Background: EST regulates estrogen homeostasis by sulfonating and deactivating estrogens.
Results: Liver ischemia and reperfusion (I/R) induced the expression of EST and comprised estrogen activity in an Nrf2-dependent manner. EST ablation gender-specifically affected I/R sensitivity.
Conclusion: EST is an oxidative stress responsive gene that affects liver I/R injury.
Significance: Inhibition of EST, at least in females, represents an effective approach to manage hepatic I/R injury.
Keywords: estrogen sulfotransferase, estrogen homeostasis, liver ischemia and reperfusion, oxidative stress, Nrf2
Abstract
Estrogen sulfotransferase (EST) regulates estrogen homeostasis by sulfonating and deactivating estrogens. Liver ischemia and reperfusion (I/R) involves both hypoxia during the ischemic phase and oxidative damage during the reperfusion phase. In this report, we showed that the expression of EST was markedly induced by I/R. Mechanistically, oxidative stress-induced activation of Nrf2 was responsible for the EST induction, which was abolished in Nrf2−/− mice. EST is a direct transcriptional target of Nrf2. In female mice, the I/R-responsive induction of EST compromised estrogen activity. EST ablation attenuated I/R injury as a result of decreased estrogen deprivation, whereas this benefit was abolished upon ovariectomy. The effect of EST ablation was sex-specific because the EST−/− males showed heightened I/R injury. Reciprocally, both estrogens and EST regulate the expression and activity of Nrf2. Estrogen deprivation by ovariectomy abolished the I/R-responsive Nrf2 accumulation, whereas the compromised estrogen deprivation in EST−/− mice was associated with increased Nrf2 accumulation. Our results suggested a novel I/R-responsive feedback mechanism to limit the activity of Nrf2 in which Nrf2 induces the expression of EST, which subsequently increases estrogen deactivation and limits the estrogen-responsive activation of Nrf2. Inhibition of EST, at least in females, may represent an effective approach to manage hepatic I/R injury.
Introduction
Hepatic ischemia/reperfusion (I/R)5 injury occurs in various clinical situations when the blood flow to the liver is blocked or the liver is in a low-flow state, such as during liver resection, solid organ transplantation, cardiac and vascular surgery, massive trauma, hemorrhagic shock, and cardiogenic shock. I/R injury can cause a series of clinical manifestations ranging from asymptomatic elevation of liver enzymes to acute liver failure or even death (1).
The pathogenesis of hepatic I/R injury is a dynamic process, including the deprivation of blood and oxygen supply, followed by their restoration. The pathological events associated with I/R include direct hypoxic damage as the result of ischemia as well as a delayed and more severe oxidative damage that eventually leads to the activation of inflammatory pathways (2). During the hypoxic phase, sublethal cellular damage leads to the release of reactive oxygen species and damage-associated molecular pattern molecules from macrophages and hepatocytes. Reperfusion augments the injury by triggering sterile inflammatory responses, causing irreversible liver damage (3). Meanwhile, a series of protective pathways are also activated, and, as such, the extent of organ damage is determined by the balance between these two systems. During I/R, there are two transcriptional factors, hypoxia inducible factor 1 (HIF-1) and nuclear factor erythroid 2-related factor 2 (Nrf2), that play important roles in protecting the liver from I/R injury. Stabilization and accumulation of HIF-1 or Nrf2 lead to the activation of an array of genes to adapt the cells to hypoxic or oxidative damages and, therefore, affect numerous cellular functions, such as cell apoptosis, proliferation, survival, metabolism, and angiogenesis (2, 4).
Both clinical and animal studies have shown a sexual dimorphic response of the liver to various stresses, including liver I/R, hemorrhagic shock-resuscitation, liver cirrhosis, and endotoxemia. Many studies have suggested that female livers were more tolerant than male livers under stress conditions, suggesting estrogen as a responsible factor for this sexual dimorphism (5–8). The proposed mechanisms for the protective effect of estrogens on liver I/R include inhibition of apoptosis (9), an increased serum level of nitric oxide, a decreased serum level of tumor necrosis factor α (10), regulation of heat shock protein expression (11), and selective modulation of MAPK kinase activities (12). However, most of these experiments were performed by challenging male mice or ovariectomized female animals with pharmacological doses of estrogens, and the dynamics of the endogenous estrogen metabolism during I/R remains unknown. Considering the potential side effects associated with pharmacological estrogen therapies (13), it is necessary to develop novel therapeutic strategies to attenuate hepatic I/R injury through the regulation of endogenous estrogen metabolism.
An important metabolic pathway to deactivate estrogens is sulfotransferase (SULT)-mediated sulfation. Sulfonated estrogens cannot bind to and activate estrogen receptors, therefore losing their hormonal activities (14). Estrogen sulfotransferase (EST or SULT1E1) is the major SULT isoform responsible for estrogen sulfation because of its high affinity for estrogens (15). EST is known to express in multiple human tissues, and decreased EST expression may have led to increased availability of estrogens and contributed to the pathogenesis of hormone-dependent breast cancers (16). The basal expression of EST in the liver is low (17), but its expression is highly inducible in response to ligands for nuclear receptors, such as the liver X receptor (17), the glucocorticoid receptor (18), and the constitutive androstane receptor (19, 20). A major induction of EST has also been reported in the liver of mouse models of obesity and type 2 diabetes, and EST ablation improved the energy metabolism in female mice by increasing estrogenic activity in the liver (21).
Here we report that hepatic I/R injury induced the expression of EST, which was accounted for by the oxidative stress-responsive activation of Nrf2 and subsequent transactivation of EST, a novel Nrf2 target gene. Reciprocally, the Nrf2-responsive induction of EST facilitated the deactivation of estrogen, which is a positive regulator of Nrf2. Our results have uncovered a novel I/R-responsive and EST/estrogen-mediated feedback mechanism to prevent the sustained overactivation of Nrf2.
Experimental Procedures
Chemicals
The HIF-1 inhibitor was purchased from EMD Millipore Chemicals (San Diego, CA). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Animals
EST−/− mice have been described previously (22). Nrf2−/− mice were purchased from The Jackson Laboratory (Bar Harbor, ME). All mice were in the C57BL/6 background. The use of mice in this study complied with the relevant federal guidelines and institutional policies.
Liver Ischemia and Reperfusion
Liver warm ischemia was performed by placing a microvascular clamp across the portal vein, hepatic artery, and bile duct just above the branching to the right lateral lobe to occlude the blood supply to the left lateral and median lobes (∼70% ischemia of the whole liver). After 60 min of partial hepatic ischemia, the clamp was removed to initiate hepatic reperfusion. Sham control mice underwent the same protocol without vascular occlusion. Ischemic liver samples and blood were collected for analysis.
Primary Hepatocyte Hypoxia and Reoxygenation
Primary hepatocytes were isolated from 6- to 8-week-old female C57BL/6 mice and seeded at a density of 4 × 105 cells/well in 6-well plates. The initial culture medium was Williams medium E containing 10% FBS. Hepatocytes were allowed to attach to plates for 2 h and then changed to RPMI 1640 medium containing 10% FBS and incubated overnight. To initiate hypoxia, the medium was replaced with hypoxic medium (equilibrated with 1% O2, 5% CO2, and 94% N2) and placed into a modular incubator chamber (Billups-Rothenburg) that was flushed with the same hypoxic gas mixture. After hypoxia, plates were returned to the normal incubator to start the reoxygenation.
Histology and Immunohistochemistry
Liver paraffin sections were stained with hematoxylin-eosin. Necrosis was estimated by dividing the necrotic area by the entire histological section using ImageJ Software. For EST immunostaining, antigen retrieval was performed by conventional steam heating for 10 min in 0.01 m sodium citrate retrieval buffer. The sections were incubated with 0.3% H2O2 for 30 min to quench endogenous peroxidase activity. Then they were incubated with blocking serum for 10 min, followed by incubation with the primary polyclonal rabbit antibody against EST/SULT1E1 (1:100) from Proteintech (Chicago, IL) overnight at 4 °C. The staining was detected with the Vestastain ABC kit from Vector (Burlingame, CA). The slides were counterstained with hematoxylin.
Serum Aminotransferase Quantification
Blood samples were collected by cardiac puncture. Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were measured using assay kits from Stanbio (Boerne, TX).
Estrogen Sulfotransferase Activity Assay
Liver cytosols were prepared by homogenization, and the cytosols were then used for sulfotransferase assay by using [35S]phosphoadenosine phosphosulfate from PerkinElmer Life Sciences (Waltham, MA) as the sulfate donor, as described previously (17).
Northern Blotting, Real-time PCR, and Western Blotting
Total RNA was extracted from tissues using TRIzol reagent. Northern hybridization and real-time PCR were carried out as described previously (18). For Western blotting, 30 μg of total proteins for each sample were separated on 10% SDS-polyacrylamide gel. The primary antibody was a polyclonal rabbit antibody against SULT1E1 (1:1000) from Proteintech or Nrf2 (1:250) from Santa Cruz Biotechnology (Dallas, TX).
Cell Culture, Plasmid Constructs, and Reporter Gene Assay
HepG2 cells were maintained in DMEM supplemented with 10% FBS. The 2.5- and 3.0-kb 5′-regulatory sequences of the mouse EST gene were cloned by PCR. HepG2 cells were transiently transfected with the reporter constructs and the Nrf2 and MafG expression vectors in 48-well plates and measured for luciferase and β-gal activities 24 h after transfection. Transfection efficiency was normalized against β-gal activity from the cotransfected pCMX-β-gal plasmid.
EMSA and ChIP Assay
The Nrf2 and MafG proteins were prepared using the T7 quick-coupled transcription/translation system from Promega (Madison, WI). EMSA was performed by using 32P-labeled oligonucleotides as described previously (17). H2O2-treated primary hepatocytes were used for ChIP assays using a Nrf2 antibody as described previously (17).
Statistical Analysis
Results are expressed as mean ± S.D. Differences between two individual groups were determined by Student's t test. Differences between multiple groups were evaluated using one-way analysis of variance followed by post hoc multiple comparison according to the Student-Newman-Keuls test. Statistical significance was accepted at p < 0.05.
Results
Liver I/R Induced the Expression of EST
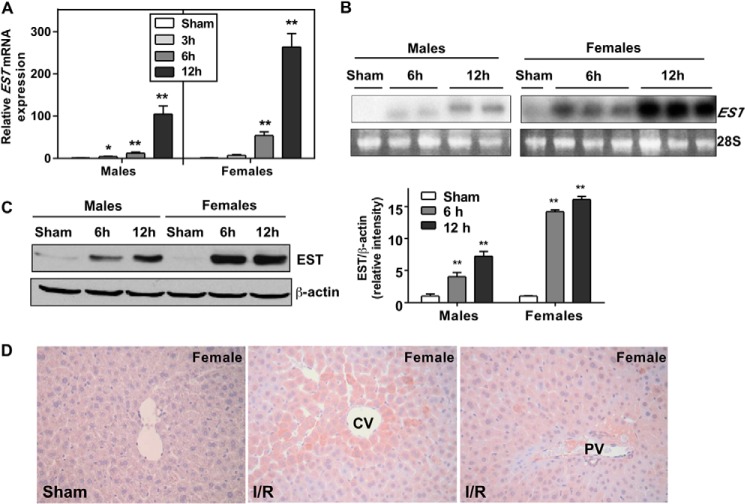
EST is known to have a low basal expression in the liver (18). Interestingly, we found a dramatic induction of hepatic EST mRNA expression upon I/R in both male and female mice in a time-dependent manner, as shown by real-time PCR (Fig. 1A) and Northern blot analysis (Fig. 1B). The induction of EST was also confirmed at the protein level by Western blotting (Fig. 1C), although the fold inductions at the mRNA and protein levels were not always consistent with each other. Immunohistochemistry showed that I/R-induced EST expression exhibited a partially zonal expression pattern, with the highest expression in hepatocytes surrounding the central veins but a reduced level in the periportal region (Fig. 1D).
FIGURE 1.
Liver I/R induced the expression of EST. Wild-type mice were subjected to I/R or a sham operation. Liver tissues were collected after 3, 6, or 12 h of reperfusion. A and B, the expression of EST mRNA levels in the liver was measured by real-time PCR with the sham groups independently set as 1 in the male and female groups (A) and Northern blotting (B). The Northern blotting results were derived from a single membrane. C, the expression of EST protein was measured by Western blotting, with the quantification of the signals shown in the right panel. D, representative EST immunostaining of liver sections from the female sham group (left panel) and I/R group (right two panels) from the same animal. CV, central vein; PV, portal vein. n = 5–6; *, p < 0.05; **, p < 0.01; all compared with the sham control.
I/R Compromised Estrogen Activity by Inducing EST, and EST Ablation Attenuated I/R Liver Injury In Female Mice
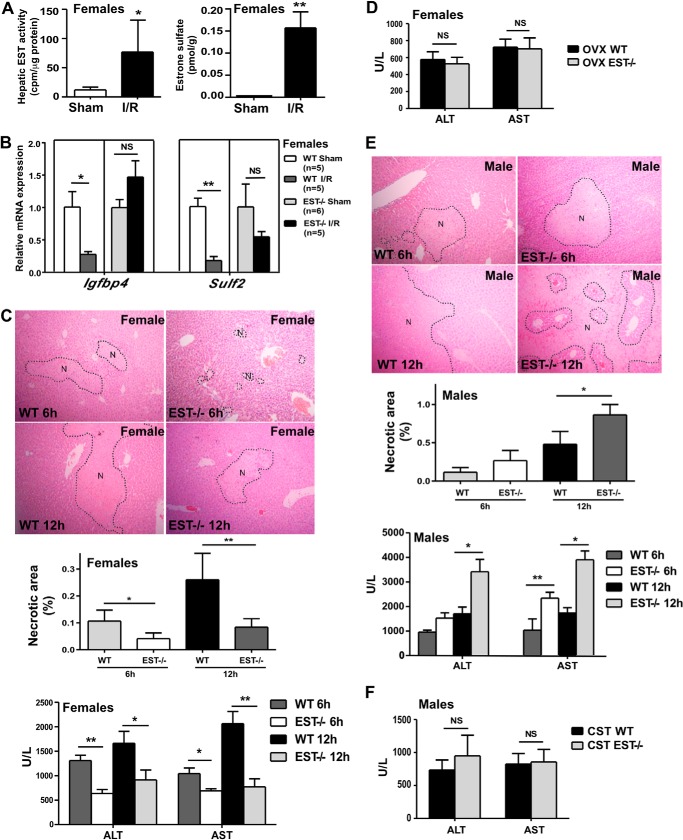
EST is known to deactivate estrogens. Consistent with their induction of EST, female mice that were subjected to I/R showed increased estrogen sulfotransferase activity (Fig. 2A, left panel) and a higher level of estrone sulfate (Fig. 2A, right panel) in their livers. Moreover, the expression of hepatic estrogen-responsive genes Igfbp4 and Sulf2 was decreased in female mice upon I/R, presumably because of increased estrogen deactivation. The I/R-responsive suppression of estrogen-responsive genes was EST-dependent because it was abolished in EST−/− mice (Fig. 2B).
FIGURE 2.
I/R compromised estrogen activity by inducing EST, and EST ablation attenuated I/R liver injury in female mice. A, female WT mice were subjected to 60-min/12-h liver I/R. Left panel, liver cytosolic extracts were measured for estrogen sulfotransferase activity using estrone as the substrate. Right panel, the liver tissue level of estrone sulfate. B, female WT and EST−/− mice were subjected to I/R. The hepatic expression of estrogen-responsive genes was measured by real-time PCR. C, mice were the same as in B. The I/R injuries were measured by histology (top panel, with the necrotic areas (N) circled) with the quantification of the necrotic area (center panel), as well as by serum ALT and AST levels. U/L, units per liter. D, female mice were subjected to ovariectomy (OVX) at 4 weeks of age before being subjected to I/R at 9 weeks of age. Shown are the serum ALT and AST levels. E, experiments were the same as in C except that male WT and EST−/− mice were used. F, male mice were subjected to castration (CST) at 4 weeks of age before being subjected to I/R at 9 weeks of age. Shown are the serum ALT and AST levels. n = 5–6; *, p < 0.05; **, p < 0.01; NS, statistically not significant.
To determine the functional relevance of EST and its regulation in I/R injury, we subjected WT and EST−/− mice to liver I/R. Female EST−/− mice were significantly protected from I/R injury compared with their WT counterparts after 6- or 12-h reperfusion, as evidenced by their decreased necrotic areas (Fig. 2C, top panel) and lower serum levels of ALT and AST (Fig. 2C, bottom panel). The protective effect of EST ablation was estrogen-dependent because ovariectomy abolished the differences in liver injury between WT and EST−/− mice (Fig. 2D). Ovariectomy seemed to weaken the ALT increase after I/R, the mechanism of which remains to be understood. Nevertheless, these results suggested that the I/R responsive EST induction may have led to the deactivation of estrogens and, therefore, contributed to the pathogenesis of I/R injury.
To our surprise, the effect of EST ablation on I/R injury was gender-specific because male EST−/− mice were more sensitive to I/R injury than WT males, which was confirmed by histology (Fig. 2E, top panel) and measurements of serum ALT and AST levels (Fig. 2E, bottom panels). The differences in the ALT and AST levels between WT and EST−/− males were abolished upon castration (Fig. 2F).
I/R-responsive Induction of EST Was Independent of HIF-1 but Nrf2-dependent
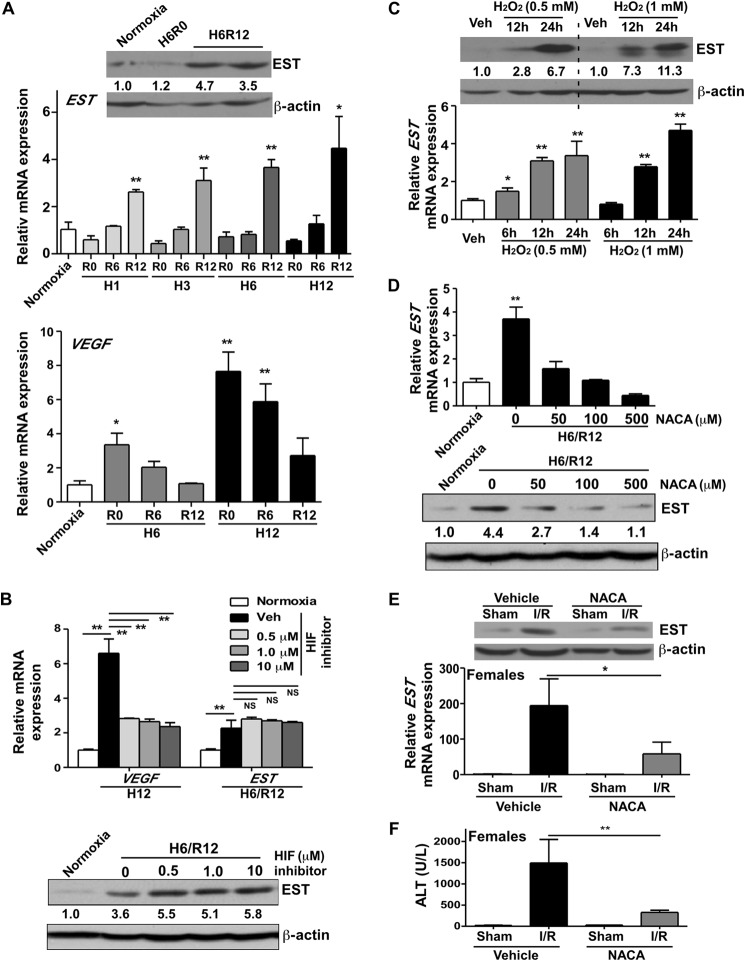
We next wanted to determine the mechanism by which I/R induced EST gene expression. Liver I/R is characterized by initial hypoxic tissue damage, followed by the return of blood flow and oxygen delivery, which accelerates the damage. To determine which stage of I/R is important for EST induction, we exposed primary mouse hepatocytes isolated from female mice to hypoxia and reoxygenation (H/R) to simulate the microenvironment of I/R. We found that 1–12 h of hypoxia alone was not sufficient to induce EST gene expression. In contrast, hypoxia followed by 12-h reoxygenation significantly induced EST expression (Fig. 3A, top panel). These results suggested that it was the reperfusion/oxidative phase, but not the ischemia/hypoxic phase, that played a dominating role in inducing EST. The expression of EST was also induced in primary human hepatocytes isolated from a female patient following 6-h hypoxia and 12-h reoxygenation (data not shown), suggesting that the effect is conserved in human hepatocytes. It is known that hypoxia increases the HIF-1 protein level by stabilizing this protein, whereas the HIF-1 protein is rapidly degraded when the oxygen is present (4). VEGF is a typical HIF-1 target gene (4). Consistent with these notions, in the same hepatocyte cultures, we found that hypoxia alone induced the expression of VEGF, whereas the expression of VEGF decreased upon reoxygenation (Fig. 3A, bottom panel). In vivo, the hepatic expression of VEGF in WT mice subjected to I/R was not induced following 6 h of reperfusion (data not shown), consistent with the hepatocyte results. Moreover, treatment of hepatocytes with HIF-1 inhibitor abolished the hypoxia-responsive induction of VEGF mRNA (Fig. 3B, top panel) but had little effect on the H/R-responsive induction of EST at the mRNA (Fig. 3B, top panel) and protein (Fig. 3B, bottom panel) levels. The results suggested that the H/R-responsive induction of EST was independent of HIF-1 activation.
FIGURE 3.
I/R-responsive induction of EST was independent of HIF-1, whereas oxidative stress was sufficient to induce EST. A, primary mouse hepatocytes were exposed to normoxia or hypoxia (H), followed by a different duration of reoxygenation (R). The mRNA expression of EST (top panel) and VEGF (bottom panel) and the protein expression of EST (top panel, inset) were measured by real-time PCR analysis and Western blotting, respectively. *, p < 0.05; **, p < 0.01; all compared with the normoxia group. B, primary mouse hepatocytes were pretreated with increasing doses of HIF-1 inhibitor before undergoing H/R. The mRNA expression of VEGF and EST (top panel) and the protein expression of EST (bottom panel) were measured by real-time PCR and Western blotting, respectively. **, p < 0.01; NS, statistically not significant; comparisons are labeled. The quantifications of the Western blotting bands are labeled. Veh, vehicle. C, primary mouse hepatocytes were treated with the indicated concentrations of H2O2 for the indicated amounts of time. The mRNA and protein expression of EST was measured by real-time PCR and Western blotting, respectively. *, p < 0.05; **, p < 0.01; all compared with the vehicle group. D, primary mouse hepatocytes were pretreated with increasing doses of NACA before undergoing H6/R12. The mRNA (top panel) and protein (bottom panel) expression of EST was measured by real-time PCR and Western blotting, respectively. **, p < 0.01 compared with the normoxia group. The quantifications of the Western blotting are labeled. E and F, female mice were pretreated with NACA (200 mg/kg) 1 h before undergoing 60-min/12-h I/R. Shown are the EST mRNA and protein expression (E) and serum ALT level (F). n = 3–5; *, p < 0.05; **, p < 0.01.
To directly test whether oxidative stress played a role in EST induction, we treated mouse primary hepatocytes isolated from female mice with a typical oxidant, H2O2, and found a time-dependent EST induction at the mRNA and protein levels (Fig. 3C). In contrast, pretreatment of hepatocytes with N-acetylcysteine amide (NACA), a membrane-penetrating antioxidant (23), abolished the H/R-responsive induction of EST mRNA (Fig. 3D, top panel) and protein (Fig. 3D, bottom panel) expression in a dose-dependent manner. In vivo, pretreatment of female mice with NACA attenuated the I/R-responsive induction of EST (Fig. 3E) and increase of serum ALT level (Fig. 3F). As expected, NACA treatment abolished the I/R-responsive Nrf2 target gene induction in the livers of female mice (data not shown), and this effect was conserved in male mice (data not shown).
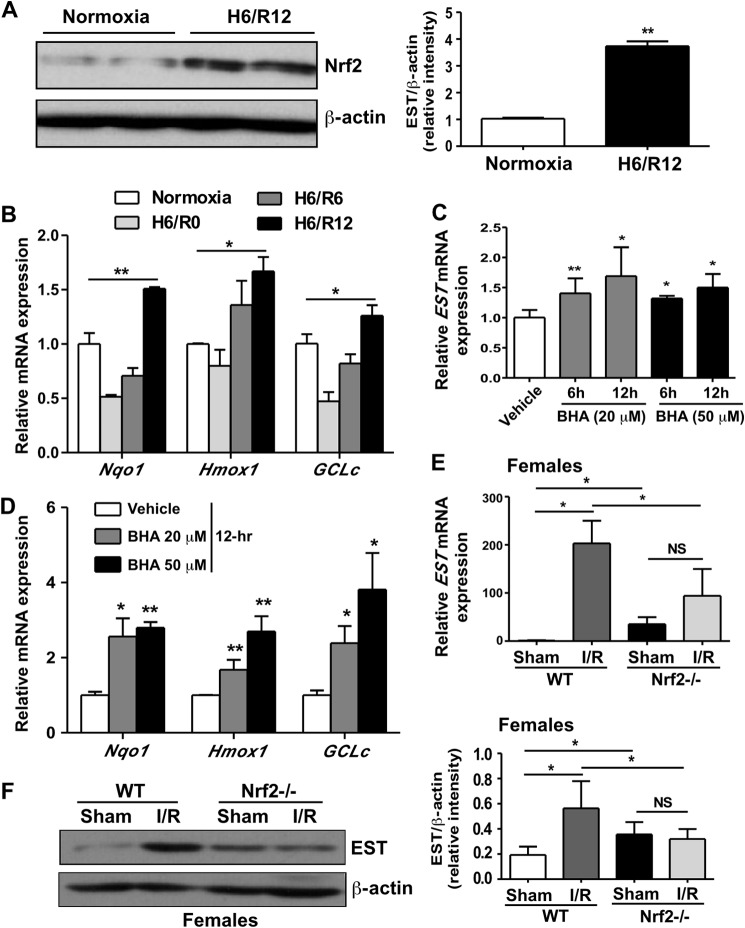
I/R-responsive oxidative stress is known to trigger multiple cellular signaling pathways (24), among which is the Nrf2-mediated regulation of cytoprotective antioxidative genes (25). Indeed, primary hepatocytes subjected to H/R showed an increased expression of Nrf2 protein (Fig. 4A). The expression of Nrf2 target genes, including Nqo1, Hmox1, and GCLc, was largely suppressed in the hypoxia-alone environment but induced significantly after 12-h reoxygenation (Fig. 4B). The pattern of induction of Nrf2 and its target genes was mirroring that of EST. Moreover, treatment of primary hepatocytes with the Nrf2 activator butylated hydroxyanisole (25) was sufficient to induce the expression of EST (Fig. 4C), along with the expected induction of several known Nrf2 target genes (Fig. 4D). In vivo, the I/R-responsive induction of EST mRNA (Fig. 4E) and protein (Fig. 4F) was abolished in Nrf2−/− mice, suggesting that the induction was Nrf2-dependent. It was noted that the basal expression of EST was higher in sham-operated Nrf2−/− mice compared with their wild-type counterparts.
FIGURE 4.
The I/R-responsive induction of EST was Nrf2-dependent. A and B, primary mouse hepatocytes were exposed to 6-h hypoxia followed by 12-h reoxygenation. Nrf2 protein expression was measured by Western blotting, with the quantification shown in the right panel (A). The mRNA expression of Nrf2 target genes was measured by real-time PCR (B). *, p < 0.05; **, p < 0.01; all compared with the normoxia groups. C and D, primary mouse hepatocytes were treated with indicated doses of butylated hydroxyanisole (BHA) for the indicated amounts of time. The mRNA expression of EST (C) and Nrf2 target genes (D) was measured by real-time PCR. *, p < 0.05; **, p < 0.01; all compared with the vehicle group. E and F, female WT and Nrf2−/− mice were subjected to 60-min/12-h liver I/R. The EST mRNA (E) and protein (F) levels were measured by real-time PCR and Western blotting, respectively. n = 4–6. *, p < 0.05; NS, statistically not significant.
EST Is a Transcriptional Target of Nrf2
Nrf2 is a transcriptional factor that regulates gene expression by binding to the antioxidant response element (ARE) in its target gene promoters (26). In determining whether EST is a transcriptional target of Nrf2, we found two putative AREs, ARE1 and ARE2, in the −5.0 kb and −2.7 kb positions of the mouse EST gene promoter, respectively (Fig. 5A). The binding of these two AREs to Nrf2 was evaluated by EMSA. Nrf2 can bind to DNA as homodimers or as heterodimers with its partner protein MafG (27). As shown in Fig. 5B, Nrf2 alone bound to both ARE1 and ARE2, and the bindings were enhanced when the MafG protein was added to the binding reactions. The binding was specific because efficient competition was achieved by adding an excess unlabeled wild-type competitor, whereas the unlabeled mutant competitor failed to compete (Fig. 5B). A ChIP assay was used to determine whether Nrf2 could be recruited onto the EST gene promoter. As shown in Fig. 5C, treatment of primary hepatocytes with H2O2 increased the recruitment of Nrf2 to both AREs. The recruitment of Nrf2 to a known ARE in the Nqo1 gene promoter (28) was included as a positive control. We also cloned the mouse EST gene promoter and evaluated its transactivation by Nrf2 by transient transfection and luciferase reporter gene assay. As shown in Fig. 5D, the 3.0-kb EST promoter that contained ARE2 was activated by the cotransfection of Nrf2 and MafG. In contrast, the ARE-less 2.5-kb promoter was not activated but modestly suppressed by Nrf2-MafG. These results suggest that the mouse EST gene is a transcriptional target of Nrf2.
FIGURE 5.
EST is a transcriptional target of Nrf2. A, two AREs were predicted in the mouse EST gene promoter. The sequences of the two WT and mutant AREs are shown in boldface with the mutated nucleotides underlined. The ARE from the Nqo1 gene promoter is also shown. B, the binding of two 32P-labeled putative AREs to Nrf2 homodimers or Nrf2-MafG heterodimers was confirmed by EMSA using in vitro-synthesized Nrf2 and MafG proteins. mt, mutant. C, ChIP assay to show the recruitment of Nrf2 protein onto the EST gene promoter. Primary hepatocytes were treated with vehicle or H2O2 (500 μm) for 12 h before ChIP using an anti-Nrf2 antibody. ChIP on Nqo1 was included as a positive control. The input contains the identical amount of DNA template that was used for the IgG or anti-Nrf2 antibody immunoprecipitation. Veh, vehicle. D, co-transfection of Nrf2 and MafG activated 3.0-kb but not 2.5-kb EST promoter reporter gene. Transfected cells were cultured for 24 h before the luciferase assay. *, p < 0.05 compared with the vector control.
Nrf2 Ablation Protected Female Mice from I/R Liver Injury
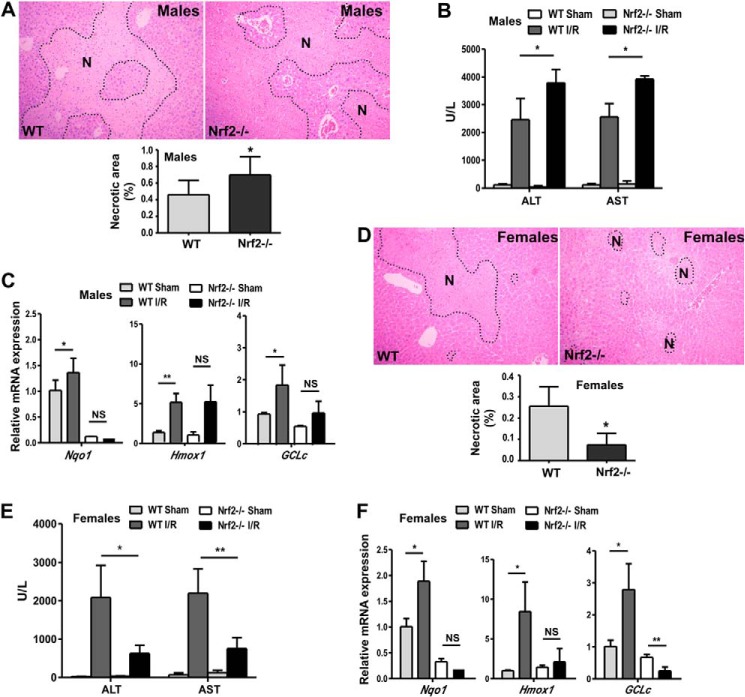
Having shown that Nrf2 is a positive regulator of EST and that EST plays a role in I/R-responsive liver injury, we went on to determine whether Nrf2 ablation can affect the I/R injury. We first showed that male Nrf2−/− mice had heightened liver I/R injury compared with their WT counterparts, as evidenced by increased liver necrosis (Fig. 6A) and serum levels of ALT and AST (Fig. 6B). The sensitizing effect of Nrf2 ablation was associated with the loss of I/R-responsive induction of Nrf2 target genes (Fig. 6C), which was consistent with previous reports (25, 29). Surprisingly and unexpectedly, we found that female Nrf2−/− mice were protected from I/R injury compared with WT females, as confirmed by histology (Fig. 6D) and measurements of serum ALT and AST levels (Fig. 6E). I/R-responsive Nrf2 target gene expression was similarly abolished in female Nrf2−/− mice (Fig. 6F), suggesting that the reduced antioxidant gene expression might not account for this sexual dimorphic effect of Nrf2 ablation.
FIGURE 6.
Nrf2 ablation protected female mice from I/R liver injury. A–C, WT and Nrf2−/− male mice were subjected to 60-min ischemia and 12-h reperfusion. Liver injury was measured by H&E staining of the liver paraffin sections with the necrotic areas (N) circled (A) and serum ALT and AST activities (B). The expression of the Nrf2 target gene was measured by real-time PCR (C). D–F, experiments were the same as described in A–C except that WT and Nrf2−/− female mice were used. n = 4–6; *, p < 0.05; **, p < 0.01; NS, statistically not significant.
EST Ablation and Estrogen Deprivation, Respectively, Increased and Attenuated the I/R-responsive Nrf2 Accumulation, a Potential Feedback Mechanism to Avoid the Overactivation of Nrf2
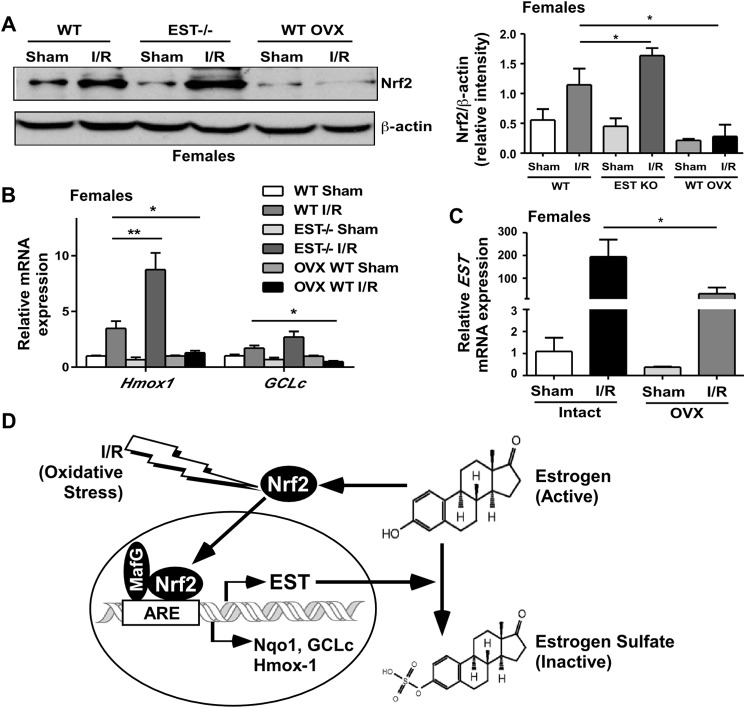
Having established EST as an Nrf2 target gene, it is interesting to note that the expression and activity of Nrf2 was also regulated by estrogens (30). These results suggest a mutual regulation between estrogens/EST and Nrf2. Indeed, we showed that the I/R-responsive accumulation of Nrf2 protein was enhanced in female EST−/− mice, likely because of compromised estrogen deprivation. In contrast, estrogen deprivation by ovariectomy abolished I/R-responsive Nrf2 accumulation (Fig. 7A). The dynamics of Nrf2 accumulation and activation in EST−/− and ovariectomized mice were confirmed by analyzing Nrf2 target gene expression (Fig. 7B). We also showed that ovariectomy attenuated the I/R-responsive induction of EST (Fig. 7C), likely as a result of the loss of estrogen-dependent Nrf2 activation. These results together suggest an estrogen-EST-Nrf2-mediated feedback mechanism to limit Nrf2 activation during I/R in which Nrf2 induces the expression of EST. The induction of EST subsequently increases estrogen deprivation and limits the estrogen-responsive induction of Nrf2 (Fig. 7D).
FIGURE 7.
EST ablation and estrogen deprivation, respectively, increased and attenuated I/R-responsive Nrf2 accumulation, a potential feedback mechanism to avoid the overactivation of Nrf2. A–C, female WT, EST−/−, and ovariectomized WT mice were subjected to 60-min/12-h liver I/R. The expression of Nrf2 protein was measured by Western blotting, with the quantification results shown in the right panel (A). Nrf2 target gene expression (B) and EST expression (C) were measured by real-time PCR. n = 4–6; *, p < 0.05; **, p < 0.01. OVX, ovariectomy. D, proposed I/R-responsive and estrogen-EST-Nrf2 mediated feedback mechanism to limit the activity of Nrf2. In this model, Nrf2 induces the expression of EST, which subsequently increases estrogen deactivation and limits the estrogen-responsive induction of Nrf2.
Discussion
Hepatic I/R injury contributes to morbidity and mortality in a wide range of clinical events, such as liver transplantation (24). In this study, we showed that the estrogen-deactivating enzyme EST was markedly induced in the mouse liver by I/R. Our results suggest that the up-regulation of EST in the liver may have played a pathogenic role in I/R injury because EST ablation in female mice attenuated I/R-responsive liver injury in an estrogen-dependent manner.
We were surprised to find that EST ablation had an opposite effect in males, because the male EST−/− mice showed heightened sensitivity to I/R injury. Previous reports suggested that estrogens protected male mice from I/R injury (10, 12). However, most of the reported protection was observed in male animals that were treated with pharmacological doses of estrogens. Our results suggest differential effects between the administration of pharmacological doses of estrogens and alteration in endogenous estrogen metabolism. The increased sensitivity to I/R in male EST−/− mice was abolished upon castration, suggesting that the sensitization was androgen-dependent. Indeed, androgens have been reported to play a role in liver I/R injury (31). The detailed mechanism for the sex-specific effect of EST ablation on I/R injury remains to be understood.
One of our interesting findings was the Nrf2-dependent but HIF-1 independent regulation of EST during I/R. HIF-1 and Nrf2 are activated during the ischemia and reperfusion stages, respectively. On the basis of the dynamics of EST regulation and the use of an HIF-1 inhibitor, our results suggest that the I/R-responsive induction of EST was HIF-1-independent. Instead, the activation of Nrf2 was found to be essential for EST induction because I/R failed to induce EST in Nrf2−/− mice. Nrf2 is a master regulator of the oxidative response (32). Most of the Nrf2 target genes encode antioxidant proteins and detoxifying enzymes (33). Consistent with the notion that Nrf2 is an oxidative responsive gene, we showed that treatment of primary hepatocytes with H2O2 induced EST, whereas the antioxidant NACA attenuated the H/R- and I/R-responsive induction of EST, further suggesting that Nrf2 activation triggered by oxidative stress is mechanistically involved in the I/R-responsive EST induction. We went on to show that EST is a direct transcriptional target of Nrf2. Interestingly, Nrf2 ablation increased the basal expression of EST, which was consistent with the data from the NCBI GEO (accession no. 55990853). These results suggested a yet to be characterized role of Nrf2 in regulating the basal expression of EST. A similar increase of the basal expression of drug-metabolizing enzyme was found in mice deficient of pregnane X receptor, a master regulator of drug-metabolizing enzymes (34).
Another intriguing finding is the I/R-responsive feedback mechanism to limit the Nrf2 activation. In this feedback mechanism, Nrf2 induces the expression of EST, the induction of which subsequently increases estrogen deactivation and limits the estrogen-responsive induction of Nrf2 (Fig. 7D). Equally intriguing is the biological significance of this feedback mechanism. Although Nrf2 has been reported to have various protective effects, activation of Nrf2 does not always lead to a favorable physiological outcome. It has been reported that enhanced Nrf2 activity worsened insulin resistance, impaired lipid accumulation in adipose tissue, and increased hepatic steatosis in leptin-deficient mice (35). In another report, oxidative stress-induced Nrf2 activation contributed to the pathogenesis of alcoholic liver disease by up-regulating the hepatic expression of very low density lipoprotein receptor (36). In this work, female Nrf2−/− mice showed attenuated sensitivity to I/R liver injury, which was reasoned to be due to the loss of Nrf2-dependent EST induction and estrogen deprivation. Our results suggest a novel estrogen/EST-mediated mechanism to prevent the overactivation or sustained activation of Nrf2, which can be potentially harmful.
Interestingly, like EST ablation, the loss of the Nrf2 effect was also gender-specific because male Nrf2−/− mice showed heightened sensitivity to I/R liver injury, as shown by others (25, 29) and by us. The sensitization in male Nrf2−/− mice was believed to be due to the loss of I/R-responsive Nrf2 target gene expression (25, 29). I/R-responsive Nrf2 target gene expression was similarly abolished in female Nrf2−/− mice, suggesting that the reduced antioxidant gene expression might not account for the sexual dimorphic effect of Nrf2 ablation on I/R injury. Instead, Nrf2−/− female mice showed a loss of Nrf2-dependent EST induction. Our results suggest that, at least in female Nrf2−/− mice, the gain of the estrogenic benefit might have outweighed the harm of loss of the induction of antioxidant genes, leading to the net outcome of attenuated I/R injury.
In summary, our results suggest that the induction of hepatic EST, which was mediated by oxidative stress-induced Nrf2 activation, may have played a pathogenic role in I/R liver injury. As such, both EST ablation and Nrf2 ablation conferred protection in female mice. Pharmacological inhibition of EST, at least in females, might represent a novel therapeutic approach to manage hepatic I/R injury. It is encouraging that major progress has been made in the identification and characterization of chemical EST inhibitors (37).
This work was supported, in whole or in part, by National Institutes of Health Grants HD073070, DK099232, and ES023438, and by the Joseph Koslow Endowed Professorship (to W. X.).
- I/R
- ischemia/reperfusion
- SULT
- sulfotransferase
- EST
- estrogen sulfotransferase
- AST
- aspartate aminotransferase
- ALT
- alanine aminotransferase
- H/R
- hypoxia and reoxygenation
- NACA
- N-acetylcysteine amide
- ARE
- antioxidant response element.
References
- 1. Serracino-Inglott F., Habib N. A., Mathie R. T. (2001) Hepatic ischemia-reperfusion injury. Am. J. Surg. 181, 160–166 [DOI] [PubMed] [Google Scholar]
- 2. Zhai Y., Busuttil R. W., Kupiec-Weglinski J. W. (2011) Liver ischemia and reperfusion injury: new insights into mechanisms of innate-adaptive immune-mediated tissue inflammation. Am. J. Transplant. 11, 1563–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klune J. R., Tsung A. (2010) Molecular biology of liver ischemia/reperfusion injury: established mechanisms and recent advancements. Surg. Clin. North Am. 90, 665–677 [DOI] [PubMed] [Google Scholar]
- 4. Déry M. A., Michaud M. D., Richard D. E. (2005) Hypoxia-inducible factor 1: regulation by hypoxic and non-hypoxic activators. Int. J. Biochem. Cell Biol. 37, 535–540 [DOI] [PubMed] [Google Scholar]
- 5. Harada H., Pavlick K. P., Hines I. N., Hoffman J. M., Bharwani S., Gray L., Wolf R. E., Grisham M. B. (2001) Selected contribution: effects of gender on reduced-size liver ischemia and reperfusion injury. J. Appl. Physiol. 91, 2816–2822 [DOI] [PubMed] [Google Scholar]
- 6. Jarrar D., Wang P., Cioffi W. G., Bland K. I., Chaudry I. H. (2000) The female reproductive cycle is an important variable in the response to trauma-hemorrhage. Am. J. Physiol. Heart. Circ. Physiol. 279, H1015–H1021 [DOI] [PubMed] [Google Scholar]
- 7. Lee C. C., Chau G. Y., Lui W. Y., Tsay S. H., King K. L., Loong C. C., Hshia C. Y., Wu C. W. (2000) Better post-resectional survival in female cirrhotic patients with hepatocellular carcinoma. Hepatogastroenterology 47, 446–449 [PubMed] [Google Scholar]
- 8. Jain A., Reyes J., Kashyap R., Dodson S. F., Demetris A. J., Ruppert K., Abu-Elmagd K., Marsh W., Madariaga J., Mazariegos G., Geller D., Bonham C. A., Gayowski T., Cacciarelli T., Fontes P., Starzl T. E., Fung J. J. (2000) Long-term survival after liver transplantation in 4,000 consecutive patients at a single center. Ann. Surg. 232, 490–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lin F. S., Shen S. Q., Chen Z. B., Yan R. C. (2012) 17β-estradiol attenuates reduced-size hepatic ischemia/reperfusion injury by inhibition apoptosis via mitochondrial pathway in rats. Shock 37, 183–190 [DOI] [PubMed] [Google Scholar]
- 10. Eckhoff D. E., Bilbao G., Frenette L., Thompson J. A., Contreras J. L. (2002) 17-β-estradiol protects the liver against warm ischemia/reperfusion injury and is associated with increased serum nitric oxide and decreased tumor necrosis factor-α. Surgery 132, 302–309 [DOI] [PubMed] [Google Scholar]
- 11. Shen S. Q., Zhang Y., Xiong C. L. (2007) The protective effects of 17β-estradiol on hepatic ischemia-reperfusion injury in rat model, associated with regulation of heat-shock protein expression. J. Surg. Res. 140, 67–76 [DOI] [PubMed] [Google Scholar]
- 12. Vilatoba M., Eckstein C., Bilbao G., Frennete L., Eckhoff D. E., Contreras J. L. (2005) 17β-estradiol differentially activates mitogen-activated protein-kinases and improves survival following reperfusion injury of reduced-size liver in mice. Transplant Proc. 37, 399–403 [DOI] [PubMed] [Google Scholar]
- 13. Staren E. D., Omer S. (2004) Hormone replacement therapy in postmenopausal women. Am. J. Surg. 188, 136–149 [DOI] [PubMed] [Google Scholar]
- 14. Song W. C. (2001) Biochemistry and reproductive endocrinology of estrogen sulfotransferase. Ann. N.Y. Acad. Sci. 948, 43–50 [DOI] [PubMed] [Google Scholar]
- 15. Zhang H., Varlamova O., Vargas F. M., Falany C. N., Leyh T. S. (1998) Sulfuryl transfer: the catalytic mechanism of human estrogen sulfotransferase. J. Biol. Chem. 273, 10888–10892 [DOI] [PubMed] [Google Scholar]
- 16. Pasqualini J. R. (2009) Estrogen sulfotransferases in breast and endometrial cancers. Ann. N.Y. Acad. Sci. 1155, 88–98 [DOI] [PubMed] [Google Scholar]
- 17. Gong H., Guo P., Zhai Y., Zhou J., Uppal H., Jarzynka M. J., Song W. C., Cheng S. Y., Xie W. (2007) Estrogen deprivation and inhibition of breast cancer growth in vivo through activation of the orphan nuclear receptor liver X receptor. Mol. Endocrinol. 21, 1781–1790 [DOI] [PubMed] [Google Scholar]
- 18. Gong H., Jarzynka M. J., Cole T. J., Lee J. H., Wada T., Zhang B., Gao J., Song W. C., DeFranco D. B., Cheng S. Y., Xie W. (2008) Glucocorticoids antagonize estrogens by glucocorticoid receptor-mediated activation of estrogen sulfotransferase. Cancer Res. 68, 7386–7393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alnouti Y., Klaassen C. D. (2008) Regulation of sulfotransferase enzymes by prototypical microsomal enzyme inducers in mice. J. Pharmacol. Exp. Ther. 324, 612–621 [DOI] [PubMed] [Google Scholar]
- 20. Sueyoshi T., Green W. D., Vinal K., Woodrum T. S., Moore R., Negishi M. (2011) Garlic extract diallyl sulfide (DAS) activates nuclear receptor CAR to induce the Sult1e1 gene in mouse liver. PLoS ONE 6, e21229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gao J., He J., Shi X., Stefanovic-Racic M., Xu M., O'Doherty R. M., Garcia-Ocana A., Xie W. (2012) Sex-specific effect of estrogen sulfotransferase on mouse models of type 2 diabetes. Diabetes 61, 1543–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Qian Y. M., Sun X. J., Tong M. H., Li X. P., Richa J., Song W. C. (2001) Targeted disruption of the mouse estrogen sulfotransferase gene reveals a role of estrogen metabolism in intracrine and paracrine estrogen regulation. Endocrinology 142, 5342–5350 [DOI] [PubMed] [Google Scholar]
- 23. Penugonda S., Mare S., Lutz P., Banks W. A., Ercal N. (2006) Potentiation of lead-induced cell death in PC12 cells by glutamate: protection by N-acetylcysteine amide (NACA), a novel thiol antioxidant. Toxicol. Appl. Pharmacol. 216, 197–205 [DOI] [PubMed] [Google Scholar]
- 24. Jaeschke H. (2003) Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am. J. Physiol. Gastrointest. Liver Physiol. 284, G15–G26 [DOI] [PubMed] [Google Scholar]
- 25. Ke B., Shen X. D., Zhang Y., Ji H., Gao F., Yue S., Kamo N., Zhai Y., Yamamoto M., Busuttil R. W., Kupiec-Weglinski J. W. (2013) KEAP1-NRF2 complex in ischemia-induced hepatocellular damage of mouse liver transplants. J. Hepatol. 59, 1200–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Malhotra D., Portales-Casamar E., Singh A., Srivastava S., Arenillas D., Happel C., Shyr C., Wakabayashi N., Kensler T. W., Wasserman W. W., Biswal S. (2010) Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acids Res. 38, 5718–5734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Katsuoka F., Motohashi H., Ishii T., Aburatani H., Engel J. D., Yamamoto M. (2005) Genetic evidence that small maf proteins are essential for the activation of antioxidant response element-dependent genes. Mol. Cell. Biol. 25, 8044–8051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lin X., Yang H., Zhou L., Guo Z. (2011) Nrf2-dependent induction of NQO1 in mouse aortic endothelial cells overexpressing catalase. Free Radic. Biol. Med. 51, 97–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kudoh K., Uchinami H., Yoshioka M., Seki E., Yamamoto Y. (2014) Nrf2 activation protects the liver from ischemia/reperfusion injury in mice. Ann. Surg. 260, 118–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gorrini C., Baniasadi P. S., Harris I. S., Silvester J., Inoue S., Snow B., Joshi P. A., Wakeham A., Molyneux S. D., Martin B., Bouwman P., Cescon D. W., Elia A. J., Winterton-Perks Z., Cruickshank J., Brenner D., Tseng A., Musgrave M., Berman H. K., Khokha R., Jonkers J., Mak T. W., Gauthier M. L. (2013) BRCA1 interacts with Nrf2 to regulate antioxidant signaling and cell survival. J. Exp. Med. 210, 1529–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Soljancic A., Ruiz A. L., Chandrashekar K., Maranon R., Liu R., Reckelhoff J. F., Juncos L. A. (2013) Protective role of testosterone in ischemia-reperfusion-induced acute kidney injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 304, R951–R958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hybertson B. M., Gao B., Bose S. K., McCord J. M. (2011) Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol. Aspects Med. 32, 234–246 [DOI] [PubMed] [Google Scholar]
- 33. Leonard M. O., Kieran N. E., Howell K., Burne M. J., Varadarajan R., Dhakshinamoorthy S., Porter A. G., O'Farrelly C., Rabb H., Taylor C. T. (2006) Reoxygenation-specific activation of the antioxidant transcription factor Nrf2 mediates cytoprotective gene expression in ischemia-reperfusion injury. FASEB J. 20, 2624–2626 [DOI] [PubMed] [Google Scholar]
- 34. Staudinger J., Liu Y., Madan A., Habeebu S., Klaassen C. D. (2001) Coordinate regulation of xenobiotic and bile acid homeostasis by pregnane X receptor. Drug. Metab. Dispos. 29, 1467–1472 [PubMed] [Google Scholar]
- 35. Xu J., Kulkarni S. R., Donepudi A. C., More V. R., Slitt A. L. (2012) Enhanced Nrf2 activity worsens insulin resistance, impairs lipid accumulation in adipose tissue, and increases hepatic steatosis in leptin-deficient mice. Diabetes 61, 3208–3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang Z., Dou X., Li S., Zhang X., Sun X., Zhou Z., Song Z. (2014) Nuclear factor (erythroid-derived 2)-like 2 activation-induced hepatic very-low-density lipoprotein receptor overexpression in response to oxidative stress contributes to alcoholic liver disease in mice. Hepatology 59, 1381–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. James M. O., Li W., Summerlot D. P., Rowland-Faux L., Wood C. E. (2010) Triclosan is a potent inhibitor of estradiol and estrone sulfonation in sheep placenta. Environ. Int. 36, 942–949 [DOI] [PMC free article] [PubMed] [Google Scholar]