Background: Glucocorticoids impair islet β-cell function via glucocorticoid receptor (GR) activation.
Results: Thiobenzothiazole-modified hydrocortisone compounds exhibit anti-inflammatory properties with reduced impact on insulin secretion.
Conclusion: Novel glucocorticoids can be engineered to reduce impact on β-cell mass and function.
Significance: Improved GR agonists will be beneficial in a variety of clinical settings.
Keywords: adipogenesis, chemokine, glucocorticoid, inflammation, insulin secretion
Abstract
Glucocorticoids signal through the glucocorticoid receptor (GR) and are administered clinically for a variety of situations, including inflammatory disorders, specific cancers, rheumatoid arthritis, and organ/tissue transplantation. However, glucocorticoid therapy is also associated with additional complications, including steroid-induced diabetes. We hypothesized that modification of the steroid backbone is one strategy to enhance the therapeutic potential of GR activation. Toward this goal, two commercially unavailable, thiobenzothiazole-containing derivatives of hydrocortisone (termed MS4 and MS6) were examined using 832/13 rat insulinoma cells as well as rodent and human islets. We found that MS4 had transrepression properties but lacked transactivation ability, whereas MS6 retained both transactivation and transrepression activities. In addition, MS4 and MS6 both displayed anti-inflammatory activity. Furthermore, MS4 displayed reduced impact on islet β-cell function in both rodent and human islets. Similar to dexamethasone, MS6 promoted adipocyte development in vitro, whereas MS4 did not. Moreover, neither MS4 nor MS6 activated the Pck1 (Pepck) gene in primary rat hepatocytes. We conclude that modification of the functional groups attached to the D-ring of the hydrocortisone steroid molecule produces compounds with altered structure-function GR agonist activity with decreased impact on insulin secretion and reduced adipogenic potential but with preservation of anti-inflammatory activity.
Introduction
Glucocorticoids (GCs)3 are lipophilic steroidal compounds synthesized endogenously in the adrenal glands. GCs have both anti-inflammatory and metabolic effects (1). Their main mode of action occurs via binding to the glucocorticoid receptor (GR; NR3C1). Ligand-bound GR translocates from the cytosol to the nucleus, where it functions as a sequence-specific DNA binding transcriptional regulator. Because of the powerful anti-inflammatory actions of GCs, they are often used in a variety of clinical situations (1). However, the clinical use of glucocorticoids is associated with many untoward side effects, including osteoporosis, Cushing syndrome, adrenal suppression, and diabetes (2).
The most common cause of drug-induced diabetes is clinical administration of GCs, and the incidence of GC-induced diabetes continues to rise (3). The distribution of the GR in virtually all tissues provides numerous targets for GC action, including effects in pancreatic β-cells. Overexpression of the GR targeted to pancreatic β-cells impairs insulin secretion (4), whereas deletion of the GR in pancreatic progenitor cells promotes β-cell mass expansion (5, 6). Thus, current GCs regulate β-cell development and suppress IL-1β-induced inflammatory signaling responses in pancreatic β-cells (7, 8) but also markedly impair adult β-cell function and mass (see Ref. 9 and the present work).
IL-1β is a key contributor to islet inflammation in both Type 1 and Type 2 diabetes mellitus, which is linked to increased expression of a number of genes involved in immune cell recruitment, nitric oxide production, and prostaglandin synthesis in pancreatic β-cells (10, 11). Chemokines are a major subset of genes induced by inflammatory stimuli, such as IL-1β and palmitate, and probably contribute to immune cell infiltration and alterations in immune cell activity in both Type 1 and Type 2 diabetes mellitus (11–15). Thus, therapeutics capable of suppressing inflammatory responses in pancreatic islets with reduced impact on insulin secretion would be extremely valuable.
GCs were removed from islet transplant regimens due to their adverse side effects (16, 17), which include β-cell dysfunction (18). However, if their powerful anti-inflammatory actions could be harnessed without, or with vastly reduced, side effects, this would greatly enhance islet transplantation protocols as well as provide therapeutic benefits in other clinical settings. In an effort to separate salutary effects from side effects, various attempts to produce GR ligands that dissociate transactivation from transrepression properties of the GR have been reported (19, 20). The generation of selective glucocorticoid receptor modulators has included both steroidal and non-steroidal compounds (21). However, more information is needed before the precise ligand structures, which offer the most beneficial properties as a GR agonist, can be determined. In addition, discovery of the most suitable GR agonist molecule with which to treat a specific pathological condition is an active area of investigation.
In this study, we report on two thiobenzothiazole-modified hydrocortisone compounds using mouse, rat, and human pancreatic β-cells. Importantly, we identified a unique steroidal compound (MS4) that had reduced impact on insulin secretion when compared with dexamethasone but retained a potent ability to suppress chemokine production. The MS4 compound could potentially be used to improve islet transplantation protocols as well as to provide therapeutic options for other clinical conditions that require long term glucocorticoid therapy.
Experimental Procedures
Cell Culture, Islet Isolation, Primary Hepatocytes, and Glucose-stimulated Insulin Secretion (GSIS)
Culture of 832/13 cells and measurements of insulin secretion have been described (22, 23). Rat islets were isolated according to previously published protocols (24). Mouse islets (C57BL6) and human islets (Integrated Islet Distribution Program) were obtained and studied as described previously (25). Six-point dose-response curves were used to generate EC50 and saturating concentrations of each steroid compound (not shown). Primary hepatocytes from Sprague-Dawley rats were purchased from Triangle Research Labs. All rats were 8–10 weeks old and weighed between 150 and 200 g at the time of the isolation procedure. For GSIS, saturating concentrations of each commercial steroid or mercapto-modified steroid were used as indicated in the figure legends.
Synthesis of MS4 and MS6
Two previously reported, yet not fully characterized, thiobenzothiazole-modified hydrocortisone analogues (MS4 and MS6) were synthesized via slight modifications of known methods (26). The complete synthetic procedures and chemical characterizations for both molecules are provided in supplemental Fig. 1.
Cell Viability Assays
832/13 cells were grown in 24-well plates, followed by exposure to the steroidal compounds as indicated in the figure legends. Viability was assessed using both 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) and adenylate kinase (ADK) assays. These procedures have been previously validated using the clonal 832/13 β-cell line (24).
Luciferase Assays, ELISA, and Real-time PCR Analysis of mRNA
832/13 cells were grown in 24-well plates to 50% confluence and then transiently transfected with 25 ng of indicated plasmid per well using TransFectin Lipid Reagent (Bio-Rad) according to the manufacturer's instructions. Cell lysis, luciferase assays, and normalization to total protein content were carried out as described previously (27). CCL2 and CCL20 secreted into the media were detected using rat Quantikine (CCL2) or DuoSet (CCL20) ELISA kits from R&D Systems, Inc. (Minneapolis, MN) according to the manufacturer's suggested protocol. Total RNA isolation, cDNA synthesis, and quantification of mRNA abundance via real-time RT-PCR were described previously (28). Primers used to detect transcript levels are shown in Table 1.
TABLE 1.
Primers used for gene expression analysis
| Gene (species) | Forward primer (5′–3′) | Reverse primer (5′–3′) |
|---|---|---|
| Adiponectin (mouse) | ggaacttgtgcaggttggat | gcttctccaggctctccttt |
| Ccl2 (rat) | atgcagttaatgccccactc | ttccttattggggtcagcac |
| Ccl20 (rat) | gcttacctctgcagccagtc | cggatcttttcgacttcagg |
| Dusp1 (mouse) | gagctgtgcagcaaacagtc | cttccgagaagcgtgatagg |
| Dusp1 (rat) | aggacaaccacaaggcagac | aatactccgcctctgcttca |
| Errfi1 (mouse) | agtgtggtccatctccaagg | agaaccccgatcacacagag |
| Errfi1 (rat) | ccattcaaagctgctcttcc | cctgccaggaacatcgtatt |
| Fabp4 (mouse) | catcagcgtaaatggggatt | tcgactttccatcccacttc |
| Mafa (mouse) | atcatcactctgcccaccat | agtcggatgacctcctcctt |
| Mafa (rat) | ttcagcaaggaggaggtcat | ccgccaacttctcgtatttc |
| Pck1 (rat) | cccaggagtcaccatcactt | ttcgtagacaagggggacac |
| Pparg (mouse) | ttttcaagggtgccagtttc | aatccttggccctctgagat |
| Pref1 (mouse) | tgtcaatggagtctgcaagg | agggagaaccattgatcacg |
| RPS9 | tccggaacaaacgtgagg | tccagcttcatcttgccc |
| Sgk1 (mouse) | catgcaaacacgctgaagtt | ccctttccgatcactttcaa |
| Sgk1 (rat) | aatggcggagagctgttcta | tgtgctcgatgttctccttg |
Adipogenesis Assay
Murine 3T3-L1 preadipocytes were plated and grown to 2 days postconfluence in DMEM containing 10% bovine serum. Medium was changed every 48–72 h. Cells were induced to differentiate by changing the medium to DMEM containing a standard MDI induction mixture of 10% fetal bovine serum (FBS), 0.5 mm 3-isobutyl-1-methylxanthine, 100 nm dexamethasone or a 100 nm concentration of MS4 or MS6, and 1.7 μm insulin.
Oil Red O Staining
An Oil Red O stock was prepared as described previously (29). Cell monolayers were aspirated and rinsed with PBS. Following incubation in a fixative solution (10% formaldehyde in PBS) for 10–15 min, the monolayers were rinsed under tap water. The remaining water was aspirated, and the cells were incubated for 1 h in the working Oil Red O solution (0.3% in isopropyl alcohol). Following incubation, the stain was aspirated, the cells were rinsed under tap water, and then the cells were examined by microscopy and scanned to produce data images.
Metabolomics
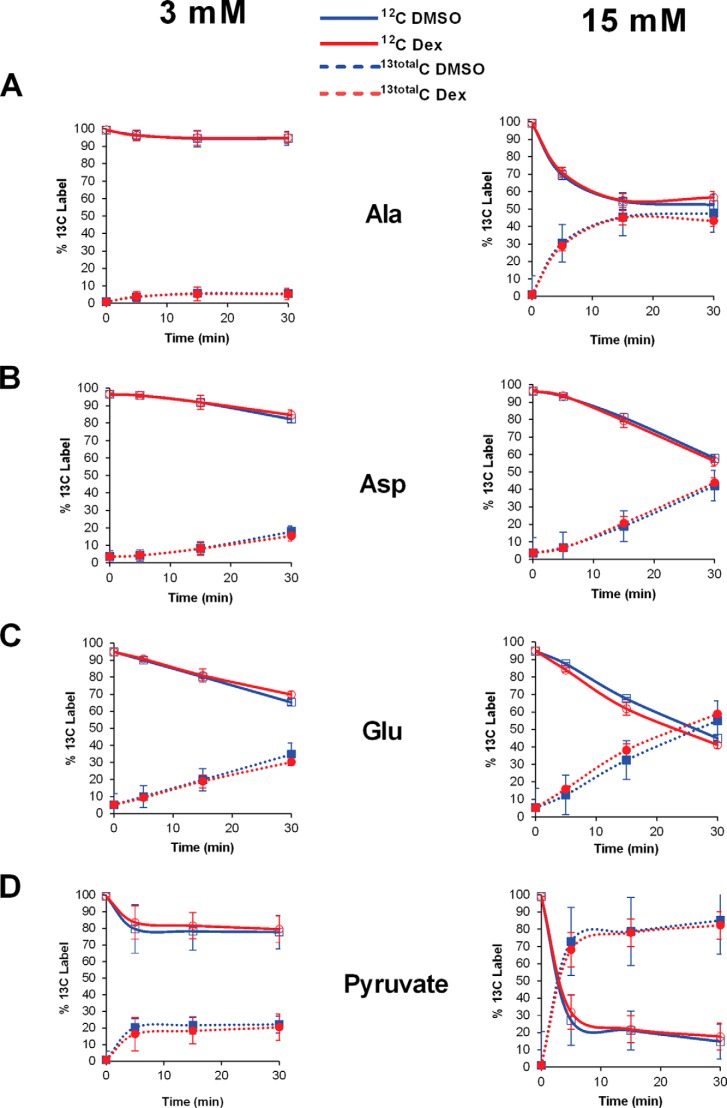
832/13 cells were grown in 15-cm dishes until 80% confluent and then cultured overnight in serum-free RPMI 1640 medium containing either DMSO (vehicle control) or dexamethasone. The following day, either 3 or 15 mm [U-13C]glucose was added to a HEPES balanced salt solution (described in Ref. 22), and the cells were harvested at the time points shown in Fig. 3. For harvesting, cells were washed with cold PBS, pelleted by centrifugation at 250 × g for 5 min, and metabolome-extracted as described (30). Sample analysis was accomplished with an ultraperformance liquid chromatography-mass spectrometric (UPLC-MS) untargeted metabolomics and flux method adapted from a previous report (31). The LC-MS system consisting of a Dionex Ultimate 3000 UPLC stack equipped with a temperature-controlled column compartment, pump, and autosampler was fitted to a Thermo Scientific Exactive Plus Orbitrap MS. Briefly, the extracted samples were stored at 4 °C in the autosampler before analysis. An aliquot of each (10 μl) was injected onto a Synergi Hydro-RP 100 column (100 × 2.00 mm, 2.5 μm; Phenomenex) maintained at 25 °C. The analytes were eluted from the column with a flow rate of 200 μl/min with Solvent A being 97:3 water/methanol, 10 mm tributylamine, and 15 mm acetic acid and Solvent B as methanol using the following 25-min gradient: t = 0 min, 100% A, 0% B; t = 2.5 min, 100% A, 0% B; t = 5 min, 80% A, 20% B; t = 7.5 min, 80% A, 20% B; t = 13 min, 45% A, 55% B; t = 15.5 min, 5% A, 95% B; t = 18.5 min, 95% A, 5% B; t = 19 min, 100% A, 0% B; t = 25 min, 100% A, 0% B. The eluent was introduced into the MS via an electrospray ionization source. Full scan data were collected in negative ionization mode using the following parameters: spray voltage = 3 kV, nitrogen sheath gas = 10 p.s.i., capillary temperature = 320 °C, AGC target = 3e6, maximum injection time = 100 ms, resolution = 140,000, and a sliding scan window of 85–800 m/z from 0 to 9 min and 110–1000 m/z from 9 to 25 min. Peaks for known metabolites and their 13C isotopomers were chosen and integrated manually using the following criteria for peak identification: exact mass within ±20 ppm (hard cut-off) and retention time within ±4 min (soft cut-off). Peak areas were exported to an Excel sheet as a .csv file for final data analysis. For flux studies, the percentage of each labeled isotopomer was determined for each time point, and fold changes were calculated by adding all isotopomer peak areas together. The samples were randomized before data acquisition. For data processing, the Orbitrap data files (generated by Xcalibur as .RAW) were converted to mzML format2 via the open source msconvert software in ProteoWizard.3 These files were then entered into Maven (also known as mzroll; see Ref. 32), which was used to automatically align the total ion chromatograms of each sample based on retention times and m/z values.
FIGURE 3.
Kinetic flux profiling by mass spectrometry reveals glucose-induced but not glucocorticoid-induced changes in metabolites associated with stimulus-secretion coupling. 832/13 cells were exposed to either DMSO or 10 nm Dex overnight. The cell culture medium was then replaced with a Hepes balanced salt solution containing either 3 or 15 mm [U-13C]glucose, and cells were harvested at the time points indicated. Cellular extracts were analyzed by UPLC-MS for 13C incorporation into water-soluble metabolites. Replicate data are shown as means ± S.D.
Measurements of Oxygen Consumption
Oxygen consumption rates (OCR) were measured in 832/13 cells using a Seahorse XF24 Analyzer (Seahorse Biosciences, North Billerica, MA). Following overnight incubation with dexamethasone (Dex), medium was removed, cells were rinsed with PBS, and 750 μl of Hepes balanced salt solution with 3 mm glucose was added for a 2-h equilibration phase to mimic established conditions used for glucose-stimulated insulin secretion (22). Basal OCR at 3 mm glucose was then measured in 6-min intervals for 3 cycles. After baseline was established, changes in OCR were measured in response to serial compound injections designed to yield the following final well concentrations: glucose (15 mm; 6 cycles), oligomycin (0.5 μg/ml; 2 cycles), carbonyl cyanide p-trifluoromethoxyphenylhydrazone (2.5 μm; 2 cycles), and antimycin A (10 μm; 2 cycles).
Statistical Analysis
One-way analysis of variance followed by Tukey's post-hoc test was performed using Prism version 6.0 software.
Results
Commercially Available Glucocorticoids Impair Glucose-stimulated Insulin Secretion
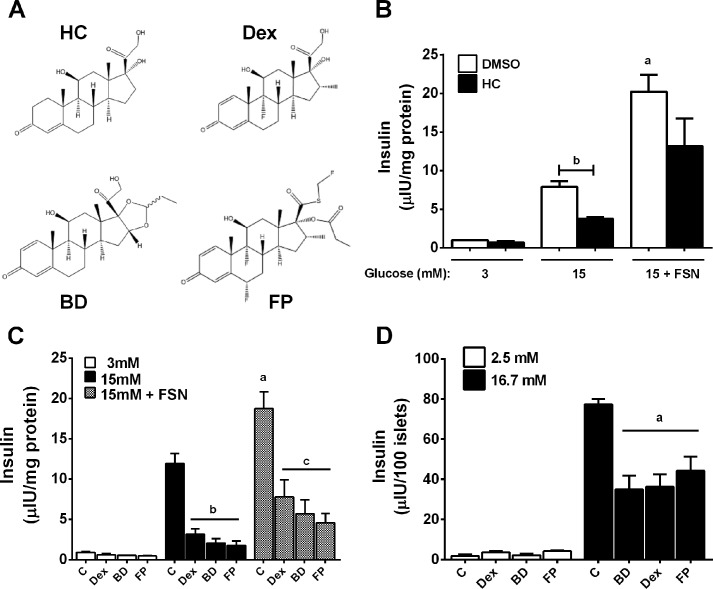
The structures of several commonly available GCs currently approved for clinical use are shown in Fig. 1A. Hydrocortisone (HC) was examined for its effect on GSIS using 832/13 rat insulinoma cells. There was a 53% decrease in GSIS following an overnight culture in the presence of HC (Fig. 1B). Moreover, there was a 2.5-fold increase in GSIS in the presence of the potentiating agent forskolin that was also blunted in the presence of HC (Fig. 1B). HC also impairs insulin secretion in isolated rat islets (not shown). We next examined several synthetic GCs, including Dex, budesonide, and fluticasone propionate, to potentially identify a compound with improved therapeutic index. We discovered that all of these molecules diminished insulin secretion in both 832/13 cells and isolated rat islets (Fig. 1, C and D).
FIGURE 1.
Glucocorticoids impair insulin secretion in 832/13 rat insulinoma cells and isolated rat islets. A, structures of commercially available synthetic glucocorticoids. B, 832/13 cells were cultured overnight (18–24 h) in the presence or absence of 100 nm hydrocortisone (HC), followed by measurements of insulin secretion in response to 3 or 15 mm glucose or 15 mm glucose plus 5 μm forskolin (FSN). a, p < 0.05 versus DMSO 15 mm glucose group; b, p < 0.01. C, 832/13 cells were incubated overnight with either DMSO vehicle control (C) or 10 nm Dex, budesonide (BD), or fluticasone propionate (FP). Insulin secretion was measured in response to 3 mm and 15 mm glucose and 15 mm glucose plus 5 μm forskolin. a, p < 0.05 versus DMSO control 15 mm glucose group; b, p < 0.05 versus DMSO control 15 mm group; c, p < 0.05 versus DMSO control 15 mm plus forskolin group. D, isolated rat islets were cultured overnight in the presence of either DMSO control (C) or 10 nm Dex, budesonide, or fluticasone propionate. Insulin secretion was measured in the presence of 2.5 and 16.7 mm glucose. a, p < 0.05 versus DMSO control 15 mm group. Error bars, S.E.
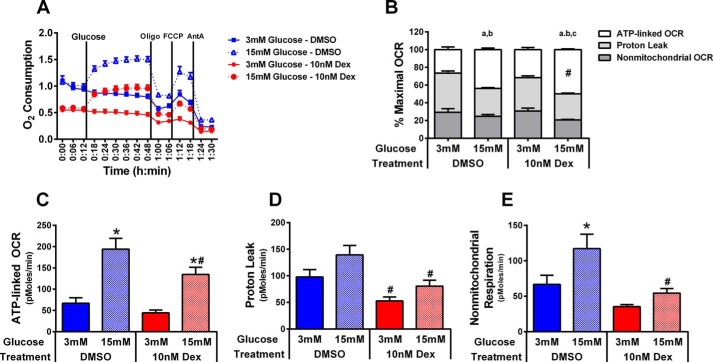
Dexamethasone Impairs Cellular Oxygen Consumption
Because the ability of each synthetic GC to impair insulin secretion was similar, we selected Dex for further investigation due to its common usage in a variety of clinical settings. Using 832/13 cells cultured for 18 h in the presence or absence of Dex, we observed a 45% decrease in the baseline OCR (Fig. 2A). Despite a lower basal OCR in the presence of Dex, the addition of high glucose increased the OCR by nearly 2-fold in both DMSO and Dex-treated cells (Fig. 2A). Using sequential injections of oligomycin, carbonyl cyanide p-trifluoromethoxyphenylhydrazone, and antimycin A, contributions of ATP-linked respiration, proton leak, and nonmitochondrial respiration to total OCR were measured. Examination of the relative contribution of each of these components to total OCR indicates that the increase in glucose-stimulated OCR preferentially supports ATP synthesis because the percentage of total OCR represented by ATP-linked OCR increased in response from 3 mm glucose to 15 mm glucose, whereas the percentage of total OCR represented by proton leak and nonmitochondrial OCR decreased (Fig. 2B). Moreover, the percentage of total OCR represented by ATP-linked OCR at 15 mm glucose was significantly higher in Dex-treated cells than in DMSO control (50% versus 44% of total OCR), supporting the possibility that, despite lower total OCR, the ability to support respiration linked to ATP generation is preferentially conserved. This contention is also partially supported by findings in Fig. 2C showing that whereas ATP-linked OCR is reduced nearly 30% in response to Dex treatment, both DMSO- and Dex-treated cells are capable of increasing ATP-linked OCR 3-fold in response to 15 mm glucose (Fig. 2C). Alternatively, Dex treatment resulted in significantly greater reductions in proton leak (∼45%; Fig. 2D) and nonmitochondrial OCR (∼50%; Fig. 2E), and the degree of glucose-stimulated induction of oxygen consumption attributed to each of these parameters (40–55% increase for proton leak; 50–75% increase for nonmitochondrial OCR) is less than the 3-fold increase observed for ATP-linked OCR. However, the decreases in proton leak and nonmitochondrial respiration represent a significant reduction in total OCR because collectively these fractions contribute nearly 70% of total OCR in the 3 mm glucose condition and roughly 50% of total OCR in the 15 mm glucose condition (Fig. 2B).
FIGURE 2.
Dexamethasone decreases oxygen consumption. 832/13 cells were seeded at a density of 50,000 cells/well on V7-PS plates to be analyzed using a Seahorse XF24 Analyzer. Cells were exposed to either DMSO control or 10 nm Dex for 18 h prior to measurements. Mitochondrial function (A) was tested as basal OCR at 3 mm glucose followed by the sequential addition of glucose (3 mm (solid lines) versus 15 mm (dashed lines)), oligomycin (Oligo; 0.5 μg/ml), carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP) (2.5 μm), and antimycin A (AntA; 10 μm). The relative contribution to maximal OCR of ATP-linked OCR, proton leak, and nonmitochondrial respiration is represented as percentage of maximal OCR (B), whereas absolute rates are presented in C–E. OCR linked to ATP production was calculated as the difference between maximal OCR (at either 3 or 15 mm glucose) minus OCR after inhibition of ATP synthase (oligomycin addition). Nonmitochondrial respiration was defined as the OCR remaining following the addition of antimycin A (Ant A), whereas proton leak was calculated as the difference in OCR after oligomycin minus antimycin A injection. Data are mean ± S.E. (error bars) for 12–16 samples. a, p < 0.05 ATP-linked OCR for 3 mm versus 15 mm glucose; b, p < 0.05 proton leak for 3 mm glucose versus 15 mm glucose; c, p < 0.05 for nonmitochondrial OCR for 3 mm versus 15 mm glucose; *, p < 0.05 3 mm versus 15 mm glucose; #, p < 0.05 DMSO versus Dex.
Glucocorticoids Do Not Alter Flux of Metabolites Associated with Insulin Secretion
Because of the decrease in OCR (Fig. 2), we attempted to identify a defect in metabolism using [U-13C]glucose and mass spectrometry-based kinetic flux profiling (30). Using this technique, we were able to quantify 118 metabolites in each glucose treatment. Within these 118 metabolites, 24 (20%) and 40 (33%) metabolites showed 13C incorporation in the 3 and 15 mm [U-13C]glucose-treated cells, respectively, with a corresponding disappearance in 12C signal over time. Although glucose-induced changes in metabolite synthesis were readily detected, which is consistent with a previous report (33), we did not observe an altered concentration and/or turnover rate (i.e. flux) with respect to Dex exposure in the 118 total metabolites analyzed (Fig. 3) (data not shown). Because the observed changes in central carbon metabolism (specifically, glycolysis and TCA cycle) are due to changes in glucose concentration, as seen previously (33), and not from Dex treatment (data not shown), the specific metabolites presented in Fig. 3 were selected based on their recognized involvement in insulin secretion (34, 35).
MS4 Has an Improved Metabolic Profile When Compared with Dexamethasone and MS6
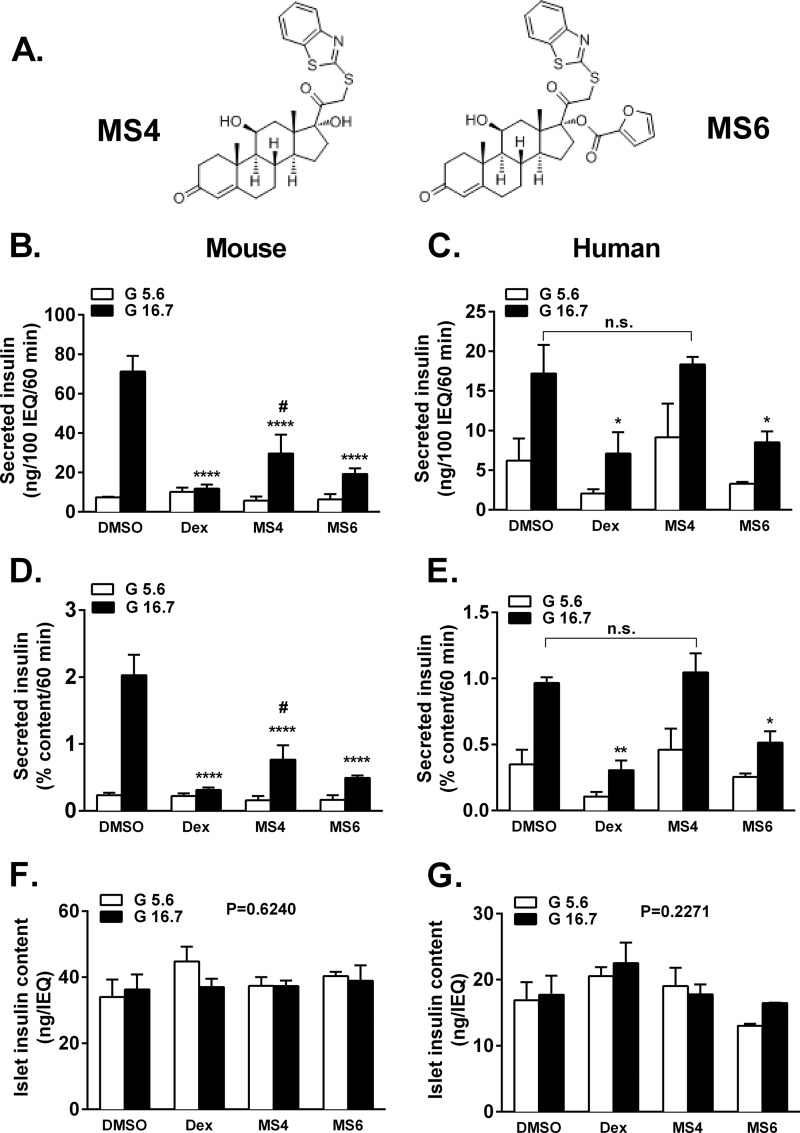
Because we did not identify an obvious metabolic defect associated with Dex-mediated impairments in insulin secretion, we turned our attention to modification of the steroid ring structure in an effort to identify compounds with altered structure-activity relationships and thus the potential for improved therapeutic value. Thiobenzathiazole modifications to the hydrocortisone backbone were reported to create compounds with dissociated activity (i.e. separation of transactivation from transrepression) (26). However, whether such molecules demonstrate improved therapeutic index (e.g. anti-inflammatory activity with retention of islet β-cell function) has not been studied using pancreatic β-cells. Therefore, two compounds ((11S,17R)-17-(2-(benzo[d]thiazol-2-ylthio)acetyl)-11,17-dihydroxy-10-methyl-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one and (11S,17R)-17-(2-(benzo[d]thiazol-2-ylthio)acetyl)-11-hydroxy-10-methyl-3-oxo-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl furan-2-carboxylate), termed mercaptosteroid 4 (MS4) and mercaptosteroid 6 (MS6), were investigated for their effects on both GSIS and anti-inflammatory activity (the chemical synthesis schemes for both MS4 and MS6 are given in supplemental Fig. 1; structures are shown in Fig. 4A and in supplemental Fig. 1).
FIGURE 4.
Glucose-stimulated insulin secretion is improved in mouse and human islets exposed to MS4 when compared with Dex and MS6. A, structures of MS4 and MS4. B, D, and F, C57BL6 islets were cultured overnight in the presence of either 100 nm Dex, MS4, or MS6 followed by static incubation in either 5.6 or 16.7 mm glucose. Insulin secreted into the culture medium and total insulin content were measured by radioimmunoassay. ****, p < 0.001 versus DMSO 16.7 mm glucose; #, p < 0.05 versus MS4 5.6 mm glucose (C, E, and G). Human islets were cultured overnight in the presence of either 100 nm Dex, MS4, or MS6 followed by static incubation in either 5.6 or 16.7 mm glucose. Insulin secreted into the culture medium and total insulin content were measured by radioimmunoassay. *, p < 0.05 versus DMSO, 16.7 mm glucose; ** p < 0.01 versus DMSO, 16.7 mm glucose. Error bars, S.E.
Using freshly isolated mouse islets, 16.7 mm glucose induced a 9-fold increase in insulin secretion when the DMSO vehicle was present, whereas Dex completely inhibited insulin secretion (Fig. 4B). Glucose-stimulated insulin secretion was 5-fold after islet exposure to MS4 and 3-fold after exposure to MS6 (Fig. 4B), indicating improved islet function with these steroidal compounds when compared with dexamethasone. In human islets, Dex impaired glucose-stimulated insulin secretion by 59%, whereas insulin secretion was maintained after overnight exposure to MS4 (Fig. 4C, black bars). MS6 reduced glucose-stimulated insulin secretion by 51% in human islets (Fig. 4C). We note that Dex and MS6 also both inhibited basal insulin secretion (Fig. 4C, white bars). When insulin secretion was measured as a percentage of islet insulin content, the patterns seen in both mouse and human islets were maintained (Fig. 4, D and E). Insulin content in mouse or human islets was not significantly different with any of the glucocorticoids used in this study (Fig. 4, F and G).
MS4 and MS6 Display Reduced Toxicity Compared with Dexamethasone
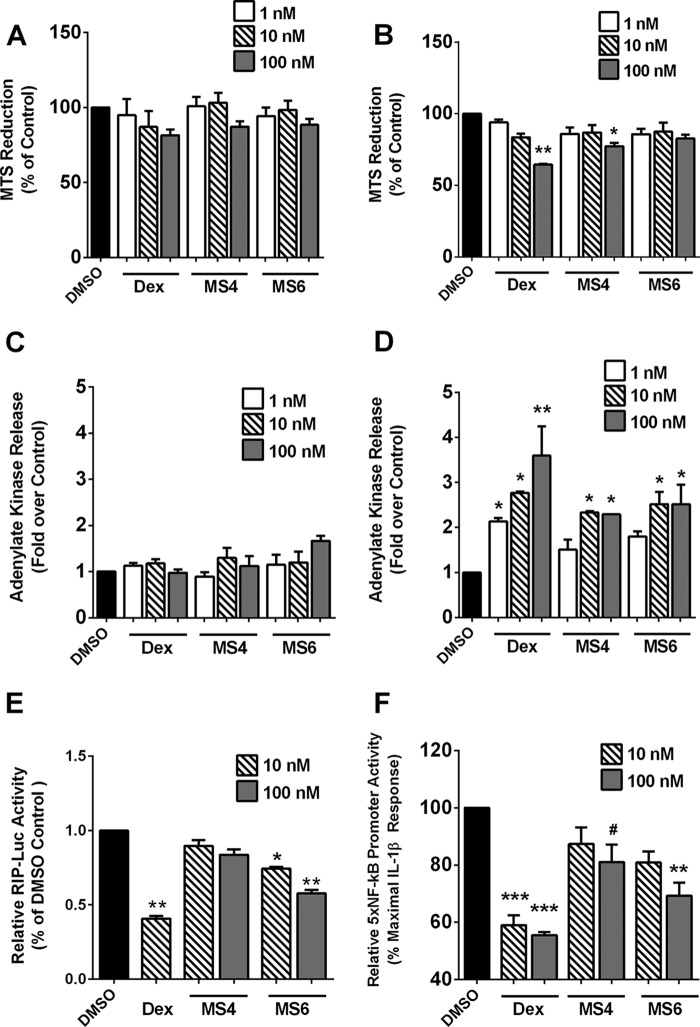
Glucocorticoids impact proliferation of pancreatic β-cells, with prolonged exposure often promoting cellular toxicity. Thus, we investigated the toxicity of Dex, MS4, and MS6 using 832/13 cells exposed for 24 or 48 h over a concentration range for each steroid. We observed a decrease in MTS reduction at 24 h at the highest concentration of all compounds tested (Fig. 5A). However, at 48 h, whereas Dex promoted a 36% decrease, MS4 and MS6 only decreased MTS reduction by 23 and 18%, respectively (Fig. 5B). Because MTS reduction can account for decreases in both proliferation and viability, we next measured ADK release to determine losses in cellular membrane integrity. There was no significant increase in ADK release by Dex, MS4, or MS6 at 24 h (Fig. 5C). However, at 48 h, we detected 2.1-, 2.8-, and 3.6-fold increases in ADK release with 1, 10, and 100 nm concentrations of Dex (Fig. 5D). MS4 and MS6 produced less than 2-fold ADK release at 1 nm (Fig. 5D). 832/13 cells cultured with 100 nm MS4 and MS6 for 48-h exposure had 2.3- and 2.5-fold elevations in ADK released into the culture medium (Fig. 5D). Thus, at the 100 nm concentration, MS4 and MS6 have 36 and 31% less toxicity, respectively, when compared with the same concentration of Dex.
FIGURE 5.
Mercaptosteroids display reduced toxicity when compared with dexamethasone. A–D, 832/13 cells were treated with the indicated concentrations of Dex, MS4, and MS6 for 24 h (A and C) or 48 h (B and D). MTS reduction (A and B) and adenylate kinase release into the culture medium (C and D) were measured after exposure to the indicated steroidal concentrations. E, 832/13 cells were transfected with a luciferase reporter gene driven by the rat insulin promoter (RIP-Luc). 12 h post-transfection, cells were exposed to either Dex (10 nm), MS4 (10 and 100 nm), or MS6 (10 and 100 nm) for 18 h. F, 832/13 cells were transduced with an adenovirus expressing the 5xNF-κB-luciferase reporter gene. 24 h after viral transduction, cells were pretreated for 1 h with Dex, MS4, and MS6, followed by exposure to 1 ng/ml IL-1β for 4 h. E and F, Promoter activity was normalized to total cellular protein content. Data are expressed as means ± S.E. (error bars) from three independent experiments. ***, p < 0.001 versus DMSO (F); **, p < 0.01 versus DMSO (B, E, and F); *, p < 0.05 versus DMSO (B, D, and E); #, p < 0.1 versus DMSO (F).
To further examine the impact of mercapto-modified steroids, we tested how these steroids affected transcription driven by a rat insulin promoter-luciferase reporter gene. Overnight exposure to Dex decreased transcription from the rat insulin promoter-luciferase reporter by 60% (Fig. 5E). MS4 had no significant effects in this assay, whereas MS6 decreased luciferase activity by 26% at the 10 nm and 42% at the 100 nm concentrations (Fig. 5E).
Using a multimerized NF-κB luciferase reporter construct, which consists of five copies of a NF-κB element upstream of the luciferase gene (5xNF-κB-Luc), we investigated the ability of each compound to suppress transcription induced by IL-1β. The 5xNF-κB-Luc is strongly activity within 4 h by 1 ng/ml IL-1β (28), producing 25–30-fold increases in luciferase activity over untreated cells. The 5xNF-κB-Luc response to IL-1β is set at 100% in Fig. 5F (black bar) to illustrate the ability of each steroidal compound to suppress NF-κB transcriptional activity. At 100 nm, Dex, MS4, and MS6 reduced NF-κB reporter activity by 45, 20, and 31%, respectively (Fig. 5F).
MS4 and MS6 Both Repress Ccl2 Promoter Activity, but Only MS6 Has Transactivation Properties
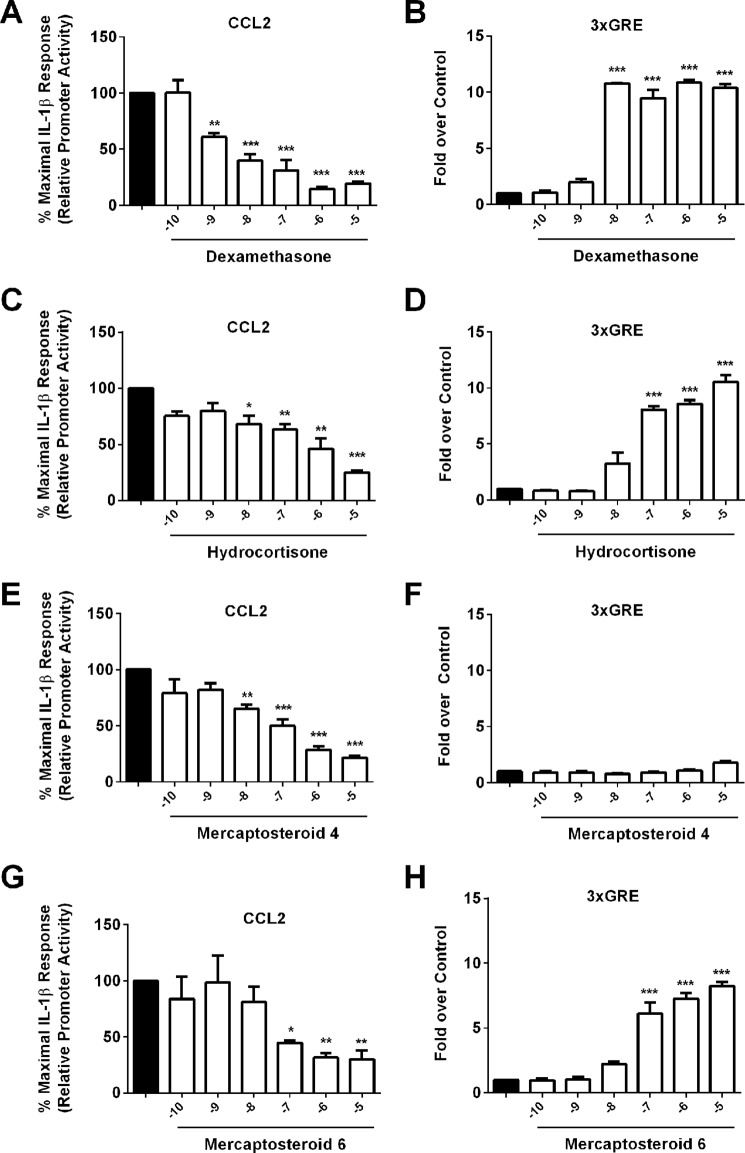
Because all compounds tested were capable of decreasing transcription from a synthetic, multimerized NF-κB reporter gene (Fig. 5F), we next investigated whether they were capable of regulating promoter activity of a specific NF-κB target gene. In addition, the ability to activate transcription from a promoter containing three copies of a glucocorticoid-response element (3xGRE) driving luciferase was also assessed. First, we compared the ability of Dex, MS4, and MS6 to repress IL-1β-mediated increases in CCL2 gene transcription. CCL2 expression is increased in both β-cells and adipose tissue during inflammatory states (7, 36) and is responsible for recruiting monocytes, macrophages, and T-lymphocytes to tissues from which it is released (12, 36). As expected, Dex and HC both repressed IL-1β-stimulated CCL2 gene transcription (Fig. 6, A and C). In addition, Dex and HC also increased transcription of the 3xGRE-containing promoter construct (Fig. 6, B and D), consistent with these GCs containing both transactivation and transrepression activities. Whereas MS4 suppressed CCL2 transcription by 50% at 100 nm (Fig. 6E), it did not activate the 3xGRE, showing no increases over DMSO at 100 nm and less than a 2-fold response at 10 μm (Fig. 6F). By contrast, MS6 reduced IL-1β-mediated transcription of CCL2 by 55% (Fig. 6G) but also increased promoter activity of the 3xGRE by over 6-fold at the 100 nm concentration (Fig. 6H). Thus, both compounds show similar efficacy in the transrepression assay (CCL2 repression), but only MS6 demonstrates transactivation potential (ability to drive transcription from the 3xGRE promoter-luciferase construct). These observations illustrate the similarity of MS6 to conventional, commercially available steroid compounds (i.e. dexamethasone), whereas MS4 displays a dissociated activity profile.
FIGURE 6.
MS6 displays both transactivation and transrepression activity in promoter-luciferase reporter assays. 832/13 cells were transfected with luciferase reporter constructs containing either −3.6 kb of the Ccl2 gene promoter (CCL2) or three copies of a consensus GRE in tandem (3xGRE). A, C, E, and G, 24 h post-transfection, cells were pretreated for 1 h with the indicated concentrations of Dex (A), HC (C), MS4 (E), and MS6 (G) and then cultured for 4 h with 1 ng/ml IL-1β. B, D, F, and H, cells were treated for 4 h with the log10 of the steroid concentrations as shown on the x axis. Data are means ± S.E. (error bars) from 3–4 individual experiments. ***, p < 0.001 versus DMSO (black bar); **, p < 0.01 versus DMSO (black bar); *, p < 0.05 versus DMSO (black bar).
Interleukin-1β-mediated CCL2 and CCL20 Transcript and Protein Abundance Are Diminished during Exposure to MS4 and MS6
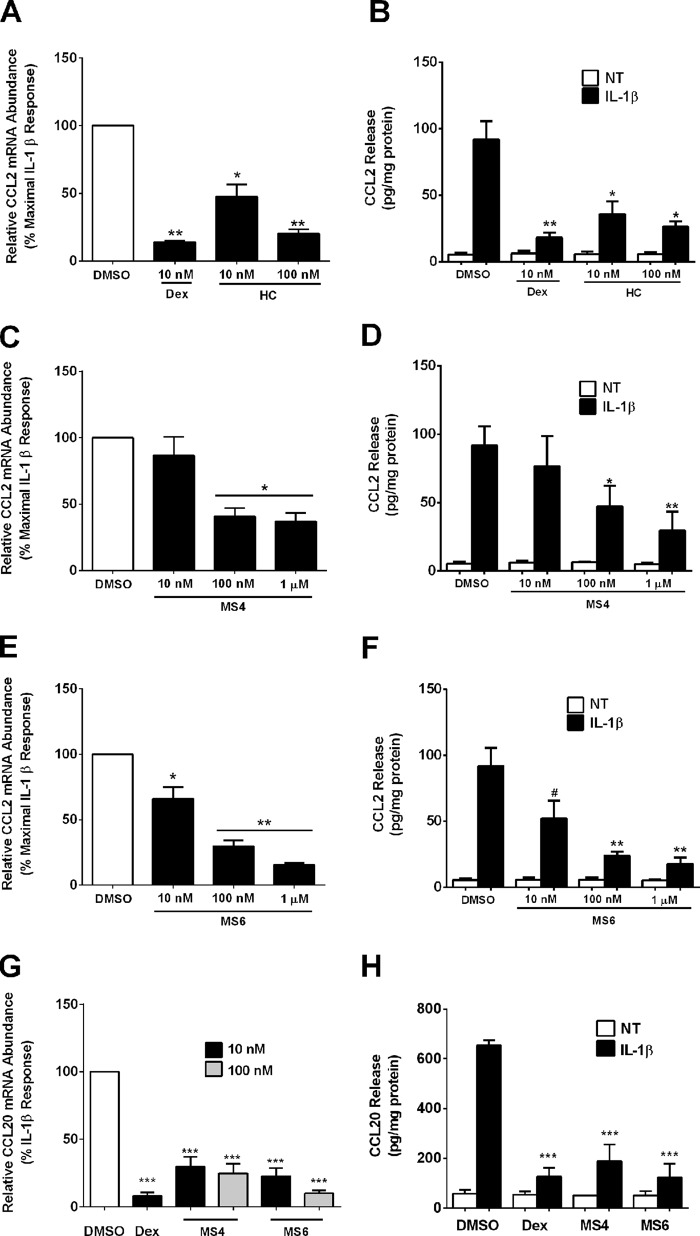
We used the concentrations of MS4 and MS6 derived from the promoter-based assays in Fig. 6 to examine the ability of these two compounds to impact synthesis of Ccl2 transcript levels and subsequent secretion of protein. The IL-1β-mediated increase in Ccl2 mRNA was diminished by 86% in the presence of 10 nm Dex, with a 53 and 80% decrease after exposure to 10 and 100 nm HC, respectively (Fig. 7A). This diminution in mRNA corresponded to a marked decrease in the release of CCL2 protein (Fig. 7B), which is consistent with a previous report (7). MS4 decreased Ccl2 mRNA by 59% at 100 nm, and increasing the concentration by 10-fold did not provide further suppression (Fig. 7C). Release of CCL2 protein decreased by 53 and 71% in the presence of 100 nm and 1 μm MS4, respectively (Fig. 7D). MS6 diminished Ccl2 mRNA levels by 35, 72, and 85% at the 10 nm, 100 nm, and 1 μm concentrations (Fig. 7E). In addition, CCL2 secretion was reduced by 48, 76, and 83% with 10 nm, 100 nm, and 1 μm concentrations of MS6 (Fig. 7F).
FIGURE 7.
Mercaptosteroids suppress Ccl2 and Ccl20 gene expression and secretion of protein. A–H, 832/13 cells were pretreated with the indicated concentrations of steroids for 1 h followed by a 6-h exposure to 1 ng/ml IL-1β. Relative mRNA abundance of Ccl2 (A, C, and E) and Ccl20 (G) was normalized to RPS9. ***, p < 0.001 versus DMSO (G); **, p < 0.01 versus DMSO (A and E); *, p < 0.05 versus DMSO (A, C, and E). CCL2 (B, D, and F) and CCL20 (H) release into the medium was measured by ELISA and normalized to total protein content. ***, p < 0.001 versus DMSO (black bar; G); **, p < 0.01 versus DMSO (black bar; B, D, and F); *, p < 0.05 versus DMSO (black bar; B and D); #, p < 0.1 versus DMSO (black bar; F). Data are expressed as means ± S.E. (error bars) from three independent experiments.
In addition to the effects on Ccl2, Dex, MS4, and MS6 all suppressed expression of the cyclooxygenase-2 gene (data not shown), which participates in the synthesis of prostaglandin E2 in islet β-cells (37, 38). We also investigated the effects of MS4 and MS6 on Ccl20, a distinct chemokine gene known to be up-regulated in islets of NOD mice and in human islets exposed to cytokines (39). Ccl20 mRNA abundance is strongly induced upon 832/13 exposure to IL-1β, and this response is prominently suppressed by Dex (92% decrease; Fig. 7G). In addition, MS4 and MS6 diminished the IL-1β-mediated induction of Ccl20 mRNA by 76 and 91%, respectively, at the 100 nm concentration (Fig. 7G). The effects on Ccl20 gene expression extend to a striking diminution in secreted CCL20 protein (Fig. 7H).
Differential Gene Expression Patterns Related to β-Cell Function Are Revealed during Exposure to MS4 and MS6
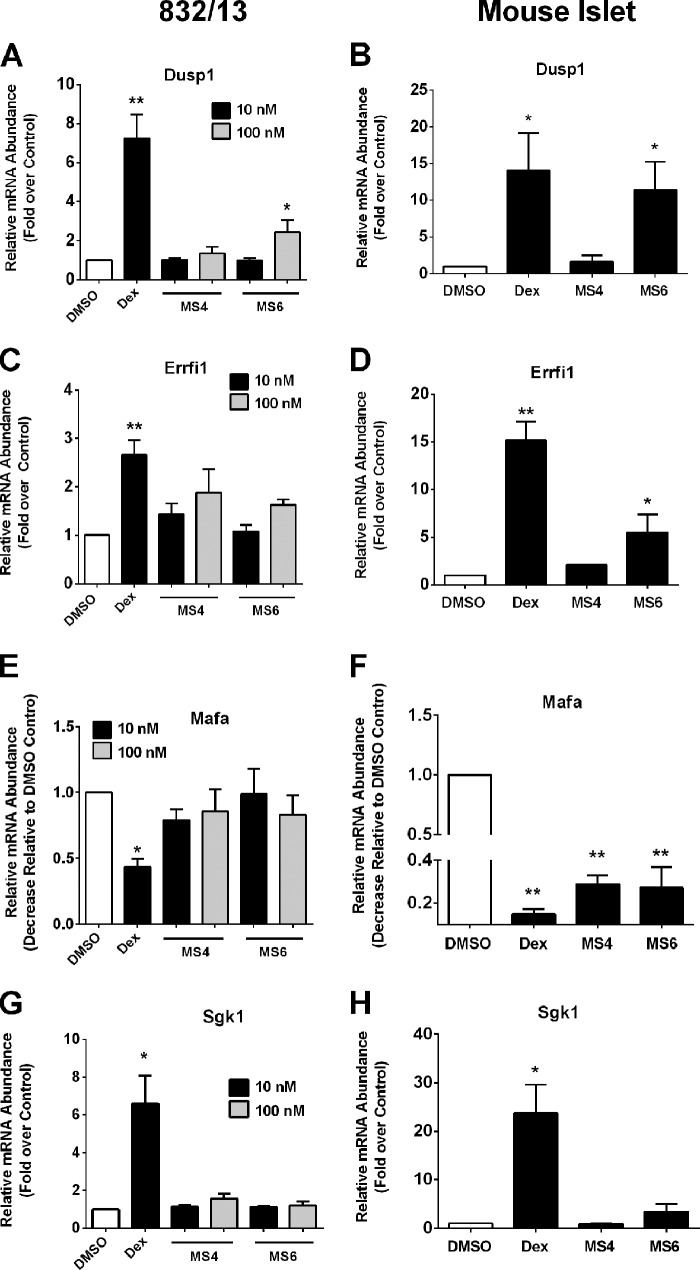
A screen of additional genes involved with β-cell identity and insulin secretion using 832/13 cells and mouse islets revealed marked differences between Dex and the thiobenzothiazole-based compounds (Fig. 8). The dual specificity phosphatase 1 (DUSP1 also known as MKP-1) is an established GR target gene in multiple tissues, including pancreatic β-cells (7, 40). The expression of Dusp1 is strongly activated by Dex in both 832/13 cells (7.2-fold) and in isolated mouse islets (14.1-fold; Fig. 8, A and B). MS4 does not induce the expression of the Dusp1 gene, which is consistent with the lack of GRE activation by this compound (Fig. 6F). By contrast, MS6 induces Dusp1 mRNA levels by 2.4-fold in 832/13 cells and 11-fold in mouse islets (Fig. 8, A and B).
FIGURE 8.
Differential expression of genes related to β-cell function by Dex and mercaptosteroids. A, C, E, and G, 832/13 cells were cultured with Dex (10 nm), MS4 (10 and 100 nm), or MS6 (10 and 100 nm) for 6 h. B, D, F, and H, isolated mouse islets were cultured with 100 nm Dex, MS4, and MS6 for 6 h. A–H, relative expression levels of Dusp1, Errfi1, Mafa, and Sgk1 were normalized to RPS9. *, p < 0.05 versus DMSO; **, p < 0.01 versus DMSO. Data are expressed as means ± S.E. (error bars).
The Errfi1 gene is linked to glucocorticoid-mediated decreases in β-cell proliferation (41). We observed a 2.7- and a 15-fold elevation in Errfi1 transcripts in 832/13 cells (Fig. 8C) and isolated mouse islets, respectively, after exposure to Dex (Fig. 8D). In 832/13 cells, 100 nm MS4 increased Errfi1 mRNA levels 1.9-fold, whereas 100 nm MS6 produced a 1.6-fold increase in the expression of this gene (Fig. 8C). In mouse islets, 100 nm MS4 enhanced Errfi1 mRNA abundance by 2.1-fold, whereas MS6 led to 5.5-fold increases in Errfi1 gene expression (Fig. 8D). Thus, the Errfi1 gene is much more strongly induced by Dex than by MS4 or MS6 (Fig. 8D).
Additionally, expression of Mafa, a transcription factor controlling the adult β-cell phenotype (42), was decreased 56% in response to Dex but was unaltered in 832/13 cells by either MS4 or MS6 (Fig. 8E). By contrast, mouse islets were much more sensitive to GR agonists in this context, with Dex suppressing Mafa transcript levels by 85%, MS4 by 71%, and MS6 by 73% (Fig. 8F). However, it is noteworthy that nearly twice as much Mafa transcript remains after exposure to MS4 versus islets exposed to Dex. This finding is consistent with MS4 having a reduced impact on insulin secretion in mouse islets (Fig. 4).
Finally, SGK1 (serum- and glucocorticoid-inducible kinase 1), which has been linked to GC-mediated impairments in insulin secretion (43), was induced 6.6-fold in 832/13 cells by Dex but not by either MS4 or MS6 (Fig. 8G). Sgk1 mRNA abundance increased 23.8-fold in mouse islets exposed to Dex but only 3.4-fold in those exposed to MS6. SGK1 is not induced by MS4 in mouse islets (Fig. 8H). The expression of Pdx-1, another transcription factor associated with β-cell function, is reduced by 48% in 832/13 cells in the presence of Dex, whereas MS4 has no effect (data not shown). Thus, altering the steroid ring with mercapto-based functional groups produces distinct gene expression patterns relative to Dex in a rat clonal β-cell line and in isolated mouse islets.
MS4 Does Not Promote Adipogenesis or Gluconeogenic Gene Expression in Primary Hepatocytes
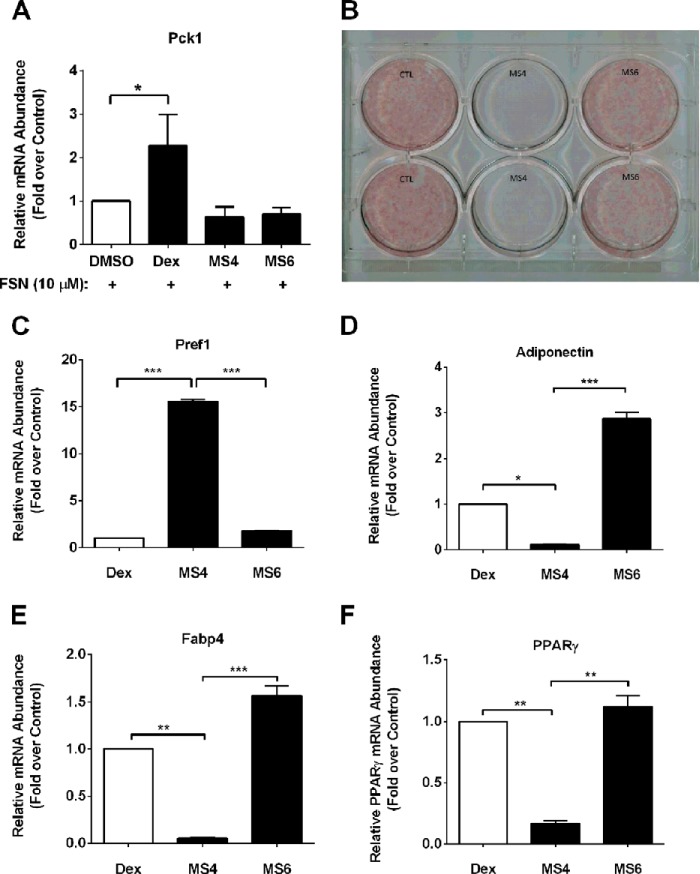
GCs are required for adipocyte differentiation (44). Excess GCs in circulation, such as occurs during Cushing disease or chronic GC therapy, promote both visceral fat deposition and hepatic insulin resistance (45, 46). An increase in hepatic insulin resistance contributes to hyperglycemia in mouse models and in human subjects (47, 48). Therefore, we examined expression of the Pck1 (Pepck) gene, one of the rate-controlling enzymes of hepatic glucose production, which is known to be regulated by glucocorticoids (49, 50). Forskolin, an activator of adenylate cyclase, increased Pck1 mRNA abundance 15–50-fold in primary rat hepatocytes (data not shown). Dexamethasone consistently augmented the forskolin-mediated induction of Pck1 gene expression (≥2-fold over DMSO control; Fig. 9A). However, neither MS4 nor MS6 was capable of activating the Pck1 gene alone (not shown) or in combination with the cAMP-generating agent forskolin (Fig. 9A).
FIGURE 9.
MS6, but not MS4, promotes adipocyte differentiation. A, primary rat hepatocytes were treated with 100 nm Dex, MS4, or MS6 in the presence 10 μm forskolin (FSN). Following RNA isolation, Pck1 (Pepck) mRNA abundance was measured and normalized to RPS9. B, murine 3T3-L1 preadipocytes were induced to differentiate using the standard MDI induction mixture containing 100 nm Dex (CTL) or 100 nm each of either MS4 or MS6. Cell monolayers were subjected to Oil Red O staining 5 days after the induction of adipogenesis. RNA was isolated from the conditions indicated in B, followed by detection of transcript levels of Pref1 (C), adiponectin (D), Fabp4 (E), and Pparg (F), followed by normalization to the housekeeping gene RPS9. Data are shown as means + S.E. (error bars) for two independent experiments, each performed in duplicate. ***, p < 0.001; **, p < 0.01; *, p < 0.05.
We next investigated the ability of both MS4 and MS6 to support adipocyte differentiation in 3T3-L1 cells. As shown in Fig. 9B, the combination of insulin, IBMX, and Dex, a typical formulation used to promote development of lipid-containing cells, promoted robust Oil Red O staining, indicative of adipocyte formation. We observed that MS4 was unable to substitute for Dex in this assay, whereas MS6 fully supported differentiation of adipocytes (Fig. 9B). Five days after the addition of the differentiation mixture, we also examined gene expression. The decrease in Pref-1 expression by Dex is set at a value of 1 to illustrate that although MS6 also suppressed Pref-1 expression, MS4 was unable to recapitulate this response (Fig. 9C). This is significant because a decrease in Pref-1 expression is reported to initiate the adipogenic program (51). Thus, the failure of MS4 to decrease Pref-1 gene expression is consistent with this compound being unable to support the adipogenic program. Moreover, whereas Dex and MS6 increased the expression of genes consistent with mature adipocytes, including adiponectin (Fig. 9D), Fabp4 (Fig. 9E), and Pparg (Fig. 9F), MS4 did not reproduce this phenotype. Therefore, MS6 effectively substitutes for Dex during adipocyte maturation, whereas MS4 does not.
Discussion
GCs are lipophilic steroid molecules that impact a variety of cellular processes, ultimately influencing reproductive function, the immune system, and glucose and lipid homeostasis. Because of the powerful anti-inflammatory properties of these molecules, they have been very useful in a variety of clinical settings (19). However, the vast array of side effects associated with prolonged GC usage has led to efforts to find new compounds that retain anti-inflammatory activities but have an improved therapeutic index (2, 21).
To our knowledge, this is the first report testing thiobenzothiazole-modified hydrocortisone molecules using rodent and human islets and clonal β-cell lines. MS4, one of the compounds in this study, retained anti-inflammatory activity (Figs. 6 and 7), with reduced impact on pancreatic β-cell function (Fig. 4). Importantly, MS4 also did not support adipogenesis or induce the expression of the Pck1 gene (Fig. 9). These findings are consistent with MS4 having the potential to reduce inflammation without increasing fat deposition or promoting hepatic insulin resistance. We note that, although GR translocation from cytoplasm to nucleus was not detectably different between Dex, MS4, and MS6 (not shown), there was a discernable difference between MS4, MS6, and Dex in assays of transactivation and transrepression (e.g. Figs. 6–8). These properties may represent critical factors influencing the ability of MS4 to diminish inflammatory responses while either partially (mouse islets) or fully (human islets) preserving insulin secretion. The molecular distinctions between transactivation and transrepression properties of the GR have been reviewed previously (20, 52) and are important biological considerations for developing novel GC-based therapeutics.
This is the first study documenting the robust transactivation potential of MS6, which is an important property to consider when examining phenotypes observed during cellular exposure to MS4 versus MS6. Thus, the conclusions based around observations reported herein may provide an emerging strategy upon which future design and development of additional unique molecules can be based. Engineering new compounds, either steroidal or non-steroidal, with biological improvements over the majority of the commercially available glucocorticoids is one approach to increase the number of therapeutic options available to treat diseases associated with inflammation. This is important because the existing synthetic GCs all markedly impair β-cell function (see Fig. 1 and Refs. 4 and 9) and promote hepatic insulin resistance (47, 53).
The Edmonton Protocol, hailed for its improved success with islet transplantation procedures, removed GCs from the immunosuppressive regimen (16). However, long term islet transplantation success using this protocol and other procedures are limited by both donor pancreas availability and continued problems with existing immunosuppressive drug toxicity to islet β-cells (54, 55). Indeed, sirolimus and tacrolimus, used as immunosuppressants in the Edmonton protocol, block β-cell regeneration in rodents (56). Moreover, lymphopenia, a common side of effect of immunosuppression, can trigger homeostatic proliferation of specific T-cell subsets, which in turn contribute to autoimmunity recurrence and graft rejection (57). The development of new molecules with anti-inflammatory activity, such as the MS4 compound presented in the present study, could potentially help enhance human islet transplantation outcomes in the future. Toward this end, we have determined herein that MS4 and MS6 also have reduced toxicity toward cultured β-cells (Fig. 5), which may be another beneficial property of mercapto-modified steroids.
Use of a thiobenzothiazole-modified hydrocortisone, such as MS4, in combination with existing clinically approved therapeutic agents, represents a novel approach to suppress inflammation with reduced effects on β-cell function. For example, anakinra, which interferes with IL-1β signaling, and etanercept, which targets the TNF-α pathway, have been combined with greater efficacy over either intervention alone in a promising preclinical report (58). New compounds, such as MS4, may thus expand the repertoire of available anti-inflammatory molecules, giving the possibility of additional combinations with even greater efficacy than available currently. Another potential advantage of MS4 over existing GCs is that it does not promote adipogenesis or contribute to hepatic expression of Pck1 (Fig. 9), which may reduce some of the negative side effects of chronic glucocorticoid usage (i.e. fat deposition and hepatic insulin resistance). Moreover, the strong repression of Ccl2 and Ccl20 synthesis and secretion by MS4 and MS6 has excellent potential for reducing inflammation-associated tissue dysfunction. As proof of principle, elevated CCL2 (also known as MCP-1) secretion is negatively associated with long lasting insulin independence after islet transplantation (59, 60).
We also discovered that GCs did not appear to impair insulin secretion by altering metabolic pathway flux (Fig. 3), which is consistent with previous studies (9). However, the mass spectrometry-based kinetic flux profiling using [U-13C]glucose did reveal robust glucose-dependent changes in metabolites associated with insulin secretion, which is complementary to a recent report (33). Interestingly, whereas GC exposure did not significantly alter overall [U-13C]glucose incorporation into metabolites of central carbon metabolism (not shown), it did decrease cellular oxygen consumption rates by 50% (Fig. 2A). Broadly, we found that OCR attributed to ATP production (Fig. 2C), proton leak (Fig. 2D), and nonmitochondrial respiration (Fig. 2E) are all significantly reduced at high glucose concentrations in response to Dex; however, the reduction in ATP-linked OCR is less substantial. Thus, we interpret these data collectively as supporting a model wherein Dex treatment reduces oxygen consumption by preferentially limiting nonmitochondrial respiration and proton leak while attempting to conserve the ability to increase oxygen consumption linked to ATP generation. Of note, because ATP is intimately involved in controlling the secretion of insulin (61), the 30% decrease in ATP-linked OCR during glucocorticoid exposure is also consistent with the observed decrease in β-cell function (Fig. 1). Moreover, it seems likely that the decrease in OCR, which was independent of changes in metabolite flux, may be indicative of a shift in the cellular bioenergetics to direct reducing equivalents toward processes other than the electron transport chain, such as maintaining redox status.
One limitation of the current study is that we currently have no data on the systemic in vivo effects of the thiobenzothiazole-modified hydrocortisone compounds. Because GC administration typically impairs insulin sensitivity in adipose tissue, liver, and skeletal muscle, in addition to impairing β-cell function, it will be important to determine whether or not MS4 or MS6 promote peripheral insulin resistance in vivo. In addition, assessment of the impact of mercapto-modified steroids on immune cell function will be necessary. Future studies will address these and other possible outcomes associated with systemic glucocorticoid delivery.
In summary, we have described two thiobenzothiazole-modified hydrocortisone compounds that may prove useful either as general anti-inflammatory molecules or as specific reagents to improve existing treatments aimed at countering inflammation with reduced negative effects on islet β-cell function. Forthcoming studies will address additional modifications to the steroid backbone as well as systemic effects of the presently described compounds in an attempt to further improve the repertoire of available anti-inflammatory therapeutic molecules.
Supplementary Material
Acknowledgments
This project used the PBRC Genomics Core Facilities that are supported in part by COBRE (Grant NIH8 P20-GM103528) and NORC (Grant 1P30-DK072476) center grants from the National Institutes of Health. Islet isolation and insulin secretion studies were performed in collaboration with the Vanderbilt Islet Procurement and Analysis Core (supported by the Vanderbilt Diabetes Research and Training Center, National Institutes of Health Grant P30 DK020593).
This work was supported, in whole or in part, by National Institutes of Health Grants P20-GM103528 (to J. J. C. and R. C. N.), R44-GM099207 (to J. J. C. and M. D. K.), R01-DK103860 (R. C. N.). This work was also supported by Veterans Affairs Merit Review Grants DK089572 and DK097829 (to A. C. P.).

This article contains supplemental Fig. 1.
- GC
- glucocorticoid
- GSIS
- glucose-stimulated insulin secretion
- OCR
- oxygen consumption rate
- GR
- glucocorticoid receptor
- GRE
- glucocorticoid-response element
- MTS
- 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- ADK
- adenylate kinase
- UPLC
- ultraperformance liquid chromatography
- Dex
- dexamethasone
- HC
- hydrocortisone
- PEPCK
- phosphoenolpyruvate carboxykinase.
References
- 1. Kadmiel M., Cidlowski J. A. (2013) Glucocorticoid receptor signaling in health and disease. Trends Pharmacol. Sci. 34, 518–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schäcke H., Döcke W. D., Asadullah K. (2002) Mechanisms involved in the side effects of glucocorticoids. Pharmacol. Ther. 96, 23–43 [DOI] [PubMed] [Google Scholar]
- 3. van Raalte D. H., Diamant M. (2014) Steroid diabetes: from mechanism to treatment? Neth. J. Med. 72, 62–72 [PubMed] [Google Scholar]
- 4. Delaunay F., Khan A., Cintra A., Davani B., Ling Z. C., Andersson A., Ostenson C. G., Gustafsson J., Efendic S., Okret S. (1997) Pancreatic beta cells are important targets for the diabetogenic effects of glucocorticoids. J. Clin. Invest. 100, 2094–2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blondeau B., Sahly I., Massouridès E., Singh-Estivalet A., Valtat B., Dorchene D., Jaisser F., Bréant B., Tronche F. (2012) Novel transgenic mice for inducible gene overexpression in pancreatic cells define glucocorticoid receptor-mediated regulations of beta cells. PLoS One 7, e30210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gesina E., Tronche F., Herrera P., Duchene B., Tales W., Czernichow P., Breant B. (2004) Dissecting the role of glucocorticoids on pancreas development. Diabetes 53, 2322–2329 [DOI] [PubMed] [Google Scholar]
- 7. Burke S. J., Goff M. R., Updegraff B. L., Lu D., Brown P. L., Minkin S. C., Jr., Biggerstaff J. P., Zhao L., Karlstad M. D., Collier J. J. (2012) Regulation of the CCL2 gene in pancreatic beta-cells by IL-1β and glucocorticoids: role of MKP-1. PLoS One 7, e46986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Corbett J. A., Wang J. L., Misko T. P., Zhao W., Hickey W. F., McDaniel M. L. (1993) Nitric oxide mediates IL-1β-induced islet dysfunction and destruction: prevention by dexamethasone. Autoimmunity 15, 145–153 [DOI] [PubMed] [Google Scholar]
- 9. Lambillotte C., Gilon P., Henquin J. C. (1997) Direct glucocorticoid inhibition of insulin secretion: an in vitro study of dexamethasone effects in mouse islets. J. Clin. Invest. 99, 414–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Padgett L. E., Broniowska K. A., Hansen P. A., Corbett J. A., Tse H. M. (2013) The role of reactive oxygen species and proinflammatory cytokines in type 1 diabetes pathogenesis. Ann. N.Y. Acad. Sci. 10.1111/j.1749-6632.2012.06826.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burke S. J., Collier J. J. (2014) Insulitis and diabetes: a perspective on islet inflammation. Immunome Res. 10, e002 [Google Scholar]
- 12. Martin A. P., Rankin S., Pitchford S., Charo I. F., Furtado G. C., Lira S. A. (2008) Increased expression of CCL2 in insulin-producing cells of transgenic mice promotes mobilization of myeloid cells from the bone marrow, marked insulitis, and diabetes. Diabetes 57, 3025–3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sarkar S. A., Lee C. E., Victorino F., Nguyen T. T., Walters J. A., Burrack A., Eberlein J., Hildemann S. K., Homann D. (2012) Expression and regulation of chemokines in murine and human type 1 diabetes. Diabetes 61, 436–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burke S. J., Lu D., Sparer T. E., Masi T., Goff M. R., Karlstad M. D., Collier J. J. (2014) NF-κB and STAT1 control CXCL1 and CXCL2 gene transcription. Am. J. Physiol. Endocrinol. Metab. 306, E131–E149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martin A. P., Grisotto M. G., Canasto-Chibuque C., Kunkel S. L., Bromberg J. S., Furtado G. C., Lira S. A. (2008) Islet expression of M3 uncovers a key role for chemokines in the development and recruitment of diabetogenic cells in NOD mice. Diabetes 57, 387–394 [DOI] [PubMed] [Google Scholar]
- 16. Shapiro A. M., Lakey J. R., Ryan E. A., Korbutt G. S., Toth E., Warnock G. L., Kneteman N. M., Rajotte R. V. (2000) Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 343, 230–238 [DOI] [PubMed] [Google Scholar]
- 17. Scharp D. W., Lacy P. E., Santiago J. V., McCullough C. S., Weide L. G., Falqui L., Marchetti P., Gingerich R. L., Jaffe A. S., Cryer P. E. (1990) Insulin independence after islet transplantation into type I diabetic patient. Diabetes 39, 515–518 [DOI] [PubMed] [Google Scholar]
- 18. van Raalte D. H., Kwa K. A., van Genugten R. E., Tushuizen M. E., Holst J. J., Deacon C. F., Karemaker J. M., Heine R. J., Mari A., Diamant M. (2013) Islet-cell dysfunction induced by glucocorticoid treatment: potential role for altered sympathovagal balance? Metabolism 62, 568–577 [DOI] [PubMed] [Google Scholar]
- 19. Vandevyver S., Dejager L., Tuckermann J., Libert C. (2013) New insights into the anti-inflammatory mechanisms of glucocorticoids: an emerging role for glucocorticoid-receptor-mediated transactivation. Endocrinology 154, 993–1007 [DOI] [PubMed] [Google Scholar]
- 20. Clark A. R., Belvisi M. G. (2012) Maps and legends: the quest for dissociated ligands of the glucocorticoid receptor. Pharmacol. Ther. 134, 54–67 [DOI] [PubMed] [Google Scholar]
- 21. Rosen J., Miner J. N. (2005) The search for safer glucocorticoid receptor ligands. Endocr. Rev. 26, 452–464 [DOI] [PubMed] [Google Scholar]
- 22. Hohmeier H. E., Mulder H., Chen G., Henkel-Rieger R., Prentki M., Newgard C. B. (2000) Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 49, 424–430 [DOI] [PubMed] [Google Scholar]
- 23. Collier J. J., White S. M., Dick G. M., Scott D. K. (2004) Phosphatidylinositol 3-kinase inhibitors reveal a unique mechanism of enhancing insulin secretion in 832/13 rat insulinoma cells. Biochem. Biophys. Res. Commun. 324, 1018–1023 [DOI] [PubMed] [Google Scholar]
- 24. Collier J. J., Fueger P. T., Hohmeier H. E., Newgard C. B. (2006) Pro- and antiapoptotic proteins regulate apoptosis but do not protect against cytokine-mediated cytotoxicity in rat islets and beta-cell lines. Diabetes 55, 1398–1406 [DOI] [PubMed] [Google Scholar]
- 25. Dai C., Brissova M., Hang Y., Thompson C., Poffenberger G., Shostak A., Chen Z., Stein R., Powers A. C. (2012) Islet-enriched gene expression and glucose-induced insulin secretion in human and mouse islets. Diabetologia 55, 707–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Biju P., McCormick K., Aslanian R., Berlin M., Solomon D., Chapman R., McLeod R., Prelusky D., Eckel S., Kelly G., Natiello M., House A., Fernandez X., Bitar R., Phillips J., Anthes J. (2011) Steroidal C-21 mercapto derivatives as dissociated steroids: discovery of an inhaled dissociated steroid. Bioorg. Med. Chem. Lett. 21, 6343–6347 [DOI] [PubMed] [Google Scholar]
- 27. Collier J. J., Burke S. J., Eisenhauer M. E., Lu D., Sapp R. C., Frydman C. J., Campagna S. R. (2011) Pancreatic beta-cell death in response to pro-inflammatory cytokines is distinct from genuine apoptosis. PLoS One 6, e22485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Burke S. J., Collier J. J. (2011) The gene encoding cyclooxygenase-2 is regulated by IL-1β and prostaglandins in 832/13 rat insulinoma cells. Cell. Immunol. 271, 379–384 [DOI] [PubMed] [Google Scholar]
- 29. Richard A. J., Fuller S., Fedorcenco V., Beyl R., Burris T. P., Mynatt R., Ribnicky D. M., Stephens J. M. (2014) Artemisia scoparia enhances adipocyte development and endocrine function in vitro and enhances insulin action in vivo. PLoS One 9, e98897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yuan J., Bennett B. D., Rabinowitz J. D. (2008) Kinetic flux profiling for quantitation of cellular metabolic fluxes. Nat. Protoc. 3, 1328–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lu W., Clasquin M. F., Melamud E., Amador-Noguez D., Caudy A. A., Rabinowitz J. D. (2010) Metabolomic analysis via reversed-phase ion-pairing liquid chromatography coupled to a stand alone orbitrap mass spectrometer. Anal. Chem. 82, 3212–3221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Melamud E., Vastag L., Rabinowitz J. D. (2010) Metabolomic analysis and visualization engine for LC-MS data. Anal. Chem. 82, 9818–9826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lorenz M. A., El Azzouny M. A., Kennedy R. T., Burant C. F. (2013) Metabolome response to glucose in the beta-cell line INS-1 832/13. J. Biol. Chem. 288, 10923–10935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lu D., Mulder H., Zhao P., Burgess S. C., Jensen M. V., Kamzolova S., Newgard C. B., Sherry A. D. (2002) 13C NMR isotopomer analysis reveals a connection between pyruvate cycling and glucose-stimulated insulin secretion (GSIS). Proc. Natl. Acad. Sci. U.S.A. 99, 2708–2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Prentki M., Matschinsky F. M., Madiraju S. R. (2013) Metabolic signaling in fuel-induced insulin secretion. Cell. Metab. 18, 162–185 [DOI] [PubMed] [Google Scholar]
- 36. Kanda H., Tateya S., Tamori Y., Kotani K., Hiasa K., Kitazawa R., Kitazawa S., Miyachi H., Maeda S., Egashira K., Kasuga M. (2006) MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Invest. 116, 1494–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Heitmeier M. R., Kelly C. B., Ensor N. J., Gibson K. A., Mullis K. G., Corbett J. A., Maziasz T. J. (2004) Role of cyclooxygenase-2 in cytokine-induced beta-cell dysfunction and damage by isolated rat and human islets. J. Biol. Chem. 279, 53145–53151 [DOI] [PubMed] [Google Scholar]
- 38. Oshima H., Taketo M. M., Oshima M. (2006) Destruction of pancreatic beta-cells by transgenic induction of prostaglandin E2 in the islets. J. Biol. Chem. 281, 29330–29336 [DOI] [PubMed] [Google Scholar]
- 39. Cardozo A. K., Proost P., Gysemans C., Chen M. C., Mathieu C., Eizirik D. L. (2003) IL-1β and IFN-γ induce the expression of diverse chemokines and IL-15 in human and rat pancreatic islet cells, and in islets from pre-diabetic NOD mice. Diabetologia 46, 255–266 [DOI] [PubMed] [Google Scholar]
- 40. Shipp L. E., Lee J. V., Yu C. Y., Pufall M., Zhang P., Scott D. K., Wang J. C. (2010) Transcriptional regulation of human dual specificity protein phosphatase 1 (DUSP1) gene by glucocorticoids. PLoS One 5, e13754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Colvin E. S., Ma H. Y., Chen Y. C., Hernandez A. M., Fueger P. T. (2013) Glucocorticoid-induced suppression of β-cell proliferation is mediated by Mig6. Endocrinology 154, 1039–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang C., Moriguchi T., Kajihara M., Esaki R., Harada A., Shimohata H., Oishi H., Hamada M., Morito N., Hasegawa K., Kudo T., Engel J. D., Yamamoto M., Takahashi S. (2005) MafA is a key regulator of glucose-stimulated insulin secretion. Mol. Cell. Biol. 25, 4969–4976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ullrich S., Berchtold S., Ranta F., Seebohm G., Henke G., Lupescu A., Mack A. F., Chao C. M., Su J., Nitschke R., Alexander D., Friedrich B., Wulff P., Kuhl D., Lang F. (2005) Serum- and glucocorticoid-inducible kinase 1 (SGK1) mediates glucocorticoid-induced inhibition of insulin secretion. Diabetes 54, 1090–1099 [DOI] [PubMed] [Google Scholar]
- 44. Sarjeant K., Stephens J. M. (2012) Adipogenesis. Cold Spring Harb. Perspect. Biol. 4, a008417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chanson P., Salenave S. (2010) Metabolic syndrome in Cushing's syndrome. Neuroendocrinology 92, 96–101 [DOI] [PubMed] [Google Scholar]
- 46. Petersons C. J., Mangelsdorf B. L., Jenkins A. B., Poljak A., Smith M. D., Greenfield J. R., Thompson C. H., Burt M. G. (2013) Effects of low-dose prednisolone on hepatic and peripheral insulin sensitivity, insulin secretion, and abdominal adiposity in patients with inflammatory rheumatologic disease. Diabetes Care 36, 2822–2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Laskewitz A. J., van Dijk T. H., Bloks V. W., Reijngoud D. J., van Lierop M. J., Dokter W. H., Kuipers F., Groen A. K., Grefhorst A. (2010) Chronic prednisolone treatment reduces hepatic insulin sensitivity while perturbing the fed-to-fasting transition in mice. Endocrinology 151, 2171–2178 [DOI] [PubMed] [Google Scholar]
- 48. Collier J. J., Scott D. K. (2004) Sweet changes: glucose homeostasis can be altered by manipulating genes controlling hepatic glucose metabolism. Mol. Endocrinol. 18, 1051–1063 [DOI] [PubMed] [Google Scholar]
- 49. O'Brien R. M., Printz R. L., Halmi N., Tiesinga J. J., Granner D. K. (1995) Structural and functional analysis of the human phosphoenolpyruvate carboxykinase gene promoter. Biochim. Biophys. Acta 1264, 284–288 [DOI] [PubMed] [Google Scholar]
- 50. Mitchell J., Noisin E., Hall R., O'Brien R., Imai E., Granner D. (1994) Integration of multiple signals through a complex hormone response unit in the phosphoenolpyruvate carboxykinase gene promoter. Mol. Endocrinol. 8, 585–594 [DOI] [PubMed] [Google Scholar]
- 51. Wang Y., Kim K. A., Kim J. H., Sul H. S. (2006) Pref-1, a preadipocyte secreted factor that inhibits adipogenesis. J. Nutr. 136, 2953–2956 [DOI] [PubMed] [Google Scholar]
- 52. Beck I. M., Vanden Berghe W., Vermeulen L., Yamamoto K. R., Haegeman G., De Bosscher K. (2009) Crosstalk in inflammation: the interplay of glucocorticoid receptor-based mechanisms and kinases and phosphatases. Endocr. Rev. 30, 830–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Miyazaki Y., DeFronzo R. A. (2009) Visceral fat dominant distribution in male type 2 diabetic patients is closely related to hepatic insulin resistance, irrespective of body type. Cardiovasc. Diabetol. 8, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rother K. I., Harlan D. M. (2004) Challenges facing islet transplantation for the treatment of type 1 diabetes mellitus. J. Clin. Invest. 114, 877–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Harlan D. M., Kenyon N. S., Korsgren O., Roep B. O. (2009) Current advances and travails in islet transplantation. Diabetes 58, 2175–2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nir T., Melton D. A., Dor Y. (2007) Recovery from diabetes in mice by beta cell regeneration. J. Clin. Invest. 117, 2553–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Monti P., Scirpoli M., Maffi P., Ghidoli N., De Taddeo F., Bertuzzi F., Piemonti L., Falcone M., Secchi A., Bonifacio E. (2008) Islet transplantation in patients with autoimmune diabetes induces homeostatic cytokines that expand autoreactive memory T cells. J. Clin. Invest. 118, 1806–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. McCall M., Pawlick R., Kin T., Shapiro A. M. (2012) Anakinra potentiates the protective effects of etanercept in transplantation of marginal mass human islets in immunodeficient mice. Am. J. Transplant. 12, 322–329 [DOI] [PubMed] [Google Scholar]
- 59. Piemonti L., Leone B. E., Nano R., Saccani A., Monti P., Maffi P., Bianchi G., Sica A., Peri G., Melzi R., Aldrighetti L., Secchi A., Di Carlo V., Allavena P., Bertuzzi F. (2002) Human pancreatic islets produce and secrete MCP-1/CCL2: relevance in human islet transplantation. Diabetes 51, 55–65 [DOI] [PubMed] [Google Scholar]
- 60. Schröppel B., Zhang N., Chen P., Chen D., Bromberg J. S., Murphy B. (2005) Role of donor-derived monocyte chemoattractant protein-1 in murine islet transplantation. J. Am. Soc. Nephrol. 16, 444–451 [DOI] [PubMed] [Google Scholar]
- 61. Newgard C. B., McGarry J. D. (1995) Metabolic coupling factors in pancreatic beta-cell signal transduction. Annu. Rev. Biochem. 64, 689–719 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.