Background: Promoter usage determines the peptide preceding the lipid-binding KalSec14 domain.
Results: Kalirin C-promoter encodes an amphipathic helix, which interacts with phosphoinositides, localizes to the trans-Golgi network, alters KalSec14 interactions with cell membranes, and stimulates secretion.
Conclusion: Sec14 domain function is altered by the preceding phosphoinositide-binding amphipathic helix.
Significance: Kalirin function is expected to vary with promoter choice.
Keywords: cholesterol, Golgi, liposome, phosphoinositide, secretion, CRAL_TRIO, PI(4)P, trans-Golgi network
Abstract
Previous studies revealed an essential role for the lipid-binding Sec14 domain of kalirin (KalSec14), but its mechanism of action is not well understood. Because alternative promoter usage appends unique N-terminal peptides to the KalSec14 domain, we used biophysical, biochemical, and cell biological approaches to examine the two major products, bKalSec14 and cKalSec14. Promoter B encodes a charged, unstructured peptide, whereas promoter C encodes an amphipathic helix (Kal-C-helix). Both bKalSec14 and cKalSec14 interacted with lipids in PIP strip and liposome flotation assays, with significantly greater binding by cKalSec14 in both assays. Disruption of the hydrophobic face of the Kal-C-helix in cKalSec14KKED eliminated its increased liposome binding. Although cKalSec14 showed significantly reduced binding to liposomes lacking phosphatidylinositol phosphates or cholesterol, liposome binding by bKalSec14 and cKalSec14KKED was not affected. When expressed in AtT-20 cells, bKalSec14-GFP was diffusely localized, whereas cKalSec14-GFP localized to the trans-Golgi network and secretory granules. The amphipathic C-helix was sufficient for this localization. When AtT-20 cells were treated with a cell-permeant derivative of the Kal-C-helix (Kal-C-helix-Arg9), we observed increased secretion of a product stored in mature secretory granules, with no effect on basal secretion; a cell-permeant control peptide (Kal-C-helixKKED-Arg9) did not have this effect. Through its ability to control expression of a novel, phosphoinositide-binding amphipathic helix, Kalrn promoter usage is expected to affect function.
Introduction
Kalirin is a Rho family GDP/GTP exchange factor (GEF)2 with widespread cell signaling functions. Alternative splicing of the Kalrn gene gives rise to multiple isoforms that are developmentally regulated and tissue-specific. Kalirin7 (Kal7), found exclusively in the nervous system, is the major isoform in the adult brain and is essential for synaptic structure and function (1–6). Kal9 and Kal12 are expressed throughout development, both within and outside of the nervous system. Kal9/12 are crucial for normal neurite outgrowth, endocrine and bone homeostasis, and smooth muscle cell migration (7–10). Kalirin proteins have multiple functional domains, with major splice variants differing at their C termini. Kal7, -9, and -12 share identical N-terminal Sec14 domains, which are followed by nine spectrin repeats and tandem Dbl and pleckstrin homology domains (GEF1), activating Rac1 and RhoG (Fig. 1A). Only Kal7 terminates in a PDZ binding motif. Kal9 and Kal12 have a second GEF domain (GEF2), which activates RhoA, and Kal12 includes a putative kinase domain.
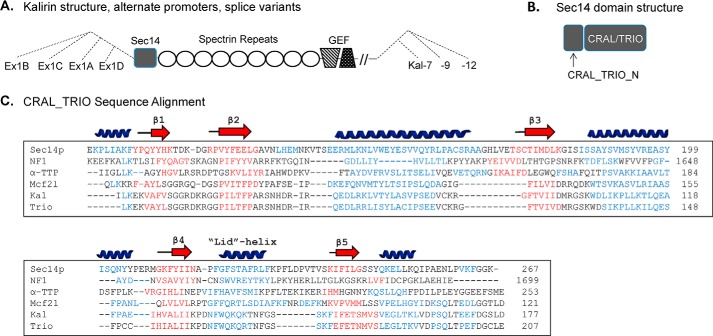
FIGURE 1.
Kalirin and the Sec14 superfamily. A, schematic illustrating use of four different Kalrn promoters, which encode alternate first exons (Ex1A, Ex1B, Ex1C, and Ex1D); the CRAL_TRIO domain begins in exon 2, which is common to transcripts initiated at each of the promoters. Alternative splicing generates transcripts encoding Kal7, Kal9, and Kal12, which each terminate with exons encoding unique 3′-untranslated regions. B, Sec14 superfamily members include a CRAL_TRIO domain, which typically binds hydrophobic ligands; some Sec14 superfamily members also include a CRAL_TRIO_N domain. C, multiple sequence/structural alignment for several CRAL_TRIO domains; residues known or predicted to form α-helices (blue) and β-sheets (red) are identified. Structures are known for yeast Sec14p (ID, 6323725; Protein Data Bank entry 1AUA; helices and β-strands shown above the alignment), neurofibromin 1 (NF1) (ID: 119600680; Protein Data Bank entry 3P7Z), and α-TTP (ID: 4507723; Protein Data Bank entry 1OIP). Secondary structures for the CRAL_TRIO regions of kalirin (ID: 295054252), Mcf2l (ID: 341940943; also known as Dbs and Ost), and Trio (ID: 257051075) were predicted using the Phyre2 modeling program. Dashes were inserted to optimize alignment; The GenBankTM position number of the final residue shown for each protein is given at the right.
In addition to its GEF domains, essential roles for the non-enzymatic domains of kalirin have been identified (11, 12). Of particular interest is the N-terminal Sec14 domain (Fig. 1, A and B). Yeast Sec14p, which catalyzes the exchange of phosphatidylcholine and phosphoinositide between cellular membranes, is required for normal post-Golgi trafficking and lipid metabolism (13–15). Based on sequence analysis, Sec14 domains have been identified in dozens of mammalian proteins as well as hundreds of proteins from other eukaryotic organisms. Sec14 domains bind to a diverse set of small lipid ligands, including phosphatidylcholine, phosphoinositides, squalene, tocopherols, and retinaldehyde. As such, members of the “Sec14 superfamily” have diverse biological functions, including roles in lipid transport, membrane trafficking, and cytoskeletal regulation (16). The Sec14 domain of α-tocopherol transfer protein (α-TTP) binds both phosphoinositides and α-tocopherol, with a single point mutation in its phosphoinositide binding cleft causing severe vitamin E deficiency and ataxia (17). Sec14 domain mutations are also associated with retinal degeneration and cancer (18, 19).
Although some Sec14 superfamily members consist almost entirely of the Sec14 domain, many are larger, multidomain GTPase regulatory proteins. The Sec14 domain of p50RhoGAP targets it to endosomes and mediates interaction with Rab11 (20). Disruption of the Sec14 domain in truncated isoforms of two other Rho GEFs (Dbl and Dbs/Ost) causes altered localization of the proteins and their effector, Cdc42 (21). The Sec14 domain of kalirin interacts with phosphoinositides but not with other more prevalent membrane lipids (11, 12). Furthermore, cultured hippocampal neurons expressing a Kal7 mutant lacking only its Sec14 domain form abnormally short dendritic spines (11).
Sequence homology among Sec14 superfamily members is typically low, but their structures are remarkably similar (17, 22, 23) (Fig. 1C). Alternating α-helix and β-sheet motifs create a lipid binding pocket with a hydrophobic core (CRAL_TRIO domain; named for cellular retinaldehyde-binding protein and Trio, a homologue of kalirin). Upstream of the lipid binding pocket, some Sec14 family members also have a four-helix subdomain, commonly referred to as a CRAL_TRIO_N domain (Fig. 1B). Recent phylogenetic analysis of the Sec14 superfamily places the Sec14-containing RhoGEFs in an intermediate position, between those that have the N-terminal domain and those that do not (24).
Alternative promoter usage generates isoforms of kalirin in which its CRAL_TRIO domain is preceded by four very different sequences encoded by unique initial exons: Ex1A, Ex1B, Ex1C, or Ex1D (Fig. 1A). The majority of Kalrn transcripts in the mouse brain contain either Ex1B or Ex1C, with very little Ex1A and Ex1D transcript detected (25). Although the peptides resulting from Ex1B and Ex1C are not identified as CRAL_TRIO_N domains, their conservation and unique features suggested that they could affect the function of the CRAL_TRIO domain. Ex1A, which encodes only four amino acids, would not be expected to form a functional domain. Here, we show that these N-terminal peptides affect Sec14 domain lipid binding, subcellular localization, and function.
Experimental Procedures
Protein Expression and Purification
KalSec14 variants were expressed and purified using the pGEX-6P vector system, as described previously (11, 26). Constructs were designed using the rat Kalrn sequence (U88157.1 numbering scheme; Ex1A) and verified by sequence analysis. GST fusion proteins were bound to a 5-ml GSTrap-4B column (GE Healthcare) and eluted by overnight cleavage with GST-HRV3C protease (GenWay Biotech, Inc., San Diego, CA). Purification was accomplished using a Q-Sepharose column (5 × 40 mm) equilibrated with 20 mm NaTES, pH 8.0, at a flow rate of 0.5 ml/min. Proteins were eluted over 180 min with a linear gradient to 500 mm NaCl in the same buffer. Proteins used for PIP strips included a rhodopsin tag at their C termini (27). For liposome assays, the rhodopsin tag was removed, and the GST fusion proteins were extended to include the first helix of spectrin repeat 1 (-EFP199) because this was found to improve protein solubility and stability.
Circular Dichroism
CD experiments were done as reported previously (26). CD spectra were recorded using a Jasco J715 spectropolarimeter (Jasco, Easton, MD) calibrated with d-(+)-10-camphor-sulfonic acid ammonium salt, with a thermostated cell housing and 1-mm path length cell at 20 ºC. Recombinant Sec14 proteins (6 μm) or synthetic peptides (20 μm) were prepared in buffer (20 mm NaTES, 150 mm NaCl, pH 7.0), and far UV CD spectra were recorded between 190 and 260 nm. An average of three runs were recorded for each protein sample. Synthetic peptides (Biomatik USA, LLC, Wilmington, DE; >90% purity) used in these studies included Kal-b (PPEGASEEGGAADSD), Kal-c (acetyl-TDRFWDQWYLWYLRLLRLLDRG-NH2), and Kal-cKKD (acetyl-TDRFKDQKYLWDLRLLRLLDRG-NH2). One tryptophan residue was left in the mutant peptide to enable tryptophan fluorescence measurement. Peptides were solubilized in 1 mm HCl and stored at −20 °C.
PIP Strips
PIP strips (Echelon Bioscience, P-6001) were used according to the manufacturer's instructions, as reported previously (11). Briefly, strips were blocked with 3% fatty acid-free BSA (Sigma-Aldrich, A6003) in Tris-buffered saline containing 0.1% Tween 20 (TTBS) for 1 h at room temperature and then incubated with 1 μg/ml of the indicated rhodopsin-tagged Sec14 protein for 1 h at room temperature. PIP strips were rinsed thoroughly, and protein binding was visualized using a mouse anti-rhodopsin monoclonal antibody and an HRP-conjugated anti-mouse secondary antibody. Signals were quantified using a GeneGnome digital imaging system and identical exposure times. Following background subtraction, densitometric data were normalized to the signal obtained for cSec14-rhodopsin binding to PI(4)P, consistently the darkest spot across groups.
Liposome Preparation and Flotation Assays
The following synthetic and natural lipids were purchased from Avanti Polar Lipids, Inc. (Alabaster, Alabama): DOPC (18:1; 850375), DOPE (18:1; 850725), DOPS (18:1; 840035), brain PI(4)P (840045), cholesterol (700000P), and LissRhod PE (18:1, emission 571 nm; 810150). The following lipids were purchased from Echelon Biosciences, Inc. (Salt Lake City, UT): phosphatidylinositol diC16 (PI diC16; P-0016); phosphatidylinositol 3-phosphate diC16 (PI(3)P diC16; P-3016); phosphatidylinositol 4-phosphate diC16 (PI(4)P diC16; P-4016); phosphatidylinositol 5-phosphate diC16 (PI(5)P diC16; P-5016); phosphatidylinositol 3,4-bisphosphate diC16 (PI(3,4)P2 diC16; P-3416); phosphatidylinositol 3,5-bisphosphate diC16 (PI(3,5)P2 diC16; P-3516); phosphatidylinositol 4,5-bisphosphate diC16 (PI(4,5)P2 diC16; P-4516); phosphatidylinositol 3,4,5-trisphosphate diC16 (PI(3,4,5)P3 diC16; P-3916). All powdered lipids were dissolved in chloroform or a mixture of chloroform, methanol, and water (20:9:1) per the manufacturer's instructions. Liposomes were prepared essentially as described by Avanti. To start, lipid stocks were mixed in the desired proportions, dried under nitrogen gas, and lyophilized overnight. Lipid mixtures were reconstituted to a final concentration of 1 mm in HN buffer (20 mm HEPES, 150 mm NaCl, pH 7.4). Mixtures underwent five freeze-thaw cycles with vigorous vortexing between each. Using a miniextruder, unilamellar liposomes were prepared by passing lipid mixtures 21 times through polycarbonate membranes of 100-nm pore size. Hydrated lipid mixtures were stored at −20 °C for up to 1 month; liposomes were freshly prepared for each experiment.
Liposome flotation assays were conducted on a density gradient in a manner similar to previous reports (28). Accudenz (Accurate Chemical and Scientific Corp., Westbury, NY) solutions (80 and 20%) were prepared in HN buffer. Unilamellar liposomes (50 μl) were combined with 0.5 μg of purified protein and incubated at room temperature for 30 min. The entire liposome/protein mixture was combined with 50 μl of 80% Accudenz at the bottom of an ultracentrifuge tube and carefully mixed to completion by pipetting. The resulting mixture was carefully overlayered with 250 μl of 20% Accudenz and then with 50 μl of HN buffer. Samples were centrifuged at 55,000 rpm at 4 °C for 30 min in a TL-100 ultracentrifuge using a Beckman TLS 55 swinging bucket rotor. Gradients were then divided into four 100-μl fractions, which were carefully collected from the bottom of the tube using gel loading micropipette tips. Each fraction was analyzed for liposome and protein content using spectrophotometry (546-nm excitation/571-nm emission) and Western blot analysis, respectively. At least 90% of the Lissamine Rhodamine PE marker was recovered in the top fraction.
For analysis of protein content, equal aliquots of each gradient fraction were denatured in 1× Laemmli sample buffer, fractionated on 4–15% acrylamide gels, and transferred to PVDF membranes. Western blot analysis was conducted using either a mouse GST antibody (for PI(4)P-Grip GST (Echelon Biosciences, Inc., G-0402)) or a rabbit KalSec14 antibody (CT302; see Ref. 7) and mouse or rabbit HRP-tagged secondary antibodies. SuperSignal West chemiluminescent substrate (Thermo Scientific) and a GeneGnome digital imaging system were used to visualize blots, with exposures adjusted to be in the linear range. After background subtraction, bands were quantified, and data were represented as a proportion of total protein recovered.
Tryptophan Fluorescence
The tryptophan blue shift assay was conducted by incubating 2 μm synthetic peptide (Kal-c or Kal-cKKD peptide) with 20 μm liposomes (diameter, 100 nm), either with or without 8% PIP (prepared as described above but without Lissamine Rhodamine PE) in HN buffer, at room temperature for 45 min. Control reactions were incubated without liposomes. Intrinsic fluorescence was measured using a F2500 spectrofluorimeter (Hitachi, Japan) with a thermostated cell holder and a 1-cm path length quartz cuvette. Slit widths with a nominal band pass of 10 nm were used for both excitation and emission beams. Fluorescence emission spectra were recorded from 300 to 400 nm after excitation at 280 nm; the blue shift graphs were constructed using averaged values from three continuous scans.
Cell Culture and Transfection
AtT-20 mouse corticotrope tumor cells were maintained at 37 °C in a 5% CO2 atmosphere in DMEM/F-12 cell culture medium containing 10% fetal calf serum (Hyclone), 10% NuSerum (Corning, Inc.), penicillin/streptomycin, and 25 mm HEPES. Transient transfections were performed in serum-free Opti-MEM medium using Lipofectamine 2000 (Invitrogen) at 2 μl/μg of DNA. To reveal differences in late/recycling endocytic trafficking, cells were treated with a low dose of nocodazole (10 μm) in growth medium for a short time (30 min) prior to fixation; this dose of nocodazole has been shown to disrupt microtubule-dependent endosomal trafficking without completely disrupting the TGN (29). New expression vectors included bKalSec14-GFP and cKalSec14-GFP (both end with -LDYNH162), Kal-C-helix-GFP (Ex1C appended to the N terminus of EGFP), and Kal-C-helixKKED-GFP (WWWY → KKED). PEAK-His.Myc-Kal7a (11) was used as a template to introduce a silent mutation (NruI site) at nucleotide 93 (GenBankTM AAT39517.1 numbering) using site-directed mutagenesis with the QuikChange kit (Stratagene). The Kal-a front without the HisMyc tag was created by PCR using this vector and a reverse primer including the NruI site. The Kal-b and Kal-c fronts were created using rat brain cDNA with sequence-specific forward primers and the common reverse primer introducing the NruI site. All three fronts were cut with BamHI and NruI and inserted into pEGFP-rKalSec14 (11) cut with BglII and NruI. The A206K substitution in EGFP-N2 was also produced using the QuikChange kit (30). All Kal-Sec14 sequences end with LRLSL172 fused to EGFP. Long synthetic oligonucleotides were hybridized to create the amphipathic wild type Kal-c front (MTDRFWDQWYLWYLRLLRLL) and the mutant Kal-cKKED front (MTDRFKDQKYLEDLRLLRLL) flanked by restriction sites for SacII and BamHI; these fragments were then inserted into pEGFP-N2(A206K). All constructs were verified by DNA sequencing.
Transferrin Uptake
A transferrin uptake assay was conducted using AtT-20 corticotropic tumor cells and Alexafluor-546-transferrin (Invitrogen) as reported previously (11, 12). Briefly, AtT-20 cells were plated on glass coverslips and transfected with vectors encoding EGFP or KalSec14-GFP variants. Twenty-four hours after transfection, cells were serum-starved for 30 min and then incubated with 0.25 mg/ml Alexafluor-546-transferrin in serum-free medium for 5 min at 37 °C/5% CO2. Cells were then quickly rinsed and immediately fixed with 4% paraformaldehyde in phosphate-buffered saline. Coverslips were stained using the GFP antibody, and nuclei were visualized using the Hoechst stain (Invitrogen).
Immunofluorescence, Imaging, and Analysis
Cell cultures were fixed in 4% paraformaldehyde, permeabilized with 0.075% Triton X-100, and blocked with 2 mg/ml BSA. Primary antibodies used for immunostaining included rat anti-GFP (1:1000; NacalaiTesque, Kyoto, Japan), rabbit-anti-TGN38 (1:1000; JH 1479 (31)), and rabbit-anti-ACTH (Kathy (31)). Hoechst nuclear stain (Invitrogen) and TRITC-phalloidin (Sigma) were used as indicated. Primary antibodies were visualized with appropriate secondary antibodies from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA). Images were obtained using a Zeiss Axiovert 200M microscope with ×63 oil objective and AxioVision software. Optical sectioning was achieved with the Zeiss ApoTome module. Images used for analysis were compressed Z-stack series through the entire cell, with identical settings within experiments. Image quantification was performed using Metamorph image analysis software. For transferrin uptake, individual cells were traced, and average 546-Tf (red) signal was determined. Intensity values were averaged within groups and then compared. For quantification of KalSec14-GFP localization, boxes were drawn around the indicated compartment, and average GFP intensity was compared with average cytosolic intensity.
Cell-permeant Peptides
To investigate the biological activity of the amphipathic Kal-C-helix, we designed a cell-permeant peptide in which the C-helix was followed by nine arginine residues (C-helix-Arg9; acetyl-TDRFWDQWYLWYLRLLRLLD-RRRRRRRRR) (32, 33). A control peptide in which four of the hydrophobic residues were replaced with charged residues (underlined) was also designed (C-helixKKED-Arg9; acetyl-TDRFKDQKYLEDLRLLRLLD-RRRRRRRRR). Synthetic peptides (Biomatik USA, LLC; >90% purity) were dissolved in DMSO, yielding stock concentrations of 2 mm.
Live Cell Imaging
AtT-20 cells were plated at low density on poly-l-lysine-coated glass bottom MatTek dishes and grown in serum-containing DMEM/F-12 for 2 days. At least 24 h before imaging, cells were transfected with the indicated plasmids as described above. Prior to imaging, cells were allowed to acclimate to imaging medium (DMEM/F-12 air without phenol red) at 37 °C for at least 20 min. While on a stage warmer held at 37 °C, cells were imaged using a Nikon Eclipse TE300 inverted epifluorescence microscope with a ×40/0.60 numerical aperture plan fluor air objective and associated NIS-Elements software. Live cell imaging was performed at a rate of 1 frame/20 s for 30–40 min. After at least 10 min of baseline imaging, a 10 μm working solution of either C-helix-Arg9 or control peptide in imaging medium was added to the cells on the stage warmer, producing a final concentration of 5 μm peptide in imaging medium. A final concentration of 10 μm peptide was also tested and gave similar results.
Secretion Experiments
Secretion experiments were conducted as reported previously (34). Briefly, AtT-20 cells were plated at high density in 12- or 6-well dishes and maintained for 2 days in growth medium. Prior to treatment, cells were equilibrated in an air incubator in complete serum-free medium lacking bicarbonate (CSFM-air); CSFM-air consists of DMEM/F-12 containing insulin/transferrin/selenium, 25 mm HEPES, pH 7.4, and 50 μg/ml bovine serum albumin. Cells were incubated in prewarmed CSFM-air at least twice for exactly 30 min at 37 °C; these samples of medium allowed us to assess “basal” secretion. After the final period of “basal” secretion, cells were incubated in CSFM-air medium containing either secretagogue (BaCl2, 0.5 mm), 5 μm cell-permeant peptide (either WT C-helix or mutant control), or both 5 μm cell-permeant peptide and 0.5 mm BaCl2. After 30 min, medium was collected (stimulated secretion), and cells were extracted into SDS lysis buffer (0.5% (w/v) SDS, 50 mm Tris·HCl, pH 8.0, 1 mm dithiothreitol, 2 mm EDTA, 50 mm NaF) and immediately heated at 95 ºC for 5 min. Protease inhibitors were added to all samples immediately following collection. Samples were separated using SDS-PAGE, and protein content was evaluated by Western blot analysis. A rabbit antibody to prohormone convertase 1 (JH 888 (35)) was used to evaluate secretion. Data are presented as -fold change over basal.
Results
Alternative Promoter Usage Creates Two Structurally Distinct KalSec14 Domains
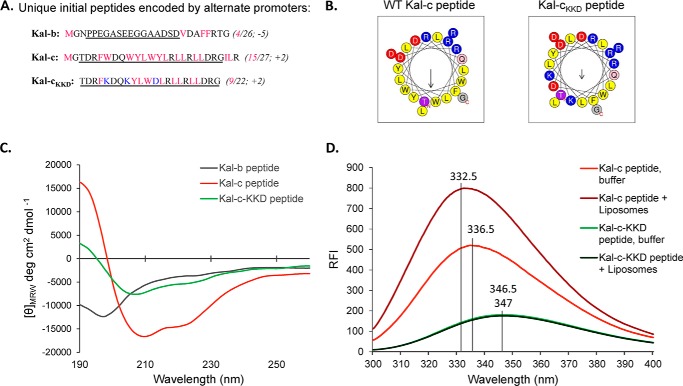
Full-length kalirin transcripts are initiated at one of four promoters, termed A, B, C, and D (Fig. 1A); transcripts generated from the different promoters encode 4–38 amino acids that precede exon 2. Proteins that extend from the N terminus to a specified point beyond the end of the CRAL_TRIO domain (which extends from the 16th residue of exon 2 (I20LK) to SLD159) are referred to as aKalSec14, bKalSec14, cKalSec14, or dKalSec14. In this study, we focused our attention on bKalSec14 and cKalSec14, the major isoforms in the adult brain (25); previous studies focused only on aKalSec14 (1). Although similar in length, the sequences and predicted structures of the initial peptides encoded by Ex1B and Ex1C are remarkably different (Fig. 2, A and B). Whereas the Kal-b peptide is negatively charged and predicted to be unstructured, the Kal-c peptide is highly hydrophobic (15 of 27 hydrophobic residues) and predicted to form an α-helix. In fact, a helical wheel projection predicts that the Kal-c peptide forms a nearly perfect amphipathic helix (Fig. 2B). Synthetic Kal-b and Kal-c peptides were used to confirm these structural predictions using circular dichroism. As expected, the Kal-b peptide was unstructured (i.e. a random coil), whereas the Kal-c peptide had spectral properties characteristic of a helical protein (high at 190 nm, minima at 208 nm and 222 nm; Fig. 2C).
FIGURE 2.
Kalirin Ex1C encodes an amphipathic helix. A, peptide sequences generated by transcripts initiated from rat promoters A, B, and C. Hydrophobic residues are shown in pink; number of hydrophobic residues/total number of residues and net charge predicted are shown in parenthesis. Underlining indicates the synthetic peptides (Kal-b, Kal-c, and Kal-cKKD) used for structural studies. In the Kal-cKKD peptide, residues shown in blue were mutated in order to maintain helicity but disrupt the amphipathic nature of the helix. B, helical wheel projections for the synthetic peptides were obtained using HeliQuest with an 18-amino acid analysis window; Kal-b was not predicted to form an α-helix (Quick2D, Max-Planck Institute for Developmental Biology Bioinformatics Toolkit). Yellow, hydrophobic residues; red, negatively charged; blue, positively charged. Arrows point to the hydrophobic face, with the length of the arrow indicative of the calculated hydrophobic moment. C, CD spectra for Kal-b, Kal-c, and Kal-cKKD peptides are shown. D, tryptophan fluorescence measurements for Kal-c and Kal-cKKD peptides with and without liposomes. As expected for an amphipathic helix, λmax for the Kal-c peptide showed a blue shift in the presence of liposomes (100-nm Golgi mix liposomes containing 8% PI(4)P) from 336.5 to 332.5 nm. The λmax for Kal-cKKD shifted by only 0.5 nm in the presence of liposomes, from 347 to 346.5 nm. RFI, relative fluorescence intensity.
Because the Kal-c peptide includes three tryptophan residues, we took advantage of the characteristic spectral shift in tryptophan fluorescence emission that occurs as a function of solvent polarity to assess its interaction with membranes (Fig. 2D). A significant change in both fluorescence intensity and emission maximum was observed upon mixing Kal-c peptide with unilamellar liposomes. A mutated version of the Kal-c peptide (Kal-cKKD) was designed to disrupt its amphipathic nature by replacing three hydrophobic residues with three polar residues (WWY → KKD; Fig. 2B). Kal-cKKD, which is predicted to adopt a helical structure with greatly reduced amphipathic moment, exhibited the features expected of a helical structure when examined by circular dichroism (Fig. 2C). However, Kal-cKKD failed to show any changes in either fluorescence intensity or emission maximum following the addition of unilamellar liposomes (Fig. 2D). Similar constructs were used as controls in later experiments. Given the unique features of the Kal-b and Kal-c peptides, which immediately precede the lipid-binding CRAL_TRIO domain of kalirin (Fig. 1), we decided to investigate functional differences between bKalSec14 and cKalSec14.
Promoter Usage Alters Lipid Interactions by KalSec14 Variants
Because exogenous aKalSec14-GFP decreased the ability of a well characterized neuroendocrine cell line (AtT-20s) to internalize fluorescently tagged transferrin (11), we first asked whether bKalSec14-GFP and cKalSec14-GFP had a similar effect (Fig. 3A). When expressed at similar levels, bKalSec14-GFP and cKalSec14-GFP inhibited the uptake of fluorescently tagged transferrin to a similar extent; the magnitude of this effect was similar to that observed when aKalSec14-GFP was expressed (11).
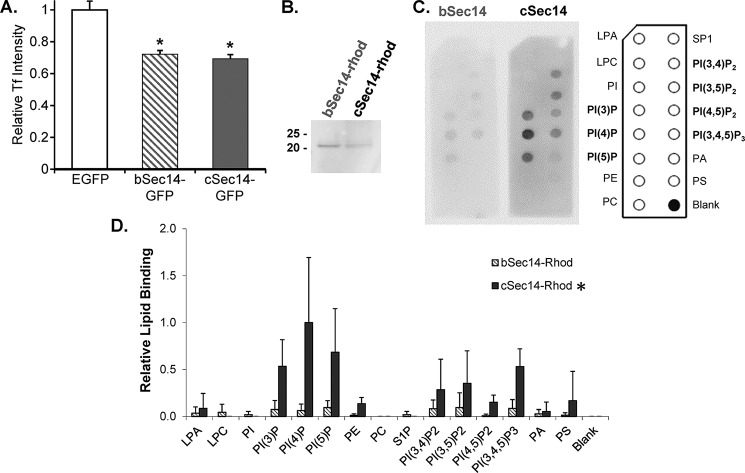
FIGURE 3.
The bSec14 and cSec14 domains of kalirin both inhibit transferrin uptake but exhibit differential binding to PIP strips. A, AtT-20 cells transiently expressing EGFP, bSec14-GFP, or cSec14-GFP were rinsed in serum-free medium, exposed to fluorescently tagged transferrin for 5 min, and then rinsed and fixed. The intensity of the transferrin signal in cells expressing EGFP, bSec14-GFP, or cSec14-GFP was quantified (n = 25, 51, and 49 cells, respectively). Two-way ANOVA statistical analysis was used; *, p < 0.005 versus EGFP. B, Coomassie-stained membrane showing purified bSec14-rhod and cSec14-rhod. C, the binding of bSec14-rhod and cSec14-rhod to PIP strips was quantified using an antibody to the epitope tag. The lipids on the PIP strips are identified in the schematic to the right. D, group data from 2–4 experiments calculated as spot intensity relative to cSec14-rhod binding to PI(4)P, the preferred interactor; error bars, S.D. cSec14 binding to PIPs was significantly higher than bSec14 binding; *, p < 0.02 by two-way ANOVA.
Like many other Sec14 family members, aKalSec14, binds to PIPs (11). Here, we used PIP strips (Echelon) to evaluate the lipid binding capabilities of bKalSec14 and cKalSec14. Rhodopsin-tagged bKalSec14 and cKalSec14 were expressed and purified, as described previously for aKalSec14 (11) (Fig. 3B). Whereas bKalSec14-rhod showed some binding to phosphoinositides, we saw significantly more binding by cKalSec14-rhod to almost all PIPs (Fig. 3, C and D). Neither protein interacted appreciably with the other lipids tested. We found previously that aKalSec14-rhod binds specifically to phosphoinositides, with the most binding to PI(3,4,5)P3 (11). Interestingly, cKalSec14-rhod showed the most binding to PI(4)P, suggesting that presence of the Kal-C-helix contributed to both the affinity and specificity of KalSec14-lipid interactions.
cKalSec14-GFP Localizes to the TGN in Neuroendocrine Cells
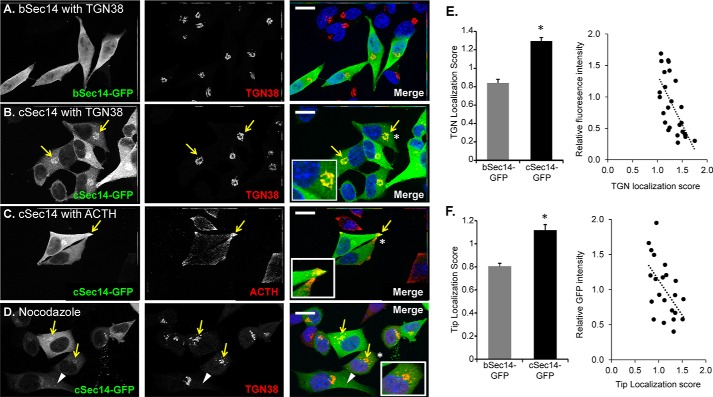
The phosphoinositides are a critically important class of cell signaling lipids which are known to have somewhat discrete subcellular localizations (for a review, see Refs. 36 and 37). Because phosphoinositide binding often contributes to effector protein localization to specific subcellular compartments, we wondered whether the alternate initial peptides influenced protein localization. To test this, we expressed KalSec14-GFP fusion proteins in AtT-20 cells. Whereas bKalSec14-GFP was diffusely distributed in these cells (Fig. 4A), cKalSec14-GFP localized to the perinuclear region, where it co-localized extensively with staining for TGN38, a marker for the trans-Golgi network (Fig. 4B). We also observed significant cKalSec14-GFP localization at the tips of processes, where mature secretory granules accumulate; secretory granules were identified by staining with an antibody to adrenocorticotropic hormone (ACTH), which is synthesized and stored in these cells.
FIGURE 4.
Differential localization of bSec14- and cSec14-GFP in AtT-20 cells. Immunostaining of AtT-20 cells transiently transfected with vectors encoding bSec14-GFP or cSec14-GFP revealed that bSec14-GFP was diffusely localized (A), whereas cSec14-GFP co-localized with the TGN marker, TGN38 (B; yellow arrows) and with ACTH (C; yellow arrows), a marker for the mature secretory granules that accumulate at the tips of cellular processes. Insets, higher magnification of areas marked by white asterisks. D, cells were treated with a low dose of nocodazole (10 μm) for a short time (30 min) to disrupt the microtubule-dependent trafficking of late/recycling endosomes into the perinuclear region (38). Much of the cSec14-GFP continued to co-localize with TGN38 (yellow arrows), but some cSec14-GFP-positive vesicular structures that lacked TGN38 could be identified (white arrowheads). The merged images show GFP in green, and TGN38 (A, B, and D) or ACTH (C) in red, and Hoechst in blue. Scale bar, 20 μm. E and F, quantification of Sec14-GFP localization. Localization scores were calculated by taking the ratio of GFP signal intensity in the indicated area (TGN or tips) over cytosolic signal intensity. For TGN localization, n = 18 and 25 cells for bSec14- and cSec14-GFP, respectively. For localization to tips, n = 26 and 22 cells for bSec14- and cSec14-GFP, respectively. For both parameters, localization scores for cSec14-GFP were inversely related to relative GFP intensity; least squares best fit lines are shown. Both localization scores (TGN and tip) differ for cSec14-GFP and bSec14-GFP; p < 0.001 (Student's t test). Error bars, S.E.
GFP signal intensity in the TGN region (marked by TGN38) or at the tips of processes was quantified and compared with cytosolic signal intensity (Fig. 4, E and F), demonstrating enrichment of cKalSec14-GFP at both sites. In cells expressing higher levels of cKalSec14-GFP, diffuse staining throughout the cytosol became more pronounced; a scatterplot relating expression level (relative GFP intensity) to TGN or tip localization score confirmed this relationship, suggesting the presence of a limited number of binding sites for cKalSec14-GFP at both locations.
The perinuclear region of AtT-20 cells that contains the Golgi and trans-Golgi network is also enriched in late endosomes (38). To better distinguish the TGN from late endosomes, we treated cKalSec14-GFP-expressing cells for a short time with a low concentration of nocodazole to destabilize microtubules and disrupt microtubule-dependent endosomal trafficking; as expected, TGN38 staining, although more fragmented than in control cells, remained concentrated in the perinuclear region (Fig. 4D). We found that most of the cKalSec14-GFP signal was still associated with TGN38-containing compartments (Fig. 4D, yellow arrows), indicating that it was largely localized to the TGN. Although a few cKalSec14-GFP-positive puncta were visible throughout the cell after nocodazole treatment (white arrowhead), our data indicate that only a small fraction of the cKalSec14-GFP was associated with endosomes.
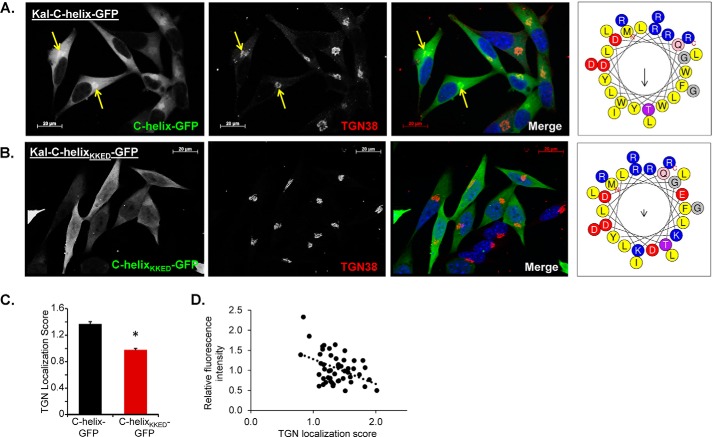
The Kal-C-Helix Alone Localizes to the TGN
We next wondered whether the amphipathic Kal-C-helix was sufficient to localize GFP to the TGN area. To test this, we transfected AtT-20 cells with expression vectors encoding GFP fusion proteins in which the wild type or mutant Kal-C-helix preceded GFP (Kal-C-helix-GFP and Kal-C-helixKKED-GFP). Remarkably, we found that Kal-C-helix-GFP localized to the TGN region in AtT-20 cells (Fig. 5A). Furthermore, disruption of the amphipathic nature of the helix (as in Fig. 2) was sufficient to prevent this localization because Kal-C-helixKKED-GFP was diffusely distributed throughout the cytosol (Fig. 5B). Quantification of GFP intensity in the TGN area (as defined by TGN38 staining) versus the cytosol confirmed the difference between Kal-C-helix-GFP and Kal-C-helixKKED-GFP (Fig. 5C); as seen with the intact KalSec14-GFP proteins, the TGN localization score for Kal-C-helix-GFP declined as its level of expression rose (Fig. 5D), suggesting saturation of a limited number of binding sites.
FIGURE 5.
The amphipathic C-helix of kalirin is sufficient to localize GFP to the TGN area in AtT-20 cells. Immunostaining of AtT-20 cells transiently transfected with either GFP fused to the C-helix of kalirin (A) or with GFP fused to Kal-C-helixKKED (B); mutations were introduced to maintain helicity but eliminate the amphipathic nature of the helix. Helical wheel projection diagrams (HeliQuest) for the two peptides are shown to the right. The merged images show GFP in green, TGN38 in red, and Hoechst in blue. Scale bar, 20 μm. The TGN localization score, calculated as described in the legend to Fig. 4, was higher in cells expressing Kal-C-helix-GFP than in cells expressing Kal-C-helixKKED-GFP (p < 0.001, Student's t test) (C) and was negatively correlated with the GFP expression level (D). Error bars, S.E.
The Amphipathic Kal-C-Helix Is Essential for cKalSec14 Lipid Interactions
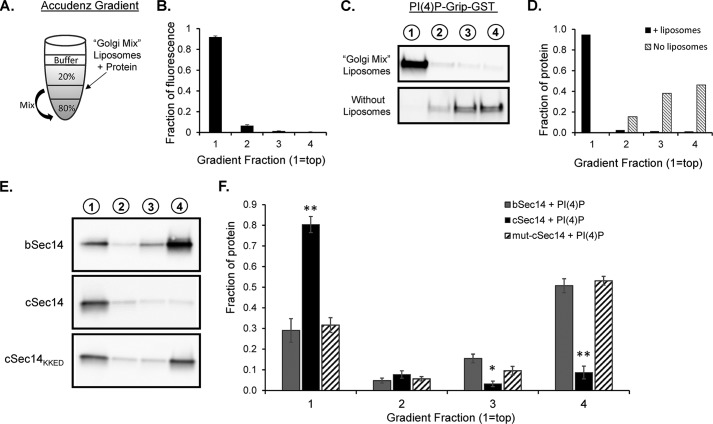
To better evaluate the role of the Kal-C-helix in KalSec14-mediated interactions with membranes, we utilized a liposome binding assay. Based on the localization of cSec14-GFP to the TGN area, synthetic and natural lipids were combined in proportions that mimicked the membranes of the TGN (28, 39). The resulting “Golgi mix” consisted of 52% DOPC, 20% DOPE, 5% DOPS, 15% cholesterol, 8% PI(4)P, and a trace amount of fluorescent lipid. Lipids were dried, reconstituted, and extruded through a 100-nm pore diameter membrane. Unilamellar liposomes were incubated for 30 min with purified KalSec14; KalSec14 bound to liposomes was separated from free KalSec14 based on its decreased density using an Accudenz gradient (Fig. 6A). After centrifugation, liposomes and any protein bound to them were recovered from the top of the gradient (“fraction 1”). Using this approach, over 90% of the fluorescently tagged unilamellar liposomes were recovered in the top fraction, as assayed by spectrophotometry for the fluorescent lipid (Fig. 6B). When liposomes were combined with PI(4)P-Grip-GST (Echelon, positive control protein), we found over 90% of the protein in the top fraction (Fig. 6, C and D). When PI(4)P-Grip-GST was loaded onto an Accudenz gradient without liposomes, no protein was detectable in the top tube (Fig. 6, C and D).
FIGURE 6.
Liposome binding assays reveal an important role for the amphipathic C-helix in KalSec14 membrane interactions. A, schematic illustrating Accudenz gradients used for the liposome flotation assays conducted in Figs. 6 and 7. The indicated liposome/protein mix (50 μl) was combined with 50 μl of 80% Accudenz and then overlayered with 250 μl of 20% Accudenz and 50 μl of buffer. Gradients were centrifuged in a swinging bucket rotor for 30 min, and 100-μl fractions were removed and analyzed for lipid and protein content. B, relative lipid fluorescence in gradient fractions; spectrophotometry data from 21 separate gradients were averaged, showing that >90% of the lipid was found in the top fraction of the gradient after centrifugation. C, purified PI(4)P-Grip-GST (positive control) was analyzed on gradients in the presence (top) or absence (bottom) of Golgi mix liposomes; gradient fractions are indicated above each lane. Protein content, detected using a GST antibody, was quantified as shown in D. E, representative blots from liposome assays with KalSec14 variants. cSec14 bound significantly more to Golgi mix liposomes than bSec14, and this difference was eliminated when the C-helix was mutated to disrupt its amphipathic nature; group data are shown in F. Error bars, S.E.; n = 4–6 experiments/protein. Asterisks indicate significant difference from bSec14 + PI(4)P liposomes by two-way ANOVA; **, p < 0.001; *, p < 0.02.
The liposome binding abilities of purified bKalSec14, cKalSec14, and cKalSec14KKED (helix mutant) were compared (Fig. 6E). All three proteins showed significant binding to liposomes (Fig. 6, E and F). Consistent with the PIP strip assays, we found significantly more cKalSec14 than bKalSec14 in the liposome fraction (Fig. 6, E and F). This difference could be due to conformational differences of the putative lipid binding pocket due to the inclusion of different N-terminal peptides (Figs. 1 and 2). We found that disruption of the Kal-C-helix was sufficient to abolish the difference in liposome binding between bKalSec14 and cKalSec14 (Fig. 6, E and F), so that cKalSec14KKED behaved like bKalSec14. These data indicated that the N-terminal amphipathic helix in cSec14 enhanced the ability of this domain to interact with membranes and that this increase was probably due to interactions mediated by the amphipathic helix itself.
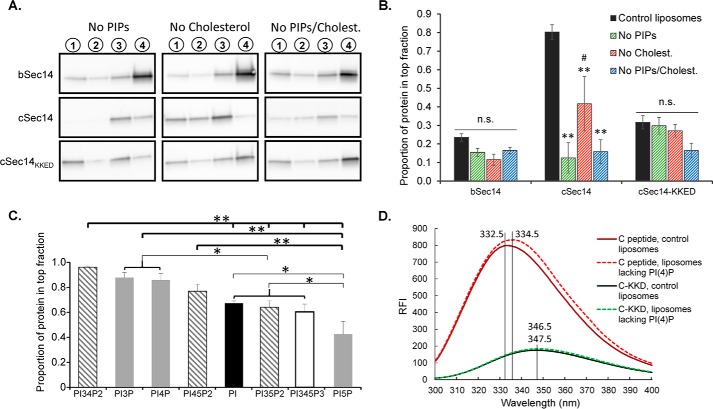
Membrane Interactions by cKalSec14 Require Phosphoinositides and Cholesterol
Because our PIP strip data indicated that KalSec14 bound specifically to phosphoinositides, we wanted to determine whether PIP content affected the KalSec14-liposome interaction. When flotation assays were conducted with liposomes lacking PIPs (Fig. 7A), we found that cKalSec14 binding was reduced by 85%, completely eliminating the difference between bKalSec14 and cKalSec14 (Fig. 7B, green hatched bars). This suggested that cKalSec14 membrane interactions were sensitive to PIP content. Although bKalSec14 binding appeared slightly reduced in the absence of PIPs, data from six separate experiments did not reach statistical significance. Because several Sec14 proteins bind lipids other than phospholipids and Golgi/TGN membranes are enriched in cholesterol (40), we wondered whether the interaction of KalSec14 with liposomes was sensitive to cholesterol content. cKalSec14 binding was significantly reduced when cholesterol was excluded from the liposomes (Fig. 7B, red hatched bars) although to a lesser extent than was seen in the absence of PIPs. bKalSec14 binding was not significantly affected by cholesterol content. Interestingly, removing both PI(4)P and cholesterol from liposomes was not sufficient to abolish completely the interaction of either protein with liposomes (Fig. 7B, blue hatched bars), suggesting that other lipids also contribute to the KalSec14-membrane interaction.
FIGURE 7.
cSec14/liposome interactions require phosphoinositides and cholesterol. A, representative blots from flotation assays using liposomes of different lipid content. B, group data showing protein content of the top fraction only for each condition. Values are relative to total protein recovery from each gradient. Data for control Golgi mix liposomes (black bars) are the same data shown in Fig. 6F, fraction 1. Error bars, S.E.; n = 5–6 experiments for “no PIP” and “no cholesterol” conditions, n = 3 experiments for “no PIP/cholesterol” condition. All statistics are by two-way ANOVA; **, p < 0.001 versus control (black bars); #, p < 0.02 versus no PIP condition (green hatched bars); n.s., not statistically significant. C, results from flotation assays using liposomes prepared with using 8% of the indicated phosphoinositide with 52% DOPC, 20% DOPE, 5% DOPS, 15% cholesterol. The bar graph shows the proportion of total cSec14 protein recovered in the top fraction. Assay conditions were as in Fig. 6. D, incubation of Kal-c peptide with Golgi mix liposomes (with PI(4)P) versus liposomes lacking PI(4)P resulted in a shift of λmax from 332.5 to 336.5 nm. The λmax for the Kal-cKKD peptide shifted slightly (from 346.5 to 347.5 nm) when PI(4)P was eliminated from liposomes; both values were within 0.5 nm of the buffer-only condition (347.0 nm) in Fig. 2D, and all were considerably red-shifted when compared with λmax values for the wild type Kal-c peptide.
To determine the role of the amphipathic C-helix in liposome interactions, we conducted the same experiments with the Kal-C-helix mutant protein, cKalSec14KKED. Remarkably, this protein did not display the same sensitivity to lipid content that was seen with cKalSec14; neither the PI(4)P nor cholesterol content of the liposomes affected cKalSec14KKED binding to liposomes (Fig. 7B).
Our PIP strip experiments suggested that cKalSec14 interacted with multiple phosphoinositides (Fig. 3). To further examine the sensitivity of cSec14-mediated lipid binding, we evaluated the ability of cKalSec14 to interact with liposomes containing different phosphoinositides by replacing the PI(4)P in the Golgi mix liposomes with PI or with one of the other phosphoinositides (Fig. 7C). Although cKalSec14 bound to all phosphoinositide-containing liposomes, we observed significant differences in the level of binding with the different phosphoinositides. Maximal binding of cKalSec14 was observed with PI(3,4)P2, PI(3)P, and PI(4)P, which did not differ significantly from each other. Minimal binding of cKalSec14 was observed to liposomes containing PI(5)P, which bound significantly less cKalSec14 than any of the other phosphoinositides. Notably, the phosphoinositide binding specificity of cKalSec14 observed using liposomes differed dramatically from that observed using PIP strips (Fig. 3D); when assayed using liposomes, cKalSec14 interacted maximally with PI(3,4)P2, which promoted little binding of cKalSec14 to PIP strips. Together, our liposome data demonstrate that the amphipathic Kal-C-helix enhanced KalSec14-mediated membrane interactions in a phosphoinositide-specific manner.
We returned to tryptophan fluorescence to determine whether the Kal-c peptide interacted directly with the different phosphoinositides (Fig. 7D and Table 1). Data for both peptides incubated with liposomes containing PI(4)P and liposomes lacking any PIPs are shown in Fig. 7D. Liposomes of different composition were incubated with the wild type Kal-c peptide or with the mutant Kal-cKKD peptide (Table 1). Blue shifts of similar magnitude were observed when the Kal-c peptide was incubated with liposomes containing PI or any of the PIPs. No blue shift was observed when the mutant Kal-cKKD peptide was incubated with any of the liposomes. These data led us to conclude that the amphipathic helix encoded by Ex1C interacted with PI and with each of the PIPs. When appended to the CRAL_TRIO domain of kalirin, this amphipathic helix creates a bipartite lipid-binding domain with some phosphoinositide specificity.
TABLE 1.
Effect of liposome composition on Kal-c peptide Trp fluorescence
Synthetic peptides (2 μm Kal-c or Kal-cKKD peptide) were incubated with liposomes (20 μm) containing PI or the indicated PIP as described under “Experimental Procedures.” The control sample (None) contained no liposomes. Spectra were recorded from 300 to 400 nm after excitation at 280 nm; data shown were averaged from three scans ± S.D. Blue shift gives difference between λmax with liposomes of a given composition versus no liposomes. ND, not done.
| PI or PIP | Kal-c peptide λmax | Blue shift | Kal-cKKD peptide λmax | Blue shift |
|---|---|---|---|---|
| nm | nm | nm | nm | |
| None | 336.5 ± 0.5 | 345 ± 0.5 | ||
| PI | 332 ± 0.5 | 4.5 | ND | ND |
| PI(3)P | 333 | 3.5 | 346.5 | −1.5 |
| PI(4)P | 332.5 ± 0.5 | 4 | 346 ± 0.5 | −1 |
| PI(5)P | 333 ± 0.5 | 3.5 | 345.5 ± 0.5 | −0.5 |
| PI(3,4)P2 | 334 ± 0.5 | 2.5 | 346 | −1 |
| PI(3,5)P2 | 333 ± 0.5 | 3.5 | 346.5 ± 0.5 | −1.5 |
| PI(4,5)P2 | 333 ± 0.5 | 3.5 | 346 ± 0.5 | −1 |
| PI(3,4,5)P3 | 333 ± 0.5 | 3.5 | 346.5 ± 0.5 | −1.5 |
The Kal-c Peptide Promotes Secretion in AtT-20 Cells
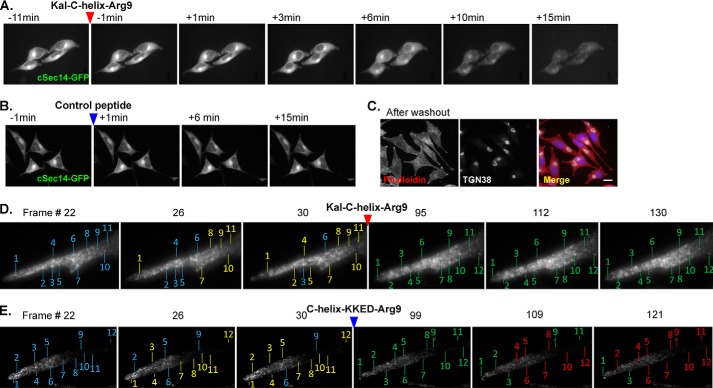
The trans-Golgi network serves as the cellular hub for protein sorting and transport as well as for secretory granule assembly and secretion. These processes are controlled by a myriad of protein and lipid interactions, many of which are mediated by phosphoinositides. TGN-enriched PI(4)P, for example, aids in recruiting effector proteins necessary for Golgi vesicle budding (41). Because Kal-C-helix-GFP localized to the TGN, whereas Kal-C-HelixKKED-GFP did not, we hypothesized that the presence of the Kal-c peptide might affect TGN-mediated trafficking and secretion. To test this, we utilized AtT-20 cells, a well studied neuroendocrine cell line, and a cell-permeant version of the amphipathic Kal-C-helix (Kal-C-helix-Arg9); in this way, we could evaluate the acute effects of the C-helix on secretion.
In order to verify that the peptide was able to penetrate cell membranes, we used time lapse imaging. cSec14-GFP was expressed transiently in AtT-20 cells; as expected, it localized to the TGN area. We reasoned that the presence of Kal-C-helix-Arg9 peptide would displace cSec14-GFP from its binding sites in the TGN. cSec14-GFP localization was disrupted within 6 min of introducing 5 μm Kal-C-helix-Arg9 peptide (Fig. 8A). There was no effect on cSec14-GFP localization following treatment with a control cell-permeant peptide (mutant R7-Kal7CT (42)) (Fig. 8B). Continued treatment with Kal-C-helix-Arg9 peptide resulted in a marked reduction in total fluorescence intensity, which was not observed in the control condition and thus not due to photobleaching. Importantly, Kal-C-helix-Arg9 peptide was not toxic; cells appeared morphologically normal and continued to divide after washout of the peptide (Fig. 8C).
FIGURE 8.
Cell-permeant Kal-c peptide. A, time lapse imaging of cSec14-GFP-expressing AtT-20 cells before and after treatment with Kal-C-helix-Arg9 peptide (red arrow). cSec14-GFP localization was disrupted, and fluorescence intensity declined over time. B, treatment with inactive control peptide (blue arrow) had no effect on cSec14-GFP localization or fluorescence intensity, indicating that the loss of signal intensity in A was not due to bleaching. C, AtT-20 cells treated with 5 μm Kal-C-helix-Arg9 peptide for 30 min were rinsed and returned to normal growth medium. Cells were fixed 72 h later and stained for filamentous actin (phalloidin, red) and TGN38 (white); nuclei were visualized using the Hoechst stain (blue). After washout, treated cells were indistinguishable from control cells, with the usual number of dividing cells (not shown). Scale bar, 20 μm. AtT-20 cells stably expressing PHM-GFP, which traverses the secretory pathway and is stored in secretory granules (30), were imaged every 20 s for 10 min before and 30 min after the addition of 5 μm Kal-C-helix-Arg9 peptide (D) or 5 μm Kal-C-helixKKED-Arg9 peptide (E). Selected high magnification frames of processes where vesicles accumulate are shown. In the first image of each series, blue lines indicate individual numbered fluorescent puncta; yellow lines in the second and third images indicate that the numbered puncta have moved, whereas blue lines show stationary puncta. After peptide addition, numbered puncta are first identified by green lines; those that moved are identified by red lines in subsequent images. Following the addition of the Kal-C-helix-Arg9 peptide, puncta stopped moving (D).
In order to assess the effects of introducing a PIP-binding cell-permeant peptide, we imaged the response of AtT-20 cells with GFP-tagged secretory granules (30) to the introduction of Kal-C-helix-Arg9 peptide or Kal-C-helixKKED-Arg9 peptide (Fig. 8, D and E). Before the addition of peptide, GFP-positive structures moved rapidly; within a few min of introducing the Kal-C-helix-Arg9 peptide, the GFP-positive structures stopped moving; introduction of the Kal-C-helixKKED-Arg9 peptide did not have the same effect.
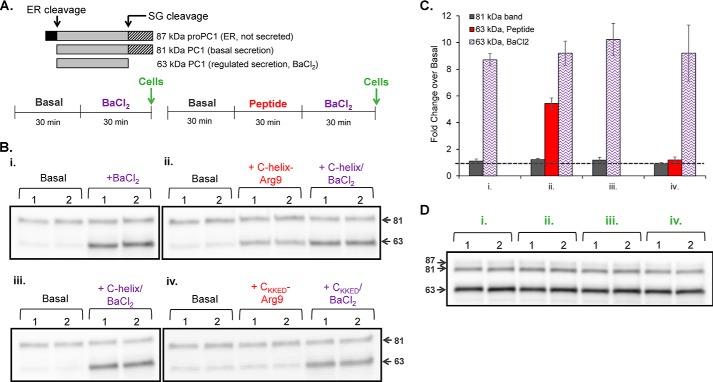
AtT-20 cells have been used extensively to study secretory pathway function, making them an excellent model system for evaluating the effects of the Kal-C-helix-Arg9 peptide on cell function. Prohormone convertase 1 (PC1) produces ACTH from proopiomelanocortin, and product peptides like ACTH are stored in secretory granules for release in response to secretagogue stimulation (35). Products that are not efficiently stored in granules are secreted basally; the products of pro-PC1 cleavage provide a means of monitoring both basal and stimulated secretion (Fig. 9A); the 81-kDa product of autoproteolytic pro-PC1 cleavage is secreted basally, whereas its more active C-terminally truncated product (63-kDa PC1) is stored in granules and released only in response to secretagogues like BaCl2.
FIGURE 9.
Kal-C-helix-Arg9 stimulates secretion by AtT-20 cells. A, secretion of PC1 cleavage products (top) was monitored to measure basal and stimulated secretion by AtT-20 cells following 30 min of treatment with Kal-C-helix-Arg9 peptide, Kal-C-helixKKED-Arg9 control peptide, and/or BaCl2, as indicated. The 81-kDa form of PC1 is secreted basally, whereas the 63-kDa form is stored in mature secretory granules and released in response to secretagogues like BaCl2 (61). After two 30-min washes to allow acclimation to serum-free medium, basal secretion was evaluated for 30 min. In duplicate, AtT-20 cells were then treated with one of four paradigms: 0.5 mm BaCl2 alone (Bi); 5 μm Kal-C-helix-Arg9 alone, followed by Kal-C-helix-Arg9 with 0.5 mm BaCl2 (Bii); 5 μm Kal-C-helix-Arg9 and 0.5 mm BaCl2 (Biii); or 5 μm Kal-C-helixKKED-Arg9 alone, followed by Kal-C-helixKKED-Arg9 and 0.5 mm BaCl2 (Biv). B, medium samples were separated by SDS-PAGE and evaluated for PC1 protein content. The addition of the Kal-C-helix-Arg9 peptide, but not the control peptide, stimulated secretion of 63-kDa PC1; secretion of 81-kDa PC1 was not affected by either peptide or by BaCl2. C, group data showing medium levels of 81- and 63-kDa PC1 as -fold change over basal (dashed line) under the four paradigms; x axis labels correspond to conditions i, ii, iii, and iv as illustrated in A and B. n = 4–6 from three separate experiments. D, Western blot showing PC1 content in cell lysates harvested after the indicated treatment paradigm. Error bars, S.E.
Basal and stimulated secretion were monitored by quantifying release of 81- and 63-kDa PC1. Duplicate wells of AtT-20 cells were first equilibrated in CSFM; sequential 30-min collection periods allowed us to assess basal and stimulated secretion and the effect of the cell-permeant peptides on each. Cell-permeant peptide (5 μm C-helix-Arg9 or C-helixKKED-Arg9), BaCl2 (0.5 mm), or both cell-permeant peptide and BaCl2 were added as indicated, and cell extracts were prepared after the final collection (Fig. 9B, top). Compared with basal levels, secretion of 63-kDa PC1 increased more than 5-fold in response to Kal-C-helix-Arg9 peptide (Fig. 9, B (ii) and C). In comparison, BaCl2 increased 63-kDa PC1 secretion 8.5-fold (Fig. 9, B (i) and C). When Kal-C-helix-Arg9-treated cells were subsequently stimulated with BaCl2, secretion was further stimulated (Fig. 9, B (ii) and C). When cells were treated with both Kal-C-helix-Arg9 peptide and BaCl2, secretion levels were comparable with those achieved with BaCl2 alone (Fig. 9, B (iii) and C). Incubation with Kal-C-helixKKED-Arg9 control peptide had no effect on secretion (Fig. 9, B (iv) and C), signifying a specific effect of Kal-C-helix-Arg9 peptide. Importantly, secretion of 81-kDa PC1, which is not stored in secretory granules, was not affected by BaCl2 or by either Kal-C-helix peptide (Fig. 9, B and C). AtT-20 cells are “professional” secretory cells, and a 30-min exposure to secretagogue releases only a small proportion of the total cell content. As such, we did not observe significant differences in PC1 cell content between conditions (Fig. 9D), further demonstrating that Kal-C-helix-Arg9 peptide treatment did not cause a generalized release of cell content.
Discussion
As important mediators of cytoskeletal regulation, the Rho family GEFs have major roles in cell motility, morphology, and polarity (for a review, see Refs. 43 and 44). Often large, multidomain proteins, it is likely that many of these proteins are highly specialized and capable of integrating different cellular signaling events. Furthermore, the sheer number of GTPase regulatory proteins has made it clear that normal cell signaling relies on their tight spatial and temporal control. In the present study, we identify the Sec14 domain of kalirin as a lipid binding module that may facilitate the integration of cytoskeletal and secretory pathway function. We show that the manner in which it does so is dictated by promoter usage, which determines the sequence of the short peptide immediately preceding the Sec14 domain.
Kalrn Ex1C Generates an Amphipathic Helix That Binds Phosphoinositides
The existence of multiple Kalrn promoters was reported previously (25), but no functional significance was attributed to the short peptides encoded by Ex1A, -1B, -1C, and -1D. Each promoter contributes an initial protein-coding exon that precedes the lipid-binding CRAL_TRIO domain of kalirin. Given that a CRAL_TRIO_N domain precedes the CRAL_TRIO domain in many Sec14 superfamily members (24), we explored the hypothesis that Kalrn promoter usage is functionally significant. Whereas Ex1B generates a negatively charged, unstructured peptide, Ex1C generates a positively charged amphipathic helix. A peptide similar to the Ex1B peptide is found in all vertebrates; although its sequence is not perfectly conserved, its length and hydrophilic nature are conserved. A peptide similar to the Ex1C peptide is found only in mammals and is perfectly conserved in over two dozen species. Notable features include the prevalence of bulky residues (3 Trp, 2 Tyr, and 1 Phe) along its hydrophobic face and spatially segregated stretches of positively (4 Arg) and negatively (3 Asp) charged residues along the opposing face.
Membrane binding amphipathic helices are used in many ways, generally adopting an orientation parallel to the plane of the membrane. Although many amphipathic helices form only in the presence of a lipid bilayer, the Kal-c peptide adopted a helical conformation in aqueous solution. Strikingly, the Kal-C-helix alone, without the CRAL_TRIO region of the Sec14 domain, localized GFP to the TGN area. The helical conformation was conserved when two of the Trp residues and one of the Tyr residues on the hydrophobic face were replaced by charged residues; these changes, which preserved the charged face of the helix, eliminated the ability of the peptide to localize a GFP fusion protein to membranes in cells.
An amphipathic helix buried within the core of Arf-GDP is exposed as a result of the conformational change that occurs when GTP replaces GDP, making the helix available to interact with membrane phospholipids (45). Amphipathic helices can serve as membrane curvature sensors; whereas BAR domains can induce and stabilize membrane curvature, adjacent amphipathic helices are thought to play a role in curvature sensation (46, 47). Amphipathic lipid packing sensor motifs, which are generally unfolded until they interact with membranes, have a hydrophobic face rich in bulky residues, with the opposing face free of charged residues (47, 48). The Kal-C-helix has a hydrophobic face rich in bulky residues, but its opposing face is highly charged. Although amphipathic lipid packing sensor motifs often recognize lipid-packing defects and areas of membrane curvature, we saw no indication that the ability of cKalSec14 to interact with liposomes was curvature-sensitive (data not shown). The ability of the 23-amino acid peptide that follows the second transmembrane domain of atlastin to form an amphipathic helix in the presence of membranes destabilizes the bilayer, facilitating membrane fusion without altering the GTPase activity of the protein (49).
Based on the ability of phosphoinositide-containing liposomes to shift the Trp emission maximum for the Kal-c peptide to a lower wavelength, we concluded that this peptide interacts directly with PI and with each of the PIPs. A similar shift was not observed with the Kal-cKKD peptide or with liposomes composed of DOPC, DOPS, DOPE, and cholesterol. Phosphoinositide-binding peptides have also been identified in gelsolin and villin (50, 51); when added to permeabilized cells at similar levels (5 μm), these positively charged peptides disrupt actin assembly at the cytoskeleton/membrane interface. Additional mutagenesis studies will be needed to characterize the interaction between the Kal-c peptide and these lipids.
Kalrn Promoter Usage Affects Sec14 Domain-mediated Membrane Interactions
Whether evaluated using PIP strips or liposomes, cKalSec14 exhibited a more robust interaction with lipids than bKalSec14. Whereas recombinant bKalSec14 and cKalSec14 interacted with multiple phosphoinositides on PIP strips, neither replicated the lipid binding specificity of aKalSec14 (11) or GST-Sec14 (12). Using liposomes, we found that the binding of cKalSec14 was sensitive to both PIP content and cholesterol content. In contrast, the binding of bKalSec14 was affected by neither PIP content nor cholesterol content. Remarkably, disrupting the amphipathic nature of the Kal-C-helix (cSec14KKED) also eliminated the effect of PIP content and cholesterol content on binding. Taken together, we conclude that the amphipathic helix that precedes the CRAL_TRIO domain when Ex1C is used plays an essential role in the ability of cKalSec14 to interact with membranes.
Using liposomes containing phosphatidylinositol or a single phosphoinositide, we observed a graded effect of phosphorylation on the binding of cKalSec14. Significant binding to phosphatidylinositol was observed, with enhanced binding to PI(3)P, PI(4)P, and PI(3,4)P2 and less binding to PI(5)P; this profile differs markedly from the binding pattern observed using PIP strips, suggesting that cKalSec14 may need access to the lipid bilayer for optimal binding. The fact that cholesterol enhanced the binding of cKalSec14 to liposomes may reflect its ability to affect bilayer structure, altering access of the amphipathic helix to the hydrophobic interior.
To our knowledge, this is the first demonstration of a Sec14 superfamily member that uses an amphipathic helix in conjunction with its lipid-binding CRAL_TRIO domain. Several Sec14 superfamily members are known to bind multiple lipid ligands. Yeast Sec14p binds phosphatidylcholine and PI, and both interactions are necessary for function (15). The Sec14 domain of α-tocopherol transfer protein (α-TTP) has distinct binding sites for α-tocopherol (vitamin E) and PIPs, and disruption of PIP binding impairs cellular α-tocopherol transport (17). How the lipid-binding Kal-C-helix and the lipid-binding CRAL_TRIO domain of kalirin interact is not yet clear.
Consistent with the liposome binding data, bKalSec14-GFP and cKalSec14-GFP behaved differently when expressed in AtT-20 cells. bKalSec14-GFP was diffusely distributed, whereas cKalSec14-GFP co-localized with markers for the trans-Golgi network and secretory granules. The fact that the TGN and tip enrichment of cKalSec14-GFP declined at high levels of expression suggests the presence of saturable binding sites. As in many other cases, the ability of cKalSec14 to interact with both proteins and lipids would allow it to serve as a coincidence detector. Phosphoinositide levels are highest in the endoplasmic reticulum and decline through the Golgi complex to the plasma membrane and into endosomal compartments; conversely, cholesterol levels are high in the plasma membrane and lowest in the endoplasmic reticulum (28, 39). Phosphoinositide identity is also expected to affect the interaction of cKalSec14 with membranes; altered cell surface levels of ionotropic glutamate receptors in Kal7KO mice and altered uptake of transferrin following expression of exogenous KalSec14 are consistent with a role for kalirin in endocytic trafficking, where PI(3)P is more prevalent (28, 39).
The Kal-c Peptide Amphipathic Helix Promotes Secretion
The multiple roles of phosphoinositides in cytoskeletal organization, membrane trafficking, and secretion (36, 37) suggest many ways in which the ability of the Kal-C-helix to bind phosphoinositides could affect cell function. Because prolonged expression of cKalSec14-GFP clearly had an impact on cell morphology, we used a cell-permeant Kal-c peptide to explore its acute effects. Although we focus here on its effects on secretion, changes in cytoskeletal organization were also observed, as would be expected for a peptide that binds PIPs (50). For example, when applied to platelets or neutrophils, a cell-permeant derivative of the 10-amino acid PIP(4,5)P2 binding region of gelsolin blocks cell motility, disassembles actin filaments, and prevents actin assembly (50).
The fact that the control peptides (a different Arg9 peptide and Kal-cKKED-Arg9) were without effect minimizes concerns about the nonspecific effects of Kal-c-Arg9, as does its selective effect on regulated, but not basal, secretion (52). Understanding what the Kal-c peptide can do when not tethered to the CRAL_TRIO domain will inform future studies. The stimulatory effect of Kal-c-Arg9 on granule release could reflect its interaction with the many PIPs known to affect the control of filamentous actin and cytoskeletal/membrane interactions or its interaction with specific PIPs known to affect calcium and potassium channels (53), retromer-dependent cargo transport (54), Ca2+-dependent secretion (55, 56), or the readily releasable pool of vesicles (57–59). The priming step that precedes calcium-triggered vesicle fusion involves a phosphatidylinositol transfer protein. Like other Sec14 superfamily members, KalSec14 may function as a lipid transport protein or may affect PIP metabolism (14, 15).
How promoter usage and Sec14 domain interactions affect the function of full-length kalirin proteins is currently under investigation. In addition to its Sec14 domain, kalirin may interact with membrane lipids through the interaction of its spectrin repeat region with sorting nexins 1 and 2 (SNX1 and SNX2) (60). Members of the SNX-BAR subfamily of sorting nexins use their phosphoinositide-binding PX domain and a curvature-sensitive BAR domain to tie cargo sorting to membrane tubulation during endocytic trafficking. Since molecular interactions and localization greatly influence the signaling cascades to which a protein contributes, it is possible that alternate promoter usage creates kalirin subpopulations, which are segregated for action in discrete signaling cascades.
Acknowledgments
We thank Darlene D'Amato and Yanping Wang for help with cell culture, biochemistry, and molecular biology and the University of Connecticut Health Center Biophysical Core for providing access to essential equipment.
This work was supported, in whole or in part, by National Institutes of Health Grant DK-032948.
- GEF
- GDP/GTP exchange factor
- α-TTP
- α-tocopherol transfer protein
- TES
- 2-{[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]amino}ethanesulfonic acid
- PIP
- phosphatidylinositol phosphate
- PI
- phosphatidylinositol
- DOPC
- 1,2-dioleoyl-sn-glycero-3-phosphocholine
- DOPE
- 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine
- DOPS
- 1,2-dioleoyl-sn-glycero-3-phosphatidylserine
- TGN
- trans-Golgi network
- EGFP
- enhanced GFP
- CSFM
- complete serum-free medium
- PC1
- prohormone convertase 1
- ANOVA
- analysis of variance
- TRITC
- tetramethylrhodamine isothiocyanate.
References
- 1. Penzes P., Johnson R. C., Sattler R., Zhang X., Huganir R. L., Kambampati V., Mains R. E., Eipper B. A. (2001) The neuronal Rho-GEF kalirin-7 interacts with PDZ domain-containing proteins and regulates dendritic morphogenesis. Neuron 29, 229–242 [DOI] [PubMed] [Google Scholar]
- 2. Penzes P., Johnson R. C., Alam M. R., Kambampati V., Mains R. E., Eipper B. A. (2000) An isoform of kalirin, a brain-specific GDP/GTP exchange factor, is enriched in the postsynaptic density fraction. J. Biol. Chem. 275, 6395–6403 [DOI] [PubMed] [Google Scholar]
- 3. Lemtiri-Chlieh F., Zhao L., Kiraly D. D., Eipper B. A., Mains R. E., Levine E. S. (2011) Kalirin-7 is necessary for normal NMDA receptor-dependent synaptic plasticity. BMC Neurosci. 12, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kiraly D. D., Lemtiri-Chlieh F., Levine E. S., Mains R. E., Eipper B. A. (2011) Kalirin binds the NR2B subunit of the NMDA receptor, altering its synaptic localization and function. J. Neurosci. 31, 12554–12565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ma X. M., Kiraly D. D., Gaier E. D., Wang Y., Kim E. J., Levine E. S., Eipper B. A., Mains R. E. (2008) Kalirin-7 is required for synaptic structure and function. J. Neurosci. 28, 12368–12382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xie Z., Srivastava D. P., Photowala H., Kai L., Cahill M. E., Woolfrey K. M., Shum C. Y., Surmeier D. J., Penzes P. (2007) Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron 56, 640–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yan Y., Eipper B. A., Mains R. E. (2014) Kalirin-9 and kalirin-12 play essential roles in dendritic outgrowth and branching. Cereb. Cortex 10.1093/cercor/bhu182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mandela P., Yankova M., Conti L. H., Ma X. M., Grady J., Eipper B. A., Mains R. E. (2012) Kalrn plays key roles within and outside of the nervous system. BMC Neurosci. 13, 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang S., Eleniste P. P., Wayakanon K., Mandela P., Eipper B. A., Mains R. E., Allen M. R., Bruzzaniti A. (2014) The Rho-GEF kalirin regulates bone mass and the function of osteoblasts and osteoclasts. Bone 60, 235–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu J. H., Fanaroff A. C., Sharma K. C., Smith L. S., Brian L., Eipper B. A., Mains R. E., Freedman N. J., Zhang L. (2013) Kalirin promotes neointimal hyperplasia by activating Rac in smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 33, 702–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ma X. M., Miller M. B., Vishwanatha K. S., Gross M. J., Wang Y., Abbott T., Lam T. T., Mains R. E., Eipper B. A. (2014) Nonenzymatic domains of kalirin7 contribute to spine morphogenesis through interactions with phosphoinositides and Abl. Mol. Biol. Cell 25, 1458–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schiller M. R., Ferraro F., Wang Y., Ma X. M., McPherson C. E., Sobota J. A., Schiller N. I., Mains R. E., Eipper B. A. (2008) Autonomous functions for the Sec14p/spectrin-repeat region of kalirin. Exp. Cell Res. 314, 2674–2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Curwin A. J., Fairn G. D., McMaster C. R. (2009) Phospholipid transfer protein Sec14 is required for trafficking from endosomes and regulates distinct trans-Golgi export pathways. J. Biol. Chem. 284, 7364–7375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hama H., Schnieders E. A., Thorner J., Takemoto J. Y., DeWald D. B. (1999) Direct involvement of phosphatidylinositol 4-phosphate in secretion in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 274, 34294–34300 [DOI] [PubMed] [Google Scholar]
- 15. Schaaf G., Ortlund E. A., Tyeryar K. R., Mousley C. J., Ile K. E., Garrett T. A., Ren J., Woolls M. J., Raetz C. R., Redinbo M. R., Bankaitis V. A. (2008) Functional anatomy of phospholipid binding and regulation of phosphoinositide homeostasis by proteins of the sec14 superfamily. Mol. Cell 29, 191–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saito K., Tautz L., Mustelin T. (2007) The lipid-binding SEC14 domain. Biochim. Biophys. Acta 1771, 719–726 [DOI] [PubMed] [Google Scholar]
- 17. Kono N., Ohto U., Hiramatsu T., Urabe M., Uchida Y., Satow Y., Arai H. (2013) Impaired α-TTP-PIPs interaction underlies familial vitamin E deficiency. Science 340, 1106–1110 [DOI] [PubMed] [Google Scholar]
- 18. He X., Lobsiger J., Stocker A. (2009) Bothnia dystrophy is caused by domino-like rearrangements in cellular retinaldehyde-binding protein mutant R234W. Proc. Natl. Acad. Sci. U.S.A. 106, 18545–18550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nile A. H., Bankaitis V. A., Grabon A. (2010) Mammalian diseases of phosphatidylinositol transfer proteins and their homologs. Clin. Lipidol. 5, 867–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sirokmány G., Szidonya L., Káldi K., Gáborik Z., Ligeti E., Geiszt M. (2006) Sec14 homology domain targets p50RhoGAP to endosomes and provides a link between Rab and Rho GTPases. J. Biol. Chem. 281, 6096–6105 [DOI] [PubMed] [Google Scholar]
- 21. Ueda S., Kataoka T., Satoh T. (2004) Role of the Sec14-like domain of Dbl family exchange factors in the regulation of Rho family GTPases in different subcellular sites. Cell. Signal. 16, 899–906 [DOI] [PubMed] [Google Scholar]
- 22. Sha B., Phillips S. E., Bankaitis V. A., Luo M. (1998) Crystal structure of the Saccharomyces cerevisiae phosphatidylinositol-transfer protein. Nature 391, 506–510 [DOI] [PubMed] [Google Scholar]
- 23. D'Angelo I., Welti S., Bonneau F., Scheffzek K. (2006) A novel bipartite phospholipid-binding module in the neurofibromatosis type 1 protein. EMBO Rep. 7, 174–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gupta A. B., Wee L. E., Zhou Y. T., Hortsch M., Low B. C. (2012) Cross-species analyses identify the BNIP-2 and Cdc42GAP homology (BCH) domain as a distinct functional subclass of the CRAL_TRIO/Sec14 superfamily. PLoS One 7, e33863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mains R. E., Kiraly D. D., Eipper-Mains J. E., Ma X. M., Eipper B. A. (2011) Kalrn promoter usage and isoform expression respond to chronic cocaine exposure. BMC Neurosci. 12, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vishwanatha K. S., Wang Y. P., Keutmann H. T., Mains R. E., Eipper B. A. (2012) Structural organization of the nine spectrin repeats of kalirin. Biochemistry 51, 5663–5673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Spijker S., Smit A. B., Eipper B. A., Malik A., Mains R. E., Geraerts W. P. (1999) A molluscan peptide α-amidating enzyme precursor that generates five distinct enzymes. FASEB J. 13, 735–748 [DOI] [PubMed] [Google Scholar]
- 28. Manneville J. B., Leduc C., Sorre B., Drin G. (2012) Studying in vitro membrane curvature recognition by proteins and its role in vesicular trafficking. Methods Cell Biol. 108, 47–71 [DOI] [PubMed] [Google Scholar]
- 29. Steveson T. C., Zhao G. C., Keutmann H. T., Mains R. E., Eipper B. A. (2001) Access of a membrane protein to secretory granules is facilitated by phosphorylation. J. Biol. Chem. 276, 40326–40337 [DOI] [PubMed] [Google Scholar]
- 30. Sobota J. A., Ferraro F., Bäck N., Eipper B. A., Mains R. E. (2006) Not all secretory granules are created equal: partitioning of soluble content proteins. Mol. Biol. Cell 17, 5038–5052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Milgram S. L., Kho S. T., Martin G. V., Mains R. E., Eipper B. A. (1997) Localization of integral membrane peptidylglycine α-amidating monooxygenase in neuroendocrine cells. J. Cell Sci. 110, 695–706 [DOI] [PubMed] [Google Scholar]
- 32. Milletti F. (2012) Cell-penetrating peptides: classes, origin, and current landscape. Drug Discov. Today 17, 850–860 [DOI] [PubMed] [Google Scholar]
- 33. Rothbard J. B., Garlington S., Lin Q., Kirschberg T., Kreider E., McGrane P. L., Wender P. A., Khavari P. A. (2000) Conjugation of arginine oligomers to cyclosporin A facilitates topical delivery and inhibition of inflammation. Nat. Med. 6, 1253–1257 [DOI] [PubMed] [Google Scholar]
- 34. Bonnemaison M., Bäck N., Lin Y., Bonifacino J. S., Mains R., Eipper B. (2014) AP-1A controls secretory granule biogenesis and trafficking of membrane secretory granule proteins. Traffic 15, 1099–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhou A., Paquet L., Mains R. E. (1995) Structural elements that direct specific processing of different mammalian subtilisin-like prohormone convertases. J. Biol. Chem. 270, 21509–21516 [DOI] [PubMed] [Google Scholar]
- 36. Wenk M. R., De Camilli P. (2004) Protein-lipid interactions and phosphoinositide metabolism in membrane traffic: insights from vesicle recycling in nerve terminals. Proc. Natl. Acad. Sci. U.S.A. 101, 8262–8269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Di Paolo G., De Camilli P. (2006) Phosphoinositides in cell regulation and membrane dynamics. Nature 443, 651–657 [DOI] [PubMed] [Google Scholar]
- 38. Bäck N., Rajagopal C., Mains R. E., Eipper B. A. (2010) Secretory granule membrane protein recycles through multivesicular bodies. Traffic 11, 972–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Meer G., Voelker D. R., Feigenson G. W. (2008) Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 9, 112–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Orci L., Montesano R., Meda P., Malaisse-Lagae F., Brown D., Perrelet A., Vassalli P. (1981) Heterogeneous distribution of filipin-cholesterol complexes across the cisternae of the Golgi apparatus. Proc. Natl. Acad. Sci. U.S.A. 78, 293–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang Y. J., Wang J., Sun H. Q., Martinez M., Sun Y. X., Macia E., Kirchhausen T., Albanesi J. P., Roth M. G., Yin H. L. (2003) Phosphatidylinositol 4 phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell 114, 299–310 [DOI] [PubMed] [Google Scholar]
- 42. Ma X. M., Wang Y., Ferraro F., Mains R. E., Eipper B. A. (2008) Kalirin-7 is an essential component of both shaft and spine excitatory synapses in hippocampal interneurons. J. Neurosci. 28, 711–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Miller M. B., Yan Y., Eipper B. A., Mains R. E. (2013) Neuronal Rho GEFs in synaptic physiology and behavior. Neuroscientist 19, 255–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kiraly D. D., Eipper-Mains J. E., Mains R. E., Eipper B. A. (2010) Synaptic plasticity, a symphony in GEF. ACS Chem. Neurosci. 1, 348–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Antonny B., Beraud-Dufour S., Chardin P., Chabre M. (1997) N-terminal hydrophobic residues of the G-protein ADP-ribosylation factor-1 insert into membrane phospholipids upon GDP to GTP exchange. Biochemistry 36, 4675–4684 [DOI] [PubMed] [Google Scholar]
- 46. Bhatia V. K., Madsen K. L., Bolinger P. Y., Kunding A., Hedegård P., Gether U., Stamou D. (2009) Amphipathic motifs in BAR domains are essential for membrane curvature sensing. EMBO J. 28, 3303–3314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Drin G., Antonny B. (2010) Amphipathic helices and membrane curvature. FEBS Lett. 584, 1840–1847 [DOI] [PubMed] [Google Scholar]
- 48. Vanni S., Vamparys L., Gautier R., Drin G., Etchebest C., Fuchs P. F., Antonny B. (2013) Amphipathic lipid packing sensor motifs: probing bilayer defects with hydrophobic residues. Biophys. J. 104, 575–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Faust J. E., Desai T., Verma A., Ulengin I., Sun T. L., Moss T. J., Betancourt-Solis M. A., Huang H. W., Lee T., McNew J. A. (2015) The atlastin C-terminal tail is an amphipathic helix that perturbs the bilayer structure during endoplasmic reticulum homotypic fusion. J. Biol. Chem. 290, 4772–4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cunningham C. C., Vegners R., Bucki R., Funaki M., Korde N., Hartwig J. H., Stossel T. P., Janmey P. A. (2001) Cell permeant polyphosphoinositide-binding peptides that block cell motility and actin assembly. J. Biol. Chem. 276, 43390–43399 [DOI] [PubMed] [Google Scholar]
- 51. Janmey P. A., Lamb J., Allen P. G., Matsudaira P. T. (1992) Phosphoinositide-binding peptides derived from the sequences of gelsolin and villin. J. Biol. Chem. 267, 11818–11823 [PubMed] [Google Scholar]
- 52. Martin T. F. (2015) PI(4,5)P-binding effector proteins for vesicle exocytosis. Biochim. Biophys. Acta 1851, 785–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hille B., Dickson E. J., Kruse M., Vivas O., Suh B. C. (2015) Phosphoinositides regulate ion channels. Biochim. Biophys. Acta 1851, 844–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Niu Y., Zhang C., Sun Z., Hong Z., Li K., Sun D., Yang Y., Tian C., Gong W., Liu J. J. (2013) PtdIns(4)P regulates retromer-motor interaction to facilitate dynein-cargo dissociation at the trans-Golgi network. Nat. Cell Biol. 15, 417–429 [DOI] [PubMed] [Google Scholar]
- 55. Eberhard D. A., Cooper C. L., Low M. G., Holz R. W. (1990) Evidence that the inositol phospholipids are necessary for exocytosis. Loss of inositol phospholipids and inhibition of secretion in permeabilized cells caused by a bacterial phospholipase C and removal of ATP. Biochem. J. 268, 15–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hay J. C., Fisette P. L., Jenkins G. H., Fukami K., Takenawa T., Anderson R. A., Martin T. F. (1995) ATP-dependent inositide phosphorylation required for Ca2+-activated secretion. Nature 374, 173–177 [DOI] [PubMed] [Google Scholar]
- 57. Milosevic I., Sørensen J. B., Lang T., Krauss M., Nagy G., Haucke V., Jahn R., Neher E. (2005) Plasmalemmal phosphatidylinositol-4,5-bisphosphate level regulates the releasable vesicle pool size in chromaffin cells. J. Neurosci. 25, 2557–2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gong L. W., Di Paolo G., Diaz E., Cestra G., Diaz M. E., Lindau M., De Camilli P., Toomre D. (2005) Phosphatidylinositol phosphate kinase type I γ regulates dynamics of large dense-core vesicle fusion. Proc. Natl. Acad. Sci. U.S.A. 102, 5204–5209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Di Paolo G., Moskowitz H. S., Gipson K., Wenk M. R., Voronov S., Obayashi M., Flavell R., Fitzsimonds R. M., Ryan T. A., De Camilli P. (2004) Impaired PtdIns(4,5)P2 synthesis in nerve terminals produces defects in synaptic vesicle trafficking. Nature 431, 415–422 [DOI] [PubMed] [Google Scholar]
- 60. Prosser D. C., Tran D., Schooley A., Wendland B., Ngsee J. K. (2010) A novel, retromer-independent role for sorting nexins 1 and 2 in RhoG-dependent membrane remodeling. Traffic 11, 1347–1362 [DOI] [PubMed] [Google Scholar]
- 61. Milgram S. L., Mains R. E. (1994) Differential effects of temperature blockade on the proteolytic processing of three secretory granule-associated proteins. J. Cell Sci. 107, 737–745 [DOI] [PubMed] [Google Scholar]