Abstract
Glycosphingolipids are a subgroup of glycolipids that contain an amino alcohol sphingoid base linked to sugars. They are found in the membranes of cells ranging from bacteria to vertebrates. This group of lipids is known to stimulate the immune system through activation of a type of white blood cell known as natural killer T cell (NKT cell). Here we summarize the extensive research that has been done to identify the structures of natural glycolipids that stimulate NKT cells and to determine how these antigens are recognized. We also review studies designed to understand how glycolipid variants, both natural and synthetic, can alter the responses of NKT cells, leading to dramatic changes in the global immune response.
Keywords: ceramide, cytokine, glycolipid structure, immunology, lipid
Introduction
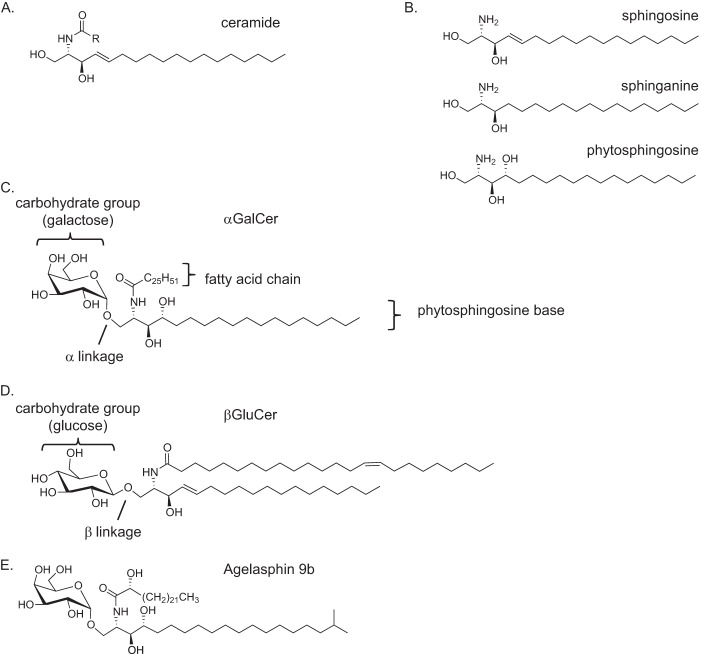
T lymphocytes are important cells of the adaptive immune response, and natural killer T cells (NKT cells)2 are a type of T lymphocyte. NKT cells were originally characterized as having cell surface markers expressed by innate immune cells such as NK1.1 in mice (1) and CD161, CD57, and CD56 in humans (2–4), as well as a T cell antigen receptor (TCR), a protein expressed by adaptive immune cells (5, 6). However, recent studies have indicated that only a portion of the NKT cells express NK1.1 in mice or CD161 and the other markers in humans, but all NKT cells are defined by a particular TCR specificity (7). Although most T lymphocytes recognize peptide fragments bound to or presented by MHC-encoded class I molecules, a subset of T cells, described below, recognizes glycosphingolipids (GSLs) and some other types of glycolipids. These GSLs are recognized when they are presented by CD1d, a cell surface protein homologous to MHC class I molecule (7, 8). The GSLs fall within the larger family of sphingolipids, or lipids that have ceramide as a core entity (9). Ceramides contain a sphingoid base with an amide linkage to a fatty acid chain (10) (Fig. 1A). Both of these chains can vary in saturation, length, branching, and hydroxylation. Natural sphingoid bases consist of sphingosines, sphinganines, or phytosphingosines (Fig. 1B); however, synthetic variants can deviate from these structures. The most common, natural fatty acid chain lengths are C:16 and C:18, but the lengths of natural and synthetic GSL fatty chains range from very few carbons to greater than C:30. The sphingoid base of GSLs has a 1″-1 glycosidic linkage to the carbohydrate head group, which is most commonly galactose or glucose, with gangliosides having more complex oligosaccharide structures (11). Because the 1″ carbon is asymmetric, it might be oriented in either an α or a β linkage (Fig. 1, C and D); however, the β orientation is the dominant linkage in mammals (12).
FIGURE 1.
Components and structures of some GSL antigens. A, ceramide. B, common types of sphingoid bases that differ at the C4 position C, αGalCer. D, β-d-glucopyranosyl ceramide (βGluCer). E, Agelasphin 9b.
There are several types of lipid-reactive NKT cells in mammals that can respond within hours of GSL stimulation by producing cytokines (7). The kinetic properties of this response are similar to innate immune cells. Type I, or invariant NKT (iNKT) cells, are one type of NKT cell. The moniker “invariant” for iNKT cells arises because these cells have an essentially identical TCR α gene rearrangement, using the Vα14 segment in mice and the homologous Vα24 (TRAV10) in humans (8). The invariant α chain is co-expressed with a restricted diversity of β chains, Vβ11 (TRBV25-1) in humans and Vβ8.2, Vβ7, and Vβ2 in mice (8). Type II NKT cells have a wider diversity of α and β chains. Because of their diverse TCRs and the relative lack of reagents to detect them, Type II NKT cells have been less studied (13). Here we will discuss the knowledge (alpha to omega) of studies on GSL recognition by both Type I and Type II NKT cells.
Presentation of GSLs by CD1d
The first GSL antigen for iNKT cells with a defined structure was α-galactosylceramide (αGalCer), which has a galactose in 1″-1 α linkage to a phytosphingosine base (Fig. 1C). To date, it remains the most studied antigen for iNKT cells, and it is among the most potent that have been identified. αGalCer was identified from structure activity relationship studies around Agelasphin 9b (Fig. 1E) by Kirin Pharmaceuticals in a screen for naturally occurring molecules that prevented tumor metastases in mice (14). This synthetic version, also known as KRN7000, retains the activity of Agelasphin 9b while being much easier to synthesize (15). The strategic simplifications included removing the fatty acid C2 hydroxyl group and the terminal branching of the sphingoid base. By also elongating the sphingoid base chain to C:18 and the fatty acid chain to C:26, the stimulatory activity of Agelasphin 9b was maintained for αGalCer.
GSLs such as αGalCer bind to the CD1d antigen-presenting molecule in a defined orientation (16). CD1d has two hydrophobic pockets termed A′ and F′. The sphingoid base chain localizes to the F′ pocket in the CD1d hydrophobic groove, and the fatty acid localizes to the A′ pocket (17) (Fig. 2A). This allows for optimal hydrogen bonding as well as optimal orientation of the saccharide head group for recognition by the iNKT cell TCR. The sugar linked to the sphingoid base plays a prominent role in the molecular determinant recognized by the iNKT cell TCR, with a galactose, in most cases, being the most potent moiety (Fig. 1C) (18). Although α-linked sugars (Fig. 1C) provide a much more potent activation of iNKT cells, the glycosidic bond to lipids is likely a β-linkage in mammals (Fig. 1D) (19, 20). Recent evidence suggests, however, that there are some GSLs with α-linked sugars in mammals (21), although their complete structure has not been determined and these GSLs are not abundant (22).
FIGURE 2.
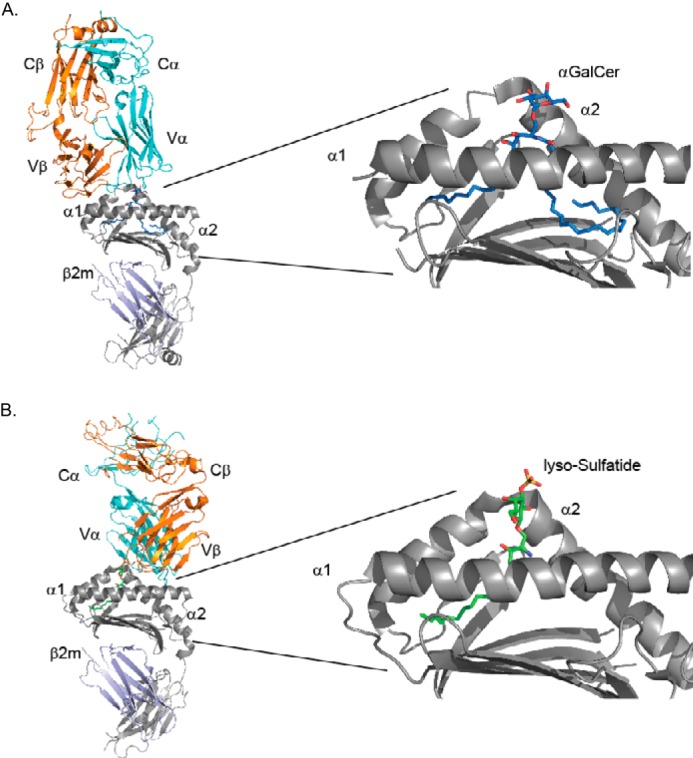
Structures of GSL-mouse CD1d complexes and trimolecular structures with the TCR. A, left, structure of the iNKT cell TCR binding to αGalCer-CD1d. The TCR α chain is in cyan, and the β chain is orange. The CD1d heavy chain is in gray, and the associated β2-microglobulin light chain is in violet. Right, expanded view of αGalCer (blue and red) in the CD1d binding groove (gray). The α1 and α2 helices of CD1d are labeled; the F′ pocket binding the phytosphingosine is on the left, and the acyl chain-binding A′ pocket is on the right. Taken from PDB code 3HE6. B, left, structure of a Type II NKT cell TCR binding to lyso-sulfatide-CD1d. Color scheme is the same as in panel A with lysosulfatide hydrocarbons in green. Right, expanded view of lysosulfatide (green, red, and yellow) in the CD1d binding groove (gray). Taken from PDB code 4ELM.
GSL Activation of iNKT Cells Alters the Immune Response
Relatively minor changes in the structure of the activating GSL antigen can cause very different types of immune responses (17). For example, certain GSLs can lead to a Th1 immune response (23). The Th1 response is characterized by the secretion of cytokines such as IFN-γ. IFN-γ is crucial for defense against intracellular pathogens, and it is important in the response against cancers. Conversely, other GSLs can cause the immune system to skew more toward a Th2 response (24), which is responsible for extracellular pathogen destruction and is characterized by secretion of the cytokine IL-4 and other cytokines. An excessive Th1 response can lead to autoimmunity or chronic inflammation, and an excessive Th2 response can lead to allergies and asthma (25). Therefore, both of these immune responses must be tightly regulated for immune homeostasis, and interestingly, Th1 and Th2 responses inhibit one another. The diverse outcomes following immunization with particular GSLs make them potential therapeutic agents for regulating immune responses and preventing immune-mediated disorders.
Although it is not known how subtle variations in GSL structure affect the immune response, several alternative hypotheses have been proposed. Some data suggest that Th1 responses depend on prolonged antigenic stimulation of iNKT cells, and this may be due to several factors, including enhanced GSL chemical stability in vivo, more stable binding of the GSL to CD1d, or increased TCR affinity for the GSL complex with CD1d (17). Alternatively, GSLs may have differential effects on antigen-presenting cells (APCs), for example, by trafficking to different components of the cell and inducing the expression of different cell surface molecules that influence immunity (27).
Among the factors that might contribute to prolonged antigenic stimulation, increased TCR affinity for the GSL-CD1d complex is not a good predictor of the type of immune response that will result (28, 29). Multiple studies have demonstrated that it is difficult to obtain a GSL with a higher affinity than αGalCer for the iNKT cell TCR by altering the sugar head group or by modifying the ceramide lipid in either the sphingoid base or the carboxylic acid. Crystallization studies have identified a similar docking motif of the iNKT TCR on the GSL-CD1d complex regardless of the modifications that have been analyzed (Fig. 2A) (30, 31). The iNKT cell TCR, in each case, is oriented over the F′ pocket of CD1d in a parallel orientation with the CD1d α helices, and the TCR uses germline-encoded residues in the CDR1α, CDR3α, and CDR2β loops to recognize the CD1d-presented lipid. A version of αGalCer derivatized at the 6″ carbon, α-GalCer-6″-(pyridin-4-yl) carbamate, promoted a very strong Th1 response as compared with αGalCer, and crystallographic analysis showed that this GSL bound to CD1d made extra contacts with the TCR. Despite this, SPR measurement of TCR affinity for this GSL-CD1d complex did not demonstrate more avid binding as compared with αGalCer-CD1d complexes (32).
As compared with TCR affinity for GSL-CD1d, there is a stronger correlation of a Th1 immune response with the strength of the interaction of the lipid with CD1d. GSL antigens that have more contacts with CD1d, and/or that are packed more tightly within the A′ and F′ pockets, are likely to be presented for a longer time by APCs. For example, the alteration of the sphingoid base chain to contain a bulkier cyclopropyl group (compound SMC124, Fig. 3A) (33) or phenyl group (7DW8-5) (34) led to a stronger Th1 response in mouse or human models, respectively. CD1d presentation of SMC124 by APC in vivo was more stable over 22 h as compared with αGalCer, perhaps due to an enhanced GSL-CD1d interaction. Crystal structure analysis of the SMC124-CD1d complex indicated that this lipid may bind in a more compact orientation within the F′ groove of CD1d. Another example of a GSL promoting a Th1 cytokine response that might be related to enhanced interaction with CD1d is provided by the lipid naphthyl-urea-αGalCer, which has a naphthyl urea group linked to the 6″ position of the saccharide (Fig. 3B). Crystal structure analysis demonstrated that the naphthyl urea group folds over and makes additional contacts with the surface of the CD1d molecule when the TCR is engaged (35). Conversely, the GSL OCH (Fig. 3C), which causes primarily a Th2 response, has a shortened sphingoid base chain and thus lacks some GSL-CD1d contacts that could stabilize the complex (24). Although these results are persuasive with regard to the important role of the GSL-CD1d interactions, the ability to promote a Th1 response depends on the interactions of iNKT cells with multiple cell types, and the causes of a Th1 cytokine response are likely to be multifactorial.
FIGURE 3.
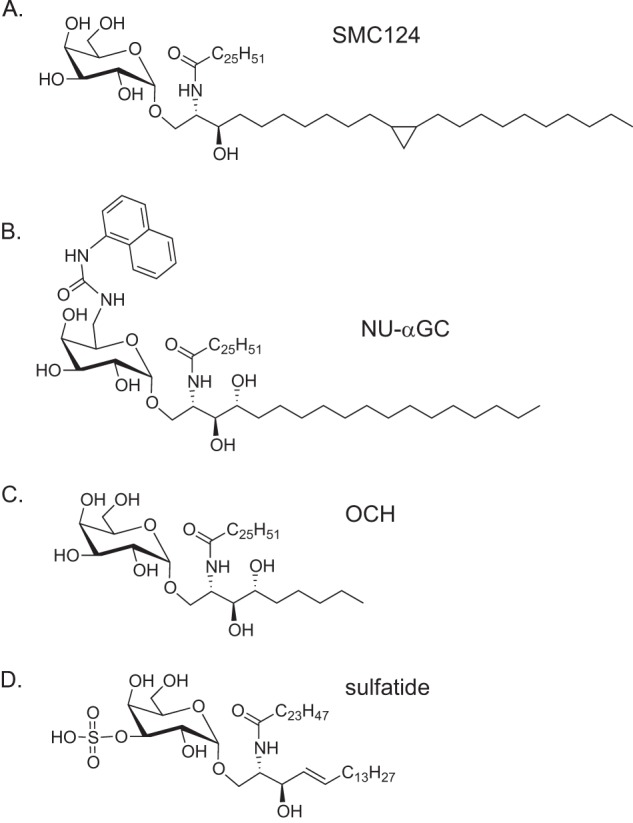
Structures of iNKT activating GSLs. A, SMC124. B, naphthyl-urea-αGalCer (NU-αGC). C, OCH. D, sulfatide.
Microbial GSL Antigens for iNKT Cells
Several microbial GSLs have been shown to activate iNKT cells. The microbiome has been a highly researched area in recent years, and we are only beginning to understand the role that commensal bacteria play in the immune system. Research has shown that the development of iNKT cells is influenced by the microbiome directly or indirectly through other cell types (36, 37). Germ-free mice had a slightly reduced iNKT cell population in the liver, spleen, and thymus as compared with normal mice, and iNKT cells from germ-free mice were hypo-responsive, whereas mice colonized with a restricted flora had an even more significant decrease in iNKT cells (36). These data suggest that the large differences in the frequency of iNKT cells in the peripheral blood of humans could be related to microflora. In contrast, germ-free mice had increased numbers of iNKT cells in the colon, lamina propria, and lungs (37, 38), and these cells are hyper-responsive, which led to exacerbated inflammation in models of inflammatory bowel disease and allergic asthma. Early life exposure to microbes could reverse the increased number and hyper-reactivity of iNKT cells and the susceptibility to inflammatory disease. These data lend support to theories that relate early childhood exposure to microbes to a decrease in immune-mediated diseases, the so-called hygiene hypothesis (39).
The commensal bacteria Sphingomonas spp. and Bacteroides fragilis are two microbial species that have GSL antigens that activate iNKT cells. Sphingomonas spp., which are α-proteobacteria, were discovered to have two GSL antigens for iNKT cells, GSL-1 and GSL-1′, which have either a glucuronic or a galacturonic saccharide, respectively, linked to a ceramide backbone having a sphinganine base (40, 41). Different Sphingomonas species produce variable GSLs, in some cases with oligosaccharide moieties containing three or four sugars, but GSLs with more complex sugars do not strongly activate iNKT cells (42, 43).
B. fragilis have an assortment of membrane phospholipids including sphingolipids. When the repertoire of sphingolipids was assessed, an isoform of αGalCer with methyl branches in the lipid chains was identified. This compound can activate both mouse and human iNKT cells (44), although in another study, it was reported that this GSL can serve as an antagonist (45).
Mammalian GSL Antigens for iNKT Cells
Mammalian GSLs represent potential self-antigens. Like other T lymphocytes, the TCR of iNKT cells must interact with ligands in the thymus to survive (46). Unlike other T cells, iNKT cells also are self-reactive as mature cells, but this self-reactivity is controlled, in part, through the expression of inhibitory receptors (47). The nature of the thymic self-ligands and stimulating self-antigens for mature iNKT cells is controversial, but some data suggest that they include both GSLs and other types of lipids (19, 20, 48). Nonetheless, certain mammalian or self-GSLs stimulate iNKT cells. Although initially it was thought that only GSLs with α-anomeric lipids could be antigens for iNKT cells, β-linked GSLs were also shown to activate them (49, 50), although they are weaker antigens than their α-anomeric counterparts. The crystal structure of β-galactosylceramide (βGalCer) bound to mouse CD1d in complex with the iNKT cell TCR revealed that the TCR was able to squash or push the orientation of the β-linked galactose to a similar orientation as the galactose in the αGalCer CD1d-GSL-iNKT cell TCR trimolecular complex (51). The closely related β-d-glucopyranosylceramide, a sphingosine containing GSL with a C24:1 fatty acid (Fig. 1D), may activate both human and mouse iNKT cells (52), although recent studies indicate that this activation is due to a possible natural α-anomeric GSL (21). The GSL isoglobotrihexosylceramide (iGb3), a trisaccharide containing GSL with glucose in β-1″-1 linkage to the sphingosine base, also activated iNKT cells. This antigen was discovered after noting that mice lacking β-hexosaminidase b, which removes the terminal β-linked GalNAc residue of isoglobotetrahexosylceramide (iGb4) to make iGb3, had a reduced number of iNKT cells (53). Although iGb3 can participate along with other self-antigens, the analysis of mice deficient for iGb3 synthase indicates that it is not essential for iNKT cells (54).
Type II NKT Cells and the Sulfatide GSLs
Type II NKT cells, as mentioned earlier, do not express an invariant TCR α chain, and consequently, they have diverse specificities. However, a number of Type II NKT cells recognize sulfatide (Fig. 3D), a GSL composed of βGalCer with the galactose sulfated at the 3″ position. In a mouse model of multiple sclerosis, sulfatide-reactive Type II NKT cells were specifically recruited to the central nervous system (55). Natural isoforms of sulfatide differ with regard to the fatty acid and sphingoid base, and it was a lyso-sulfatide that showed the greatest antigenic potency when tested with a particular Type II NKT cell hybridoma (56). Interestingly, the Type II NKT cell TCR, which has been crystalized in complex with sulfatide antigen bound to CD1d, has a completely different binding mode as compared with the iNKT cell TCR. The sulfatide-reactive Type II NKT cell TCR is oriented over the A′ pocket of the CD1d molecule, with predominant binding interactions with the TCR β chain (26) (Fig. 2B).
Conclusions
The relationship of GSLs and the T cell branch of the immune system has been studied extensively through studies of Type I and Type II NKT cells. Many synthetic, microbial, and mammalian GSLs have been tested, and some have been shown to activate one or the other type of NKT cells and influence the overall immune response. The exact mechanism whereby activated iNKT cells can skew the immune response in either the Th1 or the Th2 direction is not completely understood, but efforts are underway to develop compounds that give a strong and predictable cytokine response in humans so that GSLs can be used in clinical settings.
This work was supported by National Institutes of Health Grants R37 AI71922 and R01 AI105215 (to M. K.). This is the first article in the Thematic Minireview series “Novel Bioactive Sphingolipids.” The authors declare that they have no conflicts of interest with the contents of this article.
- NKT
- natural killer T cell
- iNKT
- invariant natural killer T cell
- αGalCer
- α-galactosylceramide
- βGalCer
- β-galactosylceramide
- iGB3
- isoglobotrihexosylceramide
- TCR
- T cell receptor
- Th1
- T helper type 1
- Th2
- T helper type 2
- GSL
- glycosphingolipid
- APC
- antigen-presenting cell.
References
- 1. MacDonald H. R. (1995) NK1.1+ T cell receptor-α/β+ cells: new clues to their origin, specificity, and function. J. Exp. Med. 182, 633–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schmidt R. E., Murray C., Daley J. F., Schlossman S. F., Ritz J. (1986) A subset of natural killer cells in peripheral blood displays a mature T cell phenotype. J. Exp. Med. 164, 351–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Exley M., Porcelli S., Furman M., Garcia J., Balk S. (1998) CD161 (NKR-P1A) costimulation of CD1d-dependent activation of human T cells expressing invariant Vα24JαQ T cell receptor α chains. J. Exp. Med. 188, 867–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harada K., Isse K., Tsuneyama K., Ohta H., Nakanuma Y. (2003) Accumulating CD57 + CD3 + natural killer T cells are related to intrahepatic bile duct lesions in primary biliary cirrhosis. Liver Int. 23, 94–100 [DOI] [PubMed] [Google Scholar]
- 5. Makino Y., Kanno R., Ito T., Higashino K., Taniguchi M. (1995) Predominant expression of invariant Vα14+ TCR α chain in NK1.1+ T cell populations. Int. Immunol. 7, 1157–1161 [DOI] [PubMed] [Google Scholar]
- 6. Porcelli S., Yockey C. E., Brenner M. B., Balk S. P. (1993) Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4−8− α/β T cells demonstrates preferential use of several Vβ genes and an invariant TCR α chain. J. Exp. Med. 178, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Godfrey D. I., MacDonald H. R., Kronenberg M., Smyth M. J., Van Kaer L. (2004) NKT cells: what's in a name? Nat. Rev. Immunol. 4, 231–237 [DOI] [PubMed] [Google Scholar]
- 8. Salio M., Silk J. D., Jones E. Y., Cerundolo V. (2014) Biology of CD1- and MR1-restricted T cells. Annu. Rev. Immunol. 32, 323–366 [DOI] [PubMed] [Google Scholar]
- 9. Lahiri S., Futerman A. H. (2007) The metabolism and function of sphingolipids and glycosphingolipids. Cell. Mol. Life Sci. 64, 2270–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hannun Y. A., Obeid L. M. (2011) Many ceramides. J. Biol. Chem. 286, 27855–27862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Degroote S., Wolthoorn J., van Meer G. (2004) The cell biology of glycosphingolipids. Semin. Cell Dev. Biol. 15, 375–387 [DOI] [PubMed] [Google Scholar]
- 12. Ilan Y. (2009) α versus β: are we on the way to resolve the mystery as to which is the endogenous ligand for natural killer T cells? Clin. Exp. Immunol. 158, 300–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Terabe M., Berzofsky J. A. (2014) The immunoregulatory role of Type I and Type II NKT cells in cancer and other diseases. Cancer Immunol. Immunother. 63, 199–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Natori T., Morita M., Akimoto K., Koezuka Y. (1994) Agelasphins, novel antitumor and immunostimulatory cerebrosides from the marine sponge Agelas mauritianus. Tetrahedron 50, 2771–2784 [Google Scholar]
- 15. Morita M., Motoki K., Akimoto K., Natori T., Sakai T., Sawa E., Yamaji K., Koezuka Y., Kobayashi E., Fukushima H. (1995) Structure-activity relationship of α-galactosylceramides against B16-bearing mice. J. Med. Chem. 38, 2176–2187 [DOI] [PubMed] [Google Scholar]
- 16. Zajonc D. M., Kronenberg M. (2007) CD1 mediated T cell recognition of glycolipids. Curr. Opin. Struct. Biol. 17, 521–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. East J. E., Kennedy A. J., Webb T. J. (2014) Raising the roof: the preferential pharmacological stimulation of Th1 and Th2 responses mediated by NKT cells. Med. Res. Rev. 34, 45–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sidobre S., Hammond K. J. L., Bénazet-Sidobre L., Maltsev S. D., Richardson S. K., Ndonye R. M., Howell A. R., Sakai T., Besra G. S., Porcelli S. A., Kronenberg M. (2004) The T cell antigen receptor expressed by Vα14i NKT cells has a unique mode of glycosphingolipid antigen recognition. Proc. Natl. Acad. Sci. U.S.A. 101, 12254–12259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Facciotti F., Ramanjaneyulu G. S., Lepore M., Sansano S., Cavallari M., Kistowska M., Forss-Petter S., Ni G., Colone A., Singhal A., Berger J., Xia C., Mori L., De Libero G. (2012) Peroxisome-derived lipids are self antigens that stimulate invariant natural killer T cells in the thymus. Nat. Immunol. 13, 474–480 [DOI] [PubMed] [Google Scholar]
- 20. Pei B., Speak A. O., Shepherd D., Butters T., Cerundolo V., Platt F. M., Kronenberg M. (2011) Diverse endogenous antigens for mouse NKT cells: self-antigens that are not glycosphingolipids. J. Immunol. 186, 1348–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brennan P. J., Tatituri R. V. V., Heiss C., Watts G. F. M., Hsu F.-F., Veerapen N., Cox L. R., Azadi P., Besra G. S., Brenner M. B. (2014) Activation of iNKT cells by a distinct constituent of the endogenous glucosylceramide fraction. Proc. Natl. Acad. Sci. U.S.A. 111, 13433–13438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kain L., Webb B., Anderson B. L., Deng S., Holt M., Constanzo A., Zhao M., Self K., Teyton A., Everett C., Kronenberg M., Zajonc D. M., Bendelac A., Savage P. B., Teyton L. (2014) The identification of the endogenous ligands of natural killer T cells reveals the presence of mammalian α-linked glycosylceramides. Immunity 41, 543–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schmieg J., Yang G., Franck R. W., Tsuji M. (2003) Superior protection against malaria and melanoma metastases by a C-glycoside analogue of the natural killer T cell ligand α-galactosylceramide. J. Exp. Med. 198, 1631–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oki S., Chiba A., Yamamura T., Miyake S. (2004) The clinical implication and molecular mechanism of preferential IL-4 production by modified glycolipid-stimulated NKT cells. J. Clin. Invest. 113, 1631–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Raphael I., Nalawade S., Eagar T. N., Forsthuber T. G. (2014) T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 10.1016/j.cyto.2014.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Girardi E., Maricic I., Wang J., Mac T.-T., Iyer P., Kumar V., Zajonc D. M. (2012) Type II natural killer T cells use features of both innate-like and conventional T cells to recognize sulfatide self antigens. Nat. Immunol. 13, 851–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arora P., Baena A., Yu K. O. A., Saini N. K., Kharkwal S. S., Goldberg M. F., Kunnath-Velayudhan S., Carreño L. J., Venkataswamy M. M., Kim J., Lazar-Molnar E., Lauvau G., Chang Y.-T., Liu Z., Bittman R., Al-Shamkhani A., Cox L. R., Jervis P. J., Veerapen N., Besra G. S., Porcelli S. A. (2014) A single subset of dendritic cells controls the cytokine bias of natural killer T cell responses to diverse glycolipid antigens. Immunity 40, 105–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mallevaey T., Clarke A. J., Scott-Browne J. P., Young M. H., Roisman L. C., Pellicci D. G., Patel O., Vivian J. P., Matsuda J. L., McCluskey J., Godfrey D. I., Marrack P., Rossjohn J., Gapin L. (2011) A molecular basis for NKT cell recognition of CD1d-self-antigen. Immunity 34, 315–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sullivan B. A., Nagarajan N. A., Wingender G., Wang J., Scott I., Tsuji M., Franck R. W., Porcelli S. A., Zajonc D. M., Kronenberg M. (2010) Mechanisms for glycolipid antigen-driven cytokine polarization by Vα14i NKT cells. J. Immunol. 184, 141–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Girardi E., Zajonc D. M. (2012) Molecular basis of lipid antigen presentation by CD1d and recognition by natural killer T cells. Immunol. Rev. 250, 167–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rossjohn J., Pellicci D. G., Patel O., Gapin L., Godfrey D. I. (2012) Recognition of CD1d-restricted antigens by natural killer T cells. Nat. Rev. Immunol. 12, 845–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aspeslagh S., Nemcovic M., Pauwels N., Venken K., Wang J., Van Calenbergh S., Zajonc D. M., Elewaut D. (2013) Enhanced TCR footprint by a novel glycolipid increases NKT-dependent tumor protection. J. Immunol. 191, 2916–2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tyznik A. J., Farber E., Girardi E., Birkholz A., Li Y., Chitale S., So R., Arora P., Khurana A., Wang J., Porcelli S. A., Zajonc D. M., Kronenberg M., Howell A. R. (2011) Glycolipids that elicit IFN-γ-biased responses from natural killer T cells. Chem. Biol. 18, 1620–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li X., Fujio M., Imamura M., Wu D., Vasan S., Wong C.-H., Ho D. D., Tsuji M. (2010) Design of a potent CD1d-binding NKT cell ligand as a vaccine adjuvant. Proc. Natl. Acad. Sci. U.S.A. 107, 13010–13015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aspeslagh S., Li Y., Yu E. D., Pauwels N., Trappeniers M., Girardi E., Decruy T., Van Beneden K., Venken K., Drennan M., Leybaert L., Wang J., Franck R. W., Van Calenbergh S., Zajonc D. M., Elewaut D. (2011) Galactose-modified iNKT cell agonists stabilized by an induced fit of CD1d prevent tumour metastasis. EMBO J. 30, 2294–2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wei B., Wingender G., Fujiwara D., Chen D. Y., McPherson M., Brewer S., Borneman J., Kronenberg M., Braun J. (2010) Commensal microbiota and CD8+ T cells shape the formation of invariant NKT cells. J. Immunol. 184, 1218–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Olszak T., An D., Zeissig S., Vera M. P., Richter J., Franke A., Glickman J. N., Siebert R., Baron R. M., Kasper D. L., Blumberg R. S. (2012) Microbial exposure during early life has persistent effects on natural killer T cell function. Science 336, 489–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wingender G., Stepniak D., Krebs P., Lin L., McBride S., Wei B., Braun J., Mazmanian S. K., Kronenberg M. (2012) Intestinal microbes affect phenotypes and functions of invariant natural killer T cells in mice. Gastroenterology 143, 418–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Leslie M. (2012) Gut microbes keep rare immune cells in line. Science 335, 1428–1428 [DOI] [PubMed] [Google Scholar]
- 40. Kinjo Y., Wu D., Kim G., Xing G.-W., Poles M. A., Ho D. D., Tsuji M., Kawahara K., Wong C.-H., Kronenberg M. (2005) Recognition of bacterial glycosphingolipids by natural killer T cells. Nature 434, 520–525 [DOI] [PubMed] [Google Scholar]
- 41. Mattner J., Debord K. L., Ismail N., Goff R. D., Cantu C., 3rd, Zhou D., Saint-Mezard P., Wang V., Gao Y., Yin N., Hoebe K., Schneewind O., Walker D., Beutler B., Teyton L., Savage P. B., Bendelac A. (2005) Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 434, 525–529 [DOI] [PubMed] [Google Scholar]
- 42. Long X., Deng S., Mattner J., Zang Z., Zhou D., McNary N., Goff R. D., Teyton L., Bendelac A., Savage P. B. (2007) Synthesis and evaluation of stimulatory properties of Sphingomonadaceae glycolipids. Nat. Chem. Biol. 3, 559–564 [DOI] [PubMed] [Google Scholar]
- 43. Kinjo Y., Pei B., Bufali S., Raju R., Richardson S. K., Imamura M., Fujio M., Wu D., Khurana A., Kawahara K., Wong C.-H., Howell A. R., Seeberger P. H., Kronenberg M. (2008) Natural Sphingomonas glycolipids vary greatly in their ability to activate natural killer T cells. Chem. Biol. 15, 654–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wieland Brown L. C., Penaranda C., Kashyap P. C., Williams B. B., Clardy J., Kronenberg M., Sonnenburg J. L., Comstock L. E., Bluestone J. A., Fischbach M. A. (2013) Production of α-galactosylceramide by a prominent member of the human gut microbiota. PLoS Biol. 11, e1001610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. An D., Oh S. F., Olszak T., Neves J. F., Avci F. Y., Erturk-Hasdemir D., Lu X., Zeissig S., Blumberg R. S., Kasper D. L. (2014) Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell 156, 123–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Koch U., Radtke F. (2011) Mechanisms of T cell development and transformation. Annu. Rev. Cell Dev. Biol. 27, 539–562 [DOI] [PubMed] [Google Scholar]
- 47. Issazadeh-Navikas S. (2012) NKT cell self-reactivity: evolutionary master key of immune homeostasis? J Mol. Cell. Biol. 4, 70–78 [DOI] [PubMed] [Google Scholar]
- 48. Fox L. M., Cox D. G., Lockridge J. L., Wang X., Chen X., Scharf L., Trott D. L., Ndonye R. M., Veerapen N., Besra G. S., Howell A. R., Cook M. E., Adams E. J., Hildebrand W. H., Gumperz J. E. (2009) Recognition of lyso-phospholipids by human natural killer T lymphocytes. PLoS Biol. 7, e1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Parekh V. V., Singh A. K., Wilson M. T., Olivares-Villagómez D., Bezbradica J. S., Inazawa H., Ehara H., Sakai T., Serizawa I., Wu L., Wang C. R., Joyce S., Van Kaer L. (2004) Quantitative and qualitative differences in the in vivo response of NKT cells to distinct α- and β-anomeric glycolipids. J. Immunol. 173, 3693–3706 [DOI] [PubMed] [Google Scholar]
- 50. Ortaldo J. R., Young H. A., Winkler-Pickett R. T., Bere E. W., Jr., Murphy W. J., Wiltrout R. H. (2004) Dissociation of NKT stimulation, cytokine induction, and NK activation in vivo by the use of distinct TCR-binding ceramides. J. Immunol. 172, 943–953 [DOI] [PubMed] [Google Scholar]
- 51. Pellicci D. G., Clarke A. J., Patel O., Mallevaey T., Beddoe T., Le Nours J., Uldrich A. P., McCluskey J., Besra G. S., Porcelli S. A., Gapin L., Godfrey D. I., Rossjohn J. (2011) Recognition of β-linked self glycolipids mediated by natural killer T cell antigen receptors. Nat. Immunol. 12, 827–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Brennan P. J., Tatituri R. V. V., Brigl M., Kim E. Y., Tuli A., Sanderson J. P., Gadola S. D., Hsu F.-F., Besra G. S., Brenner M. B. (2011) Invariant natural killer T cells recognize lipid self antigen induced by microbial danger signals. Nat. Immunol. 12, 1202–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhou D., Mattner J., Cantu C., 3rd, Schrantz N., Yin N., Gao Y., Sagiv Y., Hudspeth K., Wu Y.-P., Yamashita T., Teneberg S., Wang D., Proia R. L., Levery S. B., Savage P. B., Teyton L., Bendelac A. (2004) Lysosomal glycosphingolipid recognition by NKT cells. Science 306, 1786–1789 [DOI] [PubMed] [Google Scholar]
- 54. Porubsky S., Speak A. O., Luckow B., Cerundolo V., Platt F. M., Gröne H. J. (2007) Normal development and function of invariant natural killer T cells in mice with isoglobotrihexosylceramide (iGb3) deficiency. Proc. Natl. Acad. Sci. U.S.A. 104, 5977–5982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jahng A. (2004) Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J. Exp. Med. 199, 947–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Blomqvist M., Rhost S., Teneberg S., Löfbom L., Osterbye T., Brigl M., Månsson J. E., Cardell S. L. (2009) Multiple tissue-specific isoforms of sulfatide activate CD1d-restricted Type II NKT cells. Eur. J. Immunol. 39, 1726–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]