Background: Excessive endogenous glucose production is a major contributing factor for fasting hyperglycemia in diabetes.
Results: FoxO6 deficiency attenuates hepatic gluconeogenesis and protects against fat-induced glucose disorder in mice.
Conclusion: FoxO6 plays a significant role in regulating gluconeogenesis in the liver.
Significance: FoxO6 is a potential therapeutic target for improving glucose metabolism in diabetes.
Keywords: diabetes, gluconeogenesis, glucose metabolism, liver, obesity, FoxO6, glucose metabolism, mice
Abstract
Excessive endogenous glucose production contributes to fasting hyperglycemia in diabetes. FoxO6 is a distinct member of the FoxO subfamily. To elucidate the role of FoxO6 in hepatic gluconeogenesis and assess its contribution to the pathogenesis of fasting hyperglycemia in diabetes, we generated FoxO6 knock-out (FoxO6-KO) mice followed by determining the effect of FoxO6 loss-of-function on hepatic gluconeogenesis under physiological and pathological conditions. FoxO6 depletion attenuated hepatic gluconeogenesis and lowered fasting glycemia in FoxO6-KO mice. FoxO6-deficient primary hepatocytes were associated with reduced capacities to produce glucose in response to glucagon. When fed a high fat diet, FoxO6-KO mice exhibited significantly enhanced glucose tolerance and reduced blood glucose levels accompanied by improved insulin sensitivity. These effects correlated with attenuated hepatic gluconeogenesis in FoxO6-KO mice. In contrast, wild-type littermates developed fat-induced glucose intolerance with a concomitant induction of fasting hyperinsulinemia and hyperglycemia. Furthermore, FoxO6-KO mice displayed significantly diminished macrophage infiltration into liver and adipose tissues, correlating with the reduction of macrophage expression of C-C chemokine receptor 2 (CCR2), a factor that is critical for regulating macrophage recruitment in peripheral tissues. Our data indicate that FoxO6 depletion protected against diet-induced glucose intolerance and insulin resistance by attenuating hepatic gluconeogenesis and curbing macrophage infiltration in liver and adipose tissues in mice.
Introduction
Gluconeogenesis is a pivotal metabolic pathway for converting non-carbohydrate metabolites (lactate, glycerol, and amino acids) to glucose. Gluconeogenesis takes place mainly in the liver (1, 2), accounting for up to 80% of total glucose production in healthy individuals during a prolonged fast (3). Gluconeogenesis is tightly regulated by hormonal and nutritional cues (2, 4–8). In response to postprandial insulin secretion, gluconeogenic activity is suppressed to limit glucose production (9–11). This effect serves to prevent prolonged postprandial glucose excursion and replenish glycogen content in the liver. In response to fasting, gluconeogenesis is stimulated to increase glucose production in the liver (12–15). Such a reciprocal mechanism, orchestrated by two functionally opposing hormones (insulin and glucagon), is crucial for rapid adaptation by the liver to metabolic shift between fed and fasting states for maintaining blood glucose levels within the physiological range (2). In response to impaired insulin signaling in the liver, hepatic gluconeogenesis becomes unabated. This effect accounts for excessive glucose production, contributing to fasting hyperglycemia in diabetes. Nevertheless, the molecular basis that links impaired insulin action to unrestrained hepatic gluconeogenesis remains incompletely understood.
Critical for insulin-dependent regulation of hepatic gluconeogenesis is FoxO1,5 known as a substrate of Akt/PKB (16–19). In the absence of insulin, FoxO1 stimulates hepatic expression of PEPCK and G6Pase, two key enzymes that catalyze the first and last steps in the gluconeogenesis pathway, contributing to the induction of hepatic gluconeogenesis (20–22). This effect is amplified by peroxisome proliferator-activated receptor γ coactivator 1-α (PGC1α) that acts as a co-activator of FoxO1 to enhance gluconeogenic activity in the liver (9). Consistent with its function in gluconeogenesis, FoxO1 binds to a highly conserved DNA motif (T(G/A)TTT(T/G)), known as insulin-responsive element (IRE), in the promoters of PEPCK and G6Pase (23–27). In response to insulin, FoxO1 is phosphorylated by Akt/PKB and translocated from the nucleus to cytoplasm, resulting in inhibition of gluconeogenic gene expression (16–18, 28–32). These data underscore the importance of FoxO1 in orchestrating insulin action on hepatic gluconeogenesis. However, FoxO1 depletion in the liver does not abolish hormonal regulation of gluconeogenesis (20, 33, 34), implying that FoxO1 accounts partially for insulin-dependent regulation of hepatic gluconeogenesis. In keeping with this notion, Kamagate et al. (35) report that mice with FoxO1 deficiency in the liver are associated with partial loss of gluconeogenic activities. FoxO1 loss-of-function attenuates, but does not abrogate, the responsiveness of liver to insulin or glucagon (via cAMP) (35). These results imply that there are additional factors that integrate insulin signaling to gluconeogenesis in the liver (20, 35, 36).
Kim et al. (37) recently characterized FoxO6 as an important regulator of hepatic gluconeogenesis. FoxO6 is produced in the liver of rodents and humans. FoxO6 stimulates gluconeogenic activity in cultured hepatocytes and in the liver. This effect is enhanced by glucagon (via cAMP) and inhibited by insulin. Interestingly, FoxO6 mediates insulin action on hepatic gluconeogenesis via a distinct mechanism. Unlike FoxO1, which is translocated from the nucleus to cytoplasm in response to insulin (16–18, 38, 39), FoxO6 does not undergo insulin-dependent nucleocytoplasmic trafficking. Instead, insulin inhibits FoxO6 function by promoting its phosphorylation and disabling its DNA binding activity in the nucleus without altering its subcellular distribution (37). Consistent with its role in hepatic gluconeogenesis, FoxO6 activity is maintained in the liver at basal levels and is markedly induced in fasted mice (37). FoxO6 activity becomes abnormally higher in insulin-resistant liver, correlating with fasting hyperglycemia in dietary obese mice or diabetic db/db mice. FoxO6 transgenic mice develop pre-diabetes, culminating in the induction of fasting hyperglycemia, glucose intolerance, and hyperinsulinemia (37). Nonetheless, it remains an open question as to whether FoxO6 plays an independent role in mediating insulin action on hepatic gluconeogenesis. Likewise, it remains unknown whether FoxO6 deregulation couples impaired insulin signaling to unchecked hepatic gluconeogenesis in obesity and diabetes.
To determine the obligatory role of FoxO6 in hepatic gluconeogenesis and assess its contribution to the pathogenesis of fasting hyperglycemia in obesity and diabetes, we ablated the FoxO6 gene in mice. We showed that FoxO6 knock-out mice developed normally and grew with a similar weight gain as wild-type littermates. In contrast, FoxO1-null mice die of abnormal embryogenesis (40). Thus, FoxO6 knock-out mice provided a viable model for determining the ability of FoxO6-deficient liver to undergo gluconeogenesis in response to insulin under physiological and pathological conditions. We hypothesized that FoxO6 depletion would attenuate hepatic gluconeogenesis, and this effect would protect mice from developing insulin resistance and glucose intolerance in response to high fat feeding.
Experimental Procedures
Animal Studies
To delete the FoxO6 gene, we used the C57BL/6N mouse-derived FoxO6_BF6 embryonic stem cells with genetic deletion of the entire FoxO6 coding region from the UC Davis KOMP Repository (University of California Davis) (Fig. 1A). We verified FoxO6 gene deletion using primers flanking the first and second exons of the FoxO6 gene (forward 5′-CAGGAGTAGCCGAGGGTTCC-3′ and reverse 5′-AGCGGACCATCCAGTCGTAG-3′) (Fig. 1B). As the control, the gene cassette containing the LacZ and Neo in replacement of the FoxO6 allele was confirmed using primers specific for LacZ gene (forward 5′-GGTAAACTGGCTCGGATTAGGG-3′ and reverse 5′-TTGACTGTAGCGGCTGATGTTG-3′) and Neo cDNA (forward 5′-TTCGGCTATGACTGGGCACAACAG-3′ and reverse 5′-TACTTTCTCGGCAGGAGCAAGGTG-3′) (Fig. 1B). FoxO6_BF6 embryonic stem cells were micro-injected into BALB/c blastocysts followed by implantation into pseudo-pregnant foster mice. Chimeric mice containing targeted FoxO6 gene deletion were re-derived in C57BL/6J background followed by back-crossing with C57BL/6J mice for seven generations. Mice were fed standard rodent chow or high fat diet (fat content, 60 kcal%; Research Diets, Inc., New Brunswick, NJ) and water ad libitum in sterile cages with a 12-h light/dark cycle. Mice were fasted for 16 h, and tail vein blood was collected into capillary tubes pre-coated with potassium-EDTA (Sarstedt, Nümbrecht, Germany) for determining blood glucose levels using Glucometer Elite (Bayer, IN) or plasma insulin levels using the ultrasensitive mouse insulin enzyme-linked immunosorbent assay (ALPCO, Windham, NH). The homeostasis model for insulin resistance (HOMA-IR) was determined by multiplying fasting blood glucose (mmol/liter) and fasting plasma insulin (μIU/ml) levels, divided by 22.5. All experiments were performed in male mice to avoid the potential impact of hormonal fluctuation associated with the estrous cycle on glucose metabolism in female mice. All procedures were approved by the Institutional Animal Care and Use Committee of University of Pittsburgh.
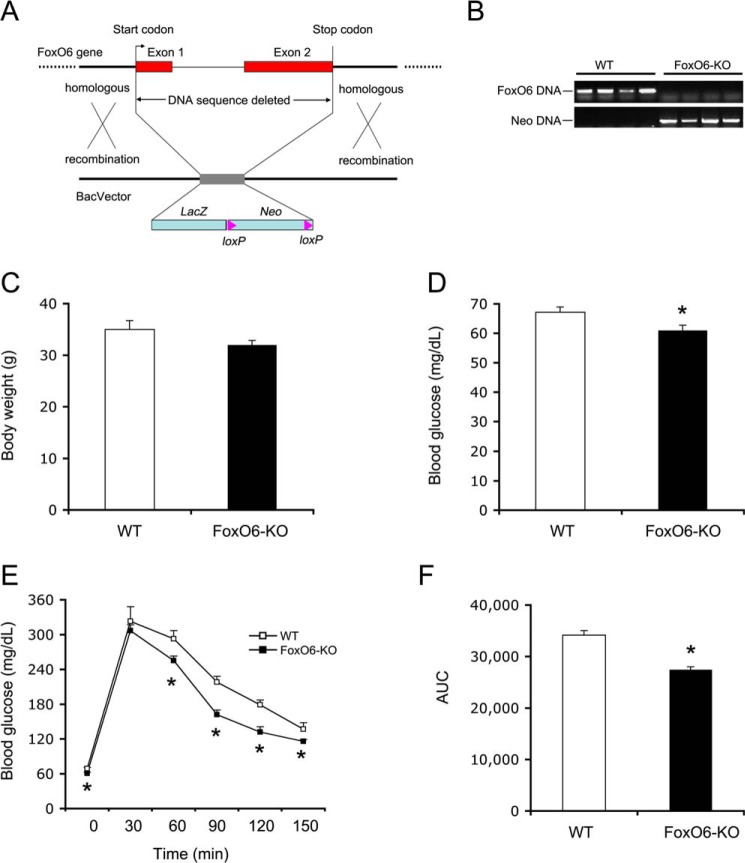
FIGURE 1.
Effect of FoxO6 deletion on glucose metabolism. A, schematic depiction of FoxO6 gene deletion. Shown is the FoxO6 locus on the chromosome 4. Neo stands for the neomycin-resistant gene. LacZ encodes bacterial β-galactosidase. B, genotyping of WT and FoxO6-KO mice. Genomic DNA isolated from mouse tail was subjected to PCR analysis using primers specific for FoxO6 or Neo gene. PCR products were resolved by agarose gel electrophoresis. C, body weight. D, blood glucose. Blood glucose levels were determined after 16-h fasting. E, glucose tolerance test. Mice were fasted for 16 h followed by intraperitoneal injection of glucose (2 g/kg body weight). Blood glucose levels before and at different times post glucose injection were determined. F, area under curve. AUC was calculated from blood glucose profiles during the glucose tolerance test. Data in panels C–F were obtained from male FoxO6-KO mice (n = 14) and age/sex-matched WT littermates (n = 7) on regular chow at 4 months of age. *, p < 0.05 versus WT control.
Glucose Tolerance Test
Mice were fasted for 16 h followed by intraperitoneal injection of glucose (2 g/kg body weight) as described (22).
Pyruvate Tolerance Test
Mice were fasted for 16 h followed by intraperitoneal injection of pyruvate (2 g/kg body weight) as described (41).
Insulin Tolerance Test
Mice were injected intraperitoneally with regular human insulin (1.5 IU/kg; Lilly) as described (22).
Fat Mass Determination
Fat mass and lean mass of mice were determined using the EchoMRI-100 system (Echo Medical Systems, Houston, TX).
Energy Expenditure Determination
Mice were placed individually in metabolic cages with free access to food and water in the Oxymax Lab Animal Monitoring System (Columbus Instruments, Columbus, OH). After acclimation for 2 days, oxygen consumption and respiratory exchange ratio of mice were determined during a 48-h period.
Plasma Cytokine and Chemokine Measurement
Plasma TNFα, IL-6 and CCL2 were determined using the mouse cytokine/chemokine magnetic bead panel (EMD Millipore). Plasma IL-1β levels were determined using the mouse IL-1β ELISA kit (BD Biosciences).
Mouse Primary Hepatocytes
Mouse primary hepatocytes were isolated as described (42). Hepatocytes were plated in collagen-coated 12-well plates at 2 × 105 cells/well and cultured in hepatocyte maintenance medium (Lonza) supplemented with dexamethasone, insulin, and GA-1000 (Lonza) as described (43).
Glucose Production Assay
Mouse primary hepatocytes were cultured in 12-well collagen-coated microplates for 12 h. After washing twice with pre-warmed PBS buffer, hepatocytes were incubated in 2 ml of glucose-free glutamine-containing DMEM supplemented with 1 mm pyruvate. After a 6-h incubation in the absence or presence of 8-cpt-cAMP (cAMP analog, 500 μm) and dexamethasone (100 μm), conditioned medium were harvested to determine glucose levels using the glucose oxidase reagent (Sigma).
RNA Isolation and Real Time RT-PCR
RNA was purified from liver tissues (20 mg) using the RNeasy Mini kit (Qiagen, Valencia, CA) and subjected to real-time qRT-PCR assay for quantifying mRNA concentrations as described (35). The primers are FoxO6 forward (5′-GAAGAGCTCCCGACGGAACG-3′) and reverse (5′-TTCAGCATCCACCATGAACT-3′) and FoxO1 forward (5′-AAGAGCGTGCCCTACTTCAA-3′) and reverse (CTCTTGCCCAGACTGGAGAG-3′). Primers for PEPCK and G6Pase mRNA and 18S RNA have been described (22).
Preparation of Stromal Vascular Cells
Epididymal adipose tissues were procured and minced in 10-ml KRB (Kreb-Ringer bicarbonate, pH 7.4) containing collagenase II (C6885, Sigma) for preparing stromal vascular cells as described (44).
G6Pase Activity Assay
40-mg liver tissues were homogenized in 400 μl of microsome buffer (20 mm Tris-Cl, pH 7.0, 1 mm EDTA, 0.25 m sucrose). After centrifugation at 4,000 × g for 10 min, the supernatant was centrifuged at 100,000 rpm for 30 min in the Sorvall Discovery M150SE ultracentrifuge (Hitachi) for preparing microsomes (37). Aliquots of 50-μl microsomes (protein concentration, 500 μg/ml) were used for determining G6Pase activity, defined as the production of Pi (in μmole) per μg of cellular microsomes per unit time (in minutes) as described (37).
Glucose 6-Phosphate (G6P) Assay
Aliquots of liver tissues (20 mg) were homogenized in 80 μl of ice-cold PBS. The homogenized samples were transferred into individual 10-kDa molecular weight cutoff spin filters (YM-10 from Millipore) followed by centrifugation at 13,000 × g for 30 min to remove proteins and insoluble materials. Aliquots of the flow-through samples (10 μl) were used for determining G6P using the colorimetric G6P assay kit (catalog no. MAK014, Sigma). Hepatic G6P content, expressed as ng of G6P per mg of hepatic proteins, was compared between FoxO6-KO and WT littermates.
Oil Red O Staining
Liver tissue was embedded in the Histoprep tissue embedding media and snap-frozen. Frozen sections (6 μm) were cut for fat staining with oil red O as described (22).
Hepatic Fat Content
Aliquots of liver tissue (20 mg) were homogenized in 500 μl of HPLC grade acetone. Aliquots (50 μl) of acetone-extracted lipid suspension were used for determining TG concentrations as described (22).
Immunohistochemistry
Liver tissues were fixed in 4% paraformaldehyde and cryopreserved in 30% sucrose. Frozen sections (6 μm) were subjected to immunohistochemistry using rat anti-F4/80 antibody (Invitrogen) and Cy3-conjugated donkey anti-rat IgG (Jackson ImmunoResearch Laboratories). The nuclei of cells were stained with 4′,6-diamidino-2-phenylindole (DAPI, Sigma) before visualization in the Aviovert 200 fluorescent microscope (Zeiss, Oberkochen, Germany). Adipose tissues fixed in 4% paraformaldehyde were embedded in paraffin. Paraffin sections (6 μm) were subjected to immunohistochemistry using rat anti-F4/80 antibody (Invitrogen) and biotinylated anti-rat IgG (BA-4001, Vector Laboratories). F4/80-positive cells were visualized using diaminobenzidine (DAB kit, SK-4100, Vector Laboratories) as the chromogen.
Statistics
Statistical analyses of data were performed by Student's t test. Data are expressed as the mean ± S.E. p values <0.05 were considered statistically significant.
Results
Blood Glucose Metabolism in FoxO6-KO Mice
To characterize the role of FoxO6 in glucose metabolism, we bred FoxO6+/− heterozygous mice to generate homozygous knock-out mice (FoxO6-KO). FoxO6-KO mice were viable. When fed regular chow, FoxO6-KO mice (n = 14) grew with similar a weight gain as wild-type (WT) littermates (n = 7) (Fig. 1C). However, FoxO6-KO mice, as opposed to WT control mice, exhibited significantly lower fasting blood glucose levels (Fig. 1D). A small insignificant reduction in fed blood glucose levels was also detected in FoxO6-KO mice (113 ± 16 versus 130 ± 20 mg/dl in WT littermates, p = 0.06). In response to glucose tolerance, FoxO6-KO mice displayed significantly improved blood glucose profiles (Fig. 1E) and reduced area under the curve (AUC) (Fig. 1F).
Effect of FoxO6 Deletion on Hepatic Gluconeogenesis
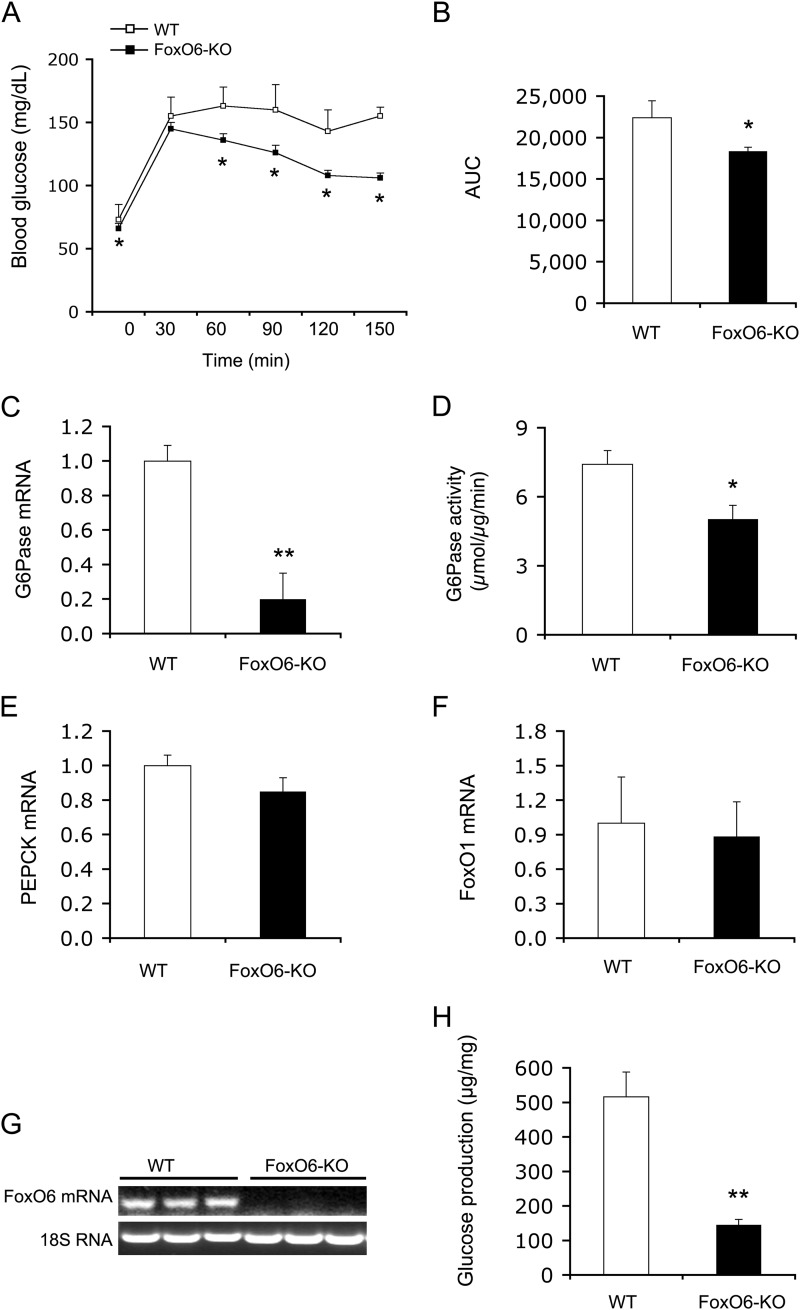
To understand the mechanism by which FoxO6 deletion lowered blood glucose levels in response to prolonged fasting and during glucose tolerance, we performed a pyruvate tolerance test to determine the ability of FoxO6-deficient liver to convert pyruvate to glucose. FoxO6-KO mice displayed significantly lower blood glucose levels and AUC (Fig. 2, A and B), suggesting that FoxO6 depletion attenuates gluconeogenesis in the liver. To support this notion, we determined hepatic mRNA levels of PEPCK and G6Pase, two key enzymes in the gluconeogenesis pathway. We detected a significant reduction in hepatic G6Pase mRNA levels (Fig. 2C), correlating with significantly reduced G6Pase activity in the liver of FoxO6-KO mice (Fig. 2D). These findings are in accordance with Kim et al. (37), who show that FoxO6 targets the G6Pase gene for trans-activation. In contrast, hepatic PEPCK mRNA levels remained unchanged in FoxO6-KO versus WT mice (Fig. 2E). Likewise, no differences were detectable in hepatic FoxO1 mRNA levels between FoxO6-KO and WT groups (Fig. 2F), precluding the possibility that the effect of FoxO6 depletion on gluconeogenesis was secondary to altered hepatic FoxO1 activity in FoxO6-KO mice. To confirm FoxO6 depletion, we determined hepatic FoxO6 mRNA levels, demonstrating that FoxO6 mRNA expression was undetectable in the liver of FoxO6-KO mice (Fig. 2G).
FIGURE 2.
Effect of FoxO6 deletion on hepatic gluconeogenesis. A, pyruvate tolerance test. B, AUC. The AUC was calculated from blood glucose profiles during pyruvate tolerance test. C, G6Pase mRNA levels. D, hepatic G6Pase activity. E, PEPCK mRNA levels. F, FoxO1 mRNA levels. G, hepatic FoxO6 mRNA. Mice were sacrificed after 16-h fasting. Liver tissues were used for isolating total RNA, which was subjected to real-time qRT-PCR analysis. Hepatic G6Pase, PEPCK, FoxO1, and FoxO6 mRNA levels were determined using 18S RNA as control. Aliquots of liver tissues (40 mg) were used for the preparation of microsomes, which were subjected to G6Pase activity assay for determining hepatic G6Pase activity, defined as the production of Pi (in μmole) per unit time (in minutes) per μg of cellular microsomes. H, glucose production in mouse primary hepatocytes. Mouse primary hepatocytes of FoxO6-KO and WT mice were cultured in the absence or presence of 8-cpt-cAMP (cAMP analog, 500 μm) and dexamethasone (100 μm). Each condition was run in six replicates. After 24-h of incubation, the amount of glucose released from hepatocytes into culture medium was determined. Data in panels A–G were obtained from male FoxO6-KO mice (n = 14) and age/sex-matched WT littermates (n = 7) on regular chow at 4 months of age. p < 0.05 (*) and p < 0.001 (**) versus WT control.
To bolster the notion that FoxO6 deficiency attenuates the ability of liver to undergo gluconeogenesis, we isolated hepatocytes from FoxO6-KO mice and WT littermates followed by the determination of glucose production in primary hepatocytes in the absence or presence of cAMP and dexamethasone in culture medium, a condition that is analogous to fasting in mice. FoxO6-deficient hepatocytes were associated with reduced capacity of producing glucose in response to cAMP and dexamethasone (Fig. 2H).
Effect of FoxO6 Depletion on Glucose Metabolism in Dietary Obese Mice
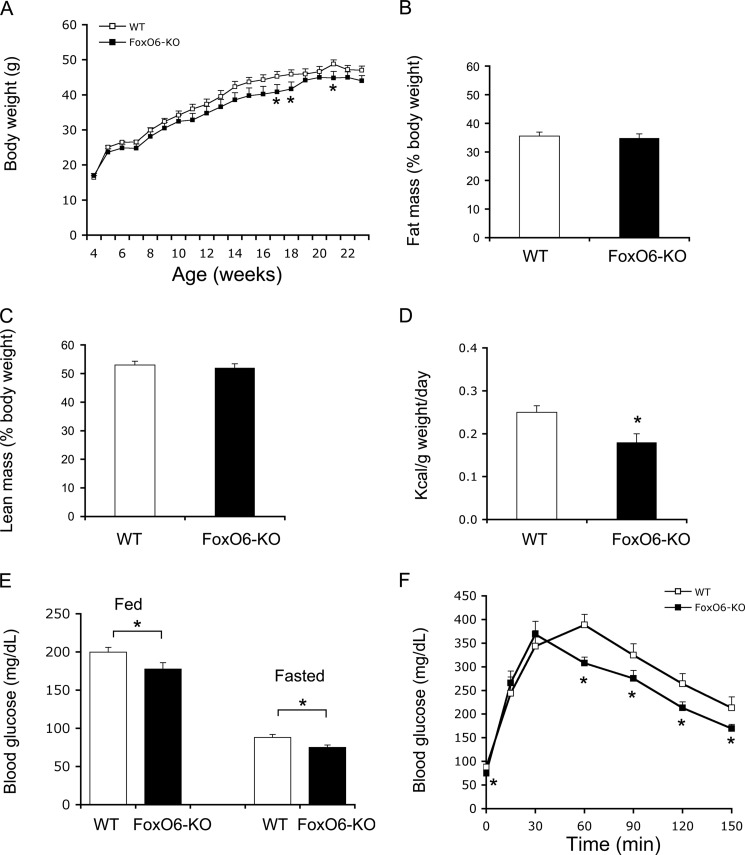
The observations that FoxO6 depletion attenuates hepatic gluconeogenesis and improves fasting blood glucose profiles spurred the hypothesis that FoxO6 deficiency would protect mice from developing fat-induced insulin resistance and glucose intolerance. Implicit in this postulation is that hepatic FoxO6 expression becomes abnormally higher, coinciding with the pathogenesis of fasting hyperglycemia in dietary obese mice and diabetic db/db mice (37). To address this hypothesis, we fed FoxO6-KO mice and WT littermates (n = 8–9/group) a high fat diet at 4 weeks of age for 4 months. Both groups of mice became obese in response to high fat feeding. FoxO6-KO mice, as opposed to WT littermates, gained less weight (Fig. 3A). There were no significant differences in fat mass (Fig. 3B) and lean mass (Fig. 3C), after normalizing to body weight, between FoxO6-KO and WT control mice. Consistent with less weight gain, FoxO6-KO mice had a small reduction in food intake (Fig. 3D).
FIGURE 3.
Glucose metabolism in FoxO6-KO mice on high fat diet. A, growth curve. Mice were fed a high fat diet at 4 weeks of age for 16 weeks. Body weight was determined weekly. B, fat mass. Fat mass was determined by MRI. C, lean mass. Lean mass was determined by MRI. D, food intake. Food intake per mouse during a 24-h period was determined for two consecutive days. E, blood glucose levels. Fed blood glucose levels were determined in fed states, and fasting blood glucose levels were determined after 16-h fasting. F, glucose tolerance test. Data were obtained from high fat-fed male FoxO6-KO (n = 9) and age/sex-matched WT littermates (n = 8) at 20–22 weeks of age. *, p < 0.05 versus WT control.
As expected, a 16-week high fat feeding resulted in fasting hyperglycemia and glucose intolerance in WT control mice (Fig. 3, E and F). These metabolic abnormalities were improved in FoxO6-KO mice, culminating in significantly reduced blood glucose levels at both fed and fasting conditions (Fig. 3E) and enhanced glucose tolerance (Fig. 3F). Plasma lipid profiling did not reveal significant differences in circulating triglyceride and free fatty acid levels (Table 1). Instead, we detected a significant reduction of total plasma cholesterol levels in high fat-fed FoxO6-KO mice (Table 1).
TABLE 1.
Plasma lipid and cytokine profiles and hepatic G6P levels in high fat-fed FoxO6-KO and WT mice
High fat-fed FoxO6-KO (n = 9) and WT mice (n = 8) at 22 weeks of age were fasted for 16 h. Blood was sampled for the determination of plasma lipid and cytokine levels. Intracellular G6P levels in the liver, expressed as ng of G6P per mg of hepatic proteins, were determined by colorimetric G6P assay, as described under “Experimental Procedures.”
| Blood parameters | WT | FoxO6-KO |
|---|---|---|
| Triglyceride (mg/dl) | 64.8 ± 8.4 | 56.7 ± 2.8 |
| Cholesterol (mg/dl) | 201.2 ± 15.6 | 156.4 ± 10.7a |
| Free fatty acid (meq/liter) | 0.66 ± 0.02 | 0.72 ± 0.04 |
| CCL2 (pg/ml) | 199 ± 42 | 184 ± 38 |
| IL-6 (pg/ml) | 9.3 ± 1.6 | 12.2 ± 2.7 |
| TNFα (pg/ml) | 4.8 ± 0.3 | 6.3 ± 1.2 |
| G6P (ng/mg) | 7,225 ± 451 | 6,055 ± 355a |
a p < 0.05 versus WT control.
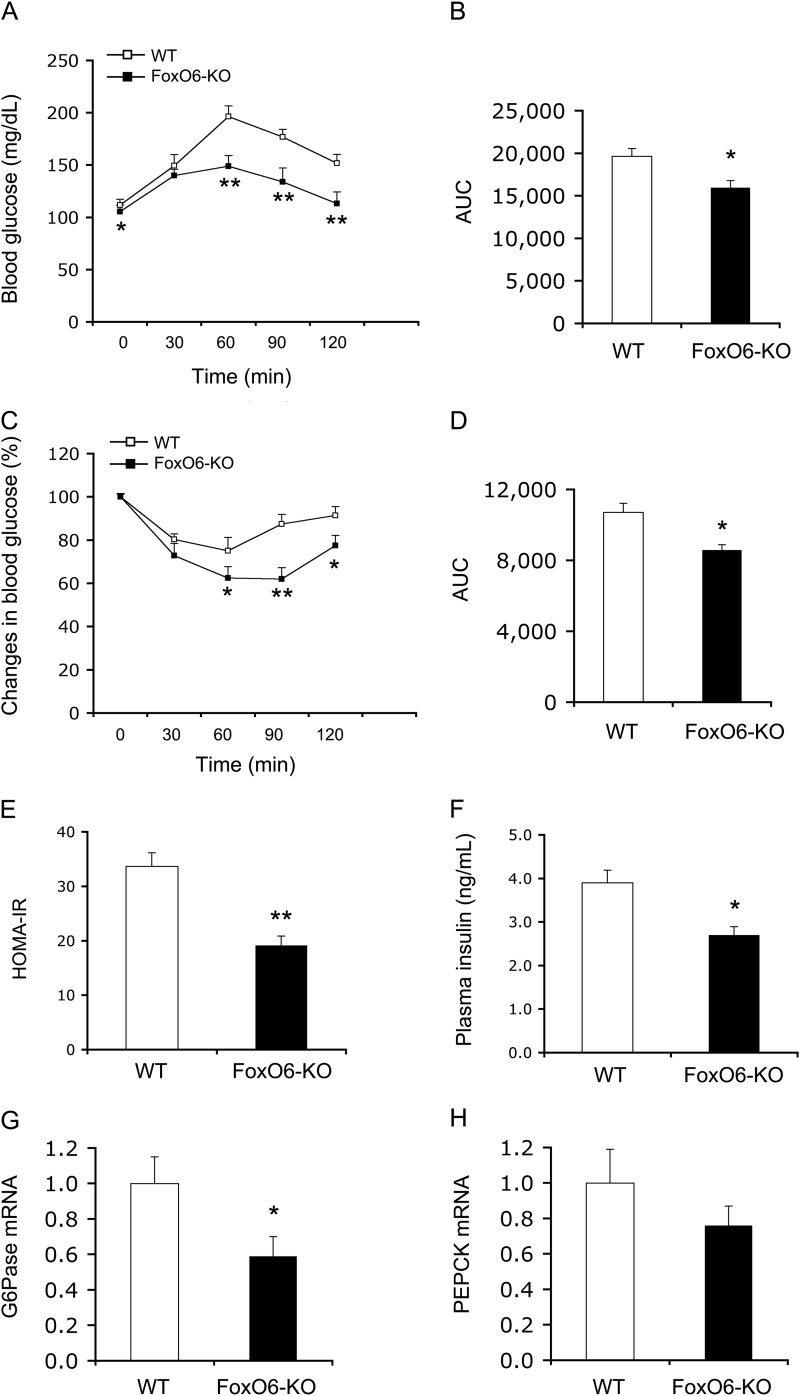
To account for the improved blood glucose metabolism in high fat-fed FoxO6-KO mice, we performed a pyruvate tolerance test. We detected significantly lower blood glucose levels (Fig. 4A) and AUC in FoxO6-KO mice during pyruvate tolerance test (Fig. 4B), consistent with the idea that FoxO6 deficiency suppresses gluconeogenesis in the liver and improves postprandial blood glucose profiles. To preclude the possibility that the reduction of gluconeogenesis is due to impaired mobilization of G6P in hepatocytes, we determined intracellular G6P levels in the liver (Table 1). A small, but significant, reduction in hepatic G6P levels was detected, correlating with attenuated gluconeogenesis in FoxO6-KO mice. To determine the potential beneficial effect of FoxO6 depletion on insulin sensitivity in high fat-fed FoxO6-KO mice, we performed an insulin tolerance test. FoxO6-KO mice exhibited significantly lower blood glucose levels and AUC in response to insulin tolerance (Fig. 4, C and D). These results were correlated with insulin resistance index, as determined by HOMA-IR. When compared with WT control mice, FoxO6-KO mice had significantly lower HOMA-IR (Fig. 4E), with a concomitant reduction of fasting hyperinsulinemia (Fig. 4F).
FIGURE 4.
Effect of FoxO6 depletion on hepatic gluconeogenesis and insulin sensitivity in high fat-fed FoxO6-KO mice. A, pyruvate tolerance test. B, AUC. AUC was calculated from blood glucose profiles during pyruvate tolerance test. C, insulin tolerance test. Mice were injected intraperitoneally with regular human insulin (1.5 IU/kg) followed by determination of blood glucose levels. D, AUC. AUC was calculated from blood glucose profiles during insulin tolerance test. E, HOMA-IR. The HOMA-IR was determined by multiplying fasting blood glucose (mmol/liter) and fasting plasma insulin (μIU/ml) levels, divided by 22.5. F, fasting plasma insulin levels. Plasma insulin levels were determined after 16-h fasting. G, G6Pase mRNA levels. H, PEPCK mRNA levels. Data were obtained from high fat-fed male FoxO6-KO (n = 9) and age/sex-matched WT littermates (n = 8) at 20–22 weeks of age. Hepatic G6Pase and PEPCK mRNA levels were determined by real-time qRT-PCR using 18S RNA as control. *, p < 0.05 versus WT control.
To gain insight into the mechanism by which FoxO6 deficiency improved glucose metabolism, we determined the effect of FoxO6 depletion on gluconeogenic gene expression. We detected a 2-fold reduction in hepatic G6Pase mRNA levels (Fig. 4G), consistent with attenuated gluconeogenesis in the liver in FoxO6-KO mice. FoxO6 depletion also reduced hepatic PEPCK mRNA expression to a lesser extent (Fig. 4H).
Energy Homeostasis in FoxO6-KO Mice on High Fat
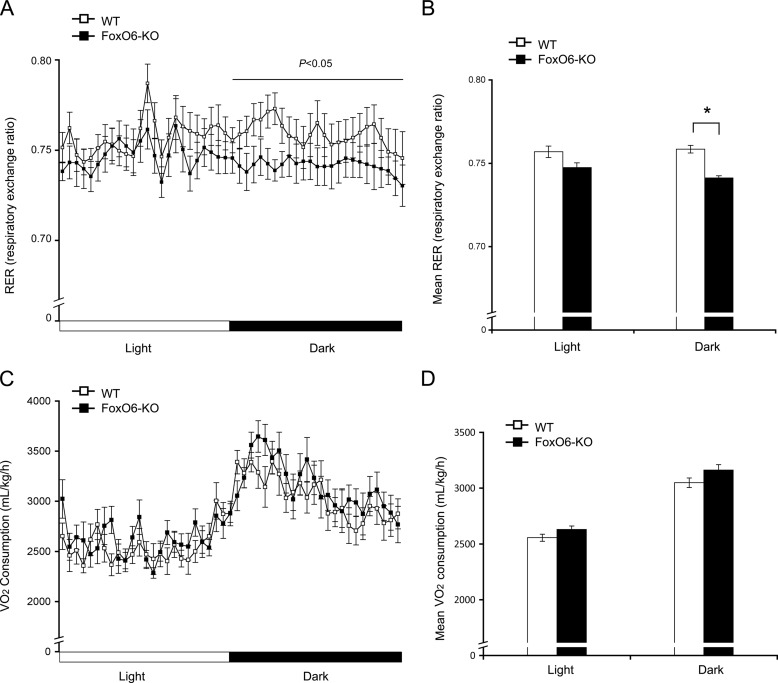
To provide further physiological underpinning of the notion that FoxO6 deficiency attenuates gluconeogenesis and improves glucose metabolism in obesity, we subjected high fat-fed FoxO6-KO mice and WT littermates (n = 8–9/group) to metabolic cage studies for the determination of energy homeostasis. FoxO6-KO mice, as opposed to WT littermates, had significantly reduced respiratory exchange ratio (RER) during the dark cycle, indicative of an increased contribution of fat to energy production in FoxO6-KO mice (Fig. 5, A and B). Consistent with these results, we detected a relatively higher mean rate of oxygen consumption in FoxO6-KO mice (Fig. 5, C and D).
FIGURE 5.
Effect of FoxO6 depletion on energy homeostasis in high fat-fed FoxO6-KO mice. Male FoxO6-KO (n = 9, 22 weeks old) and age/sex-matched WT littermates (n = 8) after 16-weeks of high fat feeding were subjected to metabolic cage studies. Mice were placed individually in metabolic cages with free access to food and water in the Oxymax Lab Animal Monitoring System. After acclamation for 2 days, oxygen consumption and respiratory exchange ratio of mice were determined during a 48-h period. A, the respiratory exchange ratio (RER) in a 12-h light/dark cycle. B, the mean respiratory exchange ratio. C, the oxygen consumption rate. D, the mean oxygen consumption rate. *, p < 0.05 versus WT control.
Effect of FoxO6 Depletion on Kupffer Cell Content in the Liver
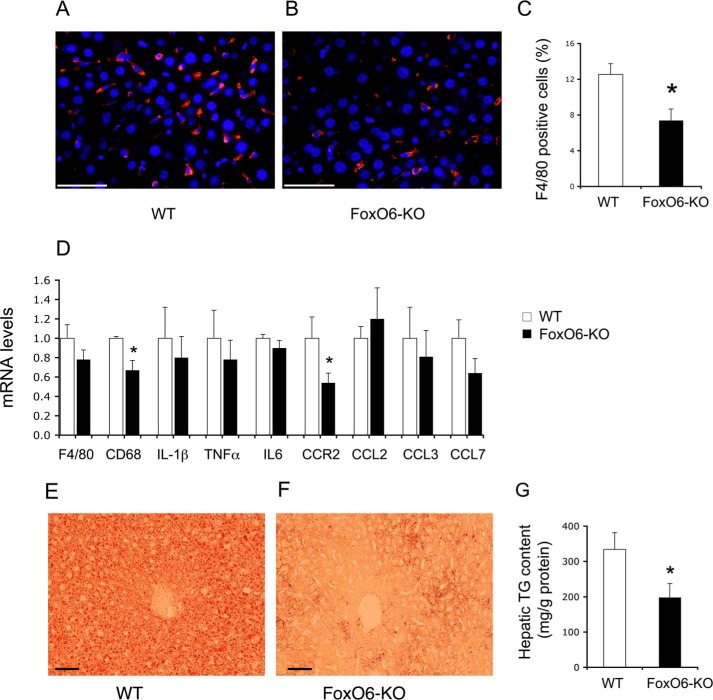
To deepen our understanding of the mechanism by which FoxO6 depletion enhances insulin sensitivity and improves glucose metabolism, we performed anti-F4/80 immunohistochemistry to determine hepatic infiltration by Kupffer cells, known as resident macrophages in the liver (45). Increased Kupffer cell infiltration into the liver is associated with insulin resistance in obesity and type 2 diabetes (46, 47). We showed that FoxO6 depletion resulted in a significant reduction in Kupffer cell content in the liver of high fat-fed FoxO6-KO mice (n = 8–9) (Fig. 6, A–C). This effect correlated with a significant decrease in hepatic expression of the C-C chemokine receptor 2 (CCR2), a factor that plays a key role in regulating macrophage cell recruitment in peripheral tissues (Fig. 6D). Consistent with reduced Kupffer cell content in the liver, FoxO6-KO mice also exhibited lower hepatic expression of F4/80 and CD68, two genetic markers of macrophages (Fig. 6D).
FIGURE 6.
Effect of FoxO6 depletion on Kupffer cell content in the liver. Liver tissues were obtained from high fat-fed WT control (A) and FoxO6-KO mice (B) as described in Fig. 5. Frozen sections of liver tissues were subjected to anti-F4/80 immunohistochemistry. F4/80 positive cells (stained red) were counted and normalized to the total number of cells (with nuclei stained blue by DAPI) in liver sections (C). Aliquots of liver tissues (20 mg) were used for the preparation of total RNA, which was subjected to real-time qRT-PCR using 18S RNA as the control for determining F4/80, CD68, IL-1β, TNFα, IL6, CCR2, CCL2, CCL3, and CCL7 mRNA levels (D). Frozen sections (6 μm) of liver tissues from high fat-fed WT (E) and FoxO6-KO (F) mice were subjected to oil red O staining. In addition, aliquots of liver tissues (20 mg) were used for determining hepatic lipid content, which was defined as mg of triglyceride (TG) per gram of liver protein (G). *, p < 0.05 versus WT control. n = 8–9 per group. Bar, 50 μm.
In addition, we studied hepatic expression profiles of proinflammatory cytokines TNFα, IL-6, and IL-1β as well as chemokines CCL2, CCL3, and CCL7 in high fat-fed FoxO6-KO versus WT littermates (n = 8–9/group). FoxO6-KO mice were associated with reduced hepatic TNFα and IL-1β mRNA levels, although the extent of reduction did not reach significant levels in comparison to WT littermates (Fig. 6D). No significant differences were seen in hepatic IL-6, CCL2, CCL3, and CCL7 mRNA levels in FoxO6-KO versus WT littermates on high fat diet (Fig. 6D).
Effect of FoxO6 Depletion on Hepatic Fat Content
To investigate the potential beneficial effect on hepatic lipid metabolism, we subjected cryosections of liver tissues to oil red O staining. FoxO6-KO mice, as opposed to WT littermates (n = 8–9/group), had significantly lower hepatic fat deposition after a 16-week high fat feeding (Fig. 6, E and F). To confirm these findings, we quantified hepatic fat content. WT mice on high fat diet developed steatosis with hepatic lipid content of 334 ± 48 mg/g of liver protein (Fig. 6G). This effect was significantly ameliorated in high fat-fed FoxO6-KO mice (hepatic lipid content, 197 ± 40 mg/g of liver protein, p < 0.05 versus WT), consistent with the reduction of Kupffer cell infiltration in the liver and improvement of glucose metabolism in FoxO6-KO mice.
Effect of FoxO6 Depletion on Macrophage Infiltration in Adipose Tissues
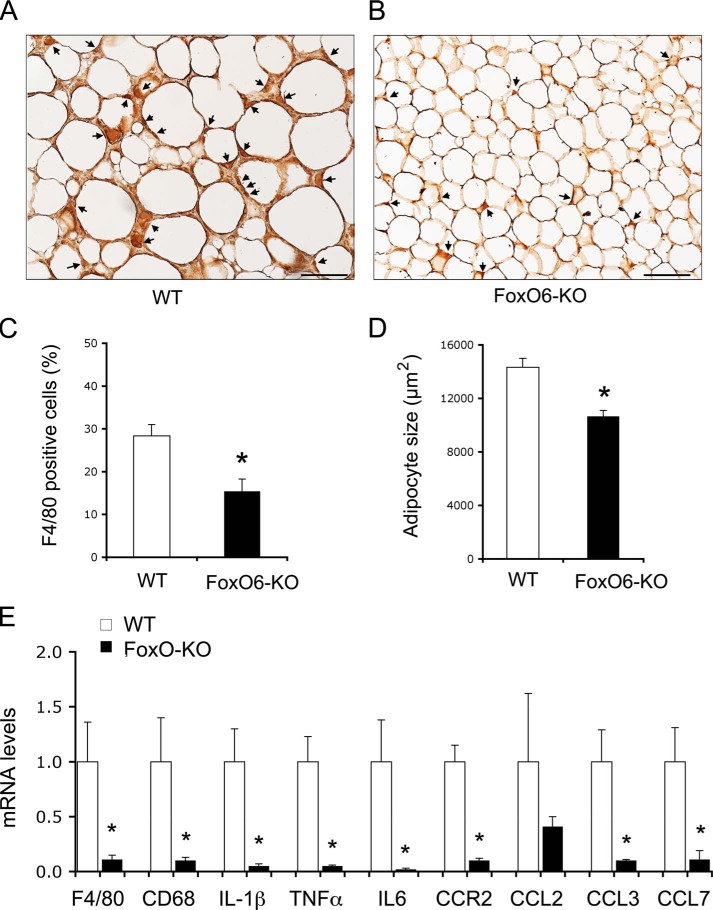
To corroborate the above findings, we performed anti-F4/80 immunohistochemistry to determine macrophage infiltration in adipose tissues. FoxO6-KO mice exhibited significantly lower macrophage content in adipose tissues when compared with WT littermates on a high fat diet (Fig. 7, A–C). FoxO6-KO mice had significantly smaller adipocytes in adipose tissues (Fig. 7D), coinciding with the improvement in glucose metabolism and whole-body insulin sensitivity. To correlate macrophage infiltration with CCR2 expression in adipose tissues, we procured epididymal adipose tissues for the preparation of stromal vascular cells, which were subjected to real-time qRT-PCR. Significantly lower levels of CCR2 mRNA along with decreased F4/80 and CD68 mRNA levels were detected in stromal vascular cells from adipose tissues of FoxO6-KO mice (Fig. 7E). Likewise, we detected a marked reduction in stromal vascular cell expression of cytokines TNFα, IL-6, and IL-1β and chemokines CCL2, CCL3, and CCL7 in FoxO6-KO versus WT littermates on high fat diet (Fig. 7E).
FIGURE 7.
Effect of FoxO6 depletion on macrophage infiltration in adipose tissues. Adipose tissues were procured from high fat-fed WT control (A) and FoxO6-KO mice (B) as described in Fig. 5. Paraffin-embedded adipose tissues were subjected to anti-F4/80 immunohistochemistry. F4/80-positive cells (stained brown) were scored and normalized to the total number of adipose cells (C). In addition, the size of adipose cells was determined and compared between control and FoxO6-KO groups (D). Epididymal adipose tissues were minced for the preparation of stromal vascular cells. Total RNA was isolated from stromal vascular cells and subjected to real-time qRT-PCR using 18S RNA as control for determining F4/80, CD68, IL-1β, TNFα, IL-6, CCR2, CCL2, CCL3, and CCL7 mRNA levels (E). *, p < 0.05 versus WT control. n = 8–9 per group. Bar, 100 μm.
Impact of FoxO6 Depletion on Systemic Inflammation
To determine the effect of FoxO6 depletion on systemic inflammation, we determined plasma TNFα, IL-1β, and IL-6 levels. No significant differences were detectable in plasma levels of proinflammatory cytokines TNFα and IL-6 between FoxO6-KO and WT littermates (n = 8–9/group) on a high fat diet (Table 1). Plasma IL-1β levels in FoxO6-KO and WT littermates were below the detection limit of IL-1β ELISA (2 pg/ml). We also measured chemokine CCL2 levels by ELISA. This assay did not reveal any differences in plasma CCL2 levels in FoxO6-KO versus WT littermates (Table 1). FoxO6 depletion did not seem to impact systemic inflammation in FoxO6-KO mice.
Discussion
In this study we determined the effect of FoxO6 depletion on hepatic gluconeogenesis and blood glucose metabolism in mice. We showed that FoxO6 depletion suppressed hepatic gluconeogenesis, contributing to a significant improvement of postprandial and fasting blood glucose profiles. We recapitulated these findings in FoxO6-KO mice on both regular chow and high fat diet. Moreover, we showed that FoxO6-deficient primary hepatocytes were associated with significantly reduced glucose production in response to glucagon (via cAMP) stimulation. As the control, we determined hepatic FoxO1 expression in FoxO6-KO mice, demonstrating that FoxO6 depletion did not result in compensatory changes in hepatic FoxO1 expression. These data suggest that FoxO6 plays an independent role in regulating hepatic gluconeogenesis. Consistent with this interpretation, FoxO6 gain-of-function is shown to augment hepatic gluconeogenesis and raise fasting blood glucose levels in FoxO6 transgenic mice independently of FoxO1 (37).
Excessive glucose production in the liver is a chief contributing factor for fasting hyperglycemia in obesity and type 2 diabetes. This effect stems from impaired abilities of insulin to suppress hepatic gluconeogenesis. Nevertheless, factors that effectively couple impaired insulin action to unchecked hepatic gluconeogenesis remain incompletely characterized. Our present studies showed that FoxO6 depletion was able to curb hepatic glucose production and prevent the development of glucose intolerance in high fat-fed FoxO6-KO mice. Notably, hepatic FoxO6 expression is up-regulated, coinciding with the onset of fasting hyperglycemia and glucose intolerance in mice with dietary obesity or genetic type 2 diabetes (37). Selective FoxO6 knockdown in insulin-resistant liver is able to suppress hepatic gluconeogenesis and ameliorate fasting hyperglycemia in diabetic db/db mice (37). Our present data along with previous results underscore the importance of FoxO6 in the pathogenesis of fasting hyperglycemia. It follows that FoxO6 deregulation serves as a knot that links aberrant insulin action to unrestrained hepatic gluconeogenesis in obesity and type 2 diabetes.
Although FoxO6 is akin to FoxO1, FoxO6 differs fundamentally from FoxO1 (48). FoxO6 and FoxO1 share a high degree of dissimilarity (>70%) in amino acid sequence homology. FoxO6 contains two consensus AKT/PKB phosphorylation sites at Thr-26 and Ser-184 within its amino DNA binding domain, whereas there are three AKT/PKB phosphorylation sites Thr-24, Ser-256, and Ser-319 in FoxO1 amino domain. FoxO6 lacks the nuclear export signal, a characteristic motif that is present in FoxO1 carboxyl domain (16). FoxO6 mediates insulin action on target gene expression without altering its nuclear localization. This is different from FoxO1, which undergoes insulin-dependent nuclear export (37). Our present studies revealed another fundamental difference: FoxO6-null mice are viable, whereas FoxO1-null mice die at embryonic day 11.5 due to defective angiogenesis in the embryos (40). These results support the notion that FoxO6 is evolutionally divergent from FoxO1 (48).
Despite becoming obese in response to high fat feeding, FoxO6-KO mice exhibited significantly enhanced insulin sensitivity when compared with obese control littermates. This was derived in part from the mitigation of fasting hyperglycemia and hyperinsulinemia, due to attenuated hepatic gluconeogenesis in FoxO6-deficient liver. FoxO6-KO mice, as opposed to WT littermates, had significantly smaller adipocytes in adipose tissues, a phenotype that is reminiscent of adiponectin-transgenic ob/ob mice. Despite increased adiposity, ob/ob mice with transgenic adiponectin overproduction have smaller adipocytes in adipose tissues, correlating with improved insulin sensitivity (49). Furthermore, we demonstrated that high fat-fed FoxO6-KO mice were associated with relatively increased energy expenditure when compared with WT littermates. This also contributed to the improvement of insulin sensitivity in high fat-fed FoxO6-KO mice.
Increased macrophage infiltration into peripheral tissues such as the liver and adipose tissues is linked to insulin resistance and steatosis in obesity and type 2 diabetes (46, 47). Indeed, Huang et al. (50) showed that depletion of Kupffer cells from the liver prevents the development of diet-induced insulin resistance and glucose intolerance in rats. Recent studies by Obstfeld et al. (51) implicate CCR2 as a key regulator of macrophage recruitment into peripheral tissues. CCR2-deficient mice are associated with improved whole-body insulin sensitivity and glucose homeostasis (52). In this study we showed that FoxO6 depletion attenuated macrophage infiltration into the liver and adipose tissues, in accordance with the improvement of glucose metabolism and insulin sensitivity in high fat-fed FoxO6-KO mice. FoxO6 depletion also mitigated fat-induced hepatic steatosis in FoxO1-KO mice. These results underscore the importance of FoxO6 in regulating macrophage infiltration into peripheral tissues. Consistent with this interpretation, we showed that macrophage expression of CCR2 was significantly decreased in the liver and adipose tissues in FoxO6-KO mice.
Although macrophage expression of TNFα, IL-1β, IL-6, and CCL2 mRNAs was down-regulated in adipose tissues, this effect did not result in a corresponding reduction in plasma levels of inflammatory cytokines TNFα, IL-1β, and IL-6 as well as chemokine CCL2 in FoxO6-KO mice. It is plausible that a reduction in macrophage expression of TNFα, IL-1β, IL-6, and CCL2 in adipose tissues was compensated by their expression in other peripheral tissues such as the liver and lung. Apart from their expression in macrophages, TNFα, IL-1β, IL-6, and CCL2 are produced in fibroblasts and endothelial cells, contributing to their circulating levels (53). We also acknowledge the limitation of FoxO6-KO mice with global FoxO6 depletion in our studies. Further studies are warranted to interrogate the role of FoxO6 in macrophage expression of cytokines and chemokines and its impact on macrophage recruitment into peripheral tissues in obesity and diabetes.
We noted that FoxO6-KO mice had a small, but significant, reduction in food intake. This effect translated into a slight reduction (5–10%) in weight gain in FoxO6-KO versus WT littermates between weeks 16 and 22 on a high fat diet. To remove the potential impact of the confounding factor associated with variable food intake and weight gain on hepatic gluconeogenesis, we isolated primary hepatocytes from FoxO6-KO and WT littermates and determined the effect of FoxO6 depletion on gluconeogenesis ex vivo. We demonstrated that FoxO6-depleted hepatocytes were associated with reduced capacities to undergo gluconeogenesis in response to glucagon. Nonetheless, we could not preclude the possibility that the observed reduction in food intake and weight gain also contributed to attenuated hepatic gluconeogenesis in vivo. Pair-feeding studies are warranted to determine whether less weight gain is a contributing factor for improved glucose metabolism and insulin sensitivity in FoxO6-KO mice.
In conclusion, we showed that FoxO6 plays an independent role in orchestrating insulin-dependent regulation of gluconeogenesis. FoxO6 depletion attenuated hepatic gluconeogenesis and lowered fasting blood glucose levels in mice. Furthermore, FoxO6-deficient liver and adipose tissues were associated with significantly reduced macrophage infiltration. These effects helped ameliorate fasting hyperglycemia and hyperinsulinemia, protecting FoxO6-KO mice from developing insulin resistance and glucose intolerance in response to high fat feeding. Our data provide in vivo evidence that FoxO6 inhibition is beneficial for curbing excessive hepatic glucose production and improving glycemic control in obesity and type 2 diabetes.
Acknowledgment
We thank Dr. Kevin Kelley (Mount Sinai Medical Center, New York) for providing assistance in generating FoxO6-KO mice.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 DK087764. The authors declare that they have no conflicts of interest with the contents of this article.
- FoxO1
- Forkhead box O1
- FoxO6
- Forkhead box O6
- PEPCK
- phosphoenolpyruvate carboxykinase
- PKB
- protein kinase B
- G6P
- glucose 6-phosphate
- G6Pase
- glucose-6-phosphatase
- CD68
- Cluster of Differentiation 68
- CCR2
- C-C chemokine receptor 2
- CCL2
- chemokine C-C motif ligand 2
- CCL3
- chemokine C-C motif ligand 3
- CCL7
- chemokine C-C motif ligand 7
- HOMA-IR
- homeostasis model for insulin resistance
- AUC
- area under the curve
- qRT
- quantitative real-time
- 8-cpt-cAMP
- 8-(4-chlorophenylthio)-adenosine-3′,5′-cyclic monophosphate.
References
- 1. Edgerton D. S., Johnson K. M., Cherrington A. D. (2009) Current strategies for the inhibition of hepatic glucose production in type 2 diabetes. Front. Biosci. 14, 1169–1181 [DOI] [PubMed] [Google Scholar]
- 2. Wahren J., Ekberg K. (2007) Splanchnic regulation of glucose production. Annu. Rev. Nutr. 27, 329–345 [DOI] [PubMed] [Google Scholar]
- 3. Ekberg K., Landau B. R., Wajngot A., Chandramouli V., Efendic S., Brunengraber H., Wahren J. (1999) Contributions by kidney and liver to glucose production in the postabsorptive state and after 60 h of fasting. Diabetes 48, 292–298 [DOI] [PubMed] [Google Scholar]
- 4. Tonelli J., Kishore P., Lee D. E., Hawkins M. (2005) The regulation of glucose effectiveness: how glucose modulates its own production. Curr. Opin. Clin. Nutr. Metab. Care 8, 450–456 [DOI] [PubMed] [Google Scholar]
- 5. Rossetti L., Massillon D., Barzilai N., Vuguin P., Chen W., Hawkins M., Wu J., Wang J. (1997) Short term effects of leptin on hepatic gluconeogenesis and in vivo insulin action. J. Biol. Chem. 272, 27758–27763 [DOI] [PubMed] [Google Scholar]
- 6. Massillon D., Chen W., Hawkins M., Liu R., Barzilai N., Rossetti L. (1995) Quantitation of hepatic glucose fluxes and pathways of hepatic glycogen synthesis in conscious mice. Am. J. Physiol. 269, E1037–E1043 [DOI] [PubMed] [Google Scholar]
- 7. Pilkis S. J., Granner D. K. (1992) Molecular physiology of the regulation of hepatic gluconeogenesis and glycogenolysis. Annu. Rev. Physiol. 54, 885–909 [DOI] [PubMed] [Google Scholar]
- 8. Yoon J. C., Puigserver P., Chen G., Donovan J., Wu Z., Rhee J., Adelmant G., Stafford J., Kahn C. R., Granner D. K., Newgard C. B., Spiegelman B. M. (2001) Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 413, 131–138 [DOI] [PubMed] [Google Scholar]
- 9. Puigserver P., Rhee J., Donovan J., Walkey C. J., Yoon J. C., Oriente F., Kitamura Y., Altomonte J., Dong H., Accili D., Spiegelman B. M. (2003) Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1α interaction. Nature 423, 550–555 [DOI] [PubMed] [Google Scholar]
- 10. Fisher S. J., Kahn C. R. (2003) Insulin signaling is required for insulin's direct and indirect action on hepatic glucose production. J. Clin. Invest. 111, 463–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Edgerton D. S., Ramnanan C. J., Grueter C. A., Johnson K. M., Lautz M., Neal D. W., Williams P. E., Cherrington A. D. (2009) Effects of insulin on the metabolic control of hepatic gluconeogenesis in vivo. Diabetes 58, 2766–2775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koo S. H., Flechner L., Qi L., Zhang X., Screaton R. A., Jeffries S., Hedrick S., Xu W., Boussouar F., Brindle P., Takemori H., Montminy M. (2005) The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature 437, 1109–1111 [DOI] [PubMed] [Google Scholar]
- 13. Liu Y., Dentin R., Chen D., Hedrick S., Ravnskjaer K., Schenk S., Milne J., Meyers D. J., Cole P., Yates J., 3rd, Olefsky J., Guarente L., Montminy M. (2008) A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature 456, 269–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jiang G., Zhang B. B. (2003) Glucagon and regulation of glucose metabolism. Am. J. Physiol. Endocrinol. Metab. 284, E671–E678 [DOI] [PubMed] [Google Scholar]
- 15. Zhang L., Rubins N. E., Ahima R. S., Greenbaum L. E., Kaestner K. H. (2005) Foxa2 integrates the transcriptional response of the hepatocyte to fasting. Cell Metab. 2, 141–148 [DOI] [PubMed] [Google Scholar]
- 16. Accili D., Arden K. C. (2004) FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 117, 421–426 [DOI] [PubMed] [Google Scholar]
- 17. Barthel A., Schmoll D., Unterman T. G. (2005) FoxO proteins in insulin action and metabolism. Trends Endocrinol. Metab. 16, 183–189 [DOI] [PubMed] [Google Scholar]
- 18. Kamagate A., Dong H. H. (2008) FoxO1 integrates insulin signaling to VLDL production. Cell Cycle 7, 3162–3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dong X. C., Copps K. D., Guo S., Li Y., Kollipara R., DePinho R. A., White M. F. (2008) Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell Metab. 8, 65–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matsumoto M., Pocai A., Rossetti L., Depinho R. A., Accili D. (2007) Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor foxo1 in liver. Cell Metab. 6, 208–216 [DOI] [PubMed] [Google Scholar]
- 21. Altomonte J., Richter A., Harbaran S., Suriawinata J., Nakae J., Thung S. N., Meseck M., Accili D., Dong H. (2003) Inhibition of Foxo1 function is: associated with improved fasting glycemia in diabetic mice. Am. J. Physiol. Endocrinol. Metab. 285, E718–E728 [DOI] [PubMed] [Google Scholar]
- 22. Qu S., Altomonte J., Perdomo G., He J., Fan Y., Kamagate A., Meseck M., Dong H. H. (2006) Aberrant Forkhead box O1 function is associated with impaired hepatic metabolism. Endocrinology 147, 5641–5652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ayala J. E., Streeper R. S., Desgrosellier J. S., Durham S. K., Suwanichkul A., Svitek C. A., Goldman J. K., Barr F. G., Powell D. R., O'Brien R. M. (1999) Conservation of an insulin response unit between mouse and human glucose-6-phosphatase catalytic subunit gene promoters. Diabetes 48, 1885–1889 [DOI] [PubMed] [Google Scholar]
- 24. Streeper R. S., Svitek C. A., Chapman S., Greenbaum L. E., Taub R., O'Brien R. M. (1997) A multicomponent insulin response sequence mediates a strong repression of mouse glucose-6-phosphatase gene transcription by insulin. J. Biol. Chem. 272, 11698–11701 [DOI] [PubMed] [Google Scholar]
- 25. Vander Kooi B. T., Streeper R. S., Svitek C. A., Oeser J. K., Powell D. R., O'Brien R. M. (2003) The three insulin response sequences in the glucose-6-phosphatase catalytic subunit gene promoter are functionally distinct. J. Biol. Chem. 278, 11782–11793 [DOI] [PubMed] [Google Scholar]
- 26. Scott D. K., O'Doherty R. M., Stafford J. M., Newgard C. B., Granner D. K. (1998) The repression of hormone-activated PEPCK gene expression by glucose is insulin-independent but requires glucose metabolism. J. Biol. Chem. 273, 24145–24151 [DOI] [PubMed] [Google Scholar]
- 27. Barthel A., Schmoll D. (2003) Novel concepts in insulin regulation of hepatic gluconeogenesis. Am. J. Physiol. Endocrinol. Metab. 285, E685–E692 [DOI] [PubMed] [Google Scholar]
- 28. Biggs W. H., 3rd, Meisenhelder J., Hunter T., Cavenee W. K., Arden K. C. (1999) Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc. Natl. Acad. Sci. U.S.A. 96, 7421–7426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nakae J., Park B.-C., Accili D. (1999) Insulin stimulates phosphorylation of the forkhead transcription factor FKHR on serine 253 through a wortmannin-sensitive pathway. J. Biol. Chem. 274, 15982–15985 [DOI] [PubMed] [Google Scholar]
- 30. Nakae J., Barr V., Accili D. (2000) Differential regulation of gene expression by insulin and IGF-1 receptors correlates with phosphorylation of a single amino acid residue in the forkhead transcription factor FKHR. EMBO J. 19, 989–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Durham S. K., Suwanichkul A., Scheimann A. O., Yee D., Jackson J. G., Barr F. G., Powell D. R. (1999) FKHR binds the insulin response element in the insulin-like growth factor binding protein-1 promoter. Endocrinology 140, 3140–3146 [DOI] [PubMed] [Google Scholar]
- 32. Schmoll D., Walker K. S., Alessi D. R., Grempler R., Burchell A., Guo S., Walther R., Unterman T. G. (2000) Regulation of glucose-6-phosphatase gene expression by protein kinase Bα and the forkhead transcription factor FKHR. J. Biol. Chem. 275, 36324–36333 [DOI] [PubMed] [Google Scholar]
- 33. Lu M., Wan M., Leavens K. F., Chu Q., Monks B. R., Fernandez S., Ahima R. S., Ueki K., Kahn C. R., Birnbaum M. J. (2012) Insulin regulates liver metabolism in vivo in the absence of hepatic Akt and Foxo1. Nat. Med. 18, 388–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Haeusler R. A., Hartil K., Vaitheesvaran B., Arrieta-Cruz I., Knight C. M., Cook J. R., Kammoun H. L., Febbraio M. A., Gutierrez-Juarez R., Kurland I. J., Accili D. (2014) Integrated control of hepatic lipogenesis versus glucose production requires FoxO transcription factors. Nat. Commun. 5, 5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kamagate A., Qu S., Perdomo G., Su D., Kim D. H., Slusher S., Meseck M., Dong H. H. (2008) FoxO1 mediates insulin-dependent regulation of hepatic VLDL production in mice. J. Clin. Invest. 118, 2347–2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rodgers J. T., Haas W., Gygi S. P., Puigserver P. (2010) Cdc2-like kinase 2 is an insulin-regulated suppressor of hepatic gluconeogenesis. Cell Metab. 11, 23–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim D. H., Perdomo G., Zhang T., Slusher S., Lee S., Phillips B. E., Fan Y., Giannoukakis N., Gramignoli R., Strom S., Ringquist S., Dong H. H. (2011) FoxO6 integrates insulin signaling with gluconeogenesis in the liver. Diabetes 60, 2763–2774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang X., Gan L., Pan H., Guo S., He X., Olson S. T., Mesecar A., Adam S., Unterman T. G. (2002) Phosphorylation of serine 256 suppresses transactivation by FKHR (FOXO1) by multiple mechanisms. Direct and indirect effects on nuclear/cytoplasmic shuttling and DNA binding. J. Biol. Chem. 277, 45276–45284 [DOI] [PubMed] [Google Scholar]
- 39. Zhao X., Gan L., Pan H., Kan D., Majeski M., Adam S. A., Unterman T. G. (2004) Multiple elements regulate nuclear/cytoplasmic shuttling of FOXO1: characterization of phosphorylation- and 14-3-3-dependent and -independent mechanisms. Biochem. J. 378, 839–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Furuyama T., Kitayama K., Shimoda Y., Ogawa M., Sone K., Yoshida-Araki K., Hisatsune H., Nishikawa S., Nakayama K., Nakayama K., Ikeda K., Motoyama N., Mori N. (2004) Abnormal angiogenesis in Foxo1 (Fkhr)-deficient mice. J. Biol. Chem. 279, 34741–34749 [DOI] [PubMed] [Google Scholar]
- 41. Foretz M., Hébrard S., Leclerc J., Zarrinpashneh E., Soty M., Mithieux G., Sakamoto K., Andreelli F., Viollet B. (2010) Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J. Clin. Invest. 120, 2355–2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee P., Peng H., Gelbart T., Beutler E. (2004) The IL-6- and lipopolysaccharide-induced transcription of hepcidin in HFE-, transferrin receptor 2-, and β2-microglobulin-deficient hepatocytes. Proc. Natl. Acad. Sci. U.S.A. 101, 9263–9265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kamagate A., Kim D. H., Zhang T., Slusher S., Gramignoli R., Strom S. C., Bertera S., Ringquist S., Dong H. H. (2010) FoxO1 links hepatic insulin action to endoplasmic reticulum stress. Endocrinology 151, 3521–3535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nishimura S., Manabe I., Nagasaki M., Eto K., Yamashita H., Ohsugi M., Otsu M., Hara K., Ueki K., Sugiura S., Yoshimura K., Kadowaki T., Nagai R. (2009) CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 15, 914–920 [DOI] [PubMed] [Google Scholar]
- 45. Baffy G. (2009) Kupffer cells in non-alcoholic fatty liver disease: the emerging view. J. Hepatol. 51, 212–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Weisberg S. P., McCann D., Desai M., Rosenbaum M., Leibel R. L., Ferrante A. W., Jr. (2003) Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lumeng C. N., Bodzin J. L., Saltiel A. R. (2007) Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kim D. H., Zhang T., Lee S., Dong H. H. (2013) FoxO6 in Glucose Metabolism. J. Diabetes 5, 233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kim J. Y., van de Wall E., Laplante M., Azzara A., Trujillo M. E., Hofmann S. M., Schraw T., Durand J. L., Li H., Li G., Jelicks L. A., Mehler M. F., Hui D. Y., Deshaies Y., Shulman G. I., Schwartz G. J., Scherer P. E. (2007) Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Invest. 117, 2621–2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Huang W., Metlakunta A., Dedousis N., Zhang P., Sipula I., Dube J. J., Scott D. K., O'Doherty R. M. (2010) Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes 59, 347–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Obstfeld A. E., Sugaru E., Thearle M., Francisco A. M., Gayet C., Ginsberg H. N., Ables E. V., Ferrante A. W., Jr. (2010) C-C chemokine receptor 2 (CCR2) regulates the hepatic recruitment of myeloid cells that promote obesity-induced hepatic steatosis. Diabetes 59, 916–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Weisberg S. P., Hunter D., Huber R., Lemieux J., Slaymaker S., Vaddi K., Charo I., Leibel R. L., Ferrante A. W., Jr. (2006) CCR2 modulates inflammatory and metabolic effects of high fat feeding. J. Clin. Invest. 116, 115–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Marra F., Tacke F. (2014) Roles for chemokines in liver disease. Gastroenterology 147, 577–594 [DOI] [PubMed] [Google Scholar]