ABSTRACT
Powassan virus (POWV) is an encephalitic tick-borne flavivirus which can result in serious neuroinvasive disease with up to a 10% case fatality rate. The study objective was to determine whether the salivary gland extract (SGE) from Ixodes scapularis ticks facilitates the transmission and dissemination of POWV in a process known as saliva-activated transmission. Groups of BALB/c mice were footpad inoculated with either a high dose of POWV with and without SGE or a low dose of POWV with and without SGE. Mice from each group were sacrificed daily. Organ viral loads and gene expression profiles were evaluated by quantitative real-time PCR. Both groups of mice infected with high-dose POWV showed severe neurological signs of disease preceding death. The presence of SGE did not affect POWV transmission or disease outcome for mice infected with the high dose of POWV. Neuroinvasion, paralysis, and death occurred for all mice infected with the low dose of POWV plus SGE; however, for mice infected with the low dose of POWV in the absence of SGE, there were no clinical signs of infection and no mice succumbed to disease. Although this group displayed low-level viremias, all mice were completely healthy, and it was the only group in which POWV was cleared from the lymph nodes. We conclude that saliva-activated transmission occurs in mice infected with a low dose of POWV. Our study is the first to demonstrate virus dose-dependent saliva-activated transmission, warranting further investigation of the specific salivary factors responsible for enhancing POWV transmission.
IMPORTANCE Powassan virus (POWV) is a tick-borne flavivirus that continues to emerge in the United States, as is evident by the surge in number and expanding geographic range of confirmed cases in the past decade. This neuroinvasive virus is transmitted to humans by infected tick bites. Successful tick feeding is facilitated by a collection of pharmacologically active factors in tick saliva. In a process known as saliva-activated transmission, tick bioactive salivary molecules are thought to modulate the host environment, making it more favorable for the transmission and establishment of a pathogen. This phenomenon has been demonstrated for several tick-borne pathogens; however, a systematic investigation of the role of tick saliva on dissemination and pathogenesis of a tick-borne viral disease has never been attempted before. This study will fill that gap by systematically examining whether the presence of tick saliva contributes to the transmission and dissemination of POWV in mice.
INTRODUCTION
Powassan virus (POWV) is a tick-borne flavivirus that continues to emerge in the United States, as is evident by the surge in the number and expanding geographic range of confirmed human cases in the past decade (http://diseasemaps.usgs.gov/pow_us_human.html). POWV first was recognized as a human pathogen in 1958, when it was isolated from the brain of a young boy who died of encephalitis in Powassan, Ontario (1). From 1958 through 1998, only 27 human POWV encephalitis cases were reported (2); however, from 2001 to 2014, 64 human cases of POWV encephalitis were documented in the United States (http://diseasemaps.usgs.gov/pow_us_human.html). After the 1999 introduction of West Nile virus into North America, there has been a heightened awareness of arthropod-borne encephalitic viruses as well as increased surveillance for these viruses. These factors may have contributed to the apparent increase in POWV encephalitis cases. The most common clinical presentations for human disease caused by POWV infection are encephalitis, meningoencephalitis, and aseptic meningitis, with an incubation period that varies from 8 to 34 days. POWV infections in humans typically involve febrile illness followed by neurological involvement, resulting in a 10% case fatality rate with permanent and severe neurological sequelae displayed in 50% of survivors. Long-term neurological sequelae in patients who survive POWV encephalitis include hemiplegia, muscle atrophy, chronic severe headaches, and memory problems. Although few studies have examined potential animal models for POWV infection, intraperitoneal delivery with 1.25 × 104 PFU of POWV to 4-week-old female BALB/c mice resulted in a biphasic course of disease, similar to what is seen in human infections (3). Mice in that study displayed initial febrile signs of illness at 5 to 6 days postinfection followed by severe neurological involvement and 100% mortality (3).
In nature, POWV is maintained in cycles between small- to medium-sized rodents and ixodid ticks; however, spillover transmission to humans also occurs (4). We have demonstrated that successful transmission of the neuroinvasive POWV can occur within 3 h of Ixodes scapularis tick attachment to the host (5). For ticks to successfully attach to a vertebrate host and take a blood meal, tick salivary proteins/factors must overcome a range of host responses. A complex repertoire of bioactive molecules is found in the salivary glands of ticks, and these can modulate host hemostasis, pain/itch responses, wound healing, and innate and adaptive immunity (6–9). Transmission of a pathogen from the salivary glands of a tick followed by pathogen establishment in the host is a complex process. Tick saliva, along with pathogenic agents such as POWV, is delivered to the tick feeding site on a vertebrate host. The repertoire of pharmacologically active molecules in tick saliva not only overcomes host immune responses but also affects pathogen transmission efficiency, pathogen establishment, and disease pathogenesis (6, 8, 10).
In a process known as saliva-activated transmission (SAT), tick bioactive salivary molecules are thought to modulate the host environment, making it more favorable for the transmission and establishment of a pathogen (11). This phenomenon has been shown for multiple tick-borne pathogens, such as tick-borne encephalitis virus (11), Thogoto virus (12), Francisella tularensis (13), and several Borrelia species (14). To date, few studies have reported on the role that tick saliva plays in virus proliferation, transmission, and dissemination in the host. In this study, we sought to determine whether the presence of I. scapularis saliva contributes to the transmission and dissemination of POWV in BALB/c mice. We also examined whether differences in the quantity of virus delivered with and without tick salivary gland extract (SGE) would affect/alter host immune responses, POWV replication, spread from the inoculation site, and disease progression.
MATERIALS AND METHODS
Mice.
Five-week-old, female BALB/c mice were purchased from The Jackson Laboratory (Bar Harbor, ME). All mouse experiments were conducted in accordance with an animal use protocol approved by the University of Texas Medical Branch (UTMB) Institutional Animal Care and Use Committee (IACUC). Mice were allowed to adapt to the local environment before being used in experiments, at which point the mice were approximately 6 weeks old.
Tick salivary gland extract (SGE).
Pathogen-free Ixodes scapularis ticks were maintained as described previously (15). The salivary glands from pathogen-free I. scapularis adult females were dissected and stored in phosphate-buffered saline (PBS). The salivary glands were homogenized and quantified using the micro-bicinchoninic acid protein assay kit (Thermo Scientific).
Virus and infections.
Stock of POWV (LB strain) was prepared from cell culture medium of Vero cells that was infected with a previously prepared virus stock in suckling mouse brains, provided by Robert Tesh at the World Reference Center for Emerging Viruses and Arboviruses. Each BALB/c mouse was infected under isoflurane anesthesia in the left hind footpad with 25 μl of 106 PFU of POWV plus 30 μg of SGE, 106 PFU of POWV alone, 103 PFU of POWV plus 30 μg of SGE, or 103 PFU of POWV alone. Mice from each treatment group were sacrificed at the following time points (n = 5): 3, 6, and 12 h postinfection (hpi) and 1, 2, 3, 4, 5, 6, 7, 8, and 9 days postinfection (dpi). An additional 10 mice were mock infected with 25 μl of media to serve as controls. Mice were considered moribund when they exhibited hind limb paralysis or total paralysis. When compiling data for the survival curve, mice that were moribund were counted as having succumbed to disease on that day.
Blinded clinical observations, including weight change, hunched posture with ruffled fur, weak grip, hind-limb paralysis, or total paralysis, were recorded for each mouse at every time point. The superficial footpad injection performed in this study was chosen as it better represents the natural tick feeding process and delivery of a tick-borne pathogen than other types of needle inoculation, such as intraperitoneal (i.p.), which was described previously (3).
RNA extractions.
RNA extraction was performed using a combination of TRIzol reagent (Life Technologies) and Qiagen protocols, as we have previously optimized the combination of these protocols to yield high-quality and high-integrity RNA (15). Whole blood (harvested by terminal cardiac puncture) was collected from each sacrificed mouse and stored in 1 ml of TRIzol LS reagent (Life Technologies). Two hundred microliters of chloroform was added to each whole-blood sample and vortexed. The samples were incubated for 3 min and then centrifuged at 12,000 × g at 4°C for 15 min. The aqueous phase was retained, and one volume of 100% ethanol was added to each sample. The blood samples then were applied to QIAamp viral RNA Mini (Qiagen) columns, and the kit protocol was followed for RNA binding, washing, and elution steps. Viral RNA from each blood sample was eluted from the spin column by adding 30 μl of nuclease-free water. Popliteal lymph node and brain also were collected from each sacrificed mouse and stored in 1 ml of TRIzol reagent (Life Technologies). These tissue samples were homogenized using a TissueLyser II (Qiagen) and incubated at room temperature for 5 min. Two hundred microliters of chloroform was added, and the samples were vortexed and then incubated for 3 min. Samples underwent centrifugation at 12,000 × g at 4°C for 15 min. The aqueous phase was retained, and one volume of 70% ethanol was added to each sample. The lymph node and brain samples then were applied to RNeasy Plus Mini (Qiagen) columns and the kit protocol was followed. Total RNA from each lymph node and brain sample was eluted from the spin column by adding 30 μl of nuclease-free water. After extraction, all RNA samples were quantified spectrophotometrically using a NanoDrop ND-1000 (NanoDrop Technologies).
Viral load determination by quantitative real-time PCR.
Ten micromolars forward and reverse primers specific for POWV (16) were mixed with reagents from the iTaq Universal SYBR green one-step kit (Bio-Rad) and loaded into iCycler IQ PCR 96-well plates (Qiagen). One microgram of each RNA sample was loaded into a well of the PCR plate. These plates were sealed and run on an iCycler iQ5 real-time PCR instrument (Bio-Rad) with the following cycling protocol: 10 min at 50°C, 1 min at 95°C, and 10 s at 95°C, followed by 30 s at 60°C for 45 cycles and then an 81-cycle (+0.5°C/cycle) 55 to 95°C melt curve.
Creation of the standard curve for viral load quantification.
RNA was extracted from a sample of known infectivity (6.5 × 107 PFU), and serial dilutions were made from the resulting RNA. A linear equation was generated by plotting the threshold cycle (CT) values of the standard curve and the log of the viral concentration. Viral load CT values from the unknown tissue samples were determined by real-time PCR and converted to log10 PFU equivalents using the linear equation determined for the standard curve (17). Statistically significant differences in viral loads at each time point between groups that received 106 PFU POWV plus SGE and mice that received 106 PFU POWV only or between the groups that received 103 PFU POWV plus SGE and those that received 103 PFU POWV only were determined by the Student t test. P values that were less than 0.05 were considered significant. SPSS statistical software was used.
Host gene expression profiling by real-time PCR.
Fourteen genes associated with the immune response were selected for analysis by real-time PCR (see Table S1 in the supplemental material). The majority of these genes were shown to be modulated in a prior study where POWV-infected I. scapularis ticks were fed on mice for 3 or 6 h (5). The remaining genes selected for profiling in this study were shown to be modulated during uninfected I. scapularis feeding (18). For brain and lymph node samples, 1 μg of total RNA from each individual mouse was converted into cDNA using the RT2 first-strand kit (Qiagen). Preoptimized primer pairs for these genes were purchased from IDT (see Table S1). Primers and cDNA were mixed with IQ SYBR green supermix (Bio-Rad) and loaded into iCycler IQ PCR 96-well plates to create customized PCR arrays. Each array plate was run on an iCycler iQ5 PCR system (Bio-Rad) with the following cycling protocol: 10 min at 95°C; 40 cycles of 15 s at 95°C and then 1 min at 60°C; and an 80-cycle (+0.5°C/cycle) 55 to 95°C melt curve. Every array included the gene for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a housekeeping gene and a no-template control. The iCycler's software was used to calculate the CT values for all analyzed genes.
PCR array data analysis.
The ΔΔCT method was used to calculate fold changes in gene expression between test groups (infected with 103 PFU of POWV plus SGE) and control groups (infected with 103 PFU of POWV alone). Data normalization was achieved by correcting all CT values to the average CT values of the GAPDH housekeeping gene. Data are presented as the average fold change for three individual animals per time point for immune gene expression. Statistical significance was calculated by the Student t test comparing the tissue-specific immune response in groups infected with 103 PFU of POWV plus SGE versus groups infected with 103 PFU of POWV alone. Significant values were those with P ≤ 0.05 and a fold change in gene expression of ≥2.
RESULTS
The presence of I. scapularis SGE did not affect the outcome of disease for mice that received 106 PFU of POWV.
Following high-dose footpad injections of 106 PFU POWV, both with and without SGE, all mice succumb to the disease at 6 dpi (Fig. 1A). The mice in both groups that received 106 PFU POWV appeared completely healthy through 3 dpi; however, at 4 dpi, mice started to display ruffled fur and slight weak grips (Table 1). At 5 dpi, all mice that received a high dose of POWV displayed ruffled fur, weak grips, and lethargy. In the group receiving 106 PFU POWV plus SGE, over 50% of these mice had hunched postures at 5 dpi. For all mice in both high-dose groups, the severe neurological signs appeared at 6 dpi. All mice that received 106 PFU POWV, both with and without SGE, displayed hind-limb paralysis followed by total paralysis and ultimately succumbed to disease at 6 dpi. The average percent change in weight loss for mice in these two groups severely decreased between 4 and 6 dpi (Fig. 1B).
FIG 1.

(A) Survival curves for mice in each treatment cohort. (B) Average percent weight change at the indicated time points compared with the day 0 weight were calculated for mice in each group. (A and B) Every data point is representative of five mice. For the mock-infected mice, data were collected through 8 dpi.
TABLE 1.
Clinical observations for mice in each treatment groupa
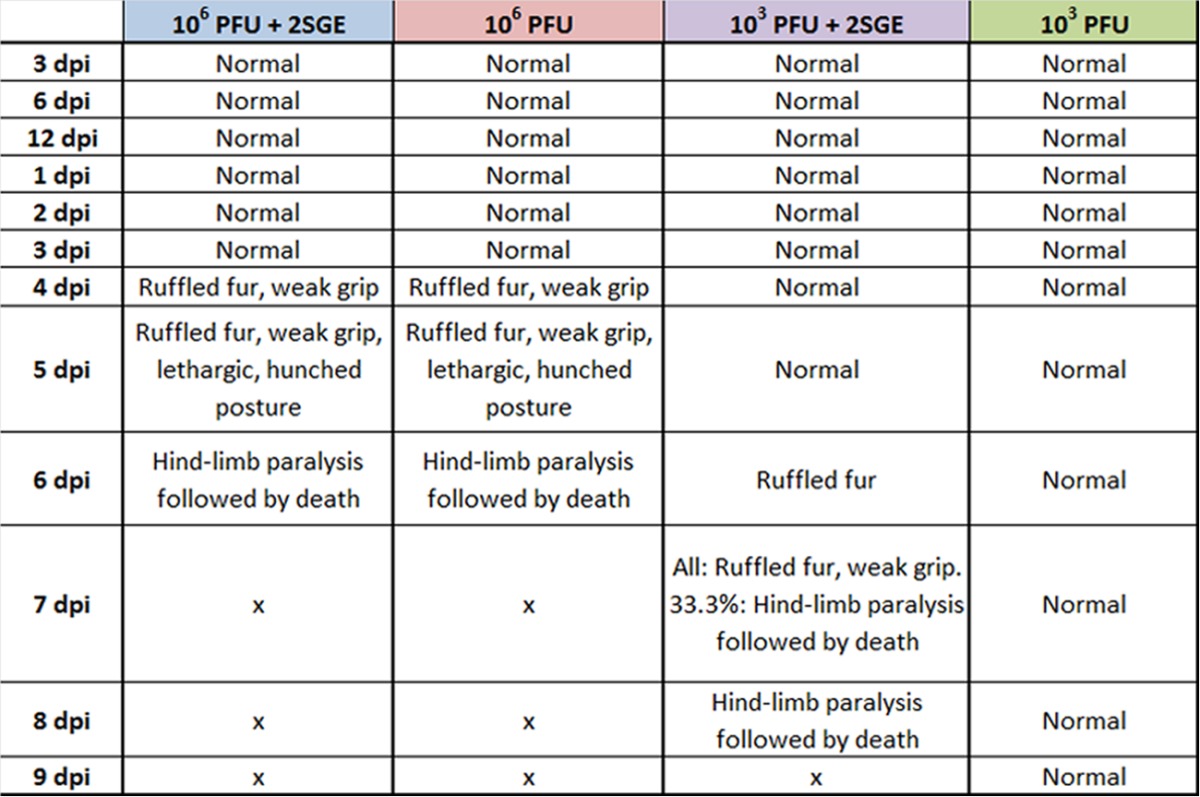
See Materials and Methods for details on how these clinical observations were made.
Mice that received 103 PFU of POWV in the absence of SGE were the only group that survived infection with POWV.
While there was no difference in clinical observations or survival for mice that received 106 PFU POWV, with and without SGE, there were clear differences between the group of mice that received 103 PFU POWV plus SGE versus the group that received only 103 PFU POWV (Table 1). All mice in the group that received 103 PFU POWV plus SGE succumbed to the disease between 7 and 8 dpi (Fig. 1A). These mice began displaying ruffled fur at 6 dpi, but before this time point all mice in the group were healthy. In the presence of SGE, all of the mice that were surviving at 7 dpi displayed ruffled fur and weak grips. One-third (33.3%) of the mice in this treatment group displayed hind-limb paralysis and succumbed to disease at 7 dpi. By 8 dpi, the remaining mice that received 103 PFU POWV plus SGE all had succumbed. For mice in this group, the average percent change in weight loss suddenly decreased between 6 and 8 dpi, with mild weight loss occurring between 4 and 6 dpi (Fig. 1B).
Interestingly, mice in the group that received 103 PFU POWV and no SGE displayed no clinical signs of infection (Table 1). All mice in this group survived the infection and appeared completely healthy at 11 dpi when the experiment was terminated (Fig. 1A). After 5 dpi, mice in this group were gaining weight compared to their day 0 weights (Fig. 1B).
Mice in all four treatment groups, including those that remained healthy, displayed clear viremias for a minimum of 5 days.
At 3 and 6 hpi, mice in the group that received 106 PFU POWV displayed viremias that were significantly higher than those of the mice that received 106 PFU POWV plus SGE (Fig. 2A). Both of the high-dose groups followed the same pattern at the early time points, where the viremias decreased between 3 and 6 hpi but then increased at 12 hpi. By 24 hpi, the viremias peaked at approximately 104 PFU/μg RNA for all mice infected with a high dose of POWV. For all time points after 1 dpi, viremias steadily decreased for mice in the high-dose groups. At 4 dpi there was a significantly higher load of virus in the blood of mice infected with 106 PFU POWV only.
FIG 2.
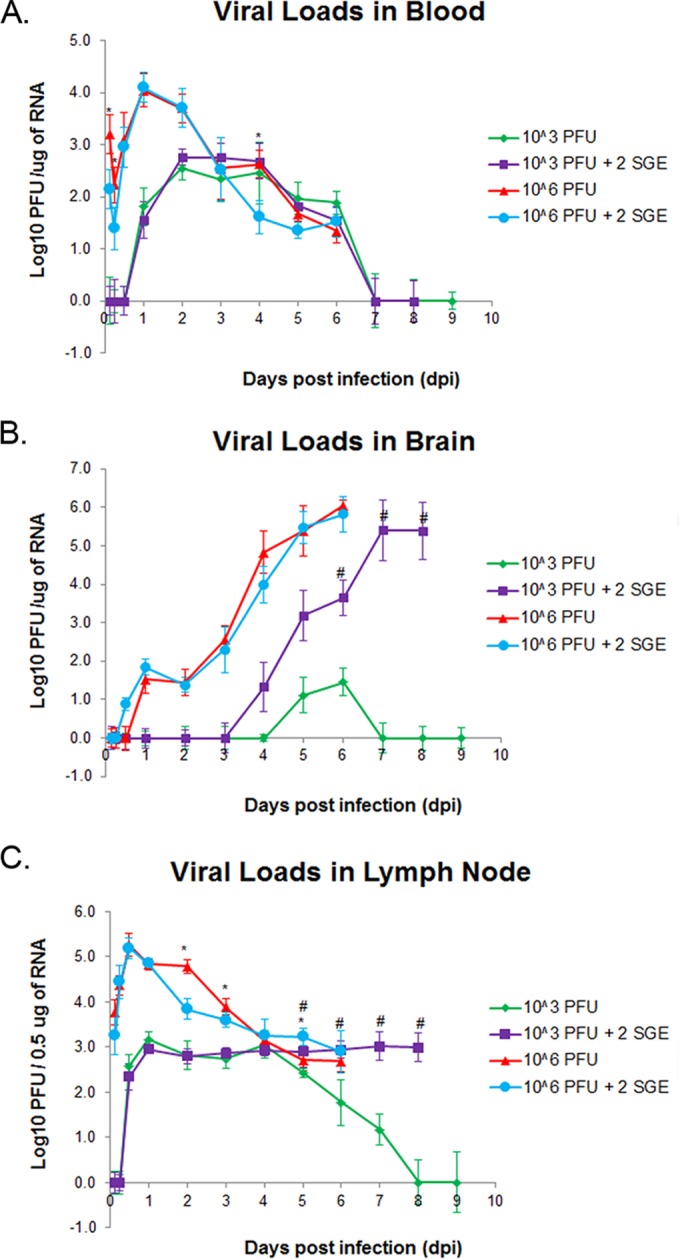
Viral load detection in blood (A), brain (B), and popliteal lymph node (C). n = 5 mice per time point and infection condition. *, P < 0.05 for 106 PFU versus 106 PFU plus 2 SGE; #, P < 0.05 for 103 PFU versus 103 PFU plus 2 SGE. The limit of detection is 10 PFU/μg RNA. Error bars indicate standard deviations. 2SGE, mice were injected with salivary gland extract from two I. scapularis adult female ticks.
All mice that were infected with the low dose of POWV displayed viremias between 1 and 6 dpi (Fig. 2A). There were no significant differences between viremias for mice in the group that received only 103 PFU POWV versus mice that received 103 PFU POWV plus SGE. The peak viremias for the two low-dose groups were more than 1 log lower than the peak viremias for the two high-dose groups.
Mice infected with 103 PFU POWV in the absence of SGE displayed levels of POWV in the brain at 5 to 6 dpi that were barely above the limit of detection, while viral loads were much higher in the brains of mice from all other groups.
Brain viral loads were examined at every time point to illustrate the timeline of neuroinvasiveness for each treatment group of mice. There were no significant differences for viral loads between the two groups of mice that received the high dose of POWV with and without SGE (Fig. 2B). The brain viral loads for these two groups of mice steadily increased over the course of infection, peaking at 6 dpi, when all mice succumbed to disease. The peak viral loads for both high-dose groups were approximately 106 PFU/μg RNA.
Mice that were infected with 103 PFU POWV plus SGE also demonstrated steadily increasing brain viral loads over the course of infection. The brain viral loads for mice in this group peaked at 7 dpi at 105.4 PFU/μg RNA, plateauing through 8 dpi. The major difference between the high-dose groups and the group infected with 103 PFU POWV plus SGE is that the latter's pattern of increasing brain viral load was delayed by 2 days compared to that of the former. Mice infected with 103 PFU POWV in the absence of SGE displayed very low levels of POWV in the brain at 5 to 6 dpi. The limit of detection using quantitative real-time PCR was considered to be 10 PFU/μg RNA, so these brain titers were barely above the limit of detection.
The group of mice infected with 103 PFU POWV in the absence of SGE is the only group in which POWV was cleared from the lymph nodes.
At each time point the popliteal lymph node was harvested from the left hind limb of every mouse. This organ was of interest because it is the major draining lymph node nearest to the site of infection. Detection of POWV in these lymph nodes indicates that it is one of the first organs infected after dissemination of POWV from the infection site. All mice that were infected with a high dose of POWV with and without SGE displayed viral loads in the popliteal lymph nodes that peaked at 12 hpi (Fig. 2C). There were significant differences at 2, 3, and 5 dpi between the high-dose groups treated with and without SGE, but the overall trend for both treatment groups was a decrease in lymph node viral load over the course of infection. By 6 dpi, when all high-dose mice had succumbed, approximately 103 PFU/0.5 μg RNA POWV was detectable in the popliteal lymph nodes of mice in both high-dose treatment groups.
Viral loads were detected in the popliteal lymph nodes of both low-dose treatment groups of mice as early as 12 hpi. From 1 dpi through 4 dpi, the viral loads in both low-dose groups were very similar, plateauing at approximately 103 PFU/0.5 μg RNA. From 5 dpi through the end of the experiment, the popliteal lymph node viral loads for both low-dose groups differed significantly. The group of mice infected with 103 PFU plus SGE maintained lymph node viral loads of approximately 103 PFU/0.5 μg RNA until all mice succumbed by 8 dpi. On the other hand, the mice infected with 103 PFU POWV in the absence of SGE displayed steadily decreasing popliteal lymph node viral loads. No virus was detected in the lymph nodes of mice at 8 and 9 dpi, demonstrating that POWV had been cleared from the lymph nodes between 7 and 8 dpi.
Before undergoing RNA extraction, all popliteal lymph nodes were imaged to compare the relative sizes of lymph nodes at various time points from each treatment group. For both high-dose treatment groups the lymph nodes steadily increased in size over the course of infection (data not shown), and there was no apparent difference in the sizes of lymph nodes between these groups. In contrast, there was a clear difference in size patterns between the two low-dose treatment groups. The lymph nodes in the group that received 103 PFU of POWV plus SGE steadily increased in size through the end of the experiment (Fig. 3). However, for the group that received 103 PFU of POWV in the absence of SGE, the lymph nodes increased in size through 4 dpi. At 5 dpi the lymph nodes in this treatment group began to decrease in size. By 8 dpi the lymph nodes had returned to a size similar to that observed at 3 hpi.
FIG 3.
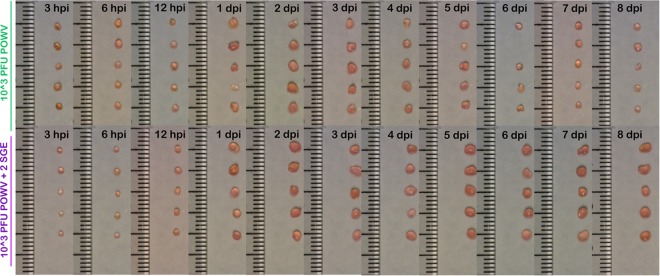
Popliteal lymph nodes were harvested from mice at the indicated time points. The sizes of lymph nodes were compared for the treatment group that received 103 PFU of POWV versus the group that received 103 PFU of POWV plus 2 SGE.
Immune response in the lymph nodes.
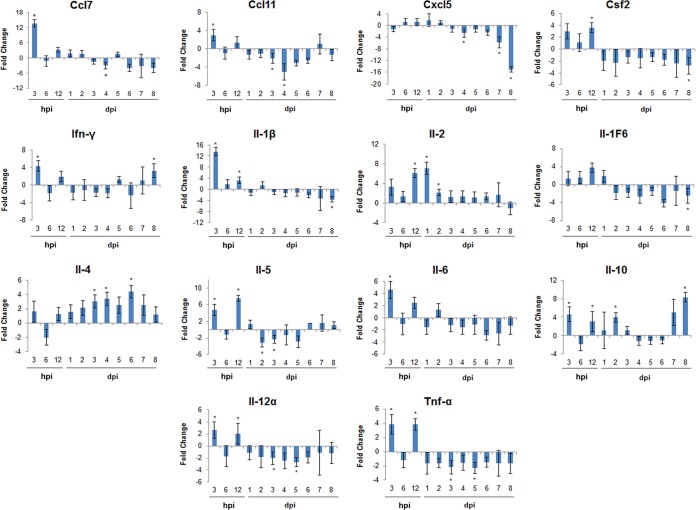
To better understand the role of SGE during POWV transmission and dissemination, we examined the lymph node immune response of mice in the low-dose groups. We only studied the immune response at the low dose of POWV infection because at this dose, and not at the high dose, the viral loads and overall disease outcome differed in the presence versus absence of SGE. Fourteen genes associated with the immune response were monitored for changes in expression at each time point. Prior studies have shown these genes to be modulated during POWV-infected I. scapularis feeding (5) or during uninfected I. scapularis feeding (18). The following comparison was made for the immune response in lymph nodes: genes modulated in mice infected with 103 PFU of POWV plus SGE versus mice infected with 103 PFU of POWV in the absence of SGE (Fig. 4). At 3 hpi, expression levels for 9 out of the 13 upregulated genes were significantly different between SGE-treated and untreated animals. CCL7 and interleukin-1β (IL-1β) were the most highly upregulated genes at 3 hpi, with values approximately 3-fold greater than those of any of the other upregulated genes. At 6 hpi the gene expression returned to basal levels, while at 12 hpi all genes were upregulated, with 7 of these genes being significant. Starting at 1 dpi and continuing through 8 dpi, there was a combination of upregulated and downregulated genes on each day.
FIG 4.
Temporal changes in popliteal lymph node immune gene expression in the presence of 103 PFU POWV plus SGE versus 103 PFU POWV in the absence of SGE. Positive fold change indicates an upregulation in gene expression in the presence of SGE compared to that in the absence of SGE. Negative fold change indicates a downregulation in gene expression. Data are presented as the average fold change for three individual animals per time point in lymph node immune gene expression. *, P ≤ 0.05 for a fold change of ≥2.
IL-2 was upregulated from 3 hpi through 7 dpi, with significant upregulation at 12 hpi, 1 dpi, and 2 dpi. IL-1β was significantly upregulated from 3 hpi through 12 hpi but was downregulated for the majority of the remaining time points. In addition to IL-1β, tumor necrosis factor alpha (TNF-α) and IL-6 also are considered to be proinflammatory cytokines (19). TNF-α and IL-6 followed the same overall pattern as IL-1β, whereby they were both upregulated at 3 hpi and at 12 hpi, but they were mostly downregulated for the remaining days. IL-5 had a similar pattern, with significant upregulation at 3 hpi and 12 hpi, followed by downregulated expression through 5 dpi; however, from 6 dpi through 8 dpi, IL-5 expression was slightly upregulated. IL-4 was upregulated at all time points except 6 hpi, with significant upregulation occurring at 1, 3, 4, and 6 dpi. IL-10 and IFN-γ both were significantly upregulated at 3 hpi and at 8 dpi, with IL-10 also being significantly upregulated at 12 hpi and 2 dpi. Although CCL7 and CCL11 were significantly upregulated at 3 hpi and slightly upregulated at 12 hpi, for the remaining time points these chemokines were either expressed at basal levels or were downregulated.
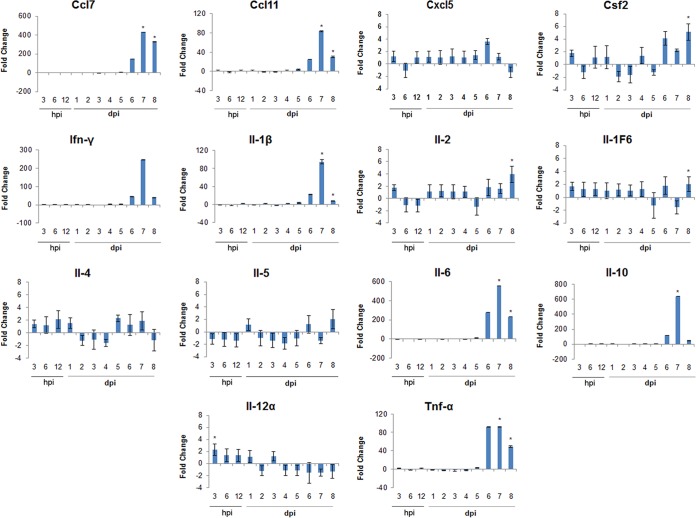
Immune response in the brain.
The same 14 immune genes monitored in the lymph nodes also were examined in the brains of mice with the goal of better understanding the course of disease for when POWV is delivered in the presence and absence of SGE. Temporal changes in brain immune gene expression were examined in the presence of 103 PFU POWV plus SGE versus 103 PFU POWV in the absence of SGE (Fig. 5). From 3 hpi through 4 dpi, all genes were very slightly up- or downregulated and seemed to be at relatively basal levels of expression. At 5 dpi CCL7, CCL11, IFN-γ, and IL-1β all were moderately upregulated, although no values were significant. IL-6 was upregulated approximately 12-fold at 5 dpi. By 6 dpi all genes, except IL-12α, were upregulated. Although none of the upregulation at 6 dpi was significant, CCL7, CCL11, IFN-γ, IL-10, IL-1β, IL-6, and TNF-α all were highly upregulated. By 7 and 8 dpi, results for several highly upregulated genes were significant. At 7 dpi and 8 dpi, CCL7, CCL11, IL-1β, IL-6, and TNF-α all were significantly upregulated. At 8 dpi, IL-1F6 and IL-2 also were significantly upregulated, although not to high levels like CCL7, CCL11, IL-1β, IL-6, and TNF-α. Mice infected with 103 PFU POWV in the absence of SGE displayed very low levels of POWV in the brain at 5 to 6 dpi, while mice infected with 103 PFU POWV plus SGE began showing detectable levels of POWV in the brain at 4 dpi (Fig. 2B). The brain immune response began to show some gene modulation other than basal-level activity as early as 5 dpi (Fig. 5). This pattern is comparable to the timeline of when POWV was introduced into the brain (Fig. 2B).
FIG 5.
Temporal changes in brain immune gene expression in the presence of 103 PFU POWV plus SGE versus 103 PFU POWV in the absence of SGE. Positive fold change indicates an upregulation in gene expression in the presence of SGE compared to that in the absence of SGE. Negative fold change indicates a downregulation in gene expression. Data are presented as the average fold changes for three individual animals per time point in brain immune gene expression. *, P ≤ 0.05 for a fold change of ≥2.
DISCUSSION
It has long been recognized that pathogens vectored by hematophagous arthropods are injected into the bite site on a vertebrate host along with arthropod saliva. Various studies have demonstrated that arthropod saliva, particularly from ticks, sandflies, and mosquitoes, contributes to enhanced infection in the host (11–14, 20–22). From these findings, arthropod-associated potentiation of infection has become a topic of interest for arthropod-borne diseases. I. scapularis is a competent vector of the neuroinvasive POWV (23). The incidence of POWV encephalitis cases is increasing in North America, especially in regions infested by I. scapularis (24); therefore, it would be worthwhile to better understand the factors that potentially enhance POWV transmission to a host. In an effort to determine whether the presence of I. scapularis saliva contributes to the transmission and dissemination of POWV, we examined whether tick saliva coinoculated with POWV increases viral loads and alters the overall disease outcome compared to that of mice inoculated with only POWV.
In the present study, we showed that a high dose (106 PFU) of POWV combined with SGE did not affect the course of disease. When mice were infected by 106 PFU of POWV with and without SGE, there was no difference in survival times, weight loss, clinical signs of infection, or virus dissemination. Conversely, SGE affected the transmission, dissemination, and overall disease outcome when administered in the presence of a low dose (103 PFU) of POWV. The combination of 103 PFU of POWV plus SGE resulted in death by 8 dpi, neuroinvasion, and paralysis for all mice in the treatment group. A much different outcome was observed when 103 PFU of POWV alone (no SGE) was administered. Mice in this treatment group displayed very low viral loads in the brain at 5 to 6 dpi and otherwise were completely healthy. None of the mice inoculated with 103 PFU of POWV alone demonstrated any overt clinical signs of infection, and none of them succumbed to disease. Therefore, we propose that when a high dose of POWV is administered in the presence of SGE, any potential effect that I. scapularis SGE has on POWV dissemination and the course of disease is saturated by the high virus titer. The overall conclusion from the present study is that at low doses of POWV, tick saliva facilitates infection and influences the disease outcome of BALB/c mice. Our data suggest that the effect SGE has on the course of disease is virus dose dependent.
Under experimental conditions it was demonstrated that I. scapularis ticks are competent vectors for POWV. All developmental stages of I. scapularis were capable of orally transmitting POWV to uninfected hosts (23). When studying tick-borne viruses, it is also important to consider the time required for virus transmission to the host and the amount of virus a single tick is capable of delivering within a certain feeding time. In our preliminary studies, we harvested 4-mm skin punch biopsy specimens from the bite site of a feeding tick and demonstrated that within 3 h of feeding, a POWV-infected I. scapularis nymph typically delivered between 102 and 103 TCID50/ml of POWV to the bite site. A prior study demonstrated that POWV was transmitted from infected I. scapularis ticks to naive mice after 15 min of feeding when ticks were infected with average titers of approximately 103.5 PFU/tick (25). Consequently, the low dose of POWV administered in the present study is representative of the amount of POWV infected I. scapularis nymphs typically deliver to the tick bite site after minimal feeding time.
In the present study, we inoculated the footpads of mice with 103 PFU of POWV in addition to the 106-PFU dose. The amount of POWV delivered during the early feeding phase of a POWV-infected tick was represented by the low dose of POWV used in our study. There is very little published data regarding animal model development for POWV, but numerous studies have been conducted with the closely related tick-borne encephalitis virus (TBEV). Typically in studies involving peripheral inoculation of TBEV, a 106-PFU dose is included because it consistently results in neuroinvasion and mortality (26); therefore, we included the high-dose group in our study design to ensure that mice infected with 106 PFU of POWV would succumb to disease. Indeed, all mice that received a high dose of POWV, both with and without SGE, showed severe neurological signs of disease preceding death at 6 dpi. Three out of the four treatment groups of mice in this study succumbed to disease: the group that received 106 PFU of POWV plus SGE, the group that received 106 PFU of POWV alone, and the group that received 103 PFU of POWV plus SGE. In all three of these treatment groups, signs of neurological involvement (weak grip, hind-limb paralysis, or total paralysis) appeared clearly and with rapid onset 1 to 2 days before mice succumbed to disease. This pattern of nonspecific clinical signs of disease followed by rapid onset of severe neurological involvement was comparable to findings of other mouse studies with POWV and TBEV (3, 26).
The objective of our study was to determine whether tick SGE, not the tick feeding process, plays a role in POWV transmission and dissemination. The present study did not use infected ticks to deliver POWV to the host but instead relied on footpad inoculations with combinations of POWV and SGE; therefore, we emphasize that the virus and SGE were instantly delivered to the skin of the host. This allowed us to examine only the role SGE plays in POWV dissemination without the additional complications of variable time and quantity of delivered virus dose that are associated with infection via tick feeding. Furthermore, we used a footpad injection because it better represents the tick feeding process than the more commonly used intraperitoneal/intracranial injections seen in many flavivirus studies. Footpad injections are a combination of intradermal and subcutaneous injection. The mouthparts of I. scapularis nymphs were observed to penetrate through the dermis, reaching the subcutaneous layers of the skin (18); therefore, our use of footpad injections delivered tick saliva and POWV to essentially the same regions of the skin as would be expected to occur during tick feeding.
In the present study, the popliteal lymph node was harvested because it is the nearest major draining lymph node to the infection site. We examined the time course of POWV infection in the popliteal lymph node, as it is likely the first tissue to which POWV disseminates from the footpad infection site. Only one treatment group of mice was able to clear POWV from its popliteal lymph node. By 8 dpi, virus had been cleared from the popliteal lymph nodes of mice that received 103 PFU of POWV. By 6 dpi, lymph nodes of mice in this group had returned to their initial size, suggesting there is a correlation between viral loads and overall lymph node size due to inflammation. Based on these observations, we sought to examine the popliteal lymph node immune response in mice that were infected with 103 PFU of POWV in the presence versus the absence of SGE. At 3 and 12 hpi, the majority of lymph node immune genes were significantly upregulated in the presence of SGE, but for the remaining time points there was a combination of upregulation and downregulation occurring on each day. In the presence of SGE, proinflammatory cytokines, including IL-1β, IL-6, and TNF-α, were upregulated at early days postinfection and downregulated at later days postinfection. This suggests that during POWV infection in the presence of SGE, a proinflammatory environment initially exists in draining lymph nodes located near the site of infection. Also noteworthy were the expression levels of anti-inflammatory cytokines IL-4 and IL-10. When SGE was present, IL-4 and IL-10 were upregulated at several early and late days postinfection. Interestingly, at later days postinfection, the upregulation of anti-inflammatory IL-4 and IL-10 seems to coincide with the downregulation of the proinflammatory IL-1β, IL-6, and TNF-α when tick SGE is present. This overall immune response pattern correlates with the decrease in lymph node size that began at 6 dpi for the mice infected with 103 PFU of POWV.
Daily immune responses were examined in the brains of mice infected with 103 PFU of POWV in the presence versus the absence of SGE. In the presence of SGE, basal gene expression levels were observed until 4 dpi, followed by moderate upregulation of several genes at 5 dpi. By 7 to 8 dpi, CCL7, CCL11, IL-1β, IL-6, IL-10, and TNF-α all were significantly upregulated. At later days postinfection, an inflammatory environment clearly exists in the brains of mice infected with 103 PFU of POWV plus SGE. This correlates with the viral load data. At 4 and 5 dpi, POWV was detected in the brains of mice infected with 103 PFU POWV plus SGE and in mice infected with only 103 PFU POWV, respectively. From 5 to 8 dpi the increase in proinflammatory cytokine expression in the brains of mice infected with 103 PFU POWV plus SGE was marked. This occurred in tandem with the steadily increasing brain viral loads for mice treated with SGE. The enhanced expression of proinflammatory cytokines, specifically TNF-α and IFN-γ, has been implicated in neuroinvasion and development of encephalitis (27–29). Our data suggest that the proinflammatory environment facilitates neuroinvasion and virus replication, leading to encephalitis and death. Future work examining the brain pathology for mice in the group infected with 103 PFU of POWV plus SGE would supplement our immune response data.
Our data show that following a 103 PFU inoculation of POWV, the transmission and dissemination of virus is enhanced by the presence of I. scapularis saliva. This phenomenon was associated with accelerated disease progression, decreased survival times, and enhanced virus dissemination for low-dose POWV-infected mice that received SGE. Therefore, we conclude that saliva-activated transmission occurs in mice that are infected with quantities of POWV consistent with those that would be delivered during tick feeding. Our study was the first to demonstrate virus dose-dependent SAT, as this phenomenon did not occur at the high dose of POWV but did take place at the low dose.
To date, no SAT factor has been directly identified for any of the tick-borne viruses, and it is not known whether one or more than one salivary factor is linked to SAT of these viruses (30). Tick saliva contains a repertoire of antihemostatic, anti-inflammatory, and immunomodulatory factors. As a tick takes a blood meal, salivation is not a continuous process (31); thus, the repertoire of salivary proteins changes during the course of feeding (32, 33). Specific proteins responsible for enhancing tick-borne virus transmission have yet to be identified and characterized. Future identification of SAT factors would help build a foundation for developing salivary protein immunogens used to block POWV transmission. This ultimately could lead to the development of rational strategies to control POWV and perhaps other tick-borne flaviviruses.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by start-up funds to S.T. from the National Research Fund for Tick-Borne Disease (NRFTD), NIAID/NIH UC7 AIO94660. M.E.H. is supported by NIAID/NIH T32AI007526.
We thank David Beasley for prereviewing the manuscript and providing valuable suggestions.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.01056-15.
REFERENCES
- 1.McLean DM, Donahue W. 1959. Powassan virus: isolation of virus from a fatal case of encephalitis. Can Med Assoc J 80:708–711. [PMC free article] [PubMed] [Google Scholar]
- 2.Hinten S, Beckett GA, Gensheimer KF, Pritchard E, Courtney TM, Sears SD, Woytowicz JM, Preston DG, Smith RP Jr, Rand PW, Lacombe EH, Holman MS, Lubelczyk CB, Kelso PT, Beelen AP, Stobierski MG, Sotir MJ, Wong S, Ebel G, Kosoy O, Piesman J, Campbell GL, Marfin AA. 2008. Increased recognition of Powassan encephalitis in the United States, 1999-2005. Vector Borne Zoonotic Dis 8:733–740. doi: 10.1089/vbz.2008.0022. [DOI] [PubMed] [Google Scholar]
- 3.Holbrook M, Aronson J, Campbell G, Jones J, Feldmann H, Barrett A. 2005. An animal model for the tickborne flavivirus–Omsk hemorrhagic fever virus. J Infect Dis 191:100–108. doi: 10.1086/426397. [DOI] [PubMed] [Google Scholar]
- 4.Ebel GD. 2010. Update on Powassan virus: emergence of a North American tick-borne flavivirus. Annu Rev Entomol 55:95–110. doi: 10.1146/annurev-ento-112408-085446. [DOI] [PubMed] [Google Scholar]
- 5.Hermance ME, Thangamani S. 2014. Proinflammatory cytokines and chemokines at the skin interface during Powassan virus transmission. J Investig Dermatol 134:2280–2283. doi: 10.1038/jid.2014.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kazimirova M, Stibraniova I. 2013. Tick salivary compounds: their role in modulation of host defenses and pathogen transmission. Front Cell Infect Microbiol 3:43. doi: 10.3389/fcimb.2013.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schoeler G, Wikel S. 2001. Modulation of host immunity by haematophagous arthropods. Ann Trop Med Parasitol 95:755–771. doi: 10.1080/0003498012011118. [DOI] [PubMed] [Google Scholar]
- 8.Wikel S. 2013 Ticks and tick-borne pathogens at the cutaneous interface: host defenses, tick countermeasures, and a suitable environment for pathogen establishment. Front Microbiol 4:337. doi: 10.3389/fmicb.2013.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brossard M, Wikel S. 2004. Tick immunobiology. Parasitology 129:S161–S176. doi: 10.1017/S0031182004004834. [DOI] [PubMed] [Google Scholar]
- 10.Titus R, Bishop J, Mejia J. 2006. The immunomodulatory factors of arthropod saliva and the potential for these factors to serve as vaccine targets to prevent pathogen transmission. Parasite Immunol 28:131–141. doi: 10.1111/j.1365-3024.2006.00807.x. [DOI] [PubMed] [Google Scholar]
- 11.Labuda M, Jones L, Williams T, Nuttall P. 1993. Enhancement of tick-borne encephalitis virus transmission by tick salivary gland extracts. Med Vet Entomol 7:193–196. doi: 10.1111/j.1365-2915.1993.tb00674.x. [DOI] [PubMed] [Google Scholar]
- 12.Jones L, Hodgson E, Nuttall P. 1989. Enhancement of virus transmission by tick salivary glands. J Gen Virol 70:1895–1898. doi: 10.1099/0022-1317-70-7-1895. [DOI] [PubMed] [Google Scholar]
- 13.Krocová Z, Macela A, Hernychová L, Kroca M, Pechová J, Kopecký J. 2003. Tick salivary gland extract accelerates proliferation of Franciscella tularensis in the host. J Parasitol 89:14–20. doi: 10.1645/0022-3395(2003)089[0014:TSGEAP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 14.Horká H, Cerná-Kýcková K, Skallová A, Kopecký J. 2009. Tick saliva affects both proliferation and distribution of Borrelia burgdorferi spirochetes in mouse organs and increases transmission of spirochetes to ticks. Int J Med Microbiol 299:373–380. doi: 10.1016/j.ijmm.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Heinze DM, Wikel SK, Thangamani S, Alarcon-Chaidez FJ. 2012. Transcriptional profiling of the murine cutaneous response during initial and subsequent infestations with Ixodes scapularis nymphs. Parasit Vectors 5:26. doi: 10.1186/1756-3305-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson JF, Armstrong PM. 2012. Prevalence and genetic characterization of Powassan virus strains infecting Ixodes scapularis in Connecticut. Am J Trop Med Hyg 87:754–759. doi: 10.4269/ajtmh.2012.12-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider BS. 2006. Ph.D. dissertation The University of Texas Graduate School of Biomedical Sciences at Galveston, Galveston, TX. [Google Scholar]
- 18.Heinze DM, Carmical JR, Aronson JF, Thangamani S. 2012. Early immunologic events at the tick-host interface. PLoS One 7:e47301. doi: 10.1371/journal.pone.0047301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, An J. 2007. Cytokines, inflammation and pain. Int Anesthesiol Clin 45:27–37. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Titus R, Ribeiro J. 1988. Salivary gland lysates from the sand fly Lutzomyia longipalpis enhance Leishmania infectivity. Science 239:1306–1308. doi: 10.1126/science.3344436. [DOI] [PubMed] [Google Scholar]
- 21.Styer LM, Lim PY, Louie KL, Albright RG, Kramer LD, Bernard KA. 2011. Mosquito saliva causes enhancement of West Nile virus infection in mice. J Virol 85:1517–1527. doi: 10.1128/JVI.01112-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneider B, Higgs S. 2008. The enhancement of arbovirus transmission and disease by mosquito saliva is associated with modulation of the host immune response. Trans R Soc Trop Med Hyg 102:400–408. doi: 10.1016/j.trstmh.2008.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costero A, Grayson M. 1996. Experimental transmission of Powassan virus (Flaviviridae) by Ixodes scapularis ticks (Acari: Ixodidae). Am J Trop Med Hyg 55:536–546. [DOI] [PubMed] [Google Scholar]
- 24.American Committee on Arthropod-Borne Viruses. 2013. Reaction to the introduction of Chikungunya virus in St. Martin. American Society of Tropical Medicine and Hygiene, Deerfield, IL. [Google Scholar]
- 25.Ebel G, Kramer L. 2004. Short report: duration of tick attachment required for transmission of Powassan virus by deer ticks. Am J Trop Med Hyg 71:268–271. [PubMed] [Google Scholar]
- 26.Hayasaka D, Nagata N, Fujii Y, Hasegawa H, Sata T, Suzuki R, Gould E, Takashima I, Koike S. 2009. Mortality following peripheral infection with Tick-borne encephalitis virus results from a combination of central nervous system pathology, systemic inflammatory and stress responses. Virology 390:139–150. doi: 10.1016/j.virol.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 27.Orandle M, MacLean A, Sasseville V, Alvarez X, Lackner A. 2002. Enhanced expression of proinflammatory cytokines in the central nervous system is associated with neuroinvasion by simian immunodeficiency virus and the development of encephalitis. J Virol 76:5797–5802. doi: 10.1128/JVI.76.11.5797-5802.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang T, Town T, Alexopoulou L, Anderson J, Fikrig E, Flavell R. 2004. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med 10:1366–1373. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- 29.Ryman K, Gardner C, Meier K, Biron C, Johnston R, Klimstra W. 2007. Early restriction of alphavirus replication and dissemination contributes to age-dependent attenuation of systemic hyperinflammatory disease. J Gen Virol 88:518–529. doi: 10.1099/vir.0.82359-0. [DOI] [PubMed] [Google Scholar]
- 30.Nuttall P, Labuda M. 2004. Tick-host interactions: saliva-activated transmission. Parasitology 129:S177–S189. doi: 10.1017/S0031182004005633. [DOI] [PubMed] [Google Scholar]
- 31.Kaufman W. 1989. Tick-host interaction: a synthesis of current concepts. Trends Parasitol 5:47–56. [DOI] [PubMed] [Google Scholar]
- 32.McSwain J, Essenber R, Sauer J. 1982. Protein changes in the salivary glands of the female lone star tick, Amblyomma americanum, during feeding. J Parasitol 68:100–106. doi: 10.2307/3281330. [DOI] [PubMed] [Google Scholar]
- 33.Ribeiro JM, Alarcon-Chaidez F, Francischetti IM, Mans BJ, Mather TN, Valenzuela JG, Wikel SK. 2006. An annotated catalog of salivary gland transcripts from Ixodes scapularis ticks. Insect Biochem Mol Biol 36:111–129. doi: 10.1016/j.ibmb.2005.11.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.