Abstract
Multicentric carpotarsal osteolysis syndrome (MCTO), an autosomal dominant disorder that often presents sporadically, features carpal-tarsal lysis frequently followed by nephropathy and renal failure. In 2012, mutations in the single-exon gene MAFB were reported in 13 probands with MCTO. MAFB is a negative regulator of RANKL-mediated osteoclastogenesis. We studied 9 MCTO patients (7 sporadic patients and one affected mother and son) for MAFB mutation. We PCR-amplified and selectively sequenced the MAFB region that contains the transactivation domain in this 323 amino acid protein, where mutations were previously reported for MCTO. We found 5 different heterozygous missense defects among 8 probands: c.176C>T, p.Pro59Leu; c.185C>T, p.Thr62Ile; c.206C>T, p.Ser69Leu (4 had this defect); c.209C>T, p.Ser70Leu; and c.211C>T, p.Pro71Ser. All 5 mutations are within a 13 amino acid stretch of the transactivation domain. Four were identical to the previously reported mutations. Our unique mutation (c.185C>T, p.Thr62Ile) involved the same domain. DNA available from 7 parents of the 7 sporadic patients did not show their child’s MAFB mutation. The affected mother and son had an identical defect. Hence, the mutations for 7/8 probands were suspected to have arisen spontaneously as there was no history of features of MCTO in either parent. Penetrance of MCTO seemed complete. Lack of nonsense or other truncating mutations suggested a dominant-negative pathogenesis. Our findings indicate that only a few transactivation domain-specific mutations within MAFB cause MCTO.
Keywords: dysplasia, nephropathy, osteoclast
INTRODUCTION
Multicentric carpotarsal osteolysis syndrome (MCTO, OMIM # 166300), also called idiopathic multicentric osteolysis with nephropathy, is an autosomal dominant (AD) disorder often arising spontaneously and causing carpal-tarsal destruction and renal failure [Shurtleff et al., 1964; Whyte et al., 1978]. We have cared collectively for nine unrelated children with sporadic (n = 8) or familial (n = 1) MCTO during the past 36 years [Whyte et al., 1978; Wenkert et al., 2007; Wenkert et al., 2008; Goldfarb et al., 2012]. In 2012, mutations within the single-exon MAFB [v-maf musculoaponeurotic fibrosarcoma oncogene ortholog B (avian)] were reported in 13 probands with MCTO [Zankl et al., 2012]. MAFB is a member of the MAF family of transcription factors, and is expressed in the kidney [Moriguchi et al., 2006] and in the monocyte/macrophage lineage, but not in other hematopoietic cells [Kim et al., 2007].
The RANK signaling pathway is essential for osteoclast differentiation and activation [Whyte and Mumm, 2004]. RANK, a cell surface receptor on osteoclast precursors, is stimulated by RANKL, resulting in osteoclastogenesis and activation of mature osteoclasts. This process is regulated by OPG, which binds RANKL acting as a decoy receptor. Genetic defects that either constitutively activate RANK or deactivate OPG by altering TNFRSF11A or TNFRSF11B, cause high-turnover bone diseases, such as familial expansile osteolysis and juvenile Paget disease, respectively [Hughes et al., 2000; Whyte et al., 2002; Cundy et al., 2002]. These molecular defects occur at the “top” of the RANK signaling pathway. Mutations in MAFB feature lytic bone disease (MCTO), apparently by downstream disruption of RANK signaling where transcription factors normally induce gene expression [Kim et al., 2007]. MAFB is a negative regulator of RANKL-induced osteoclast differentiation [Kim et al., 2007] by binding to MAF recognition DNA elements (MAREs) to induce target genes [Kim et al., 2007; Matsushima-Hibiya et al., 1998]. MAFB also inhibits the DNA binding ability of transcription factors c-Fos and Mitf, suppressing NFATc2 and OSCAR expression, two genes induced by RANKL/RANK signaling and important for osteoclastogenesis [Kim et al., 2007].
Hence, defects in MAFB would affect RANKL-mediated osteoclast differentiation, causing an imbalance in bone remodeling, apparently leading to the significant osteolysis seen in MCTO [Kim et al., 2007]. We investigated our MCTO cohort for mutations in MAFB.
PATIENTS AND METHODS
Patients
After obtaining written consent sanctioned by the Human Research Protection Office at the Washington University School of Medicine, St. Louis, we studied 8 unrelated children with MCTO at Shriners Hospital for Children, St. Louis, USA (SHC) during the past 36 years [Whyte et al., 1978; Wenkert et al., 2007; Wenkert et al., 2008; Goldfarb et al., 2012]. DNA was available for seven of eight probands. These seven patients (Patients 1–7) generally presented with joint pain in the wrists or feet starting between birth and age 3 years (Table I). Most were diagnosed erroneously with juvenile idiopathic arthritis (JIA) before referral to us. MCTO was established for 4/7 patients between ages 1–5 years based on characteristic radiographic changes showing carpal and tarsal dissolution (Table I). The other three were diagnosed with MCTO between ages 6 and 14 years. All 7 individuals were considered to have sporadic MCTO. An example of their osteolysis is shown for Patient 1 (Fig 1). Lysis could be evident in other bones. Proteinuria was documented between ages 1 – 12 years in six of seven probands; three required renal transplantation (Table I). Based on previous reports of corneal opacity in MCTO [Shinohara et al., 1991; Malecha and Wilroy, 2003], we recommended eye exams for our patients and corneal clouding was noted in three (Table I).
Table I.
MAFB Mutations and Clinical Features for Our Cohort
| Patients | MAFB Mutation | Symptom Onset; Initial Diagnosis | Age at MCTO Diagnosis (Years) | Other Joints Affected | Renal Findings‡ | Eye Problems |
|---|---|---|---|---|---|---|
| 1 | c.209C>T, p.Ser70Leu^ | Birth; JIA @ 2.5 years | 3 | Shoulders, Knees, Elbows | Trace proteinuria (3 years) | Corneal clouding |
| 2 | c.185C>T, p.Thr62Ile* | 6 months; JIA | 1.5 | Elbows | Proteinuria (4 years), Renal Transplant (11 years) | Corneal clouding |
| 3 | c.211C>T, p.Pro71Ser | 2 months | 1.5 | Elbows, Knees | Proteinuria (21 months), Unsuccessful Renal transplant (9 years) | None |
| 4 | c.176C>T, p.Pro59Leu | 2 months; rickets | 3 | TMJ, Shoulders, Elbows, Knees | Proteinuria (5 years) | None |
| 5 | c.206C>T, p.Ser69Leu | 2.5 years; JIA | 14 | Elbows | Proteinuria (7 years), Renal transplant (17 years) | None |
| 6 | c.206C>T, p.Ser69Leu | Not known; JIA @ 6 years (no records before 6 years) | 11 | Elbows | Proteinuria (14 years) | None |
| 7 | c.206C>T, p.Ser69Leu | 12 months; JIA | 6.5 | Elbows | None | Corneal clouding |
| 8 | c.206C>T, p.Ser69Leu | 20 months for symptoms; thought to have JIA at 5 years; carpal osteolysis at 6 years | 5.5 | Elbow | None | None |
| 9 (mother of Pt. 8) | c.206C>T, p.Ser69Leu | 3 years; JIA | 38 | Cervical Spine, Elbows, PIP Joints of Hands | Renal failure, shrunk kidney | None |
clear de novo mutation
unique mutation
Proteinuria – age when first detected
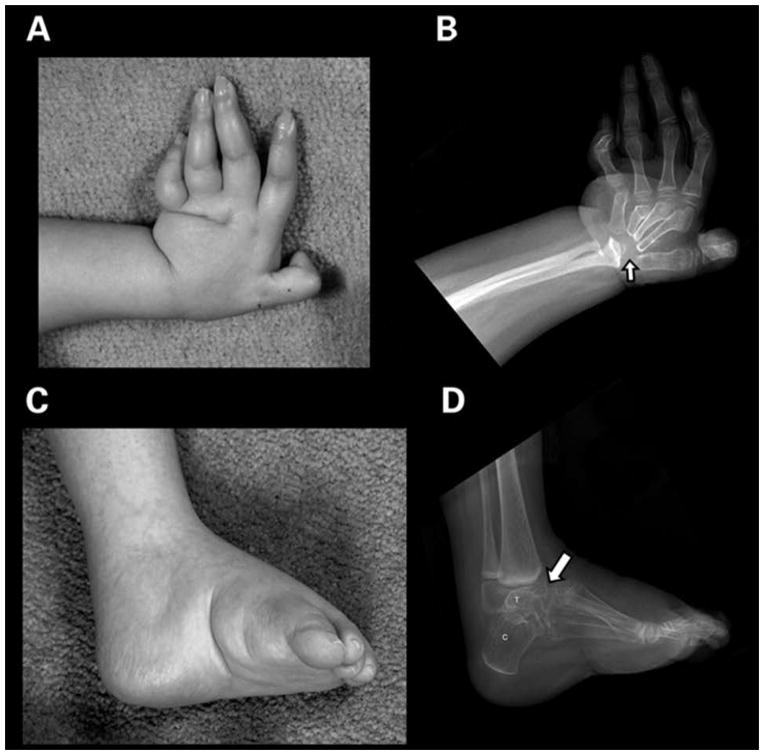
Figure 1.
A and B. Left hand: There is radial deviation of this osteopenic hand that has flexion contractures, and complete destruction of the carpal bones (arrow). The proximal metacarpals are eroded and tapered. There is distortion of the distal radius with loss of a majority of the radial epiphysis and erosions of the distal ulna. All of the distal metacarpal heads exhibit erosion and deformity. Several proximal interphalangeal joints have osteolysis and distortion.
C and D. Lateral left foot: There is marked osteolysis of the tarsal bones (arrow) and the distal tibial epiphysis in this osteopenic cavovarus foot. Only the posterior portions of the calcaneus (C) and talus (T) are still recognizable. The ankle joint is lost. There is rounding of the anterior end of the tibia from bone loss. The provisional zones of calcification in the distal tibia and fibula are dense from bisphosphonate therapy.
Most recently, we studied patient #8 from The Hospital for Sick Children, Toronto, Canada, and his affected mother. He was first seen at age 20 months for the same symptoms experienced by his mother as a toddler. Physical examination and radiographs of his hands and feet were normal at that time. His mother had been diagnosed with JIA at age 3 years. At age 5 years, Patient 8 developed pain, swelling, and loss-of-motion of the right wrist; an MRI showed synovitis and tenosynovitis. Nonsteroidal anti-inflammatory agents, intra-articular corticosteroids, and intravenous pulse methylprednisolone were ineffective. Then, a radiograph at age 5½ years showed osteolysis of the right carpus, and a diagnosis of MCTO was established. With these findings, the mother’s radiographs were reviewed and found to be typical for MCTO. She was referred to a nephrologist for evaluation who discovered that she was severely hypertensive and had significant renal dysfunction.
METHODS
Genomic DNA was isolated from blood leukocytes using the Puregene DNA Purification System (Gentra, Minneapolis, MN, USA). We amplified by PCR and sequenced the segment of the single exon MAFB, containing mutations previously reported by Zankl et al. [2012] to cause MCTO. Two sets of PCR primers and conditions were used to amplify and sequence MAFB (available on request). Twenty DNA samples from healthy Caucasians (Coriell Life Sciences, Camden, NJ) were PCR amplified and sequenced as an experimental control. The patients’ DNA sequence electropherograms were analyzed by visual examination and by aligning patient and control sequences using VectorNTI-AlignX software (Invitrogen, Carlsbad, CA) to identify any DNA changes. Mutations were validated by their absence (as common polymorphisms) from the SNP database (dbSNP). Of note, two mutations are listed in dbSNP (rs387907006 and rs387907007) as “pathogenic”.
RESULTS
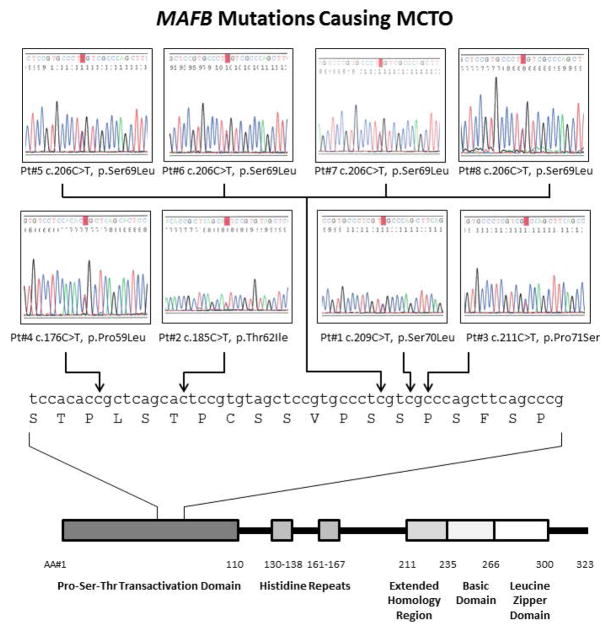
We analyzed MAFB in eight probands with MCTO by PCR amplification and sequencing of the region where mutations were reported in 2012 [Zankl et al., 2012]. We discovered five different heterozygous missense mutations [c.176C>T, p.Pro59Leu; c.185C>T, p.Thr62Ile; c.206C>T, p.Ser69Leu in 4 probands; c.209C>T, p.Ser70Leu; and c.211C>T, p.Pro71Ser]. All localized within a 13 amino acid stretch of the transactivation domain of the 323 amino acid MAFB protein (Fig 2). Four mutations were recurrent as they had been identified by Zankl et al [2012]; one was unique (c.185C>T, p.Thr62Ile) (Table II). One parent was affected and shared her son’s mutation. DNA available from seven of the other 15 parents (5 mothers and one mother-father set for Pt. 1), who were all considered unaffected, did not show their child’s MAFB mutation. Hence, the mutations for 7/8 probands were considered to have arisen spontaneously as there was no history of features of MCTO in either parent. We found no evidence of incomplete penetrance. Remarkably, all mutations were C to T transitions (pyrimidine to pyrimidine).
Figure 2.
MAFB mutations causing MCTO in our patient cohort. Top: DNA sequence electropherograms show heterozygous MAFB mutations for each proband. Bottom: MAFB gene shows amino acid number and important protein domains. The region containing mutations is expanded showing nucleotide and amino acid sequence, based on Wang et al. [1999].
Table II.
MAFB Mutations Causing MCTO
| Mutation | # Probands | Reference |
|---|---|---|
| c.161C>G, p.Ser54Trp | 1 | Mehawej et al., 2013 |
| c.161C>T, p.Ser54Leu | 2 | Zankl et al., 2012; Zankl et al., 2014 |
| c.167C>T, p.Ser56Phe | 1 | Dworschak et al., 2013 |
| c.176C>T, p.Pro59Leu | 3 | Mumm et al.; Mehawej et al., 2013; Zankl et al., 2012 |
| c.184A>C, p.Thr62Pro | 1 | Zankl et al., 2012 |
| c.185C>T, p.Thr62Ile | 1 | Mumm et al. |
| c.188C>G, p.Pro63Arg | 2 | Mehawej et al., 2013; Zankl et al., 2012 |
| c.188C>T, p.Pro63Leu | 2 | Mehawej et al., 2013 |
| c.194G>T, p.Ser65Ile | 1 | Mehawej et al., 2013 |
| c.197C>G, p.Ser66Cys | 1 | Zankl et al., 2012 |
| c.206C>T, p.Ser69Leu | 5 | Mumm et al.; Zankl et al., 2012 |
| c.208T>G, p.Ser70Ala | 1 | Zankl et al., 2012 |
| c.209C>T, p.Ser70Leu | 3 | Mumm et al.; Zankl et al., 2012 |
| c.211C>T, p.Pro71Ser | 3 | Mumm et al.; Zankl et al., 2012 |
| c.212C>T, p.Pro71Leu | 1 | Zankl et al., 2012 |
The parental age ranges at the time of birth of their affected child (excluding our multigeneration patients) were: fathers (n = 5) 29 – 38 years, and mothers (n = 6) 23 – 37 years.
DISCUSSION
From our study of 8 probands with MCTO, we identified five different heterozygous missense mutations in MAFB, one present in each. Four of these MAFB defects were recurrent when compared to the 10 mutations reported in 2012 [Zankl et al., 2012]. One was unique. Our mutations are all located within a 36 bp (13 amino acid) segment of the single exon MAFB. Our results: i) validate our previous [Wenkert et al., 2007; Wenkert et al., 2008; Goldfarb et al., 2012] phenotypic characterizations of MCTO, ii) are consistent with the results of Zankl et al. [2012], and iii) suggest that only a few domain-specific defects within MAFB cause MCTO. Autosomal dominant inheritance has been documented several times for MCTO [Whyte et al., 1978; Zankl et al., 2012; Pai and Macpherson, 1988], and was confirmed in our affected family.
Since our preliminary report of these findings [Mumm et al., 2012], mutations in this same region of MAFB (including 4 novel missense defects) were reported in 2013 in 7 additional unrelated MCTO patients/families [Dworschak et al., 2013; Mehawej et al., 2013]. Because all 15 known MAFB mutations (Table II) occur in a specific region (52 bp of the 969 bp total coding region = ~5% of the MAFB coding region), and all are missense, the pathogenesis of MCTO likely involves a dominant-negative effect. Haploinsufficiency seems unlikely to explain MCTO because nonsense mutations, and other mutations throughout the MAFB coding region have not been reported.
We note that all MAFB mutations detected in our 8 MCTO probands are C>T transitions, suggesting a shared molecular mechanism of the mutagenesis, which occurred spontaneously in seven of eight probands, and was dominantly inherited in one. In fact, transition mutations may be more common generally than transversions, due to differential repair of mismatched bases during DNA replication [Strachen and Read, 2003]. In particular, C>T transitions are the most frequent single base-pair substitutions in the human genome, because cytosine is commonly methylated at CpG dinucleotides, which in turn is spontaneously deaminated to thymine [Strachen and Read, 2003]. Indeed, six of eight probands are mutated at CpG, and otherwise one at CpT, and one at CpC. Four probands share the recurrent CpG to TpG mutation at c.206C>T. p.Ser69Leu, suggesting this is the leading cause of MCTO. In fact, using the CpG island function of the UCSC Genome Browser, we noted that much of MAFB (including its transactivation domain) constitutes a CpG island, a region of higher than average content of the dinucleotide CpG. CpG dinucleotides can be “hyper-mutated” in heritable diseases, due to methylation of cytosine in the germ line [Strachen and Read, 2003]. Oocytes exhibit low methylation whereas sperm exhibit higher methylation rates [Strachen and Read, 2003]. Therefore, spontaneous mutations often arise in the paternal germline, but we did not test paternal sperm for MAFB mutations. In our cohort, we found that the parental ages of our patients were younger than those associated with germline mutation [Toriello and Meck, 2008]. In fact, Zankl et al. [2012] reported C>T transitions in 9/13 MCTO simplex patients or families, and transversions in the remainder (A>C, C>G in 2 patients, T>G). Mehawej et al. [2013] report 3/6 C>T transitions, and the one proband reported by Dworschak et al. [2013] also had a C>T transition.
The mutations in MAFB that cause MCTO are concentrated in a small region of the Pro-Ser-Thr rich acidic transcriptional activation domain in the N-terminus [Wang et al., 1999]. Zankl et al. [2012] noted that these mutations lie within a region of MAFB that is phosphorylated. Therefore, MCTO mutations may affect MAFB phosphorylation and thereby alter the protein’s action. Functional studies and creation of mouse models based on MCTO mutations should improve our understanding of the pathophysiology of this disorder. Such studies will elucidate how defects in MAFB lead to lytic bone disease. A refined understanding of the pathogenesis may clarify why osteolysis targets the carpal and tarsal bones. Based on mouse studies, MAFB is essential for renal development, including podocyte differentiation and renal tubule survival [Moriguchi et al., 2005]. Therefore, the nephropathy of MCTO likely represents a fundamental renal developmental defect caused by MAFB mutations, and is not a secondary effect of hypercalcemia or hypercalciuria from osteolysis.
In conclusion, clinical and molecular investigation of our patient cohort confirms the reports of Zankl et al. [2012], Dworschak et al. [2013], Mehawej et al. [2013], demonstrating that only a few domain-specific MAFB mutations cause MCTO.
Acknowledgments
This study was made possible by the skill and dedication of the nursing and laboratory staff at the Center for Metabolic Bone Disease and Molecular Research, Shriners Hospital for Children, St. Louis, MO, USA. Patient # 8 was referred by Dr. Earl Silverman. Sharon McKenzie provided expert secretarial help.
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number DK067145. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This project was also funded in part by Shriners Hospitals for Children, The Clark and Mildred Cox Inherited Metabolic Bone Disease Research Fund, The Hypophosphatasia Research Fund, The Barnes-Jewish Hospital Foundation, and The Frederick S. Upton Foundation.
Footnotes
Presented in part at the 34th Annual Meeting of the American Society for Bone and Mineral Research, Minneapolis, MN, USA, October 12 – 15, 2012 [J Bone Miner Res 27 (Suppl 1). Available at http://www.asbmr.org/Meetings/AnnualMeeting/AbstractDetail.aspx?aid=2dc05f42-bb2e-45ca-99b5-6656d74aa1f3.]
Disclosures: The authors have no conflict of interest. Dr. Deborah Wenkert has since become an employee of Amgen Inc. and has received salary, stock, and stock options.
References
- Cundy T, Hegde M, Naot D, Chong B, King A, Wallace R, Mulley J, Love DR, Seidel J, Fawkner M, Banovic T, Callon KE, Grey AB, Reid IR, Middleton-Hardie CA, Cornish J. A mutation in the gene TNFRSF11B encoding osteoprotegerin causes an idiopathic hyperphosphatasia phenotype. Hum Mol Genet. 2002;11:2119–2127. doi: 10.1093/hmg/11.18.2119. [DOI] [PubMed] [Google Scholar]
- Dworschak GC, Draaken M, Hilger A, Born M, Reutter H, Ludwig M. An incompletely penetrant novel MAFB (p.Ser56Phe) variant in autosomal dominant multicentric carpotarsal osteolysis syndrome. Int J Mol Med. 2013;32:174–178. doi: 10.3892/ijmm.2013.1373. [DOI] [PubMed] [Google Scholar]
- Goldfarb CA, Steffen JA, Whyte MP. Idiopathic multicentric osteolysis: upper extremity manifestations and surgical considerations during childhood. J Hand Surg Am. 2012;37:1677–1683. doi: 10.1016/j.jhsa.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Hughes AE, Ralston SH, Marken J, Bell C, MacPherson H, Wallace RG, van Hul W, Whyte MP, Nakatsuka K, Hovy L, Anderson DM. Mutations in TNFRSF11A, affecting the signal peptide of RANK, cause familial expansile osteolysis. Nat Genet. 2000;24:45–48. doi: 10.1038/71667. [DOI] [PubMed] [Google Scholar]
- Kim K, Kim JH, Lee J, Jin HM, Kook H, Kim KK, Lee SY, Kim N. MafB negatively regulates RANKL-mediated osteoclast differentiation. Blood. 2007;109:3253–3259. doi: 10.1182/blood-2006-09-048249. [DOI] [PubMed] [Google Scholar]
- Malecha MA, Wilroy RS., Jr Bilateral corneal opacities associated with idiopathic multicentric osteolysis. Cornea. 2003;22:377–378. doi: 10.1097/00003226-200305000-00019. [DOI] [PubMed] [Google Scholar]
- Matsushima-Hibiya Y, Nishi S, Sakai M. Rat maf-related factors: the specificities of DNA binding and heterodimer formation. Biochem Biophys Res Commun. 1998;245:412–418. doi: 10.1006/bbrc.1998.8447. [DOI] [PubMed] [Google Scholar]
- Mehawej C, Courcet JB, Baujat G, Mouy R, Gérard M, Landru I, Gosselin M, Koehrer P, Mousson C, Breton S, Quartier P, Le Merrer M, Faivre L, Cormier-Daire V. The identification of MAFB mutations in eight patients with multicentric carpo-tarsal osteolysis supports genetic homogeneity but clinical variability. Am J Med Genet A. 2013 Aug 16; doi: 10.1002/ajmg.a.36151. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Moriguchi T, Hamada M, Morito N, Terunuma T, Hasegawa K, Zhang C, Yokomizo T, Esaki R, Kuroda E, Yoh K, Kudo T, Nagata M, Greaves DR, Engel JD, Yamamoto M, Takahashi S. MafB is essential for renal development and F4/80 expression in macrophages. Mol Cell Biol. 2006;26:5715–5727. doi: 10.1128/MCB.00001-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumm S, Huskey M, Wenkert D, Gottesman GS, Madson K, McAlister W, Whyte M. A limited number of mutations in MAFB, a negative regulator of RANKL-induced osteoclastogenesis, cause idiopathic multicentric osteolysis with nephropathy. 2012 doi: 10.1002/ajmg.a.36641. http://www.asbmr.org/Meetings/AnnualMeeting/AbstractDetail.aspx?aid=2dc05f42-bb2e-45ca-99b5-6656d74aa1f3. [DOI] [PMC free article] [PubMed]
- Pai GS, Macpherson RI. Idiopathic multicentric osteolysis: report of two new cases and a review of the literature. Am J Med Genet. 1988;29:929–936. doi: 10.1002/ajmg.1320290425. [DOI] [PubMed] [Google Scholar]
- Shinohara O, Kubota C, Kimura M, Nishimura G, Takahashi S. Essential osteolysis associated with nephropathy, corneal opacity, and pulmonary stenosis. Am J Med Genet. 1991;41:482–486. doi: 10.1002/ajmg.1320410421. [DOI] [PubMed] [Google Scholar]
- Shurtleff DB, Sparkes RS, Clawson DK, Guntheroth WG, Mottet NK. Hereditary osteolysis with hypertension and nephropathy. JAMA. 1964;188:363–368. doi: 10.1001/jama.1964.03060300025005. [DOI] [PubMed] [Google Scholar]
- Strachen T, Read AP. Chapter 11 Garland Science. Vol. 3. London and New York: Human Molecular Genetics; 2003. Instability of the human genome: mutation and DNA repair; pp. 315–349. [Google Scholar]
- Toriello HV, Meck JM. Statement on guidance for genetic counseling in advanced paternal age. Genetics in Medicine. 2008;10:457–460. doi: 10.1097/GIM.0b013e318176fabb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PW, Eisenbart JD, Cordes SP, Barsh GS, Stoffel M, Le Beau MM. Human KRML (MAFB): cDNA cloning, genomic structure, and evaluation as a candidate tumor suppressor gene in myeloid leukemias. Genomics. 1999;59:275–81. doi: 10.1006/geno.1999.5884. [DOI] [PubMed] [Google Scholar]
- Wenkert D, McAlister WH, Whyte MP. Additional Clinical Features of Idiopathic Multicentric Osteolysis with Nephropathy (OMIM %166300). Presented at the 30th Annual Meeting of the American Society for Bone and Mineral Research; September 12–16, 2008; Montreal, Quebec. 2008. Available Online at: http://www.abstractsonline.com/viewer/SearchResults.asp\. [Google Scholar]
- Wenkert D, Mumm S, Wiegand SM, McAlister WH, Whyte MP. Absence of MMP2 mutation in idiopathic multicentric osteolysis with nephropathy. Clin Orthop Relat Res. 2007;462:80–6. doi: 10.1097/BLO.0b013e3180d09db8. [DOI] [PubMed] [Google Scholar]
- Whyte MP, Mumm S. Heritable disorders of the RANKL/OPG/RANK signaling pathway. J Musculoskelet Neuronal Interact. 2004;4:254–67. [PubMed] [Google Scholar]
- Whyte MP, Murphy WA, Kleerekoper M, Teitelbaum SL, Avioli LV. Idiopathic multicentric osteolysis: report of an affected father and son. Arthritis Rheum. 1978;21:367–376. doi: 10.1002/art.1780210313. [DOI] [PubMed] [Google Scholar]
- Whyte MP, Obrecht SE, Finnegan PM, Jones JL, Podgornik MN, McAlister WH, Mumm S. Osteoprotegerin deficiency and juvenile Paget’s disease. N Engl J Med. 2002;347:175–184. doi: 10.1056/NEJMoa013096. [DOI] [PubMed] [Google Scholar]
- Zankl A, Duncan EL, Leo PJ, Clark GR, Glazov EA, Addor MC, Herlin T, Kim CA, Leheup BP, McGill J, McTaggart S, Mittas S, Mitchell AL, Mortier GR, Robertson SP, Schroeder M, Terhal P, Brown MA. Multicentric carpotarsal osteolysis is caused by mutations clustering in the amino-terminal transcriptional activation domain of MAFB. Am J Hum Genet. 2012;90:494–501. doi: 10.1016/j.ajhg.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zankl A, Duncan EL, Leo PJ, Clark GR, Glazov EA, Addor M-C, Herlin T, Kim CA, Leheup BP, McGill J, McTaggart S, Mittas S, Mitchell AL, Mortier GR, Robertson SP, Schroeder M, Terhal P, Brown MA. ERRATUM: Multicentric Carpotarsal Osteolysis Is Caused by Mutations Clustering in the Amino-Terminal Transcriptional Activation Domain of MAFB. Am J Hum Genet. 2014;94:643. doi: 10.1016/j.ajhg.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]