Background: CETP expression mediates cholesterol metabolism and the development of atherosclerosis.
Results: Inhibition of Topo II activates CETP expression in HepG2 cells and CETP transgenic mouse liver, which was associated with increased reverse cholesterol transport in vivo.
Conclusion: Topo II inhibitors induced CETP expression and RCT by activating LXR pathway.
Significance: We found an important function of Topo II inhibition in regulating cholesterol metabolism.
Keywords: CETP, Topo II inhibitor, LXR, liver, RCT
Abstract
Cholesteryl ester transfer protein (CETP) transfers cholesteryl esters from high density lipoprotein to triglyceride-rich lipoproteins. CETP expression can be transcriptionally activated by liver X receptor (LXR). Etoposide and teniposide are DNA topoisomerase II (Topo II) inhibitors. Etoposide has been reported to inhibit atherosclerosis in rabbits with un-fully elucidated mechanisms. In this study we determined if Topo II activity can influence cholesterol metabolism by regulating hepatic CETP expression. Inhibition of Topo II by etoposide, teniposide, or Topo II siRNA increased CETP expression in human hepatic cell line, HepG2 cells, which was associated with increased CETP secretion and mRNA expression. Meanwhile, inhibition of LXR expression by LXR siRNA attenuated induction of CETP expression by etoposide and teniposide. Etoposide and teniposide induced LXRα expression and LXRα/β nuclear translocation while inhibiting expression of receptor interacting protein 140 (RIP140), an LXR co-repressor. In vivo, administration of teniposide moderately reduced serum lipid profiles, induced CETP expression in the liver, and activated reverse cholesterol transport in CETP transgenic mice. Our study demonstrates a novel function of Topo II inhibitors in cholesterol metabolism by activating hepatic CETP expression and reverse cholesterol transport.
Introduction
Cholesteryl ester transfer protein (CETP)4 transfers cholesteryl ester from high density lipoprotein (HDL) to apoB-containing lipoproteins, such as low density lipoprotein (LDL) and very low density lipoprotein, in exchange for triglycerides (TG), thereby exerting a key role in cholesterol metabolism (1). Although the homozygotes with CETP deficiency in humans demonstrate elevated HDL-cholesterol (HDL-C) levels, some of the patients still suffer from increased risk of coronary heart disease (2). The clinical trial evaluations indicate that CETP inhibitors can raise serum HDL-C levels but not reduce the risk of coronary heart disease (1). Mice do not express CETP naturally. However, studies with CETP transgenic mice suggest that CETP expression may increase a direct removal of liver HDL cholesteryl esters in the manners that are independent of the established lipoprotein receptors (3). Therefore, CETP may contribute to reverse cholesterol transport (RCT) in humans through a direct hepatic selective CE uptake or indirect transfer of HDL-CE to apoB-containing lipoproteins and subsequent receptor-mediated liver uptake.
CETP is predominantly expressed by the liver and secreted into plasma where CETP is mainly associated with HDL and apoB-containing lipoproteins. CETP expression can be transcriptionally activated by liver X receptors (LXR) α and β, the ligand-activated transcription factors, as there is an LXR-responsive element (LXRE) in the CETP promoter (4).
Activation of LXR leads to formation of a heterodimer of LXR with another transcription factor, retinoid X receptor (RXR). The heterodimer of LXR/RXR can bind to LXRE in the promoter of target genes to initiate their transcription (5). LXR activates expression of macrophage ATP binding cassette transporter A1 (ABCA1) (6), which can increase cholesterol efflux and RCT and generation of nascent HDL. Therefore, synthetic LXR ligands inhibit atherosclerosis in the animal models (7, 8).
DNA topoisomerases IIA and IIB (Topo II) are ubiquitous enzymes and regulate topological rearrangement of DNA during replication, transcription, and separation of daughter chromosomes at mitosis (9). Topo II is vital for cell survival, and tumor cells always exert an abnormal Topo II activity. Thus, Topo II is a promising molecular target for cancer chemotherapy (10). In fact, some Topo II inhibitors, such as etoposide (VP-16) and teniposide (VM-26), have been used to treat various cancers for many years (11).
Although the functions of etoposide and teniposide have been well defined in cancer biology and immunology, it remains unclear if Topo II inhibitors can play an important role in other diseases. Etoposide has been reported to reduce atherosclerosis in hypercholesterolemia rabbits with un-fully elucidated mechanisms (12–14). Meanwhile, high expressing CETP can reduce atherosclerosis in the animal models (15–17), indicating that the anti-atherogenic properties of Topo II inhibitors are related to regulation of cholesterol metabolism, such as hepatic CETP expression. Therefore, in this study we determined if Topo II inhibitors can activate CETP expression in a human hepatic cell line, HepG2 cells, and the underlying molecular mechanisms. In vivo, we determined if teniposide is able to induce CETP expression in the liver and enhance RCT in the CETP transgenic mice.
Experimental Procedures
Materials
Etoposide and teniposide were purchased from Alexis Biochemicals (San Diego, CA). Anti-CETP, LXRα, LXRβ, p21, and E2F1 rabbit polyclonal antibodies were purchased from Proteintech Group (Chicago, IL). Anti-RIP140 and phosphorylated Rb (pRb) rabbit polyclonal and anti-Topo IIA mouse monoclonal antibodies were purchased from Santa Cruz Biotechnology (Dallas, Texas). All other chemicals were purchased from Sigma.
Cell Culture
HepG2 cells, a human hepatic cell line, and 293T cells, a human embryonic kidney cell line, were purchased from ATCC (Manassas, VA) and cultured in complete minimum Eagle's medium and RPMI1640 medium containing 10% fetal bovine serum, 50 μg/ml penicillin, 50 μg/ml streptomycin, and 2 mm glutamine, respectively. Cells were switched to serum-free medium at ∼90% confluence to receive treatment.
In Vivo Study
The protocol for the animal study was approved by The Animal Ethics Committee of Nankai University. C57BL/6 wild type mice (∼8 weeks, males) or the age-matched CETP transgenic (C57BL/6 background) mice expressing a human CETP minigene (CETPtg, males) under the control of its natural flanking sequences were subcutaneously injected with teniposide solution in DMSO that was diluted with PBS (1:10) just before injection (15 mg/kg of body weight, ∼100 μl/mouse). Mice in control groups were injected with an equal volume of solvent (10% DMSO in PBS). The injection was repeated 4 days later. All mice were free to access chow diet and water.
After 8 days of the initial teniposide injection, mice were conducted RCT assay as described (18). Briefly, peritoneal macrophages isolated from untreated wild type or CETPtg mice in suspension were radiolabeled in serum-free RPMI 1640 medium containing 50 μg/ml acetylated LDL and 2 μCi/ml [3H]cholesterol for 24 h, washed twice, equilibrated in RPMI 1640 medium containing 0.2% BSA for 4 h, spun down, and resuspended in serum-free RPMI 1640 medium before immediate injection. Wild type or CETPtg mice receiving teniposide or solvent treatment were then intraperitoneally injected with the radiolabeled macrophages (∼2 × 106 cells/mouse containing 1.2 × 106 cpm) of the corresponding type mice and transferred into metabolic chambers. Feces from individual mouse were collected at 24, 32, 40, and 48 h after the cell injection. At the end of the experiment (48 h after the cell injection), the mice were anesthetized and euthanized in a CO2 chamber followed by collection of liver (for CETPtg mice only) and blood (for both wild type and CETPtg mice) samples. Serum was isolated from blood and used to determine lipid profiles. CETP expression in the liver of CETPtg mouse was determined by real time RT-PCR, Western blot, and immunohistochemical staining (19).
Determination of CETP, LXRα/β, Receptor Interacting Protein 140 (RIP140), p21, pRb, E2F1 Protein Expression and CETP Secretion by Western Blot
After treatment, a piece of liver from CETPtg mouse or HepG2 cells was used to extract whole cellular protein. Expression of CETP, LXR, RIP140, p21, pRb, or E2F1 protein was determined by Western blot as described (20).
To determine CETP secretion, HepG2 cells in 100-mm dishes were treated with 0.5 μm teniposide for different times. The treatment medium was collected and spun for 5 min at 2600 × g at 4 °C to remove the floating cells. The supernatant was transferred into 10K Centrifugal Filter Devices (Millipore, Billerica, MA) and spun for 60 min at 2000 × g at 4 °C. The floating cells and the cells in the dish were combined and lysed. The cellular protein content was determined and used to normalize the amount of concentrated medium that was used to determine secreted CETP protein by Western blot.
Inhibition of LXRα/β and Topo IIA Expression in HepG2 Cells by siRNA
siRNA against LXRα, LXRβ, and Topo IIA and scrambled siRNA were purchased from Santa Cruz Biotechnology. HepG2 cells were transfected with scrambled or target siRNA using Lipofectamine 2000 in minimum Eagle's medium. After 6 h of transfection, the cells were added with same volume of the medium and continued transfection for 24 h. The transfected cells were then switched into complete medium and cultured for 24 h followed by treatment in serum-free medium.
Isolation of Total Cellular RNA and Real Time RT-PCR Analysis of CETP, LXRα/β, RIP140, p21, Retinoblastoma (Rb), E2F1, and Microsomal Triglyceride Transfer Protein (MTTP) mRNA Expression
After treatment, total RNA was extracted from a piece of mouse liver or HepG2 cells and used to determine mRNA expression by real time RT-PCT as described (20) with the primers listed in Table 1. Expression of CETP, LXRα, LXRβ, RIP140, p21, Rb, E2F1, and MTTP mRNA was normalized by the corresponding GAPDH mRNA.
TABLE 1.
The sequences of primers for real time RT-PCR analysis
| Gene | Sense | Anti-sense |
|---|---|---|
| CETP | 5′-CTGCCTGGTGGCTGGGTATT-3′ | 5′-GGCATCGGTCCGCACTCTAC-3′ |
| LXRα | 5′-GAAGAAACTGAAGCGGCAAGA-3′ | 5′-ACTCGAAGCCGGTCAGAAAA-3′ |
| LXRβ | 5′-TGCCTGGTTTCCTGCAGCT-3′ | 5′-AGATGTTGATGGCGATGAGCA-3′ |
| RIP140 | 5′-TTCTTCTCCTCCTCCTTGCGTAG-3′ | 5′-GGTGCACATCAGAGCCAAGC-3′ |
| Topo IIA | 5′-CTAGTTAATGCTGCGGACAACA-3′ | 5′-CATTTCGACCACCTGTCACTT-3′ |
| p21 | 5′-AGTCAGTTCCTTGTGGAGCC-3′ | 5′-CATGGGTTCTGACGGACAT-3′ |
| Rb | 5′-TGTGAACATCGAATCATGGAA-3′ | 5′-TCAGTTGGTCCTTCTCGGTC-3′ |
| E2F1 | 5′-GACCCTGACCTGCTGCTCT-3′ | 5′-GGCCAGGTACTGATGGTCA-3′ |
| MTTP | 5′-CTGGATCTCCATATTGGCCT-3′ | 5′-TGATGTCCAAAATGCTGTCG-3′ |
| GAPDH | 5′-GGTGGTCTCCTCTGACTTCAACA-3′ | 5′-GTTGCTGTAGCCAAATTCGTTGT-3′ |
Preparation of Plasmid DNA and Determination of CETP Promoter Activity
The human CETP promoter (from −834 to +28, pCETP) was constructed by PCR with human genomic DNA and the following primers: forward, 5′-GCCGCTCGAGGCACTTGGTCATCTGGTCAC-3′; reverse, 5′-GGCAAGATCTGGTTATCAGGCAGTGGTGTG-3′. After the sequence was confirmed, the PCR product was digested with XhoI and BglII followed by ligation with pGL4 luciferase reporter vector and then transformed into Escherichia coli to amplify. To generate luciferase expression plasmid pGL-TK-Luc, pRL-TK vector (Promega, Madison, WI) was first digested with BglII/HindIII and then subcloned into pGL4 luciferase reporter vector.
The promoter containing three copies of the LXRE motif in the CETP gene (PGL-3xLXRE-TK-Luc) or three copies of the E2F1 motif in the RIP140 gene (PGL-3xE2F1-TK-Luc) was constructed with pGL-TK-Luc plasmid and the following oligonucleotides: CETP, forward (5′-TCGAGGGGTCATTGTCGGGCACAAGCTTGGGGTCATTGTCGGGCACAAGCTTGGGGTCATTGTCGGGCAGAT-3′) and reverse (5′-ATCTGCCCGACAATGACCCCAAGCTTGTGCCCGACAATGACCCCAAGCTTGTGCCCGACAATGACCCC-3′; the underlined sequences are the LXRE motif in the CETP promoter); RIP140, forward (5′-TCGAGTTGAGCGCCTCGCTCACCGCCCGCTTGAGCGCCTCGCTCACCGCCCGCTTGAGCGCCGAT-3′) and reverse (5′-ATCGGCGCTCAAGCGGGCGGTGAGCGAGGCGCTCAAGCGGGCGGTGAGCGAGGCGCTCAAC-3′; the underlined sequences are the E2F1 motif in the RIP140 promoter).
To analyze promoter activity, 293T cells in 24-well plates were transfected with DNA for promoter and Renilla (for internal normalization). After 24 h of transfection plus treatment, cells were lysed, and the cellular lysate was determined activity of firefly and Renilla luciferases using the Dual-Luciferase Reporter Assay System (Promega).
Extraction of Nuclear Protein and Chromatin Immunoprecipitation (ChIP) Assay of LXR DNA Binding Activity
After treatment HepG2 cells were lifted and washed twice with cold PBS, resuspended in 400 μl of cold fresh buffer A (10 mm HEPES, pH 7.9, 10 mm KCl, 0.1 mm EDTA, 0.1 mm EGTA, 1 mm DTT, 0.5 mm PMSF), and incubated for 15 min on ice. 50 μl of 10% Nonidet P-40 was added to the suspension with vortexing for 10 s. After spinning for 30 s at 16,200 × g at 4 °C, the pellet was saved and resuspended in 100 μl of cold fresh buffer B (20 mm HEPES, pH 7.91, 400 mm KCl, 1 mm EDTA, 1 mm EGTA, 1 mm DTT, 1 mm PMSF, 10 μg/ml leupeptin, and 10 μg/ml aprotinin) and rocked for 15 min at 4 °C. The mixture was centrifuged again for 5 min at 16,200 × g at 4 °C, and the supernatant containing nuclear proteins was collected and kept at −80 °C until assays were performed.
The nuclear proteins were used to determine LXRα/β protein by Western blot or LXRα/β DNA binding activity by ChIP assay as described (21) with anti-LXRα, LXRβ, or SREBP1 (used as a negative control) polyclonal antibody. The primers for ChIP assays are: LXRE (from −384 to −399), 5′-ATCAGAGCAAGGGAAAGGTC-3′ (forward) and 5′-GCACCATTTTTGCCATCCCT-3′ (reverse); SRE (sterol responsive element, from −191 to −207), 5′-GGGCAACAGTATCTGGCAAG-3′ (forward) and 5′-GAGATTCACCTCCTTCCTGC-3′ (reverse).
Immunofluorescent Staining
After treatment, HepG2 cells on coverslips in a 24-well plate were washed twice with PBS and then fixed with 4% paraformaldehyde (400 μl/well) for 30 min at room temperature. The fixed cells were incubated in 0.5% Triton X-100, PBS for 10 min and then blocked with 2% BSA, PBS for 2 h at room temperature followed by incubation with anti-LXR or CETP rabbit polyclonal antibody overnight at 4 °C. After the primary antibody was removed, the cells were incubated with a TRITC-conjugated goat anti-rabbit IgG for 2 h at room temperature. After washing with PBS, the sections were stained with a DAPI solution for nuclei. Images of the sections were obtained with a fluorescence microscope (Leica).
Data Analysis
All experiments were repeated at least three times, and the representative results are presented. The data are presented as the mean ± S.D. and were analyzed by Student's t test using Prism (GraphPad Software). The differences were considered significant if p < 0.05.
Results
Etoposide and Teniposide Induce CETP Expression and Secretion from HepG2 Cells
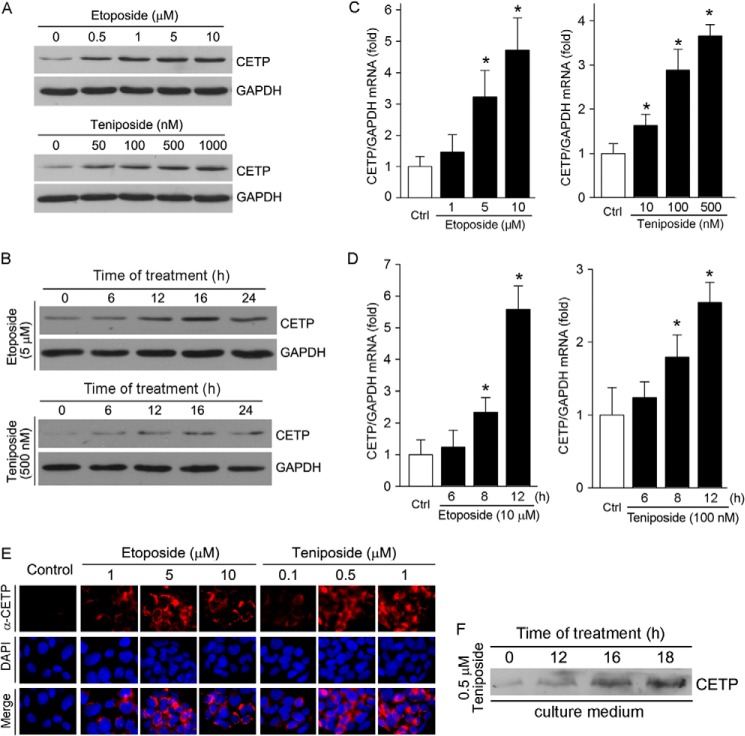
To test if Topo II inhibitors can influence CETP expression in hepatocytes, HepG2 cells were treated with etoposide or teniposide at different concentrations for 16 h followed by determination of CETP protein expression. Fig. 1A demonstrates that both etoposide and teniposide increased CETP expression in a semi-concentration-dependent manner. The maximal induction of CETP expression was observed with 5 μm etoposide and 500 nm teniposide. The time course study demonstrates that induction occurred quickly after treatment (Fig. 1B) with the maximum at 16 h after treatment. Associated with increased CETP protein expression, both etoposide and teniposide induced CETP mRNA expression in a concentration- and a time-dependent manner (Fig. 1, C and D), respectively. The induction of CETP expression by Topo II inhibitors was further confirmed by immunofluorescent staining (Fig. 1E), which shows that etoposide or teniposide increased HepG2 CETP expression in a concentration-dependent manner.
FIGURE 1.
Etoposide and teniposide induce CETP expression and secretion from HepG2 cells. A–D, HepG2 cells received the following treatment, and expression of CETP protein or mRNA was determined by Western blot or real time RT-PCR. A, etoposide or teniposide at the indicated concentrations for 16 h. B, etoposide (5 μm) or teniposide (500 nm) for the indicated times. C, etoposide or teniposide at the indicated concentrations for 12 h. D, etoposide (10 μm) or teniposide (100 nm) for the indicated times. *, versus control, p < 0.05 (n = 3). E, after the indicated treatment for 16 h, CETP protein expression was determined by immunofluorescent staining. F, HepG2 cells were treated with teniposide (0.5 μm) for the indicated times. The treatment medium was collected, concentrated, and normalized by cellular protein content before the secreted CETP protein was determined by Western blot.
CETP is mainly produced by hepatocytes followed by secretion into circulation system. To determine if the induction of CETP expression by Topo II inhibitors can result in increased CETP secretion, HepG2 cells were treated with 0.5 μm teniposide. The treatment medium was collected at different time points, concentrated, and normalized by cellular protein content before determination of secreted CETP protein by Western blot. Fig. 1F demonstrates that teniposide increased CETP protein levels in the treatment medium in a time-dependent manner, indicating that the CETP secretion is enhanced. Taken together, the results in Fig. 1 demonstrate that treatment of HepG2 cells with Topo II inhibitors activates CETP expression and secretion.
Etoposide and Teniposide Activate CETP Transcription in an LXR-dependent Manner
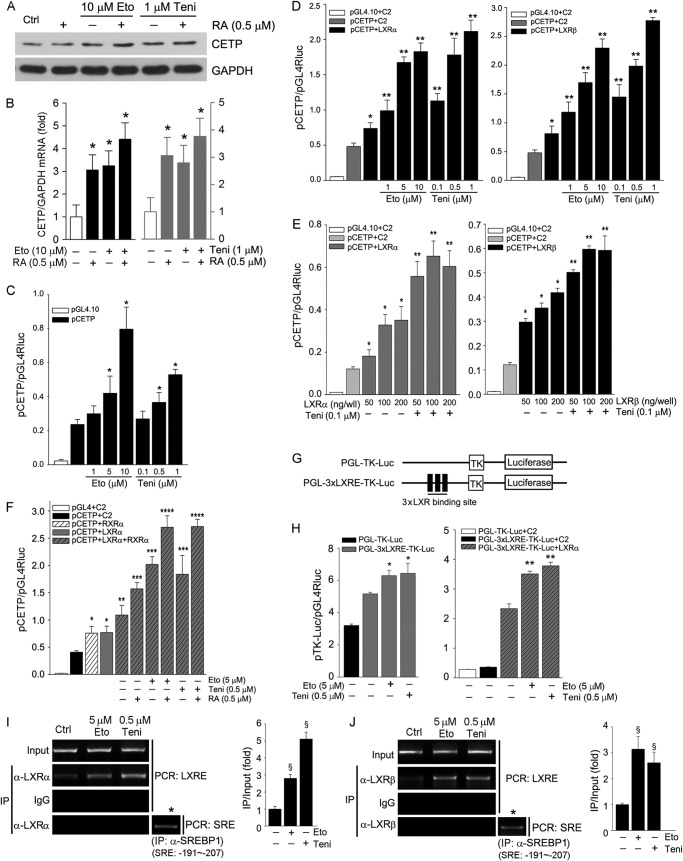
CETP is a transcriptional target of LXR. Activation of RXR by retinoic acid can coordinate LXR to induce target gene expression. To determine the role of RXR in Topo II inhibitor-induced CETP expression, HepG2 cells were treated with retinoic acid or plus etoposide/teniposide. Fig. 2, A and B, demonstrates that retinoic acid alone activated CETP mRNA expression but had little effect on CETP protein expression. However, retinoic acid clearly further increased etoposide- and teniposide-induced CETP mRNA and protein expression.
FIGURE 2.
Etoposide and teniposide induce CETP transcription in an LXR-dependent manner. A and B, HepG2 cells were treated with etoposide (Eto), teniposide (Teni), or plus 9-cis-retinoic acid (RA) as indicated for 16 h. *, versus control in the corresponding group, p < 0.05 (n = 3). C–H, 293T cells in 24-well plates were transfected with plasmid DNA for CETP promoter (pCETP), pEGFP-C2 vector (C2), pEGFP-LXRα (LXRα), pEGFP-LXRβ (LXRβ), and pEGFP-RXRα (RXRα) expression vector (100 ng/well) as indicated and Renilla luciferase (pGL4Rluc, internal control). The transfected cells received the indicated treatment for 16 h followed by determination of activity of firefly and Renilla luciferases. Significant difference at p < 0.05 (n = 3). C: *, versus pCETP alone. D: *, versus pCETP alone; **, versus pCETP+LXRα or LXRβ. E: *, versus pCETP; **, versus pCETP and pCETP+LXRα or LXRβ. F, RA, 9-cis-retinoic acid. *, versus pCETP; **, versus pCETP and pCETP+LXRα; ***, versus pCETP, pCETP+LXRα and pCETP+LXRα+RXRα; ****, versus all other groups. H: *, versus PGL-3xLXRE-TK-Luc; **, versus PGL-3xLXRE-TK-Luc+LXRα. I and J, etoposide and teniposide enhance LXR DNA binding activity. After treatment chromatin was isolated followed by immunoprecipitation (IP) with normal IgG or anti-LXRα (I) or LXRβ (J) antibody and PCR with the primers for the LXRE in the CETP promoter (from −384 to −399). *, the chromatin from control sample was immunoprecipitated with anti-SREBP-1 antibody followed by PCR with the primers for sterol responsive element (SRE; from −191 to −207) in the CETP promoter. §, versus control at p < 0.05 (n = 3).
To determine if the induction of CETP expression occurs at the transcriptional level, we constructed a CETP promoter (pCETP) that included the LXRE and determined the promoter activity in response to etoposide or teniposide treatment. Fig. 2C shows that both etoposide and teniposide increased pCETP activity in a concentration-dependent manner.
The role of LXR in Topo II inhibitor-induced CETP transcription was determined by the following experiments. 293T cells were transfected with a fixed concentration of LXRα or LXRβ expression vector plus pCETP followed by treatment with etoposide or teniposide. Fig. 2D indicates that high expressing LXRα or LXRβ increased pCETP activity, and the induction was further enhanced by etoposide and teniposide. Reciprocally, etoposide or teniposide further enhanced the high expressing LXRα- or LXRβ induced-pCETP activity (Fig. 2E). High expressing RXR also increased pCETP activity, which was further enhanced by high expressing LXR and RXR ligand (retinoic acid) and etoposide/teniposide (Fig. 2F).
Treatment of the 3xLXRE-TK-Luc plasmid (Fig. 2G), which included a three tandem of LXRE in the CETP promoter, with etoposide and teniposide increased its activity (left panel, Fig. 2H). In the presence of high expressing LXRα, the induction of 3xLXRE-TK-Luc promoter activity was enhanced by etoposide and teniposide (right panel, Fig. 2H), which suggests that Topo II inhibitors can directly target LXRE motif. For further determination, we conducted a ChIP assay to assess the binding activity of LXR protein to the LXRE in CETP promoter in response to Topo II inhibitor treatment. Fig. 2, I and J, demonstrated that the binding of LXRα and LXRβ with LXRE was increased by etoposide and teniposide, respectively.
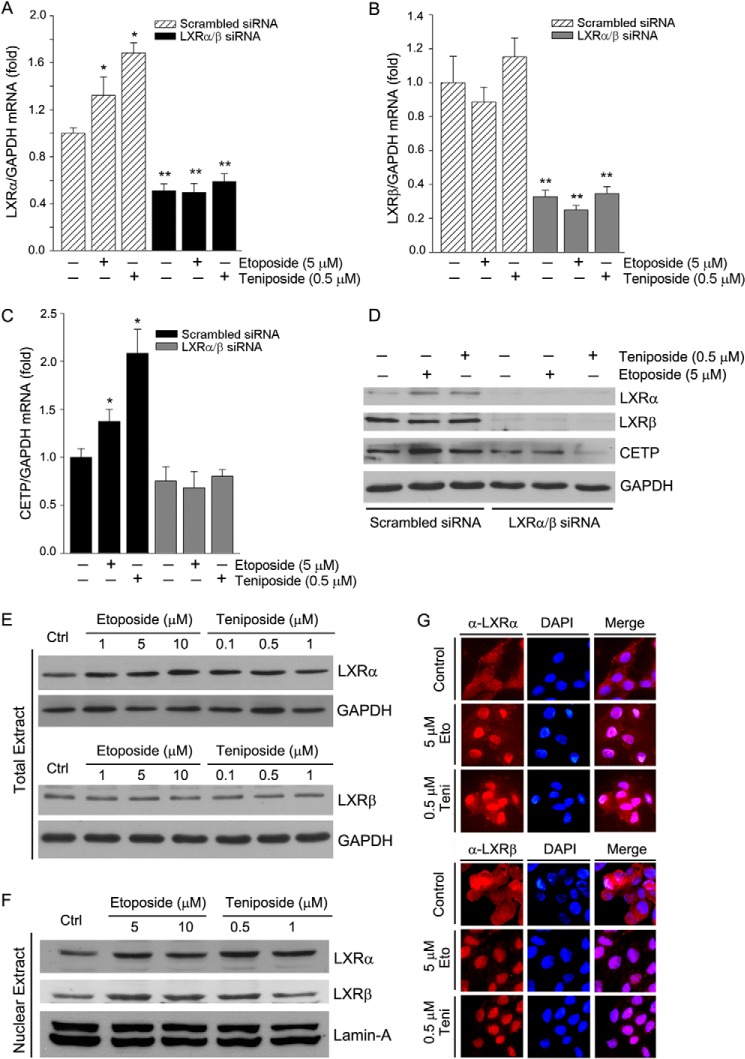
Selective inhibition of LXR expression can determine if the induction of CETP expression by Topo II inhibitor is directly through LXR. HepG2 cells were transfected with scrambled siRNA or a mixture of LXRα and LXRβ (LXRα/β) siRNA followed by treatment with etoposide and teniposide. Fig. 3, A and B, shows that both LXRα and LXRβ mRNA expression were substantially reduced by LXRα/β siRNA. Treatment of the scrambled siRNA-transfected cells with etoposide or teniposide increased expression of LXRα mRNA but not LXRβ mRNA (Fig. 3, A and B). Similar changes of LXRα and LXRβ protein expression were caused by etoposide and teniposide (Fig. 3D). Associated with induction of LXRα expression in the scrambled siRNA-transfected cells, expression of CETP mRNA and protein was similarly induced (Fig. 3, C and D). In contrast, etoposide and teniposide were not able to influence LXR (Fig. 3, A and B) and CETP (Fig. 3C) expression in the LXR siRNA-transfected cells, indicating that the existence of LXR is critical for induction of CETP expression by Topo II inhibitors.
FIGURE 3.
Etoposide and teniposide activate LXR expression or/and nuclear translocation. A–D, HepG2 cells were transfected with scrambled siRNA (100 nm) or a mixture of LXRα siRNA and LXRβ siRNA (50 nm of each) followed by treatment with etoposide or teniposide for 16 h. Expression of LXRα, LXRβ, and CETP mRNA and protein was determined by real time RT-PCR and Western blot. *, versus control in the same group; **, versus the corresponding sample in the scrambled siRNA-transfected group at p < 0.05 (n = 3). E and F, after 16 h of treatment, expression of LXRα and LXRβ protein in total extract or nuclear extract was determined by Western blot. G, expression of LXRα and LXRβ protein in intact HepG2 cells was determined by immunofluorescent staining. Eto, etoposide; Teni, teniposide.
Etoposide and Teniposide Activate LXR by Increasing LXR Expression/Nuclear Translocation and Inhibiting RIP140 Expression
Several mechanisms can be responsible for activation of LXR by Topo II inhibitors, such as induction of LXR expression or nuclear translocation and inhibition of LXR co-repressor expression. Similar to the scrambled siRNA-transfected cells (Fig. 3, A, B, and D), treatment of normal HepG2 cells with etoposide and teniposide increased LXRα, but not LXRβ levels, in whole cellular extracts (Fig. 3E), suggesting that expression of LXRα is selectively induced. However, determination of LXRα and LXRβ protein in nuclear extract shows that both LXRα and LXRβ were increased (Fig. 3F), which suggests that LXR nuclear translocation is enhanced. The effects of etoposide and teniposide on induction of LXRα expression and enhancement of LXRα/β nuclear translocation were further confirmed by immunofluorescent staining (Fig. 3G).
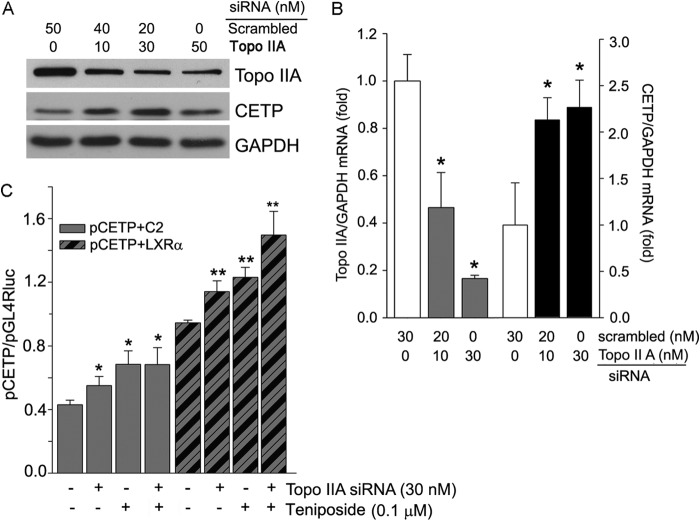
To directly link the induction of CETP expression to the inhibition of Topo II expression, we transfected HepG2 cells with Topo IIA siRNA and then determined the effect of decreased Topo II protein expression on CETP levels. The results demonstrate that Topo IIA siRNA decreased Topo IIA protein expression (top panel, Fig. 4A) while increasing CETP protein expression (middle panel, Fig. 4A). At the transcriptional level, decreased Topo IIA mRNA expression by the siRNA (left half, Fig. 4B) increased CETP mRNA expression (right half, Fig. 4B). In addition, Topo IIA siRNA activated CETP promoter activity, and the activation can be further enhanced by etoposide and teniposide (Fig. 4C).
FIGURE 4.
Induction of CETP expression is associated with inhibition of Topo IIA expression. A and B, HepG2 cells were transfected with scrambled siRNA or Topo IIA siRNA followed by determination of Topo IIA and CETP protein and mRNA expression, respectively. C, 293T cells were transfected with Topo IIA siRNA for 48 h. The cells were then transfected with pCETP plus LXRα expression vector for 6 h. After treatment with teniposide for 16 h, pCETP promoter activity was determined. C2, pEGFP-C2 vector; LXRα, pEGFP-LXRα expression vector. * and **, versus control in the corresponding groups at p < 0.05 (n = 3).
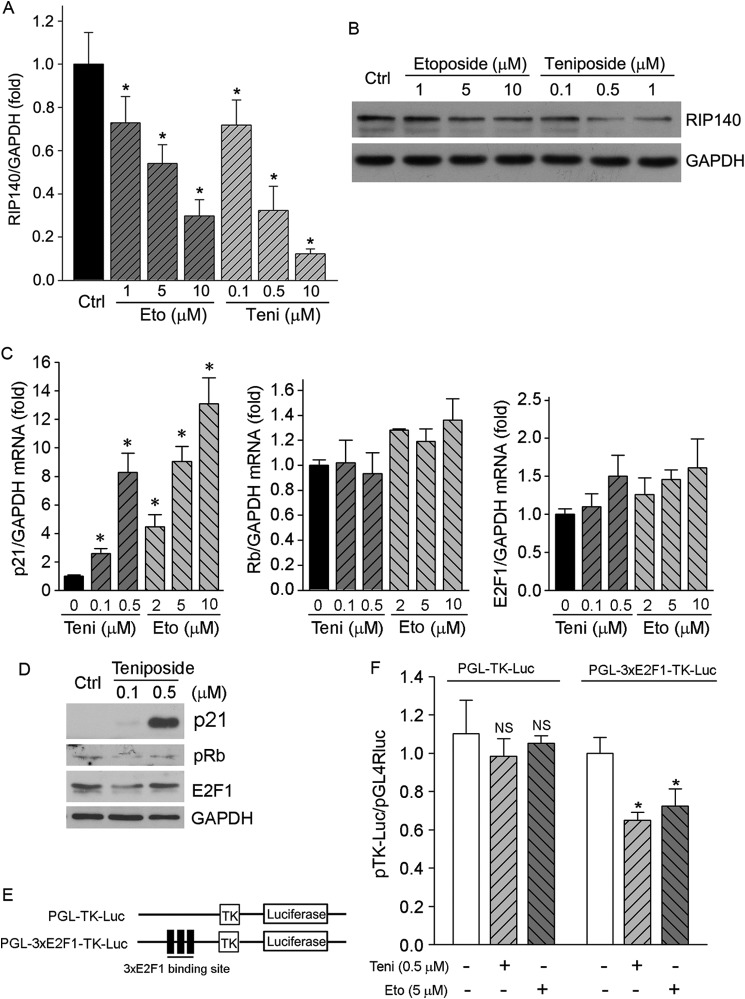
The interaction between LXR and its co-repressor, RIP140, results in decreased LXR activity (22). To determine if the induction of hepatic CETP expression by etoposide and teniposide is related to inactivation of RIP140 pathway, we initially determined RIP140 expression in response to etoposide and teniposide treatment. The results in Fig. 5, A and B, demonstrate that both etoposide and teniposide inhibited RIP140 mRNA and protein expression in a concentration-dependent manner, respectively.
FIGURE 5.
Etoposide and teniposide inactivate RIP140 pathway. A and B, HepG2 cells were treated with etoposide (Eto) or teniposide (Teni) at the indicated concentration for 12 and 16 h followed by determination of RIP140 mRNA and protein expression by real time RT-PCR and Western blot, respectively. *, versus control, p < 0.05 (n = 3). C, HepG2 cells were treated with teniposide and etoposide at the indicated concentrations for 12 h. Total cellular RNA was extracted and used to determine p21, Rb, and E2F1 mRNA expression by real time RT-PCR. *, versus control at p < 0.05 (n = 3). D, HepG2 cells were treated with teniposide at the indicated concentrations for 16 h. Expression of p21, E2F1, and pRb protein was determined by Western blot. E and F, 293T cells in 24-well plates were transfected with PGL-TK-Luc or PGL-3xE2F1-TK-Luc followed by treatment with teniposide and etoposide as indicated and determination of promoter activity. *, versus control in the corresponding group, p < 0.05 (n = 3). NS, not significant.
RIP140 has been reported as a novel target gene of the E2F1 transcription factor (23). p21 inhibits phosphorylation of Rb protein, which results in inactivation of E2F1 (24–26). At the transcriptional level, we determined that treatment of HepG2 cells with Topo II inhibitors increased p21 mRNA expression but had little effect on both Rb and E2F1 mRNA levels (Fig. 5C). Similarly, teniposide increased p21 protein expression. The increased p21 protein decreased pRb and E2F1 levels (Fig. 5D). To further determine if Topo II inhibitors can inhibit RIP140 transcription by inactivating E2F1, we constructed a plasmid that contained a three-tandem of the E2F1 motif in the RIP140 promoter (PGL-3xE2F1-TK-Luc; Fig. 5E). Both teniposide and etoposide reduced PGL-3xE2F1-TK-Luc promoter activity (Fig. 5F). Taken together, the results in Fig. 5 suggest that treatment of HepG2 cells with Topo II inhibitor induced p21 expression and subsequently inactivated the RIP140 pathway, which can result in release of RIP140 suppression on LXR activity. Thus, the induction of CETP expression by Topo II inhibitors may partially be contributed by inactivation of RIP140 pathway.
Administration of Teniposide Induces Liver CETP Expression and Enhances RCT in CETP Transgenic Mice
Wild type mice do not express CETP naturally; therefore, we used CETP transgenic mice (CETPtg) to determine the effect of teniposide-induced CETP expression on cholesterol metabolism. Both wild type mice and CETPtg mice (background matched) were subcutaneously injected with teniposide solution once every 4 days twice. After 8 days of the initial injection, mice were conducted an RCT assay by intraperitoneal injection of pre-radiolabeled corresponding peritoneal macrophages, and following determination of excreted radioactivity in feces.
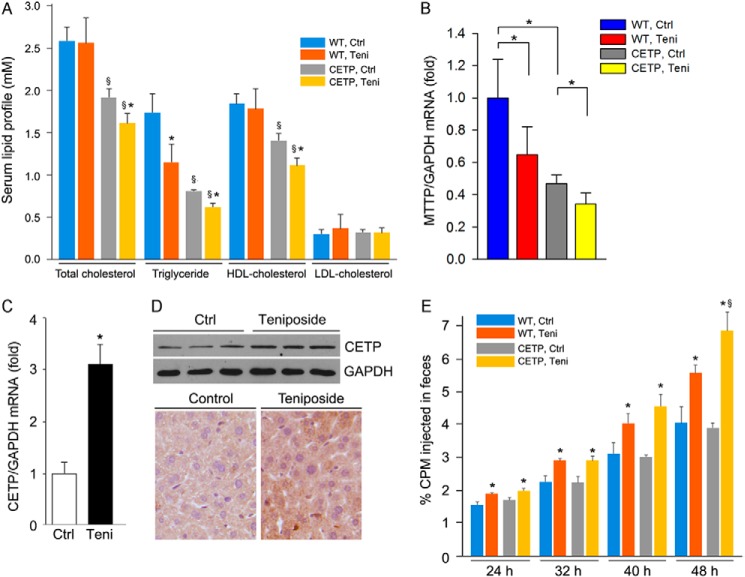
In control animals, compared with wild type mice, expression of CETP results in reduced serum total cholesterol levels, which is mainly due to decreased HDL levels (Fig. 6A), and the decreased HDL levels are in agreement with the function of CETP in vivo. Serum TG levels were also reduced by CETP expression in control CETPtg mice. Administration of teniposide to wild type mice had little effect on serum total, LDL- and HDL-cholesterol levels but showed decreased serum TG levels. In CETPtg mice, serum total, HDL-cholesterol and TG levels were reduced by teniposide. The decreased total cholesterol levels were also mainly contributed by the decreased HDL-cholesterol levels because the changes in both of total and HDL-C levels are close (Fig. 6A). MTTP is a molecule regulating TG secretion from the liver. Compared with wild type mice, expression of MTTP in CETPtg mouse liver was reduced. Meanwhile, treatment of the animals with teniposide reduced MTTP expression in both wild type and CETPtg mouse liver. The decreased MTTP expression may lead to reduced TG secretion from the liver and contribute to the reduced serum TG levels. The decreased HDL-cholesterol levels in CETPtg mice by teniposide may be attributed to the induction of hepatic CETP expression. Indeed, teniposide increased both CETP mRNA and protein expression in CETPtg mouse liver, which were determined by real time RT-PCR, Western blot, and immunohistochemical staining (Fig. 6, C and D).
FIGURE 6.
Teniposide induces liver CETP expression and RCT in CETP transgenic mice. Both wild type (WT) and CETPtg (CETP) mice (5/group) were subcutaneously injected with teniposide solution twice (15 mg/kg bodyweight, once every 4 days). 8 days after of the first injection of teniposide, all the mice were conducted in an RCT assay by intraperitoneal injecting with radiolabeled peritoneal macrophages isolated from the corresponding type mice. Both blood (wild type and CETPtg mice) and liver (CETPtg mice) samples were collected in the following assays. A, serum lipid profiles in both wild type and CETPtg mice. Teni, teniposide. B, expression of MTTP mRNA in the liver of both wild type and CETPtg mice was determined by real time RT-PCR. *, p < 0.05 (n = 5). C and D, expression of CETP mRNA and protein in the liver of CETPtg mice was determined by real-time RT-PCR, Western blot, and immunohistochemical staining. E, fecal samples from each mouse were individually collected at the indicated times after injection of radiolabeled macrophages, and excreted radioactivity was determined. §, versus wild type mice; *, versus control within same type of mice at p < 0.05 (n = 5).
Furthermore, we determined if teniposide can increase RCT in vivo. Fig. 6E shows that teniposide increased RCT in both wild type and CETPtg mice. The increased RCT in wild type mice should be due to the induction of ABCA1 expression by teniposide (14). However, the overall induction of RCT by teniposide was greater in CETPtg mice than in wild type mice, which should be contributed by induction of CETP expression.
Discussion
In this study we determined that inactivation of Topo II induced hepatic CETP expression and secretion. Mechanically, Topo II inhibitors increased LXR expression/nuclear translocation and the binding activity of LXR with the LXRE in CETP promoter but inhibited the RIP140 pathway. These findings suggest that induction of CETP expression by Topo II inhibitor is mediated by activation of LXR pathway. In vivo, administration of teniposide to CETPtg mice induced CETP expression in the liver and RCT more than wild type mice. Thus, our study suggests that Topo II inhibitors can play an important role in regulation of cholesterol metabolism by activating hepatic CETP expression.
The overall role of CETP expression in atherosclerosis is still controversial. The inverse relationship between serum CETP and HDL-C levels leads to the development of CETP pharmacological inhibitors as a potential therapy for atherosclerosis (27). Four Topo II inhibitors have been in phase III clinical trial evaluations. However, two of them (torcetrapib and dalcetrapib) failed, and the third one (anacetrapib) did not show benefits to the patients with coronary heart disease after the preliminary studies. Although these inhibitors raise HDL-C levels while decreasing LDL-C levels, compared with placebo, they either increase or have no effect on the severe cardiovascular events (1). In humans, natural CETP deficiency increases HDL-C levels, which is associated with increased coronary heart disease prevalence in this population (2). In animal models, CETP transgenic mice demonstrate reduced both HDL-C levels and atherosclerotic lesions (15). Expression of CETP also attenuates ovariectomization or testosterone deficiency-induced lesions in mice (16, 17).
The inhibitory effects of CETP on atherosclerosis might be related to the enhanced RCT. Lack of SR-BI expression results in decreased RCT. However, the impaired RCT can be restored to normal by CETP expression (28). The patients with CETP deficiency or receiving CETP inhibitor treatment demonstrate slower apoA-I catabolism (29, 30). Furthermore, the plasma isolated from subjects with high endogenous CETP activity displays increased the capacity to mediate macrophages RCT, which implies that CETP can prevent macrophage/foam cell formation (31). The transfer of cholesteryl esters from HDL to apoB-containing lipoproteins by CETP also leads to reduction of HDL size and generation of lipid-poor HDL or pre-β-HDL (32, 33), a form that is a preferred acceptor of ABCA1-mediated cholesterol efflux from macrophages (34). In addition, CETP can enhance liver delivery and selective uptake of HDL-CE that may further benefit cholesterol metabolism (3, 35). Serum HDL-cholesterol levels and TG levels are reciprocal usually. However, in CETPtg mice it has been reported that changes of serum HDL-cholesterol levels are not associated with serum TG levels reciprocally. For instance, treatment of CETPtg mice with CETP antisense oligonucleotide or CETP inhibitor, anacetrapib, increased serum HDL-cholesterol levels, whereas results were slightly increased or had no effect on serum TG levels (36). In APOE*3-Leiden.CETP transgenic mice, activation of farnesoid X receptor by taurocholic acid increased CETP expression, which was associated with decreased HDL-C and TG levels in serum (37). Similarly, we determined that Topo II inhibitor decreased both serum HDL-C and TG levels in CETPtg mice in our study.
Synthetic LXR ligands, such as T0901317 and GW3965, can induce macrophage ABCA1 expression and cholesterol efflux, thereby inhibiting atherosclerosis. However, LXR ligands also induce hepatic lipogenesis, fatty liver, and hypertriglyceridemia. The severity of lipogenesis is correlated to the doses of LXR ligand used (7). Compared with the ligands with full LXR functions, the selective LXRβ modulators may exert anti-atherogenic properties with minimal side effects. Unfortunately, the progress to discover selective LXRβ modulators is very slow due to the high similarities in both ligand and DNA binding domains between LXRα and LXRβ isoforms (38). We previously reported that etoposide and teniposide can induce macrophage ABCA1 expression. By completing a “molecular docking study,” we determined a much weaker interaction between etoposide or teniposide and amino acid residues within the ligand binding pocket of LXR than T0901317 (14). In this study both etoposide and teniposide induce CETP expression, which is also associated with activation of LXR pathway. Recently, some of cholesterol oxidized derivatives, such as cholesteryl-5α,6α-epoxide, has been reported to specifically inhibit Topo II activity and suppress human cancer cell growth (39), which implied the similar properties between Topo II inhibitors and cholesterol-derived oxysterols on LXR activation.
Etoposide and teniposide also inhibit RIP140 expression (Fig. 5, A and B), a co-repressor of LXR. RIP140 has demonstrated as a transcriptional target of E2F1, a member of E2F transcription factor family regulating a broad spectrum of genes involved in major cellular processes, such as DNA replication, apoptosis, differentiation, and cell cycles (23). Transcriptional activity of E2F1 is regulated by pRb, whereas pRb is inversely regulated by p21. In our study we observed that etoposide and teniposide activated p21 expression and subsequently reduced pRb and E2F1, which may lead to inhibition of RIP140 transcription (Fig. 5, C–F). In fact, a few studies have reported that p21 expression can be activated by Topo II inhibitor or LXR ligand in different cell types (40, 41). Thus, induction of hepatic CETP expression by Topo II inhibitors is partially contributed by inactivation of RIP140 pathway. In addition, we observed that inhibition of Topo IIA expression by siRNA activated CETP expression (Fig. 4, A–C). Altogether, these results indicate that etoposide and teniposide activate the LXR pathway by multiple mechanisms which might distinguish the ligands with full LXR activities. Indeed, in our previous study we observed that teniposide demonstrates a greater effect than T0901317 on induction of macrophage ABCA1 expression at the same concentration (14). Although the interaction between LXR and Topo II protein and the involved precise mechanisms by which to influence LXR activity require more investigation, we anticipate that this interaction may suppress LXR activity. Thus, in the presence of Topo II inhibitors, such as etoposide or teniposide, the interaction will be reduced and consequently activates expression of LXR-targeted genes including CETP.
Compared with mice, the importance of CETP in RCT implies that Topo II inhibitors may enhance RCT with a greater effect in species expressing CETP, such as humans. Indeed, more RCT was induced in CETP transgenic mice than wild type mice by teniposide (Fig. 6E), which might be another mechanism responsible for anti-atherogenic properties of Topo II inhibitors.
This work was supported by Ministry of Science and Technology of China Grant 2010CB945003, the Program for Changjiang Scholars and Innovative Research Team in University (no. IRT13023), and 111 Project B08011 (to J. H.) and National Science Foundation of China (NSFC) Grants 81272460 and 81473204 (to J. H.) and 31400694 (to Y. C.).
- CETP
- cholesteryl ester transfer protein
- LXR
- liver X receptor
- HDL-C
- HDL-cholesterol
- LXRE
- LXR-responsive element
- ABCA1
- ATP binding cassette transporter A1
- RCT
- reverse cholesterol transport
- Topo
- topoisomerase
- MTTP
- microsomal triglyceride transfer protein
- TRITC
- tetramethylrhodamine isothiocyanate
- RIP140
- receptor interacting protein 140
- Rb
- retinoblastoma
- pRb
- phosphorylated Rb
- TG
- triglyceride
- RXR
- retinoid X receptor.
References
- 1. Rader D. J., deGoma E. M. (2014) Future of cholesteryl ester transfer protein inhibitors. Annu. Rev. Med. 65, 385–403 [DOI] [PubMed] [Google Scholar]
- 2. Zhong S., Sharp D. S., Grove J. S., Bruce C., Yano K., Curb J. D., Tall A. R. (1996) Increased coronary heart disease in Japanese-American men with mutation in the cholesteryl ester transfer protein gene despite increased HDL levels. J. Clin. Invest. 97, 2917–2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhou H., Li Z., Silver D. L., Jiang X. C. (2006) Cholesteryl ester transfer protein (CETP) expression enhances HDL cholesteryl ester liver delivery, which is independent of scavenger receptor BI, LDL receptor related protein and possibly LDL receptor. Biochim. Biophys. Acta 1761, 1482–1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Luo Y., Tall A. R. (2000) Sterol up-regulation of human CETP expression in vitro and in transgenic mice by an LXR element. J. Clin. Invest. 105, 513–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Viennois E., Pommier A. J., Mouzat K., Oumeddour A., El Hajjaji F. Z., Dufour J., Caira F., Volle D. H., Baron S., Lobaccaro J. M. (2011) Targeting liver X receptors in human health: deadlock or promising trail? Expert. Opin. Ther. Targets 15, 219–232 [DOI] [PubMed] [Google Scholar]
- 6. Repa J. J., Turley S. D., Lobaccaro J. A., Medina J., Li L., Lustig K., Shan B., Heyman R. A., Dietschy J. M., Mangelsdorf D. J. (2000) Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science 289, 1524–1529 [DOI] [PubMed] [Google Scholar]
- 7. Terasaka N., Hiroshima A., Koieyama T., Ubukata N., Morikawa Y., Nakai D., Inaba T. (2003) T-0901317, a synthetic liver X receptor ligand, inhibits development of atherosclerosis in LDL receptor-deficient mice. FEBS Lett. 536, 6–11 [DOI] [PubMed] [Google Scholar]
- 8. Joseph S. B., McKilligin E., Pei L., Watson M. A., Collins A. R., Laffitte B. A., Chen M., Noh G., Goodman J., Hagger G. N., Tran J., Tippin T. K., Wang X., Lusis A. J., Hsueh W. A., Law R. E., Collins J. L., Willson T. M., Tontonoz P. (2002) Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc. Natl. Acad. Sci. U.S.A. 99, 7604–7609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heisig P. (2009) Type II topoisomerases: inhibitors, repair mechanisms, and mutations. Mutagenesis 24, 465–469 [DOI] [PubMed] [Google Scholar]
- 10. Chikamori K., Grozav A. G., Kozuki T., Grabowski D., Ganapathi R., Ganapathi M. K. (2010) DNA topoisomerase II enzymes as molecular targets for cancer chemotherapy. Curr. Cancer Drug Targets 10, 758–771 [DOI] [PubMed] [Google Scholar]
- 11. Rabinovich G. A., Toscano M. A. (2009) Turning “sweet” on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat. Rev. Immunol. 9, 338–352 [DOI] [PubMed] [Google Scholar]
- 12. de la Llera-Moya M., Rothblat G. H., Glick J. M., England J. M. (1992) Etoposide treatment suppresses atherosclerotic plaque development in cholesterol-fed rabbits. Arterioscler. Thromb. 12, 1363–1370 [DOI] [PubMed] [Google Scholar]
- 13. Tavares E. R., Freitas F. R., Diament J., Maranhão R. C. (2011) Reduction of atherosclerotic lesions in rabbits treated with etoposide associated with cholesterol-rich nanoemulsions. Int. J. Nanomedicine 6, 2297–2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang L., Jiang M., Shui Y., Chen Y., Wang Q., Hu W., Ma X., Li X., Liu X., Cao X., Liu M., Duan Y., Han J. (2013) DNA topoisomerase II inhibitors induce macrophage ABCA1 expression and cholesterol efflux-an LXR-dependent mechanism. Biochim. Biophys. Acta 1831, 1134–1145 [DOI] [PubMed] [Google Scholar]
- 15. Hayek T., Masucci-Magoulas L., Jiang X., Walsh A., Rubin E., Breslow J. L., Tall A. R. (1995) Decreased early atherosclerotic lesions in hypertriglyceridemic mice expressing cholesteryl ester transfer protein transgene. J. Clin. Invest. 96, 2071–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cazita P. M., Berti J. A., Aoki C., Gidlund M., Harada L. M., Nunes V. S., Quintão E. C., Oliveira H. C. (2003) Cholesteryl ester transfer protein expression attenuates atherosclerosis in ovariectomized mice. J. Lipid Res. 44, 33–40 [DOI] [PubMed] [Google Scholar]
- 17. Casquero A. C., Berti J. A., Salerno A. G., Bighetti E. J., Cazita P. M., Ketelhuth D. F., Gidlund M., Oliveira H. C. (2006) Atherosclerosis is enhanced by testosterone deficiency and attenuated by CETP expression in transgenic mice. J. Lipid Res. 47, 1526–1534 [DOI] [PubMed] [Google Scholar]
- 18. Naik S. U., Wang X., Da Silva J. S., Jaye M., Macphee C. H., Reilly M. P., Billheimer J. T., Rothblat G. H., Rader D. J. (2006) Pharmacological activation of liver X receptors promotes reverse cholesterol transport in vivo. Circulation 113, 90–97 [DOI] [PubMed] [Google Scholar]
- 19. Duan Y., Chen Y., Hu W., Li X., Yang X., Zhou X., Yin Z., Kong D., Yao Z., Hajjar D. P., Liu L., Liu Q., Han J. (2012) Peroxisome proliferator-activated receptor γ activation by ligands and dephosphorylation induces proprotein convertase subtilisin kexin type 9 and low density lipoprotein receptor expression. J. Biol. Chem. 287, 23667–23677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhou X., Yin Z., Guo X., Hajjar D. P., Han J. (2010) Inhibition of ERK1/2 and activation of liver X receptor synergistically induce macrophage ABCA1 expression and cholesterol efflux. J. Biol. Chem. 285, 6316–6326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Q., Ma X., Chen Y., Zhang L., Jiang M., Li X., Xiang R., Miao R., Hajjar D. P., Duan Y., Han J. (2014) Identification of interferon-γ as a new molecular target of liver X receptor. Biochem. J. 459, 345–354 [DOI] [PubMed] [Google Scholar]
- 22. Jakobsson T., Osman W., Gustafsson J. A., Zilliacus J., Wärnmark A. (2007) Molecular basis for repression of liver X receptor-mediated gene transcription by receptor-interacting protein 140. Biochem. J. 405, 31–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Docquier A., Augereau P., Lapierre M., Harmand P. O., Badia E., Annicotte J. S., Fajas L., Cavaillès V. (2012) The RIP140 gene is a transcriptional target of E2F1. PloS ONE 7, e35839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sherr C. J., Roberts J. M. (1999) CDK inhibitors: positiver and negative regulators of G1-phase progression. Genes Dev. 13, 1501–1512 [DOI] [PubMed] [Google Scholar]
- 25. Connell-Crowley L., Harper J. W., Goodrich D. W. (1997) Cyclin D1/Cdk4 regulates retinoblastoma protein-mediated cell cycle arrest by site-specific phosphorylation. Mol. Biol. Cell 8, 287–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Park S. H., Lim J. S., Jang K. L. (2011) All-trans retinoic acid induces cellular senescence via up-regulation of p16, p21, and p27. Cancer Lett. 310, 232–239 [DOI] [PubMed] [Google Scholar]
- 27. Shah P. K. (2007) Inhibition of CETP as a novel therapeutic strategy for reducing the risk of atherosclerotic disease. Eur. Heart J. 28, 5–12 [DOI] [PubMed] [Google Scholar]
- 28. Tanigawa H., Billheimer J. T., Tohyama J., Zhang Y., Rothblat G., Rader D. J. (2007) Expression of cholesteryl ester transfer protein in mice promotes macrophage reverse cholesterol transport. Circulation 116, 1267–1273 [DOI] [PubMed] [Google Scholar]
- 29. Ikewaki K., Rader D. J., Sakamoto T., Nishiwaki M., Wakimoto N., Schaefer J. R., Ishikawa T., Fairwell T., Zech L. A., Nakamura H. (1993) Delayed catabolism of high density lipoprotein apolipoproteins A-I and A-II in human cholesteryl ester transfer protein deficiency. J. Clin. Invest. 92, 1650–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brousseau M. E., Diffenderfer M. R., Millar J. S., Nartsupha C., Asztalos B. F., Welty F. K., Wolfe M. L., Rudling M., Björkhem I., Angelin B., Mancuso J. P., Digenio A. G., Rader D. J., Schaefer E. J. (2005) Effects of cholesteryl ester transfer protein inhibition on high-density lipoprotein subspecies, apolipoprotein A-I metabolism, and fecal sterol excretion. Arterioscler. Thromb. Vasc. Biol. 25, 1057–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Villard E. F., El Khoury P., Duchene E., Bonnefont-Rousselot D., Clement K., Bruckert E., Bittar R., Le Goff W., Guerin M. (2012) Elevated CETP activity improves plasma cholesterol efflux capacity from human macrophages in women. Arterioscler. Thromb. Vasc. Biol. 32, 2341–2349 [DOI] [PubMed] [Google Scholar]
- 32. Rye K. A., Barter P. J. (2004) Formation and metabolism of prebeta-migrating, lipid-poor apolipoprotein A-I. Arterioscler. Thromb. Vasc. Biol. 24, 421–428 [DOI] [PubMed] [Google Scholar]
- 33. Rye K. A., Hime N. J., Barter P. J. (1997) Evidence that cholesteryl ester transfer protein-mediated reductions in reconstituted high density lipoprotein size involve particle fusion. J. Biol. Chem. 272, 3953–3960 [DOI] [PubMed] [Google Scholar]
- 34. Favari E., Lee M., Calabresi L., Franceschini G., Zimetti F., Bernini F., Kovanen P. T. (2004) Depletion of pre-beta-high density lipoprotein by human chymase impairs ATP-binding cassette transporter A1- but not scavenger receptor class B type I-mediated lipid efflux to high density lipoprotein. J. Biol. Chem. 279, 9930–9936 [DOI] [PubMed] [Google Scholar]
- 35. Gauthier A., Lau P., Zha X., Milne R., McPherson R. (2005) Cholesteryl ester transfer protein directly mediates selective uptake of high density lipoprotein cholesteryl esters by the liver. Arterioscler. Thromb. Vasc. Biol. 25, 2177–2184 [DOI] [PubMed] [Google Scholar]
- 36. Bell T. A., 3rd, Graham M. J., Lee R. G., Mullick A. E., Fu W., Norris D., Crooke R. M. (2013) Antisense oligonucleotide inhibition of cholesteryl ester transfer protein enhances RCT in hyperlipidemic, CETP transgenic, LDLr−/− mice. J. Lipid Res. 54, 2647–2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gautier T., de Haan W., Grober J., Ye D., Bahr M. J., Claudel T., Nijstad N., Van Berkel T. J., Havekes L. M., Manns M. P., Willems S. M., Hogendoorn P. C., Lagrost L., Kuipers F., Van Eck M., Rensen P. C., Tietge U. J. (2013) Farnesoid X receptor activation increases cholesteryl ester transfer protein expression in humans and transgenic mice. J. Lipid Res. 54, 2195–2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ratni H., Wright M. B. (2010) Recent progress in liver X receptor-selective modulators. Curr. Opin. Drug Discov. Devel. 13, 403–413 [PubMed] [Google Scholar]
- 39. Ishimaru C., Yonezawa Y., Kuriyama I., Nishida M., Yoshida H., Mizushina Y. (2008) Inhibitory effects of cholesterol derivatives on DNA polymerase and topoisomerase activities, and human cancer cell growth. Lipids 43, 373–382 [DOI] [PubMed] [Google Scholar]
- 40. Kim J. H., Chae M., Kim W. K., Kim Y. J., Kang H. S., Kim H. S., Yoon S. (2011) Salinomycin sensitizes cancer cells to the effects of doxorubicin and etoposide treatment by increasing DNA damage and reducing p21 protein. Br. J. Pharmacol. 162, 773–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Blaschke F., Leppanen O., Takata Y., Caglayan E., Liu J., Fishbein M. C., Kappert K., Nakayama K. I., Collins A. R., Fleck E., Hsueh W. A., Law R. E., Bruemmer D. (2004) Liver X receptor agonists suppress vascular smooth muscle cell proliferation and inhibit neointima formation in balloon-injured rat carotid arteries. Circ. Res. 95, e110–e123 [DOI] [PubMed] [Google Scholar]