Background: Mutations in SLC4A11 result in corneal endothelial dystrophies; however, the transport characteristics of this membrane protein remain unclear.
Results: SLC4A11 showed NH4Cl-dependent currents indicative of a NH3/H+ electrogenic co-transport mode.
Conclusion: SLC4A11 is a novel NH3/H+ co-transporter, uncharacteristic of the SLC4 bicarbonate transporter family.
Significance: SLC4A11 ammonia transport capacity should be considered along with other NH3 transporters/channels when examining tissue nitrogen homeostasis.
Keywords: ammonia, cornea, electrophysiology, membrane transport, proton transport, NH3 transport, NH4+, SLC4A11
Abstract
SLC4A11 has been proposed to be an electrogenic membrane transporter, permeable to Na+, H+ (OH−), bicarbonate, borate, and NH4+. Recent studies indicate, however, that neither bicarbonate or borate is a substrate. Here, we examined potential NH4+, Na+, and H+ contributions to electrogenic ion transport through SLC4A11 stably expressed in Na+/H+ exchanger-deficient PS120 fibroblasts. Inward currents observed during exposure to NH4Cl were determined by the [NH3]o, not [NH4+]o, and current amplitudes varied with the [H+] gradient. These currents were relatively unaffected by removal of Na+, K+, or Cl− from the bath but could be reduced by inclusion of NH4Cl in the pipette solution. Bath pH changes alone did not generate significant currents through SLC4A11, except immediately following exposure to NH4Cl. Reversal potential shifts in response to changing [NH3]o and pHo suggested an NH3/H+-coupled transport mode for SLC4A11. Proton flux through SLC4A11 in the absence of ammonia was relatively small, suggesting that ammonia transport is of more physiological relevance. Methylammonia produced currents similar to NH3 but with reduced amplitude. Estimated stoichiometry of SLC4A11 transport was 1:2 (NH3/H+). NH3-dependent currents were insensitive to 10 μm ethyl-isopropyl amiloride or 100 μm 4,4′- diisothiocyanatostilbene-2,2′-disulfonic acid. We propose that SLC4A11 is an NH3/2H+ co-transporter exhibiting unique characteristics.
Introduction
SLC4A11 is a recently identified transporter ubiquitously expressed in somatic cells (1, 2), with significant expression in the corneal endothelium, cochlea, and kidney (3). Several SLC4A11 mutations have been implicated in the pathogenesis of Fuchs endothelial corneal dystrophy, congenital hereditary endothelial dystrophy, and Harboyan syndrome (corneal dystrophy with perceptive deafness) (4, 5). Three strains of SLC4A11 knock-out mice exhibited variable phenotypes that included corneal endothelial dysfunction, perceptive deafness, and polyuria or low urine osmolality (6–8). Although SLC4A11 is classified as a member of the SLC4 (solute-linked co-transporter 4) family of bicarbonate transporters, it shares rather low (14–20%) homology with other family members. Phylogenetic analysis of SLC4A11 indicates a separate branch of early evolutionary divergence in the SLC4 family, suggesting a divergent function (9).
Despite high physiological significance of SLC4A11, its transport characteristics remain unclear. Initial studies suggested that SLC4A11 is an electrogenic 2Na+-OH− (or borate) co-transporter (10). However, more recent studies provided evidence that human SLC4A11 transports neither borate nor bicarbonate (9, 11). These studies suggested ethyl-isopropyl-amiloride (EIPA)3-sensitive Na+ and H+ (or OH−) permeability through SLC4A11; however, a precise stoichiometry could not be determined. A more recent report has suggested H+ permeability through SLC4A11 but independent of Na+ (12).
Interestingly, ammonium permeability (9) and water permeability (13) have also been suggested for SLC4A11, and the across-species highly conserved asparagine-proline-X (NPX) water channel motif has been identified in a transmembrane domain of SLC4A11 (N639PS) (13). Because several water channels, such as AQP3, AQP7, AQP8, AQP9, and AQP10, are ammonia-permeable (14), and a single-point mutation in the aromatic/arginine region (ar/R) can induce ammonia permeability in the ammonia-impermeable water channel AQP1 (15), we hypothesized that SLC4A11 may also exhibit ammonia permeability similar to AQPs. To test this hypothesis, we generated two Na+/H+ exchanger (NHE)-deficient Chinese hamster PS120 fibroblast cell lines stably expressing high or low levels of SLC4A11. Employing whole-cell patch clamp electrophysiology, we found that NH4Cl generated significant EIPA- and 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS)-insensitive currents in SLC4A11-expressing PS120 cells. These currents were sensitive to [NH3] and [H+] gradients but were relatively unaffected by Na+, K+, or Cl− at physiological pH. The displayed current-voltage relations are consistent with an electrogenic NH3- and H+-coupled co-transport model.
Experimental Procedures
Transfection and Cell Culture
Human SLC4A11-HA-tagged or empty vector (EV) were stably transfected into NHE-deficient PS120 Chinese hamster CCL39 fibroblast cell line as described previously (9). The SLC4A11 (NP_001167561.1) cDNA was subcloned into a plasmid containing a neor gene for Geneticin G418 selection and a C-terminal HA tag (13). PS120 cells were cultured in DMEM (Gibco®, catalog no. 21063, Life Technologies, Inc.) supplemented with 5% fetal bovine serum (Gibco®, catalog no. 10082, Life Technologies), 1% antibiotic/antimycotic (100 units/ml penicillin and 0.25 μ/ml fungizone), and 1 mg/ml Geneticin® G418 (catalog no. 10131, Life Technologies). The cells were cultured at 37 °C in a 5% CO2 water-jacketed incubator. Culture medium was changed every other day.
Western Blot
SLC4A11- or EV- PS120 cells were grown on 6-well plates for 3 days before protein extraction. Cells were rinsed with ice-cold PBS, scrapped off from the wells, and sonicated in ice-cold radioimmune precipitation assay buffer (50 mm Tris base, 150 mm NaCl, 0.5% deoxycholic acid-sodium salt, 2% SDS, and 1% Nonidet P-40, pH 7.5) supplemented with complete protease inhibitor mixture (Roche Diagnostics, Mannheim, Germany). The extract was centrifuged for 20 min at 12,000 rpm at 4 °C, and the supernatant was collected. Protein concentration was determined using the 280-nm absorbance assay with a NanoDrop spectrophotometer (Thermo Scientific, Rockford, IL). Protein (15 μg/well) in Protein Loading Buffer Blue (2×) (National Diagnostics, catalog no. EC-886, Atlanta, GA) was resolved on 9% SDS-polyacrylamide gels and wet-transferred to PVDF membranes (Bio-Rad). Membranes were blocked with 5% nonfat milk in TBST (25 mm Tris base, 137 mm NaCl, 0.1% Tween 20) and probed with primary antibodies in the same buffer overnight at 4 °C. The following primary antibodies were used: 1) rabbit polyclonal anti-SLC4A11 (1:1000; 16B12, Covance); 2) mouse monoclonal anti-HA (1:2000; Santa Cruz Biotechnology); or 3) mouse monoclonal α-tubulin antibody (1:5000; DM1A, Novus Biologicals). Next, membranes were probed with secondary antibody (goat anti-mouse IgG peroxidase-conjugated antibody, 1:5000, catalog no. A8924 (Sigma) or goat anti-rabbit IgG peroxidase-conjugated antibody, 1:5000, catalogue no. A0545 (Sigma)) for 1 h at room temperature. Bound secondary antibodies were detected using an enhanced chemiluminescence assay (Supersignal West Pico, Pierce). Band densities and background were quantified using UN-SCAN-IT gel 6.1 (Silk Scientific).
Intracellular pH Measurements
Intracellular pH measurements were performed as described previously (9). Briefly, PS120 cells were cultured on poly-l-lysine-coated 25-mm diameter glass coverslips (Neuvitro Corp., El Monte, CA) for 2–3 days. Before each experiment, cells were incubated with 10 μm pH-sensitive fluorescent dye 2′7′-bis(carboxyethy)-5(6)-carboxyfluorescein (BCECF)-acetoxymethyl ester (Molecular Probes, Inc., Eugene, OR) in Ringer's solution for 30 min at room temperature and washed in dye-free Ringer's solution for another 30 min. The Ringer's solution contained 143.5 mm Na+, 4 mm K+, 0.6 mm Mg2+, 1.4 mm Ca2+, 118 mm Cl−, 1 mm HPO42−, 10 mm HEPES, 30 mm gluconate−, and 5 mm glucose, pH 7.5. Na+-free Ringer's solution contains 143.5 mm NMDG+ instead of Na+. All of the experimental solutions were equilibrated with air and adjusted to pH 7.5 with 1 n NaOH at 37 °C. Osmolarity of all solutions was adjusted to 295–300 mosm with sucrose. Coverslips with subconfluent cells were mounted into a perfusion chamber. The chamber was then placed on a stage warmer (37 °C) of an inverted microscope (Eclipse TE200, Nikon, Japan). Solutions were kept at 37 °C in a warming box, and the flow of the perfusate (∼0.5 ml/min) was achieved by gravity. Cells were imaged with an oil immersion objective (×40; Nikon). BCECF fluorescence was excited alternately at 495 ± 10 and 440 ± 10 nm, and the emitted light was collected through a bandpass filter (520–550 nm). Fluorescence ratios (495 nm/440 nm) were obtained at 1 Hz and converted into pHi using the high K+/nigericin calibration approach (16).
Electrophysiological Recordings of PS120 Cells
SLC4A11- and EV-PS120 cells were cultured on 25-mm glass coverslips as described above to ∼10–20% confluence. High expression Col4 SlC4A11-PS120 cells were used in all electrophysiology experiments. Whole-cell currents were amplified using an EPC7 amplifier controlled by pCLAMP 10 software (Molecular Devices, Sunnyvale, CA) in the episodic mode. The holding potential was set to −60 mV unless otherwise indicated. The currents were filtered at 3 kHz and sampled at 1 kHz. Each episode lasted 2 s and included a 100-ms voltage ramp from −100 to +100 mV. Series resistance compensation was set at 50%. CLAMPFIT 10 software (Molecular Devices, Sunnyvale, CA) was used for data analysis. Only recordings obtained with a series resistance of less than 10 megaohms were analyzed. Background currents were subtracted when analyzing current amplitude and consequent current density data. No background current subtraction was done when the reversal potential values were determined. The liquid junction potential was subtracted before reporting reversal potential values. An agar salt bridge was used to connect reference electrode and the bath to minimize possible Ag/AgCl reference electrode potential changes during solution exchanges. Complete solution exchange required about 10–15 s in the recording chamber used for electrophysiological experiments (22–23 °C).
Solutions for Electrophysiology
The compositions of extracellular (E) and pipette (intracellular; I) solutions are listed in Tables 1 and 2, respectively. Extracellular solutions were adjusted to desired pH (pH 6.5, 7.5, and 8.5) with 1 n HCl, 1 n NaOH, or 1 m NMDG base solution (for Na+-free E5). Intracellular solution pH was adjusted with tris(hydroxymethyl)aminomethane base to pH 7.2. Osmolality of all solutions was adjusted to 300–310 mosm with mannitol. 10 mm NH4Cl, methylammonium, tetraethylammonium, or 5 mm (NH4)2SO4 salt was added fresh at the day of the experiment. The (NH4)2SO4 salt was used instead of NH4Cl for Cl−-free E6 solution. Equal 0.17 mm NH3, pH 6.5, pH 7.5, and pH 8.5 E2 solutions were made by equal molar substitution of NMDG-Cl in base E2 solution by 98.5 mm, 10 mm, and 1.16 mm NH4Cl, respectively. Solution E8 was used to make 1, 3, 10, 30, and 100 mm NH4Cl extracellular solutions by equal molar substitution of NMDG-Cl with NH4Cl.
TABLE 1.
Composition of extracellular solutions
| E1 | E2 | E4 (0 K+) | E5 (0 Na+, 0 K+) | E6 (0 Cl−, 0 K+) | E8 | |
|---|---|---|---|---|---|---|
| m | m | m | m | m | m | |
| NaCl | 143.5 | 45 | 143.5 | 80 | ||
| NMDG-Cl | 98.5 | 143.5 | 100 | |||
| K2HPO4 | 1 | |||||
| KCl | 3 | |||||
| Calcium gluconate | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 | 1.4 |
| MgCl2 | 1.22 | 1.22 | 1.22 | 1.22 | 1.22 | 1.22 |
| HEPES | 10 | 10 | 10 | 10 | 10 | 10 |
| Glucose | 5.1 | 5.1 | 5.1 | 5.1 | 5.1 | 5.1 |
| NaMeSO3 | 143.5 |
TABLE 2.
Composition of intracellular solutions
| I1 | I1-NH4 | I2 | |
|---|---|---|---|
| mm | mm | mm | |
| CsMeSO3 | 125 | 125 | 145 |
| CaCl2 | 5 | 5 | |
| CsCl | 10 | ||
| MgCl2 | 2 | 2 | 2.2 |
| HEPES | 10 | 30 | 10 |
| EGTA | 10 | 10 | 0.5 |
| NH4Cl | 10 | ||
| pH | 7.2 | 7.2 | 7.2 |
Statistical Analysis
Statistical analysis was performed using SPSS Statistics version 21 (IBM Corp., Armonk, NY). Stoichiometry data were fitted to linear regression in SigmaPlot version 12 (Systat Software Inc., San Jose, CA). Throughout, data are presented as mean ± S.E., in which significance level is indicated as follows: p < 0.05 (*), p < 0.01 (**), and p < 0.001 (***).
Results
Ammonium Permeability through SLC4A11 Is Expression-dependent
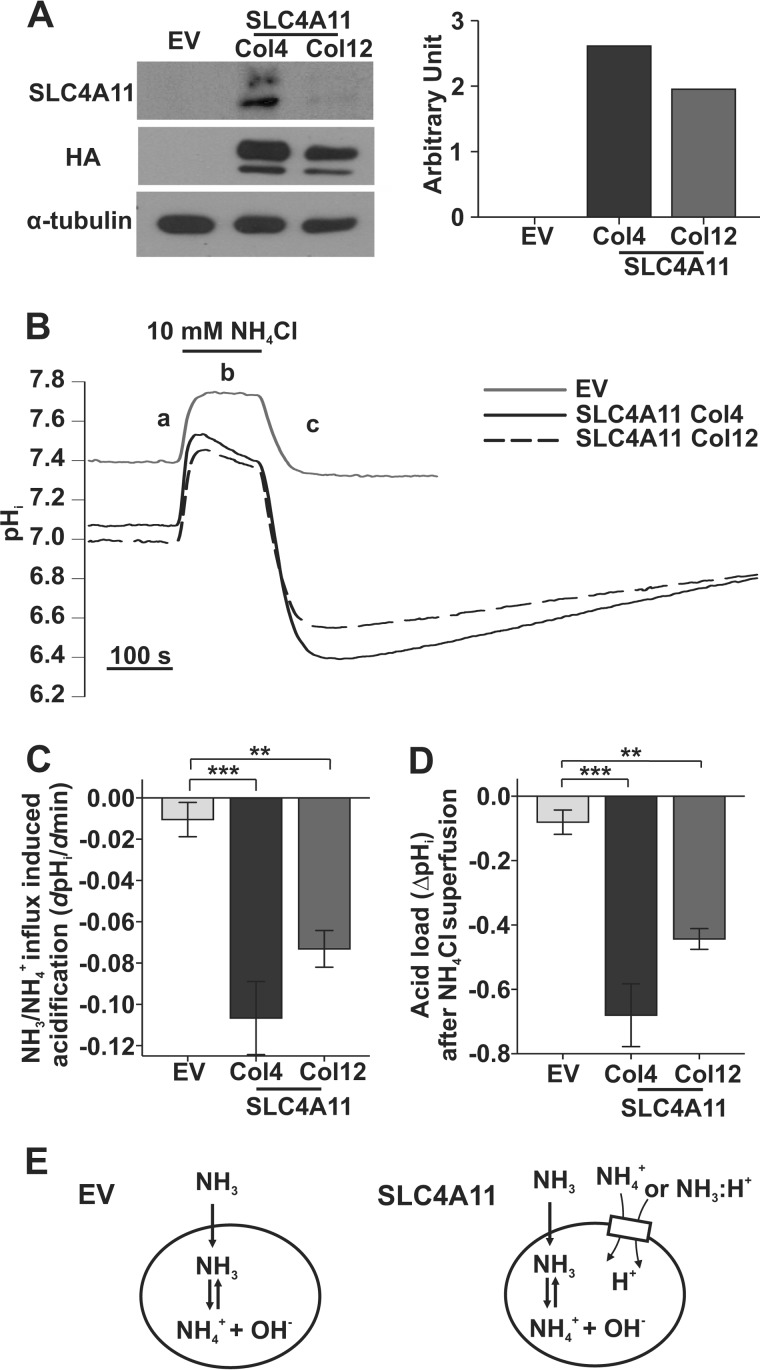
Previously, using BCECF pHi measurements, we observed increased Na+ and NH4+ permeability and higher capacity to recover from acid loads in SLC4A11-expressing HEK293 or PS120 cells (9). To further clarify the underlying mechanisms, we generated two stably transfected PS120 cell lines (Na+/H+ exchanger-deficient), high expression colony 4 (Col4) and low expression colony 12 (Col12). We determined, by Western blot, that Col4 expressed 1.34 times more SLC4A11 than Col12 (anti-HA; Fig. 1A), whereas no endogenous SLC4A11 can be detected in EV-PS120 cells (anti-SLC4A11; Fig. 1A). Employing these two colonies of SLC4A11-PS120 cells, we examined the pHi response to extracellular 10 mm NH4Cl, relative to EV. Fig. 1B shows that NH4Cl application caused a rapid pHi rise in both SLC4A11-PS120 and EV-PS120 (labeled a) due to NH3 gas diffusion and intracellular production of NH4+ and OH− (17). In phase b, pHi plateaued in EV-PS120 cells (−0.01 ± 0.02 ΔpH/Δmin), whereas a significant acidification occurred in SLC4A11-PS120 cells. The acidification rate (ΔpH/Δmin) was 1.57 times higher in Col4 (−0.11 ± 0.02, n = 3) than in Col12 cells (−0.07 ± 0.01, n = 3) (Fig. 1C). Removing NH4Cl (phase c) caused a prominent acidification: ΔpHi −0.68 ± 0.10 in Col4 (n = 3) and 1.55 times less ΔpHi −0.44 ± 0.03 in Col12 (n = 3), whereas essentially no acidification occurred (−0.08 ± 0.04, n = 5) in EV-PS120 cells (Fig. 1D). Phase b acidification is generally attributed to a finite permeability to NH4+, and consequent phase c acidification is a H+ loading effect from the finite NH4+ permeability in phase b (17). The extents of acidification during phases b and c were both positively associated with the SLC4A11 expression level (Fig. 1, C and D). Thus, we reasoned that SLC4A11 expression increased the apparent NH4+ permeability in PS120 cells. This permeability could be solo NH4+ transport or co-transport of NH3-H+ (14), but it cannot be differentiated by BCECF pHi measurements.
FIGURE 1.
NH4Cl superfusion induced differential intracellular acidification in SLC4A11-PS120 relative to EV-PS120 cells. A, Western blot verification of SLC4A11 expression in EV (empty vector), Col4, and Col12 using anti-SLC4A11 and anti-HA antibodies. A doublet shows glycosylated and non-glycosylated forms of SLC4A11 protein (40). The bar graph on the right shows the density quantification of HA-SLC4A11 normalized to α-tubulin. B, pHi response to 10 mm NH4Cl pulse in EV and SLC4A11 PS120 cells. Each trace is the average of n = 5 EV, n = 3 SLC4A11 Col4, and n = 3 SLC4A11 Col12, respectively. The traces show the rapid alkalinization phase (a), slow acidification phase (b), and rapid acid loading phase (c). C, phase b apparent ammonium influx (ΔpHi/Δmin) in SLC4A11 Col4 (n = 3, p = 0.001) and Col12 (n = 3, p = 0.01) is higher than in the EV control (n = 5) (one-way ANOVA with Tukey honest significant difference post hoc test, p = 0.001). D, phase c ammonium-induced peak acidification (ΔpHi) during wash is higher in SLC4A11 Col4 (n = 3, p < 0.001) and Col12 (n = 3, p = 0.004) than in the EV control (n = 5) (one-way ANOVA with Tukey honest significant difference post hoc test, p < 0.001). E, left, EV, simple NH3 diffusion equilibrates and raises pHi (phase a in Fig. 1B), but no secondary acidification occurs due to lack of NH4+ permeability (phase b). Right, SLC4A11, NH3 diffusion caused similar pHi rise as in EV (phase a); additional NH4+ (or NH3-H+) permeability from SLC4A11 adds net H+ influx and causes gradual acidification (phase b). Error bars, S.E. **, p < 0.01; ***, p < 0.001.
[NH3]o Not [NH4+]o Drives Extracellular NH4Cl (eNH4Cl)-dependent Inward Currents through SLC4A11
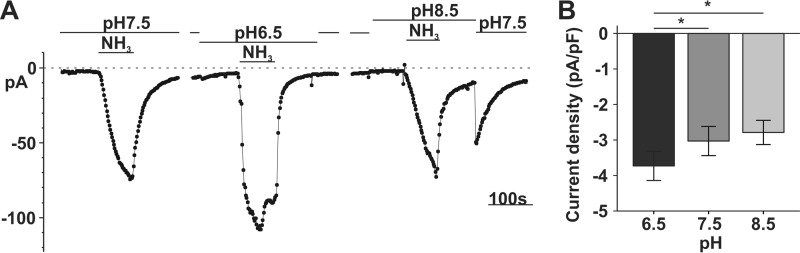
A previous study provided evidence that SLC4A11 may function as an electrogenic transporter in SLC4A11-HEK293 cells (10). Therefore, we next used the whole-cell patch clamp approach to determine whether the apparent NH4+ (or NH3-H+) permeability creates charge movements by applying 10 mm eNH4Cl to SLC4A11-PS120 Col4 cells. NH4Cl solutions were adjusted to three different extracellular pH levels (7.5, 6.5, and 8.5) to vary the proportion of NH3 to NH4+. No currents were observed in EV-PS120 during any of these NH4Cl exposures (Fig. 2A, top). In contrast, in SLC4A11-PS120 shown in Fig. 2A (bottom), pH 7.5 eNH4Cl application caused inward currents of −6.18 ± 1.09 pA/pF, (n = 7), whereas pH 6.5 eNH4Cl induced significantly smaller inward currents (−0.22 ± 0.19 pA/pF, n = 4), and pH 8.5 eNH4Cl elicited markedly larger inward currents (−18.43 ± 3.43 pA/pF, n = 4. Fig. 2B shows a summary bar graph of current density for EV- and SLC4A11-PS120 cells. In Fig. 2C, we plotted the current density against [NH3]o (left), [H+]o (middle), and [NH4+]o (right), respectively. The eNH4Cl-dependent current density varied directly with [NH3]o but inversely with [H+]o or [NH4+]o. From pH 6.5 to 8.5, the 84-fold increase in current density was concomitant with a 75-fold increase in [NH3]o; conversely, there was essentially no change (17% decrease) in [NH4+]o and a 100-fold decrease in [H+]o.
FIGURE 2.
eNH4Cl-dependent inward currents through SLC4A11. A, sample trace of whole-cell current recording (−60 mV holding potential) in response to 10 mm eNH4Cl at pHo 6.5, 7.5, and 8.5 in EV- (top trace) and SLC4A11-PS120 cells (bottom trace). Bath pH changes themselves did not elicit significant currents (open arrows). A pH 8.5 wash after pH 8.5 NH4Cl elicited outward current (star), and the pH 8.5 to 7.5 transition after NH4Cl pulse elicited inward currents (filled arrow). Inset, schematic diagram showing currents carried by NH3 and an unknown charge carrier by SLC4A11. B, bar graph summary of NH4Cl-dependent current density in EV (n = 3) and SLC4A11 cells (n = 4) (repeated measure ANOVA, cell type, p = 0.005; pH, p = 0.009; Tukey honest significant difference post hoc test comparing currents at pH 6.5 versus pH 7.5, pH 7.5 versus pH 8.5, and pH 6.5 versus pH 8.5, p = 0.085, 0.001, and 0.017, respectively). C, SLC4A11 inward current density versus extracellular [NH3], [H+], or [NH4+] in 10 mm NH4Cl at pH 6.5, 7.5, and 8.5. NH4+ pKa 9.25 was used for concentration calculation. Error bars, S.E. *, p < 0.05; ***, p < 0.001.
These results suggest that NH3 is the actual transported species and that the [NH3] gradient contributes to the driving force in SLC4A11 transport. To further test this hypothesis, we adjusted the NH4Cl concentration to achieve equal [NH3]o = 0.17 mm in the three different pH solutions. This required 98.5 mm NH4Cl in the pH 6.5 E2 solution, 10 mm NH4Cl in pH 7.5 E2, and 1.16 mm NH4Cl in pH8.5 E2, calculated from NH4+ pKa = 9.25 (equal molar substitution with NMDG-Cl). Fig. 3A shows a sample trace of current recording, and Fig. 3B shows the summary bar graph of current density. In this series, with [NH3] being equal (0.17 mm), we observed similar amplitude in current density under pH 6.5 and 8.5 compared with pH 7.5. This result strongly supports the notion that [NH3]o rather than [NH4+]o drives the inward currents through SLC4A11. Interestingly, further analysis of current density revealed small differences between each pH condition (n = 3; repeated ANOVA, p = 0.001). It appears that [H+] gradients or extracellular pH affects SLC4A11 activity, whereas the inward current density is primarily determined by [NH3]o.
FIGURE 3.
eNH4Cl-dependent inward currents when holding [NH3]o constant in SLC4A11-PS120. A, sample current trace in response to 0.17 mm NH3 at pHo 6.5, 7.5, and 8.5 in SLC4A11-PS120 cells. Current amplitude is similar across three pH solutions, with slightly larger current in pH 6.5 and slightly smaller current at pH 8.5. B, bar graph summary of NH3/H+ maximum current density at pH 6.5, 7.5, and 8.5 after a 40-s exposure to NH4Cl. Current density shows small differences under the three pH conditions, largest in pH 6.5 NH3 and smallest in pH 8.5 (n = 3, repeated measure ANOVA, p = 0.001; pairwise comparison pH 6.5 versus pH 7.5, p = 0.022; pH 7.5 versus pH 8.5, p = 0.051; pH 6.5 versus pH 8.5, p = 0.012). Error bars, S.E. *, p < 0.05.
SLC4A11 eNH4Cl-dependent Current Is Independent of K+, Cl−, and Na+
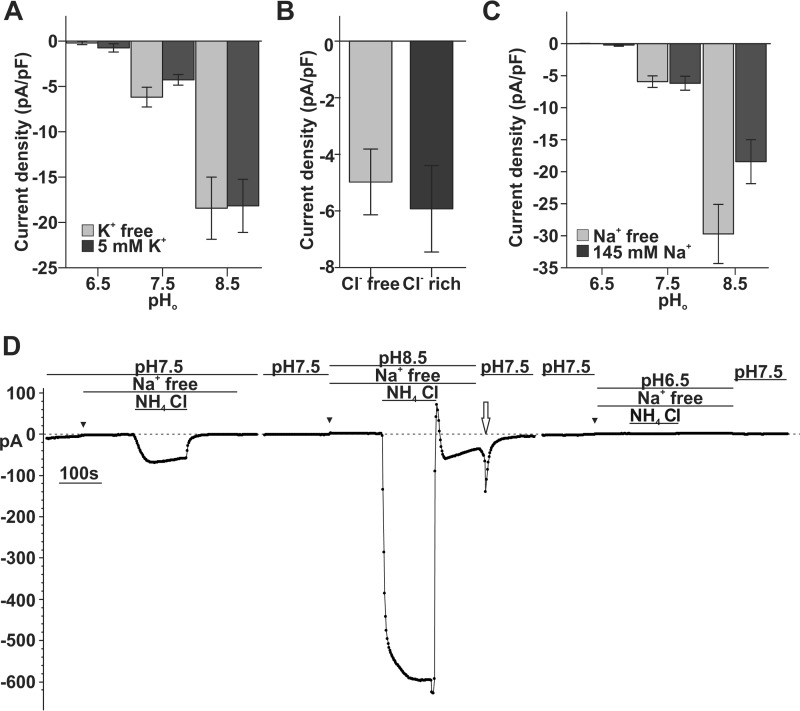
Given that NH3 is an uncharged molecule, we next tested whether K+, Cl−, or Na+ is the charge carrier of the eNH4Cl-dependent currents. Refer to Table 2 for solution composition. Removal of K+ (E1 K+ versus E4 K+-free) had no effect on eNH4Cl-dependent currents at pH 6.5, 7.5, and 8.5 (Fig. 4A; factorial ANOVA, p = 0.716). Similarly, removal of extracellular Cl− (5 mm (NH4)2SO4 was added in both Cl−-free E6 and Cl−-rich E4 solutions for comparison) did not affect the eNH4Cl-dependent current at pH 7.5 (independent t test, p = 0.65; Fig. 4B). Removal of Na+ (E4 Na+-rich versus E5 Na+-free) did not significantly affect eNH4Cl-dependent currents at pH 6.5 or 7.5 (Fig. 4C; factorial ANOVA Na+, p = 0.108; Na+/pH interaction, p = 0.058), but interestingly, the absence of Na+ enhanced eNH4Cl-dependent inward currents by 38% at pH 8.5 (Fig. 4, C and D). At pH 8.5, eNH4Cl-dependent current density in 143.5 mm Na+ E4 was −18.43 ± 3.43 pA/pF (n = 4), whereas it was −29.71 ± 4.62 pA/pF (n = 6) in Na+-free E5 (independent t test, p = 0.115; Fig. 4C). In summary, it is unlikely that K+, Cl−, or Na+ carries the eNH4Cl-dependent currents. However, there is some interaction with Na+ at high pHo.
FIGURE 4.
K+, Cl−, and Na+ are not substrates for SLC4A11 under physiological pH. A, the eNH4Cl-dependent current is not affected by removal of extracellular K+ at pH 6.5, 7.5, and 8.5. Sample size (n) under each condition is as follows: K+-free, pH 6.5, n = 4; pH 7.5, n = 7; pH 8.5, n = 4; 5 mm K+, pH 6.5, n = 3; pH 7.5, n = 6; pH 8.5, n = 4 (factorial ANOVA, 5 mm K+ versus K+-free, p = 0.716; K+/pH interaction, p = 0.763). B, the eNH4Cl-dependent current is not affected by removal of extracellular Cl− at pH 7.5. (Cl−-free n = 5 versus Cl−-rich n = 6; independent t test, p = 0.645). C, bar graph shows that eNH4Cl-dependent current is not affected by removal of extracellular Na+ at pH 6.5 and 7.5 but is enhanced at pH 8.5. Sample size (n) under each condition is as follows: Na+-free, pH 6.5 n = 4, pH 7.5 n = 6, and pH 8.5 n = 6; 145 mm Na+, pH 6.5 n = 4, pH 7.5 n = 7, and pH 8.5 n = 4 (factorial ANOVA, Na+ versus Na+-free, p = 0.108; Na+/pH interaction, p = 0.058). D, sample trace of whole-cell recording to test Na+ sensitivity. Removal of Na+ does not significantly change eNH4Cl-dependent current and slightly reduces baseline current (triangles). Bath pH transition after pH 8.5 NH4Cl elicited inward current (open arrow). Error bars, S.E.
SLC4A11 Co-transports NH3 and H+
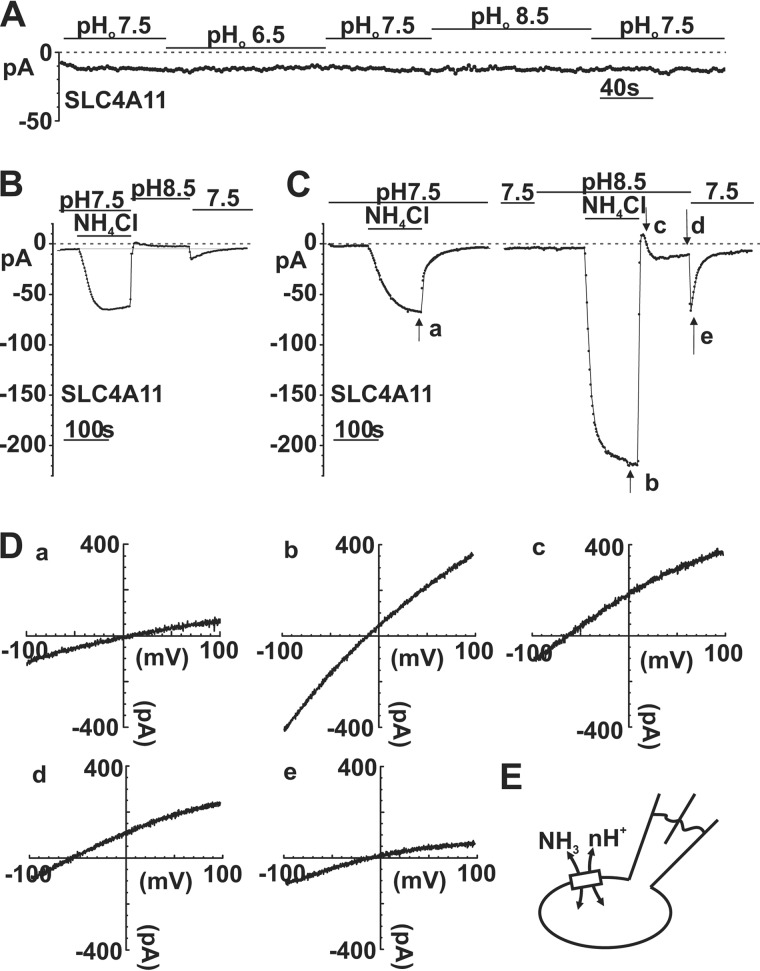
In Fig. 2A (bottom trace, solid arrow) and Fig. 4D (open arrow), we observed inward current induced by a bath pH decrease (8.5 → 7.5) after a pH 8.5 NH4Cl pulse, suggesting that SLC4A11 can provide H+ conductance. Fig. 3 shows that [H+]o affects SLC4A11 inward current density and kinetics in the presence of NH3. Fig. 5A (and Figs. 2A and 4D), however, shows that simple bath pH changes do not induce noticeable current. These data suggest that H+ current is only measurable when there is NH3 present. In Fig. 2A, a pH 8.5 wash after pH 8.5 NH4Cl pulse induced an outward current (labeled with a star). We reasoned that this outward current is the efflux of NH3 coupled to H+ resulting from outward NH3 and H+ gradient during wash. To validate, we applied a pH 8.5 wash following a pH 7.5 NH4Cl pulse, creating a greater outward [H+] gradient than a pH 7.5 wash. Fig. 5B shows smaller but measureable outward current (relative to the initial baseline) during this pH 8.5 wash, supporting the notion of NH3-coupled H+ efflux. In contrast, no outward current is observed during a pH 7.5 wash after pH 7.5 eNH4Cl pulse, indicating that an outward H+ gradient is needed. Fig. 5C (right trace) shows that later in the same cell, a pH 8.5 eNH4Cl pulse was followed by a pH 8.5 wash. The outward current (point c) during the wash is consistent with outward gradients of both [NH3] and [H+]. Interestingly, a return to pH 7.5 bath produced a significant inward current (point e), consistent with an increased [H+] inward gradient. These data show that significant H+ current through SLC4A11 can occur after an NH4Cl pulse.
FIGURE 5.
Current-voltage relations of eNH4Cl-dependent SLC4A11 currents. A, sample trace showing that bath pH transitions do not generate significant currents in the absence of NH4Cl. B, sample trace showing that transient outward current was induced after pH 7.5 eNH4Cl by a pH 8.5 wash, and transient inward currents were elicited during pH 8.5 → 7.5 bath transition. Light gray line, baseline current. C, same trace as in Fig. 2A, highlighting points for Erev comparisons. D, corresponding I-V plot at the time points a, b, c, d, and e. E, schematic diagram showing current in SLC4A11 cells is carried by NH3 and an unknown charge ion.
To provide further insight into this potential NH3-H+ co-transport model, we examined the reversal potentials at key points in Fig. 5C. The liquid junction potential was estimated to be 8.9 mV in this E4-I1 solution pairing and was subtracted from raw Erev values before analysis. Fig. 5D (a) shows the I-V relation at point a in Fig. 5C. The Erev was −4.16 ± 1.47 mV (n = 10), inconsistent with solo NH4+ conductance, which will otherwise result in large positive Erev (14). Furthermore, we analyzed Erev at time points b, c, d, and e (during and after pH 8.5 NH4Cl pulse). Fig. 5D (b), during pH 8.5 NH4Cl, Erev was −21.56 ± 1.98 mV (n = 4) at the inward current peak. Ten seconds into the pH 8.5 wash (point c), Erev shifted to −66.24 ± 2.03 mV (n = 4). Assuming that [H+]o, [H+]i, and [NH3]i changed very little from b to c in this short time frame, the decreasing [NH3]o should result in a left shift of Erev to a more negative value and result in outward current. Indeed, we observed a negative shift in Erev and NH3-H+ outward currents, representing efflux of NH3-H+ following NH3 and H+ outward gradient. Returning to pH 7.5 bath, Erev shifted from −66.37 ± 2.23 mV (n = 4, point d) to −37.60 ± 5.30 mV (n = 4, point e). This Erev rightward shift was induced by increased [H+]inward gradient and resulted in inward currents. In summary, these data suggest that [NH3] and [H+] changes synergistically affect current direction and apparent Erev in SLC4A11-PS120 cells, supporting the hypothesis that NH3 and H+ are transported in a coupled manner.
SLC4A11 H+ Permeability in the Absence of Ammonia Is Very Small
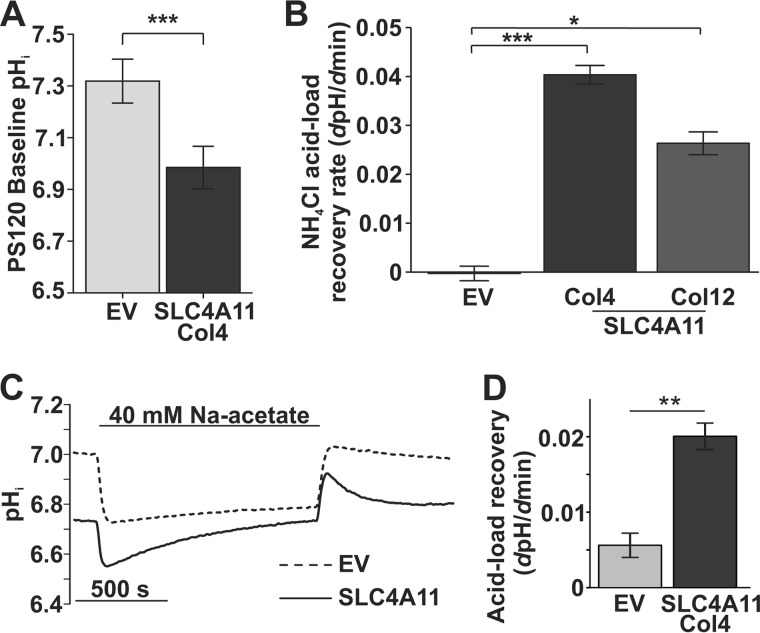
Fig. 1B suggests that the resting pHi in SLC4A11-transfected cells is lower than EV. Analysis of data from more cells (Fig. 6A) shows that the resting pHi was significantly lower in SLC4A11-PS120 Col4 (pHi 6.98 ± 0.08, n = 20) relative to EV-PS120 (pHi 7.32 ± 0.08, n = 23), suggesting that SLC4A11 provides some H+ permeability in the absence of NH3, where a negative membrane potential could drive H+ influx, as proposed in a recent report (12). Figs. 2A and 5A, however, do not reveal any measureable H+ currents in the absence of NH3. Nevertheless, increased H+ permeability is evident in SLC4A11-transfected cells during the acid recovery following NH4Cl pulses in previous reports (9, 12). Fig. 6B shows that acid recovery rates after NH4Cl pulse were significantly greater in Col4 and Col12 cells relative to EV. Next, to avoid cell exposure to NH3, we used sodium acetate to acidify these cells and monitored acid recovery. Fig. 6, C and D, show a similarly enhanced acid recovery in SLC4A11 transfected cells during acidification with sodium acetate, indicating that this apparent H+ permeability is independent of NH3. The H+ efflux during sodium acetate experiments was 4.7 μm/s (ΔpH/Δs × 14 mm/pH buffering capacity), similar to that reported recently for SLC4A11 H+ permeability (12). However, this efflux rate is quite small, only about 2.4% of Na+/H+ exchanger 1 (NHE1)-facilitated H+ flux (0.2 mm/s) (19), and ∼7.3 times smaller than H+ flux in NH4Cl (see Fig. 1C). Moreover, the effects of this added H+ permeability on resting pHi are not evident in bicarbonate-rich conditions (i.e. high buffering capacity) (9, 12) or NHE-expressing cells (9).
FIGURE 6.
H+ permeability through SLC4A11 in the absence of NH3 is small. A, baseline pHi in SLC4A11-PS120 cells (6.98 ± 0.37, n = 20) is significantly lower than in EV-PS120 cells (7.32 ± 0.41, n = 23) (independent t test, p = 0.008). B, acid recovery rates following NH4Cl pulses (see Fig. 1) in Col4 (n = 3) and Col12 (n = 3) SLC4A11-expressing cells relative to EV (n = 5). (one-way ANOVA, p = 0.001; post hoc Bonferroni EV versus Col4, p = 0.001; EV versus Col12, p = 0.012). C, pHi response to sodium acetate in EV and SLC4A11 cells. D, bar graph summary of acid load recovery rate in SLC4A11- (n = 4) or EV-PS120 (n = 4) cells during 40 mm sodium acetate pulse (independent t test, p = 0.011). Error bars, S.E. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Intracellular NH3 Decreases SLC4A11 NH3/H+ Inward Currents
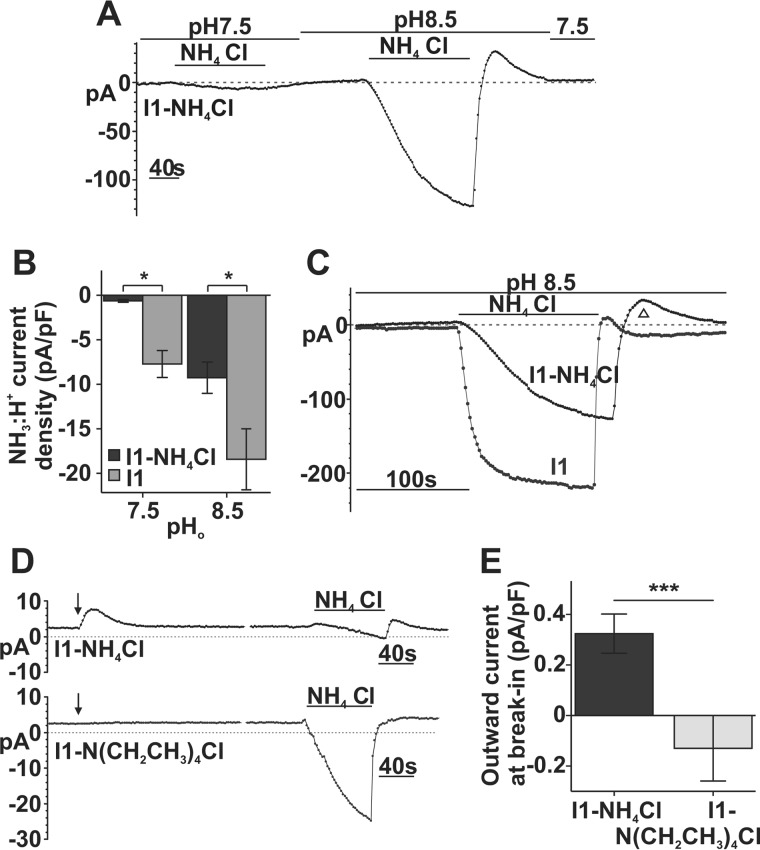
Fig. 5C (d) demonstrates that an outward [NH3] gradient can produce an outward current in SLC4A11-PS120 cells. Therefore, we next tested whether reversing or dissipating the transmembrane [NH3] gradient by increasing intracellular [NH3] would reverse any SLC4A11 electrogenic activity. Previously, we used a standard pipette solution containing 0 mm NH4Cl (I1), whereas here we used 10 mm NH4Cl pipette solution (I1-NH4Cl). Liquid junction potential was estimated to be 8.5 mV in this E4-I1-NH4Cl solution pairing and was subtracted from raw Erev values before analysis.
We dialyzed I1-NH4Cl into SLC4A11-PS120 cells reversing the transmembrane [NH3] gradient, hoping this would be sufficient to stimulate outward current. However, when the cell was held at −60 mV, we observed no significant current at pH 7.5 or 8.5 (E4 bath solution) (Fig. 7A). During NH4Cl application, inward current (Fig. 7A) was smaller than that with standard 0 mm [NH3] intracellular solution. Fig. 7B shows current densities amounting to −0.63 ± 0.16 pA/pF (n = 3) at pH 7.5 NH4Cl and −9.26 ± 1.73 pA/pF at pH 8.5 NH4Cl (n = 3); both were significantly smaller than that in I1 dialysis (p = 0.03), consistent with a reduced driving force for NH3-H+ inward currents because of reduced [NH3]o/[NH3]i gradient. Interestingly, the presence of intracellular NH3 and/or the reduced NH3 gradient also slows current development. Fig. 7C shows a significant difference in the time constants between I1-NH4Cl, τ = 58.44 ± 17.58 s (n = 3), and I1, τ = 9.00 ± 0.88 s (n = 4). In addition, the outward current at the pH 8.5 wash was prolonged and more prominent with I1-NH4Cl dialysis than I1 (Fig. 7C, ▵), indicating that increased [NH3]i can produce larger outward current.
FIGURE 7.
eNH4Cl-dependent currents through SLC4A11 are sensitive to transmembrane [NH3] gradient. A, sample trace of a whole-cell current recording in SLC4A11 cells with intracellular 10 mm NH4Cl and 10 mm eNH4Cl superfusion at −60 mV holding potential. B, bar graph summary of responses to eNH4Cl in I1-NH4Cl- (n = 3) versus I1-dialyzed cells (n = 4) (repeated measure ANOVA; intracellular NH4Cl, p = 0.03; in NH4Cl/pH interaction, p = 0.610). C, overlay of NH3/H+ current development in I1-NH4Cl and I1 pipette solution. Data were fitted to a standard exponential equation, τ = 58.44 ± 17.58 s for I1-NH4Cl (n = 3) versus τ = 9.00 ± 0.88 s for I1 (n = 4) (independent t test, p = 0.02, equal variance not assumed). D, representative trace of whole-cell current recording in SLC4A11-PS120 cells with intracellular 10 mm NH4Cl or N(CH2CH3)4Cl at break-in (arrows) and during 10 mm eNH4Cl superfusion at −10 mV holding potential. E, bar graph summary of break-in outward current density in cells with I1-NH4Cl (n = 7), or I1-N(C2H5)4Cl (n = 4) (independent t test, p = 0.009). Error bars, S.E. *, p < 0.05; ***, p < 0.001.
Whereas we could not see outward currents at baseline in I1-NH4Cl dialysis with −60 mV holding potential, Fig. 7, D and E, shows that at −10 mV holding potential, outward currents can be seen on break-in (arrow). As a control, dialysis of I1-N(CH2CH3)4Cl (tetraethylammonium chloride; Fig. 7D, bottom trace) did not activate any currents on break-in (arrow). (Extracellular N(CH2CH3)4Cl superfusion does not generate currents in SLC4A11-PS120 cells (Fig. 8D).) Moreover, Fig. 7D shows that inward currents at pH 7.5 NH4Cl were similarly reduced in an I1-NH4Cl-dialyzed cell compared with an I1-N(CH2CH3)4Cl-dialyzed cell. Also, Fig. 7D (top trace) shows outward currents at the beginning of the pH 7.5 wash in I1-NH4Cl-dialyzed cells. This outward current, although small, is consistent with an increased [NH3]i.
FIGURE 8.
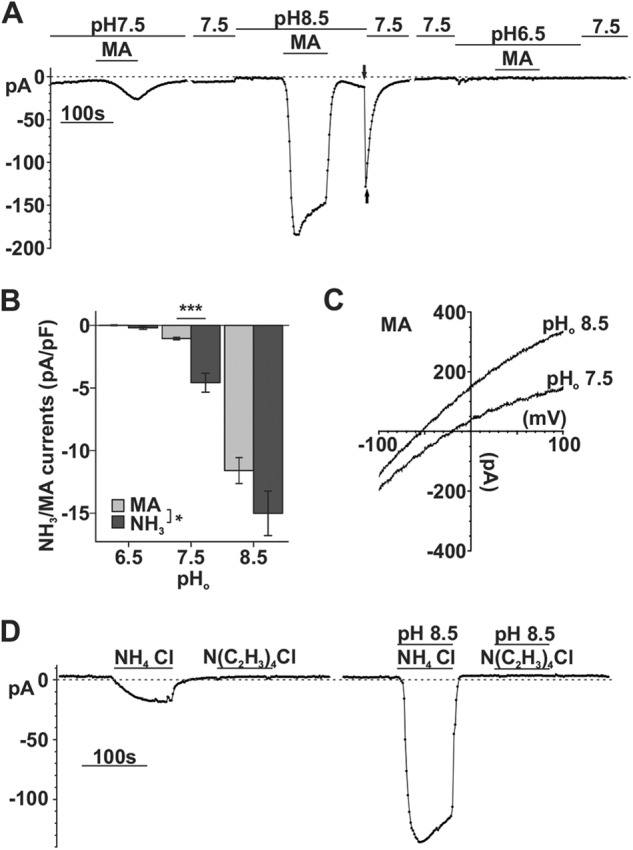
SLC4A11 can also transport methylammonia. A, sample trace of whole-cell current recording in response to extracellular 10 mm MA and change of extracellular pH in SLC4A11-PS120 cells. B, bar graph showing a comparison of MA- and NH3-induced current density in SLC4A11 cells (factorial ANOVA, NH3 versus MA, p = 0.048; pH, p < 0.001; substrate/pH interaction, p = 0.498; independent t test comparing NH3 (n) and MA (n′) current density at pH 6.5 (n = 6 versus n′ = 3), pH 7.5 (n = 21 versus n′ = 6), and pH 8.5 (n = 10 versus n′ = 5), p = 0.293, <0.001, and 0.221, respectively). C, representative reversal potential shift after MA perfusion. D, sample trace of whole-cell recording showing that tetramethylammoniun N(C2H3)4Cl does not generate currents in SLC4A11-PS120 cells. Error bars, S.E. ***, p < 0.001.
Methylammonia N(CH3)H2 Can Also Be Transported by SLC4A11
Because ammonia-permeable Rh and AQPs can also transport methylammonia (MA) (20), we next examined whether this ammonia derivative can be transported by SLC4A11. N(CH3)H3Cl application produced similar pH-sensitive inward currents, smaller at low pH and larger at high pH (Fig. 8A). Average current densities (pA/pF) at extracellular pH 6.5, 7.5, and 8.5 were −0.01 ± 0.03, −1.05 ± 0.10, and −11.60 ± 1.03, respectively, which were significantly smaller than NH4Cl-dependent current density (p = 0.048). This is consistent with lower free [MA] due to the higher pKa of MA+ (pKa 10.64) than pKa of NH4+ (pKa 9.25). Fig. 8, A and C, shows that the transient currents elicited at the pH 8.5 → 7.5 transition after pH 8.5 MA were similar to what was observed with NH4Cl. The Erev shift from −56.88 ± 3.37 mV (n = 4) at pH 8.5 to −24.39 ± 2.93 mV (n = 4) at pH 7.5 was also similar to that observed with NH4Cl. As we indicated above, application of the bulkier tetraethylammonium N(CH2CH3)4+ did not induce any current (Fig. 8D), indicating that there is a size limit for substrates of SLC4A11.
SLC4A11 Stoichiometry
We next set out to determine the stoichiometry of NH3/H+ transport through SLC4A11 at physiological pH 7.5. According to the equation,
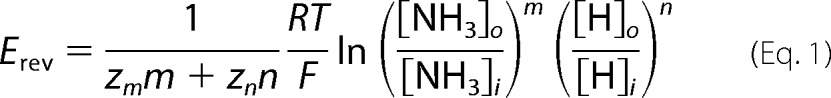 |
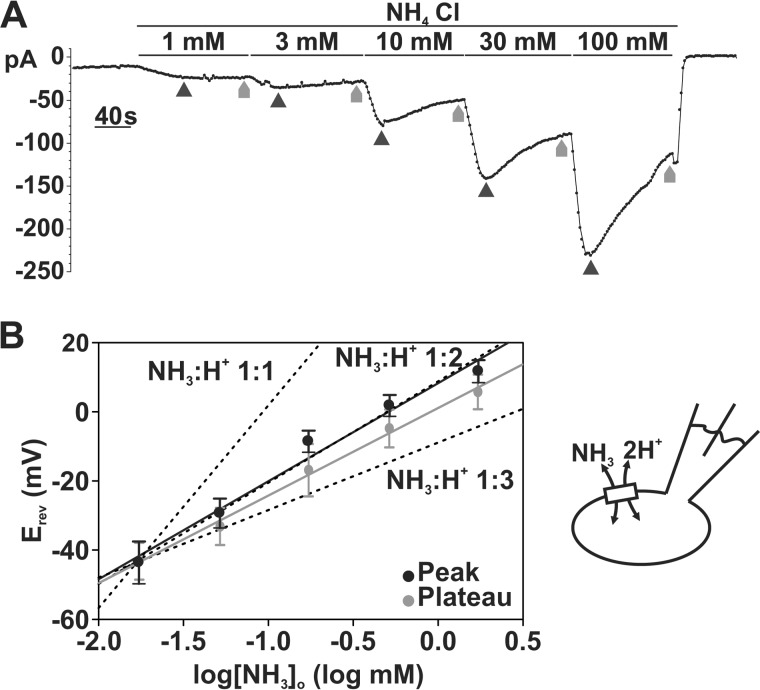
where m is the stoichiometry of NH3, n is the stoichiometry of H+, Zm is the charge of NH3 (Zm = 0), Zn is the charge of H+ (Zn = +1), R is the gas constant 8.314 J/mol*K, F is the Faraday constant 96,485 C/mol, and T is temperature in K (295 K), there is a linear relationship between Erev and log[NH3]o with a slope of ln(10) × RT/F × m/n if we assume that [NH3]i, [H]i, and [H]o are constant (21). Using this equation, we first predicted the values of linear slopes at each possible m (NH3)/n (H+) stoichiometry (at 22 °C): (i) 58.5 mV/log mm if NH3/H+ stoichiometry is 1:1; (ii) 29.25 mV/log mm if NH3/H+ stoichiometry is 1:2; and (iii) 19.50 mV/log if NH3/H+ stoichiometry is 1:3 (dashed lines in Fig. 9B). We then experimentally determined the Erev of SLC4A11 NH3/H+ currents at different [NH3]o by sequentially superfusing SLC4A11-PS120 cells with 1, 3, 10, 30, and 100 mm NH4Cl E8 solutions (I2 pipette solution; Fig. 8A). Liquid junction potential was estimated to be 13.8 mV and was subtracted from Erev values before analysis. Using the adjusted Erev at the current peak at each concentration, the plot was linear (r2 = 0.840) with a slope of 28.30 ± 2.78 mV/log mm (p < 0.0001), closest to the 1:2 NH3/H+ stoichiometry. As shown in Fig. 8A, we observed some current decay at higher NH4Cl concentrations, which could be due to an increased [NH3]i leading to decreased [NH3]o/[NH3]i gradient and in turn diminishing the current and underestimating the actual Erev. To examine this possibility, we also plotted Erev obtained at the eNH4Cl-current steady-state phase (plateaus). Indeed, we observed decreased Erev values at high NH4Cl conditions. Fig. 9B shows the plot of Erev at plateau versus log[NH3]o. The slope is 25.24 ± 3.46 mV/log mm (r2 = 0.699, p < 0.0001) still closest to the 1:2 NH3/H+ stoichiometry slope.
FIGURE 9.
Stoichiometry of SLC4A11 estimated to be 1:2 NH3/H+. A, sample trace of whole-cell current recording in response to sequentially increasing extracellular (pHo 7.5) NH4Cl concentration in SLC4A11-PS120 cell at −60 mV holding potential. Erev data for maximum “peak” current was obtained at points indicated by dark black arrows, whereas Erev for “plateau” currents were obtained at gray arrows. B, reversal potentials obtained at maximum current (“peak”) were plotted against extracellular log[NH3]o. The plot shows a linear relationship between Erev and log[NH3]o with a slope of 28.30 mV/log mm (r2 = 0.840, p < 0.0001). Dashed lines, predicted NH3/H+ 1:1, 1:2, and 1:3 slope. Reversal potentials obtained at “plateau” currents were also plotted against extracellular log[NH3]o, with a linear slope of 25.24 mV/log mm (r2 = 0.699, p < 0.0001). Error bars, S.E.
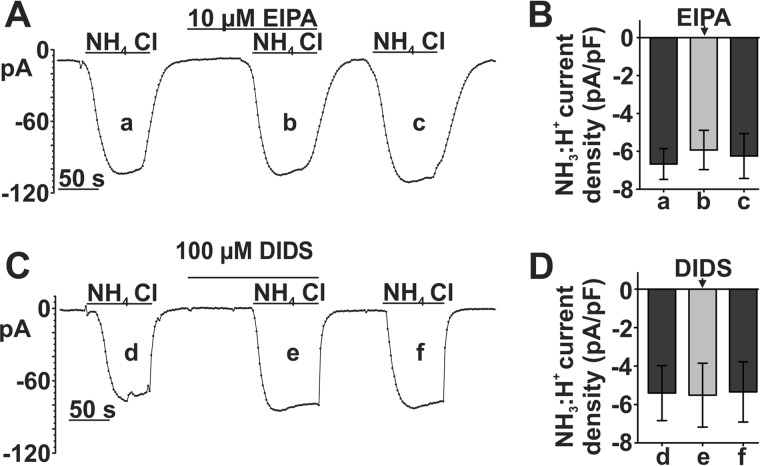
NH3/H+ current is not sensitive to DIDS or EIPA
Previous reports indicated that EIPA inhibits SLC4A11 Na+-H+ permeability (9). In addition, it has been reported that DIDS has no effect on SLC4A11 Na+-H+ permeability (10), whereas another group reported that DIDS enhances SLC4A11 H+ permeability (12). Therefore, we tested whether these two compounds affect the SLC4A11 NH3/H+ current in our model system. However, we found that neither 100 μm DIDS nor 10 μm EIPA affected NH3/H+ inward currents in SLC4A11-PS120 cells (Fig. 10). It is well known that DIDS is a broad spectrum inhibitor of anion transporters, whereas EIPA is a potent Na+/H+ exchanger inhibitor. These data suggest unique pharmacological properties for SLC4A11 expressed in NHE-deficient PS120 cells and indicate that the previous studies may be complicated by the presence of endogenous transporters influenced by SLC4A11 activity.
FIGURE 10.
SLC4A11 eNH4Cl-dependent currents are inhibited by neither 10 μm EIPA nor 100 μm DIDS. A, sample whole-cell current recording of eNH4Cl-dependent current in SLC4A11 cells before (a), during (b), and after (c) 10 μm EIPA application. B, bar graph summary of eNH4Cl-dependent current density before (a), during (b), and after (c) EIPA application (n = 6) (repeated measure ANOVA, p = 0.298). C, representative whole-cell current recording of eNH4Cl-dependent current in SLC4A11 cells before (d), during (e), and after (f) 100 μm DIDS application. D, bar graph summary of eNH4Cl-dependent current density before (d), during (e), and after (f) DIDS application (n = 7) (repeated measure ANOVA, p = 0.847). Error bars, S.E.
Discussion
In this study, we examined the potential mechanism for apparent NH4+ permeability of SLC4A11 (9). Using the NHE-deficient PS120 fibroblast cell line, we found evidence indicating that human SLC4A11 is an electrogenic NH3/H+ transporter. As such, SLC4A11 should be considered when examining ammonia: H+ balance along with other recently identified NH3 channels/transporters, namely selected AQPs (AQP8, AQP3, AQP7, AQP9, and AQP10) (14), Amt proteins, methylamine permease (22), and rhesus protein (23). In addition, our findings may also provide insight into the pathophysiology of corneal endothelial dystrophy (24, 25) and an explanation for altered renal function in SLC4A11 knock-out mice (7, 8).
We initially observed apparent SLC4A11 NH4+ permeability during studies of acid load recovery using an ammonium pulse approach. Significant acidification during the ammonium pulse was only seen in SLC4A11-PS120 cells. When Boron et al. (17) first studied NH3-induced intracellular pH transients in the squid giant axon, it was calculated that the intracellular acidification during NH4Cl pulse was due to a finite permeability to NH4+, and the acidification rate is determined mainly by the absolute NH4+ permeability. Also, the extent of the acid loading effect in the wash phase positively correlates with NH4+ permeability (17). Our data show that SLC4A11 transfection increased the apparent NH4+ permeability in PS120 cells relative to the low endogenous level in EV. SLC4A11 high expression Col4 and low expression Col12 showed higher and lower acidification rates, indicating that the apparent NH4+ (or NH3-H+ as equivalent) permeability is directly associated with SLC4A11 expression level.
The electrophysiological recordings revealed that SLC4A11-induced NH4+ (or NH3-H+) permeability results in NH4Cl-dependent currents. The inward currents vary directly with solution [NH3] but not [NH4+] and were relatively unaffected by removal of Na+, K+, or Cl−. This is very similar to that observed in AQP8, which was proposed to be permeable to NH3 rather than NH4+ due to the hydrophobic pore inside the channel (14, 23). Interestingly, there is evidence that SLC4A11 has water channel properties and shares one NPX motif with AQPs (13). NH3 and H2O share similar size and dipole moment (1.5 versus 1.8 debye), and the aromatic/arginine regions in AQPs provide selectivity of NH3 over H2O (15). In the current study, we observed an 84-fold increase in current in response to a 75-fold increase in [NH3]o (Fig. 2), and when [NH3] is equalized in different pH solutions by adding more NH4Cl at lower pH and less at high pH (Fig. 3), we observed similar current amplitude, strongly supporting NH3 as the actual species transported. Furthermore, the ammonia analog MA has a similar molecular radius as NH3 (26) but a significantly higher pKa. So there is less free uncharged [MA] than [NH3] at the same pH, and as a result, MA generated less current in SLC4A11 than NH3.
A distinctive characteristic of SLC4A11 that we observed here, which differentiates SLC4A11 from other ammonia channels/transporters, is that NH3 is evidently co-transported with H+. Unlike the ammonia- and H+-permeable AQP1 Arg-195 mutant that shows H+ currents in the absence of NH3 (15), SLC4A11 shows no currents in response to proton gradient changes alone (Fig. 5A). Proton gradient changes can induce currents only after NH4Cl pulse (Fig. 2, filled arrow, and Fig. 5, B and D). When equalizing [NH3]o (Fig. 3), extracellular pH or [H+] gradient differences can affect apparent kinetics and current density of eNH4Cl-dependent current. Reversal potential analysis of currents during and after NH4Cl pulse is also consistent with an NH3/H+ co-transport hypothesis. Although H+ is the putative charge carrier in the currents of all electrogenic NH3 channels/transporters, we show here, for the first time, evidence that H+ is co-transported with NH3 in SLC4A11. A coupled transport of H+ and NH3 may be more beneficial for the cell because it would produce smaller cytosolic pHi transients than if NH3 were transported alone.
As an NH3/2H+ co-transporter, the direction of SLC4A11 transport depends on the [NH3] gradient, [H+] gradient, and cell membrane potential. In most of the experiments, the holding potential was −60 mV, and we introduced an inward [NH3] gradient that resulted in inward currents. However, we observed outward currents on multiple occasions: 1) during pH 8.5 wash after pH 8.5 NH4Cl (Fig. 5C, time c); 2) prominent and prolonged outward currents during wash periods in cells dialyzed with NH4Cl (Fig. 7A); 3) smaller outward currents observed during pH 8.5 wash following pH 7.5 eNH4Cl (Fig. 5B); 4) outward break-in currents observed with I1-NH4Cl dialysis if the holding potential was reduced to −10 mV (Fig. 7, D and E); 5) at −10 mV with I1-NH4Cl dialysis, outward currents were observed in pH 7.5 wash after pH 7.5 NH4Cl (Fig. 7D). Given a 1:2 NH3/H+ stoichiometry of SLC4A11 at pHo 7.5, in a typical cell with pHi 7.1 and membrane potential of −50 mV, an ∼15-fold [NH3] outward gradient is needed to drive SLC4A11 transport in the outward direction. This could potentially be achieved, for example, in glutamine-metabolizing cells that generate significant amounts of NH3 (27, 28). Although NH3 can diffuse across most cell membranes, a NH3/H+ co-transporter like SLC4A11 may offer an advantage of directional regulated ammonia transport, which could play an important role in the function of ammonia detoxification like other ammonia channels (e.g. AQP8 (27)).
Although the net movement of NH3/H+ is physiologically equivalent to NH4+, transport of NH3 over NH4+ offers the advantage of high selectively. K+ and NH4+ ions are similar in several characteristics that are important for membrane transport: 1) they share an almost identical radius (NH4+ 0.148 nm versus K+ 0.141 nm) (29); 2) they have similar charge (15); and 3) hydration energies are also similar (30). Therefore, it is not surprising that multiple K+ transporters/channels allow substitution of NH4+ for K+ (e.g. in the Na-K-2Cl co-transporter NKCC2 and barium-sensitive K+ channels (31)). Reverse substitution could possibly occur in NH4+-selective transporters to carry K+ nonspecifically (15). To avoid this, the Amt NH4+ channel uses a two-step translocation process involving a hydrophobic pore to discriminate NH4+ from K+ (32). For SLC4A11, transport of NH3 instead of NH4+ may also help resolve the selectivity problem. Because K+ free extracellular and intracellular solutions were used in most of the experiments presented here, K+ involvement in the recorded currents is unlikely. Indeed, K+ is not a substrate for SLC4A11 shown in Fig. 4A comparing current density in 5 mm K+ E1 versus K+ free E4. This suggests that SLC4A11 utilizes a mechanism ensuring its relative selectivity. However, greater understanding of the molecular mechanisms underlying the processes would require further investigation.
Park et al. (10) reported increased baseline pHi in SLC4A11-transfected HEK293 cells, whereas our previous study reported no difference (9). Consistent with a more recent report (12), here we observed reduced baseline pHi in SLC4A11-PS120 cells (Fig. 6A). Although the H+ permeability is evident, in the absence of NH3, the H+ fluxes are very small, and no measureable H+ current can be recorded. Interestingly, the effect on baseline pHi is made most evident in PS120 cells because of the lack of Na+/H+ exchangers. Previous studies show that when a more robust bicarbonate buffering system is present, baseline pHi differences disappear (9, 10, 12). This suggests that SLC4A11 H+ permeability in the absence of ammonia is a relatively small contributor to pHi regulation, whereas ammonia transport through SLC4A11 is of more physiological relevance.
Park at al. (10) also concluded that SLC4A11 is an Na+-coupled borate transporter (with a capability to transport hydroxyl ions (OH−) in the absence of borate). Interestingly, boric acid is a weak acid with a pKa of 9.24, which is very close to that of NH3 (pKa = 9.25), making it largely exist as uncharged H3BO3 at physiological pH (33). The structure of boric acid is comparable with that of NH3, with the central boron atom possessing three valence electrons, as nitrogen does in NH3. The molecular radius of boric acid (2.573 Å) is comparable with that of urea (2.618 Å), which is also permeable through some ammonia-permeable AQPs (14). If a hydrophobic environment does exist in SLC4A11 supporting NH3 transport, it may provide a path for the uncharged boric acid.
This study has several limitations. Because of the ability of Na+ to modulate SLC4A11 activity at pH 8.5, we only determined the apparent stoichiometry of NH3/H+ transport at the physiological pH 7.5, under which Na+ does not appear to have any effect on eNH4Cl-dependent currents. We are also aware of the limitation of sequential NH4Cl superfusion during which the assumption of constant [NH3]i and [H+]i is uncertain. Indeed, there was current inhibition at high NH4Cl concentrations, possibly due to increased [NH3]i, which could lead to an underestimation of Erev. Notably, plotting the plateau Erev data versus log[NH3]o yielded a smaller slope, however still closest to the 1:2 NH3/H+ model. Also, because NH3 is diffusible through the PS120 cell membrane (shown in Fig. 1), we are not able to control [NH3]i and [H+]i more tightly. In addition, the possibility of NH4+-H+-coupled transport cannot be completely eliminated. Therefore, future investigations, such as SLC4A11 protein reconstitution into a planar lipid bilayer or SLC4A11 expression in an NH3-impermeable cell line, would be useful to clarify the uncertainties with the 1:2 NH3/H+ model.
In healthy individuals, urine ammonia concentration is around 30–50 mm, whereas plasma ammonia concentration ranges between 0.005 and 0.02 mm. Plasma ammonia can rise to 0.1 mm under pathophysiological conditions. The fact that we were able to record currents from 1 to 100 mm eNH4Cl (0.0017–17 mm (NH3)) with no saturation indicates that SLC4A11 can efficiently function in the physiological ammonia range. Ammonia channels/transporters play important roles in nitrogen homeostasis, not only in the major nitrogen handling organs like liver and kidney (27, 34), but also in lung (35), male reproductive system (36), and gastrointestinal tract (37). Directional ammonia transport capacity offered by SLC4A11 can play essential roles in ammonia detoxification in a wide range of tissues/cells. Especially in cells that actively metabolize glutamine and glutamate, such as neurons, skeletal muscle cells, and gastrointestinal tract epithelium (38, 39), ammonia is a major waste product that needs to be extruded. Indeed, SLC4A11 is not only associated with corneal endothelial dystrophy, but also may play roles in other physiological and pathophysiology processes. The ubiquitous expression of SLC4A11, in cornea endothelium, kidney, inner ear, salivary glands (submandibular and parotid), thyroid, mammary gland, testis, trachea, esophagus, pancreas, liver, spleen, and cerebellum (1, 6, 10) suggests a diverse physiological role. Because SLC4A11 is highly expressed in cornea endothelium, fibrocytes of the inner ear, and the loop of Henle, these three tissues could be the starting point for investigating SLC4A11 physiological function in regulating tissue nitrogen levels. It is well known that cornea and inner ear function depends on tightly controlled fluid and ion transport. At least 60 mutations have been identified as being associated with corneal endothelial dystrophy and/or perceptive deafness (40). A recent clinical study suggests that homozygous SLC4A11 mutated congenital hereditary endothelial dystrophy patients progress to Harboyan syndrome at a later age (perceptive deafness and corneal endothelial dystrophy (41)), whereas another study associated SLC4A11 mutants with the corneal ectasia disorder keratoconus (42). In the kidney, SLC4A11 is highly expressed in the thin descending limb of loop of Henle (7). Henle's loop epithelium has the capacity to reabsorb ammonia into interstitial spaces and, through the countercurrent multiplication mechanism, create a cortical-medullary increasing ammonium gradient to regulate urine ammonium excretion (18). In SLC4A11 knock-out mice, an impairment in the countercurrent multiplication process was suggested based on polyuria and urine hypo-osmolality (7). The ammonia transport property of SLC4A11 suggests that it can potentially play a role in medullary interstitial ammonia handling and ammonia countercurrent multiplication (18, 31), filling the missing piece of renal ammonia transport (31).
In summary, we have identified SLC4A11 as an additional member of the subfamily of ammonia channels and transporters and as an H+-coupled ammonia transporter with an apparent stoichiometry of 1:2 NH3/H+. The SLC4A11 ammonia current is independent of K+, Na+, and Cl− at physiological pH and is uniquely insensitive to DIDS and EIPA. These findings provide mechanistic insights into the physiological and pathophysiological processes that may occur in the cornea, kidney, and inner ear.
Acknowledgment
We thank Dr. Eranga Vithana (Singapore Eye Research Institute) for the SLC4A11-HA plasmid.
Author Contributions—W. Z., J. A. B., and A. G. O. conceived and coordinated the study and wrote the paper. W. Z., D. G. O., and J. A. B. designed and W. Z. and D. G. O. performed and analyzed the experiments shown in Figs. 1 and 6. W. Z., J. A. B., and A. G. O. designed and W. Z. performed and analyzed the electrophysiological experiments shown in Figs. 2–5 and 7–10. All authors reviewed the results and approved the final version of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants 5R01EY008834 (to J. A. B.), P30EY019008 (Core Grant of Vision Science), and R01HL115140 (to A. G. O). The authors declare that they have no conflicts of interest with the contents of this article.
- EIPA
- ethyl-isopropyl-amiloride
- AQP
- aquaporin
- DIDS
- 4,4′- diisothiocyanatostilbene-2,2′-disulfonic acid
- EV
- empty vector
- NHE
- Na+/H+ exchanger
- BCECF
- 2′7′-bis(carboxyethy)-5(6)-carboxyfluorescein
- eNH4Cl
- extracellular NH4Cl
- pF
- picofarads
- ANOVA
- analysis of variance
- MA
- methylammonia.
References
- 1. Parker M. D., Ourmozdi E. P., Tanner M. J. A. (2001) Human BTR1, a new bicarbonate transporter superfamily member and human AE4 from kidney. Biochem. Biophys. Res. Commun. 282, 1103–1109 [DOI] [PubMed] [Google Scholar]
- 2. Damkier H. H., Nielsen S., Praetorius J. (2007) Molecular expression of SLC4-derived Na+-dependent anion transporters in selected human tissues. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R2136–R2146 [DOI] [PubMed] [Google Scholar]
- 3. Shei W., Liu J., Htoon H. M., Aung T., Vithana E. N. (2013) Differential expression of the Slc4 bicarbonate transporter family in murine corneal endothelium and cell culture. Mol. Vision 19, 1096–1106 [PMC free article] [PubMed] [Google Scholar]
- 4. Aldave A. J., Han J., Frausto R. F. (2013) Genetics of the corneal endothelial dystrophies: an evidence-based review. Clin. Genet. 84, 109–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gottsch J. D., Bowers A. L., Margulies E. H., Seitzman G. D., Kim S. W., Saha S., Jun A. S., Stark W. J., Liu S. H. (2003) Serial analysis of gene expression in the corneal endothelium of Fuchs' dystrophy. Invest. Ophthalmol. Vis. Sci. 44, 594–599 [DOI] [PubMed] [Google Scholar]
- 6. Lopez I. A., Rosenblatt M. I., Kim C., Galbraith G. C., Jones S. M., Kao L., Newman D., Liu W., Yeh S., Pushkin A., Abuladze N., Kurtz I. (2009) Slc4a11 gene disruption in mice: cellular targets of sensorineuronal abnormalities. J. Biol. Chem. 284, 26882–26896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gröger N., Fröhlich H., Maier H., Olbrich A., Kostin S., Braun T., Boettger T. (2010) SLC4A11 prevents osmotic imbalance leading to corneal endothelial dystrophy, deafness, and polyuria. J. Biol. Chem. 285, 14467–14474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Han S. B., Ang H. P., Poh R., Chaurasia S. S., Peh G., Liu J., Tan D. T., Vithana E. N., Mehta J. S. (2013) Mice with a targeted disruption of Slc4a11 model the progressive corneal changes of congenital hereditary endothelial dystrophy. Invest. Ophthalmol. Vis. Sci. 54, 6179–6189 [DOI] [PubMed] [Google Scholar]
- 9. Ogando D. G., Jalimarada S. S., Zhang W., Vithana E. N., Bonanno J. A. (2013) SLC4A11 is an EIPA-sensitive Na+-permeable pHi regulator. Am. J. Physiol. Cell Physiol. 305, C716–C727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Park M., Li Q., Shcheynikov N., Zeng W., Muallem S. (2004) NaBC1 is a ubiquitous electrogenic Na+-coupled borate transporter essential for cellular boron homeostasis and cell growth and proliferation. Mol. Cell 16, 331–341 [DOI] [PubMed] [Google Scholar]
- 11. Jalimarada S. S., Ogando D. G., Vithana E. N., Bonanno J. A. (2013) Ion transport function of SLC4A11 in corneal endothelium. Invest. Ophthalmol. Vis. Sci. 54, 4330–4340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kao L., Azimov R., Abuladze N., Newman D., Kurtz I. (2015) Human SLC4A11-C functions as a DIDS-stimulatable H+(OH−) permeation pathway: partial correction of R109H mutant transport. Am. J. Physiol. Cell Physiol. 308, C176–C188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vilas G. L., Loganathan S. K., Liu J., Riau A. K., Young J. D., Mehta J. S., Vithana E. N., Casey J. R. (2013) Transmembrane water-flux through SLC4A11: a route defective in genetic corneal diseases. Hum. Mol. Genet. 22, 4579–4590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Litman T., Søgaard R., Zeuthen T. (2009) in Aquaporins (Beitz E., ed) pp. 327–358, Springer, Berlin [Google Scholar]
- 15. Beitz E., Wu B., Holm L. M., Schultz J. E., Zeuthen T. (2006) Point mutations in the aromatic/arginine region in aquaporin 1 allow passage of urea, glycerol, ammonia, and protons. Proc. Natl. Acad. Sci. U.S.A. 103, 269–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. (1979) Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry 18, 2210–2218 [DOI] [PubMed] [Google Scholar]
- 17. Boron W. F., De Weer P. (1976) Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J. Gen. Physiol. 67, 91–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Packer R. K., Desai S. S., Hornbuckle K., Knepper M. A. (1991) Role of countercurrent multiplication in renal ammonium handling: regulation of medullary ammonium accumulation. J. Am. Soc. Nephrol. 2, 77–83 [DOI] [PubMed] [Google Scholar]
- 19. Yao H., Ma E., Gu X.-Q., Haddad G. G. (1999) Intracellular pH regulation of CA1 neurons in Na+/H+ isoform 1 mutant mice. J. Clin. Invest. 104, 637–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nakhoul N. L., Abdulnour-Nakhoul S. M., Boulpaep E. L., Rabon E., Schmidt E., Hamm L. L. (2010) Substrate specificity of Rhbg: ammonium and methyl ammonium transport. Am. J. Physiol. Cell Physiol. 299, C695–C705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Virkki L. V., Wilson D. A., Vaughan-Jones R. D., Boron W. F. (2002) Functional characterization of human NBC4 as an electrogenic Na+-HCO cotransporter (NBCe2). Am. J. Physiol. Cell Physiol. 282, C1278–C1289 [DOI] [PubMed] [Google Scholar]
- 22. Winkler F. K. (2006) Amt/MEP/Rh proteins conduct ammonia. Pflugers Arch. 451, 701–707 [DOI] [PubMed] [Google Scholar]
- 23. Nakhoul N. L., Abdulnour-Nakhoul S. M., Schmidt E., Doetjes R., Rabon E., Hamm L. L. (2010) pH sensitivity of ammonium transport by Rhbg. Am. J. Physiol. Cell Physiol. 299, C1386–C1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ramprasad V. L., Ebenezer N. D., Aung T., Rajagopal R., Yong V. H., Tuft S. J., Viswanathan D., El-Ashry M. F., Liskova P., Tan D. T., Bhattacharya S. S., Kumaramanickavel G., Vithana E. N. (2007) Novel SLC4A11 mutations in patients with recessive congenital hereditary endothelial dystrophy (CHED2). Mutation in brief #958. Online. Hum. Mutat. 28, 522–523 [DOI] [PubMed] [Google Scholar]
- 25. Vithana E. N., Morgan P. E., Ramprasad V., Tan D. T., Yong V. H., Venkataraman D., Venkatraman A., Yam G. H., Nagasamy S., Law R. W., Rajagopal R., Pang C. P., Kumaramanickevel G., Casey J. R., Aung T. (2008) SLC4A11 mutations in Fuchs endothelial corneal dystrophy. Hum. Mol. Genet. 17, 656–666 [DOI] [PubMed] [Google Scholar]
- 26. Cohen B. N., Labarca C., Davidson N., Lester H. A. (1992) Mutations in M2 alter the selectivity of the mouse nicotinic acetylcholine receptor for organic and alkali metal cations. J. Gen. Physiol. 100, 373–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Soria L. R., Marrone J., Calamita G., Marinelli R. A. (2013) Ammonia detoxification via ureagenesis in rat hepatocytes involves mitochondrial aquaporin-8 channels. Hepatology 57, 2061–2071 [DOI] [PubMed] [Google Scholar]
- 28. Albrecht J., Norenberg M. D. (2006) Glutamine: a Trojan horse in ammonia neurotoxicity. Hepatology 44, 788–794 [DOI] [PubMed] [Google Scholar]
- 29. Lima A. S., Bocchi N., Gomes H. M., Teixeira M. F. (2009) An electrochemical sensor based on nanostructured hollandite-type manganese oxide for detection of potassium ions. Sensors 9, 6613–6625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. ten Hoopen F., Cuin T. A., Pedas P., Hegelund J. N., Shabala S., Schjoerring J. K., Jahn T. P. (2010) Competition between uptake of ammonium and potassium in barley and Arabidopsis roots: molecular mechanisms and physiological consequences. J. Exp. Bot. 61, 2303–2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Houillier P., Bourgeois S. (2012) More actors in ammonia absorption by the thick ascending limb. Am. J. Physiol. Renal Physiol. 302, F293–F297 [DOI] [PubMed] [Google Scholar]
- 32. Khademi S., O'Connell J., 3rd, Remis J., Robles-Colmenares Y., Miercke L. J., Stroud R. M. (2004) Mechanism of ammonia transport by Amt/MEP/Rh: structure of AmtB at 1.35 Å. Science 305, 1587–1594 [DOI] [PubMed] [Google Scholar]
- 33. Tanaka M., Wallace I. S., Takano J., Roberts D. M., Fujiwara T. (2008) NIP6;1 is a boric acid channel for preferential transport of boron to growing shoot tissues in Arabidopsis. Plant Cell 20, 2860–2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weiner I. D., Verlander J. W. (2010) Molecular physiology of the Rh ammonia transport proteins. Curr. Opin. Nephrol. Hypertens. 19, 471–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Han K. H., Mekala K., Babida V., Kim H. Y., Handlogten M. E., Verlander J. W., Weiner I. D. (2009) Expression of the gas-transporting proteins, Rh B glycoprotein and Rh C glycoprotein, in the murine lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 297, L153–L163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee H. W., Verlander J. W., Handlogten M. E., Han K. H., Cooke P. S., Weiner I. D. (2013) Expression of the rhesus glycoproteins, ammonia transporter family members, RHCG and RHBG in male reproductive organs. Reproduction 146, 283–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Handlogten M. E., Hong S. P., Zhang L., Vander A. W., Steinbaum M. L., Campbell-Thompson M., Weiner I. D. (2005) Expression of the ammonia transporter proteins Rh B glycoprotein and Rh C glycoprotein in the intestinal tract. Am. J. Physiol. Gastrointest. Liver Physiol. 288, G1036–G1047 [DOI] [PubMed] [Google Scholar]
- 38. Newsholme P., Lima M. M., Procopio J., Pithon-Curi T. C., Doi S. Q., Bazotte R. B., Curi R. (2003) Glutamine and glutamate as vital metabolites. Braz. J. Med. Biol. Res. 36, 153–163 [DOI] [PubMed] [Google Scholar]
- 39. Newsholme P., Procopio J., Lima M. M., Pithon-Curi T. C., Curi R. (2003) Glutamine and glutamate: their central role in cell metabolism and function. Cell Biochem. Funct. 21, 1–9 [DOI] [PubMed] [Google Scholar]
- 40. Vilas G. L., Morgan P. E., Loganathan S. K., Quon A., Casey J. R. (2011) A biochemical framework for SLC4A11, the plasma membrane protein defective in corneal dystrophies. Biochemistry 50, 2157–2169 [DOI] [PubMed] [Google Scholar]
- 41. Siddiqui S., Zenteno J. C., Rice A., Chacón-Camacho O., Naylor S. G., Rivera-de la Parra D., Spokes D. M., James N., Toomes C., Inglehearn C. F., Ali M. (2014) Congenital hereditary endothelial dystrophy caused by SLC4A11 mutations progresses to Harboyan syndrome. Cornea 33, 247–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nowak D. M., Karolak J. A., Kubiak J., Gut M., Pitarque J. A., Molinari A., Bejjani B. A., Gajecka M. (2013) Substitution at IL1RN and deletion at SLC4A11 segregating with phenotype in familial keratoconus. Invest. Ophthalmol. Vis. Sci. 54, 2207–2215 [DOI] [PubMed] [Google Scholar]