Background: Mutations in the NOTCH1 HD domain cause NRR perturbation and ligand-independent activation and are frequent in leukemia.
Results: Human NOTCH2 but not mouse Notch2 is resistant to NRR perturbation and ligand-independent activation by Adam17.
Conclusion: NOTCH homologs and paralogs differ in their sensitivity to ligand-independent activation.
Significance: Our data explain why activating NOTCH2 HD domain mutations are infrequent in diseases such as cancer.
Abstract
Cell surface receptors of the NOTCH family of proteins are activated by ligand induced intramembrane proteolysis. Unfolding of the extracellular negative regulatory region (NRR), enabling successive proteolysis by the enzymes Adam10 and γ-secretase, is rate-limiting in NOTCH activation. Mutations in the NOTCH1 NRR are associated with ligand-independent activation and frequently found in human T-cell malignancies. In mammals four NOTCH receptors and five Delta/Jagged ligands exist, but mutations in the NRR are only rarely reported for receptors other than NOTCH1. Using biochemical and functional assays, we compared the molecular mechanisms of ligand-independent signaling in NOTCH1 and the highly related NOTCH2 receptor. Both murine Notch1 and Notch2 require the metalloprotease protease Adam17, but not Adam10 during ligand-independent activation. Interestingly, the human NOTCH2 receptor is resistant to ligand-independent activation compared with its human homologs or murine orthologs. Taken together, our data reveal subtle but functionally important differences for the NRR among NOTCH paralogs and homologs.
Introduction
NOTCH signaling enables short-range intercellular communication between adjacent cells and is important for self-renewal, differentiation, proliferation, and apoptosis, in both embryonic and adult tissues. NOTCH receptors are transmembrane receptors with four homologs in mammals (NOTCH 1–4). They can interact with five membrane-anchored ligands from the Delta (Dll1, -3, and -4) - and Jagged (Jagged1 and 2) family (1). Signaling through NOTCH receptors is dependent on, and regulated by, three distinct proteolytic processing steps which each cleave the NOTCH receptor at distinct sites (2, 3). First, NOTCH proteins are cleaved by furin-like proteases at Site-1 (S1),3 in the secretory pathway generating a non-covalently associated NOTCH heterodimer (4). The mature S1-cleaved receptor is kept in an auto-inhibitory state by the NRR, composed of three Lin12/Notch repeats (LNR) and the heterodimerization (HD) domain. The HD domain contains both the S1 and S2 sites. In the autoinhibited NRR, the LNR domain protects the scissile bond at Site-2 (S2) (5, 6), and displacement of the three LNR repeats masking the S2 cleavage site (7, 8) is essential for activation (6). To keep the HD domain in a closed conformation and protect the S2 site from metalloproteinase cleavage, co-ordination of calcium is required for the integrity of each LNR module (5, 9). Upon ligand binding, receptor auto-inhibition is released by a mechanism involving unfolding of the NRR (9) allowing an ectodomain cleavage at S2 by the metalloproteinase Adam10 (5, 10–13). Notch1 S2 cleavage and activation is sensitive to inhibition of metalloproteinases (5, 11) and mice, worms, and flies lacking Adam10 resemble Notch1-deficient phenotypes (3). Next, S2 NOTCH cleaved is metabolically unstable and rapidly cleaved at Site-3 (S3) to releases the NOTCH IntraCellular Domain (NICD) (11, 12). S3 cleavage is carried out by the γ-secretase complex composed of four proteins containing the aspartyl protease Presenilin, Nicastrin, PEN-2, and APH-1 (14, 15). NICD translocates to the nucleus and binds to the DNA-binding protein CSL (gene name RBP-Jκ), assembling a transcriptional activation complex that regulates NOTCH target gene expression (1).
Recently, we reported on the similarities in ligand-dependent signaling among NOTCH receptors in mammals. We showed that NOTCH2 and NOTCH3 receptors also require shedding by the Adam10 metalloprotease prior to intramembranous cleavage by presenilins after ligand binding, following the NOTCH1 activation paradigm (12).
Although structurally similar, the mRNA expression patterns and knock-out phenotypes of the four Notch homologs are very different in mice. Whereas mice lacking Notch1 or Notch2 are embryonically lethal, mice lacking Notch3 or Notch4 are viable and fertile. Taking into account the central role of NOTCH signaling in development, it is not surprising that deregulation of all four NOTCH receptors as well as ligands has been associated with a variety of inherited developmental and adult onset syndromes (16) and malignancies (17). For example, activating mutations in NOTCH1 are frequently observed in T-cell acute lymphoblastic leukemia (T-ALL) resulting in ligand-independent NOTCH1 signaling. The majority of these mutations map to the HD domain, leading to NRR destabilization and allowing access of proteases to the S2 site (18). Besides the HD domain, mutations in NOTCH1 are frequently found in the proline (P), glutamic acid (E), serine (S), and threonine (T) (PEST) domain, and cause prolonged NOTCH signaling (19, 20). In NOTCH2, PEST domain mutations are the most commonly found mutations in cancer, whereas HD domain mutations are rarely found. In two cases of splenic marginal zone B-cell lymphoma (MZBL) such NOTCH2 HD domain mutations were found (21, 22). However, as for other HD domain mutations in NOTCH receptors, functional consequences have not been studied.
Given the current interest in the role of NOTCH1 signaling in cancers and clinical approaches for intervention, precise knowledge on the involvement and activation mechanism of other NOTCH receptors is paramount. As NOTCH receptors have both unique and redundant functions, how receptor cleavage is normally regulated or affected by mutations may be an important determinant of mechanistic specificity and may thus influence approaches for pharmacological targeting of specific NOTCH paralogs (23).
Here, we report on the mechanism of ligand-independent activation of the mammalian NOTCH2 receptor in comparison to NOTCH1. Our results show that different NOTCH homologs exhibit striking differences in their response to perturbations by mutation or metal ion chelation, and have important implications for interpreting the consequences of mutations in human cancers and other diseases.
Experimental Procedures
Cell Lines
OP9-Neg and OP9-Jag1 cells (24) were cultured in αMEM (PAA) supplemented with 20% FCS, HCO3− and β-mercaptoethanol (100 μm). HEK-293, 293T, U2OS, 3T3, and mouse embryonic fibroblasts (MEFs) cells were cultured in DMEM supplemented with 10% FCS.
Plasmids and Transfections
Full-length human NOTCH1 and NOTCH2 cDNAs used here were constructed as described previously (12). Full-length FLAG-tagged mouse Notch2 cDNA was a kind gift from Y. Hamada and was described previously (25). The pcDNA5/FRT- hN1ΔE-FLAG-HA construct codes for NOTCH1 aa 1563–2555 followed by FLAG and HA tags, still retaining the N-terminal signal peptide of NOTCH1. Based on the layout of hN1ΔE the pcDNA5/FRT, the hN2ΔE-FLAG-HA plasmid codes for NOTCH2 aa 1535–2471, followed by FLAG and HA tags, utilizing the N-terminal signal peptide of NOTCH1. All mutant NOTCH receptors were generated by site directed mutagenesis on full length constructs using the QuikChange kit according to the manufacturer's instructions (Stratagene). A construct containing 12xCSL synthetic binding sites in tandem was used for NOTCH transcription assays (pGL4.24–12xCSL), in which a thymidine kinase driven Renilla luciferase (pGL4.74 TK-hRL; Promega) was used for normalization. NOTCH1 and NOTCH2 full-length constructs, encoding an N-terminal FLAG-tag, followed by the ectodomains and the transmembrane domains of NOTCH1 or NOTCH2 fused to the Gal4 DNA binding domain and the transcriptional activation domain of NOTCH1 were cloned into the pcdna5/FRT vector (26). NOTCH2 ΔEGF cDNA, containing the transmembrane domain of NOTCH2 fused to the DNA binding domain of Gal4 and the transcriptional activation domain (TAD) of NOTCH1, was cloned into pcDNA5 and site-directed mutagenesis was used to generate a NOTCH2 ΔEGF L1566P mutant. For an overview of full-length and truncated NOTCH constructs used, see Fig. 1D. Expression constructs containing secreted forms of human NOTCH1 and human NOTCH2 NRRs were used in heterodimer stability assays. The NRRs from NOTCH1 (residues Glu-1446 to Gln-1733), NOTCH2 (residues Ala-1423 to Gln-1677) and Notch2 (residues Ala-1422 to Glu-1671) were subcloned into a modified pcDNA3 vector containing an N-terminal FLAG tag and a C-terminal HA tag (Fig. 2A). A construct containing synthetic Gal4 binding sites was used for Gal4 transcription assays (26). Transfections were performed using linear polyethylenimine (P-PEI, Polysciences Inc.) or Lipofectamin 2000 (Invitrogen).
FIGURE 1.
Calcium chelation activates NOTCH1, but not NOTCH2. A, expression and cleavage of transfected human HA-tagged NOTCH1 and NOTCH2 in U2OS cells after EDTA treatment. B, induction of RBP-jκ/CSL-luciferase reporter gene activity in U2OS cells by EDTA. C, expression and cleavage of endogenous NOTCH1 and NOTCH2 in HEK293 transfected with ectopic Myc-tagged Notch1 and treated with EGTA. D, upper panel, full-length and chimeric NOTCH1/2 receptors with a Gal4 DNA binding domain, replacing the RAM and ANK domain, fused to the NOTCH1 TAD and PEST domain (lower panel). Expression and cleavage of full-length (FL) and ΔEGF Gal4 fusions of NOTCH1 and NOTCH2 (N2L1566P (L>P, ●). E, Gal4 luciferase cleavage assay of N1-Gal4 or N2-Gal4 after Jagged1 stimulation corrected for empty vector-transfected U2OS cells stimulated with ligand. F, Gal4 reporter luciferase assays in U2OS cells showing N1-Gal4 but not N2-Gal4 is stimulated by EDTA. Reporter assays are presented as fold induction by EDTA versus vehicle (PBS) and normalized to empty vector. TMIC, transmembrane and intracellular domain. NEXT, NOTCH extracellular truncation domain. Lamin A/C is used as a loading control. RLU, relative light units. Data are represented as mean ± S.E. of three independent experiments. ev, empty vector *, significant (p < 0.05), and ns, non-significant.
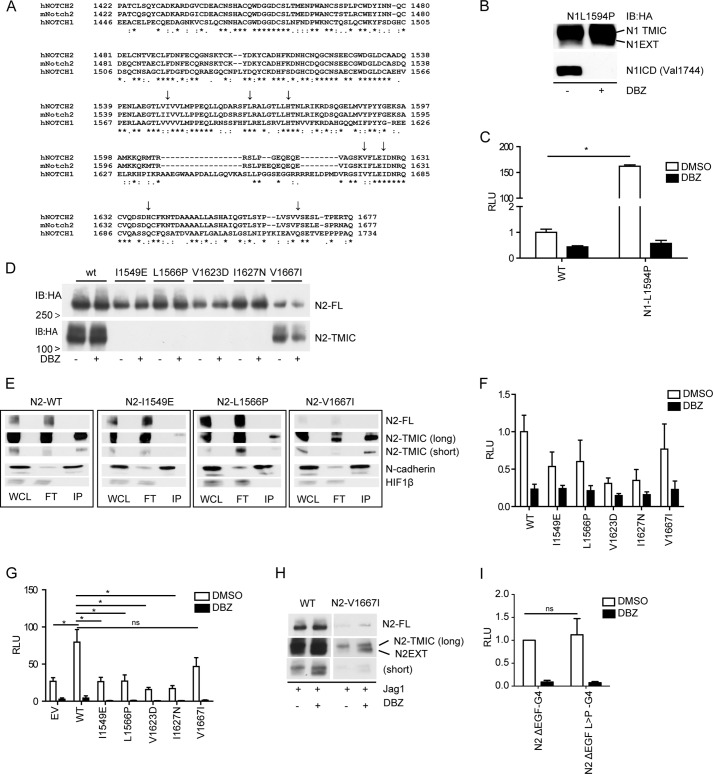
FIGURE 2.
Leukemogenic NOTCH1 mutations do not activate NOTCH2. A, clustal-W2 alignment (28) of human NOTCH1 and 2 and murine Notch2 HD domains, (*) identical residues (:) highly similar and (·) weak similarity (↓) indicate the missence mutations used in this study. B, ligand-independent cleavage of HA-N1-L1594P T-ALL mutant in transfected U2OS cells. NICD1/Val-1744 cleavage and N1EXT accumulation after GSI (DBZ) treatment and (C) RBP-jκ/CSL-luciferase reporter activity inhibited by GSI. D, HA-Immunoblots of U2OS cells transfected with either wild type (WT) or NOTCH2-HD mutants. Note loss of furin-cleaved TMIC in most NOTCH2 HD mutants. E, surface-biotinylation and streptavidin pull-down of U2OS cells transfected with wild type or HD-mutant NOTCH2 receptors. HA immunoblotting of Input (WCL) flow through (FT) and the streptavidin pulldown (IP). Only wild type and V1667I mutant NOTCH2 receptors are furin cleaved and at the cell surface. Biotinylation is specific for surface expressed proteins (i.e. N-cadherin) and does not label cytoplasmic proteins (HIF-1β). F, RBP-jκ/CSL-luciferase reporter gene activation of U2OS cells transfected with wild type or mutant NOTCH2 cultured in the absence of exogenous ligand (H) and (G) after co-culture with OP9-Jag1-expressing cells in presence or absence of GSI (DBZ). I, HA-Immunoblotting of U2OS cells expressing WT or N2-V1667I showing DBZ induces S2/N2EXT accumulation in response to ligand (OP9-Jag1). I, Gal4-luciferase reporter gene activation monitoring cleavage of wild type and N2-L1566P mutated N2ΔEGF-Gal4 proteins. RLU, relative light units. Data are represented as mean ± S.E. of three independent experiments, *, p < 0,05 and ns, non-significant.
Chemicals and Drugs
Cells were treated for 16 h with Dibenzazepine (DBZ) 0.2 μm (Syncom, The Netherlands) and DMSO (Sigma-Aldrich). 1 μg/ml of recombinant Dll4 (R&D Systems), 0.2% gelatin in PBS was coated overnight at 4 °C, rinsed once before cells were plated.
EDTA Stimulation
Cells were pre-incubated overnight with DMSO or DBZ. The next day, cells were washed in PBS and incubated with PBS in the presence or absence of 5 mm EDTA for 15 min at 25 °C, with and without DBZ. EDTA incubation was directly followed by lysate preparation for Western blotting or cells were allowed to recover for 6 h before luciferase activity was measured.
Luciferase Assays
Cells were plated 24 h before transfection, which was performed using P-PEI. 1 μg of firefly luciferase reporter plasmids containing 12xCSL binding sites or plasmids containing synthetic Gal4 binding sites (5xUAS FR-luc) were used per 6 well and 0.1 μg of thymidine kinase driven Renilla luciferase was used to normalize. Per 6 well, 0.25 μg of different NOTCH receptor plasmids were transfected. All values are given as fold Firefly/Renilla (RLU). All error bars represent mean ± S.E. of three independent experiments and statistical significance was calculated using GraphPad Prism software.
Surface Biotinylation
Surface biotinylation was performed using the ThermoFisher surface biotinylation kit according to the manufacturer's instructions (ThermoFisher). Samples were taken from input, flow through, and after surface biotinylation and prepared with Laemmli buffer for Western blot analysis.
Western Blotting and Antibodies
Cells were directly lysed in Laemmli buffer and samples were boiled prior to resolving by SDS-PAGE. Proteins were transferred onto nitrocellulose/PVDF membranes and blocked for 1 h in 5% skim milk in TBS, 0.05% Tween-20 (TBS-T). Membranes were probed overnight at 4 °C with primary antibodies and bound antibodies were visualized using HRP-linked secondary antibodies (Cell-Signaling) and ECL Luminescence (Pierce Biotechnology). Rabbit anti-HA (#H6908, 1:2500), mouse anti-FLAG-M2 (#F3165, 1:5000), and rabbit anti-Lamin A/C (#L1293, 1:1000) were from Sigma-Aldrich, rabbit anti-NOTCH2 antibody (#2420S, 1:2000), rabbit anti-NOTCH1 S3-Val1744 (#4147S, 1:1000) and secondary horse anti-mouse (#7076S, 1:2500) and goat anti-rabbit (#7074S, 1:2500) were from Cell Signaling. Mouse anti-Myc 9E10 (#sc-40, 1:5000), and rabbit anti-Gal4 (#sc-577, 1:1000) were from Santa Cruz Biotechnology, mouse anti-N-cadherin (#610920, 1:1000) and mouse anti-HIF1β (#611078, 1:5000) were from BD Transduction Laboratories.
Heterodimer Stability Assays
293T cells grown in a 10-cm dish were transfected with 3 μg of plasmids encoding the NOTCH NRRs. Upon furin cleavage during expression, the resulting polypeptides were mainly secreted as a heterodimer consisting of stably associated FLAG-LNRs-(HD-N) and (HD-C)-HA subunits. 48 h after transfection, conditioned medium was collected, cleared by centrifugation, and incubated for 2 h at 4 °C with anti-HA beads (Sigma). The beads were washed three times with DB buffer containing 50 mm Tris buffer (pH 7.5) containing 150 mm NaCl and 0.1% Nonidet P-40 in the presence or absence of 5 mm EDTA, followed by 1 h of incubation in Tris buffer or Tris buffer containing 5 mm EDTA. The stability of the heterodimers was assessed as described previously (18). In short, the immunoprecipitates were incubated for 30 min at room temperature in the DB buffer containing 0 to 5 m urea, both in the presence or absence of EDTA. Proteins bound to the beads were analyzed by SDS-PAGE and Western blot against FLAG and HA tags.
Results
NOTCH1 but Not NOTCH2 Receptors Are Activated by Calcium Chelation
We recently reported that the ligand-dependent signaling cascade for NOTCH2 and NOTCH3 in mammalian cells follow the NOTCH1 paradigm of regulated intramembrane proteolysis and the requirement of Adam10 ectodomain cleavage (12). To investigate the ligand-independent activation mechanism of NOTCH2 receptors in a comparative manner, we used EDTA, a divalent calcium chelator. EDTA is often used to induce unfolding of the NRR and formation of S2/N1EXT in the absence of ligand, as it removes the calcium, which is required for proper folding and the maintenance of the structural integrity of the NOTCH1 LNR domain, keeping the receptor in a closed and inactive state (5, 27). To determine whether EDTA activates NOTCH2, we overexpressed wild type HA-tagged human NOTCH2 receptors, stimulated these cells with EDTA, and assessed receptor proteolysis by anti-HA immunoblotting (Fig. 1A). As expected, calcium chelation by EDTA treatment induced robust S3/γ-secretase cleavage of NOTCH1, shown by immunoblotting with activated NOTCH1 ICD (Val-1744). This cleavage was γ-secretase dependent, since the γ-secretase inhibitor Dibenzazepine (DBZ) blocked cleavage, which led to the accumulation of the precursor S2 cleaved N1EXT fragment (Fig. 1A). In contrast, EDTA treatment did not lead to NOTCH2 receptor cleavage and N2EXT formation (Fig. 1A). We could not directly assess γ-secretase cleavage for NOTCH2 by immunoblotting, but only indirectly by looking at S2/NEXT accumulation, as antibodies for cleaved NOTCH2 do not exist. Next, we measured transcriptional activation of an artificial luciferase reporter gene RBP-jκ/CSL (12xCSL) after EDTA stimulation (Fig. 1B). In line with the immunoblotting data, EDTA readily induced γ-secretase-dependent RBP-Jκ/CSL reporter activity in cells transfected with NOTCH1, but not in cells transfected with NOTCH2 (Fig. 1B). Similar observations were made using EGTA which is more selective for calcium than EDTA. EGTA treatment resulted in Notch1 S3 cleavage in cells expressing Myc-tagged mouse Notch1, but not in cleavage of endogenous NOTCH2 in these same cells (Fig. 1C). Importantly, NOTCH2-expressing cells remained competent for ligand induced signaling, shown by receptor cleavage and RBP-Jκ/CSL reporter gene activation (Fig. 2H and Ref. 12). To exclude potential confounding differences in transcriptional regulation between NOTCH1 and NOTCH2 we replaced the NOTCH1 and -2 intracellular domain with the Gal4 DNA binding (G4) and the transcriptional activation domain of NOTCH1 (N1 and N2-G4, Fig. 1D) (26). Both full length N1 and N2-G4 fusion proteins were equally expressed in transfected cells (Fig. 1D) and similarly activated by co-culture with ligand (i.e. Jag1) in a γ-secretase dependent manner in cells co-transfected with a Gal4 DNA binding luciferase reporter plasmid (Fig. 1E). In line with the transcriptional reporter gene activation assays, we observed the induction of Gal4 transcriptional activity by EDTA treatment of N1-G4 but not in cells expressing N2-G4 (Fig. 1F). Taken together these results indicate that human NOTCH2 receptors are resistant to activation by calcium chelation.
NOTCH2 NRR Mutations Do Not Cause Ligand-independent Activation
Missense mutations in the HD domain of NOTCH1 are common in human T-ALL and result in ligand-independent, constitutive NOTCH1 signaling (5, 18, 20). Based on the structural conservation between the HD domains of the different NOTCH receptors, we asked whether leukemogenic mutations similar to those found in NOTCH1 in T-ALL would also lead to ligand-independent activity in NOTCH2 receptors. Site-directed mutagenesis was performed to introduce the corresponding NOTCH1 T-ALL mutations in the HD domains of NOTCH2 (Fig. 2A, using Clustal-W2 alignment (28)). The NOTCH1 T-ALL mutation L1594P, a well-described ligand-independent constitutive active NOTCH1 receptor, showed robust γ-secretase dependent RBP-Jκ/CSL reporter gene activation and the accumulation of N1EXT in the presence of DBZ, which blocked Val-1744 cleaved NICD (Fig. 2, B and C, respectively). In U2OS cells transfected with T-ALL mimicking mutations in the HD of NOTCH2, (I1549E, L1566P, V1623D, and I1627N) receptors were not efficiently furin/S1 processed, leading to reduced heterodimer/TMIC formation, as shown by HA immunoblotting (Fig. 2D). Surface biotinylation and streptavidin pull-downs followed by HA immunoblotting confirmed that HD mutant NOTCH2 receptors (I1549E and L1566P) were virtually absent from the cell surface (Fig. 2E). Specificity of the biotinylation procedure was confirmed by efficient recovery from streptavidin pull-down of membrane bound N-cadherin, while the cytoplasmic protein HIF-1β was not. Consistently, none of the introduced T-ALL like NOTCH2-HD mutants, showed neither ligand-independent (Fig. 2F) nor ligand-induced (Fig. 2H) receptor processing or induction of RBP-Jκ/CSL reporter gene activation compared with wild-type NOTCH2 proteins. One of the NOTCH2 HD mutations (NOTCH2 V1667I), found in splenic marginal zone B lymphoma (21), was normally processed into full-length and TMIC forms similar to wild type NOTCH2 and expressed at the cell surface (Fig. 2, D and E). This mutant NOTCH2 receptor showed a modest γ-secretase dependent accumulation of N2EXT and transcriptional reporter gene activity upon ligand stimulation (Fig. 2, G and H) comparable to wild type NOTCH2. These results suggest that V1667I could be a polymorphism rather than a functional activator. We also tested whether truncated human NOTCH2-Gal4 chimeric proteins lacking the extracellular ligand binding EGF repeats, but containing an intact NRR (ΔEGF-G4, Fig. 1D) were sensitive to relief of auto-inhibition by mutations analogous to oncogenic activating mutations of the NOTCH1 NRR. Such truncated proteins are ligand insensitive but undergo activating proteolysis in response to calcium chelation or HD mutations (11, 18, 29). The wild type N2ΔEGF-G4 protein is processed efficiently at S1 by furin. In contrast, S1 processing of the L1566P mutant is markedly reduced (Fig. 1D, lanes 4 and 5), and the ligand-independent signal of the L1566P mutant is no greater than the basal signal from the wild-type receptor (Fig. 2I). Thus, mutations that are activating in the context of NOTCH1 fail to show ligand-independent activation in the context of NOTCH2. Because all of the inactive NOTCH2 proteins studied here showed markedly reduced cell surface expression compared with the signaling competent receptors (in NOTCH1 or NOTCH2), it is certainly possible that their failure to signal results from attenuated cell surface expression.
The NOTCH2 NRR Responds Differently to Perturbations When Compared with NRR1
One of the most striking structural differences between the NOTCH2 and NOTCH1 NRR is at the junction between the LNR-C repeat and the HD domain. Here, NOTCH2 contains a zinc coordination site that includes two histidines (His-1574 and His-1638), whereas in NOTCH1 the interaction between the domains is mediated by a histidine and a hydrogen bond with arginine (7). We therefore asked whether these histidines mediated the resistance to calcium chelation in NOTCH2. To investigate this, we substituted both histidines to the respective amino acids found in NOTCH1 (H1574R) and NOTCH3 (H1638Q) at these positions. When overexpressed in U2OS cells, NOTCH2 H1574R or H1638Q receptors were normally processed into full-length and TMIC forms (Fig. 3A) and stimulation by Jagged1 led to the formation of N2EXT and transcriptional activation, indistinguishable from wild type NOTCH2 (Fig. 3, B and C). In the absence of ligand, neither mutant was S2/S3 processed or transcriptionally active. Furthermore, none of the NOTCH2 histidine mutants could be stimulated to produce N2EXT or led to NOTCH-dependent transcriptional activity in response to EDTA (Fig. 3, D and E). Interestingly, residue His-1638 was recently found mutated in marginal zone lymphoma (H1638Y) (22). Similar to NOTCH2 V1667I, this mutation did not result in enhanced NOTCH2 cleavage or transcriptional activation in the absence of ligand (Fig. 3, A and F), although it did respond to ligand stimulation leading to transcriptional activation, similar to the wild type NOTCH2.
FIGURE 3.
Zinc coordinating histidines within the NOTCH2 NRR are not essential for auto-inhibition. A, HA-Immunoblotting of U2OS cells expressing WT or histidine mutant HA-NOTCH2 showing furin/TMIC levels. B, RBP-jκ/CSL-luciferase reporter activation of WT and His-mutant NOTCH2 in U2OS after OP9-Jag1 co-culture in the presence and absence of GSI. C, HA-immunoblots of WT or his-mutant NOTCH2 after OP9-Jag1 showing WT and the His mutants accumulate S2/N2EXT in response to GSI. D, expresion of TMIC and NEXT/S2 in response to EDTA stimulation and GSI treatment in U2OS cells expressing either WT or His-mutant NOTCH2 and (E) RBP-jκ/CSL-luciferase activation of WT and His-mutants after EDTA stimulation. Data are normalized to non-transfected controls and expressed relative to WT transfected cells (set as 1) treated with vehicle (DMSO) only (F) NOTCH transcriptional activation measured by RBP-jκ/CSL-luciferase reporter gene activation in U2OS transfected with WT, H1574R (NOTCH1 mimic) and patient-derived H1638Y MBZL mutant NOTCH2 or empty vector control (EV). RLU, relative light units. Lamin A/C is used as loading control. Data are represented as mean ± S.E. of three independent experiments. *, p < 0,05.
The NRR of NOTCH2 Is More Resistant to Unfolding than NOTCH1
To directly address differences in stability between the NOTCH1 and NOTCH2 NRR, we produced FLAG-HA epitope-tagged NRRs composed of the LNR-(HD-N) and HD-C subunits of NOTCH1 and NOTCH2 (Fig. 4A), which are furin cleaved and secreted in the medium of transfected cells (18). Heterodimers were isolated by anti-HA immunoprecipitation and exposed to increased concentrations of urea and analyzed by PAGE. Anti-FLAG immunoblotting demonstrated that increasing concentrations of urea increased the dissociation of the NOTCH1 NRR1, showing reduced subunit dissociation at 3 m urea and complete dissociation at a urea concentration of 4 m (Fig. 4B). In contrast, dissociation of the NRR2 required higher urea concentrations, as FLAG expression was still observed at urea concentrations of 4 m (Fig. 4B). These results were confirmed by stepwise elution of the FLAG-NRR2 from the HA-IP with increasing concentration of urea, followed by immunoblotting against FLAG in the eluted fraction (Fig. 4C). Here, we observed still FLAG-NRR2 bound to the HA beads after incubation with 5 m urea (labeled all), whereas this could not be detected for FLAG-NRR1. Next, we determined whether pre-treatment with EDTA lowered the stability of the NRR and lower the amount of urea required for NRR dissociation (Fig. 4D). As expected, EDTA pre-treatment reduced the concentration of urea required to dissociate the NOTCH1 NRR, as FLAG expression was no longer detectable at 3 m urea. In contrast, EDTA pre-treatment did not affect urea induced dissociation of NRR2, as 5 m urea was still required for complete subunit dissociation, comparable to the concentration required in the absence of EDTA pre-treatment. As a control to confirm the role of the NRR2 in receptor auto-inhibition, we generated truncated NOTCHΔE receptors, lacking the ligand binding EGF repeats and LNR repeats in the NOTCH ectodomain. As expected, NOTCHΔE receptors showed a robust γ-secretase dependent RBP-Jκ/CSL reporter gene activation for NOTCH1 and NOTCH2, albeit to a different extent (Fig. 4E). These data therefore support the notion that the integrity of the NOTCH2 NRR is crucial to suppress ligand-independent activation.
FIGURE 4.

The NOTCH2 NRR is more resistant to unfolding than the NOTCH1 NRR. A, schematic overview of FLAG-HA tagged NRR composed of N and C-terminal HD domains and LNR A, B, and C modules, which are furin (S1) cleaved and secreted into the medium of HEK293 as heterodimers. B and D, HA-immuneprecipitation from HEK293 medium 48 h post-transfection. HA-IP pre-incubated in the absence (B) or presence (D) of EDTA followed by increasing urea and blotted for HA (total IP) and FLAG (eluted fragment). C, stepwise elution with urea (0–5 m) directly from HA-bound NRR1 and NRR2 on beads, followed by anti-FLAG IB from supernatant. Note earlier appearance of FLAG signal with NRR1 than NRR2 and at 5 m urea FLAG-NRR2 is still bound to the HA beads and can be observed in the residual eluted fraction (all). E, RBP-jκ/CSL-luciferase reporter gene activation in NIH3T3 cells transfected with ectodomain truncated NOTCH1 and NOTCH2 ΔE constructs showing a γ-secretase dependent transcriptional activation indicating that NRR domain removal from NOTCH2 is sufficient for constitutive activity. RLU, relative light units. Data are represented as mean ± S.E. of three independent experiments. Urea dissociation experiments are representative of at least three independent experiments.
Murine Notch2 Is Sensitive to NRR Perturbation by EDTA and HD Mutation
Previous studies showed that murine Notch2 could be activated by calcium chelation (23). Therefore we hypothesized that the mouse Notch2 in contrast to the human NOTCH2 receptor is sensitive to NRR destabilization. To test this, we treated mouse embryonic fibroblasts (MEFs), which express endogenous Notch1 and Notch2 with EDTA and assessed Notch1 and Notch2 receptor proteolysis by immunoblotting (Fig. 5A). We observed that in MEFs lacking Adam10, but that express Adam17, EDTA induced the formation of S3 and accumulation of S2/NEXT in the presence of GSI. In Adam17-deficient cells, EDTA did not induce Notch1 or Notch2 proteolysis or activation (Fig. 5A). Thus murine Notch1 and Notch2 ligand-dependent activation requires Adam10 (12) while Notch1 and Notch2 ligand-independent activation requires Adam17. If the mouse NRR2 is sensitive to EDTA activation NRR unfolding, do HD mutations also lead to constitutive activity? To address this we introduced the L1566P mutation in murine Notch2, which mimics the NOTCH1-L1594P T-ALL mutant. In contrast to human NOTCH2 (Fig. 2F), murine Notch2 L1566P receptors are constitutively active and γ-secretase dependent as measured by an RBP-Jκ/CSL reporter gene assay in the absence of ligand (Fig. 5B). To address if there is a difference in stability between the human and mouse Notch2 NRR, we performed urea dissociation assays using the murine NRR2. As was seen for the human NRR1 (Fig. 4B), we observed complete subunit dissociation of the murine NRR2 at 4 m urea (Fig. 5C). Furthermore, EDTA pre-treatment reduced the concentration of urea required for complete dissociation to 3 m urea, comparable to dissociation of the human NRR1. These data show marked differences between murine and human Notch2 receptor activation despite high sequence conservation within the NRR (Fig. 2A).
FIGURE 5.
The mouse Notch2 NRR is sensitive to unfolding and EDTA activation and requires Adam17. A, immunoblotting for endogenous Notch1 and Notch2 after EDTA stimulation of Adam10- or Adam17-deficient MEFs. Cleaved-activated Notch1 (NICD Val1744) is only observed in Adam10-deficient cells and not Adam17-deficient cells. Anti Notch2 IB (upper panel) shows N2ICD and accumulation of N2EXT/S2 only in cells expressing Adam17 (Adam10−/−) and in a GSI-dependent manner. B, RBP-jκ/CSL-luciferase reporter activation in U2OS cells expressing wild type or murine HD-mutant N2L1566P showing robust ligand-independent, γ-secretase-dependent, transcriptional activation in N2L1566P mutant. C, HA-IP of transfected murine NRR2 48 h post-transfection from medium of HEK293 in the presence or absence of EDTA followed by urea dissociation. Lamin A/C is used as loading control. RLU, relative light units. Data are represented as mean ± S.E. of three independent experiments. Urea dissociation assay with murine NRR2 is representative of at least three independent experiments.
Discussion
More than 50% of acute T-cell leukemias harbor activating mutations in NOTCH1, causing constitutive ligand-independent signaling. About half of those are found in the extracellular hetero-dimerization domain that is part of the NRR, a repressor of NOTCH ectodomain shedding and Adam metalloprotease cleavage in the absence of ligand (6). In contrast to NOTCH1, very few mutations have been found in the NRR regions of NOTCH2, although these are highly similar and structurally conserved (7).
Here, we investigated the molecular mechanisms of ligand-independent activation of human and mouse NOTCH2 in comparison to NOTCH1. We find that the human NOTCH2 NRR is resistant to chemical unfolding of the NRR or mutations within the HD domain and cannot be activated in a ligand-independent manner as NOTCH1. Despite high sequence conservation (within the NRR), the murine Notch2 receptors are robustly activated in a ligand independent manner that requires Adam17, similar to Notch1. We directly attribute this difference to the NRR's of the receptors that differ significantly in their ability to dissociate under normal and calcium-depleted conditions. Compared with the human NOTCH1 and murine Notch2, the human NRR2 shows a decreased sensitivity to dissociation.
Taken together our results reveal unexpected differences in the ligand independent activation mechanism among NOTCH receptors. These results reinforce a paradigm whereby mammalian NOTCH receptor proteolysis is regulated by NRR unfolding and depending on the stimulus, follow an Adam10 (ligand-dependent) or Adam17 (EDTA, ligand-independent) triggered proteolytic cascade. While these routes appear to be dominant for wild type Notch1 and Notch2, NRR mutant NOTCH1 receptors are processed by both Adam17 and Adam10 (5, 10, 31). Furthermore using broad-spectrum metallo-protease inhibitors (targeting both Adam10 and 17) it was shown that NRR mutant NOTCH1 receptors are still active suggesting the possible involvement of additional proteases in mutant NOTCH1 processing (5). Because both cleavages lead to presenilin-dependent intramembranous cleavage and transcriptional activation it is not clear yet why both pathways exist. One possible explanation might be that the subcellular localization differs between Adam10 and Adam17 and that Notch receptors follow different secretory routes depending on the type of stimulus (32). If true, this would provide a therapeutic option of controlling ligand-independent signaling in diseases such as cancer.
Atomic force microscopy and force-unfolding simulations have been used in attempts to gain insight into the process of receptor unfolding. The simulations and AFM studies generally agree on the existence of at least four identifiable transitions, which presumably correspond to the three LNR modules and the HD domain (33–35). The simulations performed using the NOTCH2 NRR suggest that disengagement of the LNR-A:LNR-B linker from the HD domain would result in increased solvent exposure of the S2 site, which in turn might permit metalloprotease access and S2 cleavage by Adam17 (35). Cell based assays, on the other hand, indicate that the LNR-A, the LNR-A:LNR-B linker and the LNR-B all need to be removed to enable metalloprotease cleavage and activation of both NOTCH1 and NOTCH2 (8, 9), and hydrogen-exchange mass spectrometry studies suggest that solvent penetration of the beta strand containing the S2 site occurs simultaneously with increased solvent penetration into LNR-A, the A:B linker, and LNR-B. In T-ALL causing HD mutations, local unfolding of the β-5 strand may further increase cleavage propensity and NOTCH1 activation (6, 36).
Our findings indicate that the differences observed in the sensitivity of NOTCH2 to mutations and EDTA are encoded within the conserved NRR domain that protects the NOTCH ectodomain from ligand-independent activation. The data shown here provides evidence that the NRR of NOTCH1 and NOTCH2 achieve the same goal: stabilization of the auto-inhibited conformation to suppress ligand-independent proteolysis and activation, but are differentially sensitive to perturbation by mutation or EDTA treatment.
Ligand-independent NOTCH1 activation can be induced using calcium chelators such as EDTA (27), resulting in consecutive S2 and S3 cleavages, comparable to ligand-dependent Notch1 signaling (5). Genetic, biochemical and structural studies show that both the extracellular EGF and LNR repeats require calcium for proper folding and exit from the ER. Of interest here is that all three LNR modules within the NOTCH1 NRR accommodate calcium ions, which together with disulfide bonds adopt a closed and packed conformation (8, 37, 38). Breaking the disulfide bonds leads to constitutive active Notch1 proteins that are S2 processed at the cell surface indicating their crucial role in NRR stabilization (5, 11, 29). Dynamic hydrogen-exchange mass spectrometry studies on the NOTCH NRR indicate that the NOTCH1 NRR in the absence of calcium ions is in an 'open and active' conformation compared with the calcium-bound closed-inactive state (9). Here, we provide direct evidence that EDTA does not result in cleavage and activation of NOTCH2 in mammalian cells. Furthermore EGTA, which is more selective for calcium over zinc compared with EDTA, also does not result in human NOTCH2 proteolysis and activation, whereas it does for murine Notch2. Could the differential involvement of Adam proteases for ligand-independent signaling of NOTCH1 and NOTCH2 be an explanation for these observed differences? If Adam17 could not cleave NOTCH2 and EDTA or EGTA would inhibit only Adam10, this could explain why NOTCH1, but not NOTCH2 is activated in response to chelators. While not directly addressed here, our findings suggest that the impact of EDTA/EGTA on inhibition of a S2 protease is minor on Notch1 orthologs and the murine Notch2 homolog. Studies of NOTCH proteolysis under force suggest that Adam17 can process NOTCH2 under certain conditions, but it is not clear whether the in vitro results can be extrapolated to the cellular context (35). Our urea unfolding experiments confirm that the NOTCH2 NRR is more stable to denaturation than the human NOTCH1 NRR and the murine Notch2 NRR, and that subunit dissociation in this assay is remarkably insensitive to calcium chelation independent of whether or not the sensitivity of the receptors to Adam proteases varies. Another possibility might be that EDTA/EGTA treatment induces collapse of the human NRR2, preventing S2 exposure and subunit dissociation in response to the addition of denaturant.
The AFM studies measuring the response of the NOTCH2 NRR to applied force show that metal ions (calcium and zinc) increase the resistance of the NRR to unfolding under applied force in vitro (35). Indeed, the crystal structure of the NOTCH2 NRR shows a zinc ion coordinated by two histidines in the linker between LNR:B and LNR:C unique to NOTCH2 and not present in NOTCH1. His-1638 is also not conserved in murine Notch2 where it is replaced by glutamine. We found neither histidine to be essential for ligand-dependent NOTCH2 activation nor sufficient to induce ligand-independent or EDTA induced activation of NOTCH2. Thus zinc chelation alone in the NOTCH2 NRR cannot explain the differences observed here between NOTCH1 and NOTCH2. Additionally, it fails to explain the differences between human and mouse Notch2 since the mutated histidine in the H1638Q human NOTCH2 is the same residue (glutamine) as in mouse Notch2 and not ligand-independent.
We recently demonstrated that ligand induced proteolysis of NOTCH proteins is similar and all require the Adam10 protease and presenilin1/2-dependent γ-secretase activity (12). The mechanism by which leukemia associated NOTCH1 HD mutations contribute to ligand independency in T-ALL has also been amply studied and involves destabilization of the NRR (6, 18). While the functional consequences of HD destabilizing mutations are known for NOTCH1, similar activating mutations are infrequently found in NOTCH2 and those that were found lack functional evaluation. Here, we show that mutations found in marginal zone B lymphomas, where NOTCH2 PEST domain mutations are frequent (21, 22, 39) but HD mutations are infrequent, including the V1667I and H1638Y substitutions, are not detectably perturbing, and do not cause ligand-independent NOTCH2 signaling. Although we cannot exclude that these mutations act as gain of function in B-cell precursor populations in vivo and contribute to leukemia, it seems more likely they are polymorphisms without functional consequence. Furthermore, NOTCH1 T-ALL mimicking missense mutations mapping to the HD-N and HD-C domains do not lead to constitutive activation when engineered into NOTCH2.
The T-ALL mutants engineered into the NOTCH2 NRR are markedly attenuated in their cell surface expression, which might account for their failure to be activated. Perhaps the key difference is the degree to which surface transport is affected by mutation, because NOTCH1 receptors with HD missense mutations also result in reduced surface expression compared with NOTCH1 wild type receptors (5, 18, 29), yet they are ligand-independent and variably activating. The recent report that NOTCH1 HD mutant receptors are differentially sensitive to thapsigargin, a natural inhibitor of the calcium transporter Atp2a3/SERCA (40) that blocks surface transport, is consistent with this idea. The partial selectivity for mutant versus wild-type NOTCH proteins may indicate a higher requirement of the NRR of mutant NOTCH1 receptors for calcium to maintain proper folding in order to pass to the cell surface, and shows how signaling of mutant NOTCH1 receptors is prevented when surface transport is blocked.
Surprisingly, whereas furin-resistant NOTCH1 receptors are strongly attenuated in cell surface expression and ligand response, furin-resistant NOTCH2 molecules localize to the cell surface and retain activity comparable to furin cleaved NOTCH2 (4, 41). Here, we show that missense mutations in the NOTCH2 HD domain result in receptors that do not reach the cell surface. As there is no clear correlation between the extent of NOTCH TMIC cleavage and transcriptional output between NOTCH homologs the role of furin-processing still remains somewhat enigmatic in the regulation of NOTCH activity (4, 42, 43).
If NOTCH2 HD mutations were equally potent as PEST domain mutations as seen in human T-ALL, a higher frequency of HD mutations might be expected than observed in MZBL (<1%). Our results predict that most NOTCH2 HD missense mutations result in a loss of function as a result of retention in the secretory pathway. NOTCH2 nonsense mutations occur with frequencies ranging from 5–50% and are located mostly in the ligand binding EGF repeats, however some missense mutations are found within the LNR and HD domains as well (44). While no functional analysis of these mutations has been reported, we predict these might result in improper maturation of NOTCH2 and therefore act as loss of function. The same was observed for the NOTCH1 HD mutation R1594Q (in squamous cell carcinoma), which resulted in impaired ligand-dependent activation, possibly also due to a defect in cell surface expression, although this was not directly addressed (30). In contrast, NOTCH1 HD mutations surrounding this R1594Q (R1593Q in our alignment in Fig. 2A) mutation lead to ligand-independent activation in T-ALL (18). This indicates that the balance between an activating and a loss-of-function mutation in the NRR may be very subtle, with NOTCH1 mutations mostly leading to oncogenic activation, whereas for NOTCH2 most mutations are predicted to be loss-of-function. These findings are surprising given the high degree of structural and functional conservation between NOTCH1 and NOTCH2 (7).
Finally, murine Notch2, which has a 90% identity in the NRR with human NOTCH2 (Fig. 2A) is activated by EDTA and T-ALL mimicking mutations. Furthermore, the murine Notch2 NRR shows a comparable sensitivity to urea unfolding as the human NRR1, whereas the human NRR2 was more resistant to urea induced unfolding. Why is murine Notch2 so different from human NOTCH2? The NRR of mouse and human NOTCH2 differ by 20 amino acids, yet none of these stand out as likely to substantially impact the NRR structure. One such difference was tested here (H1638Q), but showed no effect, and experimental data is lacking for the others. Further analysis using human/mouse NOTCH2 chimeras and gene replacement studies in vivo may resolve this question.
Taken together, our findings highlight functionally important differences for the NRR among NOTCH homologs, paralogs and orthologs which translates specifically into their susceptibility to ligand-independent activation seen in diseases while these differences do not seem to impact on physiological ligand activation. Our findings clarify the predominance for NOTCH1 NRR mutations in human cancers, and suggest that mutations of the NOTCH2 NRR, when found in human cancers, are either polymorphisms or loss-of-function mutations.
Acknowledgments
We thank I. Prudovsky (Maine Medical Center Research Institute) for the human NOTCH2 cDNA and B. Blom (AMC, The Netherlands) for OP9 ligand-expressing cells.
This work was supported by the European Research Council (ERC) under the European Community Seventh Framework Program (FP7/2007-2013) ERC starting Grants 208259 and 334987 (to M. V.).
- S1
- Site-1
- NRR
- negative regulatory region
- LNR
- Lin12/Notch repeat
- HD
- heterodimerization
- T-ALL
- T-cell acute lymphoblastic leukemia
- TAD
- transcriptional activation domain
- DBZ
- Dibenzazepine
- NEXT
- NOTCH extracellular truncation domain
- TMIC
- transmembrane and intracellular domain.
References
- 1. Kopan R., Ilagan M. X. (2009) The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137, 216–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mumm J. S., Kopan R. (2000) Notch signaling: from the outside in. Dev. Biol. 228, 151–165 [DOI] [PubMed] [Google Scholar]
- 3. van Tetering G., Vooijs M. (2011) Proteolytic cleavage of Notch: “HIT and RUN”. Curr. Mol. Med. 11, 255–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Logeat F., Bessia C., Brou C., LeBail O., Jarriault S., Seidah N. G., Israël A. (1998) The Notch1 receptor is cleaved constitutively by a furin-like convertase. Proc. Natl. Acad. Sci. U.S.A. 95, 8108–8112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Tetering G., van Diest P., Verlaan I., van der Wall E., Kopan R., Vooijs M. (2009) Metalloprotease ADAM10 is required for Notch1 site 2 cleavage. J. Biol. Chem. 284, 31018–31027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sanchez-Irizarry C., Carpenter A. C., Weng A. P., Pear W. S., Aster J. C., Blacklow S. C. (2004) Notch subunit heterodimerization and prevention of ligand-independent proteolytic activation depend, respectively, on a novel domain and the LNR repeats. Mol. Cell. Biol. 24, 9265–9273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gordon W. R., Roy M., Vardar-Ulu D., Garfinkel M., Mansour M. R., Aster J. C., Blacklow S. C. (2009) Structure of the Notch1-negative regulatory region: implications for normal activation and pathogenic signaling in T-ALL. Blood 113, 4381–4390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gordon W. R., Vardar-Ulu D., Histen G., Sanchez-Irizarry C., Aster J. C., Blacklow S. C. (2007) Structural basis for autoinhibition of Notch. Nat. Struct. Mol. Biol. 14, 295–300 [DOI] [PubMed] [Google Scholar]
- 9. Tiyanont K., Wales T. E., Aste-Amezaga M., Aster J. C., Engen J. R., Blacklow S. C. (2011) Evidence for increased exposure of the Notch1 metalloprotease cleavage site upon conversion to an activated conformation. Structure 19, 546–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bozkulak E. C., Weinmaster G. (2009) Selective use of ADAM10 and ADAM17 in activation of Notch1 signaling. Mol. Cell. Biol. 29, 5679–5695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mumm J. S., Schroeter E. H., Saxena M. T., Griesemer A., Tian X., Pan D. J., Ray W. J., Kopan R. (2000) A ligand-induced extracellular cleavage regulates γ-secretase-like proteolytic activation of Notch1. Mol. Cell 5, 197–206 [DOI] [PubMed] [Google Scholar]
- 12. Groot A. J., Habets R., Yahyanejad S., Hodin C. M., Reiss K., Saftig P., Theys J., Vooijs M. (2014) Regulated proteolysis of NOTCH2 and NOTCH3 receptors by A-Disintegrin-And-Metalloproteinase (ADAM) 10 and Presenilins. Mol. Cell. Biol. 34, 2822–2832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Groot A. J., Cobzaru C., Weber S., Saftig P., Blobel C. P., Kopan R., Vooijs M., Franzke C. W. (2013) Epidermal ADAM17 Is Dispensable for Notch Activation. J. Invest. Dermatol. 133, 2286–2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Strooper B., Annaert W., Cupers P., Saftig P., Craessaerts K., Mumm J. S., Schroeter E. H., Schrijvers V., Wolfe M. S., Ray W. J., Goate A., Kopan R. (1999) A presenilin-1-dependent γ-secretase-like protease mediates release of Notch intracellular domain. Nature 398, 518–522 [DOI] [PubMed] [Google Scholar]
- 15. Struhl G., Greenwald I. (1999) Presenilin is required for activity and nuclear access of Notch in Drosophila. Nature 398, 522–525 [DOI] [PubMed] [Google Scholar]
- 16. Gridley T. (2003) Notch signaling and inherited disease syndromes. Human Molecular Genetics 12, R9–R13 [DOI] [PubMed] [Google Scholar]
- 17. Egloff A. M., Grandis J. R. (2012) Molecular pathways: context-dependent approaches to Notch targeting as cancer therapy. Clin Cancer Res. 18, 5188–5195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Malecki M. J., Sanchez-Irizarry C., Mitchell J. L., Histen G., Xu M. L., Aster J. C., Blacklow S. C. (2006) Leukemia-associated mutations within the NOTCH1 heterodimerization domain fall into at least two distinct mechanistic classes. Mol. Cell. Biol. 26, 4642–4651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ellisen L. W., Bird J., West D. C., Soreng A. L., Reynolds T. C., Smith S. D., Sklar J. (1991) TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell 66, 649–661 [DOI] [PubMed] [Google Scholar]
- 20. Weng A. P., Ferrando A. A., Lee W., Morris J. P., 4th, Silverman L. B., Sanchez-Irizarry C., Blacklow S. C., Look A. T., Aster J. C. (2004) Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science 306, 269–271 [DOI] [PubMed] [Google Scholar]
- 21. Kiel M. J., Velusamy T., Betz B. L., Zhao L., Weigelin H. G., Chiang M. Y., Huebner-Chan D. R., Bailey N. G., Yang D. T., Bhagat G., Miranda R. N., Bahler D. W., Medeiros L. J., Lim M. S., Elenitoba-Johnson K. S. (2012) Whole-genome sequencing identifies recurrent somatic NOTCH2 mutations in splenic marginal zone lymphoma. J. Exp. Med. 209, 1553–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Trøen G., Wlodarska I., Warsame A., Hernández Llodrà S., De Wolf-Peeters C., Delabie J. (2008) NOTCH2 mutations in marginal zone lymphoma. Haematologica 93, 1107–1109 [DOI] [PubMed] [Google Scholar]
- 23. Liu Z., Chen S., Boyle S., Zhu Y., Zhang A., Piwnica-Worms D. R., Ilagan M. X., Kopan R. (2013) The Extracellular Domain of Notch2 Increases Its Cell-Surface Abundance and Ligand Responsiveness during Kidney Development. Dev. Cell 25, 585–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dontje W., Schotte R., Cupedo T., Nagasawa M., Scheeren F., Gimeno R., Spits H., Blom B. (2006) Delta-like1-induced Notch1 signaling regulates the human plasmacytoid dendritic cell versus T-cell lineage decision through control of GATA-3 and Spi-B. Blood 107, 2446–2452 [DOI] [PubMed] [Google Scholar]
- 25. Shimizu K., Chiba S., Hosoya N., Kumano K., Saito T., Kurokawa M., Kanda Y., Hamada Y., Hirai H. (2000) Binding of Delta1, Jagged1, and Jagged2 to Notch2 rapidly induces cleavage, nuclear translocation, and hyperphosphorylation of Notch2. Mol. Cell. Biol. 20, 6913–6922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li K., Li Y., Wu W., Gordon W. R., Chang D. W., Lu M., Scoggin S., Fu T., Vien L., Histen G., Zheng J., Martin-Hollister R., Duensing T., Singh S., Blacklow S. C., Yao Z., Aster J. C., Zhou B. B. (2008) Modulation of Notch signaling by antibodies specific for the extracellular negative regulatory region of NOTCH3. J. Biol. Chem. 283, 8046–8054 [DOI] [PubMed] [Google Scholar]
- 27. Rand M. D., Grimm L. M., Artavanis-Tsakonas S., Patriub V., Blacklow S. C., Sklar J., Aster J. C. (2000) Calcium depletion dissociates and activates heterodimeric notch receptors. Mol. Cell. Biol. 20, 1825–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Larkin M. A., Blackshields G., Brown N. P., Chenna R., McGettigan P. A., McWilliam H., Valentin F., Wallace I. M., Wilm A., Lopez R., Thompson J. D., Gibson T. J., Higgins D. G. (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23, 2947–2948 [DOI] [PubMed] [Google Scholar]
- 29. Vooijs M., Schroeter E. H., Pan Y., Blandford M., Kopan R. (2004) Ectodomain shedding and intramembrane cleavage of mammalian Notch proteins is not regulated through oligomerization. J. Biol. Chem. 279, 50864–50873 [DOI] [PubMed] [Google Scholar]
- 30. Wang N. J., Sanborn Z., Arnett K. L., Bayston L. J., Liao W., Proby C. M., Leigh I. M., Collisson E. A., Gordon P. B., Jakkula L., Pennypacker S., Zou Y., Sharma M., North J. P., Vemula S. S., Mauro T. M., Neuhaus I. M., Leboit P. E., Hur J. S., Park K., Huh N., Kwok P. Y., Arron S. T., Massion P. P., Bale A. E., Haussler D., Cleaver J. E., Gray J. W., Spellman P. T., South A. P., Aster J. C., Blacklow S. C., Cho R. J. (2011) Loss-of-function mutations in Notch receptors in cutaneous and lung squamous cell carcinoma. Proc. Natl. Acad. Sci. U.S.A. 108, 17761–17766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sulis M. L., Saftig P., Ferrando A. A. (2011) Redundancy and specificity of the metalloprotease system mediating oncogenic NOTCH1 activation in T-ALL. Leukemia 25, 1564–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ebsen H., Schröder A., Kabelitz D., Janssen O. (2013) Differential surface expression of ADAM10 and ADAM17 on human T lymphocytes and tumor cells. PloS one 8, e76853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen J., Zolkiewska A. (2011) Force-induced unfolding simulations of the human Notch1 negative regulatory region: possible roles of the heterodimerization domain in mechanosensing. PloS one 6, e22837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dey A., Szoszkiewicz R. (2012) Complete noise analysis of a simple force spectroscopy AFM setup and its applications to study nanomechanics of mammalian Notch 1 protein. Nanotechnology 23, 175101. [DOI] [PubMed] [Google Scholar]
- 35. Stephenson N. L., Avis J. M. (2012) Direct observation of proteolytic cleavage at the S2 site upon forced unfolding of the Notch negative regulatory region. Proc. Natl. Acad. Sci. U.S.A. 109, E2757–E2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aster J. C., Blacklow S. C., Pear W. S. (2011) Notch signalling in T-cell lymphoblastic leukaemia/lymphoma and other haematological malignancies. J. Pathol. 223, 262–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aster J. C., Simms W. B., Zavala-Ruiz Z., Patriub V., North C. L., Blacklow S. C. (1999) The folding and structural integrity of the first LIN-12 module of human Notch1 are calcium-dependent. Biochemistry 38, 4736–4742 [DOI] [PubMed] [Google Scholar]
- 38. Vardar D., North C. L., Sanchez-Irizarry C., Aster J. C., Blacklow S. C. (2003) Nuclear magnetic resonance structure of a prototype Lin12-Notch repeat module from human Notch1. Biochemistry 42, 7061–7067 [DOI] [PubMed] [Google Scholar]
- 39. Rossi D., Trifonov V., Fangazio M., Bruscaggin A., Rasi S., Spina V., Monti S., Vaisitti T., Arruga F., Famà R., Ciardullo C., Greco M., Cresta S., Piranda D., Holmes A., Fabbri G., Messina M., Rinaldi A., Wang J., Agostinelli C., Piccaluga P. P., Lucioni M., Tabbò F., Serra R., Franceschetti S., Deambrogi C., Daniele G., Gattei V., Marasca R., Facchetti F., Arcaini L., Inghirami G., Bertoni F., Pileri S. A., Deaglio S., Foà R., Dalla-Favera R., Pasqualucci L., Rabadan R., Gaidano G. (2012) The coding genome of splenic marginal zone lymphoma: activation of NOTCH2 and other pathways regulating marginal zone development. The Journal of experimental medicine 209, 1537–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Roti G., Carlton A., Ross K. N., Markstein M., Pajcini K., Su A. H., Perrimon N., Pear W. S., Kung A. L., Blacklow S. C., Aster J. C., Stegmaier K. (2013) Complementary genomic screens identify SERCA as a therapeutic target in NOTCH1 mutated cancer. Cancer Cell 23, 390–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gordon W. R., Vardar-Ulu D., L'Heureux S., Ashworth T., Malecki M. J., Sanchez-Irizarry C., McArthur D. G., Histen G., Mitchell J. L., Aster J. C., Blacklow S. C. (2009) Effects of S1 cleavage on the structure, surface export, and signaling activity of human Notch1 and Notch2. PLoS One 4, e6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kidd S., Lieber T. (2002) Furin cleavage is not a requirement for Drosophila Notch function. Mech. Dev. 115, 41–51 [DOI] [PubMed] [Google Scholar]
- 43. Bush G., diSibio G., Miyamoto A., Denault J. B., Leduc R., Weinmaster G. (2001) Ligand-induced signaling in the absence of furin processing of Notch1. Dev. Biol. 229, 494–502 [DOI] [PubMed] [Google Scholar]
- 44. Pickering C. R., Zhou J. H., Lee J. J., Drummond J. A., Peng S. A., Saade R. E., Tsai K. Y., Curry J., Tetzlaff M. T., Lai S. Y., Yu J., Muzny D. M., Doddapaneni H., Shinbrot E., Covington K. R., Zhang J., Seth S., Caulin C., Clayman G. L., El-Naggar A. K., Gibbs R. A., Weber R. S., Myers J. N., Wheeler D. A., Frederick M. J. (2014) Mutational landscape of aggressive cutaneous squamous cell carcinoma. Clin. Cancer Res. 20, 6582–6592 [DOI] [PMC free article] [PubMed] [Google Scholar]