Summary
The thymus is the most rapidly aging tissue in the body, with progressive atrophy beginning as early as birth and not later than adolescence. Latent regenerative potential exists in the atrophic thymus, since certain stimuli can induce quantitative regrowth, but qualitative function of T lymphocytes produced by the regenerated organ has not been fully assessed. Using a genome-wide computational approach, we show that accelerated thymic aging is primarily a function of stromal cells, and that while overall cellularity of the thymus can be restored, many other aspects of thymic function cannot. Medullary islet complexity and tissue-restricted antigen expression decrease with age, representing potential mechanisms for age-related increases in autoimmune disease, but neither of these is restored by induced regrowth, suggesting that new T cells produced by the regrown thymus will probably include more autoreactive cells. Global analysis of stromal gene expression profiles implicates widespread changes in Wnt signaling as the most significant hallmark of degeneration, changes that once again persist even at peak regrowth. Consistent with the permanent nature of age-related molecular changes in stromal cells, induced thymic regrowth is not durable, with the regrown organ returning to an atrophic state within two weeks of reaching peak size. Our findings indicate that while quantitative regrowth of the thymus is achievable, the changes associated with aging persist, including potential negative implications for autoimmunity.
Keywords: thymus, aging, stromal cells, regenerative medicine, computational biology
Introduction
The thymus is the primary site of T cell lymphopoiesis, but unlike virtually all other systems of steady-state differentiation, does not contain long-term self-renewing lymphoid progenitors, and instead depends on the periodic importation of marrow-derived progenitors that circulate in the blood (in the interest of space, Petrie & Zuniga-Pflucker, 2007 serves as a general resource for this Introduction). The progenitors that home to the thymus are multipotent, although they are distinct from all conventional marrow progenitor and stem cell populations in that they have T but not B lineage potential. In any case, once inside the thymus, these cells mainly produce T cells, suggesting that the thymic microenvironment is responsible for T lineage specification and repression of other lineage potentials. The thymic microenvironment represents a spectrum of developing T lymphoid cells, as well stromal cells of hematopoietic (mainly B cells, macrophages, and dendritic cells) and non-hematopoietic (mainly epithelial and mesenchymal) origin. Epithelial cells dominate the non-hematopoietic stroma, and are of particular importance since they express essential signals for T cell differentiation, provide the scaffold for outward migration of early-stage progenitors, and control proliferation by providing a limited number of niches for proliferating thymocyte progenitors. Thus, stromal well being is a key factor in determining the absolute number of lymphoid cells produced by the thymus.
Despite the need for lifelong replenishment of T cells that are lost to senescence, bleeding, and other causes, the thymus exhibits progressive atrophy beginning as early as birth, although it is most recognizable around the time of puberty (reviewed in Haynes et al., 2000). There has been substantial debate as to whether thymic atrophy primarily represents a defect in hematopoietic progenitor cells, or whether it is mainly a stromal phenotype (reviewed in Montecino-Rodriguez & Dorshkind, 2006). In any case, since production of new T cells is essentially proportional to thymic mass, thymic atrophy results in a progressive decline in the exportation of new T cells into the periphery (Haynes et al., 2000). While homeostatic expansion of existing T cells masks the appearance of lymphopenia, the end result is a gradual drift towards a repertoire that reflects oligo-clonal, rather than pan-clonal immunity, and an age-related increase in susceptibility to infectious disease, especially viruses (reviewed in Nikolich-Zugich & Rudd, 2010). Further, the inability to make new T cells is one of the main reasons why bone marrow stem cell transplants (as opposed to bone marrow transplants) are not widely used, since reconstitution of the recipient immune system is largely dependent on homeostatic replication of donor T cells infused with the bone marrow (see Weinberg et al., 2001). An age-related increase in autoimmune disorders is also recognized, although it is not clear whether this is related to thymic atrophy, to stromal or lymphoid aging, or both. Overall, age-related thymic atrophy remains both intellectually enigmatic and clinically problematic as human lifespan continues to increase.
It is well documented that even in advanced age the thymus retains profound regenerative capacity. The most efficient means is surgical castration, the effects of which on the thymus were first realized more than 100 years ago, and androgen blockade or other forms of regenerative therapy have been proposed as interventional therapies for age-related T cell immune-insufficiency (reviewed in Hollander et al., 2010). However, although induced regrowth of the thymus does result in increased exportation of new T cells (Sutherland et al., 2005; Weinberg et al., 2001; Williams et al., 2008), it is not clear how similar these cells are to those produced by the healthy young thymus; since T lymphopoiesis is only one of the essential functions of the thymus, other functions, such as endowment of functional capacity or self-tolerance, might differ. In this manuscript, we characterize the molecular mechanisms associated with thymic atrophy and regeneration in an inducible model of regrowth. We find that the changes associated with both aging and regeneration occur primarily in stromal cells (particularly cortical stromal cells), and include widespread changes in both canonical and non-canonical Wnt signaling pathways. Surprisingly, although the atrophic thymus can be quantitatively regrown, the molecular characteristics of stromal cells in the regrown thymus are virtually indistinct from those found in the atrophic thymus. Consequently, castration-induced regrowth of the thymus is transient, due to the persistence of age-related changes. We also show that tissue-restricted antigen (TRA) expression by medullary stromal cells decreases with age, and is not restored by regrowth, suggesting that the production of new T cells in the regenerated thymus is likely to carry adverse risks, including the potential for unexpected self-reactivity.
Results
Induced thymic regrowth is robust but not durable
It has been known for more than 100 years that the aged thymus retains latent regenerative potential, since strong systemic stimuli like castration can ameliorate atrophy or induce regrowth (Goodall, 1905; Sutherland et al., 2005). Harnessing this regenerative potential is being considered as a possible intervention for age-related degeneration of thymic function (Hollander et al., 2010). However, while the regrown thymus clearly makes more T lymphocytes than its atrophic counterpart (Sutherland et al., 2005; Weinberg et al., 2001), other functional aspects of the regrown thymus, such as the ability to induce self-tolerance, have not been thoroughly evaluated. We devised a strategy, based on our published approach for differential gene expression mapping (Griffith et al., 2009), to characterize molecular changes in both stromal and lymphoid cells that accompany age-related atrophy, and regrowth induced by castration. To identify essential points in the regrowth process, we first performed a systematic characterization of the kinetics of regrowth (Fig. 1). Thymuses in 12 month-old mice were initially about 25% of the cellularity found in 4–5 week-old mice (approximately 4 x 108 cells, data not shown), and initially exhibited a further loss of cellularity after castration, reaching a nadir at about 4 days, most likely representing stress due to anesthesia and surgery. After this point, log-phase growth ensued, obtaining peak organ size (~4 x 108 cells, virtually the same as that of 4–5 week-old mice) approximately 20 days after castration. Remarkably, however, this regrowth was very short lived, and an immediate and rapid decline ensued, reaching original (pre-castration) cellularity by 14 days later. The transient nature of this process suggests that although similar in size and composition, the regrown thymus and the healthy young thymus may be qualitatively different from one another.
Figure 1. Thymic regrowth in response to castration is robust but not durable.
Male mice at 11–13 months of age (nominally, 12 months) were castrated, followed by measurement of total cellularity at various intervals, as indicated. Red circles indicate individual thymuses (n = 48 animals total for this figure); the black line indicates a polynomial curve fit to this data. Cellularity exhibited a rapid initial decrease, reaching a nadir at day 3, followed by a period of rapid growth. Growth peaked at approximately 20 days post-castration, at which time cellularity was virtually indistinguishable from that found in 5–6 week-old mice (peak thymus size, data not shown). However, regrowth in response to castration was not durable, and cellularity had degenerated to near the original (pre-castration) value by approximately 2 weeks later.
Atrophy and regrowth responses are primarily attributable to stromal cells in the cortex
Defects in both lymphoid and stromal cells have been implicated in the accelerated atrophy of the thymus that accompanies aging (reviewed in Montecino-Rodriguez & Dorshkind, 2006). While an impact of aging on hemato-lymphoid progenitors is inevitable, the onset of thymic atrophy, which may be as early as birth, but certainly occurs no later than adolescence, seems to precede any known defects in the lymphoid component. A dominant effect of stromal vs. lymphoid cells in early thymic aging has also been suggested by numerous transplant models (Doria et al., 1997; Zhu et al., 2007; Mackall et al., 1998). To assess the relative contributions of lymphoid vs. stromal cells to aging and regrowth, we utilized a recently published approach (Griffith et al., 2009) for deriving global stromal gene expression signatures in situ, using microdissected tissue and purified lymphoid cells to probe cDNA microarrays (Affymetrix MOE-430_2). Cortical or medullary stromal gene expression signatures were calculated using 7 existing datasets each for young cortex or medulla (GEO accession # GSE18281), as well as 3 additional gene chips each for cortex or medulla from thymuses of aged (12 month-old) mice, or 12 month-old mice at the peak of regrowth after castration (day 20; see Fig. 1). Lymphoid gene expression signatures were measured from 5 existing datasets each for cortical or medullary cells from young mice (same resource as above), or 3 additional gene chips each for cortical or medullary lymphoid cells from thymuses of aged or peak of regrowth mice. The total dataset was thus 48 gene chips. Each gene chip contained pooled cells or tissue from multiple mice and multiple dates of harvest. A tabular summary of PLIER signals for all gene chips is contained in Suppl. Table 1. Stromal signals were calculated from this data as described in Methods.
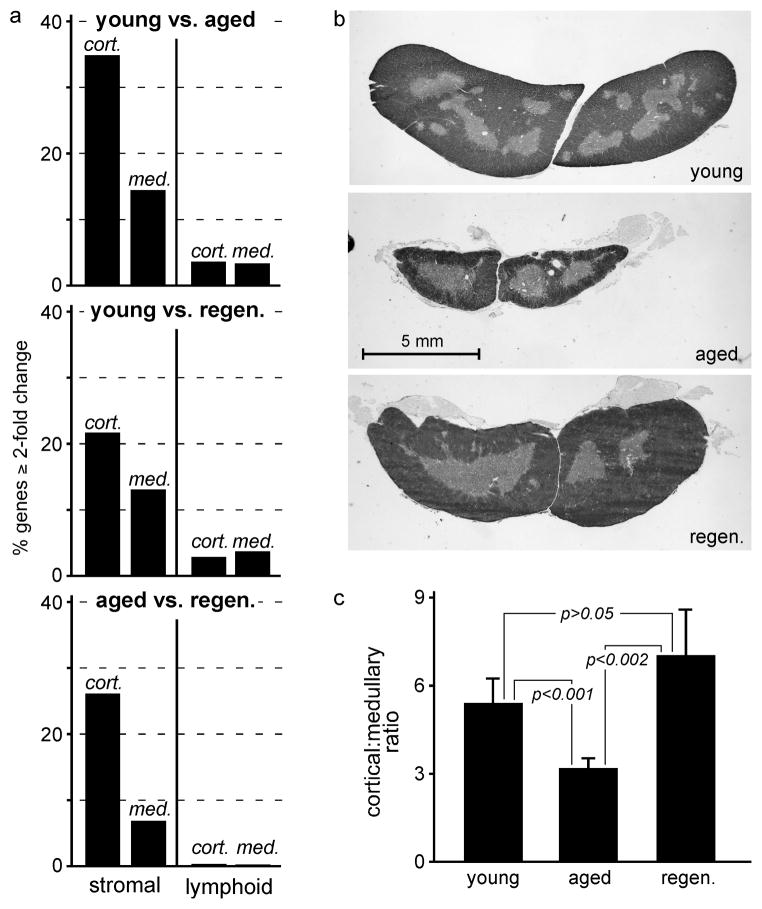
Fig. 2a shows the proportion of all genes that changed by at least twofold when cortical or medullary stromal or lymphoid cells from young, aged, or regenerated thymuses were compared. Only a small proportion (about 3–4%) of all genes were changed when lymphoid cells from young mice were compared to their counterparts from the aged or regenerated thymus; this is consistent with our findings (Suppl. Fig. 1) as well as that of others (Sutherland et al., 2005) showing that lymphoid development in the aged and regenerated thymus is essentially normal. In contrast, about 1 in every 3 cortical stromal genes was changed, and about half that many medullary stromal genes were changed (1 in 6), when young stroma was compared to aged. When young samples were compared to regenerated, this overall pattern (cortical stromal > medullary stromal > cortical or medullary lymphoid) was maintained, although the absolute magnitude of differences was less, especially in the cortex. Importantly, almost no differences were seen between lymphoid cells in the aged and regenerated thymuses, further substantiating the concept that differences between the atrophic and regrown thymus were stromal, not lymphoid, in origin. Quantitative differences between cortical and medullary stroma from aged vs. regenerated thymuses again indicated a dominant role for the cortex.
Figure 2. Aging and regeneration primarily reflect changes in stromal cells, and are dominated by changes in the cortex.
Panel a) shows the proportion of all genes (probesets) that were expressed (nominally, signal > median for PLIER signals) in at least one condition (young, aged, or regenerated) in stromal or lymphoid cells from the indicated compartments (cortex, medulla), and that also changed at least 2-fold in the indicated comparisons (young:aged, young:regenerated, aged:regenerated). Changes in cortical cells were dominant, with changes in the cortex dominating those in the medulla. Changes in lymphoid cells were minimal, indicating that the primary changes associated with early atrophy of the thymus are stromal in nature. Panel b) shows typical transverse sections from the mid-point of the thymus of young, aged, and 20 days post-castration mice. Panel c) shows relative quantitation of cortical:medullary area ratios (mean ± s.d.) for 5 thymuses of each type (significance determined by two-tailed Student’s t-test for unpaired samples). Both b) and c) substantiate the conclusion that the most profound changes occurring during both aging and regeneration occur in the cortex. Each experiment was repeated 3–7 times, as described in Materials and Methods, and each experiment contained RNA pooled from multiple mice. Actual numbers of probesets in each bar are as follows: young vs. aged cortical stroma (CS) = 14,914, medullary stroma (MS) = 6663, cortical lymphocytes (CL) = 1895, medullary lymphocytes (ML) = 1728; young vs. regenerated CS = 9940, MS = 6071, CL = 1444, ML = 1853; aged vs regenerated CS = 11,798, MS = 3114, CL = 220, ML = 89.
We further evaluated the relative contributions of cortical vs. medullary compartments to atrophy and regrowth by area measurements of histological sections. Panel b) shows representative cross-sections of young, aged (12 month), and regrown (12 month at 20 days post-castration) thymuses. Panel c) shows quantitative assessment of 5 mice for each type, revealing that the ratio of cortical:medullary area decreases substantially with age, but is restored in the regrown thymus. Overall, our studies indicate that at a time (roughly mid-life) when atrophy is already quite substantial (~75% loss of cellularity), changes in stromal cells are predominant, with cortical stromal changes being the most obvious. A dominant effect of aging on the cortical compartment has been indicated by the data of others in mice, rats, and humans (Steinmann et al., 1985; Brelinska et al., 2008; Li et al., 2003; Sutherland et al., 2005), although restoration of cortical volume in the (transiently) regenerated thymus has not been reported.
Atrophy coincides with losses in medullary complexity and tissue-restricted antigen expression that are not restored by regrowth
During our assessment of cortical:medullary volumes during aging and regrowth (Fig. 2), we noted that medullary complexity, as represented by the presence of circumscribed medullary areas, was decreased in the atrophic thymus. Quantitative assessment of large numbers of tissue sections from each type of thymus (see Methods) shows that medullary complexity is reduced approximately threefold in the atrophic thymus (Fig. 3a). It is not known whether this reflects loss or fusion of clonally distinct islets, or simply decreased branching, but importantly, the condensation of medullary islets persists in the regrown thymus (Fig. 3a, see also Fig. 2b) suggesting that these changes are permanent. Since medullary islets may each represent unique microenvironments (Rodewald et al., 2001), we asked whether the spectrum of tissue-restricted antigens (TRA) expressed by medullary stromal cells might also decrease with age. As described in Methods, we used an informatics approach to generate an objective list of TRA, using a body atlas of mouse gene expression data (biogps.org), a published method for determining the tissue specificity of any given gene (Hughes & Friedman, 2006), and our previously published list of medullary stromal-specific genes (Griffith et al., 2009). We then compared gene expression levels for the resulting 238 probesets (nominally genes) in young vs. aged, young vs. regenerated, or aged vs. regenerated medullary stroma (Fig. 3b). Most of the 148 TRA that were significantly changed (indicated in black) between young and aged were decreased, and include a broad spectrum of widely accepted non-thymic tissue antigens expressed by medullary epithelial cells (Suppl. Table 2). A smaller proportion of TRA (33 in total) increased; manual inspection of the list reveals these to be almost exclusively B cell genes. Consistent with this, we find a dramatic increase in the density of B cells in the medulla of aged mice (Fig. 3c). The relevance of this change is not known, but confirmation of the informatics approach by the presence of increased B cells in the medulla (Fig. 3c) confirms the predictive nature of our approach. When a random list of the same size was used (Fig. 3b), the number of changed genes (47) was substantially smaller, and the number of genes that went up (27) was nearly the same as the number that went down (20). Likewise, a manually curated list of known medullary epithelial cell genes (Suppl. Table 3) showed roughly equal distributions of increased and decreased genes. These findings suggest that the change in distribution of TRA genes was not likely to have occurred by chance, or from changes in the proportion of TRA-expressing epithelial cells among all medullary stroma.
Figure 3. Medullary complexity and tissue-restricted antigen (TRA) expression decrease with age, and are not restored when the thymus is regrown.
Panel a) shows medullary islet counts per lobe for young, aged, and peak regenerated thymuses; bars represent mean ± s.d. for 55 tissue sections from 5 young mice, 109 tissue sections from 6 aged mice, or 81 tissue sections from 4 castrated mice (day 20), while statistical significance was calculated using the two-tailed Student’s t-test for unpaired samples. Medullary islet number decreased approximately 3-fold with age, and did not change even after complete regrowth. Panel b) shows relative expression of TRA in medullary stromal cells from young, aged, and regenerated thymuses; statistically significant changes in expression (two-tailed Student’s t-test for unpaired samples, corrected for multiple testing, see Methods) are indicated by black points, while those that were not statistically significant are indicated in gray. Medullary stroma from aged mice showed substantial changes in TRA expression. The majority of these, representing a broad panel of non-thymic antigens (Suppl. Table 2), were decreased; those that increased exclusively represented B lineage genes, consistent with an increase in the frequency of B cells in the medulla with age (panel c, B220 staining in red, cytokeratin staining in green). The same trend was observed when young medullary stroma was compared to that in the regenerated thymus, with the specific identities of these changed genes almost completely overlapping with that found in young vs. aged. Importantly, there were no significant differences in TRA expression when aged stroma was compared to regenerated, indicating that, like medullary complexity, quantitative regrowth of thymic mass does not translate to a qualitative restoration of TRA expression. Each experiment was repeated 3–7 times, as described in Materials and Methods, and each experiment contained RNA pooled from multiple mice.
Comparison of young vs. regenerated medullary stroma bore a striking similarity to that of young vs. aged stroma (Fig. 3b); a few TRA, mostly representing B lineage genes (Suppl. Table 2), went up, but most TRA, representing a wide variety of non-thymic antigens, were decreased. Strikingly, 74 of the 79 TRA from our list that decreased in regenerated thymus when compared to young were also decreased in aged thymus when compared to young. Even more poignant, when TRA expression in aged stromal cells was compared to that of regenerated, no significant differences could be found. Identical outcomes were reached for a random gene list and the list of known medullary epithelial genes described above. Together, these data strongly support our earlier conclusion that although quantitative differences in cellularity can be induced by castration, the qualitative nature of changes in thymic stroma is not altered by this regrowth. In light of these persistent defects in TRA expression, increased T cell output from the regrown thymus (Sutherland et al., 2005) is expected to be accompanied by a proportional, and potentially dangerous, increase in self-reactive T cells.
Computer learning algorithms distinguish young from aged or young from regenerated stromal gene signatures, but cannot distinguish aged from regenerated stroma
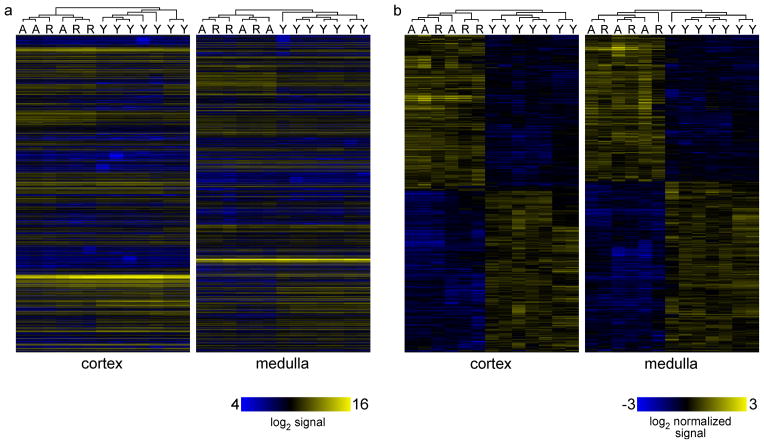
The collapse of the thymus after castration-induced regrowth (Fig. 1), together with the failure to restore medullary complexity or TRA expression on regrowth (Fig. 3) suggests that the regrown thymus is qualitatively very similar to the aged thymus. We further examined this possibility by performing unsupervised computer clustering (GeneSpring two-dimensional hierarchical clustering of MAS5 signals, Pearson-centered similarity, linkage rule “average”) of all 24,763 genes (probesets) designated as being expressed in the medulla (at least 6 out of 7 MAS5 Present calls for young tissue, OR 3/3 P calls for aged or regenerated), or 22,109 genes designated as being expressed in cortex (same criteria). The heatmap shown in Fig. 4a reinforces the conclusion that young can be distinguished from aged or regenerated, while the latter are very similar to each other. All 7 samples from young mouse thymus clustered together in either cortex or medulla, and were completely distinct from samples from aged or regenerated thymuses. In contrast, aged and regenerated failed to segregate from each other in either cortex or medulla, and instead were randomly distributed within their cluster (distinct from young thymus). To determine whether aged and regenerated samples could be distinguished using a more restricted list than all expressed genes, we performed similar two-dimensional clustering on those genes that changed significantly (Benjamini-Hochberg corrected one-way ANOVA p value < 0.05) during transition from young to aged to regenerated, representing 5717 genes (probesets) in medulla, and 3644 in cortex. The outcome of this more restricted analysis was virtually identical, with young samples being distinguished from both aged and regenerated in both cortical and medullary compartments, while aged and regenerated failed to segregate from each other. Together, these findings further support our conclusion that while castration induces quantitative regrowth, the qualitative aspects of the regenerated thymus are little changed from that of the aged thymus from which it derives.
Figure 4. Computer learning algorithms distinguish young from aged or young from regenerated stromal gene signatures, but cannot distinguish aged from regenerated.
Panel a) shows results of unsupervised two-dimensional clustering of absolute MAS5 values values (log2 signal) for all genes expressed in either young (7 gene chips), aged (3 gene chips), or regenerated (3 gene chips) medulla or cortex, as indicated. All samples from young mice clustered together, and were distinct from aged or regenerated samples. Importantly, aged and regenerated datasets failed to segregate from each other, and were essentially indistinguishable at a global level, despite quantitative changes in organ size. Even when only those genes that underwent a significant (corrected ANOVA p < 0.05) fold change were analyzed (panel b), as opposed to all expressed genes (panel a), the same result was obtained, suggesting that the differences between young and aged thymus persist in the regrown thymus, despite the apparent restoration of cellularity. Each experiment was repeated 3–7 times, as described in Materials and Methods, and each experiment contained RNA pooled from multiple mice.
Pathway analysis reveals potential mechanisms for age-related thymic atrophy, and their persistence in the regenerated thymus
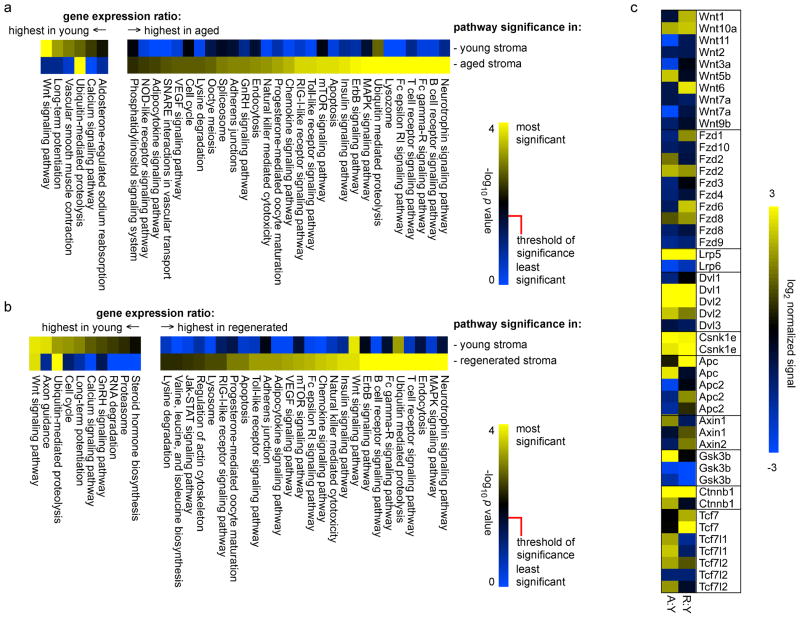
The data described above, as well as the findings of others (Steinmann et al., 1985; Brelinska et al., 2008; Li et al., 2003), suggest that the cortex plays a dominant role in thymic atrophy, and likewise dominates the regrowth response. We used our global transcriptional profiles of cortical stromal cells from young, aged, and regenerated thymuses to establish the molecular mechanisms of these processes. To do this, we mapped the most changed genes, nominally defined as the top and bottom 20% of stromal gene expression ratios between the three different states, onto KEGG signaling pathways. Pathways that were significantly changed (Benjamini-Hochberg corrected Student’s two-tailed t-test p value < 0.05) when young cortical stroma was compared to aged cortical stroma are shown in Fig. 5a, while similar comparison of young and regenerated are shown in Fig. 5b. Given the nature of global gene expression studies, it was remarkable to note that KEGG pathways associated with genes that changed the most when aged cortical stroma was compared to young were almost identical, save minor changes in statistical rank-order, when regenerated stroma was compared to young. For instance, the canonical Wnt signaling pathway was the most significant pathway characterized by genes that were down-regulated in both aged and regenerated cortical stroma as compared to young. In general, pathways represented by genes that were higher in young stroma than aged stroma were recapitulated by regenerated stroma, and likewise for those that were higher in aged than young. The likelihood of pathways that differ in young vs. aged stroma being duplicated by chance in young vs. regenerated stroma is very small (one-tailed Fisher’s exact test p value < 0.00015), further substantiating the near identity of stromal cells in the aged and regenerated thymus. Note that in some cases, pathways may appear as both higher in young than aged/regenerated and higher in aged/regenerated than young. This paradox results from the fact that most pathways consist of both negative and positive regulatory elements, and an increase (for instance) in a negative regulatory component has the same effect as a decrease in the positive component. As an example, Wnt 5b, which promotes adipogenesis (van Tienen et al., 2009), increases with age in cortical stroma, while Wnt7a and Wnt3a, which promote stem cell expansion (Le Grand et al., 2009) and epithelial cell division (Liu et al., 2010), respectively, are diminished. Thus, different pathway components can move in opposite directions while exerting the same or complimentary effects. While we make no claims for causality, the dominance of both canonical and non-canonical (calcium signaling) Wnt pathways in these non-presumptive analyses predicts a dominant role for Wnt signaling in the age-related atrophy process. This conclusion is further supported by large body of genetic evidence for the roles of individual Wnt components in thymic stromal development, survival, and/or senescence (Balciunaite et al., 2002; Liang et al., 2007; Kuraguchi et al., 2006; Kvell et al., 2010; Zuklys et al., 2009; Osada et al., 2006; Yang et al., 2009). The persistence of such age-related changes in the regrown thymus may thus explain the non-durable nature of induced regrowth, since essential survival/senescence pathways (such as Wnt signaling) would continue to be affected in the regrown organ, resulting in an inevitable collapse, as described in more detail below.
Figure 5. Biological pathways associated with transcriptional signatures in young, aged, or regenerated cortical stromal cells.
Genes represented by the top 20% of cortical stromal signal ratios (young aged, panel a; young regenerated, panel b) were mapped onto KEGG pathways, and pathways were represented at statistically significant levels (Benjamini-Hochberg corrected p value) are shown. Similar to the findings shown in Figs. 3 and 4, pathways representing differences between young and aged were very consistent with those distinguishing differences between young and regenerated, indicating that the regenerated thymus maintains most of the molecular characteristics of the aged thymus, despite an increase in size. This is further illustrated by analysis of individual components of the Wnt signaling pathway, which is the most negatively regulated pathway both during aging (panel a) and in the regrown thymus (panel b). Note that in some cases (in particular, ubiquitin-mediated proteolysis and, to a lesser extent, Wnt signaling), pathways may appear as both higher in young than aged/regenerated, and higher in aged/regenerated than young; this is because different paralogs, and/or positive vs. negative regulators of the pathway, may move in different directions, allowing statistical significance in both cases. A complete description is beyond the limitations of this manuscript, but all relevant data are contained in Suppl. Table 1. Panel c shows relative (normalized to young cortical stroma) gene expression for canonical Wnt pathway components in aged or regenerated stroma; again, the changes seen in regenerated stroma are very similar to those found in aged stroma, indicating the persistence of age related changes after regrowth.
Discussion
The rapid involution of the thymus with age is enigmatic because T cells are lost throughout life to a variety of causes, and must continuously be replaced for homeostasis and to maintain normal immune function. It is unclear what constitutes the driving force behind early thymic involution, or whether it is, in fact, a process that has been driven by selective pressure. Given that the thymus is one of the most recently evolved organs (discussed in Boehm & Bleul, 2007), found almost exclusively in jawed vertebrates, it is conceivable that it is still evolving into a more refined or efficient state. Alternatively, given the substantial body of evidence for endocrine function(s) of the thymus, including development from a shared primordium with the parathyroids (Graham, 2003), and clear functional roles in regulating reproductive function and puberty (for examples, see Besedovsky & Sorkin, 1974; Weinstein, 1978; Pierpaoli & Sorkin, 1972), it seems worthwhile to consider that T cell production may not be the original function of the thymus, but rather a recently acquired function. In this case, accelerated thymic atrophy may represent incomplete adaptation to the biological burden of lymphopoietic activity in an otherwise endocrine organ. Late acquisition of secondary lymphopoietic function by an erstwhile endocrine organ may also explain why T cells are the only blood lineage produced ectopically to bone marrow in an epithelial (rather than mesenchymal) organ, another feature of T cell biology for which the biological basis has yet to be satisfactorily explained. In any case, our data support the hypothesis that a decline in thymus size and T cell output with age is, in fact, desirable, since we find that aging results in a decrease in TRA expression (Fig. 3). Consequently, T cells produced by the aging thymus would be progressively less stringently screened against self-antigens, and would include proportionally more self-reactive cells. The tendency towards a net decrease (except for B cell antigens, see Fig. 3) in global TRA expression in our unbiased (informatics-based) list may help to explain why the frequency of autoimmune diseases increases with age, and is in agreement with other studies regarding expression of presumptive TRA (Sospedra et al., 1998). It is important to note that we do not find any correlation between Aire expression and TRA expression (Suppl. Table 3), nor do putative Aire targets (Gillard et al., 2007) exhibit a more profound change than non-Aire targets (not shown), suggesting that changes in Aire expression do not explain the loss of TRA with aging, and supporting the concept (Derbinski et al., 2005; Gillard et al., 2007) that Aire is probably not uniquely responsible for regulation of TRA in medullary stromal cells.
In addition to pronounced changes in TRA expression, we find that thymic atrophy is associated with broad alterations in multiple transcriptional profiles, especially in stromal cells. The question of whether age-related thymic atrophy results from changes in the hematopoietic or stromal compartment has been the subject of substantial debate (reviewed in Montecino-Rodriguez & Dorshkind, 2006). While the eventual aging of the hematopoietic compartment seems inevitable, our data indicates that early thymic atrophy is overwhelmingly a function of changes in thymic stromal cells, particularly cortical cells, with minimal changes in the lymphoid cells of either compartment, even at a time (12 months) when atrophy is quite substantial (~75% reduction in cellularity; see Fig. 1). Since lymphoid cellularity in the thymus is a direct function of competition for stromal niches by early progenitors (Prockop & Petrie, 2004), a decrease in cortical stromal function would directly impact lymphoid cellularity at all stages, and would also impact medullary stromal cells indirectly via lympho-stromal crosstalk (Nitta et al., 2011). Non-presumptive analysis of the differences between young and aged cortical stromal cells suggests a clear molecular basis for the degeneration process (Fig. 5a). Most notably, unbiased, non-presumptive analysis of the data, using informatics approaches, strongly implicates both canonical and non-canonical forms of the Wnt signaling pathway in thymic atrophy, with panoramic changes occurring in virtually all components of these pathways (Fig. 5b and 5c). Although our data do not prove causality, the Wnt pathway has been shown to be important for specification, proliferation, and survival of thymic stroma (Yang et al., 2009; Balciunaite et al., 2002; Kuraguchi et al., 2006; Zuklys et al., 2009; Osada et al., 2010), and preliminary evidence suggests a role in thymic senescence as well (Kvell et al., 2010). Our non-presumptive global analysis of stromal gene expression suggests that age-related, durable changes in Wnt signaling exert a dominant effect on cortical stromal cells that may explain most of the downstream phenotype resulting in atrophy. Since thymic epithelial cell development, proliferation, and survival are also critically dependent on Fgf signaling (Gillard et al., 2007; Revest et al., 2001; Kelly et al., 2010; Rossi et al., 2007), and since decreased Fgf signaling can lead directly to a decrease in Wnt function (Rossi et al., 2007), it is tempting to suggest that age-related changes in the Wnt pathway are a consequence of upstream changes in Fgf signaling. While we find little change in Fgfs or Fgf receptors with age or upon regeneration (derived from data in Suppl. Table 1), and those that do change generally tend to increase, it is possible that Fgf availability, much of which may be extrathymically derived, may nonetheless be modulated in a way that affects bioavailability in the thymus without affecting transcription in situ. Nonetheless, it seems unlikely that all of the genes in the Wnt pathway are targets of such Fgf signaling, and since almost all of them change, it would appear that Fgf-independent mechanisms of regulation must also be in play.
In addition to making comprehensive predictions regarding the mechanism of thymic atrophy, our data provide a clear explanation for why the castration-induced regrowth process is not durable (see Fig. 1). Specifically, we find that virtually all of the changes associated with aging of stromal cells in the thymus persist in the regrown thymus. For instance, our findings suggest that the lack of durable regrowth results, at least in part, from persistent changes in the Wnt signaling pathways that are virtually indistinguishable from those found to occur with aging. Thus, the inability of the thymus to sustain inducible regrowth appears to result from the fact that the stromal cells in the regenerated thymus are fundamentally indistinct from those in the atrophic thymus, and maintain all of the accumulated changes that occur as a consequence of age. While this explains the relatively rapid collapse of the regrown thymus after its peak, the mechanisms of the acute regrowth phase itself remain unknown, including whether or not it represents a direct response to the presence/absence of testosterone. While there are clear indications that both stromal and lymphoid cells can respond to testosterone, it seems logical that a direct response to the absence of testosterone would result in more sustained regrowth, since testosterone levels do not subsequently return to normal. Since this is not the case, i.e., since the thymus subsequently reverts to the atrophic state, the castration-induced regrowth response seems more likely to represent an acute systemic reaction to the sudden absence of a powerful hormonal stimulus (testosterone), followed by a gradual reversion to steady state that includes the reappearance of atrophy.
The ability of the thymus to regrow in response to stimuli such as castration leads to a proportional increase in de novo production of naïve T cells (Sutherland et al., 2005; Weinberg et al., 2001; Williams et al., 2008). Induced regrowth (via androgen blockade or other means) is being considered as an interventional approach to enhance the production of new T cells in aging and other forms of secondary immunodeficiencies, including poor vaccine responsiveness in the elderly and T cell regeneration after marrow stem cell transplantation in adults (Hollander et al., 2010). The underlying assumption is that the quantitative increase in T cell production accompanying regrowth reflects T cells that are qualitatively pristine. However, our data suggest that this concept may require further examination, and that making more T cells on aged (regenerated) stroma may have unintended negative consequences. In particular, the persistent suppression of TRA in the regrown thymus would predict that increased T cell production appears likely to be accompanied by a proportional increase in the release of self-reactive cells from the thymus, potentially resulting in the induction of autoimmune disorders and/or exacerbation of nascent autoimmune disease. In addition, numerous studies have suggested that, like new T cells produced by the aged thymus (Eaton et al., 2008; Hale et al., 2006; Clise-Dwyer et al., 2007), new T cells made by the regenerated thymus are qualitatively inferior in their ability to proliferate in response to mitogen or antigen, or to make cytokines (Dean et al., 1984; Utsuyama et al., 1995; Brunelli et al., 1992). This can be explained by the near total identity of gene expression in lymphocytes isolated from the aged vs. regenerated thymus (Fig. 2), a process that we believe reflects the persistence of age-related changes in cortical and medullary stromal cells. Overall, these combined data suggest that in the case of new T cell production by the regenerated thymus, quantity does not necessarily imply quality, and provides at least a cautionary note regarding potential negative outcomes that may accompany artificially enhanced T cell production, especially in otherwise healthy elderly individuals.
Experimental procedures
Information regarding mice, surgical castration, tissue processing, microdissection, cell sorting, microarray techniques, and computational and statistical techniques can be found in online supporting information.
Supplementary Material
Acknowledgments
This work was supported by PHS grants R01AG031576, R01AG034876, and R01AI095831 (to HTP), and F32AG032193 (to AG).
Footnotes
Author contributions. AG and HP participated in study design, data collection, data analysis, and manuscript preparation. MF and TV participated in data analysis and manuscript preparation.
- Supplemental Figure 1. Lymphoid differentiation is normal in young, atrophic, and transiently regenerated thymuses.
- Supplemental Figure 2. Validation of cross-comparison of measured and calculated gene expression in young, aged, or regenerated thymuses.
-
Supplemental Table 1. PLIER normalized complete microarray dataset.Sample legend:
- Y = young
- A = aged
- R = regenerated
- CT = cortical tissue
- MT = medullary tissue
- CL = cortical lymphocytes
- ML = medullary lymphocytes
- numbers indicate biological replicates (1, 2, 3, etc.)
- Supplemental Table 2. List of genes identified as tissue-restricted antigens and their expression in young, aged, or medullary stroma.
- Supplemental Table 3. Manually curated list of known markers of epithelial stromal cells.
Contributor Information
Ann V. Griffith, Email: agriffit@scripps.edu.
Mohammad Fallahi, Email: mfallahi@scripps.edu.
Thomas Venables, Email: tvenable@scripps.edu.
Howard T. Petrie, Email: hpetrie@scripps.edu.
References
- Balciunaite G, Keller MP, Balciunaite E, Piali L, Zuklys S, Mathieu YD, Gill J, Boyd R, Sussman DJ, Hollander GA. Wnt glycoproteins regulate the expression of FoxN1, the gene defective in nude mice. Nat Immunol. 2002;3:1102–1108. doi: 10.1038/ni850. [DOI] [PubMed] [Google Scholar]
- Besedovsky HO, Sorkin E. Thymus involvement in female sexual maturation. Nature. 1974;249:356–358. doi: 10.1038/249356a0. [DOI] [PubMed] [Google Scholar]
- Boehm T, Bleul CC. The evolutionary history of lymphoid organs. Nat Immunol. 2007;8:131–135. doi: 10.1038/ni1435. [DOI] [PubMed] [Google Scholar]
- Brelinska R, Malendowicz LK, Malinska A, Kowalska K. Characteristics of age-related changes in rat thymus: morphometric analysis and epithelial cell network in various thymic compartments. Biogerontology. 2008;9:93–108. doi: 10.1007/s10522-007-9117-3. [DOI] [PubMed] [Google Scholar]
- Brunelli R, Frasca D, Spano M, Zichella L, Doria G. Gonadectomy in old mice induces thymus regeneration but does not recover mitotic responsiveness. Ann N Y Acad Sci. 1992;673:252–255. doi: 10.1111/j.1749-6632.1992.tb27460.x. [DOI] [PubMed] [Google Scholar]
- Clise-Dwyer K, Huston GE, Buck AL, Duso DK, Swain SL. Environmental and intrinsic factors lead to antigen unresponsiveness in CD4(+) recent thymic emigrants from aged mice. J Immunol. 2007;178:1321–1331. doi: 10.4049/jimmunol.178.3.1321. [DOI] [PubMed] [Google Scholar]
- Dean DH, Hiramoto RN, Ghanta VK, Hiramoto NS, Wall CN. Effects of orchiectomy on spleen cell mitogen response and on the thymus of aged mice. Exp Aging Res. 1984;10:189–191. doi: 10.1080/03610738408258462. [DOI] [PubMed] [Google Scholar]
- Derbinski J, Gabler J, Brors B, Tierling S, Jonnakuty S, Hergenhahn M, Peltonen L, Walter J, Kyewski B. Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J Exp Med. 2005;202:33–45. doi: 10.1084/jem.20050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria G, Mancini C, Utsuyama M, Frasca D, Hirokawa K. Aging of the recipients but not of the bone marrow donors enhances autoimmunity in syngeneic radiation chimeras. Mech Ageing Dev. 1997;95:131–142. doi: 10.1016/s0047-6374(97)01871-x. [DOI] [PubMed] [Google Scholar]
- Eaton SM, Maue AC, Swain SL, Haynes L. Bone marrow precursor cells from aged mice generate CD4 T cells that function well in primary and memory responses. J Immunol. 2008;181:4825–4831. doi: 10.4049/jimmunol.181.7.4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillard GO, Dooley J, Erickson M, Peltonen L, Farr AG. Aire-dependent alterations in medullary thymic epithelium indicate a role for Aire in thymic epithelial differentiation. J Immunol. 2007;178:3007–3015. doi: 10.4049/jimmunol.178.5.3007. [DOI] [PubMed] [Google Scholar]
- Goodall A. The post-natal changes in the thymus of guinea-pigs, and the effect of castration on thymus structure. J Physiol. 1905;32:191–198. doi: 10.1113/jphysiol.1905.sp001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A. Development of the pharyngeal arches. Am J Med Genet A. 2003;119A:251–256. doi: 10.1002/ajmg.a.10980. [DOI] [PubMed] [Google Scholar]
- Griffith AV, Fallahi M, Nakase H, Gosink M, Young B, Petrie HT. Spatial mapping of thymic stromal microenvironments reveals unique features influencing T lymphoid differentiation. Immunity. 2009;31:999–1009. doi: 10.1016/j.immuni.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale JS, Boursalian TE, Turk GL, Fink PJ. Thymic output in aged mice. Proc Natl Acad Sci U S A. 2006;103:8447–8452. doi: 10.1073/pnas.0601040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes BF, Markert ML, Sempowski GD, Patel DD, Hale LP. The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection. Annu Rev Immunol. 2000;18:529–560. doi: 10.1146/annurev.immunol.18.1.529. [DOI] [PubMed] [Google Scholar]
- Hollander GA, Krenger W, Blazar BR. Emerging strategies to boost thymic function. Curr Opin Pharmacol. 2010;10:443–453. doi: 10.1016/j.coph.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AL, Friedman R. Across-tissue expression and evolution of genes controlled by the Aire transcription factor. Genomics. 2006;88:462–467. doi: 10.1016/j.ygeno.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Kelly RM, Goren EM, Taylor PA, Mueller SN, Stefanski HE, Osborn MJ, Scott HS, Komarova EA, Gudkov AV, Hollander GA, Blazar BR. Short-term inhibition of p53 combined with keratinocyte growth factor improves thymic epithelial cell recovery and enhances T-cell reconstitution after murine bone marrow transplantation. Blood. 2010;115:1088–1097. doi: 10.1182/blood-2009-05-223198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraguchi M, Wang XP, Bronson RT, Rothenberg R, Ohene-Baah NY, Lund JJ, Kucherlapati M, Maas RL, Kucherlapati R. Adenomatous polyposis coli (APC) is required for normal development of skin and thymus. PLoS Genet. 2006;2:e146. doi: 10.1371/journal.pgen.0020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvell K, Varecza Z, Bartis D, Hesse S, Parnell S, Anderson G, Jenkinson EJ, Pongracz JE. Wnt4 and LAP2alpha as pacemakers of thymic epithelial senescence. PLoS One. 2010;5:e10701. doi: 10.1371/journal.pone.0010701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Grand F, Jones AE, Seale V, Scime A, Rudnicki MA. Wnt7a activates the planar cell polarity pathway to drive the symmetric expansion of satellite stem cells. Cell Stem Cell. 2009;4:535–547. doi: 10.1016/j.stem.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Hsu HC, Grizzle WE, Stockard CR, Ho KJ, Lott P, Yang PA, Zhang HG, Mountz JD. Cellular mechanism of thymic involution. Scand J Immunol. 2003;57:410–422. doi: 10.1046/j.1365-3083.2003.01206.x. [DOI] [PubMed] [Google Scholar]
- Liang H, Coles AH, Zhu Z, Zayas J, Jurecic R, Kang J, Jones SN. Noncanonical Wnt signaling promotes apoptosis in thymocyte development. J Exp Med. 2007;204:3077–3084. doi: 10.1084/jem.20062692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Luo M, Xie W, Wells JM, Goodheart MJ, Engelhardt JF. Sox17 modulates Wnt3A/beta-catenin-mediated transcriptional activation of the Lef-1 promoter. Am J Physiol Lung Cell Mol Physiol. 2010;299:L694–710. doi: 10.1152/ajplung.00140.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackall CL, Punt JA, Morgan P, Farr AG, Gress RE. Thymic function in young/old chimeras: substantial thymic T cell regenerative capacity despite irreversible age-associated thymic involution. Eur J Immunol. 1998;28:1886–1893. doi: 10.1002/(SICI)1521-4141(199806)28:06<1886::AID-IMMU1886>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Montecino-Rodriguez E, Dorshkind K. Evolving patterns of lymphopoiesis from embryogenesis through senescence. Immunity. 2006;24:659–662. doi: 10.1016/j.immuni.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Nikolich-Zugich J, Rudd BD. Immune memory and aging: an infinite or finite resource? Curr Opin Immunol. 2010;22:535–540. doi: 10.1016/j.coi.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta T, Ohigashi I, Nakagawa Y, Takahama Y. Cytokine crosstalk for thymic medulla formation. Curr Opin Immunol. 2011;23:190–197. doi: 10.1016/j.coi.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Osada M, Ito E, Fermin HA, Vazquez-Cintron E, Venkatesh T, Friedel RH, Pezzano M. The Wnt signaling antagonist Kremen1 is required for development of thymic architecture. Clin Dev Immunol. 2006;13:299–319. doi: 10.1080/17402520600935097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada M, Jardine L, Misir R, Andl T, Millar SE, Pezzano M. DKK1 mediated inhibition of Wnt signaling in postnatal mice leads to loss of TEC progenitors and thymic degeneration. PLoS One. 2010;5:e9062. doi: 10.1371/journal.pone.0009062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie HT, Zuniga-Pflucker JC. Zoned out: functional mapping of stromal signaling microenvironments in the thymus. Annu Rev Immunol. 2007;25:649–679. doi: 10.1146/annurev.immunol.23.021704.115715. [DOI] [PubMed] [Google Scholar]
- Pierpaoli W, Sorkin E. Alterations of adrenal cortex and thyroid in mice with congenital absence of the thymus. Nat New Biol. 1972;238:282–285. doi: 10.1038/newbio238282a0. [DOI] [PubMed] [Google Scholar]
- Prockop SE, Petrie HT. Regulation of thymus size by competition for stromal niches among early T cell progenitors. J Immunol. 2004;173:1604–1611. doi: 10.4049/jimmunol.173.3.1604. [DOI] [PubMed] [Google Scholar]
- Revest JM, Suniara RK, Kerr K, Owen JJ, Dickson C. Development of the thymus requires signaling through the fibroblast growth factor receptor R2-IIIb. J Immunol. 2001;167:1954–1961. doi: 10.4049/jimmunol.167.4.1954. [DOI] [PubMed] [Google Scholar]
- Rodewald HR, Paul S, Haller C, Bluethmann H, Blum C. Thymus medulla consisting of epithelial islets each derived from a single progenitor. Nature. 2001;414:763–768. doi: 10.1038/414763a. [DOI] [PubMed] [Google Scholar]
- Rossi SW, Jeker LT, Ueno T, Kuse S, Keller MP, Zuklys S, Gudkov AV, Takahama Y, Krenger W, Blazar BR, Hollander GA. Keratinocyte growth factor (KGF) enhances postnatal T-cell development via enhancements in proliferation and function of thymic epithelial cells. Blood. 2007;109:3803–3811. doi: 10.1182/blood-2006-10-049767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sospedra M, Ferrer-Francesch X, Dominguez O, Juan M, Foz-Sala M, Pujol-Borrell R. Transcription of a broad range of self-antigens in human thymus suggests a role for central mechanisms in tolerance toward peripheral antigens. J Immunol. 1998;161:5918–5929. [PubMed] [Google Scholar]
- Steinmann GG, Klaus B, Muller-Hermelink HK. The involution of the ageing human thymic epithelium is independent of puberty. A morphometric study. Scand J Immunol. 1985;22:563–575. doi: 10.1111/j.1365-3083.1985.tb01916.x. [DOI] [PubMed] [Google Scholar]
- Sutherland JS, Goldberg GL, Hammett MV, Uldrich AP, Berzins SP, Heng TS, Blazar BR, Millar JL, Malin MA, Chidgey AP, Boyd RL. Activation of thymic regeneration in mice and humans following androgen blockade. J Immunol. 2005;175:2741–2753. doi: 10.4049/jimmunol.175.4.2741. [DOI] [PubMed] [Google Scholar]
- Utsuyama M, Hirokawa K, Mancini C, Brunelli R, Leter G, Doria G. Differential effects of gonadectomy on thymic stromal cells in promoting T cell differentiation in mice. Mech Ageing Dev. 1995;81:107–117. doi: 10.1016/0047-6374(95)01589-r. [DOI] [PubMed] [Google Scholar]
- van Tienen FH, Laeremans H, van der Kallen CJ, Smeets HJ. Wnt5b stimulates adipogenesis by activating PPARgamma, and inhibiting the beta-catenin dependent Wnt signaling pathway together with Wnt5a. Biochem Biophys Res Commun. 2009;387:207–211. doi: 10.1016/j.bbrc.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Weinberg K, Blazar BR, Wagner JE, Agura E, Hill BJ, Smogorzewska M, Koup RA, Betts MR, Collins RH, Douek DC. Factors affecting thymic function after allogeneic hematopoietic stem cell transplantation. Blood. 2001;97:1458–1466. doi: 10.1182/blood.v97.5.1458. [DOI] [PubMed] [Google Scholar]
- Weinstein Y. Impairment of the hypothalamo-pituitary-ovarian axis of the athymic “nude” mouse. Mech Ageing Dev. 1978;8:63–68. doi: 10.1016/0047-6374(78)90007-6. [DOI] [PubMed] [Google Scholar]
- Williams KM, Lucas PJ, Bare CV, Wang J, Chu YW, Tayler E, Kapoor V, Gress RE. CCL25 increases thymopoiesis after androgen withdrawal. Blood. 2008;112:3255–3263. doi: 10.1182/blood-2008-04-153627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Youm YH, Sun Y, Rim JS, Galban CJ, Vandanmagsar B, Dixit VD. Axin expression in thymic stromal cells contributes to an age-related increase in thymic adiposity and is associated with reduced thymopoiesis independently of ghrelin signaling. J Leukoc Biol. 2009;85:928–938. doi: 10.1189/jlb.1008621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Gui J, Dohkan J, Cheng L, Barnes PF, Su DM. Lymphohematopoietic progenitors do not have a synchronized defect with age-related thymic involution. Aging Cell. 2007;6:663–672. doi: 10.1111/j.1474-9726.2007.00325.x. [DOI] [PubMed] [Google Scholar]
- Zuklys S, Gill J, Keller MP, Hauri-Hohl M, Zhanybekova S, Balciunaite G, Na KJ, Jeker LT, Hafen K, Tsukamoto N, Amagai T, Taketo MM, Krenger W, Hollander GA. Stabilized beta-catenin in thymic epithelial cells blocks thymus development and function. J Immunol. 2009;182:2997–3007. doi: 10.4049/jimmunol.0713723. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.