Background: The autocrine regulatory effects of apelin on self-remodeling of adipose tissue are not known.
Results: Apelin enhances not only the differentiation and metabolic activity of brown adipocytes but also the browning of white adipocytes.
Conclusion: Apelin-APJ signaling promotes adipose tissue browning.
Significance: Apelin signaling may serve as a potential therapeutic target for obesity and associated metabolic diseases.
Keywords: apelin, adipocyte, adipose tissue, browning, uncoupling protein 1, metabolic diseases
Abstract
Brown adipose tissue expends energy in the form of heat via the mitochondrial uncoupling protein UCP1. Recent studies showed that brown adipose tissue is present in adult humans and may be exploited for its anti-obesity and anti-diabetes actions. Apelin is an adipocyte-derived hormone that plays important roles in energy metabolism. Here, we report that apelin-APJ signaling promotes brown adipocyte differentiation by increasing the expressions of brown adipogenic and thermogenic transcriptional factors via the PI3K/Akt and AMPK signaling pathways. It is also found that apelin relieves the TNFα inhibition on brown adipogenesis. In addition, apelin increases the basal activity of brown adipocytes, as evidenced by the increased PGC1α and UCP1 expressions, mitochondrial biogenesis, and oxygen consumption. Finally, we provide both in vitro and in vivo evidence that apelin is able to increase the brown-like characteristics in white adipocytes. This study, for the first time, reveals the brown adipogenic and browning effects of apelin and suggests a potential therapeutic route to combat obesity and related metabolic disorders.
Introduction
Brown adipose tissue (BAT)2 is the primary site for non-shivering thermogenesis in response to cold exposure, especially in newborns, through the actions of heat-producing brown fat cells (1, 2). Mediated by mitochondrial uncoupling protein 1 (UCP1), brown adipocytes have the ability to uncouple oxidative metabolism of energy substrates (glucose and fat) from ATP synthesis, leading to heat production and energy consumption (1). Recent studies have demonstrated that metabolically active BAT, which was thought to disappear after infancy, is present in adult humans and is inversely correlated with body mass index and visceral fat mass (2–4). Studies have shown that an increased amount of BAT is associated with weight loss in obese subjects, and increased brown fat cell differentiation leads to an increase in energy consumption and reduction in body weight gain and diet-induced obesity (5–7). BAT also regulates glucose homeostasis and improves insulin sensitivity (8, 9), and brown adipogenesis is suppressed in human subjects with insulin resistance (10). Hence, stimulating brown fat formation could be a novel route to fight against obesity and related metabolic disorders (11).
In contrast, excess accumulation of white adipose tissue (WAT) has notorious effects on the development of metabolic disorders, such as diabetes and cardiovascular diseases (12). Interestingly, brown fat-like cells (also known as beige cells) have been identified in WAT, and brown fat-like characteristics (e.g. UCP1 expression) are reduced in WAT of obese humans (13). Studies have demonstrated that an increase of beige adipocytes in WAT enhances whole-body energy expenditure and should expectedly reduce the risk of diet-induced obesity and metabolic diseases (14, 15). Differentiation of white adipocytes into beige cells occurs in response to prolonged cold exposure and can be stimulated by hormones such as β-adrenergic receptor agonists, irisin, thyroid hormone, fibroblast growth factor 21 (FGF21), etc. (15–17). On the other hand, it is increasingly recognized that WAT is not merely a passive depot for storage of excess energy as fat but also the largest endocrine organ, producing a variety of bioactive factors called adipokines (18). These adipokines play essential roles in regulating energy metabolism and also influence adipose tissue's own remodeling and functions (18–20). Emerging evidence also suggests that adipokines are important for adipose browning. For example, adiponectin and leptin modulate UCP1 expression in rodent BAT (21, 22), whereas interleukin-6 is required for cold-induced UCP1 expression in WAT of mice (23).
Apelin is a relatively recent member of the adipokine family (24). Apelin regulates various aspects of energy metabolism through APJ receptors and has been known for its anti-obesity and anti-diabetic properties (25). Apelin promotes insulin sensitivity and glucose utilization in adipose and muscle tissues (26, 27). Apelin injection decreases WAT mass and serum triglyceride level in obese mice, whereas apelin knock-out mice have augmented body adiposity and serum free fatty acid levels (28). Apelin also suppresses white adipocyte differentiation and lipolysis (28, 29). The antagonistic effects of apelin on diet-induced obesity and insulin resistance are attributable to its stimulation on UCP1 expression in BAT and body energy expenditure (30, 31). Because apelin production in adipocytes and its plasma level are elevated in obesity (24), we speculate that apelin may provide a beneficial feedback control on both brown and white adipocytes. Here, we aim for the first time to reveal the effects of apelin on adipose browning (differentiation of brown preadipocytes in BAT, metabolic activities of mature brown adipocytes, and browning of white adipocytes in WAT).
Experimental Procedures
Primary Preadipocyte Culture and Differentiation
The primary rat brown preadipocytes isolated from interscapular BAT were purchased from CosmoBio Co. Ltd. (Tokyo, Japan). In some experiments, the primary white and brown preadipocytes, isolated from mouse WAT and interscapular BAT, were also used. After digestion with 0.2% collagenase and centrifugation (1,500 rpm, 5 min), the stromal vascular fraction/preadipose cells from WAT and BAT were isolated as described before (32). Preadipose cells were seeded in 6-well plates and cultured with the basal growth medium (DMEM supplemented with 10% fetal bovine serum (FBS), 1 nm 3,5,3′-triiodothyronine, 1% penicillin and streptomycin) at 37 °C and in a humidified atmosphere containing 5% CO2 and 95% air. For adipocyte differentiation, fully confluent cells (defined as day 0) were treated for 2 days with induction medium (basal growth medium supplemented with 0.5 μg/ml insulin, 1 μm dexamethasone, 0.5 mm isobutylmethylxanthine). At day 2, the cells were incubated for another 2 days with growth medium containing insulin, and thereafter, the medium was replenished every other day for the next 6–8 days. The Transwell indirect contact co-culture system (Corning Inc.) was used to co-culture the primary rat brown preadipocytes and primary rat macrophages (isolated from adult rat bone marrow tissue; ScienCell Research Laboratories).
The human preadipocytes (Zen-Bio Inc.), collected from subcutaneous WAT (non-diabetic subject, body mass index 25–29.9), were cultured in DMEM/F-12 (1:1, v/v) containing 10% FBS until confluence. Adipocyte differentiation was then induced similarly as reported previously (33, 34). Specifically, the cells were treated for 4 days with human adipocyte growth medium (serum-free DMEM/F-12 containing 33 μm biotin, 17 μm pantothenate, 10 μg/ml transferrin, 1 nm 3,5,3′-triiodothyronine) supplemented with 0.5 μg/ml insulin, 0.5 mm isobutylmethylxanthine, 1 μm dexamethasone, and 1 μm troglitazone. The cells were then incubated in the growth medium containing insulin and dexamethasone for 12–14 days (replenishment every other day). The mouse 3T3-L1 cells (white preadipose cell line, ATCC) were cultured in DMEM supplemented with 10% bovine calf serum until confluence. The cells were then treated with the induction medium for 2 days (days 0–2), followed by growing for an additional 2 days in the growth medium containing insulin alone (days 2–4). Finally, the cells were grown for 4–6 days without insulin.
The human mesenchymal stem cells (hMSCs; Lonza Inc.) collected from bone marrow of normal subjects were grown in the basal growth medium until confluence. The stem cells were subsequently induced to differentiate into adipocytes as described previously (35). Specifically, stem cells were treated for 3 days in the induction medium (plus 200 μm indomethacin). In the next 12–14 days, the cells were maintained in the growth medium containing insulin alone (replenishment every other day). Adipocyte differentiation was confirmed by Oil Red O staining, visual appearance of intracellular lipid droplets, and immunoblot analyses of adipocyte-specific proteins (e.g. adipocyte protein 2 (ap2), leptin) as well as brown adipocyte-specific proteins (e.g. UCP1). At least two different batches (donors) of the primary cells were used for every experiment. All culture media, supplements, and sera were purchased from Life Technologies, Inc. Pyr-apelin13 (pyroglutamated 13-amino acid peptide), insulin, and troglitazone were purchased from Tocris Bioscience. All other reagents and chemicals were obtained from Sigma-Aldrich.
Animal Studies
All animal experimental protocols were approved by the SingHealth Research Facilities Institutional Animal Care and Use Committee (Singapore). Mice (C57BL/6J, 8–10 weeks old, male) were housed in light- and temperature-controlled facility (12-h light/12-h dark cycle, 21 °C), and allowed free access to standard laboratory food and water. Mice were divided into apelin-treated and non-treated (100 μl of saline, control) groups. Based on the previous studies (30, 31), apelin was given at a dose of 0.1 μmol/kg/day (dissolved in 100 μl of saline) by intraperitoneal injection for 12 days. Animals were sacrificed 24 h after the last dose, and body and fat tissue weights were measured in all animals. Inguinal WAT, epididymal WAT, and interscapular BAT were removed and frozen in liquid nitrogen for further experiments. Tissues were homogenized in radioimmunoprecipitation assay buffer (containing protease inhibitor mixture) (Roche Applied Science), and protein concentration was determined by a bicinchoninic acid (BCA) protein assay (Pierce) before the immunoblot analyses were carried out (using the same amount of the protein lysates).
siRNA Silencing
Gene silencing of apelin (APLN) or APJ receptor (APLNR) in brown preadipocytes was achieved using a rat APLN siRNA or APLNR siRNA (Ambion Silencer Select, Life Technology), respectively. Akt siRNAs (sc-43610 and sc-108059; Santa Cruz Biotechnology, Inc.) and AMPK siRNAs (sc- 45313 and sc-270142) were also used for gene silencing of Akt or AMPK in adipose cells. Transfection was done as described previously (33). Specifically, adipose cells were incubated with Opti-MEM containing a complex of Lipofectamine-RNAiMAX transfection reagent (0.5% (v/v), Life Technologies, Inc.) with siRNAs (25 nm) for 4 h, followed by the addition of basal growth medium and incubation for another 1–2 days. Knockdowns of protein expression were confirmed by immunoblot analyses.
Confocal Microscopy
Adipocytes grown on Lab-Tek II chambered coverglass were fixed with 3.7% formaldehyde in PBS for 15 min at room temperature and then washed twice with ice-cold PBS. In some experiments, the cells were permeabilized with 0.1% Triton X-100 for 10 min. After being blocked with 1% bovine albumin (BSA) in PBST (PBS with 0.1% Tween 20) at room temperature for 1 h, the cells were then incubated (4 °C) overnight in PBST with 1% BSA and primary antibodies (Santa Cruz Biotechnology): anti-apelin IgG (sc-33805), anti-APJ IgG (sc-33823), anti-UCP1 IgG (sc-6529), anti-β1-AR IgG (sc-568), or anti β3-AR IgG (sc-50436). After washing with PBS, the cells were incubated in FITC-labeled or Atto647 NHS-labeled secondary antibody (Sigma) for 1 h at room temperature, followed by washing three times with PBS. Adipocytes were then imaged using a confocal laser scanning microscope (LSM 510 Meta, Carl Zeiss GmbH). To reveal the mitochondrial membrane potential and morphology, adipocytes were incubated with the growth medium containing 0.2 μm MitoTracker Red FM (Life Technologies) (excitation/emission, 581 nm/644 nm) (at 37 °C with 5% CO2 in a humidified incubator) for 15 or 30 min, before live cell imaging with a confocal microscopy. MitoTracker Red is widely used to assess both mitochondrial morphology and membrane potential in living cells, because it is selectively localized in mitochondria, whereas its accumulation is dependent on transmembrane potential (34, 36–38).
Western Blot Analyses
The cells were washed with ice-cold PBS and collected in mammalian protein extraction reagent containing Halt protease and phosphatase inhibitor mixture (Fisher). After brief vortexing and centrifugation at 4 °C, the supernatant was collected, and the protein content was determined by a BCA protein assay (Pierce). Each sample with an equal amount of proteins was separated through 12% SDS-PAGE before it was transferred onto a nitrocellulose membrane. The membranes were then blocked for 2 h at room temperature in Superblock blocking buffer, followed by subsequent incubation for 12 h in Tris-buffered saline-Tween solution (TBST) containing primary antibody (1:100–400 dilution). After washing three times with TBST, the membrane was incubated for 6 h with horseradish peroxidase-conjugated secondary antibody (1:2,000–4,000). The protein bands were detected in a G:BOX Chemi XT4 imaging system (Syngene) using SuperSignal West Pico chemiluminescent substrate (Fisher). Antibodies against apelin (sc-33469), APJ (sc-33823), UCP1 (sc-6529), CIDE-A (sc-366814), aP2 (sc-18661), COX1 (sc-23982), C/EBPβ (sc-150), leptin (Ob, sc-9014), PPARγ (sc-7196), PGC-1α (sc-13067), PRDM16 (sc-55697), β1-AR (sc-568), β3-AR (sc-50436), Akt (sc-8312), phospho-Akt (Ser-474, sc-135651), AMPKα1/2 (sc-25792), or phospho-AMPKα1/2 (Thr-172; sc-33524) were purchased from Santa Cruz Biotechnology. Antibodies against UCP1 (PA1–24894) or PRDM16 (PA5-20872) from Fisher were also used in our experiments.
Oxygen Consumption Rate Assay
Oxygen consumption was assessed using an oxygen consumption rate assay kit (MitoXpress-Xtra HS method) (Cayman Chemical) according to the manufacturer's instructions. Briefly, preadipocytes were grown in 96-well plates (3 × 104 cells/well) until confluence and induced to differentiate into adipocytes. Adipocytes were treated with 10 μl of MitoXpress-Xtra (a phosphorescent oxygen-sensitive probe whose signal increases over time when O2 is depleted in the solution) immediately before measuring the fluorescence intensity (excitation/emission, 380 nm/650 nm at 37 °C, 0.5-min interval) using a time-resolved fluorescence plate reader (SpectraMax M5, Molecular Devices). Measurement was performed under a sealed environment (by overlaying each well with HS Mineral Oil), in which the exchange of O2 was limited. Oxygen-consuming glucose oxidase was used as a reference.
Statistical Analyses
Data were analyzed as mean ± S.E. Statistical differences between two means were assessed using Student's t test (unpaired, two-tailed). One-way analysis of variance (ANOVA) (followed by Tukey's multiple-comparison test) was used to compare differences among multiple groups. A p value of <0.05 was considered to be statistically significant.
Results
Apelin-APJ Receptor Promotes Brown Adipocyte Differentiation
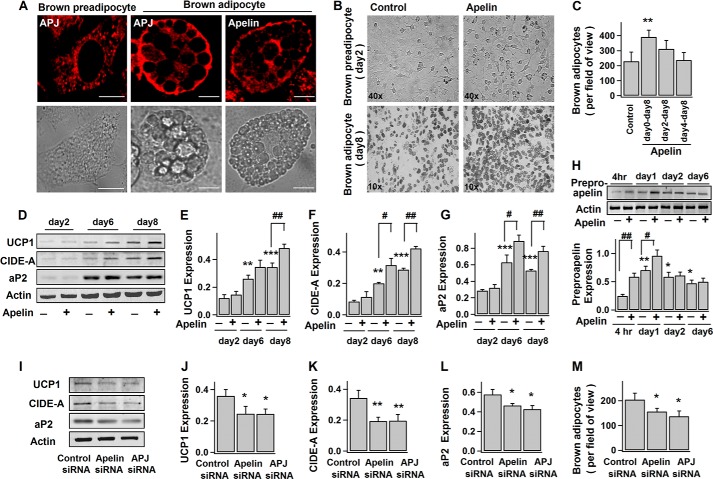
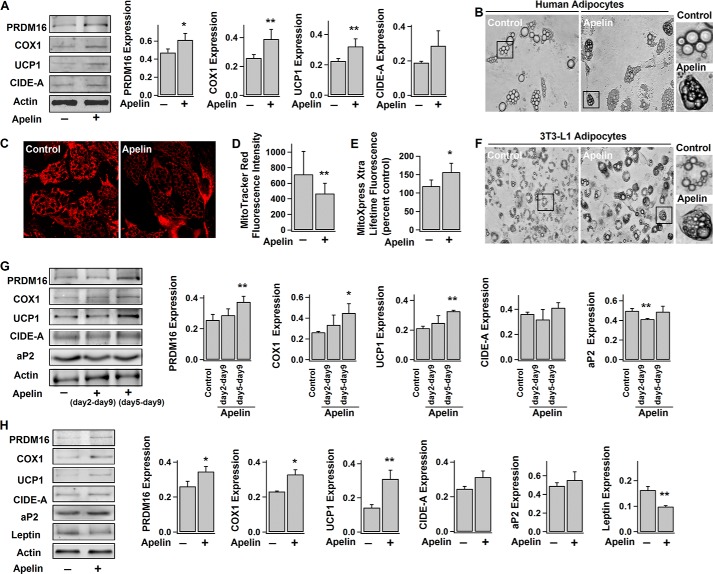
Apelin/APJ system is present in BAT (39). Here, we found that both apelin and APJ receptor are expressed in brown preadipocytes and adipocytes (Fig. 1, A and H). As shown in Fig. 1, B and C, exogenous application of apelin (since the induction of differentiation at day 0) significantly increased the number of differentiated brown adipocytes indicated by their multilocular appearance. Pyr-apelin13 (referred to as apelin) was used because it is the most active and major apelin isoform enzymatically cleaved from preproapelin (40, 41). Consistently, apelin also promoted the progressive expressions of UCP1 (thermogenesis marker), CIDE-A (brown adipocyte-specific marker), and aP2 (late marker of differentiated adipocyte) (Fig. 1, D–G) during brown adipogenesis.
FIGURE 1.
Apelin-APJ receptor enhances brown adipocyte differentiation. A, confocal images of immunostained apelin and APJ receptor in brown fat cells and their corresponding bright field images. Scale bars, 10 μm. B–H, brown preadipocytes were induced to differentiate into adipocytes without (−) (control) or (+) with exposure to 100 nm pyr-apelin13 for different periods of time. B, representative images of brown preadipocytes (day 2) and adipocytes (day 8). C, number of brown adipocytes (multiloculated cells) (at day 8) per field of view (×10); mean ± S.E. (error bars) (n = 4). D–G, time course of the protein expression levels during adipogenesis: UCP1 (∼33 kDa), CIDE-A (∼26 kDa), aP2 (∼16 kDa), and actin (∼42 kDa). The representative immunoblots and the statistics (mean ± S.E., n = 4) of the blot densities normalized to actin density (protein expressions at day 2 regarded as control). H, representative immunoblot of preproapelin (∼8 kDa) expression in brown fat cells at different stages of differentiation and the statistics (mean ± S.E., n = 4; blot densities were normalized to that of actin) (expression at 4 h as control). I–M, brown preadipocytes were transfected with control, apelin, or APJ siRNA 2–3 days before the induction of differentiation. The representative immunoblot of protein expressions (day 10) and the statistics (mean ± S.E., n = 4; blot densities were normalized to that of actin) are shown in I–L. The number of brown adipocytes (day 8) per field of view (×10) (mean ± S.E., n = 4) is shown in M. Each sample contains the same amount of total proteins (as the loading control). Using Student's t test, *, p < 0.05; **, p < 0.01 versus control; #, p < 0.05; ##, p < 0.01 between the indicated pairs.
However, apelin applied at the later stages of differentiation (at day 2 or 4) failed to increase the number of brown adipocytes (Fig. 1C), suggesting the importance of apelin signaling in the initiation of brown adipogenesis. As shown in Fig. 1H, after the initial burst increase (first day), preproapelin expression remained steady during brown adipogenesis, and the initial increase of preproapelin can be largely enhanced by exogenous apelin, suggesting that apelin may serve as the positive feedback signal to initiation of differentiation. In support of this notion, the number of mature brown adipocytes as well as expressions of UCP1, CIDE-A, and aP2 are considerably reduced when apelin and APJ in brown preadipocytes were knocked down by siRNAs (Fig. 1, I–M). Taken together, we provide evidence that apelin-APJ promotes brown adipocyte differentiation through autocrine feedback regulation.
Apelin Remedies Inflammation-impaired Brown Adipogenesis
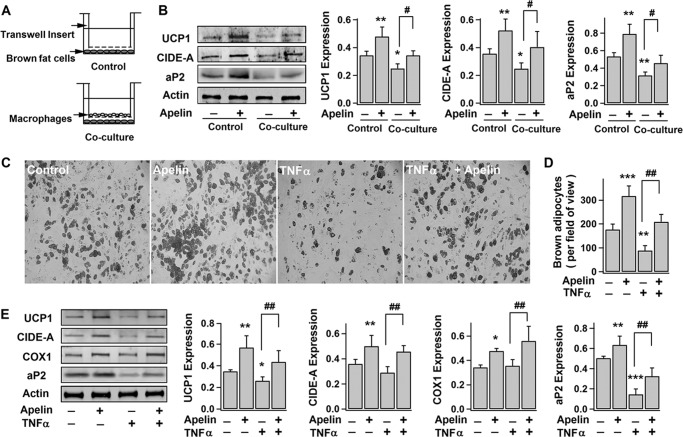
BAT mass is lower in obese humans (3, 4). Studies also suggest that adipose tissue inflammation (as characterized by accumulation of macrophages and inflammatory cytokines, such as TNFα) found in obesity may be responsible for reduced brown fat by inhibiting differentiation and inducing apoptosis of brown adipocytes (42–45). To further investigate this, indirect co-culturing of brown preadipocytes and macrophages (primary rat macrophages) was performed (Fig. 2A) (46). As shown in Fig. 2B, co-culturing with macrophages (days 0–2) significantly decreased the expressions of UCP1, CIDE-A, and aP2 in brown fat cells (at day 10), implying that inflammatory factors released from macrophages antagonize brown adipocyte differentiation. This is supported by the observation that direct application of TNFα (major inflammatory factor released from macrophages) reduced the number of brown adipocytes and expressions of brown adipocyte markers (Fig. 2, C–E). Interestingly, apelin was able to relieve the inhibitory effects of macrophages and TNFα (Fig. 2, B–E). Together with the fact that apelin expression in adipose tissue and plasma apelin level are elevated in obesity (24), our results suggest a compensatory increase in apelin to inhibited brown adipogenesis by adipose inflammation.
FIGURE 2.
Apelin improves TNFα- and macrophage-impaired brown adipocyte differentiation. A and B, brown preadipocytes were co-cultured with primary rat macrophages during the first 2 days of differentiation, followed by continuing differentiation for 8 days. A, illustration of Transwell co-culturing system. The representative immunoblots of protein expressions (UCP1, CIDE-A, aP2, actin) (day 10) in brown adipocytes and the statistics (mean ± S.E. (error bars), n = 4) of blot densities normalized to that of actin are shown in B. C–E, brown preadipocytes were induced to differentiate into brown adipocytes for 8–10 days, without (−) (control) or with (+) exposure to pyr-apelin13 and/or 10 ng/ml TNFα. Shown are representative bright field images (C) and the number of brown adipocytes (at day 8) per field of view (×10) (mean ± S.E., n = 4) (D). The representative immunoblots of UCP1, CIDE-A, COX1 (∼57 kDa), aP2, and actin expressions (day 10) and the statistics (mean ± S.E., n = 4) of blot densities normalized to that of actin are shown in E. Using one-way ANOVA, *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus control (no treatment); #, p < 0.05; ##, p < 0.01 between the indicated pairs.
Apelin Stimulates Expressions of Brown Adipogenic and Thermogenic Transcriptional Factors
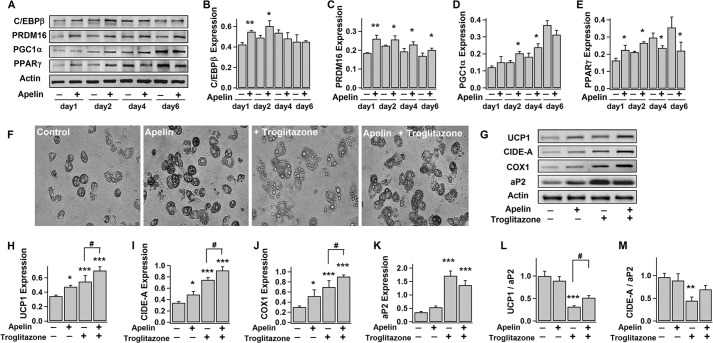
A number of transcriptional factors govern the process of adipocyte differentiation and terminal determination of brown adipocytes (e.g. UCP1 expression) (47). Among them, C/EBPβ and PPARγ are critical to initiate both white and brown adipogenesis (47, 48), whereas PRDM16 and PGC1α are essential to brown fat determination by stimulating expressions of thermogenic and brown-specific genes and repressing the expression of white-specific genes (49–51). Here, we observed that PPARγ and PGC1α exhibited a progressive increase of expression during differentiation, whereas C/EBPβ and PRDM16 levels were steady (Fig. 3, A–E). This is consistent with previous reports (49, 52). Notably, apelin treatment increased the expressions of C/EBPβ, PPARγ, and PGC1α in the early stage of differentiation and up-regulated the PRDM16 level throughout the differentiation period (Fig. 3). This is in line with the findings shown in Figs. 1 and 2 that apelin increases the number of differentiated brown adipocytes as well as the expressions of brown fat proteins required for thermogenesis and mitochondrial biogenesis (UCP1, CIDE-A, and COX1).
FIGURE 3.
Apelin increases the expressions of brown adipogenic and thermogenic transcriptional factors. A–E, time course of the expressions of C/EBPβ (∼45 kDa), PRDM16 (∼170 kDa), PGC1α (∼90 kDa), PPARγ (∼57 kDa), and actin during brown adipocyte differentiation, without (−) or with (+) exposure to pyr-apelin13. The representative immunoblots and the statistics (mean ± S.E. (error bars), n = 4; blot densities were normalized to actin density; *, p < 0.05; **, p < 0.01 versus untreated; Student's t test) are shown in A and B–E, respectively. F–M, brown preadipocytes were induced to differentiate into brown adipocytes for 8–10 days, without (−) (control) or with (+) exposure to pyr-apelin13 and/or 10 μm troglitazone (added at day 2). The representative bright field images of brown adipocytes and immunoblots of UCP1, CIDE-A, COX1, aP2, and actin expressions in brown adipocytes are shown in F and G, respectively. The statistics (mean ± S.E., n = 4) of the blot densities normalized to that of actin are shown in H–K. UCP1/aP2 and CIDE-A/aP2 are density ratios of UCP1, CIDE-A, and aP2 (L and M). Using one-way ANOVA, *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus untreated; #, p < 0.05 between the indicated pairs.
PPARγ is well described as an important regulator of brown adipocyte differentiation (48). However, recent studies have suggested that PPARγ does not control the terminal determination of brown adipocytes because PPARγ overexpression in hMSCs only induces white (not brown) fat phenotype (53); UCP1 expression was not suppressed in brown adipocytes of PPARγ-ablated mice (54); and high dose PPARγ agonist increased whitening of brown adipocyte (as indicated by enhanced lipogenesis, decreased UCP1 expression, and enlarged adipocytes with high lipid content and droplet size) (55, 56). In line with these previous reports, long term treatment with PPARγ agonist (troglitazone, 10 μm, days 2–10) induced more enlarged adipocytes with pale color and large intracellular lipid droplets (probably due to whitening of differentiated brown adipocytes; Fig. 3F).
Consistently, PPARγ agonist stimulated the expression of aP2 (adipocyte differentiation marker) to a much greater extent as compared with the simulated expressions of UCP1 and CIDE-A (brown adipocyte-specific markers) (Fig. 3, G–M). We observed that apelin only stimulated PPARγ expression at the early stage (within 2 days; Fig. 3E), and apelin co-treatment with PPARγ agonist (since day 2) significantly increased the expressions of UCP1 and CIDE-A and the number of dark color brown adipocytes. In contrast, apelin was not able to rectify the inhibited brown differentiation by PPARγ antagonist (T0070907, 1 μm) applied in the first 2 days (data not shown). Taken together, apelin promotes brown adipogenesis by 1) stimulating expression of adipogenic transcriptional factors (C/EBPβ and PPARγ) at the early stage and 2) up-regulating the transcription factors (PRDM16 and PGC1α) required for thermogenesis and mitochondrial biogenesis and counteracting PPARγ effects in the later stage to ensure terminal determination of the brown phenotype.
Apelin Raises the Basal Metabolic Activity of Brown Adipocytes
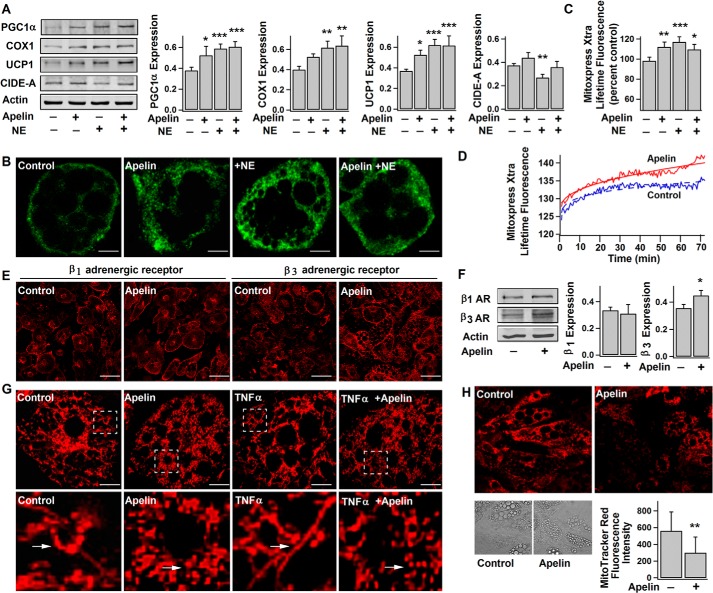
Norepinephrine (NE) released from the sympathetic nervous system activates brown adipocytes (including UCP1 expression, mitochondrial biogenesis, and respiratory activity) through β-adrenergic receptors (β-ARs), particularly β3-AR (1). In agreement with previous studies (14), NE (6-h incubation) acutely promoted the expressions of PGC1α (a key regulator of thermogenic gene expression and mitochondrial biogenesis), COX1, and UCP1 as well as the oxygen consumption rate (OCR), which reflects mitochondrial respiratory activity and metabolic rate, in brown adipocytes (Fig. 4, A–C). Similar to the NE effects, long term treatment with apelin (3–4 days) increased the expressions of PGC1α, COX1, and UCP1 in brown adipocytes (Fig. 4, A and B). Significant up-regulation of β3-AR, but not β1-AR, was also observed (Fig. 4, E and F). As shown in Fig. 4, C and D, apelin was able to stimulate OCR to a level comparable with acute stimulation by NE. However, apelin pretreatment had little influence on NE-induced protein expressions and OCR (Fig. 4, A and C), suggesting that apelin is only stimulatory to the basal metabolic activity.
FIGURE 4.
Apelin increases UCP1 expression and basal metabolic activity in brown adipocytes. After 3–4 days without (−) (control) or with (+) exposure to pyr-apelin13, brown adipocytes (day 10) were treated without (−) or with (+) 1 μm NE for 6 h. A, representative immunoblots and statistics (mean ± S.E. (error bars), n = 4, blot densities were normalized to that of actin) of PGC1α, COX1, UCP1, CIDE-A, and actin expressions in brown adipocytes. Using one-way ANOVA, *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus untreated. B, confocal images of immunostained UCP1 in differently treated brown adipocytes. Scale bars, 10 μm. C and D, lifetime fluorescence value of MitoXpress-Xtra assessed as an indication of O2 consumption rate in brown adipocytes. The statistics (mean ± S.E., n = 6 at 60 min) and the real-time responses of average fluorescence value are shown in C and D, respectively. E, confocal images of immunostained β1-AR and β3-AR in brown adipocytes. Scale bars, 50 μm. F, representative immunoblots of β1-AR (∼65 kDa) and β3-AR (∼44 kDa) expressions in brown adipocytes, and the statistics (mean ± S.E., n = 4) of the blot densities normalized to that of actin. G, the representative confocal images of mitochondria (detected by MitoTracker Red) (top lane) and their corresponding magnified images of insets (bottom lane) in brown adipocytes (day 10) after treatment without or with pyr-apelin13 and/or 10 ng/ml TNFα (2–3 days). Scale bars, 10 μm. H, the representative confocal images showing the changes of mitochondrial membrane potential (detected by MitoTracker Red) and the statistics of fluorescence intensity (mean ± S.E., n = 12 cells, from 3–4 independent experiments). Using Student's t test, *, p < 0.05; **, p < 0.01 versus untreated.
Mitochondria with abundant UCP1 are responsible for metabolic activities in brown adipocytes. Mitochondrial biogenesis and morphological change (from tubular to spherical) accompany thermogenic activation in brown adipocytes (14, 57). We and others have shown that apelin increases mitochondrial biogenesis in white adipocytes and skeletal muscle cells (31, 34). As shown in Fig. 4G, tubular mitochondria are densely packed in brown adipocytes. Apelin treatment further increased its density, whereas mitochondria appeared shorter and more spherical in shape. TNFα secreted from macrophages in adipose tissue is a deterrent to brown thermogenesis (43, 44). In comparison, tubular mitochondria became more elongated in TNFα-treated brown adipocytes. Interestingly, in some brown adipocytes, mitochondria surround small lipid droplets, and these ring-shaped mitochondria are larger and more abundant in apelin-treated cells (data not shown). As shown by a previous study, mitochondrial rings are observed in brown adipocytes treated with the uncoupling proteins that are responsible for reduced membrane potential and increased oxygen consumption in mitochondria (58). We found that apelin treatment decreased the mitochondrial membrane potential (Fig. 4H) (as evidenced by the decreased fluorescence intensity of MitoTracker Red staining), without affecting cell viability (data not shown). Taken together, the evidence indicates that apelin stimulates the basal metabolic activities in brown adipocytes.
Apelin Increases the Browning Characteristics in White Adipocytes
In addition to classical brown adipocytes, certain white adipocytes can transform into brown-like adipocytes (called beige cells) in response to various stimuli (e.g. cold exposure, etc.) (14). Beige cells are characterized by their multilocular lipid droplets, dark brown color due to high mitochondrial content, and expression of thermogenic and brown fat-specific genes (e.g. UCP1) (14). Because both apelin and APJ are abundantly expressed in white adipocytes (24, 59), we sought to investigate apelin's effects on browning of white adipocytes. Consistent with previous studies (13, 60), it was found that UCP1 expression in human white adipocytes is much lower than in brown adipocytes (Fig. 5). Similar to its stimulatory effect on brown adipocytes, apelin increased the expression of PRDM16, which is known to promote the browning of white adipocytes (14, 61) (Fig. 5, A and G). As expected, UCP1 and COX1 expressions were also significantly increased in apelin-treated human white (Fig. 5A) and 3T3-L1 adipocytes (mouse white preadipose cell line) (days 5–9) (Fig. 5G). Consistently, apelin treatment decreased the mitochondrial membrane potential and enhanced the basal activity of mitochondrial respiration (as evidenced by the increased OCR) (Fig. 5, C–E) in human white adipocytes. Some dark-colored multilocular brown adipocyte-like cells were also observed after apelin treatment (Fig. 5, B and F). All of these results indicate that apelin promotes the browning characteristics in white adipocytes.
FIGURE 5.
Apelin increases the browning characteristics in white adipocytes. A–E, human white adipocytes (day 14) were treated without (−) (control) or with (+) exposure to pyr-apelin13 for 4 days. F and G, 3T3-L1 adipocytes were treated without (−) (control) or with (+) exposure to pyr-apelin13 (days 2–9 or days 5–9). Shown are the representative immunoblots of protein expressions (PRDM16, COX1, UCP1, CIDE-A, ap2, and actin) in human (A) and 3T3-L1 adipocytes (G) with their respective statistics (mean ± S.E. (error bars), n = 4–5, normalized to actin density). B and F, representative bright field images of differently treated human and 3T3-L1 adipocytes. C and D, representative confocal images of human adipocytes showing the changes of mitochondrial membrane potential (detected by MitoTracker Red) and the statistics of fluorescence intensity (mean ± S.E., n = 12). E, lifetime fluorescence value of MitoXpress-Xtra assessed as an indication of OCR (mean ± S.E., n = 6, at 60 min) in human adipocytes. H, human mesenchymal stem cells were induced to differentiate into adipocytes without (−) or with (+) exposure to pyr-apelin13. Shown are the representative immunoblots of PRDM16, COX1, UCP1, CIDE-A, aP2, leptin (∼16 kDa), and actin expressions (day 14) and the statistics (mean ± S.E., n = 4–5, normalized to actin density). Using Student's t test, *, p < 0.05; **, p < 0.01 versus control/no treatment.
We and others have previously reported that apelin release from white adipocytes increases over time during adipocyte differentiation (24). Using 3T3-L1 adipocytes, we examined whether apelin induces the brown-like phenotype at the late stage of adipocyte differentiation. As shown in Fig. 5G, apelin treatment (days 2–9) decreased the expression of ap2 (a marker protein for differentiated adipocytes) (62), suggesting that apelin hinders the terminal differentiation of white adipocytes. This is in line with the previous observation that apelin is able to decrease 3T3-L1 adipocyte differentiation (29). On the other hand, COX1, UCP1, and CIDE-A protein levels (brown adipocyte markers) were not reduced in apelin-treated cells (Fig. 5G). On the contrary, knockdown of apelin or APJ receptor decreased the expression ratios of UCP1/aP2 and CIDE-A/aP2 (data not shown). In summary, our results suggest that apelin apparently hinders maturation of white adipocytes while promoting the brown fat-like characteristics during the terminal phase of 3T3-L1 adipocyte differentiation.
We further examined apelin's effects on browning characteristics during the adipogenic differentiation of hMSCs. A recent study showed that, unless PRDM16 is overexpressed, hMSCs will only differentiate into white adipocytes with low UCP1 expression, even under ectopic expression of PPARγ (53). Similar to human white adipocytes, only low level of UCP1 expression was detected in adipocytes differentiated from hMSCs (Fig. 5H). In comparison, UCP1 expression was dramatically increased in apelin-treated cells, together with increased expression of PRDM16 (Fig. 5H), similar to its stimulatory effect on brown adipocytes. In contrast, leptin expression was significantly reduced. Previous studies suggest that leptin is white adipocyte-specific, because unilocular UCP1-negative adipocytes are leptin-positive, whereas multilocular UCP1-positive brown adipocytes are leptin-negative (63, 64). Our experiments on hMSCs further confirm that apelin is able to promote the browning characteristics in white adipocytes, at least in part, by stimulating the expression of PRDM16.
Apelin Promotes the Browning of WAT in Vivo
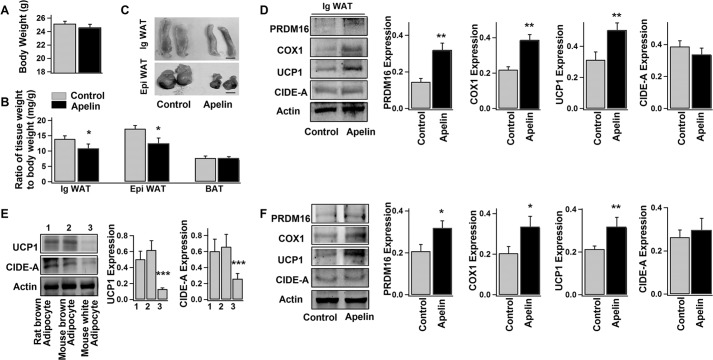
No significant difference in body weight (Fig. 6A) or food intake (data not shown) was observed in control (saline-treated) and apelin-treated mice. Interestingly, the apelin-treated mice exhibited significantly reduced weight of WAT (both inguinal and epididymal WAT), whereas the weight of interscapular BAT was similar to that of the control group (Fig. 6, B and C). Moreover, the protein levels of PRDM16, COX1, and UCP1 (brown adipocyte markers) were elevated in WAT isolated from the apelin-treated mice (Fig. 6D).
FIGURE 6.
Both in vivo and in vitro mouse experiments confirm the apelin stimulation on WAT browning. Shown are average body weights (A) and weights of inguinal WAT (Ig WAT), epididymal WAT (Epi WAT), and interscapular BAT (B) relative to the total body weights of the control and apelin-treated mice (mean ± S.E. (error bars), n = 8 mice/group). C, representative images of inguinal WAT and epididymal WAT isolated from the control and apelin-treated mice. Scale bar, 4 mm. D, representative immunoblots of protein expressions (PRDM16, COX1, UCP1, CIDE-A, and actin) in inguinal WAT isolated from the control and apelin-treated mice and the statistics of the blot density normalized to that of actin (mean ± S.E., n = 4 mice/group). E, representative immunoblots and the statistics (mean ± S.E., n = 3) of UCP1 and CIDE-A expressions in adipocytes (day 10) differentiated from different preadipose cells (1, preadipocytes isolated from rat interscapular BAT; 2, mouse interscapular BAT; 3, mouse inguinal WAT). F, representative immunoblots of protein expressions (PRDM16, COX1, UCP1, CIDE-A, and actin) and the respective statistics (mean ± S.E., n = 4, normalized to actin density) from the white adipocytes (day 8, differentiated from preadipocytes of mouse inguinal WAT) treated without (−) (control) or with (+) pyr-apelin13 for 4 days. Using Student's t test, *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus control.
To further confirm the browning effect of apelin, primary preadipocytes were isolated from mouse inguinal WAT and differentiated into white adipocytes. As shown in Fig. 6E, UCP1 (brown adipocyte-specific protein) level was much lower in these differentiated white adipocytes as compared with the brown adipocytes (differentiated from interscapular BAT preadipose cells from the same group of mice). In comparison, PRDM16, COX1, and UCP1 expressions were considerably up-regulated in apelin-treated white adipocytes (Fig. 6F), which is similar to the observations in human and 3T3-L1 white adipocytes (Fig. 5). Taken together, apelin's stimulation on WAT browning is confirmed by both in vivo and in vitro mouse experiments.
Apelin's Stimulatory Effects on Brown Adipogenesis and Browning of White Adipocytes Are Dependent on AMPK and PI3K/Akt Signaling Pathways
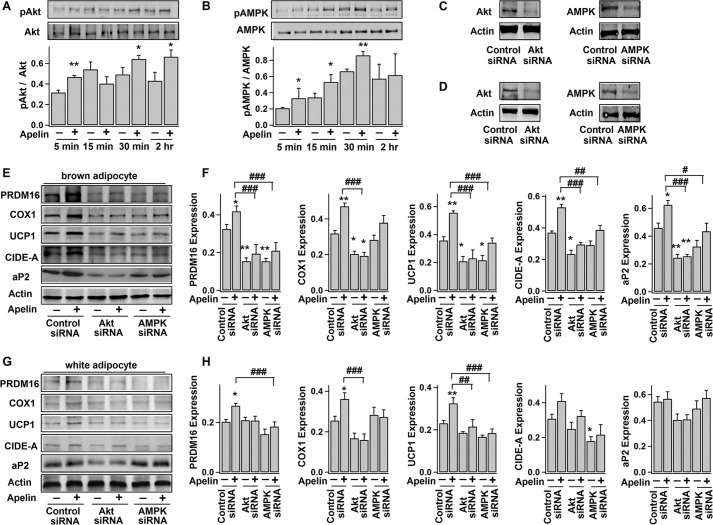
We and others have shown that apelin increases Akt and AMPK phosphorylation in white adipocytes (27, 28, 34). Here, we also found that apelin increased the activation (phosphorylation) of Akt and AMPK in primary rat brown preadipocytes (Fig. 7, A and B). As shown in Fig. 7 E and F, siRNA knockdown of Akt expression in brown preadipocytes precluded the brown adipogenesis, as evidenced by the decreased protein levels of PRDM16, COX1, UCP1, CIDE-A, and aP2. Apelin-induced brown adipogenesis was also effectively concealed in Akt-knock-out cells. In addition, AMPK protein knockdown in brown preadipocytes largely blocked the apelin-induced expressions of PRDM16, UCP1, CIDE-A, and aP2. Previous studies showed that both the PI3K/Akt and AMPK/mTOR signaling are important in brown adipogenesis (65, 66). Therefore, it may be concluded that apelin induces brown adipogenesis, at least in part, by activating the PI3K/Akt and AMPK signaling pathways.
FIGURE 7.
Signaling pathways underlying the apelin-induced brown adipocyte differentiation and browning characteristics of white adipocytes. A and B, time course of the phosphorylation and total protein levels of Akt (∼60 kDa) and AMPK (∼62 kDa) during brown adipocyte differentiation, without (−) (control) or with (+) exposure to pyr-apelin13. Top panels, representative immunoblots; bottom panels, statistics (mean ± S.E. (error bars), n = 3) of the optical density ratio between pAkt and Akt or between pAMPK and AMPK (*, p < 0.05; **, p < 0.01 versus control; Student's t test). C and D, Western blot analyses of Akt and AMPK expressions in brown preadipocytes (C) and 3T3-L1 adipocytes (D), 2 days after transfection with control siRNA, Akt siRNA, or AMPK siRNA. E and F, brown preadipocytes were transfected with control siRNA, Akt siRNA, or AMPK siRNA 1–2 days before they were induced to differentiate into adipocytes, without (−) (control) or (+) with exposure to pyr-apelin13. Shown are representative immunoblots and the statistics (mean ± S.E., n = 4, normalized to actin density) of PRDM16, COX1, UCP1, CIDE-A, aP2, and actin expressions in differentiated brown adipocytes (day 10). G and H, 3T3-L1 adipocytes (day 4) were transfected with control siRNA, Akt siRNA, or AMPK siRNA, 1–2 days before treatment without (−) (control) or with (+) pyr-apelin13 (for 4 days). The representative immunoblots and the statistics (mean ± S.E., n = 4, normalized to actin density) are shown accordingly. Using one-way ANOVA, *, p < 0.05; **, p < 0.01 versus control; #, p < 0.05; ##, p < 0.01; ###, p < 0.001 between the indicated pairs.
Similar to the browning effects on human and mouse white adipocytes (Figs. 5A and 6F), apelin treatment (days 5–9) significantly increased the expressions of PRDM16, COX1, and UCP1 in 3T3-L1 white adipocytes (Fig. 7, G and H). Also, AMPK protein knockdown significantly blocked the apelin-induced expressions of PRDM16 and UCP1 (Fig. 7, G and H). Apelin-induced UCP1 expression was also obscured in Akt-knock-out cells. This suggests that apelin's effect on browning of white adipocytes is also dependent on the AMPK signaling pathway.
Discussion
Adipose browning (including an increase of brown adipocyte differentiation in BAT and transformation of beige adipocytes in WAT) not only suppresses obesity through increased energy expenditure but also counteracts the detrimental effects of excess WAT (e.g. insulin resistance) (14, 15). An explosion of interest has been triggered because stimulating adipose browning may be a novel route to combat obesity and associated metabolic diseases. Increasing evidence is mounting to suggest that adipokines secreted by adipocytes play important roles in the self-modeling of adipose tissue (19, 29, 33). But their effects on browning are not well explored.
In this study, we present evidence that apelin secreted by both brown and white adipocytes stimulates adipose browning. Based on our experiments and previous studies, the following scenario may be proposed (Fig. 8). In obesity, adipose tissue is in chronic low grade inflammation, characterized by accumulation of macrophages and inflammatory cytokines, which contribute to the development of insulin resistance (45). Inflammatory factors (e.g. TNFα) released from macrophages suppress brown adipocyte formation (Fig. 2) (43, 44) and hence reduce BAT mass in obesity (3, 4). Consequently, decreased energy consumption further aggravates the excess energy storage in WAT (1, 15). On the other hand, apelin expression in WAT and its plasma level are elevated in obesity (24). By interacting with APJ receptor, apelin activates PI3K/Akt and AMPK signaling in brown preadipocytes (Figs. 1 and 7), leading to increased expressions of brown adipogenic and thermogenic transcription factors (Fig. 3). As brown adipogenesis progresses, apelin expression increases in BAT, creating an autocrine positive feedback loop that enhances brown adipocyte differentiation (Fig. 1) and offsets the TNFα-impaired brown adipogenesis (Fig. 2). Apelin induces browning of white adipocytes too (Figs. 5 and 6). In addition to the increased number of brown or brown-like adipocytes, apelin also stimulates metabolic activity of adipocytes (Figs. 4 and 5). As a result, the body energy consumption increases (30), and WAT masses are reduced (Fig. 6). Moreover, apelin's inhibitory effect on white adipocyte differentiation offers effective negative feedback to hinder further expansion of WAT (29) (Fig. 5G). As described, the unique co-existence of apelin's positive and negative feedback loops compensate for the imbalanced development of BAT and WAT found in obesity. On the other hand, disruption of the apelin signaling by silencing apelin or APJ receptor expression decreases the number of brown adipocytes (Fig. 1) and increases the number of hypertrophied white adipocytes (29). The above proposed notion is consistent with the previous studies in rodents showing that 1) apelin treatment increases body temperature and O2 consumption (30); 2) apelin treatment or BAT transplantation decreases body adiposity and insulin resistance (9, 25); and 3) apelin-knock-out or BAT-ablated mice exhibit increased central adiposity and insulin resistance (7, 28).
FIGURE 8.
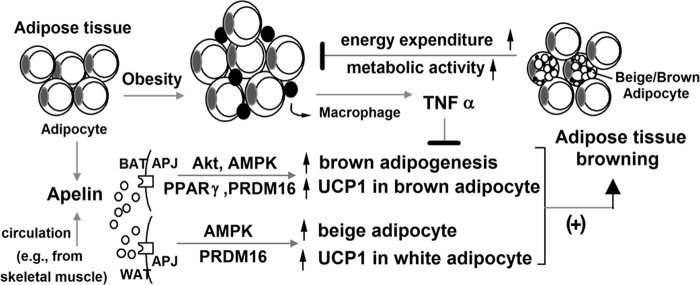
Apelin-induced adipose tissue browning.
BAT metabolic activity (and thus energy consumption) can be enhanced by acute cold exposure (a few hours) (4, 67). This is due to increased sympathetic nervous system activity, whereby NE-induced cAMP accumulation promotes UCP1 expression and its uncoupling process (1, 14). Prolonged cold exposure (several days) is known to increase BAT mass as well as browning of WAT in humans (5, 68). Interestingly, apelin expression and release from white adipocytes are up-regulated by cAMP (59), and apelin plasma level is raised upon cold exposure (69). It is conceivable that cold-induced apelin increase is an adaptive reaction to stimulate adipose browning. NE-stimulated metabolic activities of brown adipocytes, however, cannot be further augmented by apelin (Fig. 4). This is attributable to the fact that apelin and NE antagonize each other on PKA/cAMP signaling. As shown previously, apelin inhibits cAMP production via the Gi protein-coupled APJ receptor (28, 70).
Exercise training also potentiates BAT thermogenesis and browning of WAT (15). Both exercise training and cold exposure increase the circulating levels of irisin and FGF21 (16, 17, 71). These recently found myokine and hepatokine stimulate brown fat-like thermogenesis in WAT (16, 17). Interestingly, in obese people, exercise only up-regulates muscle expression of apelin, without affecting irisin and FGF21 levels (72). A number of studies show that plasma apelin level is increased after exercise training and cold exposure (69, 73, 74). Hence, like irisin and FGF21, apelin could be regarded as an exercise- and cold-induced endocrine activator to promote adipose browning. We have previously demonstrated in white adipocytes that apelin suppresses the release of fatty acids and reactive oxygen species (29, 34), which are key contributors to the pathogenesis of obesity-related metabolic disorders. Therefore, apelin is able to exert multiple beneficial effects through both white and brown adipocytes and may serve as a novel therapeutic target for obesity and related metabolic diseases.
This research is supported by the Singapore National Research Foundation under CBRG Grant (NMRC/CBRG/0070/2014) administrated by the Singapore Ministry of Health's National Medical Research Council. This work is also supported by the Singapore Ministry of Education under AcRF Tier 1 Grant RGT7/13.
- BAT
- brown adipose tissue
- WAT
- white adipose tissue
- Akt
- protein kinase B
- AMPK
- 5′-adenosine monophosphate-activated protein kinase
- APJ
- apelin receptor
- AR
- adrenergic receptor
- aP2
- adipocyte protein 2 (adipocyte-fatty acid-binding protein)
- COX1
- cytochrome c oxidase subunit I
- CIDE-A
- cell death-inducing DFFA-like effector A
- C/EBPβ
- CCAAT-enhancer binding protein β
- PPARγ
- peroxisome proliferator-activated receptor γ
- PRDM16
- PR domain-containing protein 16
- PGC-1α
- peroxisome proliferator-activated receptor γ coactivator-1-α
- NE
- norepinephrine
- OCR
- oxygen consumption rate
- UCP1
- uncoupling protein 1
- hMSCs
- human mesenchymal stem cells
- ANOVA
- analysis of variance.
References
- 1. Chechi K., Carpentier A. C., Richard D. (2013) Understanding the brown adipocyte as a contributor to energy homeostasis. Trends Endocrinol. Metab. 24, 408–420 [DOI] [PubMed] [Google Scholar]
- 2. Lidell M. E., Enerbäck S. (2010) Brown adipose tissue: a new role in humans? Nat. Rev. Endocrinol. 6, 319–325 [DOI] [PubMed] [Google Scholar]
- 3. Cypess A. M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A. B., Kuo F. C., Palmer E. L., Tseng Y. H., Doria A., Kolodny G. M., Kahn C. R. (2009) Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360, 1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Marken Lichtenbelt W. D., Vanhommerig J. W., Smulders N. M., Drossaerts J. M., Kemerink G. J., Bouvy N. D., Schrauwen P., Teule G. J. (2009) Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 360, 1500–1508 [DOI] [PubMed] [Google Scholar]
- 5. Yoneshiro T., Aita S., Matsushita M., Kayahara T., Kameya T., Kawai Y., Iwanaga T., Saito M. (2013) Recruited brown adipose tissue as an antiobesity agent in humans. J. Clin. Invest. 123, 3404–3408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vijgen G. H., Bouvy N. D., Teule G. J., Brans B., Hoeks J., Schrauwen P., van Marken Lichtenbelt W. D. (2012) Increase in brown adipose tissue activity after weight loss in morbidly obese subjects. J. Clin. Endocrinol. Metab. 97, E1229–E1233 [DOI] [PubMed] [Google Scholar]
- 7. Lowell B. B., S-Susulic V., Hamann A., Lawitts J. A., Himms-Hagen J., Boyer B. B., Kozak L. P., Flier J. S. (1993) Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature 366, 740–742 [DOI] [PubMed] [Google Scholar]
- 8. Chondronikola M., Volpi E., Børsheim E., Porter C., Annamalai P., Enerbäck S., Lidell M. E., Saraf M. K., Labbe S. M., Hurren N. M., Yfanti C., Chao T., Andersen C. R., Cesani F., Hawkins H., Sidossis L. S. (2014) Brown adipose tissue improves whole body glucose homeostasis and insulin sensitivity in humans. Diabetes 63, 4089–4099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stanford K. I., Middelbeek R. J., Townsend K. L., An D., Nygaard E. B., Hitchcox K. M., Markan K. R., Nakano K., Hirshman M. F., Tseng Y. H., Goodyear L. J. (2013) Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Invest. 123, 215–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang X., Enerbäck S., Smith U. (2003) Reduced expression of FOXC2 and brown adipogenic genes in human subjects with insulin resistance. Obesity Res. 11, 1182–1191 [DOI] [PubMed] [Google Scholar]
- 11. Cypess A. M., Kahn C. R. (2010) Brown fat as a therapy for obesity and diabetes. Curr. Opin. Endocrinol. Diabetes. Obes. 17, 143–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grundy S. M. (2004) Obesity, metabolic syndrome, and cardiovascular disease. J. Clin. Endocrinol. Metab. 89, 2595–2600 [DOI] [PubMed] [Google Scholar]
- 13. Carey A. L., Vorlander C., Reddy-Luthmoodoo M., Natoli A. K., Formosa M. F., Bertovic D. A., Anderson M. J., Duffy S. J., Kingwell B. A. (2014) Reduced UCP-1 content in in vitro differentiated beige/brite adipocytes derived from preadipocytes of human subcutaneous white adipose tissues in obesity. PLoS One 9, e91997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harms M., Seale P. (2013) Brown and beige fat: development, function and therapeutic potential. Nat. Med. 19, 1252–1263 [DOI] [PubMed] [Google Scholar]
- 15. Bartelt A., Heeren J. (2014) Adipose tissue browning and metabolic health. Nat. Rev. Endocrinol. 10, 24–36 [DOI] [PubMed] [Google Scholar]
- 16. Boström P., Wu J., Jedrychowski M. P., Korde A., Ye L., Lo J. C., Rasbach K. A., Boström E. A., Choi J. H., Long J. Z., Kajimura S., Zingaretti M. C., Vind B. F., Tu H., Cinti S., Højlund K., Gygi S. P., Spiegelman B. M. (2012) A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481, 463–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fisher F. M., Kleiner S., Douris N., Fox E. C., Mepani R. J., Verdeguer F., Wu J., Kharitonenkov A., Flier J. S., Maratos-Flier E., Spiegelman B. M. (2012) FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 26, 271–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Trayhurn P., Bing C., Wood I. S. (2006) Adipose tissue and adipokines: energy regulation from the human perspective. J. Nutr. 136, 1935S–1939S [DOI] [PubMed] [Google Scholar]
- 19. Karastergiou K., Mohamed-Ali V. (2010) The autocrine and paracrine roles of adipokines. Mol. Cell Endocrinol. 318, 69–78 [DOI] [PubMed] [Google Scholar]
- 20. Ye F., Than A., Zhao Y., Goh K. H., Chen P. (2010) Vesicular storage, vesicle trafficking, and secretion of leptin and resistin: the similarities, differences, and interplays. J. Endocrinol. 206, 27–36 [DOI] [PubMed] [Google Scholar]
- 21. Masaki T., Chiba S., Yasuda T., Tsubone T., Kakuma T., Shimomura I., Funahashi T., Matsuzawa Y., Yoshimatsu H. (2003) Peripheral, but not central, administration of adiponectin reduces visceral adiposity and upregulates the expression of uncoupling protein in agouti yellow (Ay/a) obese mice. Diabetes 52, 2266–2273 [DOI] [PubMed] [Google Scholar]
- 22. Commins S. P., Watson P. M., Padgett M. A., Dudley A., Argyropoulos G., Gettys T. W. (1999) Induction of uncoupling protein expression in brown and white adipose tissue by leptin. Endocrinology 140, 292–300 [DOI] [PubMed] [Google Scholar]
- 23. Knudsen J. G., Murholm M., Carey A. L., Biensø R. S., Basse A. L., Allen T. L., Hidalgo J., Kingwell B. A., Febbraio M. A., Hansen J. B., Pilegaard H. (2014) Role of IL-6 in exercise training- and cold-induced UCP1 expression in subcutaneous white adipose tissue. PLoS One 9, e84910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boucher J., Masri B., Daviaud D., Gesta S., Guigné C., Mazzucotelli A., Castan-Laurell I., Tack I., Knibiehler B., Carpéné C., Audigier Y., Saulnier-Blache J. S., Valet P. (2005) Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology 146, 1764–1771 [DOI] [PubMed] [Google Scholar]
- 25. Castan-Laurell I., Dray C., Attané C., Duparc T., Knauf C., Valet P. (2011) Apelin, diabetes, and obesity. Endocrine 40, 1–9 [DOI] [PubMed] [Google Scholar]
- 26. Yue P., Jin H., Aillaud M., Deng A. C., Azuma J., Asagami T., Kundu R. K., Reaven G. M., Quertermous T., Tsao P. S. (2010) Apelin is necessary for the maintenance of insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 298, E59–E67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhu S., Sun F., Li W., Cao Y., Wang C., Wang Y., Liang D., Zhang R., Zhang S., Wang H., Cao F. (2011) Apelin stimulates glucose uptake through the PI3K/Akt pathway and improves insulin resistance in 3T3-L1 adipocytes. Mol. Cell Biochem. 353, 305–313 [DOI] [PubMed] [Google Scholar]
- 28. Yue P., Jin H., Xu S., Aillaud M., Deng A. C., Azuma J., Kundu R. K., Reaven G. M., Quertermous T., Tsao P. S. (2011) Apelin decreases lipolysis via Gq, Gi, and AMPK-dependent mechanisms. Endocrinology 152, 59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Than A., Cheng Y., Foh L. C., Leow M. K., Lim S. C., Chuah Y. J., Kang Y., Chen P. (2012) Apelin inhibits adipogenesis and lipolysis through distinct molecular pathways. Mol. Cell Endocrinol. 362, 227–241 [DOI] [PubMed] [Google Scholar]
- 30. Higuchi K., Masaki T., Gotoh K., Chiba S., Katsuragi I., Tanaka K., Kakuma T., Yoshimatsu H. (2007) Apelin, an APJ receptor ligand, regulates body adiposity and favors the messenger ribonucleic acid expression of uncoupling proteins in mice. Endocrinology 148, 2690–2697 [DOI] [PubMed] [Google Scholar]
- 31. Attané C., Foussal C., Le Gonidec S., Benani A., Daviaud D., Wanecq E., Guzmán-Ruiz R., Dray C., Bezaire V., Rancoule C., Kuba K., Ruiz-Gayo M., Levade T., Penninger J., Burcelin R., Pénicaud L., Valet P., Castan-Laurell I. (2012) Apelin treatment increases complete fatty acid oxidation, mitochondrial oxidative capacity, and biogenesis in muscle of insulin-resistant mice. Diabetes 61, 310–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim H. J., Cho H., Alexander R., Patterson H. C., Gu M., Lo K. A., Xu D., Goh V. J., Nguyen L. N., Chai X., Huang C. X., Kovalik J. P., Ghosh S., Trajkovski M., Silver D. L., Lodish H., Sun L. (2014) MicroRNAs are required for the feature maintenance and differentiation of brown adipocytes. Diabetes 63, 4045–4056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Than A., Leow M. K., Chen P. (2013) Control of adipogenesis by the autocrine interplays between angiotensin 1–7/Mas receptor and angiotensin II/AT1 receptor signaling pathways. J. Biol. Chem. 288, 15520–15531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Than A., Zhang X., Leow M. K., Poh C. L., Chong S. K., Chen P. (2014) Apelin attenuates oxidative stress in human adipocytes. J. Biol. Chem. 289, 3763–3774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Scott M. A., Nguyen V. T., Levi B., James A. W. (2011) Current methods of adipogenic differentiation of mesenchymal stem cells. Stem Cells Dev. 20, 1793–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Johnson I., Spence M. T. Z. (2010) The Molecular Probes Handbook: A Guide to Fluorescent Probes and Labeling Technologies, Section 12.2, 11th Ed., Life Technologies, Inc., Carlsbad, CA [Google Scholar]
- 37. Graves J. A., Wang Y., Sims-Lucas S., Cherok E., Rothermund K., Branca M. F., Elster J., Beer-Stolz D., Van Houten B., Vockley J., Prochownik E. V. (2012) Mitochondrial structure, function and dynamics are temporally controlled by c-Myc. PLoS One 7, e37699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Buravkov S. V., Pogodina M. V., Buravkova L. B. (2014) Comparison of mitochondrial fluorescent dyes in stromal cells. Bull. Exp. Biol. Med. 157, 654–658 [DOI] [PubMed] [Google Scholar]
- 39. Butruille L., Drougard A., Knauf C., Moitrot E., Valet P., Storme L., Deruelle P., Lesage J. (2013) The apelinergic system: sexual dimorphism and tissue-specific modulations by obesity and insulin resistance in female mice. Peptides 46, 94–101 [DOI] [PubMed] [Google Scholar]
- 40. Zhen E. Y., Higgs R. E., Gutierrez J. A. (2013) Pyroglutamyl apelin-13 identified as the major apelin isoform in human plasma. Anal. Biochem. 442, 1–9 [DOI] [PubMed] [Google Scholar]
- 41. Pitkin S. L., Maguire J. J., Bonner T. I., Davenport A. P. (2010) International Union of Basic and Clinical Pharmacology. LXXIV. Apelin receptor nomenclature, distribution, pharmacology, and function. Pharmacol. Rev. 62, 331–342 [DOI] [PubMed] [Google Scholar]
- 42. Nisoli E., Briscini L., Giordano A., Tonello C., Wiesbrock S. M., Uysal K. T., Cinti S., Carruba M. O., Hotamisligil G. S. (2000) Tumor necrosis factor α mediates apoptosis of brown adipocytes and defective brown adipocyte function in obesity. Proc. Natl. Acad. Sci. U.S.A. 97, 8033–8038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Valladares A., Roncero C., Benito M., Porras A. (2001) TNF-α inhibits UCP-1 expression in brown adipocytes via ERKs. Opposite effect of p38MAPK. FEBS Lett. 493, 6–11 [DOI] [PubMed] [Google Scholar]
- 44. Sakamoto T., Takahashi N., Sawaragi Y., Naknukool S., Yu R., Goto T., Kawada T. (2013) Inflammation induced by RAW macrophages suppresses UCP1 mRNA induction via ERK activation in 10T1/2 adipocytes. Am. J. Physiol. Cell Physiol. 304, C729–C738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Heilbronn L. K., Campbell L. V. (2008) Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. Curr. Pharm. Des. 14, 1225–1230 [DOI] [PubMed] [Google Scholar]
- 46. Than A., Ye F., Xue R., Ong J. W., Poh C. L., Chen P. (2011) The crosstalks between adipokines and catecholamines. Mol. Cell Endocrinol. 332, 261–270 [DOI] [PubMed] [Google Scholar]
- 47. Seale P., Kajimura S., Spiegelman B. M. (2009) Transcriptional control of brown adipocyte development and physiological function: of mice and men. Genes Dev. 23, 788–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nedergaard J., Petrovic N., Lindgren E. M., Jacobsson A., Cannon B. (2005) PPARgamma in the control of brown adipocyte differentiation. Biochim. Biophys. Acta 1740, 293–304 [DOI] [PubMed] [Google Scholar]
- 49. Kajimura S., Seale P., Tomaru T., Erdjument-Bromage H., Cooper M. P., Ruas J. L., Chin S., Tempst P., Lazar M. A., Spiegelman B. M. (2008) Regulation of the brown and white fat gene programs through a PRDM16/CtBP transcriptional complex. Genes Dev. 22, 1397–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Seale P., Kajimura S., Yang W., Chin S., Rohas L. M., Uldry M., Tavernier G., Langin D., Spiegelman B. M. (2007) Transcriptional control of brown fat determination by PRDM16. Cell Metab. 6, 38–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Uldry M., Yang W., St-Pierre J., Lin J., Seale P., Spiegelman B. M. (2006) Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab. 3, 333–341 [DOI] [PubMed] [Google Scholar]
- 52. Matsuo K., Bettaieb A., Nagata N., Matsuo I., Keilhack H., Haj F. G. (2011) Regulation of brown fat adipogenesis by protein tyrosine phosphatase 1B. PLoS One 6, e16446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ahfeldt T., Schinzel R. T., Lee Y. K., Hendrickson D., Kaplan A., Lum D. H., Camahort R., Xia F., Shay J., Rhee E. P., Clish C. B., Deo R. C., Shen T., Lau F. H., Cowley A., Mowrer G., Al-Siddiqi H., Nahrendorf M., Musunuru K., Gerszten R. E., Rinn J. L., Cowan C. A. (2012) Programming human pluripotent stem cells into white and brown adipocytes. Nat. Cell Biol. 14, 209–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. He W., Barak Y., Hevener A., Olson P., Liao D., Le J., Nelson M., Ong E., Olefsky J. M., Evans R. M. (2003) Adipose-specific peroxisome proliferator-activated receptor γ knockout causes insulin resistance in fat and liver but not in muscle. Proc. Natl. Acad. Sci. U.S.A. 100, 15712–15717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kelly L. J., Vicario P. P., Thompson G. M., Candelore M. R., Doebber T. W., Ventre J., Wu M. S., Meurer R., Forrest M. J., Conner M. W., Cascieri M. A., Moller D. E. (1998) Peroxisome proliferator-activated receptors γ and α mediate in vivo regulation of uncoupling protein (UCP-1, UCP-2, UCP-3) gene expression. Endocrinology 139, 4920–4927 [DOI] [PubMed] [Google Scholar]
- 56. Festuccia W. T., Blanchard P. G., Turcotte V., Laplante M., Sariahmetoglu M., Brindley D. N., Richard D., Deshaies Y. (2009) The PPARγ agonist rosiglitazone enhances rat brown adipose tissue lipogenesis from glucose without altering glucose uptake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R1327–R1335 [DOI] [PubMed] [Google Scholar]
- 57. Wikstrom J. D., Mahdaviani K., Liesa M., Sereda S. B., Si Y., Las G., Twig G., Petrovic N., Zingaretti C., Graham A., Cinti S., Corkey B. E., Cannon B., Nedergaard J., Shirihai O. S. (2014) Hormone-induced mitochondrial fission is utilized by brown adipocytes as an amplification pathway for energy expenditure. EMBO J. 33, 418–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liu X., Hajnóczky G. (2011) Altered fusion dynamics underlie unique morphological changes in mitochondria during hypoxia-reoxygenation stress. Cell Death Differ. 18, 1561–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Than A., Tee W. T., Chen P. (2012) Apelin secretion and expression of apelin receptors in 3T3-L1 adipocytes are differentially regulated by angiotensin type 1 and type 2 receptors. Mol. Cell Endocrinol. 351, 296–305 [DOI] [PubMed] [Google Scholar]
- 60. Lehr L., Canola K., Léger B., Giacobino J. P. (2009) Differentiation and characterization in primary culture of white adipose tissue brown adipocyte-like cells. Int. J. Obes. 33, 680–686 [DOI] [PubMed] [Google Scholar]
- 61. Seale P., Conroe H. M., Estall J., Kajimura S., Frontini A., Ishibashi J., Cohen P., Cinti S., Spiegelman B. M. (2011) Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J. Clin. Invest. 121, 96–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gregoire F. M., Smas C. M., Sul H. S. (1998) Understanding adipocyte differentiation. Physiol. Rev. 78, 783–809 [DOI] [PubMed] [Google Scholar]
- 63. Cinti S., Frederich R. C., Zingaretti M. C., De Matteis R., Flier J. S., Lowell B. B. (1997) Immunohistochemical localization of leptin and uncoupling protein in white and brown adipose tissue. Endocrinology 138, 797–804 [DOI] [PubMed] [Google Scholar]
- 64. Cancello R., Zingaretti M. C., Sarzani R., Ricquier D., Cinti S. (1998) Leptin and UCP1 genes are reciprocally regulated in brown adipose tissue. Endocrinology 139, 4747–4750 [DOI] [PubMed] [Google Scholar]
- 65. Hinoi E., Iezaki T., Fujita H., Watanabe T., Odaka Y., Ozaki K., Yoneda Y. (2014) PI3K/Akt is involved in brown adipogenesis mediated by growth differentiation factor-5 in association with activation of the Smad pathway. Biochem. Biophys. Res. Commun. 450, 255–260 [DOI] [PubMed] [Google Scholar]
- 66. Vila-Bedmar R., Lorenzo M., Fernández-Veledo S. (2010) Adenosine 5′-monophosphate-activated protein kinase-mammalian target of rapamycin cross talk regulates brown adipocyte differentiation. Endocrinology 151, 980–992 [DOI] [PubMed] [Google Scholar]
- 67. Ouellet V., Labbé S. M., Blondin D. P., Phoenix S., Guérin B., Haman F., Turcotte E. E., Richard D., Carpentier A. C. (2012) Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J. Clin. Invest. 122, 545–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. van der Lans A. A., Hoeks J., Brans B., Vijgen G. H., Visser M. G., Vosselman M. J., Hansen J., Jörgensen J. A., Wu J., Mottaghy F. M., Schrauwen P., van Marken Lichtenbelt W. D. (2013) Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J. Clin. Invest. 123, 3395–3403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mazzucotelli A., Ribet C., Castan-Laurell I., Daviaud D., Guigné C., Langin D., Valet P. (2008) The transcriptional co-activator PGC-1 α up regulates apelin in human and mouse adipocytes. Regul. Pept. 150, 33–37 [DOI] [PubMed] [Google Scholar]
- 70. Masri B., Morin N., Pedebernade L., Knibiehler B., Audigier Y. (2006) The apelin receptor is coupled to Gi1 or Gi2 protein and is differentially desensitized by apelin fragments. J. Biol. Chem. 281, 18317–18326 [DOI] [PubMed] [Google Scholar]
- 71. Lee P., Linderman J. D., Smith S., Brychta R. J., Wang J., Idelson C., Perron R. M., Werner C. D., Phan G. Q., Kammula U. S., Kebebew E., Pacak K., Chen K. Y., Celi F. S. (2014) Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 19, 302–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Besse-Patin A., Montastier E., Vinel C., Castan-Laurell I., Louche K., Dray C., Daviaud D., Mir L., Marques M. A., Thalamas C., Valet P., Langin D., Moro C., Viguerie N. (2014) Effect of endurance training on skeletal muscle myokine expression in obese men: identification of apelin as a novel myokine. Int. J. Obes. 38, 707–713 [DOI] [PubMed] [Google Scholar]
- 73. Kadoglou N. P., Vrabas I. S., Kapelouzou A., Lampropoulos S., Sailer N., Kostakis A., Liapis C. D., Angelopoulou N. (2012) The impact of aerobic exercise training on novel adipokines, apelin and ghrelin, in patients with type 2 diabetes. Med. Sci. Monit. 18, CR290–CR295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhang J., Ren C. X., Qi Y. F., Lou L. X., Chen L., Zhang L. K., Wang X., Tang C. (2006) Exercise training promotes expression of apelin and APJ of cardiovascular tissues in spontaneously hypertensive rats. Life Sci. 79, 1153–1159 [DOI] [PubMed] [Google Scholar]