Background: Diet-induced obesity leads to a chronic low grade inflammation with production of activated macrophages associated with systemic sexually dimorphic metabolic dysfunction.
Results: Males have enhanced myelopoiesis and a proinflammatory response to obesity compared with females.
Conclusion: Sex differences in myelopoiesis result in dimorphic responses to obesity-induced inflammation.
Significance: Given differences in inflammatory responses, targeted treatment strategies are probably required for males and females.
Keywords: adipose tissue, hematopoiesis, inflammation, metabolism, obesity, adipose tissue inflammation, sexually dimorphic, diet-induced obesity, metainflammation
Abstract
Women of reproductive age are protected from metabolic disease relative to postmenopausal women and men. Most preclinical rodent studies are skewed toward the use of male mice to study obesity-induced metabolic dysfunction because of a similar protection observed in female mice. How sex differences in obesity-induced inflammatory responses contribute to these observations is unknown. We have compared and contrasted the effects of high fat diet-induced obesity on glucose metabolism and leukocyte activation in multiple depots in male and female C57Bl/6 mice. With both short term and long term high fat diet, male mice demonstrated increased weight gain and CD11c+ adipose tissue macrophage content compared with female mice despite similar degrees of adipocyte hypertrophy. Competitive bone marrow transplant studies demonstrated that obesity induced a preferential contribution of male hematopoietic cells to circulating leukocytes and adipose tissue macrophages compared with female cells independent of the sex of the recipient. Sex differences in macrophage and hematopoietic cell in vitro activation in response to obesogenic cues were observed to explain these results. In summary, this report demonstrates that male and female leukocytes and hematopoietic stem cells have cell-autonomous differences in their response to obesity that contribute to an amplified response in males compared with females.
Introduction
Obesity and its medical sequelae contribute to individual morbidity and are a burden to healthcare costs. Although obesity is rising in prevalence and incidence in the United States, not all obese individuals are at equal risk for obesity-induced cardiovascular and metabolic diseases. For example, reproductive aged women are relatively protected from cardiovascular disease compared with age-matched men (1). Estrogen receptor signaling probably contributes to many of these effects, but because estrogen replacement therapy alone is not sufficient to improve health, other mechanisms are likely involved (2). Sex differences in adipose tissue depot development, cell intrinsic differences in preadipocyte proliferation and differentiation, and modulatory effects of sex steroids on adipocytes have been proposed to contribute to the sexually dimorphic differences in obesity-associated metabolic disease (3).
Advances in the field of immunometabolism have established strong connections between the chronic inflammation induced by obesity and diseases such as atherosclerosis, type 2 diabetes, cancer, and autoimmune diseases (4). Activation of myeloid cells (monocytes, macrophages, and neutrophils) is a prominent signature of human and animal models of obesity both systemically and in tissues such as adipose tissue (5). Recent investigations have shown that enhanced myelopoiesis with high fat diet exposure in male mice is a significant mechanism in the generation of obesity-induced inflammation (6–8).
Obesity triggers an expansion of hematopoietic stem cells (HSCs)2 and myeloid progenitors with a subsequent increase in activated monocytes (Ly6chi/CCR2) (9, 10). These monocytes traffic to obese adipose tissue and form CD11c+ M1-like adipose tissue macrophages (ATMs) producing activated cytokines and an inflammatory environment that contributes to insulin resistance (11).
One aspect that has not been thoroughly investigated is the role that sex differences in inflammatory responses may play in obesity and metabolic dysfunction. Several preclinical studies in rodents identify a blunted pattern of inflammatory gene expression in female visceral adipose tissue with diet-induced obesity (DIO) (12–14). This correlates with a decrease in crown-like structures, hallmarks of adipose tissue inflammation, in obese female mice compared with males, but the mechanisms that generate this difference have not been elucidated. Sex differences in inflammatory responses in other settings agree with this and suggest that intrinsic differences in innate immunity may regulate metabolism. A wide range of reports have demonstrated sex differences in innate immune responses and hematopoiesis (15). Although females have a robust humoral immune mediated response in many autoimmune diseases (15), compared with males, females have attenuated innate immune responses to systemic infections and a lower incidence of severe sepsis (16). Peripheral blood monocytes from female patients produce less TNFα in response to LPS activation compared with males (17).
Understanding sex differences in metainflammation induced by obesity is required to improve our interpretation of preclinical studies in animal models of obesity. The majority of studies evaluating the inflammatory effects of high fat diet (HFD) report data from either male or female mice exclusively (18–20), making it difficult to understand the broader applicability of these observations. This is especially relevant to obesity research, where patients seeking bariatric surgery for obesity treatment are overwhelmingly female (21). Because few studies have directly evaluated the metainflammatory response to DIO based on gender, we compared and contrasted innate immune cell activation and hematopoiesis in response to DIO feeding in age-matched male and female C57Bl/6J mice. Our studies identify cell intrinsic enhancement in myeloid cell production from hematopoietic stem cells in male mice as a mechanism that contributes to the sexual dimorphic responses to obesity.
Experimental Procedures
Animals and Animal Care
Mice used in these experiments were male and female C57Bl/6J or CD45.1 CD57 Bl/6J mice (B6.SJL-Ptprca Pepcb/BoyJ) purchased from Jackson Laboratories. Heterozygous CD45 1.2 mice were bred for BMT experiments. Mice were fed ad libitum either a control normal diet (ND) consisting of 13% fat (5001, LabDiet) or a HFD of 60% of calories from fat (D12492, Research Diets, Inc.) starting at 6 weeks of age for 6 or 16 weeks duration. Glucose tolerance testing was performed after 6 h of fasting. Animals were housed in a specific pathogen-free facility with a 12-h light/12-h dark cycle and given free access to food and water. All animal use was in compliance with the Institute of Laboratory Animal Research Guide for the Care and Use of Laboratory Animals and approved by the University Committee on Use and Care of Animals at the University of Michigan (Animal welfare assurance number A3114-01). Comprehensive Lab Animal Monitoring System (CLAMS; Columbus Instruments) analysis of metabolism in individually housed mice was performed at the University of Michigan Metabolomics and Obesity Center Animal Phenotyping Core.
Adipose Tissue Stromal Vascular Fraction (SVF) Isolation and Flow Cytometry
Adipose tissue fractionation and flow cytometry analysis were performed as described previously (22). SVF cells were stained with CD64 PE, CD45.1 PECy7, CD45.2 e450, and CD11c-PE-Cy7 (eBioscience) (23), and gating was performed for macrophage populations and by CD45 gates to determine donor or recipient cell populations.
Flow Cytometry Assessment of HSC and Myeloid Progenitors
Bone marrow from one femur was flushed with PBS and, using a syringe, made into a single cell suspension and spun. Pellet was then treated with RBC lysis solution for 5 min. After resuspension in PBS and washing, cells were stained with lineage markers on APC (CD4, CD5, CD8, CD11b, B220 (CD45R), Gr1, Ter119, and CD41), CD117-APCCy7, Sca1-PECy7, CD16/32 PerCP5.5 (eBioscience), CD150-PE, and Endoglin-Pacific Blue (Biolegend) and gating as described by Pronk et al. (24).
Colony-forming Unit Assays
Bone marrow from a femur was flushed with Iscove's modified Dulbecco's medium. This marrow was then treated with fatty acid-free BSA or palmitic acid (10 μm; purchased from Sigma) in fatty acid-free BSA for 1 h at 37 ºC. Cells were then resuspended in methocult medium and plated at 10,000 cells/plate/protocol (Stem Cell Technologies, Inc.). After 7 days, colonies were counted.
Bone Marrow Transplantation
Bone marrow cells were isolated from donor groups (25) and injected retro-orbitally into lethally irradiated (900 rads) 6-week-old recipient mice (10 million cells/mouse). Animals were treated with antibiotics (polymyxin and neomycin) for 4 weeks post-BM transplantation. Following 2 weeks of normal chow diet, they were started on ND or HFD diets as detailed under “Results.” Glucose tolerance testing was performed as described (10) after 6 h of fasting with an intraperitoneal injection of 0.7 g/kg dextrose.
Statistics
Results are presented as mean ± S.E. Two-way analysis of variance was performed with factors of sex and diet. If there was a main effect for either factor, then t tests were performed for male versus female differences within each diet or for diet groups within each sex, respectively.
Results
Male Mice Have Increased Adiposity with DIO Compared with Females
To assess sex differences in inflammatory responses to obesity, 6-week-old male and female C57Bl/6J mice were fed control ND or HFD (60% kcal from fat) ad libitum (Fig. 1A). To assess when divergent inflammatory responses to obesity occur between male and female mice, we assessed sex differences in metabolism and inflammatory cell activation in short term (6-week) and long term (16-week) DIO paradigms.
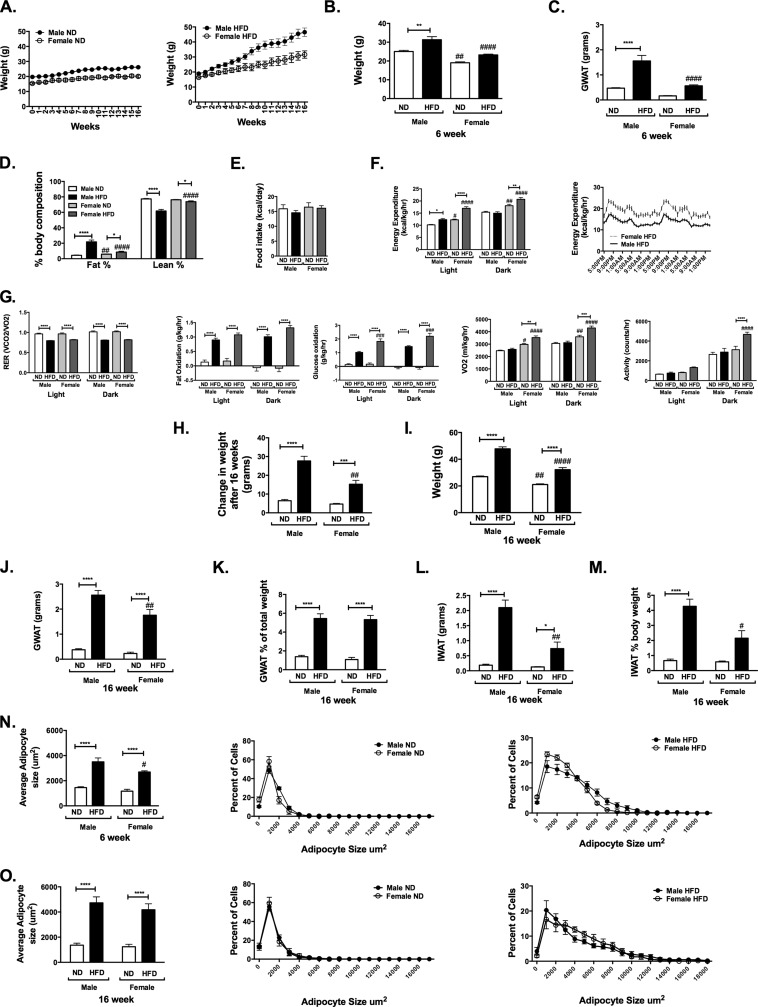
FIGURE 1.
Sexually dimorphic body composition responses to high fat diet feeding in mice. Male and female C57Bl6 mice were fed ND or 60% HFD diets starting at 6 weeks of age for up to 16 weeks. A, animals were weighed weekly. Shown are weight (B) and GWAT (C) after 6 weeks of diet. D, body composition performed based on Minispec NMR. Shown are food intake (E) and energy expenditure (F) in light and dark hours and in HFD mice over the 48-h period. G, respiratory exchange ratio (RER), fat and glucose oxidation, oxygen consumption (VO2), and activity. Shown are change in weight after 16 weeks (H) and final weight after 16 weeks of diet (I). Shown are GWAT fat pad weight (J), GWAT weight as a percentage of body weight (K), and inguinal fat in grams (L) and as a percentage of total body weight (M). N, adipocyte cross-sectional area at 6 weeks and distributions of adipocyte cross-sectional area. O, adipocyte cross-sectional area at 16 weeks and distributions of adipocyte cross-sectional area. n = 4–8/group; *, p < 0.05; **, p < 0.01; ***, p < 0.005; ***, p < 0.001. Comparisons between sexes are shown as follows: #, p < 0.05; ##, p < 0.01; ###, p < 0.005; ####, p < 0.001. Error bars, S.E.
After 6 weeks, ND-fed males and females gained similar amounts of body weight (p = 0.78). HFD-fed males gained 54% and females gained 42% of their original weight (p = 0.046) (Fig. 1B) associated with a significant increase in gonadal fat pad weight (gonadal white adipose tissue; GWAT) in males compared with females (Fig. 1C). Body composition assessment at 9 weeks showed that ND-fed females had a higher percentage of body fat compared with ND-fed males (Fig. 1D). Both HFD-fed male and female mice showed a significant increase in percentage of body fat compared with ND-fed controls. In HFD conditions, male mice had a higher percentage of body fat and lower percentage of lean mass than female mice.
To evaluate the mechanism for the differential response to HFD between sexes, mice were assessed for food intake and energy expenditure in metabolic cages. Males and females had the same daily caloric intake regardless of diet (Fig. 1E). Female mice on ND had increased total energy expenditure (Fig. 1F) compared with their male counterparts. HFD-fed female mice had higher energy expenditure compared with males (Fig. 1F).
The respiratory exchange ratio was similar between sexes and was decreased in HFD-fed mice compared with ND in both sexes. There were no significant differences in fat or glucose oxidation between sexes in ND-fed mice. However, HFD-fed female mice had increased glucose oxidation compared with male mice and a significant increase in oxygen consumption (VO2) and activity (Fig. 1G).
After 16 weeks of HFD, there was significant weight gain in both sexes (Fig. 1H). HFD-fed male mice gained more weight and were significantly heavier than female mice at the end of the study (Fig. 1I, p < 0.0001). HFD induced significant hypertrophy of GWAT in both sexes, with GWAT weighing more in males compared with females (Fig. 1J). However, when corrected for total body weight, GWAT was expanded equally between male and female mice with HFD (Fig. 1K). When subcutaneous inguinal fat pads (inguinal white adipose tissue; IWAT) were evaluated, HFD induced a significant expansion of IWAT in male mice that was more profound compared with female mice (Fig. 1L), especially when expressed as a percentage of total body weight (Fig. 1M).
Assessment of adipocyte cross-sectional area demonstrated the induction of adipocyte hypertrophy in GWAT in both sexes with HFD. After 6 weeks of diet, female mean adipocyte size was smaller then male adipocytes (Fig. 1N), but the average size and degree of adipocyte hypertrophy was similar between sexes after 16 weeks of HFD (Fig. 1O). Evaluation of the size distribution showed that HFD-fed female mice had an increase in the proportion of medium sized adipocytes compared with males (Fig. 1O). Overall, these studies demonstrate that the relative protection from DIO in female mice is evident as early as 4–6 weeks and is due to an increase in energy expenditure characterized by increased fat and glucose oxidation as well as activity.
Male Mice Are More Sensitive to DIO-induced Glucose Intolerance Compared with Females
Fasting glucose levels were elevated to a similar degree in both males and females after 4 weeks of diet (Fig. 2A). Fasting insulin levels were not changed with HFD feeding in either sex during this short term diet challenge, but they were lower for all female groups compared with males (Fig. 2B). After 10 weeks of diet challenge, serum glucose and insulin were assessed in the long term HFD feeding. HFD-fed male mice had significantly higher fasting blood glucose and insulin levels compared with ND controls (Fig. 2C). At this time, no significant differences in fasting glucose and insulin levels were seen in HFD females compared with ND-fed controls.
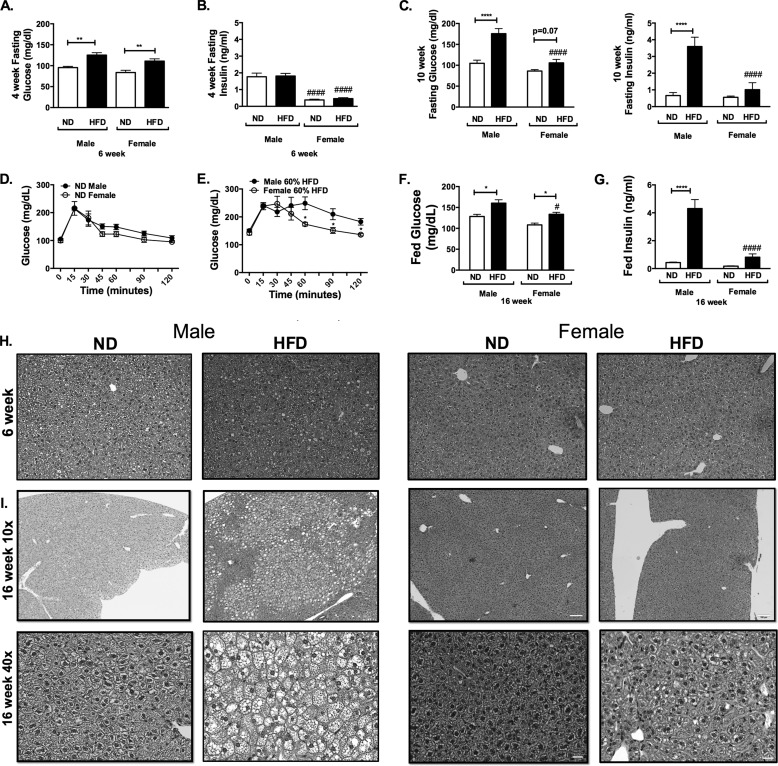
FIGURE 2.
Sexually dimorphic metabolic responses to high fat diet feeding in mice. Shown are fasting glucose (A) and insulin levels (B) assessed at 4 weeks of diet challenge and 10 weeks of diet challenge (C). Shown is glucose tolerance testing in male and female mice after 12 weeks of diet feeding in ND (D) and HFD feeding (E). 16-week-fed glucose (F) and insulin (G) are shown. Shown are H&E images of livers after 6 weeks (H) and 16 weeks (I) of HFD. n = 4; *, p < 0.05; **, p < 0.01; ***, p < 0.005; ***, p < 0.001. Comparisons between sexes are shown as follows: #, p < 0.05; ##, p < 0.01; ###, p < 0.005; ####, p < 0.001. Error bars, S.E.
A glucose tolerance test was performed at 12 weeks of feeding after 6 h of fasting. No significant differences in glucose tolerance testing were seen between ND-fed male and female mice (Fig. 2D). Both HFD-fed male and female mice had glucose intolerance compared with sex-matched ND controls (Fig. 2E). Compared with HFD females, HFD-fed male mice demonstrated more glucose intolerance, primarily in the later stages of the glucose tolerance test. At 16 weeks of HFD, serum analysis showed significantly higher fed glucose levels in HFD-fed animals (Fig. 2F). Females had lower insulin levels than males (Fig. 2G).
Liver microsteatosis was noted in male HFD mice as early as at 6 weeks of HFD challenge (Fig. 2H). Liver steatosis at 16 weeks of diet was prominent in male mice on HFD but not females (Fig. 2I).
These results demonstrate that short term DIO induces similar degrees of fasting hyperglycemia in males and females, with male mice showing increased adipose tissue hypertrophy. With long term DIO, hyperinsulinism and liver steatosis are more prominent in males despite similar increases in adipocyte hypertrophy and adiposity in females. Given this, we next examined how inflammatory responses to DIO differed between males and females as a contributor to the metabolic dysfunction seen in males.
Increased Adipose Tissue Inflammation in HFD-fed Male Mice
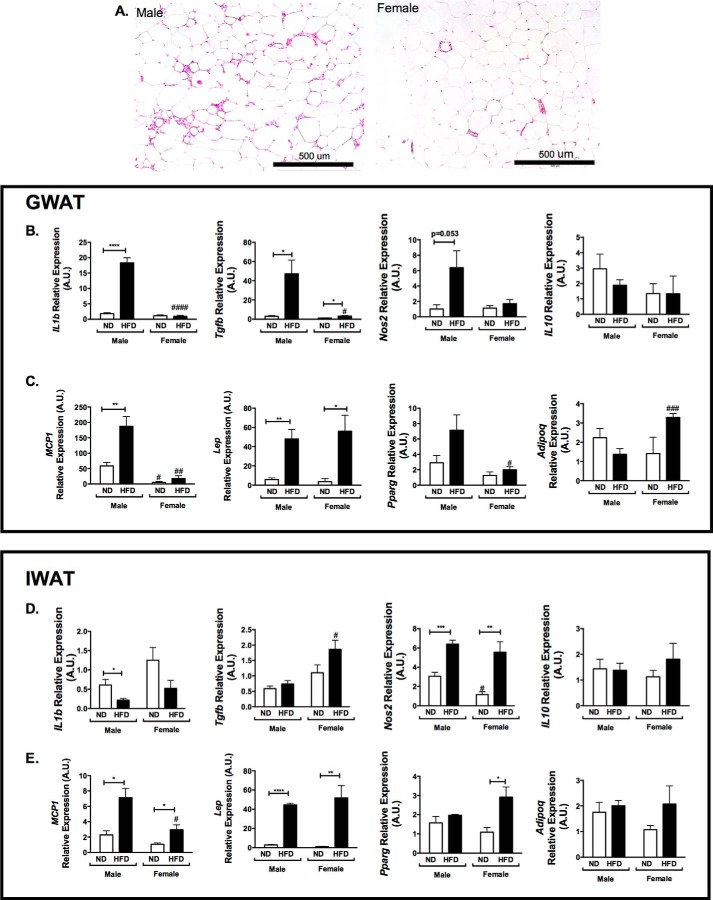
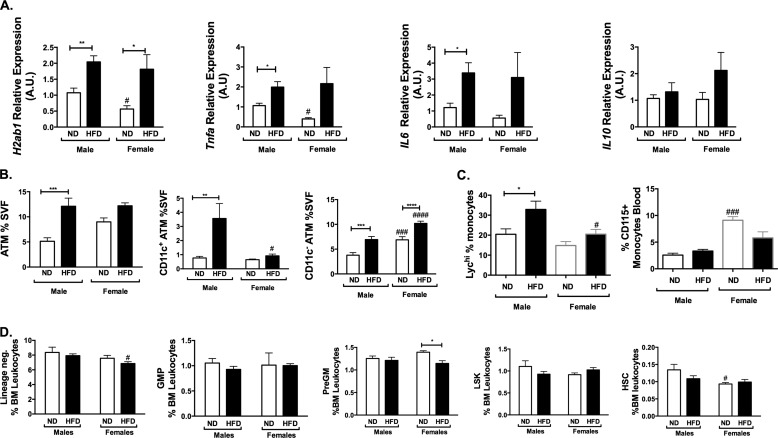
Crown-like structures are a hallmark of obesity-induced inflammation and ATM accumulation in adipose tissue. Histologic analysis of GWAT from 16-week HFD-fed mice demonstrated a significant induction of crown-like structures in male mice but not females (Fig. 3A) despite similar adipocyte hypertrophy. Gene expression analysis demonstrated an increase in several proinflammatory genes (Il1b, Nos2, and Tgfb) in GWAT in male HFD mice, whereas Il10 was unchanged (Fig. 3B). Consistent with the observed differences in the recruitment of monocyte/macrophages into GWAT, Ccl2/Mcp1 expression was increased in males on HFD but not females (Fig. 3C). Expression of leptin (Lep) was increased to similar degrees in males and females with HFD (Fig. 3C). Pparg was increased in males on HFD but not females. Adipoq was increased in HFD females but not males (Fig. 3C). In IWAT, a different pattern in gene expression was observed. Il1b was reduced on HFD, and Nos2 was increased in both males and females on HFD (Fig. 3D), with no difference in Il10. Ccl2 increased with HFD but with much lower levels of expression and lower levels in female mice (Fig. 3E). Lep had expression patterns similar to those of GWAT, but Pparg was higher in IWAT of females and Adipoq was higher in GWAT. Overall, these data show that the activation of inflammatory gene expression in GWAT with HFD is more prominent in males compared with females.
FIGURE 3.
Adipose tissue inflammatory gene expression in GWAT and IWAT. A, H&E images of GWAT in male and female mice after 16 weeks of HFD. B and C, GWAT inflammatory gene expression; D and E, IWAT gene expression. A.U., arbitrary units. n = 4; *, p < 0.05; **, p < 0.01; ***, p < 0.005; ****, p < 0.001. Comparisons between sexes are shown as follows: #, p < 0.05; ##, p < 0.01; ###, p < 0.005; ####, p < 0.001. Error bars, S.E.
CD11c+ ATMs Are Induced in Gonadal Fat in Male Mice but Not Females Despite Similar Adipocyte Hypertrophy
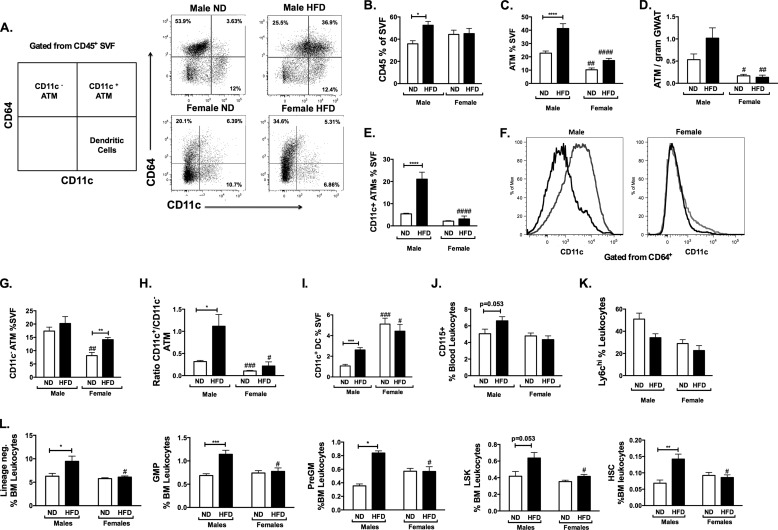
To date, few, if any, studies have systematically compared adipose tissue leukocytes in diet-matched male and female mice. To assess this, we examined ATM induction in GWAT from 16-week-fed mice by flow cytometry (Fig. 4A). ATMs were defined using CD64, a recently identified specific marker for murine macrophages (26). Total CD45+ leukocytes in the SVF were similar in male and female ND-fed mice (Fig. 4B). With HFD, CD45+ cells were induced in males but not females. In ND-fed mice, males had more total CD64+ ATMs in the SVF compared with females (Fig. 4C) even when normalized to fat pad weight (Fig. 4D). The difference between HFD male and female ATMs was primarily driven by the substantial induction of CD11c+ ATMs in male GWAT that was absent from HFD females (Fig. 4, E and F). Interestingly, female mice demonstrated an increase in CD11c− ATMs with HFD, which are primarily composed of a resident macrophage population (Fig. 4G). This induction was not seen in males. These changes resulted in an increase in the ratio of CD11c+ to CD11c− ATMs in male obese mice but not females (Fig. 4H). Dendritic cell populations were higher in females compared with males and were only induced in male GWAT with HFD (Fig. 4I). Overall, this demonstrates that HFD generates different patterns of ATM accumulation in GWAT in males and females.
FIGURE 4.
Compared with females, male mice have increased numbers of CD11c+ ATMs and myeloid progenitors with high fat diet. A, flow cytometry plots for ATM subtypes and adipose tissue dendritic cells based on CD64 and CD11c staining. Shown are quantitations of CD45+ cells in SVF (B), total ATMs (C), ATM/g of GWAT (D), and CD11c+ ATMs (E) in gonadal adipose tissue in male and female mice after 16 weeks of diet challenge. F, representative flow cytometry histogram of CD11c expression in CD64+ ATM for males and female mice (ND (black) and HFD (gray)). G, CD11c− ATMs; H, ratio of CD11c+/CD11c− ATMs; I, adipose tissue dendritic cell populations. Shown are quantitations of total circulating CD115+ monocytes (J) and Ly6chi classical monocytes (K) by flow cytometry after 12 weeks of diet challenge. L, quantitation of HSC precursors after 16 weeks of diet. n = 4; *, p < 0.05; **, p < 0.01; ***, p < 0.005; ****, p < 0.001. Comparisons between sexes are shown as follows: #, p < 0.05; ##, p < 0.01; ###, p < 0.005; ####, p < 0.001. Error bars, S.E.
Myelopoiesis Is Enhanced in Male Mice Fed HFD
The increase in CD11c+ ATMs in male mice occurred despite similar adipocyte and GWAT hypertrophy between sexes. This suggests that a mechanism of increased adipose tissue inflammation in males relates to enhancement in the myeloid machinery that promotes ATM trafficking to adipose tissue, the primary mechanism of CD11c+ ATM accumulation (9, 10). Obesity is associated with increases in circulating myeloid cells (neutrophils and monocytes) in human and mouse models (10). Since CD11c+ ATMs are primarily generated by recruitment from BM-derived monocytes, we next examined whether alterations in circulating leukocytes may be a mechanism that contributes to the differences seen with chronic HFD feeding in male and female mice. Whereas ND female and male mice had similar CD115+ monocytes in the blood (Fig. 4J), HFD increased the total circulating monocyte pool in males but not females. Classical Ly6chi monocytes trended toward being higher in lean males compared with females (Fig. 4K). HFD feeding decreased the quantity of circulating Ly6chi monocytes in males after 16 weeks but not in females.
The accumulation of ATMs might be potentiated in males based on fundamental differences in myeloid cell function or production from the bone marrow. To assess this, we quantified hematopoietic stem cells and myeloid progenitors in the bone marrow of the mice. In ND-fed male and female mice, no significant differences were noted in the quantity of lineage-negative progenitors, LSK (Lin− Sca1+ Kit+) stem cell-enriched populations, or long term HSCs (Lin− Sca+ Kit+ Endoglin+ CD150+). However, with HFD exposure, there was a significant increase in Lin−, granulocyte-macrophage progenitor (GMP), pre-granulocyte-macrophage, and HSC populations in males on HFD, with a marginally significant increase in LSK in males but not in females (Fig. 4L). This demonstrates a coupling between the HFD-induced accumulation of CD11c+ ATMs and myeloid progenitor expansion in male mice that was not observed in female mice.
Male Mice Are More Sensitive to ATM Accumulation with Short Term DIO Compared with Females
Given that during short term diet exposure, males and females had similar weight gain and hyperglycemia without the generalized obesity and obesity-induced hyperinsulinemia, we wanted to use this paradigm to assess whether inflammation occurred in a sexually dimorphic fashion before obesity. Gene expression analysis identified significant increases in Tnfa and Il6 in males but not females with short term HFD. HFD increased H2Ab1 expression in GWAT in both sexes. As in long term diets, Il10 was not changed significantly by 6 weeks of HFD (Fig. 5A).
FIGURE 5.
Male mice accumulate CD11c+ ATMs more rapidly with short term high fat compared with females. Male and female C57Bl6 mice were fed either ND or 60% HFD chow for 6 weeks. A, gonadal gene expression in arbitrary units (A.U.). Shown are flow cytometry quantitations of total and CD11c+ ATMs in GWAT (B), CD115+ monocytes and Ly6chi blood monocytes (C), and bone marrow myeloid progenitors and stem cells (D). n = 8; *, p < 0.05; **, p < 0.01; ***, p < 0.005; ****, p < 0.001. Comparisons between sexes are shown as follows: #, p < 0.05; ##, p < 0.01; ###, p < 0.005; ####, p < 0.001. Error bars, S.E.
We next evaluated ATM accumulation in this short term paradigm. At this younger age, males and females had similar numbers of total and CD11c+ ATMs on normal chow. Male mice had significant expansion of ATMs as a percentage of the SVF after 6 weeks of HFD. This was due to an accumulation of CD11c+ ATMs that was not seen in female mice at this time point. ND males and females had similar numbers of CD11c+ ATMs. In this short term diet challenge, both males and females had an expansion of CD11c− ATMs with a more prominent induction of CD11c− ATMs in females (Fig. 5B). This demonstrates that CD11c+ ATM accumulation in males is a relatively early event after the start of HFD.
To examine the impact of short term HFD feeding on circulating myeloid cells, we assessed blood monocytes in male and female mice after 6 weeks of HFD. Male mice had significantly lower circulating CD115+ monocytes, but in both sexes, this short term diet challenge did not significantly change the number of CD115+ monocytes (Fig. 5C). Males had a significantly higher proportion of Ly6chi monocytes in lean and obese states after HFD (p < 0.0001) (Fig. 5C).
We next quantified the hematopoietic stem cell and myeloid progenitors after short term diet challenge. After short term HFD, there were trends toward lower myeloid precursors (GMP, LSK, and HSC) numbers within the bone marrow compartment in both males and females (Fig. 5D). This demonstrates that with short term HFD exposure, male mice have an accentuated macrophage response in adipose tissue but do not yet have expansion of myeloid precursors within the bone marrow compartment.
HSCs from Male Mice Have a Cell-autonomous Preferential Capacity to Generate Myeloid Cells in Response to Obesity Compared with Female HSCs
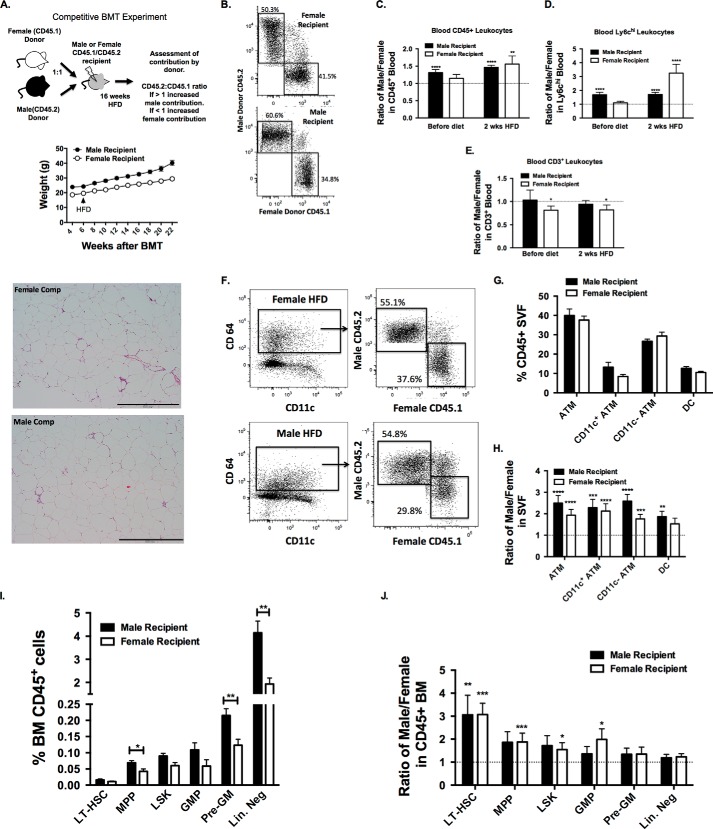
The observation of the induction of ATMs only in male mice despite similar adipocyte hypertrophy at 16 weeks of HFD in females suggests that sex differences in myeloid activation are critical to generating adipose tissue inflammation in obesity. However, given that males weight consistently more then females with HFD, it is difficult to delineate whether the sex differences in inflammation is dependent on or independent of the increased adiposity in males. To test whether sex differences in myeloid activation are intrinsic or extrinsic to HSCs, we examined the differential capacity of male and female HSCs to respond to obesity in a competitive bone marrow transplantation experiment that enables us to differentiate sex differences in hematopoietic cell function in the same recipient mouse (Fig. 6A). We used congenic CD45 variants as sources of donor marrow; male donors were CD45.2, female donors were CD45.1, and recipients (male or female) were heterozygous CD45.1/CD45.2 double-positive (27). BM from age-matched male and female donor mice were mixed in a 1:1 ratio and injected into lethally irradiated male or female recipients. Analysis of leukocytes in the chimeras was based on examining the ratio of CD45.2+ (male) to CD45.1+ (female) cells with an expected ratio of 1 if there is equal contribution from male and female donor BM. An increase in the CD45.2/CD45.1 ratio to >1 would indicate a preferential contribution of male donor cells to a particular leukocyte population, whereas a ratio of <1 would indicate a preferential contribution of female marrow. Male and female recipients were independently evaluated to identify the importance of stromal (non-hematopoietic) factors in influencing sex differences in the inflammatory response to obesity.
FIGURE 6.
In a competitive BMT model, HSCs from male mice preferentially contribute to myeloid cells and CD11c+ ATMs after high fat diet. A, experimental scheme. Lethally irradiated female and male recipients (CD45.1/CD45.2 heterozygotes) were injected with male (CD45.2) and female (CD45.1) BM mixed in a 1:1 ratio. Animals were weighed weekly. B, representative flow cytometry analysis of mice after 2 weeks of diet (8 weeks after BMT). Shown is a quantitation of the ratio of male to female (CD45.2/CD45.1) cells in total leukocytes (C), Ly6chi monocytes (D), and CD3+ cells (E) prior to HFD exposure and after 2 weeks of HFD feeding. F, representative flow cytometry plots of male and female marrow contributions to total GWAT ATMs after 16 weeks of diet challenge. G, quantitation of total ATMs, CD11c subsets, and adipose tissue dendritic cells (DC) in male and female recipients. H, ratio of male to female (CD45.2/CD45.1) cells in GWAT ATMs and dendritic cells. I, quantitation of total BM HSCs and myeloid progenitors in male and female recipient mice. J, donor contribution to HSC and myeloid progenitor subsets in the bone marrow. n = 7 males, and n = 9 females. *, p < 0.05; **, p < 0.01; ***, p < 0.005; ****, p < 0.001. Comparisons between sexes are shown as follows: #, p < 0.05; ##, p < 0.01; ###, p < 0.005; ####, p < 0.001. Error bars, S.E.
6 weeks after BM transplantation, chimeras were fed a HFD. Both male and female chimeras gained similar weight (males 69 ± 11.9% (S.D.) and females 58 ± 17% (S.D.), p = 0.16), but male mice weighed more at all time points compared with females (Fig. 6A). There were no significant differences in total leukocyte numbers (neutrophils or monocytes) between male and female recipients (not shown). Analysis of CD45+ blood leukocytes (Fig. 6B) prior to HFD exposure showed a significant increase in the CD45.2/CD45.1 ratio to >1 in male recipients but not females (Fig. 6C). This suggests that stromal signals from males, but not females, promote a preferential reconstitution of male BM cells in lethally irradiated hosts. After 2 weeks of HFD, the CD45.2/CD45.1 ratio was >1 for both male and female recipients. The male predominance in contributing to leukocyte production with HFD exposure was particularly striking in Ly6chi monocytes (Fig. 6D). The preferential contribution of male donors to leukocyte populations was limited to myeloid populations because CD3+ lymphocytes in the chimeras were derived equally from male and female donors in male mice and did not change with HFD (Fig. 6E). Skewed contribution toward the female donor lymphocytes (ratio <1) was observed in female recipients.
After 16 weeks of HFD, ATMs were assessed in the chimeric mice for donor contribution (Fig. 6F). In contrast to the non-irradiated mice (Fig. 1), male and female radiation chimeras demonstrated similar numbers of total ATMs in gonadal adipose tissue with DIO. When ATM subsets were analyzed, female recipients had a marginally significant trend toward fewer CD11c+ ATMs (p = 0.07) and dendritic cells (CD64− CD11c+) (p = 0.07) (Fig. 6G). When the donor chimerism of ATM subsets was assessed, the CD45.2/CD45.1 ratio was significant >1 for total, CD11c+, and CD11c− ATMs in both male and female recipients (Fig. 6H). For dendritic cells, the CD45.2/CD45.1 ratio was >1 for male recipients but not in females. Overall, this finding suggests that male hematopoietic cells have a cell-autonomous advantage over female hematopoietic cells to traffic to adipose tissue and generate ATMs independent of sex-specific stromal (non-hematopoietic) signals.
To examine whether the peripheral effects on ATMs could be related to changes in the reconstitution of the BM compartment, we phenotyped the chimerism of BM progenitors. Comparing male and female recipients, there was a significant increase in the quantity of Lin− progenitors and several myeloid progenitor populations (pre-granulocyte-macrophage) in male mice (Fig. 6I). This was similar to the results seen in non-irradiated mice, suggesting that the female stromal compartment is less capable of supporting hematopoiesis compared with males. When chimerism was assessed, the CD45.2/CD45.1 ratio was >1 for LT-HSCs regardless of the sex of the recipient. In female mice, the CD45.2/CD45.1 ratio was >1 in LSK, GMP, myeloid progenitor, and LT-HSC populations (Fig. 6J). Overall, these findings suggest that male hematopoietic cells have a cell-autonomous advantage over female hematopoietic cells stimulating myelopoiesis in response to DIO.
Male Bone Marrow Is Primed to Make More Activated Myeloid Cells in Response to LPS and Palmitic Acid
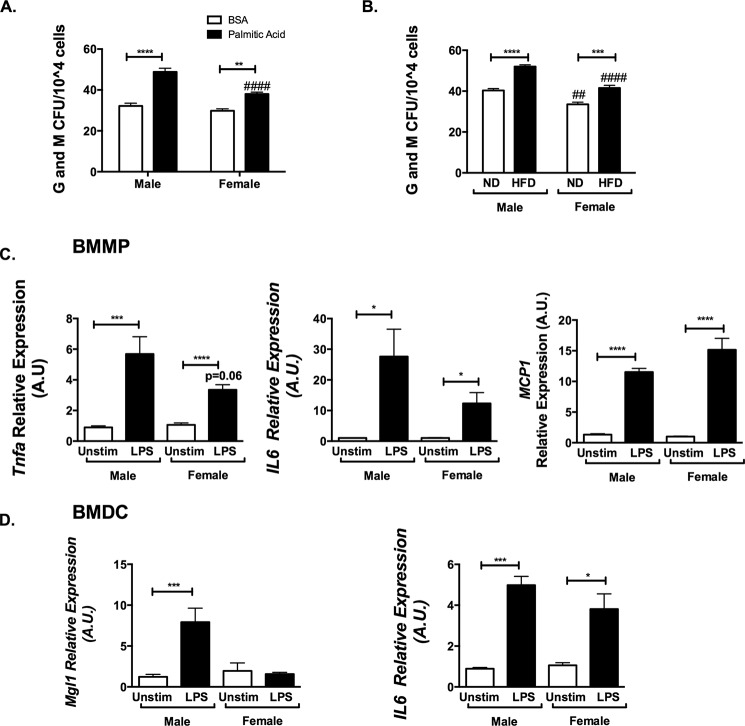
As a potential mechanism for the expansion of myeloid progenitors, we examined the hypothesis that saturated fatty acids may alter HSC progenitor production and explain differential effects between sexes. BM from male and female mice was isolated and analyzed in in vitro granulocyte-macrophage colony-forming unit (cfu-GM) assays with and without stimulation with palmitic acid. No differences in cfu-GM were observed between untreated male and female BM. Palmitic acid treatment increased cfu-GM in both female and male BM, with more cfu-GM counts in males compared with in females (Fig. 7A). Similarly, male and female BM from HFD-fed animals produced an increased quantity of myeloid colonies, but fewer cfu were generated in HFD females compared with males (Fig. 7B). Because BM leukocytes themselves appeared to be different between males and females in vivo, we evaluated whether there are intrinsic differences in myeloid cell inflammatory gene expression between males and females. Bone marrow macrophages and bone marrow-derived dendritic cells were differentiated from both sexes and examined in vitro after stimulation with LPS. Gene expression showed that male bone marrow macrophages and bone marrow-derived dendritic cells produced more Tnfa and IL6 compared with females (Fig. 7, C and D). LPS stimulation induced Mcp1 gene expression similarly in both male and female cells. Overall, these studies identify fundamental differences in proinflammatory gene expression activation profiles between sexes in macrophages that can be traced to BM progenitors as a mechanism of the protection from obesity-induced inflammation.
FIGURE 7.
Males have increased bone marrow inflammatory responses to palmitic acid, high fat diet, and LPS compared with females. A, male and female BM was isolated and plated in methocult media for granulocyte and macrophage colony-forming units. 10,000 cells were isolated and stimulated with FA free BSA or 10 μm palmitic acid with BSA. B, similarly, male and female marrow after in vivo diet challenge were plated for cfu. Bone marrow mononuclear phagocytes (C) and bone marrow-derived dendritic cells (D) of male mice have increased proinflammatory profiles after stimulation with LPS compared with females. A.U., arbitrary units. n = 8; *, p < 0.05; **, p < 0.01; ***, p < 0.005; ****, p < 0.001. Comparisons between sexes are shown as follows: #, p < 0.05; ##, p < 0.01; ###, p < 0.005; ####, p < 0.001. Error bars, S.E.
Discussion
Obesity is a leading cause of metabolic and cardiovascular disease, but there appear to be targeted populations of individuals at increased risk for these diseases. Identifying individuals protected from disease may help in identification of novel treatment approaches for those at risk. Women of reproductive age are protected from cardiovascular and metabolic disease, but it has not been clear what mechanisms drive this. It was initially thought that estrogen alone explained these differences, but given that replacement therapy did not improve disease risk (2, 28), it appears that there are other inherent differences in males and females driving risk for obesity-associated disease.
Whereas previous studies have identified differences in male and female adipose tissue biology through gene expression profiles and lipid processing, a thorough comparison of leukocyte development and adipose tissue macrophages in obese males and females has not been directly investigated. Our findings show that sex differences in myeloid cell production and maturation of proinflammatory ATMs are one factor that may explain prior observations. Despite similar adipocyte hypertrophy, female mice are protected from inflammatory changes in adipose tissue similar to other reports (29, 30). Given that short and long term HFD studies cannot distinguish whether the enhanced ATM myeloid activation in male mice is merely due to the increase in adiposity and lower energy expenditure or to insulin resistance seen in males compared with females, we performed a competitive BMT experiment that permitted us to compare myeloid cell activation between sexes in the same recipient. This study identifies fundamental cell-autonomous differences in the response of HSCs to obesity between males and females that generate differential myeloid cell production from the bone marrow niche. Reductions in HFD-induced myelopoiesis in female HSCs were observed when compared with male cells regardless of the sex of the recipient. In addition, sexually dimorphic differences in stromal/non-hematopoietic signals influenced the expansion of myeloid progenitors in the BM niche.
Animal and human studies support the idea that different immune responses by sex may explain sexually dimorphic disease risk (17, 31). Although this has not been fully explored in the context of obesity, it supports the concept that immune system responses play a role in sex differences in metabolic disease. Female mice are generally protected from adipose tissue inflammation. Hormonal factors may play a role in this protection because ovariectomy induces a partial change to a male obese phenotype (12) and increased inflammation. Inflammatory factors, such as altered MCP-1, have also been shown to play a role in sex differences in myelopoieis (32), as is seen in our studies as well with female mice having lower MCP1 and protection from insulin resistance and dampened inflammation. Our observations align with known differences in immune activation between sexes because studies have shown that females are at greater risk for autoimmune disease (31), whereas males are at greater risk for sepsis and cardiovascular disease (33). It is important to have a clear understanding of these differences in DIO models because animal studies primarily utilize male mice to investigate obesity and inflammatory mechanisms due to their increased weight gain and insulin resistance with HFD (14), which may misrepresent what is occurring in females. The need for a change in the convention of exclusively examining male mice in obesity studies has also come to light in upcoming policy changes by the National Institutes of Health requiring an improved balance of male and female animals in preclinical studies (34). This is important in obesity research, given that many of the studies examining adipose tissue obtained at the time of bariatric surgery are from cohorts that are predominantly female (21).
Although the studies presented here show intrinsic leukocyte differences, one potential pitfall of our BMT approach is that irradiation is likely to alter the estrogen production, which may alter estrogen signaling and macrophage polarization, which may not be seen here (35). Given the likely hypoestrogenic state of these animals, it is interesting that the effects were still persistent, suggesting non-estrogen-mediated influences on hematopoiesis. It is also possible that the estrogen receptor (ERα) located on hematopoietic cells (36) may be stimulated by other ligands that suppress stimulation of myelopoiesis in females other then estrogen itself. Overall, however, these inflammatory effects are probably multifactorial, given a variety of inflammatory mediators, including adipokines, altered in males and females (37) after diet exposure.
Another contributing factor is that male mice have been shown to be sensitive to TLR4 activation in obesity, whereas females appear to be relatively protected (38). Given the presence of TLR4 on HSCs and its role in activating myelopoiesis (39), this may further explain why female mice have a reduced inflammatory leukocyte production with diet-induced obesity and is consistent with our cfu-GM studies (Fig. 7, A and B). Whereas in male mice, TLR4 activation is linked to myelopoiesis, Calippe et al. (40) identified that estrogen signaling itself may influence TLR presence and activation state, and further studies have shown an effect of LPS responsiveness being reduced in females (41, 42). These studies demonstrated that ERα activation itself stimulates TLR production of cytokines, priming the system for future dietary challenge. This finding is inconsistent with other findings and emphasizes that there are probably hormonal, signaling, and immune system factors that lead to altered diet responses in females.
Our studies show that there is a sexually dimorphic process of generation of myeloid cells, with males producing inherently proinflammatory factors compared with females. Recent human studies have found that hypogonadal males have a worse body composition due to both estrogen and androgen deficiency (43). Overall, our studies emphasize the importance of sex-specific investigations, because specified treatment strategies for the immune sequelae may need to be considered in male and female obese patients. It also has implications for the design and interpretation of preclinical studies of immunometabolism in mice. Further, it is important to investigate whether these altered findings of diet-induced inflammation have ramifications for interpreting inflammation in non-obese settings.
Acknowledgments
This work utilized Core Services from the Michigan Nutrition and Obesity Research Center (supported by National Institutes of Health Grant DK089503 to the University of Michigan). This work also utilized the Microscopy Core of the Michigan Diabetes Research Center (supported by National Institutes of Health, NIDDK, Grant 2P30-DK20572).
This work was supported, in whole or in part, by National Institutes of Health Grants DK090262 and DK090262-S1 (to C. N. L.) and National Institutes of Health, NIAID, Experimental Training in Immunology Grant T32 AI007413-19 (to B. Z.). This work was also supported by American Diabetes Association Grant 7-12-CD-08 (to C. N. L.), American Heart Association Scientist Development Grant 14SDG17890004, and a Department of Pediatrics Janette Ferrantino Investigator Award (to K. S).
- HSC
- hematopoietic stem cell
- BM
- bone marrow
- BMT
- bone marrow transplantation
- ATM
- adipose tissue macrophage
- DIO
- diet-induced obesity
- ND
- normal diet
- HFD
- high fat diet
- SVF
- stromal vascular fraction
- GWAT
- gonadal white adipose tissue
- IWAT
- inguinal white adipose tissue
- LSK
- Lin− Sca1+ Kit+
- GMP
- granulocyte-macrophage progenitor.
References
- 1. Meyer M. R., Haas E., Barton M. (2006) Gender differences of cardiovascular disease: new perspectives for estrogen receptor signaling. Hypertension 47, 1019–1026 [DOI] [PubMed] [Google Scholar]
- 2. Barrett-Connor E. (2009) Women and heart disease: neglected directions for future research. J. Cardiovasc. Transl. Res. 2, 256–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Karastergiou K., Smith S. R., Greenberg A. S., Fried S. K. (2012) Sex differences in human adipose tissues: the biology of pear shape. Biol. Sex Differ. 3, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gregor M. F., Hotamisligil G. S. (2011) Inflammatory mechanisms in obesity. Ann. Rev. Immunol. 29, 415–445 [DOI] [PubMed] [Google Scholar]
- 5. Olefsky J. M., Glass C. K. (2010) Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 72, 219–246 [DOI] [PubMed] [Google Scholar]
- 6. Singer K., DelProposto J., Morris D. L., Zamarron B., Mergian T., Maley N., Cho K. W., Geletka L., Subbaiah P., Muir L., Martinez-Santibanez G., Lumeng C. N. (2014) Diet-induced obesity promotes myelopoiesis in hematopoietic stem cells. Mol. Metab. 3, 664–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nagareddy P. R., Kraakman M., Masters S. L., Stirzaker R. A., Gorman D. J., Grant R. W., Dragoljevic D., Hong E. S., Abdel-Latif A., Smyth S. S., Choi S. H., Korner J., Bornfeldt K. E., Fisher E. A., Dixit V. D., Tall A. R., Goldberg I. J., Murphy A. J. (2014) Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab. 19, 821–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nagareddy P. R., Murphy A. J., Stirzaker R. A., Hu Y., Yu S., Miller R. G., Ramkhelawon B., Distel E., Westerterp M., Huang L. S., Schmidt A. M., Orchard T. J., Fisher E. A., Tall A. R., Goldberg I. J. (2013) Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab. 17, 695–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weisberg S. P., Hunter D., Huber R., Lemieux J., Slaymaker S., Vaddi K., Charo I., Leibel R. L., Ferrante A. W., Jr. (2006) CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J. Clin. Invest. 116, 115–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Westcott D. J., Delproposto J. B., Geletka L. M., Wang T., Singer K., Saltiel A. R., Lumeng C. N. (2009) MGL1 promotes adipose tissue inflammation and insulin resistance by regulating 7/4hi monocytes in obesity. J. Exp. Med. 206, 3143–3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lumeng C. N., Deyoung S. M., Bodzin J. L., Saltiel A. R. (2007) Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes 56, 16–23 [DOI] [PubMed] [Google Scholar]
- 12. Grove K. L., Fried S. K., Greenberg A. S., Xiao X. Q., Clegg D. J. (2010) A microarray analysis of sexual dimorphism of adipose tissues in high-fat-diet-induced obese mice. Int. J. Obes. 34, 989–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nteeba J., Ortinau L. C., Perfield J. W., 2nd, Keating A. F. (2013) Diet-induced obesity alters immune cell infiltration and expression of inflammatory cytokine genes in mouse ovarian and peri-ovarian adipose depot tissues. Mol. Reprod. Dev. 80, 948–958 [DOI] [PubMed] [Google Scholar]
- 14. Estrany M. E., Proenza A. M., Gianotti M., Lladó I. (2013) High-fat diet feeding induces sex-dependent changes in inflammatory and insulin sensitivity profiles of rat adipose tissue. Cell Biochem. Funct. 31, 504–510 [DOI] [PubMed] [Google Scholar]
- 15. Pennell L. M., Galligan C. L., Fish E. N. (2012) Sex affects immunity. J. Autoimmun. 38, J282–291 [DOI] [PubMed] [Google Scholar]
- 16. Marriott I., Huet-Hudson Y. M. (2006) Sexual dimorphism in innate immune responses to infectious organisms. Immunol. Res. 34, 177–192 [DOI] [PubMed] [Google Scholar]
- 17. Imahara S. D., Jelacic S., Junker C. E., O'Keefe G. E. (2005) The influence of gender on human innate immunity. Surgery 138, 275–282 [DOI] [PubMed] [Google Scholar]
- 18. Shi H., Kokoeva M. V., Inouye K., Tzameli I., Yin H., Flier J. S. (2006) TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 116, 3015–3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mukundan L., Odegaard J. I., Morel C. R., Heredia J. E., Mwangi J. W., Ricardo-Gonzalez R. R., Goh Y. P., Eagle A. R., Dunn S. E., Awakuni J. U., Nguyen K. D., Steinman L., Michie S. A., Chawla A. (2009) PPAR-δ senses and orchestrates clearance of apoptotic cells to promote tolerance. Nat. Med. 15, 1266–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vandanmagsar B., Youm Y. H., Ravussin A., Galgani J. E., Stadler K., Mynatt R. L., Ravussin E., Stephens J. M., Dixit V. D. (2011) The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 17, 179–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pratt G. M., Learn C. A., Hughes G. D., Clark B. L., Warthen M., Pories W. (2009) Demographics and outcomes at American Society for Metabolic and Bariatric Surgery Centers of Excellence. Surg. Endosc. 23, 795–799 [DOI] [PubMed] [Google Scholar]
- 22. Morris D. L., Oatmen K. E., Wang T., DelProposto J. L., Lumeng C. N. (2012) CX3CR1 deficiency does not influence trafficking of adipose tissue macrophages in mice with diet-induced obesity. Obesity 20, 1189–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lumeng C. N., DelProposto J. B., Westcott D. J., Saltiel A. R. (2008) Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes 57, 3239–3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pronk C. J., Rossi D. J., Månsson R., Attema J. L., Norddahl G. L., Chan C. K., Sigvardsson M., Weissman I. L., Bryder D. (2007) Elucidation of the phenotypic, functional, and molecular topography of a myeloerythroid progenitor cell hierarchy. Cell Stem Cell 1, 428–442 [DOI] [PubMed] [Google Scholar]
- 25. Erickson J. C., Clegg K. E., Palmiter R. D. (1996) Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature 381, 415–421 [DOI] [PubMed] [Google Scholar]
- 26. Gautier E. L., Shay T., Miller J., Greter M., Jakubzick C., Ivanov S., Helft J., Chow A., Elpek K. G., Gordonov S., Mazloom A. R., Ma'ayan A., Chua W. J., Hansen T. H., Turley S. J., Merad M., Randolph G. J. (2012) Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat. Immunol. 13, 1118–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vander Lugt B., Tubo N. J., Nizza S. T., Boes M., Malissen B., Fuhlbrigge R. C., Kupper T. S., Campbell J. J. (2013) CCR7 plays no appreciable role in trafficking of central memory CD4 T cells to lymph nodes. J. Immunol. 191, 3119–3127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Riant E., Waget A., Cogo H., Arnal J. F., Burcelin R., Gourdy P. (2009) Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology 150, 2109–2117 [DOI] [PubMed] [Google Scholar]
- 29. Medrikova D., Jilkova Z. M., Bardova K., Janovska P., Rossmeisl M., Kopecky J. (2012) Sex differences during the course of diet-induced obesity in mice: adipose tissue expandability and glycemic control. Int. J. Obes. 36, 262–272 [DOI] [PubMed] [Google Scholar]
- 30. Pettersson U. S., Waldén T. B., Carlsson P. O., Jansson L., Phillipson M. (2012) Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PloS One 7, e46057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Whitacre C. C., Reingold S. C., O'Looney P. A. (1999) A gender gap in autoimmunity. Science 283, 1277–1278 [DOI] [PubMed] [Google Scholar]
- 32. Kim W. K., Choi E. K., Sul O. J., Park Y. K., Kim E. S., Yu R., Suh J. H., Choi H. S. (2013) Monocyte chemoattractant protein-1 deficiency attenuates oxidative stress and protects against ovariectomy-induced chronic inflammation in mice. PloS One 8, e72108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marriott I., Bost K. L., Huet-Hudson Y. M. (2006) Sexual dimorphism in expression of receptors for bacterial lipopolysaccharides in murine macrophages: a possible mechanism for gender-based differences in endotoxic shock susceptibility. J. Reprod. Immunol. 71, 12–27 [DOI] [PubMed] [Google Scholar]
- 34. Clayton J. A., Collins F. S. (2014) Policy: NIH to balance sex in cell and animal studies. Nature 509, 282–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bolego C., Cignarella A., Staels B., Chinetti-Gbaguidi G. (2013) Macrophage function and polarization in cardiovascular disease: a role of estrogen signaling? Arterioscler. Thromb. Vasc. Biol. 33, 1127–1134 [DOI] [PubMed] [Google Scholar]
- 36. Ribas V., Drew B. G., Le J. A., Soleymani T., Daraei P., Sitz D., Mohammad L., Henstridge D. C., Febbraio M. A., Hewitt S. C., Korach K. S., Bensinger S. J., Hevener A. L. (2011) Myeloid-specific estrogen receptor α deficiency impairs metabolic homeostasis and accelerates atherosclerotic lesion development. Proc. Natl. Acad. Sci. U.S.A. 108, 16457–16462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gui Y., Silha J. V., Murphy L. J. (2004) Sexual dimorphism and regulation of resistin, adiponectin, and leptin expression in the mouse. Obes. Res. 12, 1481–1491 [DOI] [PubMed] [Google Scholar]
- 38. Orr J. S., Puglisi M. J., Ellacott K. L., Lumeng C. N., Wasserman D. H., Hasty A. H. (2012) Toll-like receptor 4 deficiency promotes the alternative activation of adipose tissue macrophages. Diabetes 61, 2718–2727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Megías J., Yáñez A., Moriano S., O'Connor J. E., Gozalbo D., Gil M. L. (2012) Direct Toll-like receptor-mediated stimulation of hematopoietic stem and progenitor cells occurs in vivo and promotes differentiation toward macrophages. Stem Cells 30, 1486–1495 [DOI] [PubMed] [Google Scholar]
- 40. Calippe B., Douin-Echinard V., Delpy L., Laffargue M., Lélu K., Krust A., Pipy B., Bayard F., Arnal J. F., Guéry J. C., Gourdy P. (2010) 17β-Estradiol promotes TLR4-triggered proinflammatory mediator production through direct estrogen receptor alpha signaling in macrophages in vivo. J. Immunol. 185, 1169–1176 [DOI] [PubMed] [Google Scholar]
- 41. Iwasa T., Matsuzaki T., Kinouchi R., Gereltsetseg G., Murakami M., Munkhzaya M., Altankhuu T., Kuwahara A., Yasui T., Irahara M. (2014) Changes in central and peripheral inflammatory responses to lipopolysaccharide in ovariectomized female rats. Cytokine 65, 65–73 [DOI] [PubMed] [Google Scholar]
- 42. Iwasa T., Matsuzaki T., Matsui S., Munkhzaya M., Tungalagsuvd A., Kawami T., Murakami M., Kato T., Kuwahara A., Yasui T., Irahara M. (2014) The effects of LPS-induced endotoxemia on the expression of adiponectin and its receptors in female rats. Endocr. J. 61, 891–900 [DOI] [PubMed] [Google Scholar]
- 43. Finkelstein J. S., Lee H., Burnett-Bowie S. A., Pallais J. C., Yu E. W., Borges L. F., Jones B. F., Barry C. V., Wulczyn K. E., Thomas B. J., Leder B. Z. (2013) Gonadal steroids and body composition, strength, and sexual function in men. N. Engl. J. Med. 369, 1011–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]