Abstract
Sphingosine was named by J. L. W. Thudichum for its enigmatic properties. This descriptor has applied to sphingolipids for over a century because new enigmas continue to surface. This JBC minireview series presents articles about three novel subspecies of sphingolipids, α-galactosylceramides, 4,5-dihydroceramides, and 1-deoxysphingolipids, that have important activities but, until recently, remained undetected (or at least understudied) in the shadow of very closely related compounds. They also serve as a reminder that important metabolites still lie “off the radar screen” in reports of global and comprehensive metabolomic profiling.
Keywords: apoptosis, autophagy, cancer, cell signaling, ceramide, diabetes, glycolipid, immunology, sphingolipid, neuropathy
Introduction
Sphingolipids are one of the eight major categories of lipids (1) and are defined by the presence of a sphingoid base backbone (2, 3), with sphingosine (Fig. 1) displayed most frequently. The backbone is usually derivatized with an amide-linked fatty acid and/or a headgroup attached to the hydroxyl on carbon 1, as also depicted in Fig. 1 (4).
FIGURE 1.
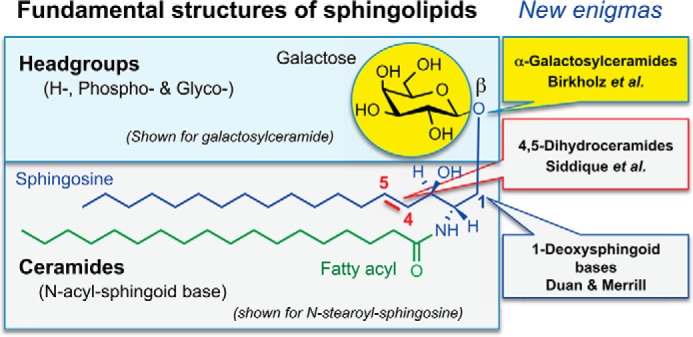
Fundamental structures of sphingolipids and the topics discussed in this minireview series. Sphingolipids are defined by having a sphingoid base (shown for sphingosine) that is often derivatized with an amide-linked fatty acid and/or headgroup of the general types shown.
These compounds have long been considered to be enigmas (mysterious and puzzling riddles) beginning with the initial naming of “sphingosin” in the 1880s by J. L. W. Thudichum (5). However, a substantial number of mysteries have been clarified over the past several decades as much has been learned about their structures and biophysical properties (6–8), biosynthesis and turnover (4, 9–11), interactions with proteins (12–14), and roles in cell-cell communication and signaling (13, 15–17). So have all the enigmas been solved?
This minireview series illustrates how subtle structural features of sphingolipids still have the capacity to surprise. The articles describe findings with naturally occurring deviations from three “hallmarks” of mammalian sphingolipid structure: the β-glycosidic linkage of the first carbohydrate attached to ceramide, the 4,5-double bond of the sphingoid base, and the hydroxyl on carbon 1 of the sphingoid base (Fig. 1).
The first minireview in the series, “The alpha and omega of galactosylceramides in T cell immune function” by Birkholz et al. (18), describes a monohexosylceramide that differs from the predominant glycosphingolipids made by mammals in having an α-linked rather than the β-linked sugar shown in the figure. A subset of T lymphocytes called natural killer T cells (NKT cells)2 recognizes glycolipids, predominantly α-linked glycosphingolipids, when they are bound to the cell surface protein CD1d. By activating NKT cells, synthetic α-linked glycosphingolipids can have profound effects on T cell immune responses. Structure-function studies have found fascinating contributions from the lipid backbone (19), and clinical trials of some of these derivative compounds as vaccine adjuvants are planned. Similar compounds are found in soil bacteria, and an interesting recent discovery has been that α-galactosylceramides are produced by Bacteroides fragilis, a prominent member of the human gut microflora (20). This expands the possible impact of these compounds beyond just their pharmaceutical (and basic research) applications into their possible roles in affecting gastrointestinal immune function and the complex relationship of the host with the microbiome. Self-glycosphingolipids with β-linked sugars are much weaker antigens, but they can also stimulate NKT cells. Recent work suggests, however, that mammalian cells may also produce small amounts of α-linked glycosphingolipids, and these may contribute to the tightly regulated but essential self-reactivity of NKT cells.
The second minireview, “Dihydroceramides: from bit players to lead actors” by Siddique et al. (21), discusses intermediates of de novo sphingolipid biosynthesis that do not have the 4,5-double bond of sphingosine (Fig. 1). Until recently, they were thought to be essentially inert (indeed, they were added to cells as controls for studies of the bioactivity of ceramides), but advanced detection techniques that facilitated the resolution of ceramides and dihydroceramides led to the surprising finding that drug effects previously attributed to ceramides were in fact driven by the dihydroceramides (22). Indeed, experimental inhibition (23) or depletion (24) of the desaturase that converts dihydroceramides to ceramides in mammalian cells revealed distinct and non-overlapping functions of these endogenous sphingolipids. Independent roles for dihydroceramides are emerging as players in autophagy, hypoxia, and metabolic control, and they have been implicated in the etiology or treatment of diabetes, cancer, and neurodegenerative diseases (24).
The third minireview, “1-Deoxysphingolipids encountered exogenously and made de novo: dangerous mysteries inside an enigma” by Duan and Merrill (25), describes a subcategory of sphingoid bases that lack the hydroxyl group on the first carbon (Fig. 1). These types of compounds were known to be produced by fungi and other organisms (3), and to be of health interest as mycotoxins (26) and as potential anticancer compounds that surfaced in screens of aquatic organisms (27). Thus, it was surprising to learn that mammals can also make 1-deoxy-sphingolipids (28) because wild-type serine palmitoyltransferase can accommodate l-alanine in addition to l-serine, and furthermore, that mutations that increase l-alanine utilization and 1-deoxysphingolipid production cause sensory neuropathies (29, 30). Elevations in these bioactive compounds have also been found in diabetes (31), non-alcoholic steatohepatitis (32), and when serine biosynthesis is defective (33), and can be envisioned for other conditions where metabolic changes or diet alter the amounts.
The findings with these novel compounds illustrate that our understanding of structure and function for sphingolipids is still in its infancy. This should be kept in mind when interpreting claims of “global” and “comprehensive” profiling of the metabolome because most of the bioactive sphingolipids, including ones yet to be discovered, still lie “off the radar screen” of such analyses.
The authors declare that they have no conflicts of interest with the contents of this article.
- NKT cells
- natural killer T cells.
References
- 1. Fahy E., Subramaniam S., Brown H. A., Glass C. K., Merrill A. H., Jr., Murphy R. C., Raetz C. R., Russell D. W., Seyama Y., Shaw W., Shimizu T., Spener F., van Meer G., VanNieuwenhze M. S., White S. H., Witztum J. L., Dennis E. A. (2005) A comprehensive classification system for lipids. J. Lipid Res. 46, 839–861 [DOI] [PubMed] [Google Scholar]
- 2. Carter H. E., Haines W. J., Ledyard W. E., Norris W. P. (1947) Biochemistry of the sphingolipides. I. Preparation of sphingolipides from beef brain and spinal cord. J. Biol. Chem. 169, 77–82 [PubMed] [Google Scholar]
- 3. Pruett S. T., Bushnev A., Hagedorn K., Adiga M., Haynes C. A., Sullards M. C., Liotta D. C., Merrill A. H., Jr. (2008) Biodiversity of sphingoid bases (“sphingosines”) and related amino alcohols. J. Lipid Res. 49, 1621–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Merrill A. H., Jr. (2011) Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics. Chem. Rev. 111, 6387–6422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thudichum J. L. W. (1884) A Treatise on the Chemical Constitution of Brain, Bailliere, Tindall, and Cox, London [Google Scholar]
- 6. Carreira A. C., Ventura A. E., Varela A. R., Silva L. C. (2015) Tackling the biophysical properties of sphingolipids to decipher their biological roles. Biol. Chem. 396, 597–609 [DOI] [PubMed] [Google Scholar]
- 7. Yu R. K., Yanagisawa M., Ariga T. (2007) Glycosphingolipid Structures. in Comprehensive Glycoscience. From Chemistry to Systems Biology (Kamerling J. P., ed), pp 73–122, Elsevier, Oxford, United Kingdom [Google Scholar]
- 8. Goñi F. M., Sot J., Alonso A. (2014) Biophysical properties of sphingosine, ceramides and other simple sphingolipids. Biochem. Soc. Trans. 42, 1401–1408 [DOI] [PubMed] [Google Scholar]
- 9. Schulze H., Sandhoff K. (2014) Sphingolipids and lysosomal pathologies. Biochim. Biophys. Acta 1841, 799–810 [DOI] [PubMed] [Google Scholar]
- 10. Astudillo L., Sabourdy F., Therville N., Bode H., Ségui B., Andrieu-Abadie N., Hornemann T., Levade T. (2015) Human genetic disorders of sphingolipid biosynthesis. J. Inherit. Metab. Dis. 38, 65–76 [DOI] [PubMed] [Google Scholar]
- 11. Taniguchi N., Honke K., Fukuda M., Narimatsu H., Yamaguchi Y., Angata T. (eds) (2014) Handbook of Glycosyltransferases and Related Genes, Springer, Japan [Google Scholar]
- 12. Snook C. F., Jones J. A., Hannun Y. A. (2006) Sphingolipid-binding proteins. Biochim. Biophys. Acta 1761, 927–946 [DOI] [PubMed] [Google Scholar]
- 13. Lopez P. H., Schnaar R. L. (2009) Gangliosides in cell recognition and membrane protein regulation. Curr. Opin. Struct. Biol. 19, 549–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Björkholm P., Ernst A. M., Hacke M., Wieland F., Brügger B., von Heijne G. (2014) Identification of novel sphingolipid-binding motifs in mammalian membrane proteins. Biochim. Biophys. Acta 1838, 2066–2070 [DOI] [PubMed] [Google Scholar]
- 15. Maceyka M., Harikumar K. B., Milstien S., Spiegel S. (2012) Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 22, 50–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schnaar R. L. (2015) Glycans and glycan-binding proteins in immune regulation: a concise introduction to glycobiology for the allergist. J. Allergy Clin. Immunol. 135, 609–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coskun Ü., Grzybek M., Drechsel D., Simons K. (2011) Regulation of human EGF receptor by lipids. Proc. Natl. Acad. Sci. U.S.A. 108, 9044–9048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Birkholz A. M., Howell A. R., Kronenberg M. (2015) The alpha and omega of galactosylceramides in T cell immune function. J. Biol. Chem. 290, 15365–15370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tyznik A. J., Farber E., Girardi E., Birkholz A., Li Y., Chitale S., So R., Arora P., Khurana A., Wang J., Porcelli S. A., Zajonc D. M., Kronenberg M., Howell A. R. (2011) Glycolipids that elicit IFN-γ-biased responses from natural killer T cells. Chem. Biol. 18, 1620–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wieland Brown L. C., Penaranda C., Kashyap P. C., Williams B. B., Clardy J., Kronenberg M., Sonnenburg J. L., Comstock L. E., Bluestone J. A., Fischbach M. A. (2013) Production of α-galactosylceramide by a prominent member of the human gut microbiota. PLoS Biol. 11, e1001610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Siddique M. M., Li Y., Chaurasia B., Kaddai V. A., Summers S. A. (2015) Dihydroceramides: from bit players to lead actors. J. Biol. Chem. 290, 15371–15379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zheng W., Kollmeyer J., Symolon H., Momin A., Munter E., Wang E., Kelly S., Allegood J. C., Liu Y., Peng Q., Ramaraju H., Sullards M. C., Cabot M., Merrill A. H., Jr. (2006) Ceramides and other bioactive sphingolipid backbones in health and disease: lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochim. Biophys. Acta 1758, 1864–1884 [DOI] [PubMed] [Google Scholar]
- 23. Gagliostro V., Casas J., Caretti A., Abad J. L., Tagliavacca L., Ghidoni R., Fabrias G., Signorelli P. (2012) Dihydroceramide delays cell cycle G1/S transition via activation of ER stress and induction of autophagy. Int. J. Biochem. Cell Biol. 44, 2135–2143 [DOI] [PubMed] [Google Scholar]
- 24. Siddique M. M., Li Y., Wang L., Ching J., Mal M., Ilkayeva O., Wu Y. J., Bay B. H., Summers S. A. (2013) Ablation of dihydroceramide desaturase 1, a therapeutic target for the treatment of metabolic diseases, simultaneously stimulates anabolic and catabolic signaling. Mol. Cell Biol. 33, 2353–2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Duan J., Merrill A. H., Jr. (2015) 1-Deoxysphingolipids encountered exogenously and made de novo: dangerous mysteries inside an enigma. J. Biol. Chem. 290, 15380–15389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang E., Norred W. P., Bacon C. W., Riley R. T., Merrill A. H., Jr. (1991) Inhibition of sphingolipid biosynthesis by fumonisins. Implications for diseases associated with Fusarium moniliforme. J. Biol. Chem. 266, 14486–14490 [PubMed] [Google Scholar]
- 27. Cuadros R., Montejo de Garcini E., Wandosell F., Faircloth G., Fernández-Sousa J. M., Avila J. (2000) The marine compound spisulosine, an inhibitor of cell proliferation, promotes the disassembly of actin stress fibers. Cancer Lett. 152, 23–29 [DOI] [PubMed] [Google Scholar]
- 28. Zitomer N. C., Mitchell T., Voss K. A., Bondy G. S., Pruett S. T., Garnier-Amblard E. C., Liebeskind L. S., Park H., Wang E., Sullards M. C., Merrill A. H., Jr., Riley R. T. (2009) Ceramide synthase inhibition by fumonisin B1 causes accumulation of 1-deoxysphinganine: a novel category of bioactive 1-deoxysphingoid bases and 1-deoxydihydroceramides biosynthesized by mammalian cell lines and animals. J. Biol. Chem. 284, 4786–4795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Penno A., Reilly M. M., Houlden H., Laurá M., Rentsch K., Niederkofler V., Stoeckli E. T., Nicholson G., Eichler F., Brown R. H., Jr., von Eckardstein A., Hornemann T. (2010) Hereditary sensory neuropathy type 1 is caused by the accumulation of two neurotoxic sphingolipids. J. Biol. Chem. 285, 11178–11187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eichler F. S., Hornemann T., McCampbell A., Kuljis D., Penno A., Vardeh D., Tamrazian E., Garofalo K., Lee H. J., Kini L., Selig M., Frosch M., Gable K., von Eckardstein A., Woolf C. J., Guan G., Harmon J. M., Dunn T. M., Brown R. H., Jr. (2009) Overexpression of the wild-type SPT1 subunit lowers desoxysphingolipid levels and rescues the phenotype of HSAN1. J. Neurosci. 29, 14646–14651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dohrn M. F., Othman A., Hirshman S. K., Bode H., Alecu I., Fähndrich E., Karges W., Weis J., Schulz J. B., Hornemann T., Claeys K. G. (2015) Elevation of plasma 1-deoxy-sphingolipids in type 2 diabetes mellitus: a susceptibility to neuropathy? Eur. J. Neurol. 22, 806-e55 [DOI] [PubMed] [Google Scholar]
- 32. Gorden D. L., Myers D. S., Ivanova P. T., Fahy E., Maurya M. R., Gupta S., Min J., Spann N. J., McDonald J. G., Kelly S. L., Duan J., Sullards M. C., Leiker T. J., Barkley R. M., Quehenberger O., Armando A. M., Milne S. B., Mathews T. P., Armstrong M. D., Li C., Melvin W. V., Clements R. H., Washington M. K., Mendonsa A. M., Witztum J. L., Guan Z., Glass C. K., Murphy R. C., Dennis E. A., Merrill A. H., Jr., Russell D. W., Subramaniam S., Brown H. A. (2015) Biomarkers of NAFLD progression: a lipidomics approach to an epidemic. J. Lipid Res. 56, 722–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Esaki K., Sayano T., Sonoda C., Akagi T., Suzuki T., Ogawa T., Okamoto M., Yoshikawa T., Hirabayashi Y., Furuya S. (April 22, 2015) l-Serine deficiency elicits intracellular accumulation of cytotoxic deoxy-sphingolipids and lipid body formation. J. Biol. Chem. 290, 14595–14609 [DOI] [PMC free article] [PubMed] [Google Scholar]


