in Japanese type 1 diabetes: contribution of class I alleles and influence of class I subdivision in susceptibility to type 1 diabetes. J. Clin. Endocrinol. Metab. 92, 1791–1795 [DOI] [PubMed] [Google Scholar]
- 20. Pugliese A., Miceli D. (2002) The insulin gene in diabetes. Diabetes Metab. Res. Rev. 18, 13–25 [DOI] [PubMed] [Google Scholar]
- 21. Huxtable S. J., Saker P. J., Haddad L., Walker M., Frayling T. M., Levy J. C., Hitman G. A., O'Rahilly S., Hattersley A. T., McCarthy M. I. (2000) Analysis of parent-offspring trios provides evidence for linkage and association between the insulin gene and type 2 diabetes mediated exclusively through paternally transmitted class III variable number tandem repeat alleles. Diabetes 49, 126–130 [DOI] [PubMed] [Google Scholar]
- 22. Dai N., Rapley J., Angel M., Yanik M. F., Blower M. D., Avruch J. (2011) mTOR phosphorylates IMP2 to promote IGF2 mRNA translation by internal ribosomal entry. Genes Dev. 25, 1159–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zeggini E., Weedon M. N., Lindgren C. M., Frayling T. M., Elliott K. S., Lango H., Timpson N. J., Perry J. R., Rayner N. W., Freathy R. M., Barrett J. C., Shields B., Morris A. P., Ellard S., Groves C. J., et al. (2007) Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 316, 1336–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chon S. J., Kim S. Y., Cho N. R., Min D. L., Hwang Y. J., Mamura M. (2013) Association of variants in PPARγ2, IGF2BP2, and KCNQ1 with a susceptibility to gestational diabetes mellitus in a Korean population. Yonsei Med. J. 54, 352–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Benrahma H., Charoute H., Lasram K., Boulouiz R., Atig R. K., Fakiri M., Rouba H., Abdelhak S., Barakat A. (2014) Association analysis of IGF2BP2, KCNJ11, and CDKAL1 polymorphisms with type 2 diabetes mellitus in a Moroccan population: a case-control study and meta-analysis. Biochem. Genet. 52, 430–442 [DOI] [PubMed] [Google Scholar]
- 26. Höög A., Sandberg-Nordqvist A. C., Abdel-Halim S. M., Carlsson-Skwirut C., Guenifi A., Tally M., Ostenson C. G., Falkmer S., Sara V. R., Efendić S., Schalling M., Grimelius L. (1996) Increased amounts of a high molecular weight insulin-like growth factor II (IGF-II) peptide and IGF-II messenger ribonucleic acid in pancreatic islets of diabetic Goto-Kakizaki rats. Endocrinology 137, 2415–2423 [DOI] [PubMed] [Google Scholar]
- 27. Devedjian J. C., George M., Casellas A., Pujol A., Visa J., Pelegrín M., Gros L., Bosch F. (2000) Transgenic mice overexpressing insulin-like growth factor-II in beta cells develop type 2 diabetes. J. Clin. Invest. 105, 731–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pelegrin M., Devedjian J. C., Costa C., Visa J., Solanes G., Pujol A., Asins G., Valera A., Bosch F. (1998) Evidence from transgenic mice that interferon-β may be involved in the onset of diabetes mellitus. J. Biol. Chem. 273, 12332–12340 [DOI] [PubMed] [Google Scholar]
- 29. Casellas A., Salavert A., Agudo J., Ayuso E., Jimenez V., Moya M., Muñoz S., Franckhauser S., Bosch F. (2006) Expression of IGF-I in pancreatic islets prevents lymphocytic infiltration and protects mice from type 1 diabetes. Diabetes 55, 3246–3255 [DOI] [PubMed] [Google Scholar]
- 30. Pfaffl M. W. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45–e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sachdeva M. M., Claiborn K. C., Khoo C., Yang J., Groff D. N., Mirmira R. G., Stoffers D. A. (2009) Pdx1 (MODY4) regulates pancreatic beta cell susceptibility to ER stress. Proc. Natl. Acad. Sci. U.S.A. 106, 19090–19095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Back S. H., Kaufman R. J. (2012) Endoplasmic reticulum stress and type 2 diabetes. Annu. Rev. Biochem. 81, 767–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vangheluwe P., Raeymaekers L., Dode L., Wuytack F. (2005) Modulating sarco(endo)plasmic reticulum Ca2+-ATPase 2 (SERCA2) activity: cell biological implications. Cell Calcium 38, 291–302 [DOI] [PubMed] [Google Scholar]
- 34. Martino L., Masini M., Novelli M., Beffy P., Bugliani M., Marselli L., Masiello P., Marchetti P., De Tata V. (2012) Palmitate activates autophagy in INS-1E β-cells and in isolated rat and human pancreatic islets. PLoS One 7, e36188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wirawan E., Vanden Berghe T., Lippens S., Agostinis P., Vandenabeele P. (2012) Autophagy: for better or for worse. Cell Res. 22, 43–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Friedrichsen B. N., Neubauer N., Lee Y. C., Gram V. K., Blume N., Petersen J. S., Nielsen J. H., Møldrup A. (2006) Stimulation of pancreatic beta-cell replication by incretins involves transcriptional induction of cyclin D1 via multiple signalling pathways. J. Endocrinol. 188, 481–492 [DOI] [PubMed] [Google Scholar]
- 37. Swenne I., Andersson A. (1984) Effect of genetic background on the capacity for islet cell replication in mice. Diabetologia 27, 464–467 [DOI] [PubMed] [Google Scholar]
- 38. Kulkarni R. N., Almind K., Goren H. J., Winnay J. N., Ueki K., Okada T., Kahn C. R. (2003) Impact of genetic background on development of hyperinsulinemia and diabetes in insulin receptor/insulin receptor substrate-1 double heterozygous mice. Diabetes 52, 1528–1534 [DOI] [PubMed] [Google Scholar]
- 39. Brüning J. C., Winnay J., Bonner-Weir S., Taylor S. I., Accili D., Kahn C. R. (1997) Development of a novel polygenic model of NIDDM in mice heterozygous for IR and IRS-1 null alleles. Cell 88, 561–572 [DOI] [PubMed] [Google Scholar]
- 40. Kulkarni R. N. (2002) Receptors for insulin and insulin-like growth factor-1 and insulin receptor substrate-1 mediate pathways that regulate islet function. Biochem. Soc Trans. 30, 317–322 [DOI] [PubMed] [Google Scholar]
- 41. Gerich J. E. (2002) Is reduced first-phase insulin release the earliest detectable abnormality in individuals destined to develop type 2 diabetes? Diabetes 51, S117–S121 [DOI] [PubMed] [Google Scholar]
- 42. Wajchenberg B. L. (2007) Beta-cell failure in diabetes and preservation by clinical treatment. Endocr. Rev. 28, 187–218 [DOI] [PubMed] [Google Scholar]
- 43. Ohneda M., Johnson J. H., Inman L. R., Chen L., Suzuki K., Goto Y., Alam T., Ravazzola M., Orci L., Unger R. H. (1993) GLUT2 expression and function in beta-cells of GK rats with NIDDM. Dissociation between reductions in glucose transport and glucose-stimulated insulin secretion. Diabetes 42, 1065–1072 [DOI] [PubMed] [Google Scholar]
- 44. Eizirik D. L., Cardozo A. K., Cnop M. (2008) The role for endoplasmic reticulum stress in diabetes mellitus. Endocr. Rev. 29, 42–61 [DOI] [PubMed] [Google Scholar]
- 45. Tuusa J. T., Markkanen P. M., Apaja P. M., Hakalahti A. E., Petäjä-Repo U. E. (2007) The endoplasmic reticulum Ca2+-pump SERCA2b interacts with G protein-coupled receptors and enhances their expression at the cell surface. J. Mol. Biol. 371, 622–638 [DOI] [PubMed] [Google Scholar]
- 46. Inaba W., Mizukami H., Kamata K., Takahashi K., Tsuboi K., Yagihashi S. (2012) Effects of long-term treatment with the dipeptidyl peptidase-4 inhibitor vildagliptin on islet endocrine cells in nonobese type 2 diabetic Goto-Kakizaki rats. Eur. J. Pharmacol. 691, 297–306 [DOI] [PubMed] [Google Scholar]
- 47. Ehses J. A., Perren A., Eppler E., Ribaux P., Pospisilik J. A., Maor-Cahn R., Gueripel X., Ellingsgaard H., Schneider M. K., Biollaz G., Fontana A., Reinecke M., Homo-Delarche F., Donath M. Y. (2007) Increased number of islet-associated macrophages in type 2 diabetes. Diabetes 56, 2356–2370 [DOI] [PubMed] [Google Scholar]
- 48. Nielsen F. C. (1992) The molecular and cellular biology of insulin-like growth factor II. Prog. Growth Factor Res. 4, 257–290 [DOI] [PubMed] [Google Scholar]
- 49. Forejt J., Gregorová S. (1992) Genetic analysis of genomic imprinting: an Imprintor-1 gene controls inactivation of the paternal copy of the mouse Tme locus. Cell 70, 443–450 [DOI] [PubMed] [Google Scholar]
- 50. Morcavallo A., Gaspari M., Pandini G., Palummo A., Cuda G., Larsen M. R., Vigneri R., Belfiore A. (2011) Research resource: new and diverse substrates for the insulin receptor isoform A revealed by quantitative proteomics after stimulation with IGF-II or insulin. Mol. Endocrinol. 25, 1456–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Okabayashi Y., Maddux B. A., McDonald A. R., Logsdon C. D., Williams J. A., Goldfine I. D. (1989) Mechanisms of insulin-induced insulin-receptor down-regulation: decrease of receptor biosynthesis and mRNA levels. Diabetes 38, 182–187 [DOI] [PubMed] [Google Scholar]
- 52. Djiogue S., Nwabo Kamdje A. H., Vecchio L., Kipanyula M. J., Farahna M., Aldebasi Y., Seke Etet P. F. (2013) Insulin resistance and cancer: the role of insulin and IGFs. Endocr. Relat. Cancer 20, R1–R17 [DOI] [PubMed] [Google Scholar]
- 53. Cornu M., Yang J. Y., Jaccard E., Poussin C., Widmann C., Thorens B. (2009) Glucagon-like peptide-1 protects beta-cells against apoptosis by increasing the activity of an IGF-2/IGF-1 receptor autocrine loop. Diabetes 58, 1816–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]