Abstract
Fontan failure can occur even with normal systolic ventricular function and often in the context of significant liver disease. We hypothesized that Fontan failure is hemodynamically distinct from traditional heart failure and characterized by low systemic vascular resistance (SVR) index and preserved cardiac index. Twenty-seven symptomatic adult Fontan (SAF) patients who underwent catheterization from 2001 to 2011 constituted our study group. Fifty-four predominantly asymptomatic pediatric Fontan (PF) patients who underwent catheterization during the same period were randomly selected to perform a control:case cohort analysis. Clinical comparisons were made between the 2 groups. The adults were more symptomatic than the PF cohort (New York Heart Association classes I and II or III and IV: 48% or 52% [SAF] vs 94% or 6% [PF], respectively, p <0.01). SAF versus PF mean catheterization findings were central venous pressure 18 ± 6 versus 14 ± 3 mm Hg (p <0.01), SVR index 1,680 ± 368 versus 1,960 ± 550 dyn s/cm5/m2 (p = 0.02), and cardiac index 2.7 ± 0.8 versus 2.8 ± 0.7 L/min/m2 (p = 0.25). By imaging, the SAF cohort demonstrated a greater incidence of abnormal liver texture changes (96% vs 75%, p = 0.04) and nodularity (77% vs 42%, p = 0.02). In conclusion, adult patients with failing Fontan circulation had a lower SVR index and similar cardiac index compared with the pediatric cohort. Liver disease in the adults was more advanced. Our data suggest that Fontan failure is a distinct circulatory derangement with hemodynamic features similar to portal hypertension, albeit with limited ability to augment cardiac output.
Single ventricle palliation, culminating in the Fontan operation, has been described as a failed strategy in terms of long-term survival and quality of life.1 Among survivors, only 70% actuarial freedom from death or cardiac transplantation 25 years after Fontan palliation has been reported.2 “Fontan failure” is a term used to characterize much of the associated morbidity; however, it is itself variably defined. Descriptions include low cardiac output in the absence of ventricular failure,3 “myocardial failure”,4 and “death, (Fontan) takedown, transplantation, or New York Heart Association (NYHA) classes III and IV”.5 Depiction of the hemodynamic profile of Fontan failure has been similar to traditional heart failure: elevated central venous pressure, pulmonary capillary wedge pressure, and systemic vascular resistance (SVR), with a low cardiac index.1,6–8 However, clinical deterioration can occur in the absence of ventricular dysfunction,3,9 suggesting that distinct mechanisms are contributive. Based on the growing evidence of liver pathology in Fontan patients over time,10,11 we hypothesized that portal hypertension might play a signifi-cant role in failing Fontan pathophysiology. We sought to define the hemodynamic phenotype of patients with Fontan failure using catheterization data from symptomatic adult Fontan (SAF) patients and then compared our data with a pediatric Fontan (PF) cohort.
Methods
We performed a retrospective review of the Emory Adult Congenital Heart Center database for patients with the single ventricle physiology status after Fontan palliation who underwent a heart catheterization from January 2001 to December 2011. Surveillance catheterizations are not performed on all patients with Fontan palliation at our adult center and instead are done because of clinical deterioration. We defined clinical deterioration (Fontan failure) as development of significant symptoms such as refractory edema, ascites, protein-losing enteropathy, or considerable exercise intolerance, regardless of ventricular function. A total of 27 patients (NYHA functional classes III and IV: 52%) met the inclusion criteria. We also identified predominantly asymptomatic Fontan patients (NYHA functional classes I and II = 94%), aged 10 to 19 years, who had a catheterization performed during the study period. At our institution, catheterizations are routinely performed in PF patients at 10 and 15 years after the Fontan palliation. A total of 54 patients were randomly selected to perform a 2:1 control: case cohort analysis.
Clinical data, echocardiograms, catheterization findings, and liver imaging were compared between the SAF and PF cohorts. Echocardiogram interpretations are those of the attending cardiologist, with the evaluation of ventricular function accomplished by subjective assessment, modified Simpson's method, and triplane 3-dimensional reconstruction. Cardiac catheterizations were performed using local anesthesia and conscious sedation. Measurements were made using an end-hole catheter and a transducer using the standard technique. Systemic blood flow was calculated using the Fick method, and SVR and cardiac output were indexed to account for discrepant body surface area between the 2 patient populations. Pulmonary vascular resistance was not calculated because of known difficulties in measuring pulmonary blood flow in the failing Fontan circulation12,13; however, transpulmonary gradients were normal in each cohort. Hepatic vein wedge pressures were not routinely obtained and are likely not useful in Fontan patients with postsinusoidal portal hypertension.14 Finally, although pertinent medications are listed for each cohort, all such medications were held on the day of catheterization in each group.
At our institution, PF patients routinely undergo hepatic magnetic resonance imaging (MRI) or ultrasound at the age of 13 years, whereas adult Fontan patients receive liver imaging as part of their intake assessment. Computed tomography and ultrasound are used only when MRI is contraindicated. Imaging findings consistent with liver disease include changes in echogenicity by ultrasound or heterogenous enhancement of the parenchyma on delayed phase postcontrast imaging by MRI. Liver nodularity is a well-described imaging finding in patients with cirrhosis, indicative of the underlying distortion of hepatic architecture by formation of regenerative nodules.15 Varices were documented by imaging and not endoscopy. Liver imaging interpretations are those of the attending radiologist.
Analysis of the comparisons was completed using paired t test for continuous variables and Fisher's exact test for categorical variables. We usedSAS, version 9.2 (SAS Institute Inc., Cary, North Carolina). Data are expressed as mean values ± SDs. Statistical significance was defined as p value ≤ 0.05.
Results
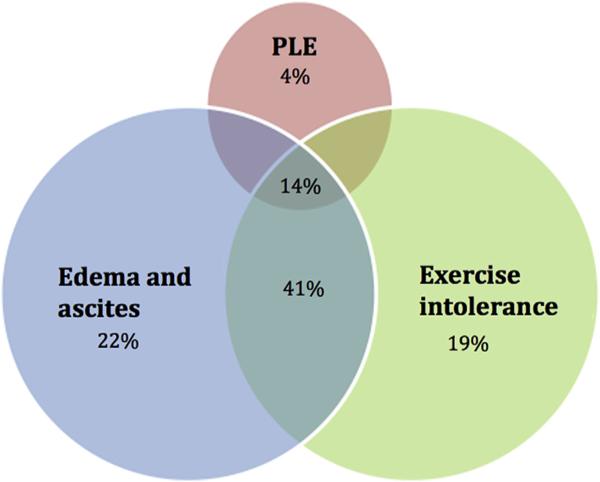
A total of 27 SAF patients (aged 21 to 55 years) underwent a catheterization during the study period. All the patients had Fontan circulatory failure, reflected by clinical deterioration, as the indication for catheterization. Symptoms at the time of catheterization are depicted in Figure 1. Pertinent mean catheterization findings of the cohort include central venous pressure of 18 ± 6 mm Hg, pulmonary capillary wedge pressure of 13 ± 5 mm Hg, systemic arteriovenous oxygen saturation difference of 24 ± 6%, SVR index of 1,680 ± 368 dyn s/cm5/m2, and cardiac index of 2.7 ± 0.8 L/min/m2.
Figure 1.
Symptoms in the adult Fontan cohort (n = 27). PLE = protein-losing enteropathy.
Clinical features of the SAF and PF cohorts are listed in Table 1. Compared with the PF cohort, the adult patients were statistically older at the time of Fontan palliation and farther removed from the Fontan at the time of catheterization. The SAF cohort was more symptomatic than the PF cohort by NYHA classification. Furthermore, of the 13 SAF patients (48%) who were in NYHA classes I and II, only 2 were in NYHA class I. Both these patients had severe fluid overload despite diuretics. NYHA class itself did not predict hemodynamic profile among the SAF cohort; there was no significant difference between patients in NYHA classes I and II and those in classes III and IV within this group. The SAF cohort contained a greater percentage of atriopulmo-nary Fontan palliations, whereas the PF group contained almost exclusively lateral tunnel and extracardiac Fontan patients. Finally, although angiotensin-converting enzyme inhibitors were more likely to be used in the PF cohort, β blockers were used with greater frequency in the SAF patients.
Table 1.
Clinical features in symptomatic adult versus pediatric Fontans
| Variable (Mean ± SD) | Adults, n = 27 (%) | Pediatric, n = 54 (%) | p |
|---|---|---|---|
| Age at catheterization (yrs) | 31.3 ± 9.3 | 13.7 ± 2.9 | <0.01 |
| Age at Fontan (yrs) | 10.6 ± 8 | 2.9 ± 2 | <0.01 |
| Time from Fontan to catheterization (yrs) | 20.7 ± 5.9 | 10.7 ± 3.8 | <0.01 |
| Male (%) | 52 | 46 | 0.71 |
| Height (cm) | 165.9 ± 33.5 | 150.8 ± 14.6 | 0.03 |
| Weight (kg) | 81.2 ± 18.8 | 45.7 ± 18.6 | <0.01 |
| Body surface area (m2) | 2 ± 0.3 | 1.4 ± 0.3 | <0.01 |
| NYHA functional class* | |||
| I and II | 48 | 94 | <0.01 |
| III and IV | 52 | 6 | |
| Atriopulmonary Fontan | 67 | 2 | <0.01 |
| Lateral tunnel Fontan | 33 | 69 | <0.01 |
| Extracardiac Fontan | 0 | 29 | <0.01 |
| Fontan fenestration | 11 | 87 | <0.01 |
| Systemic left ventricle | 81 | 44 | <0.01 |
| Systemic right ventricle | 19 | 56 | <0.01 |
| ACE inhibitor† | 41 | 74 | <0.01 |
| β Blocker† | 67 | 26 | <0.01 |
| Furosemide | 63 | 41 | 0.10 |
| Aldactone | 48 | 37 | 0.12 |
| Digoxin | 30 | 39 | 0.2 |
| Hemoglobin (g/dl) | 14.9 ± 2 | 14.6 ± 1.7 | 0.42 |
ACE = angiotensin-converting enzyme.
Among the adult Fontan cohort, 13 (48%) of the 27 patients were NYHA class I and II; however, only 2 of these were NYHA class I. Both patients had significant fluid overload despite diuretics.
ACE inhibitor and b-blocker use was not mutually exclusive. In the adult cohort, 8 patients (30%) were prescribed both medication classes, whereas 8 pediatric patients (15%) were prescribed both medicine types.
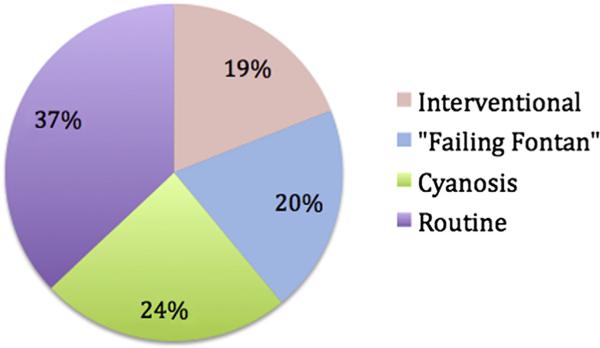
Tables 2 and 3 highlight differences in catheterization and echocardiographic findings between the SAF and PF cohorts. The hemodynamic profiles of the adult atrio-pulmonary versus lateral tunnel Fontans did not significantly differ and therefore were combined for analysis. The pediatric cohort had statistically lower values for central venous pressure and pulmonary capillary wedge pressure but greater SVR index. There was no significant difference in the cardiac index between the 2 groups. Indications for catheterization among the PF cohort are shown in Figure 2.
Table 2.
Catheterization data in symptomatic adult versus pediatric Fontans
| Parameters (Mean ± SD) | Adults (n = 27) | Pediatric (n = 54) | p |
|---|---|---|---|
| Central venous pressure (mm Hg) | 18 ± 6 | 14 ± 3 | <0.01 |
| Pulmonary capillary wedge pressure (mm Hg) | 13 ± 5 | 10 ± 3 | <0.01 |
| Aortic pressure (mm Hg) | 76 ± 12 | 79 ± 12 | 0.34 |
| Systemic arteriovenous pressure difference (mm Hg) | 57 ± 12 | 65 ± 12 | <0.01 |
| Mixed venous saturation (%) | 66 ± 8 | 68 ± 6 | 0.45 |
| Aortic saturation (%) | 91 ± 5 | 92 ± 4 | 0.46 |
| Systemic arteriovenous saturation difference (%) | 24 ± 6 | 24 ± 5 | 0.75 |
| Cardiac index (L/min/m2) | 2.7 ± 0.8 | 2.8 ± 0.7 | 0.57 |
| SVR index (dyn s/cm5/m2) | 1,680 ± 368 | 1,960 ± 550 | 0.02 |
Table 3.
Echocardiographic data in symptomatic adult versus pediatric Fontans
| Parameters | Adults (%) | Pediatric (%)* | p |
|---|---|---|---|
| At least moderate RV systolic dysfunction† | 80 | 12 | <0.01 |
| At least moderate TR† | 20 | 8 | 0.42 |
| Moderate or greater RV dysfunction or TR† | 80 | 15 | <0.01 |
| At least moderate LV systolic dysfunction‡ | 18 | 5 | 0.21 |
| At least moderate MR‡ | 23 | 19 | 0.78 |
| Moderate or greater LV dysfunction or MR‡ | 27 | 24 | 0.93 |
LV = left ventricle; MR = mitral regurgitation; RV = right ventricle; TR = tricuspid regurgitation.
Echocardiographic data were unavailable for 7 pediatric patients.
In those patients with a systemic right ventricle (n = 5 adult and n = 26 pediatric patients).
In those patients with a systemic left ventricle (n = 22 adult and n = 21 pediatric patients).
Figure 2.
Pediatric Fontan catheterization indications (n = 54).
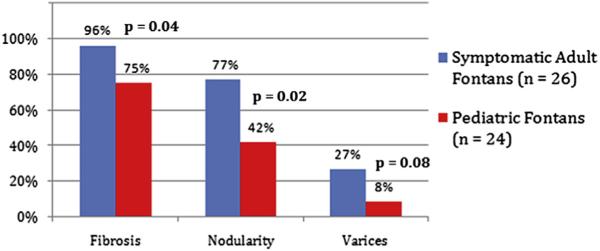
The results of liver imaging in each cohort are given in Figure 3. Liver imaging was available in 26 (96%) of 27 adults and 24 (44%) of 54 pediatric patients. In the adults, MRI (42%), computed tomography (50%), and ultrasound (8%) were used, whereas only MRI (58%) and ultrasound (42%) were used in the pediatric patients.
Figure 3.
Extent of liver disease in adult versus pediatric Fontan patients. Although fibrosis is technically a histologic diagnosis, it is used here to indicate imaging changes likely consistent with the finding. Varices were documented by imaging and not endoscopy.
Discussion
As we enter the 5th decade of caring for patients palliated with the Fontan procedure, it is apparent that adult survivors face significant morbidity and mortality. Although selected medicines may ameliorate symptoms,16 mortality benefit has not been demonstrated. Furthermore, there is evidence that circulatory failure rather than ventricular failure is most important—Fontan patients with preserved ventricular function have been shown to do worse after heart transplantation compared with patients with decreased ventricular function.3
We theorized that the hemodynamics of a SAF cohort would be divergent from those described in traditional, adult onset, systolic heart failure (elevated central venous pressure, pulmonary capillary wedge pressure, and SVR, with low cardiac index).8,17,18 In the SAF patients, although mean central venous and pulmonary capillary wedge pressures were elevated, SVR index was low and cardiac index was preserved. Although others have noted these hemodynamics,3,9 further interpretation was not included. SVR was indexed to control for cohort body size differences and is low compared with published norms.19,20
The SAF cohort had higher central venous and pulmonary capillary wedge pressures than the PF cohort, as might be expected. SVR index, however, was lower in the adults and cardiac index was preserved, even in the context of more severe symptoms. This suggests additional mechanisms influencing the hemodynamics and contributing to the symptoms of Fontan failure. Discrepant SVR index was present despite increased use of angiotensin-converting enzyme inhibitors in the PF cohort. Although β blockers were used more frequently in the adults, their effect on SVR has been shown to be neutral in large studies.21,22 Echo-cardiographic data were also informative. Among patients with a systemic left ventricle, there was no significant difference between the cohorts in the incidence of signifi-cant mitral regurgitation or left ventricular dysfunction. Adults with a systemic right ventricle, however, were more likely to have significant systolic ventricular dysfunction. Our interpretation of this data is threefold. First, a traditional heart failure model does not explain the difference in symptoms between the 2 cohorts. Second, the hemodynamic profile instead resembles that described in portal hypertension, with the exception being limited ability to augment cardiac output above a certain threshold in a Fontan circulation.23 The catalyst for this pathophysiology is most likely time-related exposure to elevated postsinusoidal Fontan pressure, leading to liver damage and eventually portal hypertensive–type circulatory derangement. Third, patients with a systemic right ventricle likely face both limitations of a systemic right ventricle and failing Fontan pathophysiology.
What evidence is there that elevated Fontan pressures can lead to liver damage and furthermore that the resulting circulatory dysfunction resembles that seen in our adult cohort? Evidence of liver pathology in Fontan patients over time is robust.10,11 Animal models, specifically data showing that rat livers exposed to elevated postsinusoidal pressure go on to develop fibrosis and portal hypertension,24 provide further support. Post-sinusoidal obstructive disease such as Budd-Chiari syndrome is a useful model for this pathophysiology and is a well-described cause of cirrhosis and portal hyper-tension.25,26 Finally, the mechanism of circulatory dysfunction seen with portal hypertension and cirrhosis has been described and resembles catheterization findings of our study.27,28 With significant portal hypertension, there is a reduction in hepatic perfusion by the portal venous system and a compensatory shift to increased hepatic arterial perfusion. This compensatory response occurs through splanchnic arterial vasodilation, likely mediated by local vasodilators such as nitric oxide, which leads to decreased SVR. As splanchnic vasodilation becomes more intense, arterial hypotension, renal hypoperfusion, and then reflexive activation of the sympathetic nervous system and renin-angiotensin-aldosterone system develop. Although an increase in cardiac output can be expected in patients with a normal heart, vasoconstriction of the renal vasculature also occurs, which leads to sodium and water retention and the eventual development of edema and ascites once entering a decompensated state. With time-related exposure to the Fontan as the catalyst, we believe failing Fontan pathophysiology has similar characteristics: elevated central venous pressure, low SVR, preserved cardiac output, and fluid retention. A final point is that, as mentioned earlier, Fontan patients cannot proportionately augment their cardiac output above a certain threshold, thus potentiating renal hypoperfusion and leading to refractory symptoms.
Understanding Fontan circulatory failure has important implications. Medicines such as systemic vasodilators used in heart failure may not be tolerated over time as SVR decreases. Befitting therapies can instead be applied by appreciating the unique hemodynamics present. As an example, systemic vasoconstrictors are often used to improve renal blood flow in patients with hepatorenal syndrome, a hemodynamically similar population.29 Finally, our study highlights the necessity of a catheterization in symptomatic Fontan patients to guide therapy.
The limitations to this study include those inherent to a retrospective review. The study design allows for us to describe the association of catheterization findings in SAF patients with liver imaging changes but does not allow for proving causation. A further limitation is the heterogenous nature of Fontan failure—it is likely that not all patients “fail” in the same way, and each patient should be evaluated individually when looking for underlying causes. Discrepant medication use should be mentioned. However, angiotensin-converting enzyme inhibitors, which may decrease SVR, were more commonly used in the adolescents and b blockers should have a neutral effect on SVR. Finally, our study cohorts were relatively small, and we lacked asymptomatic adult Fontan patients as a control group. Further studies with larger data sets are necessary to confirm and expand on our findings.
Footnotes
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Rychik J. Forty years of the Fontan operation: a failed strategy. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2010;13:96–100. doi: 10.1053/j.pcsu.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Khairy P, Fernandes SM, Mayer JE, Jr, Triedman JK, Walsh EP, Lock JE, Landzberg MJ. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation. 2008;117:85–92. doi: 10.1161/CIRCULATIONAHA.107.738559. [DOI] [PubMed] [Google Scholar]
- 3.Griffiths ER, Kaza AK, Wyler von Ballmoos MC, Loyola H, Valente AM, Blume ED, del Nido P. Evaluating failing Fontans for heart transplantation: predictors of death. Ann Thorac Surg. 2009;88:558–563. doi: 10.1016/j.athoracsur.2009.03.085. discussion 563–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michielon G, Parisi F, Di Carlo D, Squitieri C, Carotti A, Buratta M, Di Donato RM. Orthotopic heart transplantation for failing single ventricle physiology. Eur J Cardiothorac Surg. 2003;24:502–510. doi: 10.1016/s1010-7940(03)00342-7. discussion 510. [DOI] [PubMed] [Google Scholar]
- 5.d'Udekem Y, Iyengar AJ, Cochrane AD, Grigg LE, Ramsay JM, Wheaton GR, Penny DJ, Brizard CP. The Fontan procedure: contemporary techniques have improved long-term outcomes. Circulation. 2007;116:I157eI184. doi: 10.1161/CIRCULATIONAHA.106.676445. [DOI] [PubMed] [Google Scholar]
- 6.Akagi T, Benson LN, Green M, Ash J, Gilday DL, Williams WG, Freedom RM. Ventricular performance before and after Fontan repair for univentricular atrioventricular connection: angiographic and radio-nuclide assessment. J Am Coll Cardiol. 1992;20:920–926. doi: 10.1016/0735-1097(92)90194-r. [DOI] [PubMed] [Google Scholar]
- 7.Milanesi O, Stellin G, Colan SD, Facchin P, Crepaz R, Biffanti R, Zacchello F. Systolic and diastolic performance late after the Fontan procedure for a single ventricle and comparison of those undergoing operation at <12 months of age and at >12 months of age. Am J Cardiol. 2002;89:276–280. doi: 10.1016/s0002-9149(01)02227-5. [DOI] [PubMed] [Google Scholar]
- 8.Binanay C, Califf RM, Hasselblad V, O'Connor CM, Shah MR, Sopko G, Stevenson LW, Francis GS, Leier CV, Miller LW. Evaluation study of congestive heart failure andpulmonary artery catheterizationeffectiveness: the ESCAPE trial. JAMA. 2005;294:1625–1633. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]
- 9.Simpson KE, Cibulka N, Lee CK, Huddleston CH, Canter CE. Failed Fontan heart transplant candidates with preserved vs impaired ventricular ejection: 2 distinct patient populations. J Heart Lung Transplant. 2012;31:545–547. doi: 10.1016/j.healun.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Friedrich-Rust M, Koch C, Rentzsch A, Sarrazin C, Schwarz P, Herrmann E, Lindinger A, Sarrazin U, Poynard T, Schafers HJ, Zeuzem S, Abdul-Khaliq H. Noninvasive assessment of liver fibrosis in patients with Fontan circulation using transient elastography and biochemical fibrosis markers. J Thorac Cardiovasc Surg. 2008;135:560–567. doi: 10.1016/j.jtcvs.2007.09.039. [DOI] [PubMed] [Google Scholar]
- 11.Johnson JA, Cetta F, Graham RP, Smyrk TC, Driscoll DJ, Phillips SD, John AS. Identifying predictors of hepatic disease in patients after the Fontan operation: a postmortem analysis. J Thorac Cardiovasc Surg. 2012 doi: 10.1016/j.jtcvs.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell MB, Campbell DN, Ivy D, Boucek MM, Sondheimer HM, Pietra B, Das BB, Coll JR. Evidence of pulmonary vascular disease after heart transplantation for Fontan circulation failure. J Thorac Cardiovasc Surg. 2004;128:693–702. doi: 10.1016/j.jtcvs.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Carey JA, Hamilton JR, Hilton CJ, Dark JH, Forty J, Parry G, Hasan A. Orthotopic cardiac transplantation for the failing Fontan circulation. Eur J Cardiothorac Surg. 1998;14:7–13. doi: 10.1016/s1010-7940(98)00130-4. discussion 13–14. [DOI] [PubMed] [Google Scholar]
- 14.Kiesewetter CH, Sheron N, Vettukattill JJ, Hacking N, Stedman B, Millward-Sadler H, Haw M, Cope R, Salmon AP, Sivaprakasam MC, Kendall T, Keeton BR, Iredale JP, Veldtman GR. Hepatic changes in the failing Fontan circulation. Heart. 2007;93:579–584. doi: 10.1136/hrt.2006.094516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Lelio A, Cestari C, Lomazzi A, Beretta L. Cirrhosis: diagnosis with sonographic study of the liver surface. Radiology. 1989;172:389–392. doi: 10.1148/radiology.172.2.2526349. [DOI] [PubMed] [Google Scholar]
- 16.Schumacher KR, Cools M, Goldstein BH, Ioffe-Dahan V, King K, Gaffney D, Russell MW. Oral budesonide treatment for protein-losing enteropathy in Fontan-palliated patients. Pediatr Cardiol. 2011;32:966–971. doi: 10.1007/s00246-011-0029-2. [DOI] [PubMed] [Google Scholar]
- 17.Steimle AE, Stevenson LW, Chelimsky-Fallick C, Fonarow GC, Hamilton MA, Moriguchi JD, Kartashov A, Tillisch JH. Sustained hemodynamic efficacy of therapy tailored to reduce filling pressures in survivors with advanced heart failure. Circulation. 1997;96:1165–1172. doi: 10.1161/01.cir.96.4.1165. [DOI] [PubMed] [Google Scholar]
- 18.Curtiss C, Cohn JN, Vrobel T, Franciosa JA. Role of the reninangiotensin system in the systemic vasoconstriction of chronic congestive heart failure. Circulation. 1978;58:763–770. doi: 10.1161/01.cir.58.5.763. [DOI] [PubMed] [Google Scholar]
- 19.Krovetz LJ, Goldbloom S. Normal standards for cardiovascular data. II. Pressure and vascular resistances. Johns Hopkins Med J. 1972;130:187–195. [PubMed] [Google Scholar]
- 20.Krovetz LJ, McLoughlin TG, Mitchell MB, Schiebler GL. Hemodynamic findings in normal children. Pediatr Res. 1967;1:122–130. doi: 10.1203/00006450-196703000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Metra M, Giubbini R, Nodari S, Boldi E, Modena MG, Dei Cas L. Differential effects of beta-blockers in patients with heart failure: a prospective, randomized, double-blind comparison of the long-term effects of metoprolol versus carvedilol. Circulation. 2000;102:546–551. doi: 10.1161/01.cir.102.5.546. [DOI] [PubMed] [Google Scholar]
- 22.Bristow MR. Beta-adrenergic receptor blockade in chronic heart failure. Circulation. 2000;101:558–569. doi: 10.1161/01.cir.101.5.558. [DOI] [PubMed] [Google Scholar]
- 23.Shafer KM, Garcia JA, Babb TG, Fixler DE, Ayers CR, Levine BD. The importance of the muscle and ventilatory blood pumps during exercise in patients without a subpulmonary ventricle (Fontan operation). J Am Coll Cardiol. 2012;60:2115–2121. doi: 10.1016/j.jacc.2012.08.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higashiyama H, Yamaguchi M, Kumada K, Sasaki H, Yamaguchi T, Ozawa K. Functional deterioration of the liver by elevated inferior vena cava pressure: a proposed upper safety limit of pressure for maintaining liver viability in dogs. Intensive Care Med. 1994;20:124–129. doi: 10.1007/BF01707667. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez-Guerra M, Lopez E, Bellot P, Piera C, Turnes J, Abraldes JG, Bosch J, Garcia-Pagan JC. Systemic hemodynamics, vasoactive systems, and plasma volume in patients with severe Budd-Chiari syndrome. Hepatology. 2006;43:27–33. doi: 10.1002/hep.20990. [DOI] [PubMed] [Google Scholar]
- 26.Valla DC. Primary Budd-Chiari syndrome. J Hepatol. 2009;50:195–203. doi: 10.1016/j.jhep.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Arroyo V, Jimenez W. Complications of cirrhosis. II. Renal and circulatory dysfunction. Lights and shadows in an important clinical problem. J Hepatol. 2000;32:157–170. doi: 10.1016/s0168-8278(00)80423-7. [DOI] [PubMed] [Google Scholar]
- 28.Sola E, Gines P. Renal and circulatory dysfunction in cirrhosis: current management and future perspectives. J Hepatol. 2010;53:1135–1145. doi: 10.1016/j.jhep.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Esrailian E, Pantangco ER, Kyulo NL, Hu KQ, Runyon BA. Octreo-tide/Midodrine therapy significantly improves renal function and 30-day survival in patients with type 1 hepatorenal syndrome. Dig Dis Sci. 2007;52:742–748. doi: 10.1007/s10620-006-9312-0. [DOI] [PubMed] [Google Scholar]