Background: Immunological memory is maintained by antigen-independent tonic stimulation.
Results: Stress-treated dendritic cells stimulate IL-15- and IL-1β-mediated pathways, required for optimum memory T cell induction.
Conclusion: Stress agents may be responsible for the persistence of immunological memory, involving an epigenetic mechanism.
Significance: Repetitive stress may account for tonic stimulation, which maintains immunological memory.
Keywords: immunology, inflammasome, stress, T helper cells, transcription factor
Abstract
The prevailing evidence suggests that immunological memory does not require antigenic re-stimulation but is maintained by low level tonic stimulation. We examined the hypothesis that stress agents contribute to tonic cellular activation and maintain immunological memory. Stimulation of monocyte-derived dendritic cells (DC) with stress agents elicits reactive oxygen species and HSP70. NFκB is activated, which up-regulates membrane-associated (ma) IL-15, caspase-1 and IL-1β. Co-culture of stress-treated DC with mononuclear cells activates IL-15 and IL-1β receptors on CD4+ T cells, eliciting CD40L, proliferation, and up-regulation of CD45RO+ memory T cells. The transcription factors Tbethigh and RORγt are up-regulated, whereas FoxP3 is down-regulated, resulting in enhanced Th1 and Th17 expression and the corresponding cytokines. The interaction between maIL-15 expressed by DC and IL-15R on CD4+ T cells results in one pathway and the corresponding cells expressing IL-1β and IL1βR as a second pathway. Importantly, inhibition studies with IL-15 antibodies and IL-1βR inhibitor suggest that both pathways may be required for optimum CD4+ CD45RO+ memory T cell expression. Type 1 IFN expression in splenic CD11c DC of stress-treated mice demonstrated a significant increase of IFN-α in CD11c CD317+ and CD8α+ DC. Analysis of RNA in human CD4+ memory T cells showed up-regulation of type 1 IFN-stimulated genes and inhibition with histone methyltransferase inhibitor. We suggest the paradigm that stress-induced tonic stimulation might be responsible for the robust persistence of the immune response in vaccination and that epigenetic changes are involved in maintaining CD4+ T cell memory.
Introduction
Immunological memory is an essential feature of an effective vaccine. There is compelling evidence that memory T cells do not require antigen-stimulated MHC-TCR interaction to survive (1, 2). The robust smallpox vaccine efficacy is the best example of life-long memory without further antigenic stimulation (3). However, the mechanism of maintenance of immunological memory has not been elucidated, despite its immense significance in the field of preventive immunization. It has been argued that maintenance of immunological T cell memory may involve low-level antigen-independent T cell homeostatic division in lymphoid tissues (4–6). This maintains a stable CD4+ memory T cell population as demonstrated with smallpox vaccination. We have examined the hypothesis that repetitive TCR-independent activation by cellular stress may account for the low level signaling maintaining immunological memory and that epigenetic changes might be involved.
In humans thermal or oxidative stress studies in vitro (7) and in vivo experiments in BALB/c mice (8) demonstrated that stress agents activating antigen-TCR independent interaction between DC3 and CD4+ T cells, may be responsible for maintaining the homeostatic CD4+ T cell memory. HSP70 expression is the hallmark of a stress response, which functions as an endogenous danger signal to the immune system (9, 10). Oxidative stress is induced by free radicals of ROS (reactive oxygen species), which may stimulate DC to release glutathione, which is broken down to cysteine and enables T cells to maintain redox homeostasis (11). ROS affects the innate immune system through attraction of polymorphonuclear leukocytes, monocytes and macrophages, which control microorganisms, and Toll-like receptors may be involved (12, 13). ROS also functions in adaptive immunity regulating T cell proliferation (14), activating NFκB (15). Furthermore, mitochondrial ROS may activate the inflammasome pathway (16). The biological and biochemical complexities of oxidative stress and their functions have been extensively reviewed recently (17).
Human DC-CD4+-T cell interactions can be stimulated by heat, oxidative stress agents, gramicidin (K releasing agent), or dithiocarbamate. a metal ionophore, leading to NFκB membrane-associated (ma)IL-15 expression (7, 8). The latter ligates the IL-15R complex on CD4+ T cells and induces CD40L expression, T-cell proliferation, and IFN-γ production (7). Intracellular stress sensors activate inflammasomes and the production of IL-1β (18, 19). Metabolic stress is also associated with NLRP3 inflammasomes in type 2 diabetes (20) but can be independent of inflammasomes (21). Intracellular sensors involve NLR (nucleotide-binding oligomerization domain-like receptors), which sense endogenous danger signals (22), released from damaged or necrotic cells, such as HSP70, termed damage-associated molecular patterns.
The aim of this paper was to study the effect of a number of diverse stress agents on the interaction between maIL-15 expressed by DC and IL-15R on CD4+ T cells in one pathway and the corresponding cells expressing IL-1β and IL-1βR in a parallel pathway. The results suggest that stress activates the two pathways and both are required for optimum CD4+CD45RO+ memory T cells. Furthermore, we studied type 1 IFN expression in 3 subsets of splenic DC from BALB/c mice treated with the same stress agents and alum, which demonstrated a significant increase of IFN-α in the CD317+ DC and to a lesser extent in the CD11/c CD8+ DC. The transcription factors RORγt and Tbet IFN-γ were significantly up-regulated in memory T cells, eliciting Th17 and Th1 cells, respectively, and the corresponding cytokines, whereas FoxP3 expression was down-regulated. Gene expression of CD4+ CD45RO+ memory T cells was studied using Illumina and validated by Affymetrix-unbiased microarray analysis. The data demonstrate transcriptional up-regulation of type 1 IFN-stimulated genes (ISG), which may be critical both for modulating immune responses and for control of viral infections.
Experimental Procedures
Stress Agents Used to Stimulate Monocyte-derived Dendritic Cells
Three different agents were used to induce stress in DC. Sodium arsenite was selected as it is an oxidative agent, gramicidin induces K efflux, dithiocarbamate is an ionophore, and alum is the most common clinically used adjuvant, with characteristics of a stress agent (7, 8). The 3 stress agents were all purchased from Sigma, and alum from Serva Electrophoresis GmbH. Human CD14+ monocytes were prepared from PBMC (obtained from the National Blood Service) by either enriching by depletion of CD14− cells using Monocyte Isolation Kit (MACS, Miltenyi Biotec) or positively selected by antibody to CD14 using the “panning” method. The isolated CD14+ monocytes were cultured in GM-CSF (800 units/ml) and IL-4 (20 units/ml) supplemented medium for 4–5 days to differentiate into immature dendritic cells. Immature DC (2 × 105/ml) were incubated with increasing concentrations of stress agents. After 90 min at 37 °C, the cells were washed 3 times and used in comparison with untreated cells.
Assay of ROS
Assay of ROS was carried out using the Image-iT live green ROS detection kit (Molecular Probes, Invitrogen). DC were treated with increasing concentrations of sodium arsenite, gramicidin, dithiocarbamate, or 1 mg/ml of alum for 5–30 min in RPMI culture medium, supplemented with 10% FCS. After incubation, carboxy-2′,7′-dichlorodihydrofluorescein diacetate was added to the cells to a final concentration of 25 μm. Following a further 30-min incubation, the samples were analyzed by flow cytometry.
Real-time PCR for HSP70 mRNA
Aliquots of 1 × 106 untreated and stress-treated DC were incubated overnight, the RNA were isolated with the Total RNA Isolation Kit (Promega, Southampton, UK) and quantified using the spectrophotometer (GeneQuant II, Pharmacia Biotech). cDNA was generated from RNA by using the Reverse Transcription System (Promega), according to the manufacturer's instructions. The primers for human HSP70 and GAPDH were synthesized by Sigma (UK). The relative amount of HSP70 mRNA was quantified by real-time PCR (ABI Prism 5700), using the Platinum SYBR Green qPCR SuperMix-UDG with ROX (Invitrogen, Life Technologies), as described previously. The results were expressed as fold-increase over control RNA.
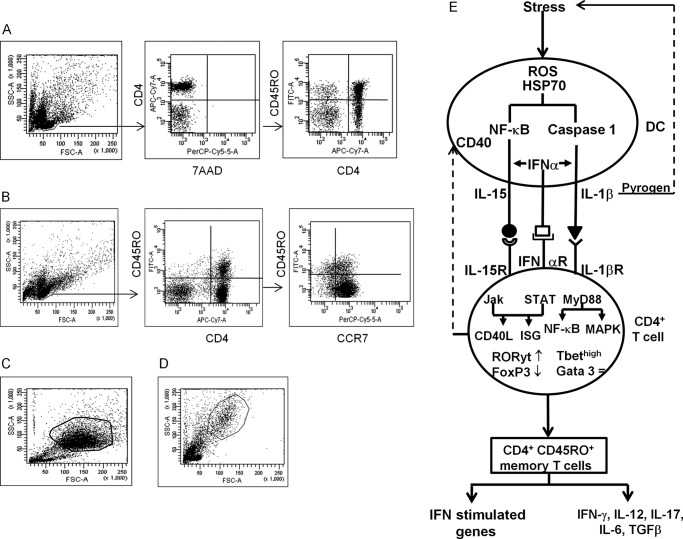
Gating Strategy of Flow Cytometry
Flow cytometry was carried out on BD FacsCantoTM II, using DIVA software and data analysis used WinMDI 2.8 software. The gating strategies used in this study are shown in Fig. 2. Fig. 2A for cell surface molecule staining of the live lymphocyte population was gated and confirmed by 7-aminoactinomycin D negative staining. CD4+ T cells were then gated for cell surface molecules and/or memory cell. Fig. 2B shows intracellular staining of cells for cell surface markers before fixation and permeabilization. The lymphocyte population was then gated, CD4 naive and CD45RO+ memory T cells were identified, and the intracellular proteins were examined within identified CD4+ or CD45RO+ central (CCR7+) or effector (CCR7−) memory T cells. Cultures of DC (Fig. 2C) or DC (Fig. 2D) co-cultured with isolated CD4+ T cells were gated based on FSC and SSC and these populations were examined for either cell surface or intracellular molecules.
FIGURE 2.
Gating strategies used in this study. A, cell surface molecule staining of the live lymphocyte population was gated and confirmed by 7-aminoactinomycin D (7ADD) negative staining. CD4+ T cells were then gated for cell surface molecules and/or CD45RO+ memory T cell. B, for intracellular staining, cells were first stained for cell surface markers, followed by fixation and permeabilization. The lymphocyte population was then gated, CD4 naive and memory T cells were identified, and various intracellular proteins were examined within the identified CD4+, CD45RO+ central (CCR7+), or effector (CCR7−) memory T cells. C, culture of DC, or D, DC co-cultured with isolated CD4+ T cells were gated, based on FSC and SSC and these populations were examined for cell surface or intracellular molecules. E, schema of the dual stress-activated pathways, leading to CD4+CD45RO+ memory T cells, cytokines, and IFN-stimulated genes.
Assay of maIL-15, IL-15Rα, and HSP70 in DC by Flow Cytometry
Cell surface expression of IL-15 or HSP70 on monocyte-derived DC without or with treatment by stress agents were determined by flow cytometry. Cells were incubated with 10 μl (10 μg/ml) of phycoerythrin-conjugated anti-HSP70 or 10 μl (100 μg/ml, Strassgene) of APC-conjugated anti-human IL-15 mAb for 30 min. After washing cells were then analyzed by flow cytometry.
For intracellular HSP70 staining DC were treated with fixation buffer (eBiosciences, London, United Kingdom) and permeabilized with permeabilization buffer (eBiosciences). Intracellular HSP70 was then stained with the anti-HSP70 mAb. In some experiments, the ROS inhibitor NAC (N-acetylcystein) was added to the stress-treated DC to study the effect of ROS on HSP70 or IL-15 production.
Assay of Inflammasome Molecules, Cytokines, and the Receptors
Caspase-1 activation in DC was identified using the FAM-FLICA caspase-1 kit (AbD Serotec). Cultured DC (2 × 104 per well) in 96-well plates were incubated with 20 μl of 1:300 diluted FAM-YVAD-FMK for 1 h at 37 °C. After washing, the cells were analyzed by flow cytometry. For the IL-1β assay, DC (2 × 104 in 100 μl per well) in 96-well plates were treated with various stress agents in the presence of 10 ng/ml of LPS. After 18 h incubation, the supernatants were collected and IL-1β was assayed using human IL-1β ELISA set (BD OptEIATM). The ROS inhibitor NAC was used as above in caspase 1 and IL-1β production.
CD4+ T cells (2 × 105 per well) were co-cultured in U-bottom 96-well plates with 2 × 104 stress-treated DC. After 5 days incubation, culture supernatants were collected and these were used in ELISA kits to detect IL-12p40 (BD OptEIATM), IL-12p70 (BD OptEIATM), IL-6 (Ready-Set-Go, eBiosciences), and TGF-β (Ready-Set-Go, eBiosciences). Expression of cytokine receptors IL-1R, IL15Rα, and IL-6R in CD4+ T cells was also analyzed by flow cytometry following co-culture of stress-treated DC with PBMC.
Co-culture of DC with Autologous Monocyte-depleted PBMC and Analyzed for CD4+ Memory T Cells, CD40L, and Cytokines
Aliquots of 2 × 105 PBMC/well or isolated CD4+ T cells in U-bottom 96-well plates were co-cultured with 2 × 104 stress-treated DC in a total volume of 200 μl/well. After 5 days CD4+ T cells were analyzed for CD45RO+, central CD45RO+CCR7+, and effector CD4+45RO+CCR7− memory markers, using mAb to CD45RO and CCR7 (both from Biolegend). For detection of cell surface CD40L, 5 μl of FITC-conjugated mAb to CD40L or isotype control antibody (BD Biosciences, BD Europe) was added to the culture and incubated for 4 h. For IFN-γ staining, Golgistop (0.7 μg/ml, BD Biosciences) was added 4 h before termination of the co-culture and the cells were fixed for 10 min with 100 μl of fixation buffer and permeabilized with 1 ml of permeabilization buffer. The cells were then washed twice and stained with 5 μl of antibody to phycoerythrin-conjugated anti-IFN-γ for 30 min at room temperature in 45 μl of Perm/Wash solution. The cells were then characterized by flow cytometry. To study the effect of stressed DC on IL-17 production, LPS-treated mature DC were used and treated with the stress agents. In addition, PBMC were stimulated with antibodies to CD3 and CD28 (1 μg/ml each). After 5 days culture, cells were fixed and permeablized and intracellular IL-17 was detected with phycoerythrin-conjugated antibodies to IL-17.
Inhibition Studies of Human CD4+ T Cells
To ascertain whether the homeostasis (IL-15-mediated) or inflammasomes (IL-1β mediated) or both pathways are involved in eliciting CD40L and CD45RO+ memory T cells, co-cultures of DC and PBMC were treated with either 10 μg/ml of anti-IL-15 neutralizing antibodies (R&D Systems) or 50 μm IL-1β receptor I antagonist (IL-1β RI, Merck) or both were used with previously determined optimum doses of inhibitors. After 5 days incubation the cells were examined for CD40L expression by flow cytometry. Similarly, CD45RO+ CD4+ memory T cells and the CCR7+ or CCR7− subsets were assayed by flow cytometry.
Examination of Transcription Factors Tbet, GATA3, RORγt, and FoxP3
DC were stimulated with stress inducing agents and co-cultured with PBMC for 1–5 days. After cell surface staining intracellular expression of T-bet, GATA-3, and RORγt in CD4+ T cells or CD45RO+ memory T cells were stained with the respective fluorochrome-conjugated antibodies to CD3 (1 μg/ml) and CD28 (1 μg/ml). After 5 days co-culture, cells were fixed and permeablized and intracellular Foxp3 was detected with phycoerythrin-conjugated antibodies to Foxp3 (Biolegend, UK).
RNA Microarray Studies
RNA was prepared from CD4CD45RO+ memory T cells by the FACSArial cell sorter to >95% purity, following co-culture for 5 days of PBMC (2 × 106 per ml) with 2 × 105 untreated DC or gramicidin-treated DC (5 μg/ml), without or with 200 μm methyltransferase inhibition (MTA). RNA was isolated using Qiagen Allprep kit. 50 ng of RNA was amplified using the NuGEN Applause 3′-Amp system and labeled with NuGEN Encore BiotinIL module. cDNA was hybridized to Illumina HT12v4 arrays and these were scanned using Illumina's iSCAN platform. Array data were subjected to quantile normalization using Illumina Genome Studio and exported for analysis within Partek Genomics Suite (Partek, St. Louis, MO) by using the gene expression workflow to identify differentially expressed genes. Briefly, robust multichip average pre-processing was performed, and genes differentially expressed were identified using the Partek ANOVAmodel. This data were then validated by the Affymetrix system. RNA was extracted using the Total RNA Isolation Kit (Promega, Southampton, UK) and quantified using NanoDrop 2000c (Thermo Scientific). The RNA was amplified using the MessageAmp Premier RNA Amplification Kit (Ambion), fragmented, then hybridized onto U133 2.0 gene chips (Affymetrix). Chips were scanned (Affymetrix GeneChip Scanner 3000) and checked using the Affymetrix Command Console (AGCC) software suite. These data were statistically analyzed using Qlucore Omics Explorer version 2.3. Gene Ontology analysis was performed using MetaCore (version 2.4, GeneGo Inc.).
The Effect of Treatment of the CD4+CD45RO+ Memory T Cells with the Histone MTA
To study the potential effect of epigenetic changes on T cell activation, MTA was used in the co-culture system. CD4+ T cells (2 × 105 per well) were co-cultured in U-bottom 96-well plates with 2 × 104 stress-treated DC in the presence of 100 or 200 μm MTA. After 5 days culture intracellular expression of IL-17 and IFN-γ were assayed, as described above. In some experiments CD4+CD45RO+ cells were isolated from the co-culture, using the memory cell separation kit (MACS), total RNA was prepared as described above and mRNA for HSP70, IL-17, and IFN-γ assayed by real-time PCR.
Statistical Analysis
The in vitro experiments were repeated 3 or more times and the data are presented as the mean ± S.E. The significance between groups was analyzed by F-ANOVA, followed by paired t test with selected groups, using GraphPad Prism 5 software.
Results
The Effect of Stress Agents on ROS, HSP70 mRNA, Cell Surface, and Intracellular HSP70 in DC
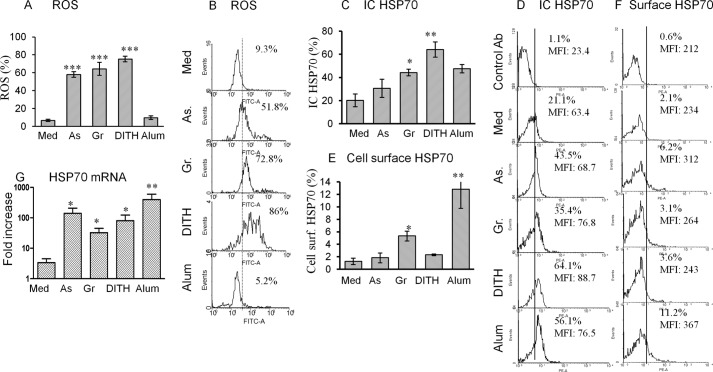
ROS induce oxidative stress that leads to a state of redox equilibrium, which stimulates the PI3K pathway to induce downstream IL-1β. ROS was assayed in human DC before and after treatment with sodium arsenite (50 μm), gramicidin (2 μm/ml), dithiocarbamate (1 μm), and alum (1%). All except alum yielded a very significant increase in ROS production (p < 0.001, Fig. 1A), from a baseline of 9.3% up to 86%; and representative flow cytometry is presented in Fig. 1B.
FIGURE 1.
Stress agents stimulate human monocyte-derived DC to up-regulate ROS, HSP70. The optimum concentration of the stress agents used were sodium arsenite (As) 50 μm, gramicidin (Gr) 2 μm, dithiocarbamate (DITH) 1 μm, or alum 1%, which up-regulated (A) ROS, flow cytometry (B), intracellular HSP70 (C), flow cytometry (D), and cell surface HSP70 (E and F). G, HSP70 mRNA. All experiments were performed with 6 different samples of blood. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Cellular stress elicits intracellular sensors and here we examined inducible HSP70 in human monocyte-derived DC. We first analyzed the proportion (and MFI) of intracellular HSP70, which were significantly up-regulated with all test agents and maximally with dithiocarbamate (Fig. 1, C and D; MFI is not presented). The production of intracellular HSP70 was broadly in line with that of ROS, except for alum, which failed to enhance ROS. However, cell surface HSP70 was significantly up-regulated only with gramicidin and alum (Fig. 1, E and F). All stress agents significantly up-regulated HSP70 mRNA (p < 0.05-<0.01, Fig. 1G). Altogether, ROS was increased with the 3 stress agents in parallel with intracellular and mRNA HSP70 but not cell surface HSP70. Alum, however, showed no effect on ROS, suggesting that a different mechanism is involved in up-regulating HSP70. The gating strategies are shown in Fig. 2, A–D, and for ease of following the DC-CD4 T cell interactions a flow sheet (Fig. 2E).
Stress-activated maIL-15 and Inflammasomes
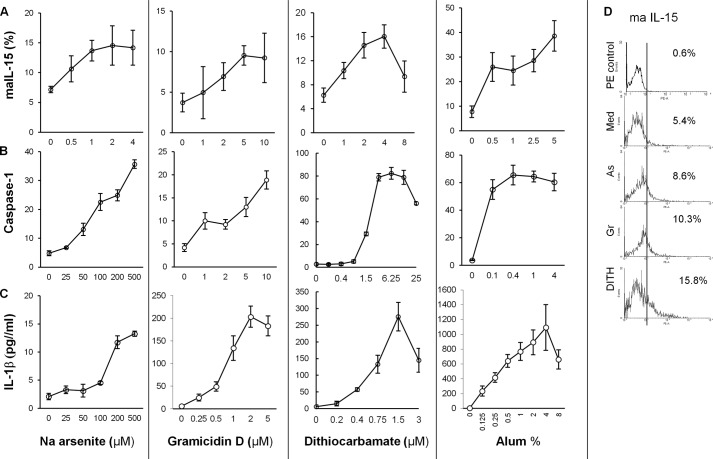
Previous studies suggest that DC exposed to stress agents activate the NFκB pathway, which acts as a transcription factor for maIL-15 molecules (7). Dose-dependent studies with increasing concentrations of sodium arsenite, gramicidin, dithiocarbamate, and alum showed concentration-dependent increases of maIL-15 in DC (Fig. 2, A and D). These were consistent with our previous results using thermal and oxidative stress only (7). IL-15Rα was up-regulated from 10.5 ± 1.1 to 15.7 ± 0.7% (p < 0.01) when treated with gramicidin but not with sodium arsenite (data not presented).
Stress agents stimulate the NLRP3 inflammasomes (18–20) and here we demonstrate that stimulation of DC elicited a dose-dependent increase in caspase-1 activation and IL-1β production with all stress agents and alum (Fig. 3, B and C). This is consistent with the data elicited in vivo in Balb/c mice, when immunized with ovalbumin (8). The dose-dependent effect of alum-treated DC showed an increase in IL-1β, which differed from caspase-1 reaching a plateau with the lowest concentration of alum. Thus, both IL-15 and inflammasomes are activated by the stress agents, but although alum also up-regulates inflammasomes it differs in kinetics.
FIGURE 3.
Dose-dependent increase of the stress agents sodium arsenite, gramicidin, and dithiocarbamate and Alum on maIl-15 expression (A), caspase-1 activation (B), and IL-1β in human monocyte-derived DC (C) (n = 6). D, flow cytometry illustrations of maIL-15 elicited by treatment of the DC with the stress agents. The concentrations of caspase-1 and IL-1β showed similar patterns with the stress agents, but alum differed. As, sodium arsenite; Gr, gramicidin; DITH, dithiocarbamate.
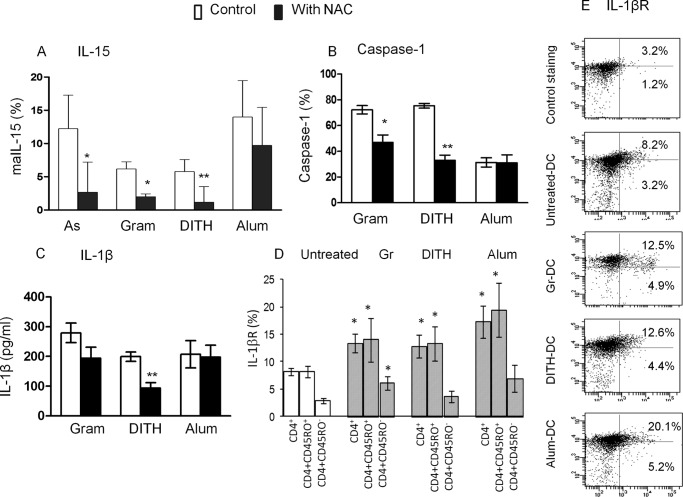
Dependence of IL-15, Caspase-1 and IL-1β on ROS by Inhibition with N-Acetylcystein
To determine whether maIL-15, caspase-1, and IL-1β production are dependent on ROS we used NAC inhibition. This showed a significant inhibition of maIL-15 in cells treated with the 3 stress agents but not with alum, consistent with the expression of maIL-15 being dependent on ROS (p < 0.05–0.01, Fig. 4A). Similar inhibition of caspase-1 and IL-1β expression with NAC was found with gramicidin and dithiocarbamate but not with alum (p < 0.05–0.01, Fig. 4, B and C) or sodium arsenite (not shown). The data suggest that two of the stress agents activating caspase-1 and inducing the IL-1β-mediated pathway are at least partly dependent on ROS. However, although alum stimulates maIL-15 and inflammasomes they are not ROS dependent.
FIGURE 4.
The effect of inhibition by NAC, without NAC (□), with 5 μm NAC (■) on stress-induced (A) maIL-15, (B) caspase-1, and (C) IL1β. Stress-mediated expression of IL-1βR stress up-regulates IL-1βR only in CD45RO+ memory and not in naive CD45RO− CD4 T cells (D and E). As, sodium arsenite; Gr, gramicidin; DITH, dithiocarbamate.
IL-15- and IL-1β-mediated Effect in Co-culture of Stress-treated DC with CD4+ T Cells
DC expressing maIL-15 interact with the IL-15 receptor complex on CD4+ T cells, which are up-regulated following co-culture with stress-treated DC and activate Jak3 and STAT5 phosphorylation to induce CD40L (7). This study extended the data and a showed a significant increase in IL-1β, which bind IL-1βR1 expressed by CD4+ T cells, and these are significantly increased (p < 0.05) (Fig. 4, D and E). This was found only in CD4+CD45 RO+ memory but not CD45RO− naive T cells, treated with gramicidin, dithiocarbamate, or alum (p < 0.05), compared with the untreated cells (Fig. 4D).
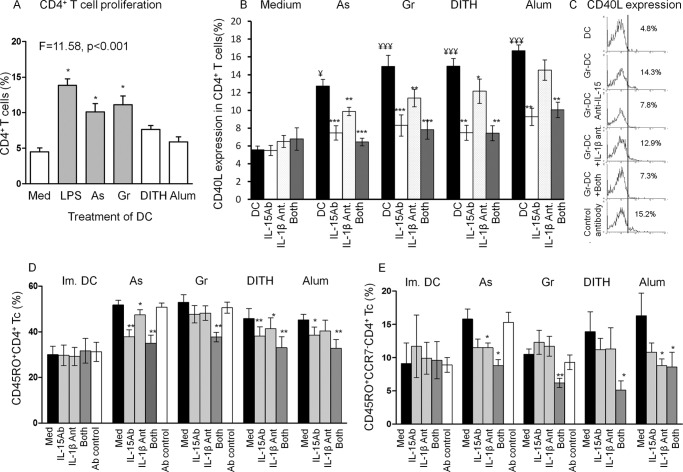
Functional Effects by the Stress Agents on CD4+ T Cell Proliferation and Expression of CD40 L (CD154)
CD4+ T cell proliferation was tested by the carboxyfluorescein succinimidyl ester method. A significant increase in proliferation was found with sodium arsenite and gramicidin (p = 0.015–0.011, Fig. 5A.), similar to that of control LPS-treated DC used to induce maturation of DC. A smaller increase with dithiocarbamate, however, was not significant and alum had no effect. The lack of stimulation of CD4+ T cells with alum is consistent with it being a B cell adjuvant. CD4+ T cell responses to the stress agents, without activation by an antigen, is of considerable significance in enhancing the helper and adjuvant functions of these cells. These findings are consistent with both in vitro and in vivo murine studies (7, 8).
FIGURE 5.
Co-culture of stress-treated DC with autologous PBMC induce CD40L and CD45RO+ memory in CD4+ T cells. These are significantly inhibited with both IL-15 antibodies and IL-1β antagonists with all stress agents. For optimum expression of human CD45RO+ or CD45RO+CCR7− memory T cells, both the homeostatic and inflammasome pathways have to be activated. To determine the role of IL-15 and IL-1β on CD40L expression in DC-PBMC co-cultures, IL-15 antibodies and IL-1β antagonist or both were added to the cultures treated with sodium arsenite (As), gramicidin (Gr), dithiocarbamate (DITH), and alum. A, CD4+ T cell proliferative responses were performed by the carboxyfluorescein succinimidyl ester method. B, CD40L expression in CD4+ T cells co-cultured with autologous DC, activated with the stress agents, and treated with IL-15 antibodies, IL-1βR antagonist or both. Similarly, isotype control antibody showed no difference from the untreated cells (data not presented). C, representative flow cytometry. Similarly the two pathways were studied for CD45RO+ memory (D) and CD45RO+CCR7− effector memory CD4+ (E) T cells inhibitions. Thus, all stress agents up-regulated CD4+ and CCR7− memory T cells and optimum inhibition resulted when both inhibition agents were used. Student's t test was used for the inhibitions against untreated cultures; *, p < 0.05; **, p < 0.01; ***, p ≤ 0.001; and those of stress treated against untreated DC: ¥, <0.05; ¥¥¥, <0.001 (n = 4); ■, DC.
CD40L is up-regulated by antigen stimulation but stress may have a similar function, as all agents used significantly enhanced CD40L expression (p < 0.01, p < 0.001, Fig. 5A). The role of IL-15- and IL-1β-mediated pathways in CD4+ T cell activation was first examined by inhibition of CD40L expression. An increase of CD40L was blocked by anti-IL-15 antibodies (p < 0.001, p < 0.01, Fig. 5B) with all agents used and to a lesser extent with IL-1β RI antagonist, limited to sodium arsenite and gramicidin (p < 0.01); representative flow cytometry is presented in Fig. 4B. With the combined inhibitors there was increased inhibition with each stress agent (p < 0.001, p < 0.01). These results suggest that both pathways can inhibit CD40L expression, but IL-15 may be more effective than the IL-1β pathway (Fig. 5, B and C). This potentially important finding suggests that CD40L expression may be antigen-independent and that both pathways may be involved.
Are Both Pathways Required to Induce CD4+ CD45RO+ Memory T Cells
The effect of stress-activated DC on the development of antigen-independent CD4+ CD45RO+ memory T cells was then studied following co-culture with autologous PBMC for 5 days. All stress agents and alum up-regulated CD45RO+ memory T cells, as compared with untreated DC (from 30 + 3.7), but only sodium arsenite (51.8 ± 2.0; p < 0.05) and gramicidin (53.0 ± 3.4, p < 0.05) reached significant levels (Fig. 5D). Further examination of the two major memory subsets, central (CD45RO+ CCR7+) and effector (CD45RO+ CCR7−) CD4+ memory T cells showed a significant increase in the effector memory T cells with sodium arsenite (p < 0.01) and dithiocarbamate, (p = 0.01) but not gramicidin or alum (Fig. 5D). To ascertain whether both pathways are activated by the stress agents to elicit CD4 CD45RO+ T cells, the co-cultures were treated with either IL-15 antibodies or IL-1β receptor I (IL-1β RI) antagonist or both, using previously determined optimum doses of inhibitors.
Whereas sodium arsenite-, dithiocarbamate-, or alum-treated DC inhibited CD45RO+ CD4+ T cells with IL-15 antibodies, without or with IL-1βRI antagonist, gramicidin showed significant inhibition only with the combined inhibitors, although each separately induced some inhibition (Fig. 5D). The CD4+ CD45RO+CCR7− effector memory T cells were inhibited significantly predominantly when treated with both inhibitors (Fig. 5E). These results suggest that to elicit optimal expression of CD45RO+ memory and CCR7− effector memory CD4+ T cells, both the homeostasis and inflammasome pathways may be engaged. The CD45RO− naive CD4+ T cells were not affected by the two inhibiting agents (data not presented). These experiments raised the issue whether cell to cell contact is essential between DC and CD4+ T cells to induce memory cells. This was studied with stress-stimulated DC in parallel with DC-derived culture supernatant, each co-cultured with CD4+ T cells, followed by assay of CD4+ T cell proliferation. Significant proliferation was found in co-cultures with DC (37.0 ± 3.0%), but not with the culture supernatant (5.0 ± 1%), suggesting that CD4+ memory T cells are cell to cell contact dependent.
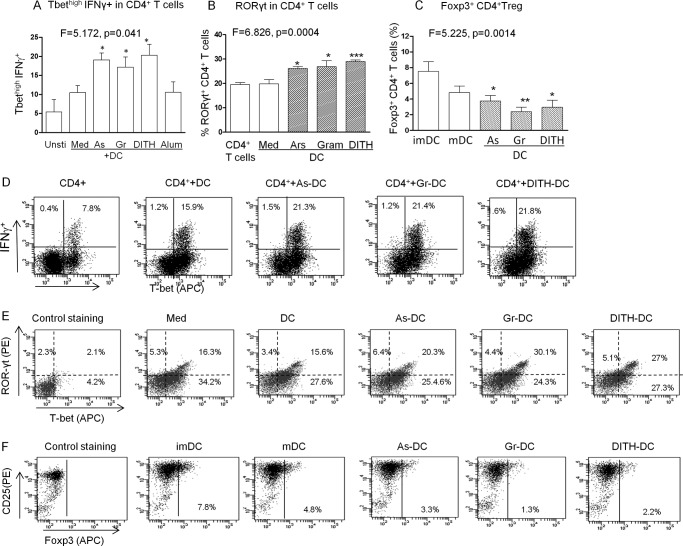
The Effect of Stress Agents on CD4+ T Cell Transcription Factors
We next examined the effect of stress on Th1, Th2, Th17, and Treg transcription factors. DC were treated with the stress agents for 90 min, then co-cultured with CD4+ T cells for 5 days and examined for the 4 transcription factors in CD4+ T cells by flow cytometry. The Tbet transcription factor of Th1 CD4+ T cells showed a significant increase with the 3 stress agents only if co-expressed with IFNγ (F = 5.172, p = 0.041, Fig. 6A), but not with alum. GATA3 failed to be up-regulated (data not shown). However, RORyt, the transcription factor for Th17 CD4+ T cells was significantly up-regulated (F = 6.826, p = 0.0004, Fig. 6B), as well as with each stress agent (p < 0.05 and p < 0.001); co-expression of Tbet with RORyt was also demonstrated (F = 7.893, p = 0.0002; Fig. 6C). In contrast the FoxP3 transcription factor of the CD25+ regulatory CD4+ T cells was significantly down-regulated (F = 5.225, p = 0.0014, Fig. 6D) with each stress agent (p < 0.05–0.01). The flow cytometry for each of the 3 transcription factors is presented in Fig. 6, E–G. The net result of these findings is that Tbet and RORyt transcription of Th1 and Th17 functions are enhanced by virtue of a decreased inhibitory effect of the FoxP3 regulatory activity. To facilitate reading the sequence of interactions a flow diagram is presented in Fig. 2E.
FIGURE 6.
The stress agents up-regulate T- bet and RORγt and down-regulate FoxP3. To study the effect of stress agents on transcription factors human DC were treated with stress agents for 90 min, followed by co-culture with autologous PBMC for 5 days. Expression of (A and D) T-bethigh IFNγ (B and E) RORγt, and (C and F) FoxP3+ CD4+ Treg cells induced by immature DC, LPS matured DC (mDC), or DC treated as above but co-cultured with autologous CD4+ T cells for 5 days in the presence of anti-CD3 (10 ng/ml) and anti-CD28 (10 ng/ml). ANOVA was used for each transcription factor in 5–6 groups, followed by the t test for each stress-treated group against DC alone (n = 5). *, p < 0.05; **, p < 0.01; ***, p < 0.01. Gata3 transcription of Th2 showed no change with the stress agents. D, production of IL-12p70; E, IL17 producing CD4+ T cells after co-cultures were treated with anti-CD3 and CD28 antibodies, followed by PMA and ionomycin; F, IL-6 in the culture supernatant from DC only □ or co-cultured CD4+ T cell supernatant ■ (n = 3–5). As, sodium arsenite; Gr, gramicidin; DITH, dithiocarbamate.
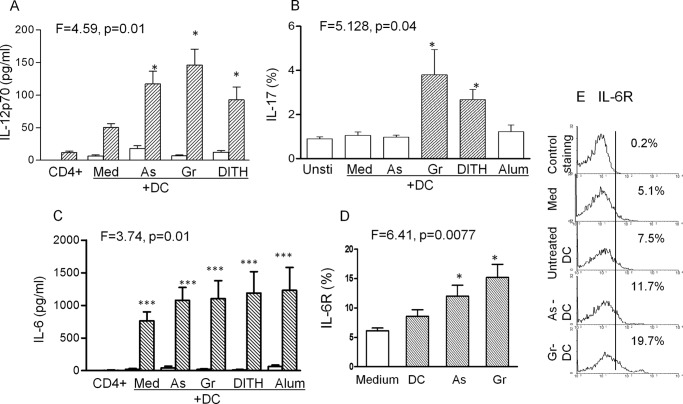
Cytokine Production
The cytokines corresponding to the transcription factors were then studied. The two subtypes, IL-12p40 and IL-12p70, showed significant enhancement when DC were co-cultured with PBMC and treated with the stress agents (Fig. 7A, only IL-12 p70 is presented). IL-17 was significantly up-regulated only by gramicidin and dithiocarbamate (Fig. 7B). Untreated DC or CD4+ T cell culture supernatants each produced very small quantities of IL-6, but co-culture of immature DC with CD4+ T cells (p < 0.001) or treatment with stress (p < 0.001, Fig. 7C) up-regulated IL-6 compared with untreated cells. IL-6R in CD4+ T cells were also up-regulated in co-cultures with DC (Fig. 7D) but only with arsenite and gramicidin. Low levels of TGFβ-1 were found in CD4+ T cell culture supernatants, however, a significant increase was induced with gramicidin or dithiocarbanate co-cultured with PBMC (p < 0.05; data not presented).
FIGURE 7.
A, production of IL-12p70; B, IL-17 producing CD4+ T cells; and C, IL-6 in the culture supernatant from DC only □ or co-cultured CD4+ T cell supernatant ■ (n = 3–5). For induction of IL-17 producing cells co-cultures of CD4+ T cells and stress agent-treated DC were treated with anti-CD3 and CD28 antibodies, followed by PMA and ionomycin, stress-treated DC-mediated IL-6R up-regulation (D) and representative flow cytometry of IL-6R expression in CD4+ T cells (E). As, sodium arsenite; Gr, gramicidin; DITH, dithiocarbamate.
Microarray Analysis
The transcriptome of CD45RO+ memory T cells (95% purity) stimulated by gramicidin-treated DC was studied by Illumina HT12v4 arrays (n = 3) validated by the Affymetrix array (n = 3) systems following 5-day cultures. The unbiased hierarchical clustering of the Illumina arrays demonstrated up-regulation of 4 groups of stress-stimulated genes (Tables 1–6) and a 5th group of repressed cholesterol genes (data not presented).
TABLE 1.
Microarray analysis of CD4+CD45RO+ memory T cells
These cells were tested from 3 subjects by the Illumina platform following co-culture of PBMC with gramicidin-treated DC and validated by the Affymetrix platform with cells from 3 other subjects. Group 1A consists of 16 IFN-stimulated genes which were also subjected to methylation inhibition by MTA.
| Group 1A genes (n = 16) | Microarray platform |
||
|---|---|---|---|
| Illumina p <0.05–0.01 | Affymetrix | MTA | |
| Interferon-stimulated genes | |||
| MX1 | 3.38 | 1.8 | −0.8 |
| MX2 | 2.0 | 1.4 | −0.2 |
| APOBEC3A | 4.70 | 2.0 | −11.4 |
| IRF7 | 2.1 | 2.2 | −0.9 |
| IRF1 | 1.6 | 1.4 | −1.4 |
| IFITM1 | 1.9 | 1.2 | −0.9 |
| IFITM2 | - | 1.4 | −1.5 |
| IFITM3 | 1.8 | 1.8 | −1.5 |
| 0AS2 | 1.7 | 2.1 | −0.33 |
| IFI35 | 2.0 | 2.1 | −3.1 |
| OAS3 | 1.60 | 2.0 | −3.0 |
| CBS | 2.1 | 2.1 | −3.2 |
| LILRA5 | 1.9 | 1.6 | −0.2 |
| AARS | 1.9 | 1.6 | −0.8 |
| SC02 | 1.8 | 1.6 | −1.2 |
| SLC6A12 | 1.8 | 1.7 | +1.9 |
TABLE 2.
Microarray analysis of CD4+CD45RO+ memory T cells
These cells were tested from 3 subjects by the Illumina platform following co-culture of PBMC with gramicidin-treated DC and validated by the Affymetrix platform with cells from 3 other subjects. Group 1B consists of 10 IFN-stimulated genes which were also subjected to methylation inhibition by MTA.
| Group 1B genes (n = 10) | Interferon-stimulated genes |
||
|---|---|---|---|
| Illumina p < 0.05 | Affymetrix | MTA | |
| IF144L | 2.0 | 4.2 | −3.4 |
| IFIT1 | 2.3 | 5.2 | −3.0 |
| IFIT2 | 1.9 | 3.5 | −2.0 |
| ISG15 | 2.3 | 2.1 | −1.2 |
| IFI44 | 2.2 | 1.7 | +1.4 |
| IFI27 | 3.3 | 6.8 | −9.7 |
| RSAD2 | 2.2 | 2.0 | −4.8 |
| HERC5 | 1.9 | 2.2 | −1.7 |
| ID01 | 2.3 | 1.9 | −8.6 |
| BST2 | 3.3 | −1.3 | |
TABLE 3.
Microarray analysis of CD4+CD45RO+ memory T cells
These cells were tested from 3 subjects by the Illumina platform following co-culture of PBMC with gramicidin-treated DC and consist of 21 IFN stimulated genes.
| Group 1C (n = 21) | IFN-stimulated genes, microarray platform, Illumina p < 0.05 |
|---|---|
| FAM26F | 2.2 |
| EPST11 | 1.8 |
| KIAA1618 | 1.8 |
| XAF1 | 1.8 |
| GRIN3A | 1.7 |
| RHOLL | 1.7 |
| MEFV | 1.7 |
| CD300LF | 1.7 |
| PLEKHO1 | 1.6 |
| HMFO | 1.6 |
| LOC1001330 | 1.6 |
| ALDH1A1 | 1.6 |
| PARP10 | 1.6 |
| GFRA2 | 1.6 |
| ACSL1 | 1.6 |
| SESN2 | 1.6 |
| WARS | 1.5 |
| RBCK1 | 1.5 |
| STAC | 1.5 |
| DDX20 | 1.5 |
| ECGF1 | 1.5 |
TABLE 4.
Microarray analysis of CD4+CD45RO+ memory T cells
These cells were tested from 3 subjects by the Illumina platform following co-culture of PBMC with gramicidin-treated DC and validated by the Affymetrix platform with cells from 3 other subjects. Group 2 consists of 6 IFN stimulated transcription factor genes also subjected to methylation inhibition by MTA.
| Group 2 genes (n = 6) | Transcription factor genes |
||
|---|---|---|---|
| Illumina | Affymetrix | MTA | |
| IRF1 | 1.6 | 1.3 | −1.3 |
| IRF7 | 2.0 | 2.2 | 0.9 |
| BATF3 | 1.5 | 1.8 | −1.3 |
| Tbet | NCa | 1.8 | −1.8 |
| ATF5 | 1.5 | NC | NC |
| NFκB | NC | 5.25 | −4.2 |
a NC, no change.
TABLE 5.
Microarray analysis of CD4+CD45RO+ memory T cells
These cells were tested from 3 subjects by the Illumina platform following co-culture of PBMC with gramicidin-treated DC and validated by the Affymetrix platform with cells from 3 other subjects. Group 3 shows complement genes (n = 3).
| Group 3 (n = 3) | Complement genes |
|
|---|---|---|
| Illumina | Affymetrix | |
| CIQC2 | 1.5 | |
| C2 | 2.0 | NIa |
| CFB | 1.6 | 1.8 |
a NI, not identified.
TABLE 6.
Microarray analysis of CD4+CD45RO+ memory T cells
These cells were tested from 3 subjects by the Illumina platform following co-culture of PBMC with gramicidin-treated DC and validated by the Affymetrix platform with cells from 3 other subjects. Group 4 shows miscellaneous genes (n = 4).
| Group 4 genes (n = 4) | Miscellaneous genes |
|
|---|---|---|
| Illumina | Affymetrix | |
| p38 (MAPK11) | NCa | 8.0 |
| Jak2 | 1.6 | 1.2 |
| TGFβ | NC | 4.4 |
| CLCN7 | 1.7 | NC |
a NC, no change.
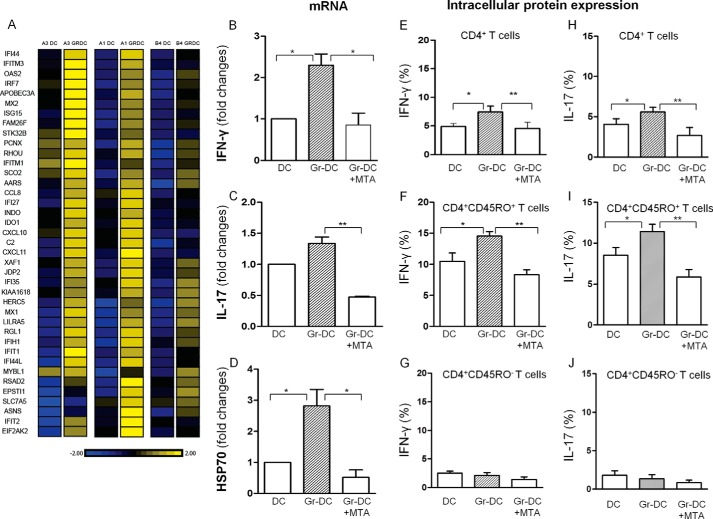
Group 1 consisted of 47 ISG, with ≥1.5-fold increase of gramicidin-treated compared with untreated CD4+ memory T cells and shown in the heat map (Fig. 8A). Of the 47 ISG, 37 showed a significant increase in the stress-stimulated compared with the unstimulated DC (p < 0.05-<0.01; Tables 1–3). 23 of the ISG were validated by the Affymetrix platform (Tables 1 and 2). Group 2 (Table 4) consisted of transcription genes, IRF1 and IRF7 (IFN regulatory factors), which are upstream regulators of many ISGs (also noted in Table 1) and BATF3, a regulator of RORγt in Th17 cells (Table 4). The NFκB gene involved in transcription of IL-15 in DC and activation of CD4+ T cells and Tbet in activation of Th1 cells were demonstrated only by the Affymetrix platform. Network analysis of the ISGs suggests that they are IRF1 and IRF7 driven, with hubs at NFκB and ISG15, OAS, and type 1 IFN.
FIGURE 8.
A, heat map of human CD4+CD45RO+ memory T cells following co-culture of gramicidin (Gr)-treated DC with PMBC demonstrating up-regulation of IFN-stimulated genes. RNA was prepared from 3 independent human samples (B–J). The effect of MTA on gramicidin-stimulated DC co-cultured with PMBC: B–D, mRNA of IFN-γ, IL-17, and HSP70 in CD4+CD45RO+ T cells; E and H, intracellular IFN-γ and IL-17 production; F–J, CD4+ CD45RO+, and CD45RO− T cells (n = 3–6); *, p < 0.05; **, p < 0.01. The data suggest that stress may influence the chromatin environment and elicit epigenetic changes.
The 3rd group included 3 early complement system genes of the classical C1Q, C2, and alternative CFB pathways, which were significantly up-regulated (Table 5, Group 3). The miscellaneous group 4 (Table 6, Group 4) included the signaling p38 (MAPK11) and JAK2 (Janus kinase 2) genes, which provides a protein involved in the JAK/STAT signaling pathway. Finally as the stress agents used to stimulate DC activates K+ channel opening, we searched for channel genes. Surprisingly, whereas the KCNQ potassium channel was not identified, the chloride channel 7 gene (CLCN7) was significantly up-regulated in the CD4 memory T cells (Table 6, Group 4).
Examination of repressed genes revealed a cluster of 22 cholesterol-regulated genes (data not presented), which produced a network, with hubs at high and low density lipids (HDL and LDL) and INSIG1. Among these genes were CD1A and CD1B, which are MHC class 1-like antigen presenting molecules specialized in presenting lipid antigens, especially mycobacterial cell wall components to CD1-specific T cells (23). Also repressed was CD1E, which facilitates loading lipids onto CD1B.
The Effect of Stress on Subsets of CD11c DC and the Expression of IFNα
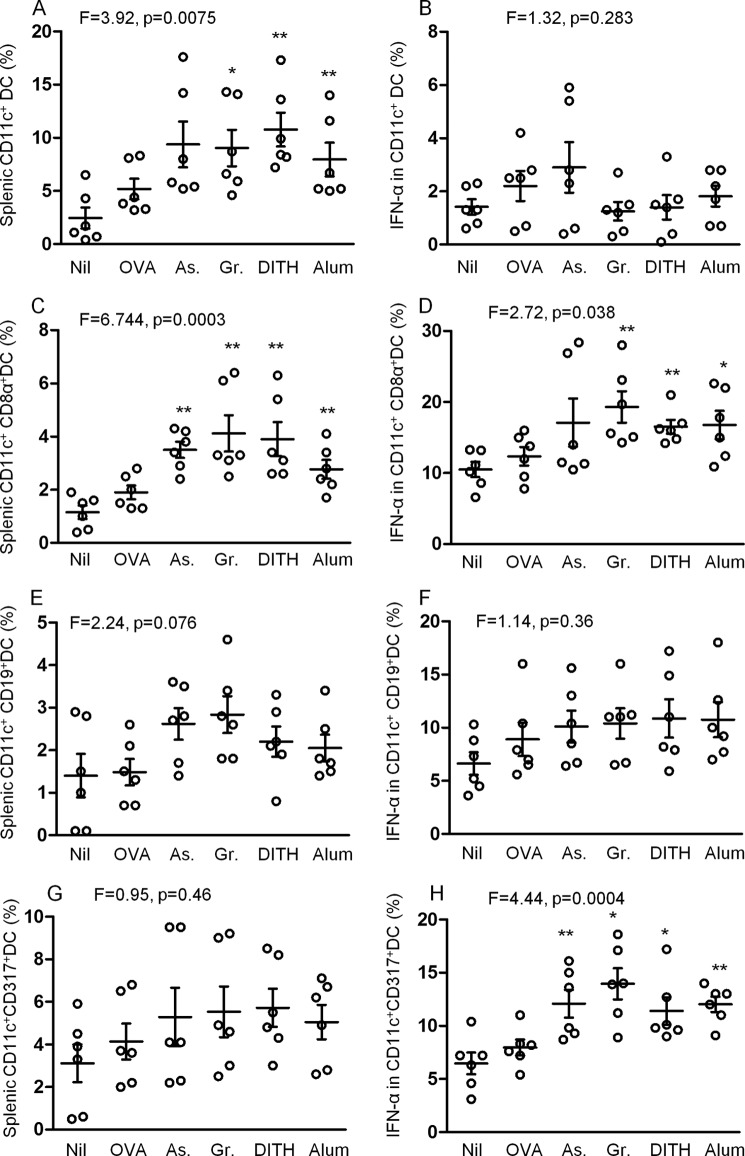
The microarray analyses of CD4 CD45RO+ memory T cells revealed a significant increase of type 1 ISGs. This raised the question whether type 1 IFN is also induced by the stress agents. We have previously demonstrated that maIL-15 and IL-1β are up-regulated in CD11c+ splenic DC when BALBc mice were treated with the stress agents and OVA (8). We followed this strategy by analyzing this effect on IFNα expression in 3 subsets of CD11c+ DC (Fig. 9, A and C).The CD11c CD19+ and CD317+ subsets were up-regulated but significant levels were not reached probably because of the large variation in the 6 samples tested (Fig. 9, E and G). However, IFNα was very significantly increased in the CD317+ subset (p < 0.0005, Fig. 9H), to a lesser extent in the CD8α+ subset (p < 0.05, Fig. 9D), but not in the CD19+ subset of CD11c+ DC. These are novel findings, in that stress agents up-regulate IFNα in plasmacytoid DC and CD8α+ DC, which may regulate immunological functions.
FIGURE 9.
Effect of stress agents on expression of splenic CD11c DC subsets and IFN-α production in corresponding cells in BALB/c mice. Mice were treated with 3 stress agents and alum 3 times at 2-week intervals. 1 week after the final treatment, splenic cells DC subsets were analyzed for expression of CD11c (A), or in combination with CD8α (C), CD19 (E), and CD317 (G). Intracellular IFN-α was also measured in the corresponding DC subsets (B, D, F, and H). As, sodium arsenite; Gr, gramicidin; DITH, dithiocarbamate.
Analysis of CD45RO+ Memory T Cells Treated with the Histone Methyltransferase Inhibitor
We next examined the paradigm that stress might induce low level tonic signaling with epigenetic changes, which may be responsible for maintaining memory. We sought evidence for an epigenetic component mediated either by histone modification or DNA methylation. To this end we co-cultured the gramicidin-treated and untreated DC with PBMC, some of which were further treated with 5-deoxy-5-methylthiodenosine (MTA) a protein methyltransferase inhibitor that inhibits both arginine (24) and lysine methylation of histone H3 (25). Treatment of CD4+, T cells with gramicidin significantly increased IFN-γ, IL-17 cytokines, and HSP70 mRNA (Fig. 8, B–D), or protein expression (Fig. 8, E and H), which was inhibited with MTA. This was observed only with CD45RO+ memory T cells but not with the CD45RO− naive T cells (Fig. 8, F–J). Microarray analysis of the effect of MTA on the CD4+CD45RO+ memory T cells showed that 14/23 of the Affymetrix-tested ISG that were up-regulated >1.5-fold with gramicidin were also down-regulated ≥1.5-fold by MTA (Tables 1 and 2). We suggest that short term culture with a stress agent may influence the chromatin environment and elicit epigenetic changes.
Discussion
We studied parallel induction of the IL-15/IL15-R and Il-1β/IL1βR pathways by co-culture stress-activated DC with autologous CD4+ T cells. Stress-induced ROS may activate HSP70 an intracellular sensor molecule. A flow sheet illustration of the effect of stress on the interactions between DC and CD4+ T cells leading to CD4+CD45RO+ memory T cells, cytokines, and ISGs are shown in Fig. 2E. The stress agents up-regulate inducible HSP70, which binds CD40, activating NFκB, inducing among others transcription of IL-15 in DC. We have demonstrated previously that stress-mediated maIL-15 in DC binds and activates the IL-15R complex on CD4+ T cells (8). In parallel the inflammasome NLRP3 pathway is stimulated, activating caspase-1, which produces pro-IL-1. Both pathways can activate Jak3 and STAT5 phosphorylation in CD4+ T cells and induce CD40L in CD4+ T cells, which may reactivate CD40 molecules on DC and create a positive feedback loop (7). CD40L (CD154) expression is commonly activated by antigens (26, 27) but here we demonstrate an antigen-independent effect stimulated by stress agents.
Investigation of key lineage-determining transcription factors in CD4+ T cells showed up-regulation of Tbet co-expressed with IFNγ and RORγt when DC were stimulated with the stress agents. Tbet regulates Th1 cytokines and RORγt Th17 cell differentiation. The GATA3 transcription factor promotes Th2 cell differentiation but was not affected by the stress agents. Because the regulatory FoxP3 transcription factor of CD4+ T cells is significantly down-regulated, the net effect is to enhance the immune functions.
The critical question raised by these results was whether both pathways are required to elicit CD4+CD45RO+ memory T cells. IL-15 has been well recognized to be significantly involved in antigen-independent CD4+ and CD8+ memory T cells (28–33). Our previous studies also demonstrated IL-15-dependent CD4+ memory T cell proliferation initiated by stressed DC (7). In an attempt to ascertain a requirement for the IL-1/IL1βR pathway, DC were treated with IL-1βR antagonist without or with IL-15 antibodies in addition to the stress agents, followed by co-culture with autologous PBMC. The results suggest that gramicidin required both pathways, as only the combination of IL-15 antibodies and IL-1βP antagonist significantly inhibited CD4+CD45RO+ memory or CD4+CD45RO+CCR7− effector memory T cells. However, sodium arsenite and dithiocarbamate down-regulated these memory cells with either of the two inhibitors. Alum also showed optimum inhibition with both inhibitors. These results are consistent with the paradigm that both homeostasis and inflammasome pathways may be involved in eliciting optimum CD4+CD45RO+ memory or CD45RO+CCR7− effector memory T cells.
The effect of one of the stress agents (gramicidin) was then studied on gene expression by microarray analysis of human CD4+CD45RO+ memory T cells. The most striking finding was up-regulation of 47 type 1 IFN-stimulated genes, which promote innate and adaptive immunity. This is a novel and potentially important finding of cellular stress eliciting ISGs in CD4+ memory T cells. ISGs control a number of viruses at any stage of their replicative cycle. There is ample evidence that a combination of ISG is more effective than any single ISG in controlling post-entry viral replication. Recently APOBEC3G and -F, Tetherin (BST2), Trim5α, and MX2 have been identified as retrovirus restriction factors, especially in CD4+ T cells (34, 35). Type 1 IFN also stimulates APOBEC3G, -3F, and -3A production in macrophages where it may prevent Vif neutralization of APOBEC (36). However, anti-HIV-1 function of APOBEC3A seems to be confined to the myeloid cells (37). Other restriction factors, such as MX1, IFIT, and ISG15 inhibit influenza A viruses, and MX1, IFI44L, OAS2, and RSAD2 may inhibit hepatitis C virus.
Type 1 IFNs are most likely produced by DC, especially plasmacytoid DC and macrophages. We addressed this issue in vivo by treatment of BALB/c mice with OVA, without or with the stress agents, and studied 3 subsets of CD11c DC. IFNα was up-regulated significantly in the CD11c CD317+ subset (p < 0.0005), consistent with the literature, as these are plasmacytoid DC. However, stress also increased the CD8α+ subset (p < 0.05), which has not been reported. They inhibit TH2 cytokines but stimulate TH1 cytokines, as found in this work. They activate maturation of DC, induce antigen processing and presentation to T cells, CD8+ T cell cross-presentation, and CD4+ T cell responses (38, 39). Whereas IFNα-mediated IL-15/IL-15R pathway stimulates, IL-1β/IL1βR inhibits CD4+ memory T cell expression (40, 41).
The question whether DC-CD4+ T cell direct contact was essential for stress-activated DC cells to induce the memory cells was studied with stress-stimulated DC in parallel with DC-derived culture supernatants, each co-cultured with CD4+ T cells, followed by assay of CD4+ T cell proliferation. Significant proliferation was found in co-cultures with DC (37.0 ± 3.0%), but not with the culture supernatant (5.0 ± 1%), suggesting that CD4+ memory T cells are cell to cell contact dependent. This finding may be of particular significance, as cell to cell transfer of HIV may represent an escape strategy from innate host defense (42).
Another cluster of up-regulated genes show transcriptional regulatory activity, of which IFN regulating factors, IRF1 and IRF7, are upstream regulators of many ISGs. They are master transcription factor genes, which are significantly up-regulated by vaccination (43), as well as by adjuvants (44). Interestingly, these two transcriptional factors of ISG were also found with the powerful MF59 and CPG adjuvants (44). We have detected increased expression of BATF3, ATF5, and JDP2 with the stress agent (gramicidin) exposed cultures. These findings are noteworthy because BATF3 and ATF are upstream regulators of RORγt and many other genes in the Th17 lineage; and BATF-deficient mice lack Th17 cells (45). The elevated expression of these genes may contribute to the observed increase in the Th17 cells. Because BATF and IRFs have also been shown to interact functionally it is likely that the BATF family in association with IRF1 and IRF7 may play a role in transcription regulation of the differentially regulated ISGs in this study. Gramicidin also induced early complement components and proteins of inflammasomes.
We suggest that the widely held view that immunological memory is maintained by tonic stimulation of the homeostatic pathway might be due to repetitive cellular stress to which DC cells are exposed. These may activate the dual homeostatic and inflammasome pathways between the IL-15 and IL1β molecules expressed by DC and their respective receptors expressed by CD4+ T cells, with optimum expression of memory CD4+ T cells requiring both pathways (Fig. 2D). This raises an important issue, whether stress-activated, antigen-independent memory is part of innate immunity, which maintains clones of existing antigen-specific memory T cells. Both pathways may self-regulate the stress-induced memory T cells, by the down-regulated CD4+ CD25+FoxP3+, enhancing immune functions. These cells are further inhibited by type 1 IFN (46). Furthermore, IL-6 may inhibit TGFβ-induced T regulatory cell differentiation (47). IFN-α also inhibits caspase-1 and the IL-1β pathway (41), but enhances the Th1 cytokines. The cellular stress response to tryptophan depletion by GEN-2 (general control of non-derepressible 2-kinase), which is activated by the up-regulated enzyme IDO (indoleamine 2–3 diogenase), regulates IFN-α production (48), and interacts through Jak/STAT signaling to induce ISG in CD4+CD45RO+ memory T cells. IDO may also be involved in microbial infections. CD40L, which binds CD40, may activate DC in a positive feedback loop. The net outcome of the positive and negative feedback is difficult to predict, but both the direct evidence and inhibition studies are consistent with the concept that tonic stimulation by the stress agents may maintain a steady state of CD4+ memory T cells.
Recent studies suggest that commensal bacteria that colonize the gut, upper respiratory system, and vagina may provide tonic stimulation of the innate immune system and elicit optimal antiviral immunity (49, 50). Among the multitude of antigens in microbes LPS in Gram-negative bacteria and HSP in most microbes are potent stimulators of innate and adaptive immunity (7, 8, 51–54). We suggest that tonic microbial stimulation and that by stress agents may share a common mechanism outlined in the present investigation.
A comparison of the above stress-induced signatures with the polyfunctional response elicited by the powerful yellow fever vaccine demonstrated a surprising degree of similarity between them (43). Both stress and the yellow fever vaccine induce innate immunity, complement components, interferon-stimulated genes, and inflammasomes. Adaptive immunity again is induced by both stress and the vaccine in T and B cell responses, although the latter is not the subject of this paper. Finally, the two diverse stimulating agents up-regulated specific transcription factors, of which IRF7 is shared by them, and may precede the development of the broad immune response. We suggest the paradigm that cellular stress might be responsible for the robust persistence of the immune response in vaccination. This will have to be demonstrated by immunization with an antigen to which the animal had not been exposed, followed by repeated treatment with a stress agent and then testing if the specific response is recalled longitudinally.
The data also raised the question, whether repetitive DC-T cell exposure to stress might elicit epigenetic changes, thereby maintaining antigen-independent memory T cells. Preliminary studies demonstrated that expression of a number of genes were inhibited by MTA, shown by flow cytometry and RT-PCR. This included IFN-γ and IL-17, produced by CD4+ memory T cells and was confirmed by RNA microarrays, which demonstrated inhibition with MTA in 14/23 ISGs up-regulated by the stress agent. Epigenetic modifications have been suggested to be responsible for the differentiation of T helper lineage, involving demethylation of DNA and acquiring permissive histone modifications (55, 56). This may apply especially to the differential regulation of Th1 (IFNγ) and Th2 (IL-4) genes. The present results suggest that during stress responses, histone methyltransferase activity is involved in increased transcription of ISGs. MTA can block methylation of lysine 4 on histone H3 and this post-transcriptional modification is a determinant of the level of gene expression (57). Because H3K4me3 levels at the transcriptional start sites of genes are related to the level of gene expression (58) and MTA can block trimethylation of H3K4, we postulate that stress induced by gramicidin may influence transcription of up-regulated genes via a histone methyltransferase. Our data suggest that stress may influence the chromatin environment, which comprises both histone and DNA components and elicit epigenetic changes, which maintain antigen independent memory.
This work was supported by the European Union Network of excellence, Europrise Grant LSHP-CT-2006-037611C), Advanced Immunization Technology Grant 280873, and King's Health Partner's Research and Challenge Fund.
- DC
- dendritic cells
- alum
- aluminum hydroxide
- HSP70
- 70-kDa heat shock protein
- ma
- membrane-associated
- ISG
- type 1 interferon-stimulated genes
- NAC
- N-acetylcystein
- ROS
- reactive oxygen species
- MTA
- 5-deoxy-5-methylthiodenosine
- PBMC
- peripheral blood mononuclear cell
- ANOVA
- analysis of variance
- FMK
- fluoromethyl ketone.
References
- 1. Goldrath A. W., Bevan M. J. (1999) Selecting and maintaining a diverse T-cell repertoire. Nature 402, 255–262 [DOI] [PubMed] [Google Scholar]
- 2. Swain S. L., Hu H., Huston G. (1999) Class II-independent generation of CD4 memory T cells from effectors. Science 286, 1381–1383 [DOI] [PubMed] [Google Scholar]
- 3. Hammarlund E., Lewis M. W., Hansen S. G., Strelow L. I., Nelson J. A., Sexton G. J., Hanifin J. M., Slifka M. K. (2003) Duration of antiviral immunity after smallpox vaccination. Nat. Med. 9, 1131–1137 [DOI] [PubMed] [Google Scholar]
- 4. Tough D. F., Sprent J. (1994) Turnover of naive- and memory-phenotype T cells. J. Exp. Med. 179, 1127–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Murali-Krishna K., Lau L. L., Sambhara S., Lemonnier F., Altman J., Ahmed R. (1999) Persistence of memory CD8 T cells in MHC class I-deficient mice. Science 286, 1377–1381 [DOI] [PubMed] [Google Scholar]
- 6. Intlekofer A. M., Wherry E. J., Reiner S. L. (2006) Not-so-great expectations: re-assessing the essence of T-cell memory. Immunol. Rev. 211, 203–213 [DOI] [PubMed] [Google Scholar]
- 7. Wang Y., Seidl T., Whittall T., Babaahmady K., Lehner T. (2010) Stress activated dendritic cells interact with CD4+ T cells to elicit homeostatic memory. Eur. J. Immunol. 40, 1628–1638 [DOI] [PubMed] [Google Scholar]
- 8. Wang Y., Rahman D., Lehner T. (2012) A comparative study of stress-mediated immunological functions with the adjuvanticity of alum. J. Biol. Chem. 287, 17152–17160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lindquist S., Craig E. A. (1988) The heat-shock proteins. Annu. Rev. Genet. 22, 631–677 [DOI] [PubMed] [Google Scholar]
- 10. Matzinger P. (2002) The danger model: a renewed sense of self. Science 296, 301–305 [DOI] [PubMed] [Google Scholar]
- 11. Angelini G., Gardella S., Ardy M., Ciriolo M. R., Filomeni G., Di Trapani G., Clarke F., Sitia R., Rubartelli A. (2002) Antigen-presenting dendritic cells provide the reducing extracellular microenvironment required for T lymphocyte activation. Proc. Natl. Acad. Sci. U.S.A. 99, 1491–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hattori H., Subramanian K. K., Sakai J., Jia Y., Li Y., Porter T. F., Loison F., Sarraj B., Kasorn A., Jo H., Blanchard C., Zirkle D., McDonald D., Pai S. Y., Serhan C. N., Luo H. R. (2010) Small-molecule screen identifies reactive oxygen species as key regulators of neutrophil chemotaxis. Proc. Natl. Acad. Sci. U.S.A. 107, 3546–3551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martinon F., Chen X., Lee A. H., Glimcher L. H. (2010) TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat. Immunol. 11, 411–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Devadas S., Zaritskaya L., Rhee S. G., Oberley L., Williams M. S. (2002) Discrete generation of superoxide and hydrogen peroxide by T cell receptor stimulation: selective regulation of mitogen-activated protein kinase activation and fas ligand expression. J. Exp. Med. 195, 59–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Los M., Schenk H., Hexel K., Baeuerle P. A., Dröge W., Schulze-Osthoff K. (1995) IL-2 gene expression and NF-κB activation through CD28 requires reactive oxygen production by 5-lipoxygenase. EMBO J. 14, 3731–3740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou R., Yazdi A. S., Menu P., Tschopp J. (2011) A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225 [DOI] [PubMed] [Google Scholar]
- 17. Nathan C., Cunningham-Bussel A. (2013) Beyond oxidative stress: an immunologist's guide to reactive oxygen species. Nat. Rev. Immunol. 13, 349–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martinon F., Mayor A., Tschopp J. (2009) The inflammsomes: guardians of the body. Annu. Rev. Immunol. 27, 229–265 [DOI] [PubMed] [Google Scholar]
- 19. Pétrilli V., Papin S., Dostert C., Mayor A., Martinon F., Tschopp J. (2007) Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 14, 1583–1589 [DOI] [PubMed] [Google Scholar]
- 20. Schroder K., Zhou R., Tschopp J. (2010) The NLRP3 inflammasome: a sensor for metabolic danger? Science 327, 296–300 [DOI] [PubMed] [Google Scholar]
- 21. Andris F., Denanglaire S., Baus E., Rongvaux A., Steuve J., Flavell R. A., Leo O. (2011) Metabolic stress boosts humoral responses in vivo independently of inflammasome and inflammatory reaction. J. Immunol. 186, 2245–2253 [DOI] [PubMed] [Google Scholar]
- 22. Mariathasan S., Monack D. M. (2007) Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat. Rev. Immunol. 7, 31–40 [DOI] [PubMed] [Google Scholar]
- 23. Brigl M., Brenner M. B. (2004) CD1: antigen presentation and T cell function. Annu. Rev. Immunol. 22, 817–890 [DOI] [PubMed] [Google Scholar]
- 24. Zhao X., Jankovic V., Gural A., Huang G., Pardanani A., Menendez S., Zhang J., Dunne R., Xiao A., Erdjument-Bromage H., Allis C. D., Tempst P., Nimer S. D. (2008) Methylation of RUNX1 by PRMT1 abrogates SIN3A binding and potentiates its transcriptional activity. Genes Dev. 22, 640–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang J., Kent J. R., Placek B., Whelan K. A., Hollow C. M., Zeng P. Y., Fraser N. W., Berger S. L. (2006) Trimethylation of histone H3 lysine 4 by Set1 in the lytic infection of human herpes simplex virus 1. J. Virol. 80, 5740–5746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Frentsch M., Arbach O., Kirchhoff D., Moewes B., Worm M., Rothe M., Scheffold A., Thiel A. (2005) Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat. Med. 11, 1118–1124 [DOI] [PubMed] [Google Scholar]
- 27. Chattopadhyay P. K., Yu J., Roederer M. (2005) A live-cell assay to detect antigen-specific CD4+ T cells with diverse cytokine profiles. Nat. Med. 11, 1113–1117 [DOI] [PubMed] [Google Scholar]
- 28. Judge A. D., Zhang X., Fujii H., Surh C. D., Sprent J. (2002) Interleukin 15 controls both proliferation and survival of a subset of memory-phenotype CD8+ T cells. J. Exp. Med. 196, 935–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ku C. C., Murakami M., Sakamoto A., Kappler J., Marrack P. (2000) Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science 288, 675–678 [DOI] [PubMed] [Google Scholar]
- 30. Becker T. C., Wherry E. J., Boone D., Murali-Krishna K., Antia R., Ma A., Ahmed R. (2002) Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J. Exp. Med. 195, 1541–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang X., Sun S., Hwang I., Tough D. F., Sprent J. (1998) Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity 8, 591–599 [DOI] [PubMed] [Google Scholar]
- 32. Lenz D. C., Kurz S. K., Lemmens E., Schoenberger S. P., Sprent J., Oldstone M. B., Homann D. (2004) IL-7 regulates basal homeostatic proliferation of antiviral CD4+ T cell memory. Proc. Natl. Acad. Sci. U.S.A. 101, 9357–9362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Purton J. F., Tan J. T., Rubinstein M. P., Kim D. M., Sprent J., Surh C. D. (2007) Antiviral CD4+ memory T cells are IL-15 dependent. J. Exp. Med. 204, 951–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Malim M. H., Bieniasz P. D. (2012) HIV Restriction factors and mechanisms of evasion. Cold Spring Harbor Perspect. Med. 2, a006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goujon C., Moncorgé O., Bauby H., Doyle T., Ward C. C., Schaller T., Hué S., Barclay W. S., Schulz R., Malim M. H. (2013) Human MX2 is an interferon-induced post-entry inhibitor of HIV-1 infection. Nature 502, 559–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Peng G., Lei K. J., Jin W., Greenwell-Wild T., Wahl S. M. (2006) Induction of APOBEC3 family proteins, a defensive maneuver underlying interferon-induced anti-HIV-1 activity. J. Exp. Med. 203, 41–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Berger G., Durand S., Fargier G., Nguyen X. N., Cordeil S., Bouaziz S., Muriaux D., Darlix J. L., Cimarelli A. (2011) APOBEC3A is a specific inhibitor of the early phases of HIV-1 infection in myeloid cells. PLoS Pathogens 7, e1002221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Le Bon A., Etchart N., Rossmann C., Ashton M., Hou S., Gewert D., Borrow P., Tough D. F. (2003) Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat. Immunol. 4, 1009–1015 [DOI] [PubMed] [Google Scholar]
- 39. Longhi M. P., Trumpfheller C., Idoyaga J., Caskey M., Matos I., Kluger C., Salazar A. M., Colonna M., Steinman R. M. (2009) Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly(IC) as adjuvant. J. Exp. Med. 206, 1589–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Santini S. M., Lapenta C., Logozzi M., Parlato S., Spada M., Di Pucchio T., Belardelli F. (2000) Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J. Exp. Med. 191, 1777–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Guarda G., Braun M., Staehli F., Tardivel A., Mattmann C., Förster I., Farlik M., Decker T., Du Pasquier R. A., Romero P., Tschopp J. (2011) Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity 34, 213–223 [DOI] [PubMed] [Google Scholar]
- 42. Vendrame D., Sourisseau M., Perrin V., Schwartz O., Mammano F. (2009) Partial inhibition of human immunodeficiency virus replication by type I interferons: impact of cell-to-cell viral transfer. J. Virol. 83, 10527–10537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gaucher D., Therrien R., Kettaf N., Angermann B. R., Boucher G., Filali-Mouhim A., Moser J. M., Mehta R. S., Drake D. R., 3rd, Castro E., Akondy R., Rinfret A., Yassine-Diab B., Said E. A., Chouikh Y., Cameron M. J., Clum R., Kelvin D., Somogyi R., Greller L. D., Balderas R. S., Wilkinson P., Pantaleo G., Tartaglia J., Haddad E. K., Sékaly R. P. (2008) Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J. Exp. Med. 205, 3119–3131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mosca F., Tritto E., Muzzi A., Monaci E., Bagnoli F., Iavarone C., O'Hagan D., Rappuoli R., De Gregorio E. (2008) Molecular and cellular signatures of human vaccine adjuvants. Proc. Natl. Acad. Sci. U.S.A. 105, 10501–10506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jordan-Williams K. L., Poston S., Taparowsky E. J. (2013) BATF regulates the development and function of IL-17 producing iNKT cells. BMC Immunol. 14, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Srivastava S., Koch M. A., Pepper M., Campbell D. J. (2014) Type I interferons directly inhibit regulatory T cells to allow optimal antiviral T cell responses during acute LCMV infection. J. Exp. Med. 211, 961–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kimura A., Kishimoto T. (2010) IL-6: regulator of Treg/Th17 balance. Eur. J. Immunol. 40, 1830–1835 [DOI] [PubMed] [Google Scholar]
- 48. Manlapat A. K., Kahler D. J., Chandler P. R., Munn D. H., Mellor A. L. (2007) Cell-autonomous control of interferon type I expression by indoleamine 2,3-dioxygenase in regulatory CD19+ dendritic cells. Eur. J. Immunol. 37, 1064–1071 [DOI] [PubMed] [Google Scholar]
- 49. Abt M. C., Artis D. (2009) The intestinal microbiota in health and disease: the influence of microbial products on immune cell homeostasis. Curr. Opin. Gastroenterol. 25, 496–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ichinohe T., Pang I. K., Kumamoto Y., Peaper D. R., Ho J. H., Murray T. S., Iwasaki A. (2011) Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl. Acad. Sci. U.S.A. 108, 5354–5359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Amit I., Garber M., Chevrier N., Leite A. P., Donner Y., Eisenhaure T., Guttman M., Grenier J. K., Li W., Zuk O., Schubert L. A., Birditt B., Shay T., Goren A., Zhang X., Smith Z., Deering R., McDonald R. C., Cabili M., Bernstein B. E., Rinn J. L., Meissner A., Root D. E., Hacohen N., Regev A. (2009) Unbiased reconstruction of a mammalian transcriptional network mediating pathogen responses. Science 326, 257–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Doyle S., Vaidya S., O'Connell R., Dadgostar H., Dempsey P., Wu T., Rao G., Sun R., Haberland M., Modlin R., Cheng G. (2002) IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity 17, 251–263 [DOI] [PubMed] [Google Scholar]
- 53. Corcoran B. M., Stanton C., Fitzgerald G., Ross R. P. (2008) Life under stress: the probiotic stress response and how it may be manipulated. Curr. Pharm. Des. 14, 1382–1399 [DOI] [PubMed] [Google Scholar]
- 54. Flahaut S., Hartke A., Giard J. C., Benachour A., Boutibonnes P., Auffray Y. (1996) Relationship between stress response toward bile salts, acid and heat treatment in Enterococcus faecalis. FEMS Microbiol. Lett. 138, 49–54 [DOI] [PubMed] [Google Scholar]
- 55. Wilson C. B., Rowell E., Sekimata M. (2009) Epigenetic control of T-helper-cell differentiation. Nat. Rev. Immunol. 9, 91–105 [DOI] [PubMed] [Google Scholar]
- 56. Youngblood B., Hale J. S., Ahmed R. (2013) T-cell memory differentiation: insights from transcriptional signatures and epigenetics. Immunology 139, 277–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wei G., Wei L., Zhu J., Zang C., Hu-Li J., Yao Z., Cui K., Kanno Y., Roh T. Y., Watford W. T., Schones D. E., Peng W., Sun H. W., Paul W. E., O'Shea J. J., Zhao K. (2009) Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity 30, 155–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Barski A., Cuddapah S., Cui K., Roh T. Y., Schones D. E., Wang Z., Wei G., Chepelev I., Zhao K. (2007) High-resolution profiling of histone methylations in the human genome. Cell 129, 823–837 [DOI] [PubMed] [Google Scholar]