Background: TAZ functions as a co-activator by up-regulating downstream transcriptional targets.
Results: TAZ can negatively regulate transcription of many genes such as ΔNp63 through TEAD transcription factor.
Conclusion: TAZ can also function as a transcriptional co-repressor.
Significance: Our findings open a new avenue for TAZ function in cancer.
Keywords: cell migration, Hippo pathway, oncogene, transcription coactivator, transcription corepressor
Abstract
Transcriptional co-activator with a PDZ binding domain (TAZ) is a WW domain-containing transcriptional co-activator and a core component of an emerging Hippo signaling pathway that regulates organ size, tumorigenesis, metastasis, and drug resistance. TAZ regulates these biological functions by up-regulating downstream cellular genes through transactivation of transcription factors such as TEAD and TTF1. To understand the molecular mechanisms underlying TAZ-induced tumorigenesis, we have recently performed a gene expression profile analysis by overexpressing TAZ in mammary cells. In addition to the TAZ-up-regulated genes that were confirmed in our previous studies, we identified a large number of cellular genes that were down-regulated by TAZ. In this study, we have confirmed these down-regulated genes (including cytokines, chemokines, and p53 gene family members) as bona fide downstream transcriptional targets of TAZ. By using human breast and lung epithelial cells, we have further characterized ΔNp63, a p53 gene family member, and shown that TAZ suppresses ΔNp63 mRNA, protein expression, and promoter activity through interaction with the transcription factor TEAD. We also show that TEAD can inhibit ΔNp63 promoter activity and that TAZ can directly interact with ΔNp63 promoter-containing TEAD binding sites. Finally, we provide functional evidence that down-regulation of ΔNp63 by TAZ may play a role in regulating cell migration. Altogether, this study provides novel evidence that the Hippo component TAZ can function as a co-repressor and regulate biological functions by negatively regulating downstream cellular genes.
Introduction
The Hippo pathway was originally discovered in Drosophila as an evolutionarily conserved tumor suppressor pathway that acts as a key regulator of organ size control (1, 2). This signaling pathway has been shown to control many biological functions such as cell proliferation, apoptosis, cell-cell contact inhibition, stem cell self-renewal, and tissue regeneration (2–10). In mammals, cell-cell contact or increased actin polymerization can activate mammalian sterile-20-like kinase 1/2, which subsequently activates adaptor proteins Mob1A/1B and scaffold protein salvador (Sav1) to promote the phosphorylation and activation of large tumor suppressor 1/2 kinases. In turn, large tumor suppressor 1/2 phosphorylate downstream transcriptional co-activators transcriptional co-activator with a PDZ binding domain (TAZ)5 and its paralog yes-associated protein (YAP) to promote their cytoplasmic retention and subsequent degradation (11–14). Conversely, dephosphorylated YAP and TAZ are able to enter the nucleus where they interact with multiple transcription factors and exert high transactivation activity.
TAZ is a widely characterized oncogene that is overexpressed or dysregulated in several cancer types including breast (15, 16), lung (17, 18), colorectal (19), and thyroid (20). It is proposed as a major regulator of cell proliferation, cell migration and invasion, epithelial-mesenchymal transition (EMT), human embryonic stem cell renewal, and drug resistance (21–27). Within the N terminus of TAZ lies a TEAD binding domain (TBD) responsible for the interaction with the TEAD family of transcription factors. Mounting evidence over the years has supported TEAD family members as one of the most common binding partners of TAZ; they play crucial roles in mediating many TAZ functions including cellular growth, proliferation, and oncogenic transformation (28–31). The mechanisms underlying TAZ-mediated transcriptional activation of downstream genes through its interaction with transcription factors have been often studied and observed by many research groups. However, there has been little interest in elucidating novel targets negatively regulated by TAZ and addressing their molecular mechanisms and functional implications in tumorigenesis.
In this study, we have identified ΔNp63, a member of the p53 tumor suppressor family, as a significantly down-regulated target in TAZ-overexpressing breast and lung epithelial cells. Moreover, we show that TAZ-induced repression of ΔNp63 transcription is mediated by the TEAD family of transcription factors and that reintroduction of ΔNp63 into TAZ-overexpressing cells partially rescues TAZ-induced cell migration. Together, our findings provide the first evidence that TAZ can directly negatively regulate cellular gene transcription by interacting with TEAD transcription factor.
Experimental Procedures
Plasmid Construction and Site-directed Mutagenesis
The promoter region of ΔNp63 (nucleotide positions −1500 to +40) was amplified by PCR from genomic DNA extracted from MCF10A human immortalized mammary cells using the following primers: ΔNp63-pr sense primer (5′-ATGGTACCTATGTGTGAAGAAATGAATGTTTTGTCTG-3′; KpnI site is underlined) and ΔNp63-pr antisense primer (5′-AATCTCGAGAAGATAACAGAACTCAAGTCCCTCTCTCTC-3′; XhoI site is underlined). The PCR products were digested with KpnI/XhoI and subsequently cloned into the KpnI/XhoI sites of the pGL3-Basic luciferase reporter vector (Promega). Human ΔNp63 cDNA (Addgene) was cloned into the XhoI/MluI sites of the doxycycline (Dox)-inducible pTRIPZ lentiviral vector (Open Biosystems). Mutation of TAZ-F52A/F53A (F, phenylalanine; A, alanine) was performed by overlapping PCR using TAZ-mutagenic primers. TAZ-S89A-F52A/F53A mutant was created using overlapping PCR and subsequently cloned into the XhoI/MluI sites of the pTRIPZ lentiviral vector.
Cell Culture
MCF10A (human immortalized epithelial breast) cells were cultured in Dulbecco's modified Eagle's medium (DMEM)/Nutrient Mixture F-12 Ham (Sigma-Aldrich) supplemented with 5% horse serum, 1% penicillin-streptomycin, 2.5 mm l-glutamine, 10 μg/ml insulin, 0.5 μg/ml hydrocortisone, 100 ng/ml cholera toxin, and 20 ng/ml human EGF. SK-BR3 (human breast cancer) cells were cultured in McCoy's 5A modified medium (Sigma-Aldrich) supplemented with 2.2 g/liter sodium bicarbonate, 10% fetal bovine serum (FBS), and 1% penicillin-streptomycin. SK-Luci6 (human anaplastic lung cancer), HEK293T (human embryonic kidney), COS7 (monkey fibroblast-like kidney), A549 (lung adenocarcinoma), and HCC38 (human ductal breast carcinoma) cells were cultured in RPMI 1640 medium (Sigma-Aldrich) supplemented with 10% FBS and 1% penicillin-streptomycin. Cells were maintained at 37 °C with 5% CO2.
Lentiviral Production, Infection, and Establishment of Cell Lines with Stable Overexpression of Cellular Genes
Lentiviral production, purification, titration, and infection of overexpressing constructs were performed as described (11). Generation of ΔNp63-overexpressing stable cell lines was performed by infecting TAZ-overexpressing MCF10A cells with lentivirus expressing Dox-inducible ΔNp63 (pTRIPZ vector) at a multiplicity of infection of 2. Generation of stable cell lines with overexpression of TEAD binding mutants of TAZ was performed by infecting TAZ-low MCF10A cells with lentivirus expressing TAZ-F52A/F53A-HA (WPI vector) or Dox-inducible TAZ-S89A-F52A/F53A-HA at a multiplicity of infection of 2. Cells were selected 48 h postinfection using 1 μg/ml puromycin.
Microarray and Data Analysis
Gene expression profile analysis by microarray and data analysis were as described (32).
Transient Knockdown of Gene Expression by Small Interfering RNA (siRNA)
To knock down TEAD1/3/4, 5 × 104 SK-BR3 cells were transfected with 50 nm TEAD1/3/4 siRNA (5′-CACAAGACGUCAAGCCUUU-3′ (sense)/5′-UUGUGGAUGAAGUUGAUCAUU-3′ (antisense)) (GE Healthcare) using Lipofectamine RNAiMAX transfection reagent (Life Technologies) according to the manufacturer's protocol. Efficiency of knockdown was assessed by Western blotting 48 h post-transfection.
Reagents, Antibodies, Western Blotting, and Co-immunoprecipitation
Trichostatin A and UNC0631 were purchased from Sigma. The mouse monoclonal antibodies used in this study were obtained from the following companies: anti-TAZ from BD Pharmingen; anti-p63 (4A4), anti-vestigial-like protein 4 (VGLL4), and anti-FLAG (M2) from Sigma-Aldrich; anti-TEAD (TEF-1) from Abcam; and anti-HA (F7) from Santa Cruz Biotechnology. Anti-histone acetylation component antibodies were obtained from Cell Signaling Technology. Protein extraction, Western blot analyses, and co-immunoprecipitation were performed as described (11).
RNA Isolation and Quantitative Reverse Transcription-PCR (qRT-PCR)
Cells were grown to about 70–90% confluence. RNA was extracted using TRIzol reagent (Invitrogen) according to the manufacturer's protocol. RNA quantitation and quality were assessed by spectrophotometry and RNase-free gel electrophoresis. qRT-PCR analyses were performed in duplicates of 200 ng/μl RNA/sample/reaction with 200 nm gene-specific forward and reverse primers (Table 1) using the SuperScript III Platinum SYBR Green One-Step qRT-PCR kit (Invitrogen) and the Applied Biosystems ViiA 7 Real-Time PCR System. 18S ribosomal RNA (rRNA) expression was used as an internal control. mRNA expression levels were calculated as described (11) and are shown as -fold change.
TABLE 1.
Primers used for qRT-PCR
F, forward; R, reverse.
| F | GGACTGTATCCGCATGCA |
| R | GACCTGGGCTGTGCGTAG |
| ΔNp63 | |
| F | GAGTTCTGTTATCTTCTTAAG |
| R | TGTTCTGCGCGTGGTCTG |
| BMP2 | |
| F | TGCGCATGCTTCCACCATGAAG |
| R | TCTGCTGSGGTGATAAACTCC |
| CXCL1 | |
| F | AGTCATAGCCACACTCAAGAATGG |
| R | GATGCAGGATTGAGGCAAGC |
| CXCL2 | |
| F | CGCCCAAACCGAAGTCATAG |
| R | AGACAAGCTTTCTGCCCATTCT |
| CXCL3 | |
| F | TCCCCCATGGTTCAGAAAATC |
| R | GGTGCTCCCCTTGTTCAGTAT |
| IL1α | |
| F | TGTGACTGCCCAAGATGAAG |
| R | CTTAGCGCCGTGAGTTTCCC |
| IL1β | |
| F | GAAGCTGATGGCCCTAAACAG |
| R | GAAGCCCTTTGCTGTAGTGGT |
| IL6 | |
| F | TCCTCGACGGGCATCTCAGCC |
| R | ATCTTTGGAAGGTTCAGGTTG |
| IL8 | |
| F | CGGAAGGAACCATCTCACTG |
| R | AGCACTCCTTGGCAAAACTG |
| GJA1 | |
| F | ACACCTTCCCTCCAGCAGTT |
| R | GGAGTTCAATCACTTGGCGT |
Dual-Luciferase Assay
Triplicates of 5 × 104 cells/well SK-BR3 or SK-LuCi6 cells were seeded in 12-well plates and transfected with ΔNp63-luc or its mutants (0.1 μg) alone or in combination with TAZ (0.2 μg), TAZ (0.2 μg) plus TEAD (0.1 μg), or their respective mutants using PolyJet reagent (SignaGen). 10 ng/well Renilla luciferase vector (pRL-TK) was used as an internal transfection control. Luciferase activity was assessed 48 h post-transfection using a Turner Biosystem 20/20 luminometer and the Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer's protocol.
Chromatin Immunoprecipitation (ChIP) Assay
A ChIP-IT Express enzymatic kit (Active Motif) was used for ChIP analysis of TAZ-S89A and ΔNp63 promoter interaction. MCF10A cells expressing WPI or TAZ-S89A were grown to 70–80% confluence on 150-mm dishes. Cells were treated with 1% formaldehyde, lysed, harvested, and homogenized using a Dounce homogenizer according to the manufacturer's protocol. DNA was enzymatically sheared, and the fragmented chromatin was incubated with 2 μg of mouse anti-HA (F7) monoclonal antibody. Chromatin was eluted, reverse cross-linked, and treated with Proteinase K. Amplification of the ΔNp63 promoter was performed by PCR using the following primers: (5′-ATGGTACCGTCTGTCTCCTGGGTTTG-3′ (sense) and 5′-GTGCACTTTCTTATGAAAGAGAC-3′ (antisense)). The PCR products were run on a 3% ethidium bromide-agarose gel and visualized under UV light using the Gel Doc system.
Wound Healing Cell Migration Assay
MCF10A-WPI, MCF10A-TAZ, and MCF10A-TAZ-ΔNp63 cells were grown to 80% confluence, serum-starved overnight in 2% horse serum, and scratch-wounded 24 h later using a P20 pipette tip. Cell migration was monitored, and pictures were taken at 0, 20, or 40 h under white light at 10× magnification using a Nikon Eclipse TE-2000U inverted microscope and a Nikon Coolpix 990 camera. Distance migrated (pixels) was measured with Adobe Photoshop software. MCF10A-TAZ-S89A, MCF10A-TAZ-S89A-F52A/F53A, MCF10A-TAZ-S89A-WWm, and HBE135-TAZ-S89A cells were untreated (−) or treated (+) with Dox (1 μg/ml); A549 and HCC38 cells were infected with siRNA against TAZ; and cells were subsequently plated and scratched-wounded as described above. Pictures were taken at 24 or 48 h.
Statistical Analysis
Significant differences were analyzed by Student's t test, and differences in mRNA levels between MCF10A-TAZ and its mutants and in promoter activities were calculated using analysis of variance tests. A p value <0.05 was regarded as statistically significant.
Results
Identification and Validation of Target Genes Negatively Regulated by TAZ
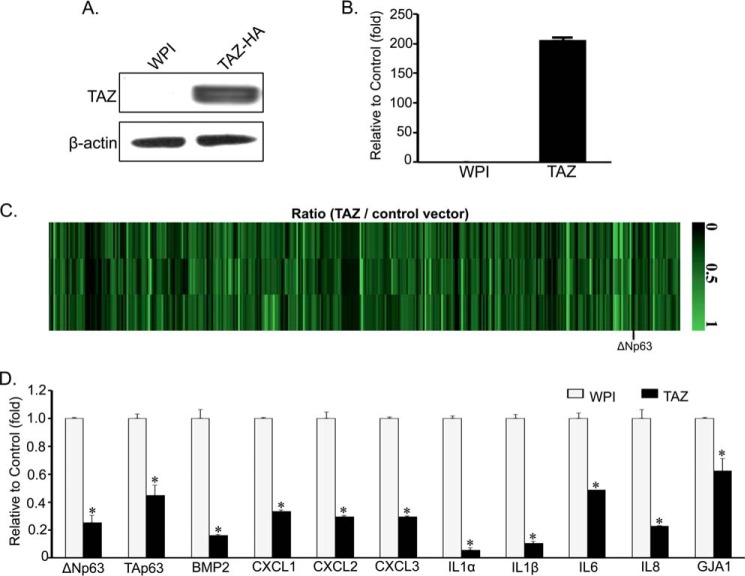
To identify downstream genes mediating TAZ function, we performed a 44,000 whole human genome microarray profiling (32) using RNAs from MCF10A stably expressing WPI empty vector control (MCF10A-WPI) or wild-type TAZ (MCF10A-TAZ). Enhanced TAZ mRNA and protein expression levels were confirmed by qRT-PCR and Western blotting (Fig. 1, A and B). Although 390 genes were up-regulated by TAZ (32), surprisingly, about 328 cellular genes were also found to be down-regulated by TAZ (Fig. 1C and supplemental Table S1). Based on their functional relevance in tumorigenesis, we confirmed several target genes from our DNA microarray results using real time qRT-PCR. Interestingly, we identified several proinflammatory cytokines and chemokines including IL1α/β, IL6, IL8, CXCL1/2/3, and BMP2 as well as p63 isoforms ΔNp63 and TAp63 as significantly down-regulated (1.5–10 ± 0–0.05-fold) targets in MCF10A-TAZ cells (Fig. 1D). Among these, proinflammatory cytokines IL1α and IL1β showed the most significant decrease in their relative mRNA expression, suggesting a possible involvement of TAZ in immune and inflammatory responses. Surprisingly as well, the p53 family members TAp63 and its N-terminal truncated form ΔNp63 showed an important TAZ-induced suppression in mRNA expression levels. Because p63 has been previously suggested as a marker of epithelial breast carcinoma and plays important roles in tumorigenesis and metastasis (33, 34), we sought to elucidate the molecular mechanisms and functional implications of TAZ-mediated repression of p63.
FIGURE 1.
Validation of target genes negatively regulated by TAZ using real time qRT-PCR. A, Western blot analysis of TAZ expression in MCF10A cells. MCF10A cells were stably infected with lentivirus expressing WPI vector control or TAZ-HA. Western blot analysis was performed by using anti-TAZ antibody. β-Actin was used as an internal loading control. B and D, qRT-PCR analysis of TAZ (B) and its down-regulated cellular gene mRNA expression (D). Total RNAs were extracted from MCF10A-WPI and MCF10A-TAZ cells. mRNA levels were measured by real time qRT-PCR using gene-specific primers (Table 1). Relative expression levels of mRNA in MCF10A-TAZ (black bars) were compared with MCF10A-WPI control cells (white bars). Data are represented as relative -fold decrease. The experiment was performed in duplicate, and error bars represent S.D. from each set of duplicates. Statistical differences in mRNA levels between MCF10A-WPI and MCF10A-TAZ cells were analyzed by Student's t test. *, statically significant difference (p < 0.05). C, heat map for genes down-regulated by TAZ.
ΔNp63 Is a Novel Down-regulated Target of TAZ
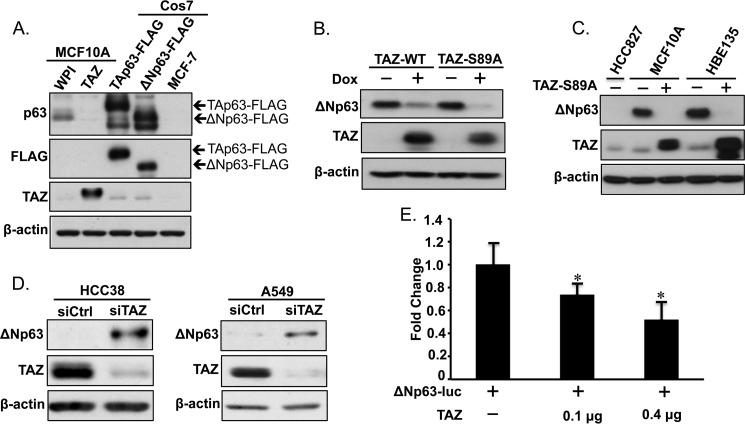
To further confirm TAZ-mediated suppression of p63 in MCF10A cells, we performed protein analysis using Western blotting. Protein lysates from MCF10A-WPI and MCF10-TAZ cells were analyzed together with MCF-7 p63 negative control and COS7 cells transfected with TAp63-FLAG and ΔNp63-FLAG plasmids. We identified ΔNp63 rather than TAp63 as the p63 protein isoform predominantly suppressed by TAZ in MCF10A-TAZ cells (Fig. 2A). Similarly, ΔNp63 protein expression was highly decreased in MCF10A cells after transient induction of wild-type TAZ (TAZ-WT) or constitutively active TAZ (TAZ-S89A) by Dox (Fig. 2B), suggesting that suppression of ΔNp63 by TAZ is not caused by viral infection or puromycin selection during establishment of stable lines. As expected, inducible expression of TAZ-S89A displayed a stronger effect on ΔNp63 repression compared with TAZ-WT. To characterize ΔNp63 as a bona fide down-regulated target gene of TAZ in other cell type, we analyzed ΔNp63 protein expression levels in HBE135 human bronchial epithelial lung cells containing inducible expression of TAZ-S89A (Fig. 2C). Interestingly, ΔNp63 expression was also repressed in HBE135 cells after TAZ induction, similar to the effect observed in MCF10A cells. Furthermore, we performed transient TAZ knockdown using siRNAs (siTAZ) in HCC38 human epithelial breast cancer cells and A549 human lung adenocarcinoma cells that expressed high levels of TAZ (35).6 ΔNp63 protein levels were significantly increased after TAZ was knocked down in both cell lines (Fig. 2D). Finally, we sought to elucidate the effects of TAZ overexpression on ΔNp63 promoter activity using the Dual-Luciferase assay. The promoter region of ΔNp63 was cloned from MCF10A genomic DNA into a luciferase reporter vector (ΔNp63-luc), which was transiently transfected into TAZ-low SK-BR3 breast cancer cells alone or in combination with increasing dosages of TAZ (Fig. 2E). Concordant with our previous results, TAZ showed a dosage-dependent suppression of ΔNp63-luc activity, suggesting that TAZ causes reduced ΔNp63 expression by suppressing its promoter activity. Together, these results strongly suggest ΔNp63 as a bona fide downstream target negatively regulated by TAZ in several breast and lung cell lines.
FIGURE 2.
Validation of ΔNp63 as a downstream transcriptional target of TAZ. A, p63 protein expression levels were assessed in MCF10A cells overexpressing WPI control or TAZ using anti-p63 antibody. MCF-7 protein cell lysate was used as a negative control for p63 staining. COS7 cells transfected with TAp63-FLAG or ΔNp63-FLAG were used as positive controls. β-Actin was used as an internal loading control. B, ΔNp63 and TAZ protein levels were assessed in MCF10A cells in the presence (+) or absence (−) of Dox-mediated inducible expression of wild-type (TAZ-WT) or constitutively active TAZ (TAZ-S89A). C, expression of ΔNp63 in MCF10A mammary and HBE135 lung epithelial cells. TAZ expression was induced with Dox (+) in MCF10A-TAZ or HBE135-TAZ-S89A cells. D, knockdown of TAZ in breast and lung cancer cells caused enhanced protein expression of ΔNp63. HCC38 breast and A549 lung cancer cells were transiently transfected with control siRNA (siCtrl) or siRNA against TAZ (siTAZ). Three days after transfection, cells were subjected to protein extraction and Western blot analysis using anti-TAZ or anti-p63 antibody. E, TAZ suppresses ΔNp63 promoter activity. SK-BR3 cells grown in a 12-well plate were transfected with ΔNp63-luc alone (0.1 μg) or in combination with increasing amounts (0, 0.1, and 0.4 μg) of TAZ followed by the Dual-Luciferase assay. -Fold changes were calculated by normalizing SK-BR3 cells transfected with ΔNp63-luc alone to those transfected with TAZ. The experiment was performed in triplicate. The experiment was performed in triplicate, and error bars represent S.D. from each set of triplicates. *, statistically significant difference (p < 0.05).
TBD Is Necessary for TAZ-induced Negative Regulation of ΔNp63
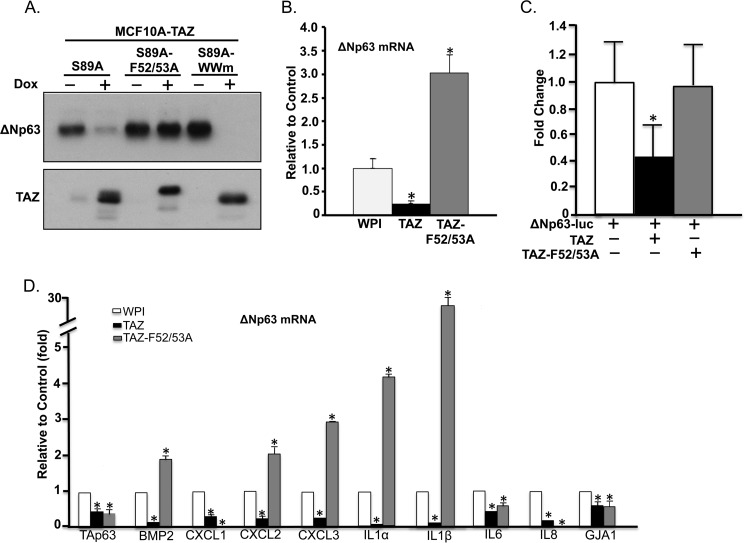
TAZ was originally identified as a transcriptional co-activator that lacks a DNA binding domain. Thus, to modulate transcription of downstream cellular genes, TAZ requires interactions with transcription factors through its TBD or WW (W, tryptophan) domain. Previous studies have shown that TAZ interacts with members of the TEAD family of transcription factors (TEAD1–4) through seven conserved residues in the TBD of TAZ (23, 28). Among these, two phenylalanine residues located at positions 52 and 53 (Phe-52/53) were shown to be critical for TAZ-TEAD binding. Therefore, we examined whether the effect of TAZ on ΔNp63 repression could be abolished in TBD mutants of TAZ. We generated a missense mutation in TAZ Phe-52 and Phe-53 residues (TAZ-F52A/F53A) to abolish TEAD interaction with TAZ. We then established MCF10A cells stably expressing TAZ-F52A/F53A and examined the effect of this TAZ mutant on mRNA and protein expression of ΔNp63. qRT-PCR and Western blot analysis showed that loss of interaction with TEAD in TAZ-F52A/F53A mutant completely abolished TAZ-induced suppression of both protein and mRNA of ΔNp63 (Fig. 3, A and B). The TAZ-F52A/F53A mutant also showed inability to suppress ΔNp63 promoter activity (Fig. 3C). Interestingly, loss of interaction with TEAD in TAZ-F52A/F53A had a dominant-negative effect and activated transcription (1–28 ± 0–1-fold) of many down-regulated genes including ΔNp63, BMP2, CXCL2, CXCL3, IL1α, and IL1β (Fig. 3, B and D). Conversely, overexpression of TAZ-F52A/F53A can still suppress TAp63 mRNA, suggesting that TAZ regulates TAp63 and ΔNp63 differently and that TAZ may down-regulate TAp63 independently of TEAD. Next, we sought to explore whether the TBD of TAZ was the only domain responsible for p63 suppression. Because the TAZ WW domain has been shown to be critical for gene transcription regulation through interaction with (L/P)PXY (L, lysine; P, proline; X, any amino acid; Y, tyrosine) motif-containing transcription factors such as TTF1 and Pax8 (36–38), we tested whether a WW domain TAZ mutant (TAZ-WWm) containing two residue mutations, W152A and P155A, had any effect on TAZ-induced suppression of ΔNp63. MCF10A cells stably expressing TAZ-S89A-WWm were established by infecting MCF10A cells with lentivirus expressing inducible TAZ-S89A-WWm (MCF10A-TAZ-S89A-WWm). Similarly to the effect observed in MCF10A-TAZ-S89A, in the presence of Dox, the WW domain mutant TAZ-WWm effectively suppressed ΔNp63 expression (Fig. 3A). Together, these findings suggest that the TBD, but not the WW domain, of TAZ is essential for TAZ-induced repression of ΔNp63.
FIGURE 3.
TEAD binding domain is essential for TAZ-induced transcriptional repression of ΔNp63. A, TEAD binding domain is critical for ΔNp63 repression. Constitutively active TAZ-S89A with mutations in TEAD binding (S89A-F52A/F53A) or WW (S89A-WWm) domains were induced with Dox in MCF10A cells. ΔNp63 and TAZ protein expression was assessed and compared with MCF10A cells with inducible expression of TAZ-S89A (S89A). β-Actin was used as an internal loading control. B, qRT-PCR analysis of ΔNp63 mRNA in MCF10A cells expressing WPI, TAZ, or TAZ-F52A/F53A. C, TEAD binding mutant of TAZ abolishes TAZ-mediated suppression of ΔNp63 promoter. SK-BR3 cells were transfected with ΔNp63-luc alone or in combination with wild-type TAZ (TAZ) or its TEAD binding mutant (TAZ-F52A/F53A). Promoter activity was measured as described in Fig. 2E. The experiment was performed in triplicate. D, qRT-PCR analysis of TAZ-down-regulated genes in MCF10A-WPI, TAZ, or TAZ-F52A/F53A cells. Procedures and data analyses were performed as described in Fig. 1B. The experiment was performed in triplicate, and error bars represent S.D. from each set of triplicates. *, statistically significant difference (p < 0.05).
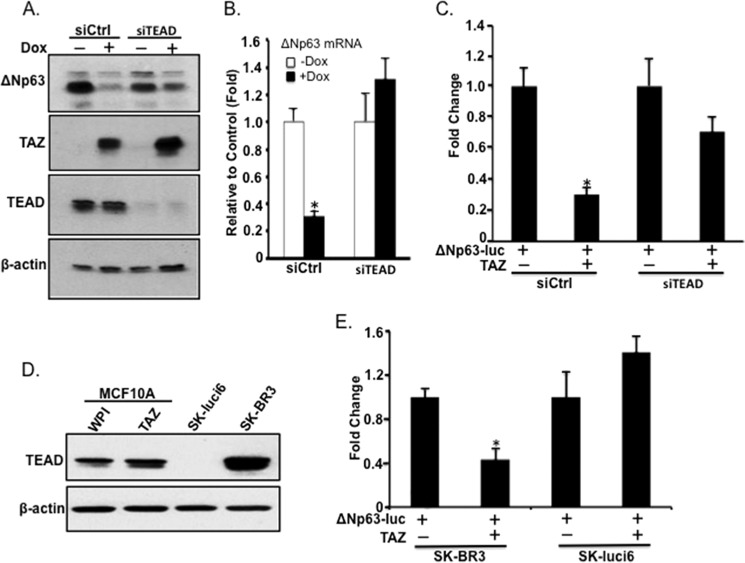
TEAD Is Essential for TAZ-induced Suppression of ΔNp63
To directly confirm whether TAZ suppresses ΔNp63 through TEAD, we performed transient knockdown of TEAD using a previously used siRNA simultaneously targeting TEAD1, TEAD3, and TEAD4 (siTEAD) in MCF10A cells with inducible expression of constitutively active TAZ-S89A. Although ΔNp63 protein and mRNA were significantly repressed by TAZ-S89A in the presence of Dox in cells expressing a siRNA negative control (siCtrl), TAZ-S89A-mediated repression of ΔNp63 seemed to be abolished in MCF10A cells with TEAD knockdown (Fig. 4, A and B). In addition, TEAD knockdown also diminished TAZ-induced suppression of ΔNp63 promoter (Fig. 4C). Moreover, although TAZ can suppress ΔNp63-luc activity in TEAD-positive SK-BR3 cells, its suppression on ΔNp63 promoter was abolished in TEAD-negative SK-Luci6 lung cancer cells (Fig. 4, D and E). Together, these studies strongly suggest that TEAD is essential for TAZ-induced suppression of ΔNp63.
FIGURE 4.
TEAD-dependent suppression of ΔNp63 by TAZ. A, TEAD knockdown diminishes TAZ-induced repression of ΔNp63 protein. Transient siRNA knockdown of TEAD1/3/4 (siTEAD) was performed in MCF10A cells with inducible expression of TAZ-S89A. An siRNA targeting a nonspecific sequence was used as a negative control (siCtrl). Twenty-four hours post-transfection, cells were induced (+) or not (−) with Dox. Protein was extracted 48 h postinduction, and ΔNp63 expression was assessed in cells with and without TAZ-S89A expression. β-Actin was used as an internal loading control. B, knockdown of TEAD abolishes TAZ-induced suppression of ΔNp63 mRNA. qRT-PCR analysis of ΔNp63 mRNA was performed. Cell lines and treatment conditions were as described in A. C, knockdown of TEAD by siRNA partially blocks TAZ-induced suppression of ΔNp63 promoter activity. D, expression of TEAD in MCF10A-WPI, MCF10A-TAZ, SK-Luci6, and SK-BR3 cells. E, TAZ fails to inhibit TAZ promoter in TEAD-negative SK-Luci6 cells. Luciferase analysis was performed as described in Fig. 2E. The experiment was performed in triplicate, and error bars represent S.D. from each set of triplicates. *, statistically significant difference (p < 0.05).
TAZ Suppresses ΔNp63 by Directly Binding to the ΔNp63 Promoter through TEAD
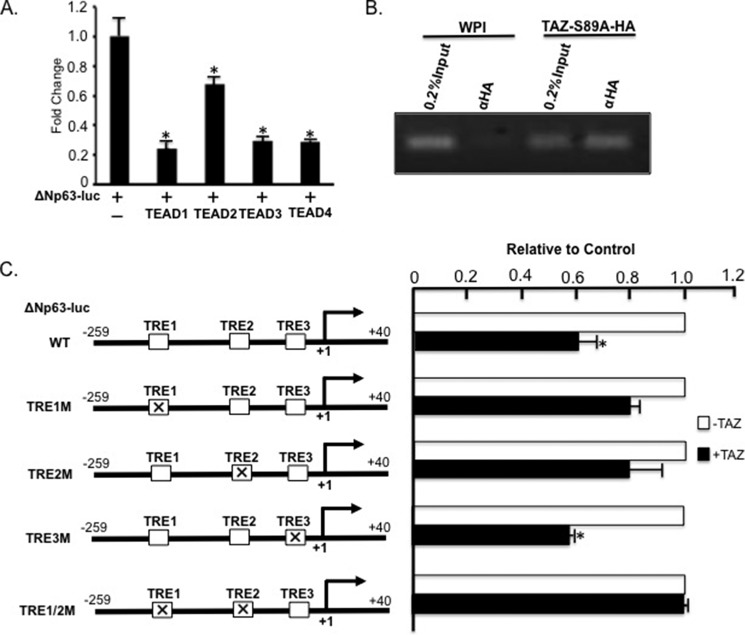
Next, we tested whether TAZ suppresses ΔNp63 transcription by directly interacting with ΔNp63 promoter through TEAD. Because TEAD is required for TAZ-induced suppression of ΔNp63 transcription, we first tested whether TEAD can directly suppress ΔNp63 promoter activity by transfecting ΔNp63-luc reporter alone or together with TEAD1, TEAD2, TEAD3, or TEAD4 into SK-BR3 cells. Significantly, all TEADs showed ΔNp63 promoter repression (Fig. 5A), although TEAD1/3/4 exerted a more dramatic effect than TEAD2 in ΔNp63 repression with over 3.5 ± 1.2-fold decrease in the promoter activity, suggesting that TEADs are involved in ΔNp63 repression. To confirm that TAZ-TEAD indeed directly binds to ΔNp63 promoter, we performed a ChIP assay in MCF10A cells expressing WPI vector or TAZ-S89A-HA using anti-HA antibody and primers flanking a TEAD response element (TRE) (Fig. 5C, TRE1). Interestingly, our ChIP assay showed that TAZ-S89A could indeed be co-immunoprecipitated with the ΔNp63 promoter DNA in vivo (Fig. 5B). After further examination of ΔNp63 promoter sequences, we identified three potential TREs (TRE1, TRE2, and TRE3) in the ΔNp63 promoter and mutated them individually (TRE1M, TRE2M, or TRE3M) or in combination (TRE1/2M; Fig. 5C). Although mutation of TRE1 or TRE2 rather than TRE3 partially blocked TAZ-induced suppression of ΔNp63 promoter, combined mutations of both TRE1 and TRE2 (TRE1/2M) completely abolished TAZ-induced suppression of ΔNp63 promoter (Fig. 5C), thus suggesting that TAZ-TEAD complex binds to TRE1 and TRE2 to suppress ΔNp63 transcription.
FIGURE 5.
TAZ and TEAD are recruited on ΔNp63 promoter through TREs to directly suppress ΔNp63 transcription. A, TEADs repress ΔNp63 promoter activity. SK-BR3 cells were transfected with ΔNp63-luc alone or in combination with TEAD1, TEAD2, TEAD3, or TEAD4, and luciferase assay was performed as described in Fig. 2E. B, ChIP analysis of TAZ interaction with the ΔNp63 promoter. DNA and protein were cross-linked after treating MCF10A-WPI and MCF10A-TAZ-S89A-HA cells with 1% formaldehyde. Chromatin and DNA-binding protein were subjected to immunoprecipitation using mouse monoclonal α-HA (F7) antibody followed by PCR and electrophoresis on a 3% agarose gel. 0.2% input chromatin extracted from MCF10A-WPI or MCF10A-TAZ-S89A-HA cells was used as a positive PCR control. C, mapping the TRE in the ΔNp63 promoter. Three potential TREs (TRE1 (GGAAT), TRE2 (CATGCC), and TRE3 (GGTAT)) in the ΔNp63 promoter were mutated (TRE1M (AAAAA), TRE2M (AAAAAA), and TRE3M (AAAAA)) alone or in combination (TRE1/2M). ΔNp63-luc containing WT, TRE1M, TRE2M, TRE3M, or TRE1/2M was transfected alone (−TAZ; control) or together with TAZ (+TAZ) into SK-BR3 cells followed by the Dual-Luciferase assay. Promoter activity is shown relative to control and was calculated as the ratio of relative luciferase units of +TAZ to −TAZ. The mean and S.D. of three experiments are shown. The mean and S.D. (error bars) of three experiments are shown. *, statistically significant difference (p < 0.05) between relative luciferase units of +TAZ and −TAZ.
Modulation of Deacetylation Is Critical for TAZ-TEAD-induced Suppression of ΔNp63
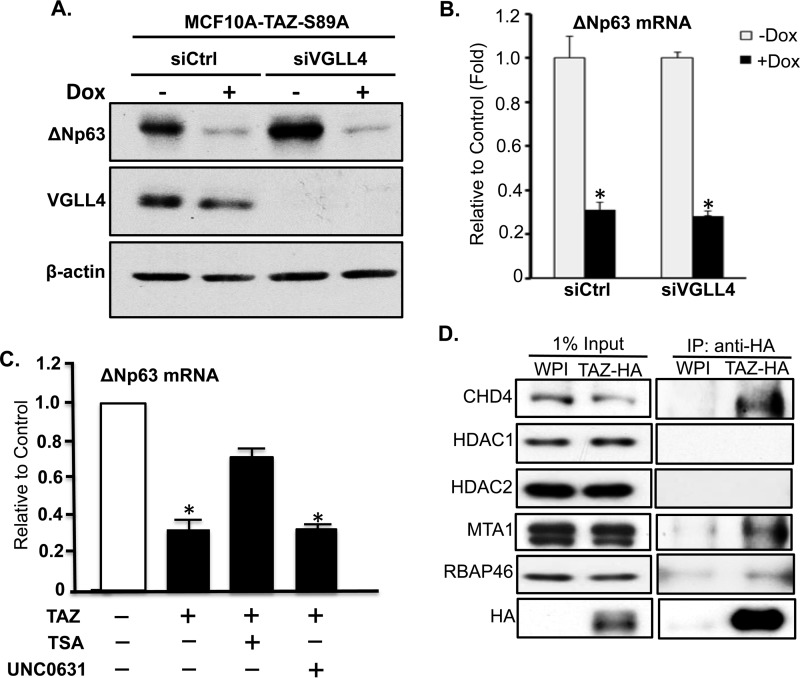
Two recent studies suggest that TEAD or its Drosophila homolog Scallop can suppress cellular gene transcription through interaction with transcription cofactor VGLL4 or Tgi, respectively (39, 40). However, VGLL4 knockdown in MCF10A cells could not block TAZ-induced suppression of ΔNp63 (Fig. 6, A and B), suggesting that VGLL4 is not involved in TAZ-TEAD-induced transcriptional repression of ΔNp63.
FIGURE 6.
Suppression of ΔNp63 transcription by TAZ through modulation of chromatin acetylation rather than VGLL4. A, knockdown of VGLL4 by siRNA. MCF10A-TAZ-S89A cells were transfected with control siRNA (siCtrl) or siRNA against VGLL4 (siVGLL4) followed by incubation in the absence (−) or presence (+) of Dox for 1 day. Cells were subjected to protein extraction and Western blot analysis using anti-p63 and anti-VGLL4 antibodies. B, qRT-PCR analysis of ΔNp63 mRNA. Experimental procedures were as described in A. C, levels of ΔNp63 mRNA after treatment of cells with histone deacetylase (HDAC) and histone methyltransferase inhibitors. MCF10A-TAZ-S89A cells were untreated or treated with trichostatin A (TSA) (300 nm) or UNC0631 (20 μm) in the absence (−) or presence (+) of Dox for 1 day followed by qRT-PCR analysis. Data analysis was described in Fig. 1B. D, interaction of TAZ with histone deacetylase complex. Co-immunoprecipitation analysis was performed by immunoprecipitation (IP) of TAZ-HA in 250 μg of protein lysates extracted from MCF10A-WPI and MCF10A-TAZ-S89A-HA cells using anti-HA antibody followed by Western blotting using each specific antibody against each protein of the histone deacetylase complex. The membrane was stripped and reprobed with anti-HA antibody to see whether TAZ-S89A-HA was pulled down from MCF10A-TAZ-S89A-HA rather than WPI cells. About of input protein lysate (2.5 μg) was also subjected to Western blotting. The experiment was performed in triplicate, and error bars represent S.D. from each set of triplicates. *, statistically significant difference (p < 0.05).
Previous studies also suggest that transcriptional suppression of some genes may depend on DNA methylation or histone deacetylation of chromatin of their promoter regions (41–43). To explore whether TAZ-TEAD-induced ΔNp63 transcriptional repression is due to chromosome methylation/acetylation, we treated breast cancer cells with inhibitors of histone modification. Significantly, treatment of cells with histone deacetylase inhibitor trichostatin A rather than histone methyltransferase inhibitor UNC0631 partially rescued TAZ-induced suppression of ΔNp63 transcription (Fig. 6C). Moreover, we have further shown that TAZ directly interacts in vivo with some components (CHD4, MTA1, and RBP46) of the histone deacetylase complex (Fig. 6D).
Functional Implications of TAZ-TEAD Interactions and ΔNp63 Suppression
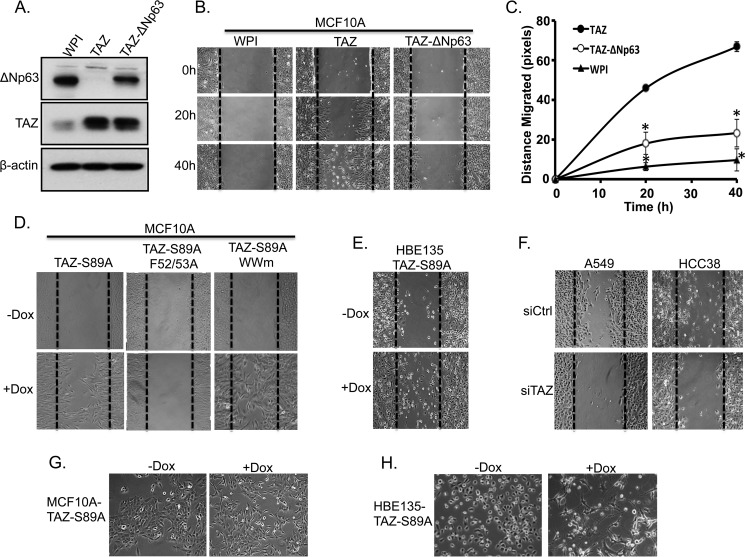
Our results have strongly elucidated the role of TEAD and its co-activator TAZ in p63 transcriptional repression, particularly ΔNp63, in breast and lung epithelial cells. Because TAZ overexpression has been previously correlated with enhanced cell migration and invasion (14, 15, 44) and ΔNp63 knockdown causes EMT and increased cell migration and metastasis in MCF10A cells or breast cancer (45–47), we sought to elucidate the functional consequences of TAZ-TEAD-mediated repression of ΔNp63 in breast cell migration. First, we reintroduced ΔNp63 expression in MCF10A-TAZ cells by using lentiviral infection. Assessment of similar TAZ and ΔNp63 protein expression levels was performed by Western blotting (Fig. 7A). Second, we sought to examine the functional implications of ΔNp63 suppression on cell migration by performing a wound healing assay in MCF10A cells expressing WPI vector control, TAZ, or TAZ plus ΔNp63. Cell migration was compared at different time points after wound induction. Compared with the WPI control (MCF10A-WPI), TAZ-overexpressing cells (MCF10A-TAZ) increased cell migration 9.0 ± 1.5- and 8.8 ± 3.5-fold at 20 and 40 h, respectively. However, this effect was partially abolished in TAZ-ΔNp63-expressing cells (MCF10A-TAZ-ΔNp63) (Fig. 7, B and C).
FIGURE 7.
Reintroduction of ΔNp63 partially recues TAZ-mediated cell migration. A, Western blot analysis of ΔNp63 expression. MCF10A-TAZ cells were infected with lentivirus expressing ΔNp63 (MCF10A-TAZ-ΔNp63). Protein was extracted from these cells, and ΔNp63 expression was compared with MCF10A-WPI and MCF10A-TAZ cells. β-Actin was used as an internal loading control. B, ΔNp63 reintroduction in TAZ-overexpressing cells partially rescues TAZ-induced increased cell migration. MCF10A-WPI, MCF10A-TAZ, and MCF10A-TAZ-ΔNp63 cells were plated to confluence and starved in 2% horse serum overnight. A wound healing assay was performed, and cell migration was analyzed between cells at different time points (0, 20, and 40 h). C, quantification of cell migration. Cell migration distance (pixels) was quantified in all cells as described in B. The experiment was performed in triplicate, and error bars represent S.D. from each set of triplicates. *, statistically significant difference (p < 0.05) between MCF10A-TAZ and MCF10A-WPI or MCF10A-TAZ-ΔNp63. D, TEAD-dependent increased cell migration by TAZ. Cell migration analyses were performed using the established cell lines and conditions described in Fig. 3A. E, overexpression of TAZ-S89A causes increased cell migration in HBE135 cells. Wound healing analyses were performed in cell lines described in Fig. 2C. F, knockdown of TAZ in HCC38 and A549 cells decreases cell migration. G and H, overexpression of TAZ-S89A induces EMT in both MCF10A (G) and HBE135 (H) cells.
Interestingly, further examination of TAZ and its two domain mutants showed that although Dox induction of TAZ-S89A (+Dox) caused increased cell migration TBD mutant TAZ-F52A/F53A completely abolished TAZ-induced increased cell migration in MCF10A cells (Figs. 3A and 7D). Conversely, WW domain mutant TAZ-WWm can still cause increased cell migration (Fig. 7D), suggesting that TEAD binding domain, rather than WW domain, is essential for TAZ-induced increased cell migration. Furthermore, enhanced cell migration is also observed when TAZ-S89A is overexpressed in the presence of Dox (+Dox) in HBE135 cells (Figs. 2C and 7E). Moreover, down-regulation of TAZ in A549 and HCC38 cells inhibits cell migration (Fig. 7F). Finally, we have further shown that overexpression of TAZ-S89A in both breast MCF10A and lung HBE135 cells causes EMT, thus suggesting that this TAZ-induced increased cell migration is due to loss of cell-cell adhesion (Fig. 7, G and H). In summary, our results suggest that TAZ interacts with TEAD to promote ΔNp63 suppression and reduced cell-cell adhesion, thus increasing the migratory capacity of cells, which can further lead to metastatic progression in breast and lung cancer cells.
Discussion
TAZ Is a Dual Regulator of Gene Transcription
Studies have widely characterized TAZ as a transcriptional co-activator of gene expression. Its ability to interact with a wide range of transcription factors accounts for its multifunctional effects in tumor development and progression. Moreover, TAZ-induced activation of protumorigenic genes such as Cyr61, CTGF, BMP4 (32, 44) and many others has been often reported in the literature. However, our DNA microarray data have uncovered a whole new perspective on TAZ transcriptional regulation. Besides its well studied role as a transcriptional co-activator, our results have suggested a novel function of TAZ in transcriptional repression. This transcriptional duality of TAZ has been previously questioned after observing that TAZ-induced activation of RUNX2 and repression of the transcriptional activity of peroxisome proliferator-activated receptor-γ were critical for mesenchymal stem cell differentiation (48). Recently, phosphorylation of Tyr-316 of TAZ has been shown to promote TAZ interaction and repression of the transcriptional activity of NFAT5 in response to hyperosmotic stress (49). Although these studies have shed light on the transcriptional repressing potential of TAZ, they have failed to elucidate the molecular mechanisms and oncogenic functions underlying TAZ-induced suppression. The fact that our microarray results have shown that transcription of over 320 cellular genes can be suppressed by TAZ has uncovered a new layer of the signaling complexity of TAZ and the Hippo pathway. Furthermore, validation analysis of several target genes including p63 and proinflammatory cytokines and chemokines has indeed confirmed that TAZ expression is also critical for transcriptional inhibition. Moreover, the strong repression of IL1α/β exerted by TAZ has suggested a novel role in modulating the inflammatory response and tumor microenvironment. In fact, TAZ was found to indeed repress IL1β promoter in the same manner as shown for ΔNp63 (data not shown). Collectively, our results have elucidated an underrated role of TAZ as a negative regulator of transcription in breast and lung epithelial cells. Moreover, we have uncovered the duality of TAZ in gene transcription regulation, a trait that has been reported previously for other transcriptional cofactors such as CCAAT-enhancer-binding protein or cAMP-response element-binding protein (CREB)-binding protein and its paralog p300 (50, 51).
ΔNp63 Is a Novel Downstream Target Negatively Regulated by TAZ
Despite the progress made toward elucidating the molecular mechanisms involved in breast cancer development and progression, metastatic cancer cells remain a major obstacle for successful breast cancer treatments. In this context, TAZ has been highly associated with cell acquisition of EMT phenotypes and subsequent metastatic dissemination of breast cells (14, 15, 32, 52). By regulating gene expression, TAZ has been shown to modulate oncogenic traits in cells. However, the transcriptional downstream targets mediating these TAZ-induced phenotypes remain mostly unexplored. By using a DNA microarray and real time qRT-PCR, we identified p63 isoforms TAp63 and ΔNp63 as transcriptional targets negatively regulated by TAZ in mammary tumorigenesis. Of these, ΔNp63 showed the most significant repression and was shown to be the predominant isoform expressed in MCF10A cells. Therefore, we characterized ΔNp63 as a bona fide negative transcriptional target of TAZ involved in cell migration. First, we have shown that TAZ overexpression in MCF10A non-tumorigenic breast cells causes a significant decrease in ΔNp63 mRNA expression levels (Fig. 1D). Second, we have shown that overexpression of both wild-type TAZ and its constitutively active mutant TAZ-S89A suppresses ΔNp63 protein expression in MCF10A cells, whereas TAZ knockdown caused increased ΔNp63 in breast and lung cancer cells (Fig. 2, A, B, and D). Finally, we have confirmed that TAZ physically interacts with ΔNp63 promoter and causes ΔNp63 transcriptional repression through interaction with TEAD and TRE (Fig. 2E).
After confirming that ΔNp63 is indeed a real downstream target negatively regulated by TAZ, we examined the functional implications of ΔNp63 down-regulation in mammary epithelial cells. After reintroducing ΔNp63 protein expression into TAZ-overexpressing MCF10A cells, we found that ΔNp63 could partially reverse TAZ-mediated cell migration (Fig. 7, A–C). Specifically, assessment of the migration distance of MCF10A cells expressing TAZ plus ΔNp63 showed a significantly slower wound closure rate that was more similar to that displayed by MCF10A-WPI control than by MCF10A-TAZ cells. Because multiple genes may regulate breast cancer cell migration, ΔNp63 re-expression could not completely reverse this TAZ-mediated phenotype. Nonetheless, our studies strongly suggest that ΔNp63 is one of the genes involved in TAZ-induced cell migration. These results are consistent with studies showing ΔNp63 as an inhibitor of cell migration, invasion, and metastatic progression in breast cells (45, 46). Moreover, p63 and particularly ΔNp63 expression has been specifically observed in normal myoepithelial breast cells, has been proposed as a marker for cell differentiation, and is shown to be down-regulated in non-metaplastic invasive breast carcinomas (33, 53–55). Importantly, TAZ overexpression is highly correlated with breast cancer invasiveness and dissemination (32). Thus, it is likely that TAZ-induced suppression of ΔNp63 enhances its metastatic potential by promoting EMT and breast cell migration. It will be interesting to further investigate the correlation between the levels of TAZ and ΔNp63 and whether these could be used as prognostic biomarkers for clinical metastatic breast cancer. Until then, our results have provided a better understanding of the molecular mechanisms of TAZ-induced metastatic dissemination that might be critical for future development of effective targeted therapies for breast and lung cancer patients.
TEADs Are Critical Binding Partners of TAZ, Which Suppresses ΔNp63 Transcription
Our results have identified the members of the TEAD/transcriptional enhancer factor family as major transcription factors mediating TAZ-induced transcriptional repression of downstream genes, particularly ΔNp63. We showed that TAZ could no longer suppress ΔNp63 promoter activity in TEAD-null SK-luci6 cells or MCF10A cells with TEAD knockdown (Fig. 4, D and E). This TAZ-TEAD-mediated suppression seems to be direct because our results have shown that TEAD itself can suppress ΔNp63 promoter activity and that TAZ can suppress ΔNp63 promoter activity by directly interacting with two TREs on the ΔNp63 promoter (Fig. 5, B and C). Consistent with our findings, through ChIP sequencing, a recent study also suggests that TEAD2 can regulate EMT-relevant genes by acting as a transcriptional activator or repressor mainly by directly binding to the promoters containing TREs (56). It is still unclear why the same TAZ-TEAD complex activates transcription of some cellular genes such as CTGF and Cyr61 but suppresses transcription of other genes such as ΔNp63. Most interestingly, several recent studies have identified components of the chromatin/chromatin-remodeling complexes (BRM, MED mediator complexes, and SWI/SNF complexes) and histone methyltransferase (Ncoa6) complexes as binding partners of TAZ or its Drosophila homolog Yki (41, 57–59). Because these complexes control transcriptional status (activation or inactivation) of a specific gene, the methylation or acetylation status of chromatin on the promoter regions of a specific gene may determine the transcriptional activation or suppression functions of the TAZ-TEAD complex. Indeed, our data further showed that TAZ can directly interact with histone deacetylation complex (Fig. 6D) and that inhibition of histone deacetylase partially releases TAZ-induced suppression of ΔNp63 transcription by directly interacting with histone deacetylation complex (Fig. 6C), suggesting that TAZ may suppress gene transcription by activating histone deacetylation and histone deacetylase-mediated chromatin tightening.
Besides TEAD-dependent transcriptional suppression by TAZ, we also observed TEAD-independent suppression of some downstream genes such as TAp63 and GJA1 (Fig. 3D). In addition, a recent study reported a ZEB2-dependent suppression of ΔNp63 promoter by YAP (TAZ paralog) during squamous transdifferentiation of lung epithelial cells (60). These studies suggest that TAZ, like YAP, may also suppress cellular gene transcription through interacting with other transcription factors. Nevertheless, our findings have shed light on the unanticipated complexity of the mechanisms underlying TAZ and TEAD interaction for gene transcriptional regulation and provide convincing evidence that TAZ can exert its function by negatively regulating transcription of downstream cellular genes such as ΔNp63 through interaction with TEAD transcription factor.
Supplementary Material
Acknowledgments
We thank Dr. Wanji Hong for providing the TAZ-WWm plasmid, Dr. David Berman for using the Applied Biosystems ViiA 7 Real-Time PCR System, and Dr. Robin Hallett for assistance in heat map design.
This work was supported in part by Canadian Institute of Health Research (CIHR) Grant 119325 and Canadian Breast Cancer Foundation, Canada (to X. Y.).

This article contains supplemental Table S1.
X. Yang, unpublished data.
- TAZ
- transcriptional co-activator with a PDZ binding domain
- YAP
- yes-associated protein
- EMT
- epithelial-mesenchymal transition
- TBD
- TEAD binding domain
- Dox
- doxycycline
- VGLL4
- vestigial-like protein 4
- qRT-PCR
- quantitative reverse transcription-PCR
- TRE
- TEAD response element.
References
- 1. Yang X., Xu T. (2011) Molecular mechanism of size control in development and human diseases. Cell Res. 21, 715–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pan D. (2010) The hippo signaling pathway in development and cancer. Dev. Cell 19, 491–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Badouel C., McNeill H. (2011) SnapShot: the hippo signaling pathway. Cell 145, 484–484.e1 [DOI] [PubMed] [Google Scholar]
- 4. Barron D. A., Kagey J. D. (2014) The role of the Hippo pathway in human disease and tumorigenesis. Clin. Transl. Med. 3, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gomez M., Gomez V., Hergovich A. (2014) The Hippo pathway in disease and therapy: cancer and beyond. Clin. Transl. Med. 3, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Halder G., Johnson R. L. (2011) Hippo signaling: growth control and beyond. Development 138, 9–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johnson R., Halder G. (2014) The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat. Rev. Drug Discov. 13, 63–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mo J. S., Park H. W., Guan K. L. (2014) The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep. 15, 642–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao B., Tumaneng K., Guan K. L. (2011) The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat. Cell Biol. 13, 877–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu H., Jiang D., Chi F., Zhao B. (2012) The Hippo pathway regulates stem cell proliferation, self-renewal, and differentiation. Protein Cell 3, 291–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hao Y., Chun A., Cheung K., Rashidi B., Yang X. (2008) Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J. Biol. Chem. 283, 5496–5509 [DOI] [PubMed] [Google Scholar]
- 12. Zhao B., Wei X., Li W., Udan R. S., Yang Q., Kim J., Xie J., Ikenoue T., Yu J., Li L., Zheng P., Ye K., Chinnaiyan A., Halder G., Lai Z. C., Guan K. L. (2007) Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 21, 2747–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chan E. H., Nousiainen M., Chalamalasetty R. B., Schäfer A., Nigg E. A., Silljé H. H. (2005) The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene 24, 2076–2086 [DOI] [PubMed] [Google Scholar]
- 14. Lei Q. Y., Zhang H., Zhao B., Zha Z. Y., Bai F., Pei X. H., Zhao S., Xiong Y., Guan K. L. (2008) TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol. Cell. Biol. 28, 2426–2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chan S. W., Lim C. J., Guo K., Ng C. P., Lee I., Hunziker W., Zeng Q., Hong W. (2008) A role for TAZ in migration, invasion, and tumorigenesis of breast cancer cells. Cancer Res. 68, 2592–2598 [DOI] [PubMed] [Google Scholar]
- 16. Cordenonsi M., Zanconato F., Azzolin L., Forcato M., Rosato A., Frasson C., Inui M., Montagner M., Parenti A. R., Poletti A., Daidone M. G., Dupont S., Basso G., Bicciato S., Piccolo S. (2011) The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 147, 759–772 [DOI] [PubMed] [Google Scholar]
- 17. Zhou Z., Hao Y., Liu N., Raptis L., Tsao M. S., Yang X. (2011) TAZ is a novel oncogene in non-small cell lung cancer. Oncogene 30, 2181–2186 [DOI] [PubMed] [Google Scholar]
- 18. Xie M., Zhang L., He C. S., Hou J. H., Lin S. X., Hu Z. H., Xu F., Zhao H. Y. (2012) Prognostic significance of TAZ expression in resected non-small cell lung cancer. J. Thorac. Oncol. 7, 799–807 [DOI] [PubMed] [Google Scholar]
- 19. Yuen H. F., McCrudden C. M., Huang Y. H., Tham J. M., Zhang X., Zeng Q., Zhang S. D., Hong W. (2013) TAZ expression as a prognostic indicator in colorectal cancer. PLoS One 8, e54211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Cristofaro T., Di Palma T., Ferraro A., Corrado A., Lucci V., Franco R., Fusco A., Zannini M. (2011) TAZ/WWTR1 is overexpressed in papillary thyroid carcinoma. Eur. J. Cancer 47, 926–933 [DOI] [PubMed] [Google Scholar]
- 21. Avruch J., Zhou D., Fitamant J., Bardeesy N., Mou F., Barrufet L. R. (2012) Protein kinases of the Hippo pathway: regulation and substrates. Semin. Cell Dev. Biol. 23, 770–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dupont S., Morsut L., Aragona M., Enzo E., Giulitti S., Cordenonsi M., Zanconato F., Le Digabel J., Forcato M., Bicciato S., Elvassore N., Piccolo S. (2011) Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 [DOI] [PubMed] [Google Scholar]
- 23. Hong W., Guan K. L. (2012) The YAP and TAZ transcription co-activators: key downstream effectors of the mammalian Hippo pathway. Semin. Cell Dev. Biol. 23, 785–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Varelas X. (2014) The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development 141, 1614–1626 [DOI] [PubMed] [Google Scholar]
- 25. Liu C., Huang W., Lei Q. (2011) Regulation and function of the TAZ transcription co-activator. Int. J. Biochem. Mol. Biol. 2, 247–256 [PMC free article] [PubMed] [Google Scholar]
- 26. Lai D., Visser-Grieve S., Yang X. (2012) Tumour suppressor genes in chemotherapeutic drug response. Biosci. Rep. 32, 361–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhao Y., Yang X. (2014) The Hippo pathway in chemotherapeutic drug resistance. Int. J. Cancer 10.1002/ijc.29293 [DOI] [PubMed] [Google Scholar]
- 28. Chan S. W., Lim C. J., Loo L. S., Chong Y. F., Huang C., Hong W. (2009) TEADs mediate nuclear retention of TAZ to promote oncogenic transformation. J. Biol. Chem. 284, 14347–14358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang H., Liu C. Y., Zha Z. Y., Zhao B., Yao J., Zhao S., Xiong Y., Lei Q. Y., Guan K. L. (2009) TEAD transcription factors mediate the function of TAZ in cell growth and epithelial-mesenchymal transition. J. Biol. Chem. 284, 13355–13362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhao B., Kim J., Ye X., Lai Z. C., Guan K. L. (2009) Both TEAD-binding and WW domains are required for the growth stimulation and oncogenic transformation activity of yes-associated protein. Cancer Res. 69, 1089–1098 [DOI] [PubMed] [Google Scholar]
- 31. Zhao B., Ye X., Yu J., Li L., Li W., Li S., Yu J., Lin J. D., Wang C. Y., Chinnaiyan A. M., Lai Z. C., Guan K. L. (2008) TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 22, 1962–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lai D., Ho K. C., Hao Y., Yang X. (2011) Taxol resistance in breast cancer cells is mediated by the hippo pathway component TAZ and its downstream transcriptional targets Cyr61 and CTGF. Cancer Res. 71, 2728–2738 [DOI] [PubMed] [Google Scholar]
- 33. Koker M. M., Kleer C. G. (2004) p63 expression in breast cancer: a highly sensitive and specific marker of metaplastic carcinoma. Am. J. Surg. Pathol. 28, 1506–1512 [DOI] [PubMed] [Google Scholar]
- 34. Deyoung M. P., Ellisen L. W. (2007) p63 and p73 in human cancer: defining the network. Oncogene 26, 5169–5183 [DOI] [PubMed] [Google Scholar]
- 35. Zhou Z., Zhu J. S., Xu Z. P., Zhang Q. (2011) Lentiviral vector-mediated siRNA knockdown of the YAP gene inhibits growth and induces apoptosis in the SGC7901 gastric cancer cell line. Mol. Med. Rep. 4, 1075–1082 [DOI] [PubMed] [Google Scholar]
- 36. Murakami M., Nakagawa M., Olson E. N., Nakagawa O. (2005) A WW domain protein TAZ is a critical coactivator for TBX5, a transcription factor implicated in Holt-Oram syndrome. Proc. Natl. Acad. Sci. U.S.A. 102, 18034–18039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Di Palma T., D'Andrea B., Liguori G. L., Liguoro A., de Cristofaro T., Del Prete D., Pappalardo A., Mascia A., Zannini M. (2009) TAZ is a coactivator for Pax8 and TTF-1, two transcription factors involved in thyroid differentiation. Exp. Cell Res. 315, 162–175 [DOI] [PubMed] [Google Scholar]
- 38. Murakami M., Tominaga J., Makita R., Uchijima Y., Kurihara Y., Nakagawa O., Asano T., Kurihara H. (2006) Transcriptional activity of Pax3 is co-activated by TAZ. Biochem. Biophys. Res. Commun. 339, 533–539 [DOI] [PubMed] [Google Scholar]
- 39. Koontz L. M., Liu-Chittenden Y., Yin F., Zheng Y., Yu J., Huang B., Chen Q., Wu S., Pan D. (2013) The Hippo effector Yorkie controls normal tissue growth by antagonizing scalloped-mediated default repression. Dev. Cell 25, 388–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang W., Gao Y., Li P., Shi Z., Guo T., Li F., Han X., Feng Y., Zheng C., Wang Z., Li F., Chen H., Zhou Z., Zhang L., Ji H. (2014) VGLL4 functions as a new tumor suppressor in lung cancer by negatively regulating the YAP-TEAD transcriptional complex. Cell Res. 24, 331–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Basu D., Reyes-Múgica M., Rebbaa A. (2013) Histone acetylation-mediated regulation of the Hippo pathway. PLoS One 8, e62478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hildmann C., Riester D., Schwienhorst A. (2007) Histone deacetylases—an important class of cellular regulators with a variety of functions. Appl. Microbiol. Biotechnol. 75, 487–497 [DOI] [PubMed] [Google Scholar]
- 43. Hublitz P., Albert M., Peters A. H. (2009) Mechanisms of transcriptional repression by histone lysine methylation. Int. J. Dev. Biol. 53, 335–354 [DOI] [PubMed] [Google Scholar]
- 44. Lai D., Yang X. (2013) BMP4 is a novel transcriptional target and mediator of mammary cell migration downstream of the Hippo pathway component TAZ. Cell. Signal. 25, 1720–1728 [DOI] [PubMed] [Google Scholar]
- 45. Lindsay J., McDade S. S., Pickard A., McCloskey K. D., McCance D. J. (2011) Role of ΔNp63γ in epithelial to mesenchymal transition. J. Biol. Chem. 286, 3915–3924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bergholz J., Zhang Y., Wu J., Meng L., Walsh E. M., Rai A., Sherman M. Y., Xiao Z. X. (2014) ΔNp63α regulates Erk signaling via MKP3 to inhibit cancer metastasis. Oncogene 33, 212–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wu J., Liang S., Bergholz J., He H., Walsh E. M., Zhang Y., Xiao Z. X. (2014) ΔNp63α activates CD82 metastasis suppressor to inhibit cancer cell invasion. Cell Death Dis. 5, e1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hong J. H., Hwang E. S., McManus M. T., Amsterdam A., Tian Y., Kalmukova R., Mueller E., Benjamin T., Spiegelman B. M., Sharp P. A., Hopkins N., Yaffe M. B. (2005) TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science 309, 1074–1078 [DOI] [PubMed] [Google Scholar]
- 49. Jang E. J., Jeong H., Han K. H., Kwon H. M., Hong J. H., Hwang E. S. (2012) TAZ suppresses NFAT5 activity through tyrosine phosphorylation. Mol. Cell. Biol. 32, 4925–4932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vo N., Goodman R. H. (2001) CREB-binding protein and p300 in transcriptional regulation. J. Biol. Chem. 276, 13505–13508 [DOI] [PubMed] [Google Scholar]
- 51. Xu J., Kawai Y., Arinze I. J. (2013) Dual role of C/EBPα as an activator and repressor of Gαi2 gene transcription. Genes Cells 18, 1082–1094 [DOI] [PubMed] [Google Scholar]
- 52. Bartucci M., Dattilo R., Moriconi C., Pagliuca A., Mottolese M., Federici G., Benedetto A. D., Todaro M., Stassi G., Sperati F., Amabile M. I., Pilozzi E., Patrizii M., Biffoni M., Maugeri-Saccà M., Piccolo S., De Maria R. (2015) TAZ is required for metastatic activity and chemoresistance of breast cancer stem cells. Oncogene 34, 681–690 [DOI] [PubMed] [Google Scholar]
- 53. Reis-Filho J. S., Milanezi F., Amendoeira I., Albergaria A., Schmitt F. C. (2003) Distribution of p63, a novel myoepithelial marker, in fine-needle aspiration biopsies of the breast: an analysis of 82 samples. Cancer 99, 172–179 [DOI] [PubMed] [Google Scholar]
- 54. Wang X., Mori I., Tang W., Nakamura M., Nakamura Y., Sato M., Sakurai T., Kakudo K. (2002) p63 expression in normal, hyperplastic and malignant breast tissues. Breast Cancer 9, 216–219 [DOI] [PubMed] [Google Scholar]
- 55. Stefanou D., Batistatou A., Nonni A., Arkoumani E., Agnantis N. J. (2004) p63 expression in benign and malignant breast lesions. Histol. Histopathol. 19, 465–471 [DOI] [PubMed] [Google Scholar]
- 56. Diepenbruck M., Waldmeier L., Ivanek R., Berninger P., Arnold P., van Nimwegen E., Christofori G. (2014) Tead2 expression levels control the subcellular distribution of Yap and Taz, zyxin expression and epithelial-mesenchymal transition. J. Cell Sci. 127, 1523–1536 [DOI] [PubMed] [Google Scholar]
- 57. Qing Y., Yin F., Wang W., Zheng Y., Guo P., Schozer F., Deng H., Pan D. (2014) The Hippo effector Yorkie activates transcription by interacting with a histone methyltransferase complex through Ncoa6. Elife 3, e02564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Skibinski A., Breindel J. L., Prat A., Galván P., Smith E., Rolfs A., Gupta P. B., Labaer J., Kuperwasser C. (2014) The Hippo transducer TAZ interacts with the SWI/SNF complex to regulate breast epithelial lineage commitment. Cell Rep. 6, 1059–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Oh H., Slattery M., Ma L., Crofts A., White K. P., Mann R. S., Irvine K. D. (2013) Genome-wide association of Yorkie with chromatin and chromatin-remodeling complexes. Cell Rep. 3, 309–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gao Y., Zhang W., Han X., Li F., Wang X., Wang R., Fang Z., Tong X., Yao S., Li F., Feng Y., Sun Y., Hou Y., Yang Z., Guan K., Chen H., Zhang L., Ji H. (2014) YAP inhibits squamous transdifferentiation of Lkb1-deficient lung adenocarcinoma through ZEB2-dependent DNp63 repression. Nat. Commun. 5, 4629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.