Background: Insulin stimulates the exocytic translocation of vesicles containing GLUT4 glucose transporters and insulin-regulated aminopeptidase (IRAP).
Results: Insulin acts through TUG proteins to control IRAP targeting, similar to GLUT4; the activity of vasopressin, an IRAP substrate, is reduced in mice with disrupted TUG action in muscle.
Conclusion: TUG regulates vasopressin action.
Significance: Exocytic translocation of vesicles in muscle coordinates vasopressin inactivation with glucose uptake.
Keywords: aminopeptidase, glucose transporter type 4 (GLUT4), insulin, membrane trafficking, skeletal muscle, translocation, vasopressin
Abstract
In adipose and muscle cells, insulin stimulates the exocytic translocation of vesicles containing GLUT4, a glucose transporter, and insulin-regulated aminopeptidase (IRAP), a transmembrane aminopeptidase. A substrate of IRAP is vasopressin, which controls water homeostasis. The physiological importance of IRAP translocation to inactivate vasopressin remains uncertain. We previously showed that in skeletal muscle, insulin stimulates proteolytic processing of the GLUT4 retention protein, TUG, to promote GLUT4 translocation and glucose uptake. Here we show that TUG proteolysis also controls IRAP targeting and regulates vasopressin action in vivo. Transgenic mice with constitutive TUG proteolysis in muscle consumed much more water than wild-type control mice. The transgenic mice lost more body weight during water restriction, and the abundance of renal AQP2 water channels was reduced, implying that vasopressin activity is decreased. To compensate for accelerated vasopressin degradation, vasopressin secretion was increased, as assessed by the cosecreted protein copeptin. IRAP abundance was increased in T-tubule fractions of fasting transgenic mice, when compared with controls. Recombinant IRAP bound to TUG, and this interaction was mapped to a short peptide in IRAP that was previously shown to be critical for GLUT4 intracellular retention. In cultured 3T3-L1 adipocytes, IRAP was present in TUG-bound membranes and was released by insulin stimulation. Together with previous results, these data support a model in which TUG controls vesicle translocation by interacting with IRAP as well as GLUT4. Furthermore, the effect of IRAP to reduce vasopressin activity is a physiologically important consequence of vesicle translocation, which is coordinated with the stimulation of glucose uptake.
Introduction
During periods of increased metabolic activity in muscle, GLUT4 2 glucose transporters translocate to the cell surface and mediate increased rates of glucose uptake (1). Insulin and exercise both mobilize GLUT4 out of internal membranes and insert these transporters into T-tubules to make these membranes permeable to extracellular glucose. The TUG protein is a critical regulator of insulin-stimulated GLUT4 translocation (2–7). TUG traps GLUT4-containing vesicles at the Golgi matrix, and insulin triggers endoproteolytic cleavage of TUG to release GLUT4 into a cell surface recycling pathway. Insulin also acts through Akt2, AS160/Tbc1D4, and Tbc1D1, specific Rab proteins, and other pathways to control overall GLUT4 movement (1, 8). The ability of insulin to stimulate GLUT4 translocation is impaired in insulin-resistant states such as type 2 diabetes.
The GLUT4 storage vesicles (GSVs) that are mobilized by insulin contain a distinct set of cargo proteins. Best characterized in adipocytes, GSVs are thought to exist as a preformed pool of vesicles in unstimulated cells (9). GSV cargos include the insulin-regulated aminopeptidase, IRAP, as well as sortilin, LRP1, and VAMP2 (10–16). During GSV formation, sortilin binds GLUT4, IRAP, and LRP1 in the lumen of the nascent vesicle so that these cargos enter the GSV as a unit during vesicle budding (16, 17). Each GSV contains, on average, 5–6 molecules of GLUT4 and more than 10 molecules of IRAP (18). The importance of IRAP for regulated GLUT4 trafficking has been well documented (19, 20). However, the importance of GSV translocation for physiological functions other than glucose uptake remains uncertain.
IRAP is a transmembrane aminopeptidase that is the rodent ortholog of human placental leucine aminopeptidase (P-LAP, encoded by the LNPEP gene) (21). IRAP is widely expressed and resides primarily on intracellular membranes; in adipose and muscle, it is translocated together with GLUT4 in response to insulin stimulation. Upon translocation, the aminopeptidase domain of IRAP is displayed extracellularly and cleaves circulating substrates including vasopressin (22). In IRAP knock-out mice, the half-life of circulating vasopressin is increased 3-fold, and plasma concentrations are increased 2-fold. Some data suggest that TUG proteins regulate vesicles that contain IRAP as well as GLUT4; however, this has not been demonstrated directly (23). Most work to understand the physiological effects of GSV translocation has focused on glucose metabolism, and the relative importance of IRAP action, when compared with GLUT4 action, remains unknown.
Experimental Procedures
Animals
Transgenic mTUGUBX-Cter mice were described previously (6). Mice were maintained on a C57BL/6J background, and heterozygote male mice were used for experiments. Controls were age-matched, male wild-type mice, usually nontransgenic littermates. Mice were maintained on a standard 12-h/12-h light-dark cycle with ad libitum access to chow (Harlan-Teklad 2018, 5% calories from fat) and water unless otherwise stated. The Yale Institutional Animal Care and Use Committee approved all procedures.
Water Homeostasis
Water intake was measured using metabolic cages (CLAMS, Columbus Instruments). Water restriction experiments were carried out in fasting animals because the effect of the transgene is observed primarily during the fasting state (6). To distinguish weight loss due to water restriction from that due to food removal, sequential measurements were made. Mice were fasted for 18 h, weight loss was recorded, and food was returned for 48–72 h for mice to recover their initial body weights. Mice were then deprived of food and water for 18 h, weight loss was again recorded, and food and water were returned. The weight loss attributable to water removal was calculated from the differences in weight loss and plotted as a percentage of the initial body weight (before food restriction) for each mouse. For measurement of renal AQP2 protein abundances, mice were sacrificed after food and water restriction, and whole kidney lysates were immunoblotted using antibodies to AQP2 (Thermo Fisher) and α-tubulin (Sigma). For measurement of plasma copeptin concentrations, mice were fasted for 4–6 h and were allowed free access to water. Mice were sacrificed, and plasma copeptin concentrations were measured using an ELISA kit (Cedarlane Labs).
Muscle Fractionation
T-tubule-enriched membrane fractions were isolated from quadriceps of fasting mice essentially as described (6, 24). Briefly, muscles (∼200 mg/sample) were minced, added to 2 ml of ice-cold Buffer A (20 mm Na4P2O7, 20 mm NaH2PO4, 1 mm MgCl2, 0.3 m sucrose, 0.5 mm EDTA, 20 mm iodoacetamide, two protease inhibitor tablets (Roche Applied Science) per 50 ml). Samples were homogenized using a Polytron tissue grinder for 10–15 s at 8000 rpm and then centrifuged at 13,000 rpm for 20 min at 4 °C using an SS-34 rotor (Sorvall). Pellets were resuspended in 1.7 ml of Buffer A, homogenized again using a Polytron (13,500 rpm for 45 s), and then centrifuged at 11,000 rpm for 20 min at 4 °C using an SS-34 rotor. The supernatant was centrifuged in a TLA-120.2 rotor (Beckman) at 18,000 rpm for 10 min at 4 °C. Pellets were resuspended in 200 μl of Buffer A. To strip myofibrillar proteins, 500 μl of Buffer B (0.3 m sucrose, 20 mm Tris, pH 7.0) and 300 μl of 4 m KCl were added (the final KCl concentration was 1.2 m), and samples were incubated at 4 °C for 1 h with gentle agitation. Samples were then centrifuged at 57,000 rpm in a TLA-120.2 rotor for 10 min at 4 °C. The T-tubule-enriched membrane pellets were resuspended in 150 μl of Buffer B and analyzed by SDS-PAGE and immunoblotting as described (6). Antibodies to IRAP were raised in rabbits using a peptide corresponding to IRAP residues 17–33 (Covance) and were also purchased from Cell Signaling Technology. An antibody to insulin receptor β-chain was purchased from Millipore.
Recombinant Protein Expression and Pulldowns
GST fusion proteins with IRAP residues 2–109, 55–108, and 2–52 were generated as described (25) and were visualized using GelCode Blue staining (Pierce). A synthetic peptide corresponding to IRAP residues 55–84, with a biotin group and two aminohexanoic acid spacers at its N terminus, was synthesized at the Yale Keck Biotechnology Resource facility. IRAP fragments or peptides were immobilized on glutathione or streptavidin beads, respectively, and used for purification of TUG proteins from 3T3-L1 adipocyte or 293 cell lysates. 293 cells were transfected to express intact or truncated TUG proteins, as described (2). Pulldowns were performed in 20 mm Tris, pH 8.0, 150 mm NaCl, 2 mm EDTA, and either 1% Triton X-100 or 1% IGEPAL CA-630 (Sigma). Eluted proteins were analyzed by SDS-PAGE and immunoblotting using an antibody to the TUG C terminus (2).
Cell Culture, Vesicle Purification, and Cell Surface Biotinylation
293 cells were cultured and 3T3-L1 cells were cultured and differentiated as described (5). To purify TUG-bound vesicles, the site-specific biotinylating enzyme, BirA, was cloned in the pBICD4 retrovirus vector (2, 26–28). A form of the TUG protein containing the biotin acceptor peptide at its C terminus was coexpressed, using the pBICD2 retrovirus vector (2, 28). Stable 3T3-L1 cells expressing both proteins were isolated using FACS. After differentiation into adipocytes, cells were treated with or without 80 nm insulin for 15 min and then homogenized in TES buffer (250 mm sucrose, 10 mm Tris, pH 7.4, 0.5 mm EDTA, and 20 mm iodoacetamide) using a Dounce-type tissue grinder. Homogenates were microcentrifuged at 13,000 rpm for 15 min at 4 °C, and then microsomes remaining in the supernatant were incubated with streptavidin-agarose (NeutrAvidin, Pierce) or, as a control, biotin-saturated streptavidin-agarose. Nonspecific binding was reduced by preincubating the agarose beads using streptavidin blocking buffer for 2–3 h (Solulink). After incubation of the lysates for 2–16 h at 4 °C, the beads were pelleted and washed, and proteins were eluted in sample buffer and analyzed by SDS-PAGE and immunoblotting. Biotinylation of cell surface proteins in 3T3-L1 adipocytes was done as described (23), except that some biotinylation reactions were allowed to proceed at 4 °C for 40 min rather than at 37 °C for 12 min.
Statistical Analysis
Data are presented as mean ± S.E. Significance was assessed using an unpaired, two-tailed t test, and differences were considered significant at p < 0.05.
Results
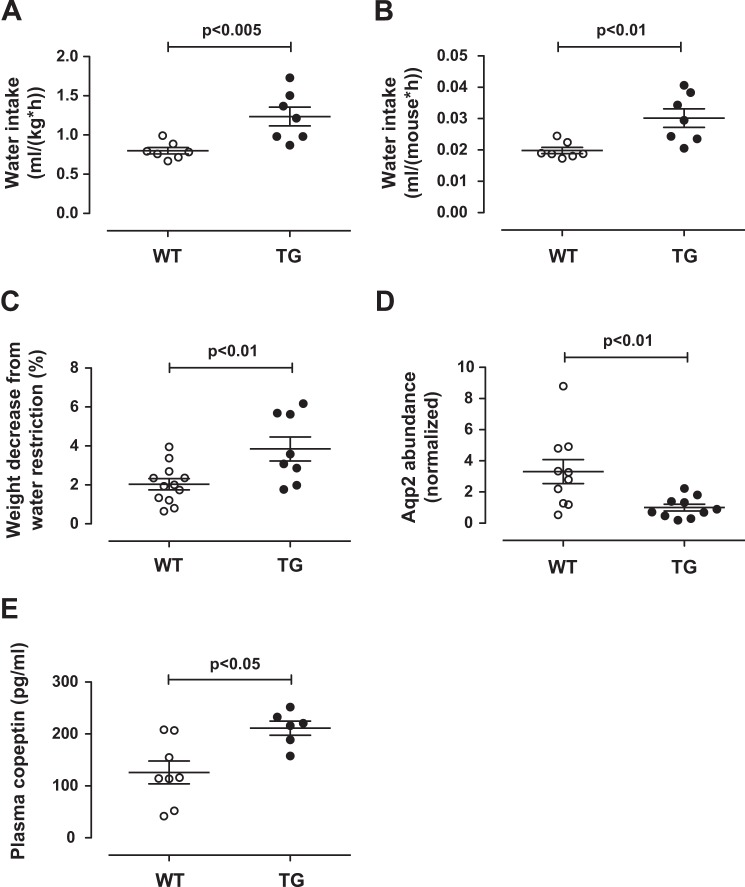
We recently described transgenic mTUGUBX-Cter mice, which have constitutive endoproteolytic cleavage of TUG proteins in skeletal muscle in the absence of insulin stimulation (6). As predicted, these mice exhibit GLUT4 translocation during the fasting state so that fasting plasma glucose and insulin concentrations are reduced and whole-body glucose turnover is increased. In these mice, the UBX-Cter transgene activates TUG proteolysis, similar to insulin, but it does so incompletely and only in a subset of muscles in which it is highly expressed. Because the transgene is not fully effective in all muscles, the increase in whole-body glucose turnover during fasting is limited to 17% (6). Unexpectedly, and in the setting of this partial effect of the transgene, metabolic cage studies showed that the mTUGUBX-Cter transgenic mice had a 55% increase in water intake, when compared with wild-type control mice (Fig. 1A). This increase in water consumption could not be attributed to an ∼3% reduction in body weight in transgenic mice (6). When analyzed on a per mouse basis, the increased water intake remained highly significant, as shown in Fig. 1B.
FIGURE 1.
Reduced vasopressin action and increased copeptin in mTUGUBX-Cter mice. A and B, water intake was measured in 12-week-old control (WT) and mTUGUBX-Cter TG (TG) mice and is plotted per body weight (A) and per mouse (B). C, WT and TG mice were treated without food for 18 h, allowed to recover, and then treated without food and water for 18 h. The weight decrease attributable to water removal is plotted as a percentage of initial body weight. D, mice were sacrificed after removal of food and water, and kidney lysates were immunoblotted to detect the vasopressin-responsive protein AQP2 and, as a control, α-tubulin. The relative abundance of AQP2 is plotted in WT and TG mice. E, plasma concentrations of copeptin, a product of the vasopressin prohormone, were measured in fasted WT and TG mice with free access to water. Data are presented as mean ± S.E.; n = 6–12 in each group.
We hypothesized that the increased water intake results from reduced vasopressin activity in the transgenic mice, when compared with wild-type controls. This could occur if TUG cleavage causes translocation of IRAP together with GLUT4 because cell surface-exposed IRAP cleaves and inactivates circulating vasopressin (22). The transgenic mice would then have an impaired ability to concentrate urine. To test the prediction that the transgenic mice would have greater weight loss during water restriction, we used an 18-h water deprivation test (29). We performed this study on fasting animals because the effect of the transgene is maximal during fasting, and we controlled for weight loss due to food removal. Fig. 1C shows that when compared with wild-type controls, mTUGUBX-Cter transgenic mice had greater weight loss attributable to water deprivation. These data support the idea that mTUGUBX-Cter transgenic mice are impaired in their ability to concentrate urine.
To further test vasopressin activity, we measured renal AQP2 abundance, which is stimulated by vasopressin and can serve as a bioassay for vasopressin activity (30, 31). Vasopressin regulates water permeability in the renal collecting system by both minute-to-minute effects on AQP2 targeting and hour-to-day-long effects on AQP2 abundance (30, 32). We considered that the longer-term effects on AQP2 abundance would be most relevant in mTUGUBX-Cter transgenic mice, which are predicted to have chronically accelerated inactivation of circulating vasopressin. Supporting this idea, Fig. 1D shows that AQP2 protein was less abundant in kidneys of water-deprived mTUGUBX-Cter mice, when compared with wild-type controls. Renal AQP2 is present exclusively in parts of the nephron where vasopressin regulates the osmotic transport of water to concentrate urine (32). Although other cells contribute material to whole kidney lysates, gross and histologic studies revealed no differences between kidneys of transgenic and wild-type mice (6). Thus, the observed difference in AQP2 abundance can be reasonably considered to reflect actions of vasopressin on the collecting duct system. Together, the data support the notion that vasopressin activity is reduced in mTUGUBX-Cter transgenic mice when compared with wild-type controls.
Vasopressin itself is a 9-residue peptide that is present in blood plasma at very low concentrations, so it is difficult to measure. Copeptin is a cleavage product of the vasopressin prohormone, analogous to the C-peptide of insulin (33, 34). Copeptin is both larger and more abundant than vasopressin, and the development of an assay to measure copeptin as a surrogate marker for vasopressin was considered an important advance (35). In fasted mTUGUBX-Cter mice with free access to water, we predicted that vasopressin secretion would be greater than that in wild-type control mice. Supporting this idea, plasma copeptin was increased in mTUGUBX-Cter mice, when compared with controls (Fig. 1E). Thus, although vasopressin activity is decreased, its secretion is increased. The data support the notion that vasopressin inactivation is accelerated in mice with constitutive translocation of TUG-regulated vesicles in muscle.
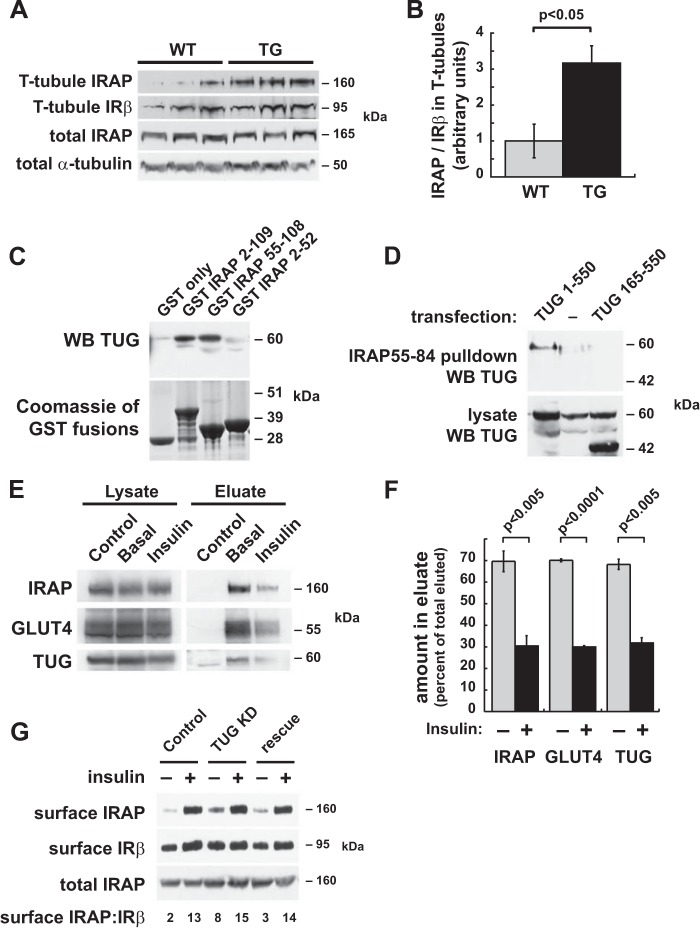
To test whether IRAP is targeted to the cell surface in muscles of mTUGUBX-Cter mice, we isolated T-tubule-enriched membrane fractions from quadriceps muscles of fasting animals, similar to our previous work on GLUT4 (6). Immunoblots of these membranes demonstrated that IRAP is more abundant in T-tubules isolated from transgenic mice, when compared with those from wild-type controls (Fig. 2, A and B). There was no effect of the transgene on the abundance of total IRAP, supporting the idea that TUG regulates IRAP targeting in muscle. The magnitude of the increase in T-tubule IRAP (3.2-fold) was similar to that observed for GLUT4 (3.6-fold) in similar experiments (6). Together with previous work showing that IRAP regulates the half-life of circulating vasopressin (22), we conclude that the targeting of IRAP to T-tubules in mTUGUBX-Cter mice can account, at least in part, for the observed effects on vasopressin and water homeostasis.
FIGURE 2.
TUG binds IRAP and controls its targeting. A and B, T-tubule membrane fractions were prepared from quadriceps muscles of fasted mTUGUBX-Cter TG and WT control mice. Immunoblots were performed to detect IRAP and insulin receptor β-chain (IRβ), as indicated. B, densitometry was done on immunoblots from A, and the relative abundance of IRAP in T-tubule fractions was plotted. C, GST fusion proteins containing the indicated regions of the IRAP cytosolic N terminus were used to purify TUG from lysates of transfected 293 cells. TUG was detected by immunoblotting (WB), and GST proteins were stained using GelCode Blue. D, a biotinylated peptide containing IRAP residues 55–84 was used to purify TUG from lysates of 293 cells transfected with full-length TUG (residues 1–550) or with a truncated form (residues 165–550). Purified proteins and lysates were immunoblotted as indicated. E, TUG-bound vesicles were purified from basal and insulin-stimulated 3T3-L1 adipocytes expressing biotin-tagged TUG, using immobilized streptavidin beads. Control precipitations were done using biotin-saturated streptavidin. Eluted proteins were immunoblotted to detect IRAP, GLUT4, and TUG as indicated. F, replicates of the experiment shown in E were analyzed using densitometry of the immunoblots, and data were quantified and plotted. Data are presented as mean ± S.E.; n = 3. G, cell surface proteins were biotinylated in control 3T3-L1 adipocytes, in cells containing an shRNA to deplete TUG (TUG KD), and in cells containing both the shRNA and the shRNA-resistant TUG (rescue). Cells were treated with insulin as indicated. Surface-exposed proteins were purified and immunoblotted to detect IRAP and, as a control, IRβ. The ratio of surface IRAP to surface IRβ band intensity is indicated below each lane.
The TUG protein is proposed to regulate GSVs and interacts with GLUT4 (3). However, insulin-regulated GSV trafficking occurs to some degree in cells lacking GLUT4, and IRAP is proposed as the more important protein for regulated GSV targeting (19, 36, 37). To test whether TUG binds IRAP, we performed pulldown experiments. As shown in Fig. 2C, recombinant IRAP proteins were able to purify TUG from transfected 293 cells. Both the entire IRAP cytosolic region (residues 2–109) and the membrane-proximal region (residues 55–108) were sufficient to bind TUG. A recombinant protein containing IRAP residues 55–82 was sufficient to redistribute GLUT4 to the cell surface when microinjected into 3T3-L1 adipocytes (38). Therefore, we tested whether a peptide corresponding to IRAP residues 55–84 binds TUG. As shown in Fig. 2D, this peptide was sufficient to pull down TUG from transfected cells. Additionally, this interaction required TUG residues 1–164 and was not observed when a truncated form of TUG containing only residues 165–550 was expressed. Insulin triggers site-specific cleavage of TUG at the bond joining residues 164–165 (5). The N-terminal product has properties of a novel ubiquitin-like protein modifier, TUGUL (TUG ubiquitin-like), and is hypothesized to help carry the GSVs to the cell periphery (7). Therefore, an interaction of TUGUL with IRAP, as well as with the large intracellular loop of GLUT4 (3), may facilitate GSV movement to the cell surface.
To further test whether IRAP is present in TUG-bound vesicles, we expressed a biotin-tagged TUG protein in 3T3-L1 adipocytes. We used retroviruses to express BirA, a site-specific biotinylating enzyme, together with a form of TUG containing the biotin acceptor peptide at its C terminus (26, 27). This approach permits the rapid, single-step purification of protein complexes or, in the absence of detergent, TUG-bound vesicles. Subcellular fractionation experiments detected no large effect of the tagged TUG protein on GLUT4 translocation and showed that the tagged protein colocalized with native TUG (not shown). As shown in Fig. 2E, IRAP and GLUT4 were present in vesicles bound to biotin-tagged TUG in 3T3-L1 adipocytes. Insulin caused similar reductions in the amounts of IRAP, GLUT4, and TUG that were purified, consistent with previous data showing that insulin stimulates TUG proteolysis to liberate GSVs (5). The data were quantified in Fig. 2F, which is also consistent with previous data showing that insulin stimulates the dissociation of intact TUG from immunoprecipitated GLUT4 (2). The results support the idea that insulin stimulates TUG cleavage in 3T3-L1 adipocytes and that the biotin-tagged TUG C-terminal product does not bind strongly to either IRAP or GLUT4. The TUG C-terminal product was not observed in the eluates, likely because it has limited stability (5). The data suggest that cytosolic TUG in 3T3-L1 adipocytes also has limited stability in the homogenates, consistent with the idea that intact TUG is stabilized by incorporation into a complex together with membrane-bound interacting proteins (5). In particular, the central region of TUG has low sequence complexity and may be unstructured in the absence of a binding partner(s). Together, the data support the notion that IRAP is present together with GLUT4 in TUG-regulated GSVs in 3T3-L1 adipocytes as well as in skeletal muscle.
To confirm that TUG regulates IRAP cell surface targeting in 3T3-L1 adipocytes, we biotinylated surface-exposed proteins and purified them on immobilized streptavidin. We used previously described control cells, “TUG KD” cells containing an shRNA to deplete TUG, and “rescue” cells containing this shRNA as well as shRNA-resistant wild-type TUG (3, 23). As shown in Fig. 2G, insulin stimulated the translocation of IRAP to the cell surface in control cells. In TUG KD cells, IRAP was observed at the cell surface in the unstimulated state. This effect of TUG depletion was similar to that observed for GLUT4 (3) and was rescued by shRNA-resistant TUG. As a control, we observed no marked effect on cell surface insulin receptor β-chain in the same samples. The data are consistent with Fig. 2A, which shows that IRAP is distributed to T-tubule-enriched membrane fractions in skeletal muscle of mTUGUBX-Cter transgenic mice. Together, the results support the idea that intact TUG is required for intracellular retention of IRAP in unstimulated 3T3-L1 adipocytes, as well as during fasting in skeletal muscle in mice.
Discussion
The data presented here show that TUG regulates IRAP translocation to the cell surface in both skeletal muscle and 3T3-L1 adipocytes. Our data support the idea that in mTUGUBX-Cter transgenic mice, IRAP translocation in muscle results in accelerated inactivation of vasopressin and systemic effects on water homeostasis. To compensate for the accelerated vasopressin turnover, the transgenic mice both drank more water and secreted more vasopressin, as indicated by plasma copeptin measurements. Together with previous results (2–7), the data show that vasopressin inactivation and glucose uptake are coordinately regulated by the translocation of TUG-bound vesicles to the cell surface.
IRAP, which is present in at least twice as many copies per vesicle as GLUT4, is likely the most abundant GSV cargo (16, 18). Our data suggest that there is a physiological correlate for this molecular stoichiometry. In mTUGUBX-Cter mice, the 55% increase in water consumption was ∼3-fold greater than the 17% increase in whole-body fasting glucose turnover (6). In these mice, the UBX-Cter transgene did not fully activate TUG cleavage and glucose uptake in all muscles, which likely mitigated effects on whole-body glucose homeostasis. By similar reasoning, if IRAP translocation was not fully activated in all muscles, then the data here may underestimate the potential whole-body effect to reduce vasopressin action. The data imply that a major physiological effect of GSV translocation is to diminish vasopressin action.
Why should vasopressin inactivation be coordinately regulated with glucose uptake? One possibility is that during periods of increased metabolic activity in muscles, termination of vasopressin action helps to clear the water that is produced from oxidation of glucose. This effect may be more important during muscle contraction, when compared with insulin stimulation, because insulin stimulates primarily nonoxidative glucose disposal. It is not known whether TUG regulates contraction-stimulated glucose uptake in muscle, or even whether the same GSVs are mobilized by contraction and insulin stimulation (39, 40). A second possibility is that inactivation of vasopressin results in dilation of terminal arterioles and helps to recruit microvascular units and increase capillary blood flow in stimulated muscles (41, 42). Although this may normally be a localized action, systemic effects of reduced vasopressin could also result because of the mass of skeletal muscle and the enormous surface area of the T-tubule system (43).
The water produced by glucose oxidation can amount to a substantial fraction of intravascular volume. Our mice consumed ∼250 mg of carbohydrate/day (6). If this is considered as glucose that is completely oxidized, it would generate ∼150 μl of water, or ∼11% of the estimated blood volume. An alternative calculation is based on whole-body glucose turnover. In fasting wild-type control mice, glucose turnover was measured and was ∼0.25 mg/mouse/min, of which 85–90% was oxidized (6). Over the course of 24 h, this amount would generate ∼200 μl of water, equivalent to ∼15% of estimated blood volume. These rough calculations make it clear that oxidation produces a substantial amount of water, which must be disposed of to maintain blood osmolality within a narrow range.
Impaired GLUT4 translocation results in insulin resistance, and impaired IRAP translocation may contribute to altered vasopressin action in insulin-resistant individuals. Indeed, plasma copeptin concentrations are increased in insulin resistance, and copeptin has been termed a “unifying factor” underlying the metabolic syndrome (44). Impaired insulin-stimulated translocation of these proteins may reflect increased abundance at the cell surface during the basal state, as well as decreased abundance at the cell surface in the insulin-stimulated state. Increased cell surface IRAP is observed in unstimulated adipose of type 2 diabetics, when compared with controls (45). In skeletal muscle, a trend toward increased IRAP in T-tubule membranes is observed in fasting individuals with insulin resistance or diabetes, when compared with control subjects (46). Therefore, increased copeptin concentrations in insulin-resistant individuals may reflect, at least in part, accelerated inactivation of vasopressin, together with a compensatory increase in vasopressin secretion. Whether this may contribute to hypertension in the setting of insulin resistance is not known, and is worthy of further study (35, 47, 48).
IRAP is more important than GLUT4 itself for the regulated trafficking of GSVs (19, 20, 36, 37). Critical to this function of IRAP is a dileucine motif and an acidic patch within its 109-residue cytosolic N terminus (49–51). A recombinant IRAP protein containing this sequence caused GLUT4 translocation when injected into 3T3-L1 adipocytes, implying that it may interact with a protein involved in vesicle targeting (38). Acyl-CoA dehydrogenases bind this sequence, but the physiological significance of this interaction remains uncertain (52). Our data show that this sequence also interacts with TUG and that TUG residues 1–164 were required for this interaction. This region of TUG forms tandem ubiquitin-like folds (5, 53). Previous work supports a model in which TUG cleavage produces a ubiquitin-like modifier, TUGUL, which contains these domains and helps to carry GSVs toward the cell surface. This model fits well with the idea that IRAP binds TUGUL (5, 7). The IRAP peptide resembles a SUMO-interacting motif, which also contains an acidic patch and a dileucine sequence, suggesting that it may interact in a similar way with a ubiquitin-like domain of TUGUL (54–57). Of note, TUGUL also binds the large intracellular loop of GLUT4 (3). Thus, the specificity of TUG binding to GSVs may result from a combinatorial interaction with multiple GSV cargos. The second ubiquitin-like domain of TUGUL does not fold stably in the absence of a binding partner (53). A possibility is that binding of IRAP, GLUT4, and possibly other proteins to TUGUL stabilizes this fold. Such stabilization could confer susceptibility to a deubiquitinase-like enzyme that catalyzes TUG cleavage and could thus contribute to the specificity of regulated translocation for GSVs and not other vesicles (5).
The data here support the idea that GSV translocation coordinately regulates glucose uptake and vasopressin inactivation. We speculate that other aspects of physiology and metabolism may also be subject to this coordinated regulation. In adipocytes, GSVs contain the LDL receptor-related protein LRP1, and sortilins are present in GSVs in both fat and muscle cells (14, 58–60). These proteins bind ApoA-V and ApoE to mediate uptake of triglyceride-rich lipoproteins; in addition, LRP1 modulates lipid metabolism by controlling Wnt signaling (58, 61–63). Consistent with the idea that GSV translocation participates in uptake of these lipoproteins, mTUGUBX-Cter mice have reduced plasma triglycerides (6). This may also result from decreased de novo lipogenesis during fasting because insulin concentrations were reduced in the transgenic animals. Even so, together with other results, the data here raise the possibility that multiple aspects of physiology and metabolism may be coordinately regulated by TUG cleavage and vesicle translocation and that defects in this pathway may contribute to multiple aspects of the metabolic syndrome in humans.
Acknowledgments
We thank Dr. Vassily Ogryzko for the BirA construct and Drs. Mark Knepper, Gerald Shulman, and Charisse Orme for helpful discussions and assistance. This work used the Cell Biology Core of the Yale Diabetes Endocrinology Research Center (supported by National Institutes of Health Grant P30 DK45735), the Yale Mouse Metabolic Phenotyping Center (National Institutes of Health Grant U24 DK059635), and the Physiology Core of the George M. O'Brien Kidney Center at Yale (National Institutes of Health Grant P30 DK079310), as well as services provided by the W. M. Keck Foundation Biotechnology Resource Laboratory at Yale University.
This work was supported, in whole or in part, by National Institutes of Health Grants R21AG041383 and R01DK092661 (to J. S. B.), by a Pilot and Feasibility grant (to J. S. B.) from the George M. O'Brien Kidney Center at Yale (P30DK079310), and by American Diabetes Association Grant 1-12-BS-16 (to J. S. B.). This work was also supported by National Institutes of Health Grants F30DK093198 (to J. P. B.) T32DK007058 (to E. N. H.) and by Department of Veterans Affairs Grants I01BX000901 (to V. T. S.) and I01BX000702 (to N. W. C.).
- GLUT4
- glucose transporter 4
- GSV
- GLUT4 storage vesicle
- IRAP
- insulin-regulated aminopeptidase
- IRβ
- insulin receptor β-chain
- TG
- transgenic
- TUG
- tether containing a UBX domain for GLUT4
- mTUG
- mouse TUG
- TUGUL
- TUG ubiquitin-like
- UBX
- ubiquitin regulatory X
- UBX-Cter
- UBX-C terminus.
References
- 1. Bogan J. S. (2012) Regulation of glucose transporter translocation in health and diabetes. Annu. Rev. Biochem. 81, 507–532 [DOI] [PubMed] [Google Scholar]
- 2. Bogan J. S., Hendon N., McKee A. E., Tsao T. S., Lodish H. F. (2003) Functional cloning of TUG as a regulator of GLUT4 glucose transporter trafficking. Nature 425, 727–733 [DOI] [PubMed] [Google Scholar]
- 3. Yu C., Cresswell J., Löffler M. G., Bogan J. S. (2007) The glucose transporter 4-regulating protein TUG is essential for highly insulin-responsive glucose uptake in 3T3-L1 adipocytes. J. Biol. Chem. 282, 7710–7722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xu Y., Rubin B. R., Orme C. M., Karpikov A., Yu C., Bogan J. S., Toomre D. K. (2011) Dual-mode of insulin action controls GLUT4 vesicle exocytosis. J. Cell Biol. 193, 643–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bogan J. S., Rubin B. R., Yu C., Löffler M. G., Orme C. M., Belman J. P., McNally L. J., Hao M., Cresswell J. A. (2012) Endoproteolytic cleavage of TUG protein regulates GLUT4 glucose transporter translocation. J. Biol. Chem. 287, 23932–23947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Löffler M. G., Birkenfeld A. L., Philbrick K. M., Belman J. P., Habtemichael E. N., Booth C. J., Castorena C. M., Choi C. S., Jornayvaz F. R., Gassaway B. M., Lee H. Y., Cartee G. D., Philbrick W., Shulman G. I., Samuel V. T., Bogan J. S. (2013) Enhanced fasting glucose turnover in mice with disrupted action of TUG protein in skeletal muscle. J. Biol. Chem. 288, 20135–20150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Belman J. P., Habtemichael E. N., Bogan J. S. (2014) A proteolytic pathway that controls glucose uptake in fat and muscle. Rev. Endocr. Metab. Disord. 15, 55–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klip A., Sun Y., Chiu T. T., Foley K. P. (2014) Signal transduction meets vesicle traffic: the software and hardware of GLUT4 translocation. Am. J. Physiol. Cell Physiol. 306, C879–C886 [DOI] [PubMed] [Google Scholar]
- 9. Xu Z., Kandror K. V. (2002) Translocation of small preformed vesicles is responsible for the insulin activation of glucose transport in adipose cells: evidence from the in vitro reconstitution assay. J. Biol. Chem. 277, 47972–47975 [DOI] [PubMed] [Google Scholar]
- 10. Kandror K. V., Pilch P. F. (1994) gp160, a tissue-specific marker for insulin-activated glucose transport. Proc. Natl. Acad. Sci. U.S.A. 91, 8017–8021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Keller S. R., Scott H. M., Mastick C. C., Aebersold R., Lienhard G. E. (1995) Cloning and characterization of a novel insulin-regulated membrane aminopeptidase from Glut4 vesicles. J. Biol. Chem. 270, 23612–23618 [DOI] [PubMed] [Google Scholar]
- 12. Lin B. Z., Pilch P. F., Kandror K. V. (1997) Sortilin is a major protein component of Glut4-containing vesicles. J. Biol. Chem. 272, 24145–24147 [DOI] [PubMed] [Google Scholar]
- 13. Morris N. J., Ross S. A., Lane W. S., Moestrup S. K., Petersen C. M., Keller S. R., Lienhard G. E. (1998) Sortilin is the major 110-kDa protein in GLUT4 vesicles from adipocytes. J. Biol. Chem. 273, 3582–3587 [DOI] [PubMed] [Google Scholar]
- 14. Jedrychowski M. P., Gartner C. A., Gygi S. P., Zhou L., Herz J., Kandror K. V., Pilch P. F. (2010) Proteomic analysis of GLUT4 storage vesicles reveals LRP1 to be an important vesicle component and target of insulin signaling. J. Biol. Chem. 285, 104–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bogan J. S., Kandror K. V. (2010) Biogenesis and regulation of insulin-responsive vesicles containing GLUT4. Curr. Opin. Cell Biol. 22, 506–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kandror K. V., Pilch P. F. (2011) The sugar is sIRVed: sorting Glut4 and its fellow travelers. Traffic 12, 665–671 [DOI] [PubMed] [Google Scholar]
- 17. Pilch P. F. (2008) The mass action hypothesis: formation of Glut4 storage vesicles, a tissue-specific, regulated exocytic compartment. Acta Physiol. (Oxf.) 192, 89–101, 10.1111/j.1748-1716.2007.01788.x [DOI] [PubMed] [Google Scholar]
- 18. Kupriyanova T. A., Kandror V., Kandror K. V. (2002) Isolation and characterization of the two major intracellular Glut4 storage compartments. J. Biol. Chem. 277, 9133–9138 [DOI] [PubMed] [Google Scholar]
- 19. Jordens I., Molle D., Xiong W., Keller S. R., McGraw T. E. (2010) Insulin-regulated aminopeptidase is a key regulator of GLUT4 trafficking by controlling the sorting of GLUT4 from endosomes to specialized insulin-regulated vesicles. Mol. Biol. Cell 21, 2034–2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yeh T. Y., Sbodio J. I., Tsun Z. Y., Luo B., Chi N. W. (2007) Insulin-stimulated exocytosis of GLUT4 is enhanced by IRAP and its partner tankyrase. Biochem. J. 402, 279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Keller S. R. (2003) The insulin-regulated aminopeptidase: a companion and regulator of GLUT4. Front. Biosci. 8, s410–s420 [DOI] [PubMed] [Google Scholar]
- 22. Wallis M. G., Lankford M. F., Keller S. R. (2007) Vasopressin is a physiological substrate for the insulin-regulated aminopeptidase IRAP. Am. J. Physiol. Endocrinol. Metab. 293, E1092–E1102 [DOI] [PubMed] [Google Scholar]
- 23. Belman J. P., Bian R. R., Habtemichael E. N., Li D. T., Jurczak M. J., Alcázar-Román A., McNally L. J., Shulman G. I., Bogan J. S. (2015) Acetylation of TUG protein promotes the accumulation of GLUT4 glucose transporters in an insulin-responsive intracellular compartment. J. Biol. Chem. 290, 4447–4463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zorzano A., Camps M. (2006) Isolation of T-tubules from skeletal muscle. Curr. Protoc. Cell Biol. Chapter 3, Unit 3.24, 10.1002/0471143030.cb0324s31 [DOI] [PubMed] [Google Scholar]
- 25. Chi N. W., Lodish H. F. (2000) Tankyrase is a Golgi-associated mitogen-activated protein kinase substrate that interacts with IRAP in GLUT4 vesicles. J. Biol. Chem. 275, 38437–38444 [DOI] [PubMed] [Google Scholar]
- 26. de Boer E., Rodriguez P., Bonte E., Krijgsveld J., Katsantoni E., Heck A., Grosveld F., Strouboulis J. (2003) Efficient biotinylation and single-step purification of tagged transcription factors in mammalian cells and transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 100, 7480–7485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mechold U., Gilbert C., Ogryzko V. (2005) Codon optimization of the BirA enzyme gene leads to higher expression and an improved efficiency of biotinylation of target proteins in mammalian cells. J. Biotechnol. 116, 245–249 [DOI] [PubMed] [Google Scholar]
- 28. Liu X., Constantinescu S. N., Sun Y., Bogan J. S., Hirsch D., Weinberg R. A., Lodish H. F. (2000) Generation of mammalian cells stably expressing multiple genes at predetermined levels. Anal. Biochem. 280, 20–28 [DOI] [PubMed] [Google Scholar]
- 29. Yang B., Zhao D., Qian L., Verkman A. S. (2006) Mouse model of inducible nephrogenic diabetes insipidus produced by floxed aquaporin-2 gene deletion. Am. J. Physiol. Renal Physiol. 291, F465–F472 [DOI] [PubMed] [Google Scholar]
- 30. Wilson J. L., Miranda C. A., Knepper M. A. (2013) Vasopressin and the regulation of aquaporin-2. Clin. Exp. Nephrol. 17, 751–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miranda C. A., Lee J. W., Chou C. L., Knepper M. A. (2014) Tolvaptan as a tool in renal physiology. Am. J. Physiol. Renal Physiol. 306, F359–F366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Knepper M. A., Kwon T. H., Nielsen S. (2015) Molecular physiology of water balance. N. Engl. J. Med. 372, 1349–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morgenthaler N. G., Struck J., Alonso C., Bergmann A. (2006) Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin. Chem. 52, 112–119 [DOI] [PubMed] [Google Scholar]
- 34. Morgenthaler N. G., Struck J., Jochberger S., Dünser M. W. (2008) Copeptin: clinical use of a new biomarker. Trends Endocrinol. Metab. 19, 43–49 [DOI] [PubMed] [Google Scholar]
- 35. Bankir L., Bouby N., Ritz E. (2013) Vasopressin: a novel target for the prevention and retardation of kidney disease? Nat. Rev. Nephrol. 9, 223–239 [DOI] [PubMed] [Google Scholar]
- 36. Gross D. N., Farmer S. R., Pilch P. F. (2004) Glut4 storage vesicles without Glut4: transcriptional regulation of insulin-dependent vesicular traffic. Mol. Cell. Biol. 24, 7151–7162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hosaka T., Brooks C. C., Presman E., Kim S. K., Zhang Z., Breen M., Gross D. N., Sztul E., Pilch P. F. (2005) p115 interacts with the GLUT4 vesicle protein, IRAP, and plays a critical role in insulin-stimulated GLUT4 translocation. Mol. Biol. Cell 16, 2882–2890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Waters S. B., D'Auria M., Martin S. S., Nguyen C., Kozma L. M., Luskey K. L. (1997) The amino terminus of insulin-responsive aminopeptidase causes Glut4 translocation in 3T3-L1 adipocytes. J. Biol. Chem. 272, 23323–23327 [DOI] [PubMed] [Google Scholar]
- 39. Cartee G. D. (2015) Roles of TBC1D1 and TBC1D4 in insulin- and exercise-stimulated glucose transport of skeletal muscle. Diabetologia 58, 19–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Richter E. A., Hargreaves M. (2013) Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol. Rev. 93, 993–1017 [DOI] [PubMed] [Google Scholar]
- 41. Wagenmakers A. J., Strauss J. A., Shepherd S. O., Keske M. A., Cocks M. (2015) Increased muscle blood supply and transendothelial nutrient and insulin transport induced by food intake and exercise: effect of obesity and ageing. J. Physiol. 10.1113/jphysiol.2014.284513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Muniyappa R., Sowers J. R. (2013) Role of insulin resistance in endothelial dysfunction. Rev. Endocr. Metab. Disord. 14, 5–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fraser J. A., Huang C. L., Pedersen T. H. (2011) Relationships between resting conductances, excitability, and t-system ionic homeostasis in skeletal muscle. J. Gen. Physiol. 138, 95–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Enhörning S., Struck J., Wirfält E., Hedblad B., Morgenthaler N. G., Melander O. (2011) Plasma copeptin, a unifying factor behind the metabolic syndrome. J. Clin. Endocrinol. Metab. 96, E1065–E1072 [DOI] [PubMed] [Google Scholar]
- 45. Maianu L., Keller S. R., Garvey W. T. (2001) Adipocytes exhibit abnormal subcellular distribution and translocation of vesicles containing glucose transporter 4 and insulin-regulated aminopeptidase in type 2 diabetes mellitus: implications regarding defects in vesicle trafficking. J. Clin. Endocrinol. Metab. 86, 5450–5456 [DOI] [PubMed] [Google Scholar]
- 46. Garvey W. T., Maianu L., Zhu J. H., Brechtel-Hook G., Wallace P., Baron A. D. (1998) Evidence for defects in the trafficking and translocation of GLUT4 glucose transporters in skeletal muscle as a cause of human insulin resistance. J. Clin. Invest. 101, 2377–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mironova E., Bugaj V., Roos K. P., Kohan D. E., Stockand J. D. (2012) Aldosterone-independent regulation of the epithelial Na+ channel (ENaC) by vasopressin in adrenalectomized mice. Proc. Natl. Acad. Sci. U.S.A. 109, 10095–10100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mironova E., Chen Y., Pao A. C., Roos K. P., Kohan D. E., Bugaj V., Stockand J. D. (2015) Activation of ENaC by AVP contributes to the urinary concentrating mechanism and dilution of plasma. Am. J. Physiol. Renal Physiol. 308, F237–F243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Johnson A. O., Lampson M. A., McGraw T. E. (2001) A di-leucine sequence and a cluster of acidic amino acids are required for dynamic retention in the endosomal recycling compartment of fibroblasts. Mol. Biol. Cell 12, 367–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hou J. C., Suzuki N., Pessin J. E., Watson R. T. (2006) A specific dileucine motif is required for the GGA-dependent entry of newly synthesized insulin-responsive aminopeptidase into the insulin-responsive compartment. J. Biol. Chem. 281, 33457–33466 [DOI] [PubMed] [Google Scholar]
- 51. Watson R. T., Hou J. C., Pessin J. E. (2008) Recycling of IRAP from the plasma membrane back to the insulin-responsive compartment requires the Q-SNARE syntaxin 6 but not the GGA clathrin adaptors. J. Cell Sci. 121, 1243–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Katagiri H., Asano T., Yamada T., Aoyama T., Fukushima Y., Kikuchi M., Kodama T., Oka Y. (2002) Acyl-coenzyme A dehydrogenases are localized on GLUT4-containing vesicles via association with insulin-regulated aminopeptidase in a manner dependent on its dileucine motif. Mol. Endocrinol. 16, 1049–1059 [DOI] [PubMed] [Google Scholar]
- 53. Tettamanzi M. C., Yu C., Bogan J. S., Hodsdon M. E. (2006) Solution structure and backbone dynamics of an N-terminal ubiquitin-like domain in the GLUT4-regulating protein, TUG. Protein Sci. 15, 498–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hecker C. M., Rabiller M., Haglund K., Bayer P., Dikic I. (2006) Specification of SUMO1- and SUMO2-interacting motifs. J. Biol. Chem. 281, 16117–16127 [DOI] [PubMed] [Google Scholar]
- 55. Ouyang J., Shi Y., Valin A., Xuan Y., Gill G. (2009) Direct binding of CoREST1 to SUMO-2/3 contributes to gene-specific repression by the LSD1/CoREST1/HDAC complex. Mol. Cell 34, 145–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Song J., Durrin L. K., Wilkinson T. A., Krontiris T. G., Chen Y. (2004) Identification of a SUMO-binding motif that recognizes SUMO-modified proteins. Proc. Natl. Acad. Sci. U.S.A. 101, 14373–14378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sun H., Hunter T. (2012) Poly-small ubiquitin-like modifier (PolySUMO)-binding proteins identified through a string search. J. Biol. Chem. 287, 42071–42083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hofmann S. M., Zhou L., Perez-Tilve D., Greer T., Grant E., Wancata L., Thomas A., Pfluger P. T., Basford J. E., Gilham D., Herz J., Tschöp M. H., Hui D. Y. (2007) Adipocyte LDL receptor-related protein-1 expression modulates postprandial lipid transport and glucose homeostasis in mice. J. Clin. Invest. 117, 3271–3282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ariga M., Nedachi T., Katagiri H., Kanzaki M. (2008) Functional role of sortilin in myogenesis and development of insulin-responsive glucose transport system in C2C12 myocytes. J. Biol. Chem. 283, 10208–10220 [DOI] [PubMed] [Google Scholar]
- 60. Tsuchiya Y., Hatakeyama H., Emoto N., Wagatsuma F., Matsushita S., Kanzaki M. (2010) Palmitate-induced down-regulation of sortilin and impaired GLUT4 trafficking in C2C12 myotubes. J. Biol. Chem. 285, 34371–34381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nilsson S. K., Christensen S., Raarup M. K., Ryan R. O., Nielsen M. S., Olivecrona G. (2008) Endocytosis of apolipoprotein A-V by members of the low density lipoprotein receptor and the VPS10p domain receptor families. J. Biol. Chem. 283, 25920–25927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Terrand J., Bruban V., Zhou L., Gong W., El Asmar Z., May P., Zurhove K., Haffner P., Philippe C., Woldt E., Matz R. L., Gracia C., Metzger D., Auwerx J., Herz J., Boucher P. (2009) LRP1 controls intracellular cholesterol storage and fatty acid synthesis through modulation of Wnt signaling. J. Biol. Chem. 284, 381–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Willnow T. E., Kjølby M., Nykjaer A. (2011) Sortilins: new players in lipoprotein metabolism. Curr. Opin. Lipidol. 22, 79–85 [DOI] [PubMed] [Google Scholar]