Abstract
Recent advances in nanotechnology have established its importance in several areas including medicine. The myriad of applications in oncology range from detection and diagnosis to drug delivery and treatment. Although nanotechnology has attracted a lot of attention, the practical application of nanotechnology to clinical cancer care is still in its infancy. This review summarizes the role that nanotechnology has played in improving cancer therapy, its potential for affecting all aspects of cancer care, and the challenges that must be overcome to realize its full promise.
Introduction
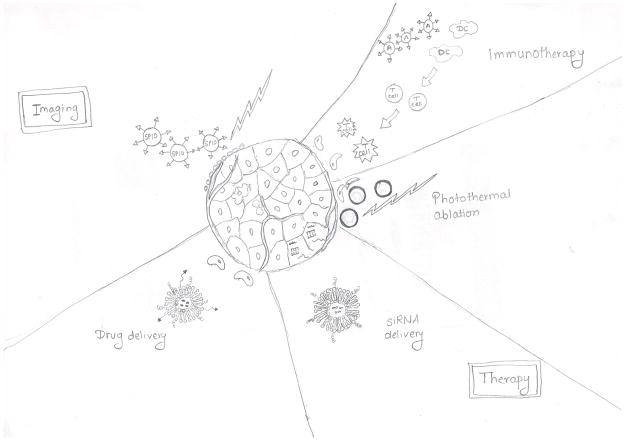
Nanomedicine offers unique opportunities for improving current ways of treating cancer and other diseases (Fig. 1). These stem from the potential of nanoformulations to improve drug delivery and achieve targeted delivery, thereby reducing systemic toxicity (1). Various nanoparticle formulations such as quantum dots, liposomes, polymeric nanoparticles, carbon nanotubes, metallic nanoparticles, or dendrimers have been investigated in preclinical and clinical settings for drug or gene delivery, photothermal therapy, immunotherapy and imaging (Table 1). Although few formulations have been approved by the FDA (Table 2), the full potential of nanotechnology in the clinical setting is yet to be realized. Here, we review the successes of nanotechnology in cancer care and provide a critical appraisal of its future applications.
Figure 1.
Shown are some of the advantages of nanoparticles over conventional therapeutic modalities
Table 1.
Types of nanoparticles
| Name | Formulation | Advantages | Disadvantages and toxicity | Application |
|---|---|---|---|---|
| Liposome | Self-assembled bilayers of phospholipid molecules |
|
Cationic liposomes can lead to hemolysis and blood coagulation | Mainly used for drug delivery (e.g., doxil, daunoxome, etc.) |
| Polymeric nanoparticles |
|
|
Toxicity at very high doses | Drug delivery (Abraxane), siRNA delivery (currently in clinical trials) |
| Dendrimers | Highly branched polymeric molecules with a central core |
|
|
Drug, siRNA or plasmid delivery |
| Gold nanoparticle | Reduction and precipitation of ions leads to nanoformulations |
|
Some particle retention after the therapy | Currently in clinical trials for photothermal application MR imaging Drug delivery |
| Iron oxide nanoparticle | Reduction and precipitation of ions lead to nanoformulation |
|
Hydrphobic surface leads to Particle aggregation | MR imaging Photothermal ablation Drug delivery |
| Carbon nanotubes | Single or multi walled cylindrical graphene sheets |
|
|
Imaging Drug delivery |
| Quantum dots | Core-shell structure, most common core- Cadmium selenium, Cadmium tellurium Shell- zinc sulfide, zinc selenium |
|
|
Imaging Drug and gene delivery |
Table 2.
Nanoformulations currently in clinical trials
| Name | Formulation | Indication | Phase status | Trial number |
|---|---|---|---|---|
| Drug delivery | ||||
| Myocet | Liposomal doxorubicin | Metastastic breast cancer | Approved | |
| Daunoxome | Liposomal daunorubicin | Kaposi’s sarcoma | Approved | |
| Doxil | PEGylated liposomal doxorubicin | Kaposi’s sarcoma Recurrent ovarian cancer Metastatic breast cancer Multiple myeloma |
Approved | |
| Marqibo | Liposomal vincristine | Acute lymphoblastic leukemia | Approved | |
| Abraxane | Albumin bound paclitaxel | Breast cancer Non-small cell lung cancer Pancreatic cancer |
Approved | |
| Paclitaxel poliglumex | Polyamino acid bound paclitaxel | Head and neck cancer Ovarian cancer Glioma Non-small cell lung cancer |
Phase I/II Phase III Phase II |
NCT00660218 NCT00269828 NCT01402063 NCT00045682 |
| Zinostatin stimalamer | Neocarzinostatin SMANCS (Polymer-protein conjugate) | Hepatocellular carcinoma | Approved | |
| Oncospar | PEG-L- asparaginase | Acute lymphoblastic leukemia | Approved | |
| siRNA delivery | ||||
| CALAA-01 | Transferrin targeted cyclodextrin nanoparticle with siRRM2 | Solid tumor | Phase 1 | NCT00689065 |
| Atu027 | Cationic liposome- siPKN3 | Solid tumor | Phase 1 |
NCT00938574 NCT01808638 |
| TKM 080301 | Stable nucleic acid lipid particle-siPLK1 | Solid tumor | Phase 1 | NCT01262235 |
| ALN-VSP02 | Stable nucleic acid lipid particle- siVEGF and siKSP | Solid tumors | Phase 1 | NCT01158079 |
| Epharna | Neutral DOPC liposome-siEphA2 | Solid tumor | Phase 1 | NCT01591356 |
| Immunotherapeutic agents | ||||
| CHP-HER2 and CHP-NY- ESO-01 | Cholesterol-Bearing hydrophobized pullulan HER2 Protein 146 (CHP- HER2) and NY- ESO-1 Protein (CHP-NY-ESO-1) in combination With OK-432 | Esophageal cancer Lung cancer Stomach cancer Breast cancer Ovarian cancer |
Phase 1 | NCT00291473 |
| CYT004- MelQbG10 | Virus like nanoparticle with antigens Melan- A/MART-1 and adjuvant CpG- oligonucleotide (ODn) | Malignant melanoma | Phase 2 | NCT00651703 |
| Photothermal and radiotherapy application | ||||
| AuroLase | Silico core with gold metal shell and near infrared laser | Head and neck cancer Lung cancer |
Phase 1 | NCT00848042, NCT01679470 |
| NBTXR3 | Hafnium oxide nanocrystals | Soft tissue sarcoma | Phase 1 | NCT01946867, NCT01433068 |
| Imaging agents | ||||
| SPIO MRI | Ultrasmall Superparamagnetic Iron Oxide Magnetic Resonance Imaging | Pancreatic cancer | Phase 4 | NCT00920023 |
| fluorescent cRGDY-PEG- Cy5.5-C dots | RGD labeled silica nanoparticle with Cy5.5 dye | Solid tumor | Phase 0 | NCT02106598 |
| Carbon nanoparticles | Carbon nanoparticles | Advanced gastric cancer | Phase 3 | NCT02123407 |
Nanoparticles for Drug Delivery
Small molecule drug delivery
Chemotherapy drugs are used for many cancer types, but conventional chemotherapy is nonspecific and can lead to intolerable toxicities, compromising patients’ quality of life. Nanotechnology has the potential to overcome such hurdles. Targeted delivery, reduced toxicity, improved pharmacokinetics and bioavailability are some of the potential advantages offered by nanotechnology.
Among various nanoparticle platforms, liposomes are the most advanced with regard to integration into clinical care. Liposomal incorporation of doxorubicin and daunorubicin increase plasma concentration, reduce clearance rate, and volume of distribution, thus, increasing bioavailability of the drug (2, 3). Moreover, there is substantial decrease in cardiac and other toxicities with liposomal doxorubicin as compared to free doxorubicin (4). Further improvement in the safety and pharmacokinetics was achieved by using polyethylene glycol (PEG) to coat liposomes (5–7).
Polymeric nanoparticles have also been instrumental in improving the therapeutic window of conventional drugs. For instance, the use of cremophor with paclitaxel contributes to hypersensitivity reactions and neuropathy, but albumin nanoparticle-based formulation of paclitaxel facilitates endothelial transcytosis to achieve significant accumulation in the tumor (8). Phase I evaluation established that maximum tolerated dose (MTD) dose of such nanoparticles was about 70% higher than traditional paclitaxel (9). This formulation is associated with lower neutropenia and hypersensitivity while achieving higher response rate than standard paclitaxel (10). Paclitaxel poliglumex is another polymeric formulation (poly(L-glutamic acid, PG) with increased water solubility of paclitaxel, increased plasma half-life, tumor uptake, increased anti-tumor activity and improved safety profile compared to free paclitaxel (11, 12).
The nanoparticle platforms discussed above rely predominantly on passive accumulation of nanoparticles at tumor sites based on enhanced permeability and retention (EPR) effect. Tumor selectivity can be further enhanced by attaching tumor-specific ligands (e.g., folic acid, HER2 antibody, aptamers, and transferrin) to nanoparticles to enhance tumor accumulation, increased cellular internalization and increased anti-tumor effects (13–17). For example, MCC-465 (PEGylated immunoliposome conjugated with F(ab′)2 fragment of GAH and encapsulates doxorubicin) was well tolerated in preclinical and early clinical testing. (18). MM-302 is a HER-2 targeted PEGylated liposome containing doxorubicin that has shown improved cardiac toxicity profile in combination with trastuzumab (19). Phase 1 testing of cetuximab conjugated doxorubicin liposome was also well tolerated (20). Additional formulations (e.g., MBP-426 and SGT53 (p53) are in clinical testing (21).
Several nanoparticle strategies have been developed for targeting stromal populations such as endothelial cells, macrophages and cancer stem cells. Paclitaxel loaded into PLGA nanoparticles decorated with CD133 antibody resulted in enhanced survival in preclinical cancer models (22). Combination therapy with epigenetic-targeted decitabine and doxorubicin nanoparticles targeting cancer stem cells was shown to be more beneficial than free decitabine and doxorubicin in chemoresistant breast cancer models (23). Chitosan nanoparticles decorated with RGD peptides localize to the tumor vasculature and exert anti-angiogenic effects (24). The next generation nanoparticles aim to achieve further selectivity by allowing spatiotemporal control over drug release. These nanoparticles are designed to selectively release drugs in response to stimuli such as an alternating magnetic field, UV or near infra-red radiation or low pH in the tumor microenvironment (25–29). However, issues related to tumor heterogeneity, cost considerations and changes in characteristics of a nanoparticle after ligand conjugation will require careful consideration during drug development.
Nucleotide delivery
Nucleotide therapies hold an important place in cancer therapy since many of the undruggable genes can be targeted using antisense oligonucleotides (ASO) or siRNAs. Several ASOs are now in clinical trials, but the success has been modest (30). SiRNAs may be a better alternative to ASOs due to ease of synthesis and ability to achieve greater silencing at lower concentration than ASOs. However, several challenges associated with siRNA (e.g., enzymatic degradation in plasma, inefficient uptake by cells, and immunostimulation) must be overcome. Several nanoparticle platforms have been investigated to overcome these hurdles in siRNA delivery. While some cationic liposomes are efficacious, these carriers can cause toxicities (e.g., activation of complement system and inflammatory responses) (31–33). Formulations such as AtuPLEX showed that toxicity can be reduced by incorporation of helper neutral lipids and PEGylation (34). The lipoplex Atu027 containing siPKN3 is currently in clinical trials for advanced solid cancers. Although preclinical studies showed anti-tumor effect, it should be noted that the PKN3 mRNA reduction was more pronounced in liver and lung compared to tumor (35). Stable nucleic acid lipid particles (SNALP) formulations such as ALN-VSP02 (first generation SNALP containing siVEGF and siKSP) showed moderate gene knockdown (36), and some liver and spleen toxicities were noted. The next generation SNALP, TKM-080301, was formulated with more stable PEG-lipids in the nanoparticles. The clinical trial with siPLK1 showed better immune profile along with increased drug exposure compared to the earlier generation of SNALPs (37). Neutral nanoliposomes (e.g., DOPC) have shown improved delivery (approximately 10-fold) of siRNA and anti-tumor effects with systemic delivery (38). Moreover, in a hepatocarcinoma mouse model, neutral liposome containing doxorubicin showed better biodistribution profile and anti-tumor efficacy compared to their cationic counterparts (39).
Once inside the tumor cells, it is important to overcome barriers such as endosomal uptake (40). Systems such as polymer-based dynamic polyconjugate (DPC) delivery system, which contains endosomolytic N-acetylgalactosamine–conjugated melittin-like peptide, may allow specific endosomal release of siRNA from its nanocarrier, thus lowering siRNA EC50 (37, 41).
Nanoparticles for Immunotherapy
One of the unmet needs in the field of immunotherapy is the lack of efficient delivery systems for cytokines and antigens. Success of systemic administration of cytokines has been limited because of their early degradation, non-specific binding to proteins, quick excretion and undesired toxic effects. Gold nanoparticles conjugated with TNF-alpha are currently being investigated in clinical trials (42). Early results suggest that such a formulation may be safer with higher MTD than free recombinant TNF-alpha (43). In addition, studies with IL-2 and IL-12 have also shown that nanoparticle incorporation increased plasma retention time (44, 45).
Successful antigen vaccination can be achieved by ensuring a) sufficient concentration of antigen in antigen presenting cells (APCs), b) sustained release of antigen for prolonged exposure to APCs and c) cytoplasmic delivery of antigen for MHC class I processing. Incorporation of an antigen into target specific nanoparticles can increase the concentration of antigens in dendritic cells (46–48). Nanoparticles can also incorporate adjuvants and antigens in the same vehicle (46, 47, 49). For example, conjugation of polyribocytidylic acid (adjuvant) with DOTAP containing a tumor lysate (antigen) not only increased toll-like receptor signaling, but also led to increased DC maturation and enhanced anti-immune response (47). Since DOTAP can lead to ROS production and apoptosis of DC cells (50), safer and more effective systems are needed.
Poly-lactic-co-glycolic acid (PLGA) nanoparticles have been shown to act as intracellular antigen reservoirs for DCs, minimizing degradation of antigens and the need of repeated dosing (51). Such sustained antigen presentation leads to stronger immune responses and consequently better anti-tumor effects. However, precise knowledge of degradation and release kinetics is essential to formulate effective sustained release nanoparticles. Once inside the cells, it is important that the antigens are released in the cytosol for preferential processing through MHC class I pathway to prime CD8+ T-cells. Use of poly-(gamma–glutamic acid), conjugation of pH responsive peptide bonds, or incorporation of cell penetrating peptide R8, are some of the strategies to achieve enhanced cytoplasmic release of antigens (52–54). Immunogenicity of certain nanomaterials is a potential concern (e.g., PEG coated nanoparticles can activate the complement system and lead to PEG specific IgM antibodies) (55, 56); strategies to overcome these unexpected side effects are needed.
Nanoparticle as an Individual Active Agent for Therapy and Imaging
Photothermal ablation for tumoricidal effect
Photothermal ablation involves exposure of tissues to high temperature for membrane lysis and subsequent cell death (57). Increased susceptibility of cancer cells to hyperthermia is on account of their higher metabolic rates than normal cells (58), however, this selectivity is minimal. The main concern with photothermal therapy (PTT) is the non-specific effect on surrounding normal tissues. Localized heating, enabled with the use of nanoparticles, can avoid toxicity to normal cells. Blood and tissues are relatively transmissive in near infra-red (NIR) range. NIR has thus been effective for PTT since it achieves optimal tissue penetration to reach deeply localized tumor tissues. Initial preclinical studies were conducted using FDA approved NIR free dyes (e.g., indocyanine green). Although it showed anti-tumor effect, the strategy mainly suffered because of the low circulation time of ICG (3 minutes) and damage to normal tissues (59). Incorporation of these dyes into polymeric nanoparticles improved solubility and stability, increased photothermal ablation while keeping toxicity at minimum (60–62).
Gold nanoparticles are widely used for PTT (57, 63, 64). Nanoshells with silica core coated with thin gold layer have been studied extensively in preclinical studies and are currently in clinical trials for head and neck and metastatic lung cancer patients (65, 66). The temperatures achieved by these nanoparticles (ΔT = 37.4 ± 6.6°C) were significantly higher than those achieved by laser treatment alone (ΔT < 10°C) (65). In the treatment arm, tumor growth was significantly lower and survival was significantly higher. Further studies demonstrated that malignant cells required less than half of the laser density (~20W/cm2) for ablation compared to normal cells (57 W/cm2) when incubated with EGFR conjugated gold nanoshells (67, 68). Smaller hollow gold nanospheres have also been developed for simultaneous laser triggered drug delivery of doxorubicin, with significantly better anti-tumor effects compared to PTT alone (69), and have a favorable safety profile (70). Copper sulfide (CuS) nanoparticles are also being investigated for PTT. PEG-CuS nanoparticles plus laser treatment resulted in significantly higher tumor tissue necrosis (~65%) compared to saline plus laser treatment (~5%) (71). Several attractive features (smaller size (<15nm), better renal clearance, ease of synthesis, and low cost) make them promising candidates for PTT (71, 72).
Clinical applicability of a nanoparticle can be further improved if it can also be used as an imaging agent for MR imaging and spatiotemporal monitoring of the nanoparticles (73). Similar to previously discussed nanoparticles, gold and iron oxide nanoparticles also have certain toxicity issues (74–77) that will require additional work.
Tumor imaging
The limitations of current imaging modalities, such as iodine and gadolinium based CT, X-ray and MRI scans, are lack of sensitivity in detecting small tumor nodules, lack of specificity, shorter imaging time, and toxic effects. Novel nanoparticle platforms may help to overcome these limitations. For example, ferumoxtran-10, an iron oxide nanoparticle showed significantly higher sensitivity (90.5% vs. 35.4%) in detecting lymph node metastasis as compared to conventional MRI scans (78, 79). Iron oxide nanoparticles, when compared with gadolinium (Gd) chelates, showed lower diffusion from tumor site, increased internalization by cancer cells, and enhanced detection of lesions in the brain (80). Polymeric dendrimers used as nanocarriers for Gd proved as a better tool for detecting lymph nodes compared to free Gd chelates; such sensitivity was achieved at 1/2500th of the molar concentration of the clinical Gd dose (81). Owing to the high atomic number and electron density, gold nanoparticles have higher absorption coefficient than conventional iodine and are better contrast agents for PET and X-rays (82). Ability to coat them with PEG and functionalize the surface with targeting ligands makes it possible to increase circulation time and achieve high tumor cell specificity (83, 84). Nanoparticle formulations have also been used to reduce the toxicity of conventional contrast agents. Gadolinium containing agents can cause nephrogenic systemic fibrosis, which can be prevented with nanoparticle incorporation (85, 86).
Certain nanoparticles such as self-assembled nanocages facilitate interaction between water molecules and Gd, allowing higher MR signal at much lower Gd concentration (87). Same was the case with free NIR dyes, where non-specific accumulation of dyes in lungs and testicles had raised concerns, but nanoparticle formulations improved biodistribution and significantly reduced toxicity (88). Carbon nanotubes (CNT) are being investigated for X-ray imaging, but may be limited by potential carcinogenic effects (89).
Oncolytic Viruses
Oncolytic viruses are natural or genetically modified viruses that specifically kill cancer cells either by intensive cytopathic effect or inducing strong immune response in tumor microenvironment. JX-594 poxvirus, genetically modified to express GM-CSF, has been shown to increase anti-tumor immune response. The intratumoral injections had limited side effects and resulted in partial remission or stable disease in patients with liver cancer (90). Talimogene laherparepvec (T-vec) is a Herpes simplex virus expressing GM-CSF, which has shown promising results in patients with advanced melanoma. ONYX-015, an adenovirus specifically targeting tumors with inactivated p53 has also shown promising results in phase 1 and 2 trials (91). A similar virus (H101) has already gained approval for the treatment of head and neck cancer in China. Systemic administration can lead to the production of neutralizing antibodies, which may require immunosuppressive treatments prior to viral therapy (92). Combination of oncolytic viral therapy with chemotherapy or radiation may further enhance its activity (91).
Challenges and Future Perspectives
Nanotechnology has transformed the field of medicine by crafting promising avenues in therapeutics and diagnosis (Fig. 2), but there is clearly room for further improvement. Considering the heterogeneity of tumor, extent of hypoxia or expression of specific enzymes required for drug release may not be the same at all metastatic sites, potentially making drug release unpredictable. A possible solution to increase tumor specificity is to use dual stimuli responsive triggers (93–96), but particular attention must be given to characterizing these systems further and improving the scalability of the formulations. Regarding the multidrug carrying nanoparticles, optimized ratiometric loading and compatibility of their efficacy and toxicity profiles are important aspects to be considered. For theranostic nanoparticles, care must be taken to avoid compromising imaging quality or therapeutic efficacy. Pharmacokinetic (PK) and pharamacodynamic (PD) requirements are also different for imaging and drug delivery vehicles. For examples, long circulation times that are ideal for effective drug delivery may not be suitable for imaging purposes and will give high background signal (97, 98). Collectively, several factors must be considered to improve the translation of nanomedicines from bench to bedside. Here, we discuss key issues and ways to accelerate the development of clinically feasible nanosystems.
Figure 2.
Nanotechnology offers a wide array of applications for drug delivery, nucleotide delivery, photothermal therapy, immunotherapy and imaging. Shown are some of the commonly used nanoformulations for each of the applications: ligand conjugated, target specific PEGylated liposomes for small molecule drug delivery; PEGylated stable nucleic acid lipid particles (SNALP) for nucleotide (e.g., siRNA) delivery; gold nanoshells for photothermal therapy; ligand conjugated, target specific antigen carrying polymeric nanoparticles for immunotherapy and super paramagnetic iron oxide nanoparticles (SPIO) for imaging. Dendritic cells (DC) are immune cells that process and present antigens to T cells.
Use of relevant preclinical models to predict EPR effect
Most nanoparticles are thought to rely on the EPR effect to accumulate in tumors. Reliable methods for assessing delivery are needed. A recent study with NIRF labelled polymeric nanoparticles showed that tumors with high vascularity accumulated a greater density of nanoparticles (99). Although the study used fairly small nanoparticles (10 nm), the concept of predicting EPR effects by simple ultrasound imaging of vasculature should be explored further in relevant pre-clinical and clinical models.
Discrepancies between pre-clinical studies and clinical trials
It is now recognized that in vitro models may not reliably predict the utility of nanoparticles. For example, a dual targeted (Tf and mAb 2C5) nanoparticle system failed to reproduce the in vitro effectiveness when tested in mouse models (100). Whether 3D biomimetic tumor models can help bridge this gap to some extent is not fully understood (101). The current 3D systems could potentially be improved by incorporating relevant stromal cells, ECM proteins or even relevant mechanical forces. These factors will help to closely simulate the tumor microenvironment and will make 3D systems a reliable platform for designing of subsequent preclinical studies.
Testing the biodistribution and efficacy of nanoparticles in relevant animal models is crucial to move the therapy into the clinic. Ideally, animal models that are reflective of human disease should be utilized for such studies. Subcutaneous models are likely to be the least reliable due to aberrant stromal and vascular biology compared to the orthotopic sites (102). Design of preclinical trials is crucial for predicting the efficacy and safety of the nanoparticles. Moreover, it is also important to assess immunological parameters (e.g., changes in cytokine levels or number of immune cells) during preclinical testing. Such comprehensive analysis will help in predicting efficacy and toxicity profile of a nano-therapy in patients. In addition, phase 0 studies should be employed to improve clinical translatability of nanoparticles. PK profile and tumor localization potential of a given nanocarrier in humans can be assessed in a timely manner in Phase 0 trials. They are much cheaper to conduct compared to phase 1 trials and researchers can also obtain feedback on the clinical feasibility of a given nanosystem much more quickly.
Selection of clinically relevant route of administration
Nanodrugs should ideally be administered the same way in the preclinical models as it is expected to be delivered in patients. For instance, pre-clinical studies with oncolytic viral therapy typically use intratumoral injections (103). Many other studies using non-viral nanocarriers also try to prove better efficacy using intratumoral injections (104, 105). However, this would have limited utility in patients with widely metastatic disease. Unlike intratumoral injections; intravenous injections can expose the particles to various biological barriers and thus will not be as effective as intartumoral route.
Choosing the right ligand for targeted delivery
Ligand targeted therapies have been shown to superior in terms of tumor specificity and low off-target effects. (106–108). In preclinical studies, greater accumulation of nanoparticles can be achieved by choosing a tumor model overexpressing the specific receptor. (105). However, there is heterogeneity in receptor expression in tumors (109, 110), and a single ligand could ultimately lead to selection of cells that lack target expression. Multi-ligand approach has been shown to be more specific and leads to better uptake of nanoparticles (111–113). With increased specificity, multi-ligand nanoparticles are less likely to be taken up by normal cells and thus have less toxicity issues (112, 114).
In summary, the versatility of formulations, targeted delivery and biocompatibility have garnered a lot of interest in nanotechnology. However, we must first address several practical issues. Every new nanomaterial and added complexities require additional controls and toxicity checks, making FDA approval potentially more difficult. Batch-to-batch variations in these cases further complicate scaling up the production. Thus, the clinical benefit and toxicity profile has to be far superior compared to the conventional drugs to justify the cost. Moreover, it is important to understand the intricacies of nanotechnology in vivo and predict the behavior, distribution, and kinetics with certainty. Then, we can develop strategies for scaling up production and distribution with the ultimate goal of direct clinical translation and patient benefit.
Acknowledgments
Grant Support
K.M. Gharpure is supported by the Altman Goldstein Discovery Fellowship. S.Y. Wu is supported by the Ovarian Cancer Research Fund, Foundation for Women’s Cancer, and Cancer Prevention Research Institute of Texas (CPRIT) training grants (RP101502, RP101489, and RP110595). C. Li and A.K. Sood are supported by the NIH under award number U54CA151668. A.K. Sood is supported by the NIH under award numbers P50CA083639, CA109298, P50CA098258, UH2TR000943, CA016672, U54CA96300, and U54CA96297; CPRIT grants (RP110595 and RP120214); an Ovarian Cancer Research Fund Program Project Development Grant; U.S. Department of Defense grants (OC120547 and OC093416); the Betty Ann Asche Murray Distinguished Professorship; the RGK Foundation; the Gilder Foundation; the Judi A. Rees Ovarian Cancer Research Fund; the Chapman Foundation; the Meyer and Ida Gordon Foundation; and the Blanton-Davis Ovarian Cancer Research Program.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Parveen S, Misra R, Sahoo SK. Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedicine. 2012;8:147–66. doi: 10.1016/j.nano.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 2.Swenson CE, Bolcsak LE, Batist G, Guthrie TH, Jr, Tkaczuk KH, Boxenbaum H, et al. Pharmacokinetics of doxorubicin administered i.v. as Myocet (TLC D-99; liposome-encapsulated doxorubicin citrate) compared with conventional doxorubicin when given in combination with cyclophosphamide in patients with metastatic breast cancer. Anticancer Drugs. 2003;14:239–46. doi: 10.1097/00001813-200303000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Gill PS, Espina BM, Muggia F, Cabriales S, Tulpule A, Esplin JA, et al. Phase I/II clinical and pharmacokinetic evaluation of liposomal daunorubicin. J Clin Oncol. 1995;13:996–1003. doi: 10.1200/JCO.1995.13.4.996. [DOI] [PubMed] [Google Scholar]
- 4.Batist G, Ramakrishnan G, Rao CS, Chandrasekharan A, Gutheil J, Guthrie T, et al. Reduced cardiotoxicity and preserved antitumor efficacy of liposome-encapsulated doxorubicin and cyclophosphamide compared with conventional doxorubicin and cyclophosphamide in a randomized, multicenter trial of metastatic breast cancer. J Clin Oncol. 2001;19:1444–54. doi: 10.1200/JCO.2001.19.5.1444. [DOI] [PubMed] [Google Scholar]
- 5.Gabizon A, Catane R, Uziely B, Kaufman B, Safra T, Cohen R, et al. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res. 1994;54:987–92. [PubMed] [Google Scholar]
- 6.O’Brien ME, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A, et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004;15:440–9. doi: 10.1093/annonc/mdh097. [DOI] [PubMed] [Google Scholar]
- 7.Gabizon A, Martin F. Polyethylene glycol-coated (pegylated) liposomal doxorubicin. Rationale for use in solid tumours. Drugs. 1997;4:15–21. doi: 10.2165/00003495-199700544-00005. [DOI] [PubMed] [Google Scholar]
- 8.Miele E, Spinelli GP, Miele E, Tomao F, Tomao S. Albumin-bound formulation of paclitaxel (Abraxane(®) ABI-007) in the treatment of breast cancer. Int J Nanomedicine. 2009;4:99–105. doi: 10.2147/ijn.s3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ibrahim NK, Desai N, Legha S, Soon-Shiong P, Theriault RL, Rivera E, et al. Phase I and pharmacokinetic study of ABI-007, a Cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin Cancer Res. 2002;8:1038–44. [PubMed] [Google Scholar]
- 10.Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23:7794–803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 11.Li C, Wallace S. Polymer-drug conjugates: recent development in clinical oncology. Adv Drug Deliv Rev. 2008;60:886–98. doi: 10.1016/j.addr.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li C, Yu DF, Newman RA, Cabral F, Stephens LC, Hunter N, et al. Complete regression of well-established tumors using a novel water-soluble poly(L-glutamic acid)-paclitaxel conjugate. Cancer Res. 1998;58:2404–9. [PubMed] [Google Scholar]
- 13.Hrkach J, Von Hoff D, Mukkaram Ali M, Andrianova E, Auer J, Campbell T, et al. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci Transl Med. 2012;4:128ra39. doi: 10.1126/scitranslmed.3003651. [DOI] [PubMed] [Google Scholar]
- 14.Ireson CR, Kelland LR. Discovery and development of anticancer aptamers. Mol Cancer Ther. 2006;5:2957–62. doi: 10.1158/1535-7163.MCT-06-0172. [DOI] [PubMed] [Google Scholar]
- 15.Park JW, Hong K, Kirpotin DB, Colbern G, Shalaby R, Baselga J, et al. Anti-HER2 immunoliposomes: enhanced efficacy attributable to targeted delivery. Clin Cancer Res. 2002;8:1172–81. [PubMed] [Google Scholar]
- 16.Werner ME, Karve S, Sukumar R, Cummings ND, Copp JA, Chen RC, et al. Folate-targeted nanoparticle delivery of chemo- and radiotherapeutics for the treatment of ovarian cancer peritoneal metastasis. Biomaterials. 2011;32:8548–54. doi: 10.1016/j.biomaterials.2011.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng Y, Yu B, Weecharangsan W, Piao L, Darby M, Mao Y, et al. Transferrin-conjugated lipid-coated PLGA nanoparticles for targeted delivery of aromatase inhibitor 7alpha-APTADD to breast cancer cells. Int J Pharm. 2010;390:234–41. doi: 10.1016/j.ijpharm.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamaguchi T, Matsumura Y, Nakanishi Y, Muro K, Yamada Y, Shimada Y, et al. Antitumor effect of MCC-465, pegylated liposomal doxorubicin tagged with newly developed monoclonal antibody GAH, in colorectal cancer xenografts. Cancer Sci. 2004;95:608–13. doi: 10.1111/j.1349-7006.2004.tb02495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reynolds JG, Geretti E, Hendriks BS, Lee H, Leonard SC, Klinz SG, et al. HER2-targeted liposomal doxorubicin displays enhanced anti-tumorigenic effects without associated cardiotoxicity. Toxicol Appl Pharmacol. 2012;262:1–10. doi: 10.1016/j.taap.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Mamot C, Ritschard R, Wicki A, Stehle G, Dieterle T, Bubendorf L, et al. Tolerability, safety, pharmacokinetics, and efficacy of doxorubicin-loaded anti-EGFR immunoliposomes in advanced solid tumours: a phase 1 dose-escalation study. Lancet Oncol. 2012;13:1234–41. doi: 10.1016/S1470-2045(12)70476-X. [DOI] [PubMed] [Google Scholar]
- 21.Senzer N, Nemunaitis J, Nemunaitis D, Bedell C, Edelman G, Barve M, et al. Phase I study of a systemically delivered p53 nanoparticle in advanced solid tumors. Mol Ther. 2013;21:1096–103. doi: 10.1038/mt.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin C, Yang Z, Yang J, Li H, He Y, An J, et al. Paclitaxel-loaded nanoparticles decorated with anti-CD133 antibody: a targeted therapy for liver cancer stem cells. J Nanopart Res. 2013;16:1–15. [Google Scholar]
- 23.Li SY, Sun R, Wang HX, Shen S, Liu Y, Du XJ, et al. Combination therapy with epigenetic-targeted and chemotherapeutic drugs delivered by nanoparticles to enhance the chemotherapy response and overcome resistance by breast cancer stem cells. J Control Release. 2014;15:00748–2. doi: 10.1016/j.jconrel.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 24.Han HD, Mangala LS, Lee JW, Shahzad MM, Kim HS, Shen D, et al. Targeted gene silencing using RGD-labeled chitosan nanoparticles. Clin Cancer Res. 2010;16:3910–22. doi: 10.1158/1078-0432.CCR-10-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amstad E, Kohlbrecher J, Muller E, Schweizer T, Textor M, Reimhult E. Triggered release from liposomes through magnetic actuation of iron oxide nanoparticle containing membranes. Nano Lett. 2011;11:1664–70. doi: 10.1021/nl2001499. [DOI] [PubMed] [Google Scholar]
- 26.Derfus AM, von-Maltzahn G, Harris TJ, Duza T, Vecchio KS, Ruoslahti E, et al. Remotely triggered release from magnetic nanoparticles. Adv Mater. 2007;19:3932–6. [Google Scholar]
- 27.Dromi S, Frenkel V, Luk A, Traughber B, Angstadt M, Bur M, et al. Pulsed-high intensity focused ultrasound and low temperature-sensitive liposomes for enhanced targeted drug delivery and antitumor effect. Clin Cancer Res. 2007;13:2722–7. doi: 10.1158/1078-0432.CCR-06-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fomina N, McFearin C, Sermsakdi M, Edigin O, Almutairi A. UV and near-IR triggered release from polymeric nanoparticles. J Am Chem Soc. 2010;132:9540–2. doi: 10.1021/ja102595j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giri S, Trewyn BG, Stellmaker MP, Lin VSY. Stimuli-responsive controlled-release delivery system based on mesoporous silica nanorods capped with magnetic nanoparticles. Angew Chem Int Ed. 2005;44:5038–44. doi: 10.1002/anie.200501819. [DOI] [PubMed] [Google Scholar]
- 30.Farooqi AA, Rehman Zu, Muntane J. Antisense therapeutics in oncology: current status. OncoTargets Ther. 2014;7:2035–42. doi: 10.2147/OTT.S49652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schreier H, Gagne L, Bock T, Erdos GW, Druzgala P, Conary JT, et al. Physicochemical properties and in vitro toxicity of cationic liposome cDNA complexes. Pharm Acta Helv. 1997;72:215–23. doi: 10.1016/s0031-6865(97)00019-8. [DOI] [PubMed] [Google Scholar]
- 32.Guo S, Huang L. Nanoparticles escaping RES and endosome: challenges for siRNA delivery for cancer therapy. J Nanomaterials. 2011;2011:12. [Google Scholar]
- 33.Filion MC, Phillips NC. Toxicity and immunomodulatory activity of liposomal vectors formulated with cationic lipids toward immune effector cells. Biochim Biophys Acta. 1997;1329:345–56. doi: 10.1016/s0005-2736(97)00126-0. [DOI] [PubMed] [Google Scholar]
- 34.Schultheis B, Strumberg D, Santel A, Vank C, Gebhardt F, Keil O, et al. First-inhuman phase I study of the liposomal RNA interference therapeutic Atu027 in patients with advanced solid tumors. J Clin Oncol. 2014;32:4141–8. doi: 10.1200/JCO.2013.55.0376. [DOI] [PubMed] [Google Scholar]
- 35.Aleku M, Schulz P, Keil O, Santel A, Schaeper U, Dieckhoff B, et al. Atu027, a liposomal small interfering RNA formulation targeting protein kinase N3, inhibits cancer progression. Cancer Res. 2008;68:9788–98. doi: 10.1158/0008-5472.CAN-08-2428. [DOI] [PubMed] [Google Scholar]
- 36.Tabernero J, Shapiro GI, LoRusso PM, Cervantes A, Schwartz GK, Weiss GJ, et al. First-in-man trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013;3:406–17. doi: 10.1158/2159-8290.CD-12-0429. [DOI] [PubMed] [Google Scholar]
- 37.Wu SY, Lopez-Berestein G, Calin GA, Sood AK. RNAi therapies: drugging the undruggable. Sci Transl Med. 2014;6:240ps7. doi: 10.1126/scitranslmed.3008362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Landen CN, Jr, Chavez-Reyes A, Bucana C, Schmandt R, Deavers MT, Lopez-Berestein G, et al. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res. 2005;65:6910–8. doi: 10.1158/0008-5472.CAN-05-0530. [DOI] [PubMed] [Google Scholar]
- 39.Zhao W, Zhuang S, Qi XR. Comparative study of the in vitro and in vivo characteristics of cationic and neutral liposomes. Int J Nanomedicine. 2011;6:3087–98. doi: 10.2147/IJN.S25399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilleron J, Querbes W, Zeigerer A, Borodovsky A, Marsico G, Schubert U, et al. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat Biotech. 2013;31:638–46. doi: 10.1038/nbt.2612. [DOI] [PubMed] [Google Scholar]
- 41.Wong SC, Klein JJ, Hamilton HL, Chu Q, Frey CL, Trubetskoy VS, et al. Co-injection of a targeted, reversibly masked endosomolytic polymer dramatically improves the efficacy of cholesterol-conjugated small interfering RNAs in vivo. Nucleic Acid Ther. 2012;22:380–90. doi: 10.1089/nat.2012.0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghosh P, Han G, De M, Kim CK, Rotello VM. Gold nanoparticles in delivery applications. Adv Drug Deliv Rev. 2008;60:1307–15. doi: 10.1016/j.addr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 43.Libutti SK, Paciotti GF, Byrnes AA, Alexander HR, Gannon WE, Walker M, et al. Phase I and pharmacokinetic studies of CYT-6091, a novel PEGylated colloidal gold-rhTNF nanomedicine. Clin Cancer Res. 2010;16:6139–49. doi: 10.1158/1078-0432.CCR-10-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimizu T, Kishida T, Hasegawa U, Ueda Y, Imanishi J, Yamagishi H, et al. Nanogel DDS enables sustained release of IL-12 for tumor immunotherapy. Biochem Biophys Res Commun. 2008;367:330–5. doi: 10.1016/j.bbrc.2007.12.112. [DOI] [PubMed] [Google Scholar]
- 45.Yao H, Ng SS, Huo LF, Chow BK, Shen Z, Yang M, et al. Effective melanoma immunotherapy with interleukin-2 delivered by a novel polymeric nanoparticle. Mol Cancer Ther. 2011;10:1082–92. doi: 10.1158/1535-7163.MCT-10-0717. [DOI] [PubMed] [Google Scholar]
- 46.Mansour M, Pohajdak B, Kast WM, Fuentes-Ortega A, Korets-Smith E, Weir GM, et al. Therapy of established B16-F10 melanoma tumors by a single vaccination of CTL/T helper peptides in VacciMax. J Transl Med. 2007;5:20. doi: 10.1186/1479-5876-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang C, Zhuang Y, Zhang Y, Luo Z, Gao N, Li P, et al. Toll-like receptor 3 agonist complexed with cationic liposome augments vaccine-elicited antitumor immunity by enhancing TLR3-IRF3 signaling and type I interferons in dendritic cells. Vaccine. 2012;30:4790–9. doi: 10.1016/j.vaccine.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Z, Tongchusak S, Mizukami Y, Kang YJ, Ioji T, Touma M, et al. Induction of anti-tumor cytotoxic T cell responses through PLGA-nanoparticle mediated antigen delivery. Biomaterials. 2011;32:3666–78. doi: 10.1016/j.biomaterials.2011.01.067. [DOI] [PubMed] [Google Scholar]
- 49.Diwan M, Tafaghodi M, Samuel J. Enhancement of immune responses by co-delivery of a CpG oligodeoxynucleotide and tetanus toxoid in biodegradable nanospheres. J Control Release. 2002;85:247–62. doi: 10.1016/s0168-3659(02)00275-4. [DOI] [PubMed] [Google Scholar]
- 50.Yan W, Chen W, Huang L. Reactive oxygen species play a central role in the activity of cationic liposome based cancer vaccine. J Control Release. 2008;130:22–8. doi: 10.1016/j.jconrel.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 51.Shen H, Ackerman AL, Cody V, Giodini A, Hinson ER, Cresswell P, et al. Enhanced and prolonged cross-presentation following endosomal escape of exogenous antigens encapsulated in biodegradable nanoparticles. Immunology. 2006;117:78–88. doi: 10.1111/j.1365-2567.2005.02268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mukai Y, Yoshinaga T, Yoshikawa M, Matsuo K, Yoshikawa T, Niki K, et al. Induction of endoplasmic reticulum-endosome fusion for antigen cross-presentation induced by poly (gamma-glutamic acid) nanoparticles. J Immunol. 2011;187:6249–55. doi: 10.4049/jimmunol.1001093. [DOI] [PubMed] [Google Scholar]
- 53.Nakamura T, Moriguchi R, Kogure K, Shastri N, Harashima H. Efficient MHC class I presentation by controlled intracellular trafficking of antigens in octaarginine-modified liposomes. Mol Ther. 2008;16:1507–14. doi: 10.1038/mt.2008.122. [DOI] [PubMed] [Google Scholar]
- 54.Yuba E, Kojima C, Harada A, Tana, Watarai S, Kono K. pH-Sensitive fusogenic polymer-modified liposomes as a carrier of antigenic proteins for activation of cellular immunity. Biomaterials. 2010;31:943–51. doi: 10.1016/j.biomaterials.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 55.Ishida T, Kiwada H. Accelerated blood clearance (ABC) phenomenon upon repeated injection of PEGylated liposomes. Int J Pharm. 2008;354:56–62. doi: 10.1016/j.ijpharm.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 56.Pham CT, Mitchell LM, Huang JL, Lubniewski CM, Schall OF, Killgore JK, et al. Variable antibody-dependent activation of complement by functionalized phospholipid nanoparticle surfaces. J Biol Chem. 2011;286:123–30. doi: 10.1074/jbc.M110.180760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jing-Liang L, Gu M. Gold-nanoparticle-enhanced cancer photothermal therapy. IEEE J Sel Top Quantum Electron. 2010;16:989–96. [Google Scholar]
- 58.Huff TB, Tong L, Zhao Y, Hansen MN, Cheng JX, Wei A. Hyperthermic effects of gold nanorods on tumor cells. Nanomedicine (Lond) 2007;2:125–32. doi: 10.2217/17435889.2.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yuan A, Wu J, Tang X, Zhao L, Xu F, Hu Y. Application of near-infrared dyes for tumor imaging, photothermal, and photodynamic therapies. J Pharm Sci. 2013;102:6–28. doi: 10.1002/jps.23356. [DOI] [PubMed] [Google Scholar]
- 60.Peng CL, Shih YH, Lee PC, Hsieh TM, Luo TY, Shieh MJ. Multimodal image-guided photothermal therapy mediated by 188Re-labeled micelles containing a cyanine-type photosensitizer. ACS Nano. 2011;5:5594–607. doi: 10.1021/nn201100m. [DOI] [PubMed] [Google Scholar]
- 61.Yu J, Javier D, Yaseen MA, Nitin N, Richards-Kortum R, Anvari B, et al. Self-assembly synthesis, tumor cell targeting, and photothermal capabilities of antibody-coated indocyanine green nanocapsules. J Am Chem Soc. 2010;132:1929–38. doi: 10.1021/ja908139y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng X, Xing D, Zhou F, Wu B, Chen WR. Indocyanine green-containing nanostructure as near infrared dual-functional targeting probes for optical imaging and photothermal therapy. Mol Pharm. 2011;8:447–56. doi: 10.1021/mp100301t. [DOI] [PubMed] [Google Scholar]
- 63.Huang X, El-Sayed MA. Gold nanoparticles: optical properties and implementations in cancer diagnosis and photothermal therapy. J Adv Res. 2010;1:13–28. [Google Scholar]
- 64.Shao J, Griffin RJ, Galanzha EI, Kim J-W, Koonce N, Webber J, et al. Photothermal nanodrugs: potential of TNF-gold nanospheres for cancer theranostics. Sci Rep. 2013:3. doi: 10.1038/srep01293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hirsch LR, Stafford RJ, Bankson JA, Sershen SR, Rivera B, Price RE, et al. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc Natl Acad Sci U S A. 2003;100:13549–54. doi: 10.1073/pnas.2232479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O’Neal DP, Hirsch LR, Halas NJ, Payne JD, West JL. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett. 2004;209:171–6. doi: 10.1016/j.canlet.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 67.El-Sayed IH, Huang X, El-Sayed MA. Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett. 2006;239:129–35. doi: 10.1016/j.canlet.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 68.Huang X, El-Sayed IH, Qian W, El-Sayed MA. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J Am Chem Soc. 2006;128:2115–20. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]
- 69.You J, Zhang R, Zhang G, Zhong M, Liu Y, Van Pelt CS, et al. Photothermal-chemotherapy with doxorubicin-loaded hollow gold nanospheres: a platform for near-infrared light-trigged drug release. J Control Release. 2012;158:319–28. doi: 10.1016/j.jconrel.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.You J, Zhou J, Zhou M, Liu Y, Robertson J, Liang D, et al. Pharmacokinetics, clearance, and biosafety of polyethylene glycol-coated hollow gold nanospheres. Part Fibre Toxicol. 2014;11:26. doi: 10.1186/1743-8977-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou M, Zhang R, Huang M, Lu W, Song S, Melancon MP, et al. A chelator-free multifunctional [64Cu]CuS nanoparticle platform for simultaneous micro-PET/CT imaging and photothermal ablation therapy. J Am Chem Soc. 2010;132:15351–8. doi: 10.1021/ja106855m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li Y, Lu W, Huang Q, Huang M, Li C, Chen W. Copper sulfide nanoparticles for photothermal ablation of tumor cells. Nanomedicine. 2010;5:1161–71. doi: 10.2217/nnm.10.85. [DOI] [PubMed] [Google Scholar]
- 73.Melancon MP, Elliott A, Ji X, Shetty A, Yang Z, Tian M, et al. Theranostics with multifunctional magnetic gold nanoshells: photothermal therapy and t2* magnetic resonance imaging. Invest Radiol. 2011;46:132–40. doi: 10.1097/RLI.0b013e3181f8e7d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alkilany AM, Murphy CJ. Toxicity and cellular uptake of gold nanoparticles: what we have learned so far? J Nanopart Res. 2010;12:2313–33. doi: 10.1007/s11051-010-9911-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Murphy CJ, Gole AM, Stone JW, Sisco PN, Alkilany AM, Goldsmith EC, et al. Gold nanoparticles in biology: beyond toxicity to cellular imaging. Acc Chem Res. 2008;41:1721–30. doi: 10.1021/ar800035u. [DOI] [PubMed] [Google Scholar]
- 76.Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26:3995–4021. doi: 10.1016/j.biomaterials.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 77.Pisanic TR, II, Blackwell JD, Shubayev VI, Fiñones RR, Jin S. Nanotoxicity of iron oxide nanoparticle internalization in growing neurons. Biomaterials. 2007;28:2572–81. doi: 10.1016/j.biomaterials.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 78.Harisinghani MG, Barentsz J, Hahn PF, Deserno WM, Tabatabaei S, van de Kaa CH, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med. 2003;348:2491–9. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]
- 79.Ross RW, Zietman AL, Xie W, Coen JJ, Dahl DM, Shipley WU, et al. Lymphotropic nanoparticle-enhanced magnetic resonance imaging (LNMRI) identifies occult lymph node metastases in prostate cancer patients prior to salvage radiation therapy. Clin Imaging. 2009;33:301–5. doi: 10.1016/j.clinimag.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 80.Neuwelt EA, Varallyay P, Bago AG, Muldoon LL, Nesbit G, Nixon R. Imaging of iron oxide nanoparticles by MR and light microscopy in patients with malignant brain tumours. Neuropathol Appl Neurobiol. 2004;30:456–71. doi: 10.1111/j.1365-2990.2004.00557.x. [DOI] [PubMed] [Google Scholar]
- 81.Kobayashi H, Kawamoto S, Sakai Y, Choyke PL, Star RA, Brechbiel MW, et al. Lymphatic drainage imaging of breast cancer in mice by micro-magnetic resonance lymphangiography using a nano-size paramagnetic contrast agent. J Natl Cancer Inst. 2004;96:703–8. doi: 10.1093/jnci/djh124. [DOI] [PubMed] [Google Scholar]
- 82.Jain S, Hirst DG, O’Sullivan JM. Gold nanoparticles as novel agents for cancer therapy. Brit J Radiol. 2012;85:101–13. doi: 10.1259/bjr/59448833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Popovtzer R, Agrawal A, Kotov NA, Popovtzer A, Balter J, Carey TE, et al. Targeted gold nanoparticles enable molecular CT imaging of cancer. Nano Lett. 2008;8:4593–6. doi: 10.1021/nl8029114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Z. Plasmon—resonant gold nanoparticles for cancer optical imaging. Sci China Phys, Mech Astron. 2013;56:506–13. [Google Scholar]
- 85.Liu Y, Chen Z, Liu C, Yu D, Lu Z, Zhang N. Gadolinium-loaded polymeric nanoparticles modified with anti-VEGF as multifunctional MRI contrast agents for the diagnosis of liver cancer. Biomaterials. 2011;32:5167–76. doi: 10.1016/j.biomaterials.2011.03.077. [DOI] [PubMed] [Google Scholar]
- 86.Zhang HW, Wang LQ, Xiang QF, Zhong Q, Chen LM, Xu CX, et al. Specific lipase-responsive polymer-coated gadolinium nanoparticles for MR imaging of early acute pancreatitis. Biomaterials. 2014;35:356–67. doi: 10.1016/j.biomaterials.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 87.Manus LM, Mastarone DJ, Waters EA, Zhang X-Q, Schultz-Sikma EA, MacRenaris KW, et al. Gd(III)-nanodiamond conjugates for MRI contrast enhancement. Nano Lett. 2009;10:484–9. doi: 10.1021/nl903264h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reul R, Tsapis N, Hillaireau H, Sancey L, Mura S, Recher M, et al. Near infrared labeling of PLGA for in vivo imaging of nanoparticles. Polym Chem. 2012;3:694–702. [Google Scholar]
- 89.Magrez A, Kasas S, Salicio V, Pasquier N, Seo JW, Celio M, et al. Cellular toxicity of carbon-based nanomaterials. Nano Lett. 2006;6:1121–5. doi: 10.1021/nl060162e. [DOI] [PubMed] [Google Scholar]
- 90.Heo J, Reid T, Ruo L, Breitbach CJ, Rose S, Bloomston M, et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med. 2013;19:329–36. doi: 10.1038/nm.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Galanis E, Okuno SH, Nascimento AG, Lewis BD, Lee RA, Oliveira AM, et al. Phase I-II trial of ONYX-015 in combination with MAP chemotherapy in patients with advanced sarcomas. Gene Ther. 2005;12:437–45. doi: 10.1038/sj.gt.3302436. [DOI] [PubMed] [Google Scholar]
- 92.Lichty BD, Breitbach CJ, Stojdl DF, Bell JC. Going viral with cancer immunotherapy. Nat Rev Cancer. 2014;14:559–67. doi: 10.1038/nrc3770. [DOI] [PubMed] [Google Scholar]
- 93.Gao W, Xiang B, Meng TT, Liu F, Qi XR. Chemotherapeutic drug delivery to cancer cells using a combination of folate targeting and tumor microenvironment-sensitive polypeptides. Biomaterials. 2013;34:4137–49. doi: 10.1016/j.biomaterials.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 94.Soppimath KS, Liu LH, Seow WY, Liu SQ, Powell R, Chan P, et al. Multifunctional core/shell nanoparticles self-assembled from pH-induced thermosensitive polymers for targeted intracellular anticancer drug delivery. Adv Funct Mater. 2007;17:355–62. [Google Scholar]
- 95.Xiao D, Jia H-Z, Zhang J, Liu C-W, Zhuo R-X, Zhang X-Z. A dual-responsive mesoporous silica nanoparticle for tumor-triggered targeting drug delivery. Small. 2014;10:591–8. doi: 10.1002/smll.201301926. [DOI] [PubMed] [Google Scholar]
- 96.Huang S, Shao K, Liu Y, Kuang Y, Li J, An S, et al. Tumor-targeting and microenvironment-responsive smart nanoparticles for combination therapy of antiangiogenesis and apoptosis. ACS Nano. 2013;7:2860–71. doi: 10.1021/nn400548g. [DOI] [PubMed] [Google Scholar]
- 97.Cheng Z, Al Zaki A, Hui JZ, Muzykantov VR, Tsourkas A. Multifunctional nanoparticles: cost versus benefit of adding targeting and imaging capabilities. Science. 2012;338:903–10. doi: 10.1126/science.1226338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Theek B, Rizzo LY, Ehling J, Kiessling F, Lammers T. The theranostic path to personalized nanomedicine. Clin Transl Imaging. 2014;2:66–76. doi: 10.1007/s40336-014-0051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Theek B, Gremse F, Kunjachan S, Fokong S, Pola R, Pechar M, et al. Characterizing EPR-mediated passive drug targeting using contrast-enhanced functional ultrasound imaging. J Control Release. 2014;182:83–9. doi: 10.1016/j.jconrel.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sawant RR, Jhaveri AM, Koshkaryev A, Qureshi F, Torchilin VP. The effect of dual ligand-targeted micelles on the delivery and efficacy of poorly soluble drug for cancer therapy. J Drug Target. 2013;21:630–8. doi: 10.3109/1061186X.2013.789032. [DOI] [PubMed] [Google Scholar]
- 101.Goodman TT, Ng CP, Pun SH. 3-D tissue culture systems for the evaluation and optimization of nanoparticle-based drug carriers. Bioconjug Chem. 2008;19:1951–9. doi: 10.1021/bc800233a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Francia G, Cruz-Munoz W, Man S, Xu P, Kerbel RS. Mouse models of advanced spontaneous metastasis for experimental therapeutics. Nat Rev Cancer. 2011;11:135–41. doi: 10.1038/nrc3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bartlett D, Liu Z, Sathaiah M, Ravindranathan R, Guo Z, He Y, et al. Oncolytic viruses as therapeutic cancer vaccines. Mol Cancer. 2013;12:103. doi: 10.1186/1476-4598-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Al-Ghananeem AM, Malkawi AH, Muammer YM, Balko JM, Black EP, Mourad W, et al. Intratumoral delivery of Paclitaxel in solid tumor from biodegradable hyaluronan nanoparticle formulations. AAPS Pharm Sci Tech. 2009;10:410–7. doi: 10.1208/s12249-009-9222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chattopadhyay N, Fonge H, Cai Z, Scollard D, Lechtman E, Done SJ, et al. Role of antibody-mediated tumor targeting and route of administration in nanoparticle tumor accumulation in vivo. Mol Pharm. 2012;9:2168–79. doi: 10.1021/mp300016p. [DOI] [PubMed] [Google Scholar]
- 106.Sahoo SK, Ma W, Labhasetwar V. Efficacy of transferrin-conjugated paclitaxel-loaded nanoparticles in a murine model of prostate cancer. Int J Cancer. 2004;112:335–40. doi: 10.1002/ijc.20405. [DOI] [PubMed] [Google Scholar]
- 107.Mitra S, Gaur U, Ghosh PC, Maitra AN. Tumour targeted delivery of encapsulated dextran-doxorubicin conjugate using chitosan nanoparticles as carrier. J Control Release. 2001;74:317–23. doi: 10.1016/s0168-3659(01)00342-x. [DOI] [PubMed] [Google Scholar]
- 108.Farokhzad OC, Cheng J, Teply BA, Sherifi I, Jon S, Kantoff PW, et al. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc Natl Acad Sci U S A. 2006;103:6315–20. doi: 10.1073/pnas.0601755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jabbour M, Massad C, Boulos F. Variability in hormone and growth factor receptor expression in primary versus recurrent, metastatic, and post-neoadjuvant breast carcinoma. Breast Cancer Res Treat. 2012;135:29–37. doi: 10.1007/s10549-012-2047-z. [DOI] [PubMed] [Google Scholar]
- 110.Gasch C, Bauernhofer T, Pichler M, Langer-Freitag S, Reeh M, Seifert AM, et al. Heterogeneity of epidermal growth factor receptor status and mutations of KRAS/PIK3CA in circulating tumor cells of patients with colorectal cancer. Clin Chem. 2013;59:252–60. doi: 10.1373/clinchem.2012.188557. [DOI] [PubMed] [Google Scholar]
- 111.Xu Q, Liu Y, Su S, Li W, Chen C, Wu Y. Anti-tumor activity of paclitaxel through dual-targeting carrier of cyclic RGD and transferrin conjugated hyperbranched copolymer nanoparticles. Biomaterials. 2012;33:1627–39. doi: 10.1016/j.biomaterials.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 112.Ko HY, Choi KJ, Lee CH, Kim S. A multimodal nanoparticle-based cancer imaging probe simultaneously targeting nucleolin, integrin alphavbeta3 and tenascin-C proteins. Biomaterials. 2011;32:1130–8. doi: 10.1016/j.biomaterials.2010.10.034. [DOI] [PubMed] [Google Scholar]
- 113.Kluza E, van der Schaft DWJ, Hautvast PAI, Mulder WJM, Mayo KH, Griffioen AW, et al. Synergistic targeting of αvβ3 integrin and galectin-1 with heteromultivalent paramagnetic liposomes for combined MR imaging and treatment of angiogenesis. Nano Lett. 2010;10:52–8. doi: 10.1021/nl902659g. [DOI] [PubMed] [Google Scholar]
- 114.Saul JM, Annapragada AV, Bellamkonda RV. A dual-ligand approach for enhancing targeting selectivity of therapeutic nanocarriers. J Control Release. 2006;114:277–87. doi: 10.1016/j.jconrel.2006.05.028. [DOI] [PubMed] [Google Scholar]