Abstract
Work rotation schedules may be used to reduce the negative effects of vibration on vascular function. This study determined how long it takes vascular function to recover after a single exposure to vibration in rats (125 Hz, acceleration 5g). The responsiveness of rat-tail arteries to the vasoconstricting factor UK14304, an α2C-adrenoreceptor agonist, and the vasodilating factor acetylcholine (ACh) were measured ex vivo 1, 2, 7, or 9 d after exposure to a single bout of vibration. Vasoconstriction induced by UK14304 returned to control levels after 1 d of recovery. However, re-dilation induced by ACh did not return to baseline until after 9 d of recovery. Exposure to vibration exerted prolonged effects on peripheral vascular function, and altered vascular responses to a subsequent exposure. To optimize the positive results of work rotation schedules, it is suggested that studies assessing recovery of vascular function after exposure to a single bout of vibration be performed in humans.
Repeated exposures to hand-transmitted vibration through the use of powered hand tools may result in vascular dysfunction characterized by cold-induced vasospasms that result in finger blanching and severe discomfort for workers. These symptoms are usually referred to as vibration-induced white finger (VWF) disease (Pelmear, 1971; 1974; Pyykko and Gemne, 1987). Left untreated, this disorder may produce hypoxia in local tissues, and in the worst cases might induce a severe loss of function and amputation of the digits. In order to reduce the risk of developing VWF, interventions need to be developed that will decrease workers’ exposure to vibration.
One intervention that might be used to reduce a worker's exposure to hand-transmitted vibration is to employ work rotation schedules. Under these types of schedules, workers would use a vibrating hand tool for a period of time and then switch to another task where they would not be exposed to vibration. However, for this approach to be successful, the rest period between repetitive bouts of vibration would have to be long enough to allow for recovery of vascular function. Although blood flow to the fingers appears to return to baseline levels more rapidly when humans are exposed to intermittent vibration in the lab (Bovenzi et al., 2004), studies using animal models of vibration-induced vascular dysfunction suggest that even acute exposures may exert prolonged effects on vascular responsiveness to vasoconstricting and dilating factors (Hughes et al., 2009; Krajnak et al., 2006) and on vascular morphology (Curry et al., 2005; Govindaraju et al., 2006)
The goal of this study was to use a rat-tail model of vibration-induced injury to determine how long it takes vascular function to recover after exposure to a single bout of vibration. The model used in this study has been well characterized, and studies demonstrated that both the physical (Dong et al., 2008) and physiological responses (Krajnak et al., 2010, 2012; Welcome et al., 2008) of the tail to single bouts of vibration exposure are similar to the responses displayed by human fingers (Welcome et al., 2008). Investigation also showed that exposure to a single bout of vibration results in a transient vasoconstriction and increased sensitivity to α2C-adrenoreceptor-mediated vasoconstriction and acetylcholine (ACh) re-dilation is altered (Hughes et al., 2009; Krajnak et al., 2006, 2009) These acute effects of vibration on vascular function are in part mediated by elevation in the generation of reactive oxygen species (ROS) (Hughes et al., 2009). Thus, in the current study, ex vivo responses to vasoconstricting and dilating factors and tissue nitric oxide (NO) levels were measured to determine the length of time it takes the vascular system to recover after exposure to a single bout of vibration.
METHODS
Animals
Male Sprague-Dawley rats [Hla:(SD) CVF] (Hilltop Lab Animals, Inc., Scottdale, PA) that were 6 wk of age and weighing approximately 250 g at arrival were used in both studies. Rats were maintained in a colony room with a 12:12-h light:dark cycle (lights on 0700 h) and with Teklad 2918 food and tap water available ad libitum, at the National Institute for Occupational Safety and Health (NIOSH) facility, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. Rats were acclimated to the facilities for 1 wk before being used in experiments. All procedures were approved by the NIOSH Animal Care and Use Committee and were in compliance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals and the NRC Guide for the Care and Use of Laboratory Animals.
Procedures
Procedures and equipment used to expose rats to vibration have been previously described (Krajnak et al., 2006; Welcome et al., 2008). Briefly, on the day of the experiment, rats were restrained in Broome style restrainers and placed in sound-attenuating chambers. Rats exposed to vibration had their tails secured to platforms attached to shakers and were exposed to vertical, sinusoidal vibration at a frequency of 125 Hz and a constant acceleration of 49 m/s2 root mean square (r.m.s.) for 4 h (n = 6–8/group). Control rats also were restrained but had their tails secured to platforms mounted on isolation blocks (n = 6–8/group). Rats were exposed to vibration or control conditions and euthanized 1, 2, 7, or 9 d after exposure by exsanguination under pentobarbital anesthesia (100 mg/kg, ip). Additional groups of rats underwent the same exposure and were allowed to recover for similar amounts of time (n = 6–8/group). However, these groups of rats were exposed to another bout of vibration and euthanized 1 h after the second exposure.
Measurement of Nitrate/Nitrite Concentrations
After euthanasia, the C13–15 region of the tail was dissected and the ventral tail artery was immediately removed. The artery was bisected and two segments were immediately frozen in separate cryovials in liquid nitrogen. Nitrate/nitrite (NOx) concentrations were measured in the distal segment using the NOx colorimetric assay (Caymen Chemical Company; Ann Arbor, MI). Both assays were performed using the manufacturer's instructions. Protein concentrations were measured using the BCA protein assay (Pierce, Rockford, IL).
In Vitro Microvessel Studies
The remaining portion of the tail was placed in cold Dulbecco's modified medium (DMEM, Invitrogen, Carlsbad, CA) and kept at 4°C until used in microvessel studies. Ventral tail arteries from approximately the C16–20 region were dissected and the proximal section was used to measure vasoconstriction induced by the α-2C-adrenoreceptor agonist, UK14304. The distal segment was used to examine vascular re-dilation to ACh after constricting the artery with the α-1 adrenoreceptor agonist phenylephrine (PE). All vasoactive factors were purchased from Sigma Chemicals (St Louis, MO USA). Re-dilation after constriction was assessed because ventral tail arteries have little endogenous basal tone (Krajnak et al., 2006). To assess responses to vasoconstricting and dilating factors, artery segments were mounted on pipettes in a microvessel chamber (Catamount Research and Development, Living Systems, St. Albans, VT) containing HEPES buffer with glucose (10%) and sodium bicarbonate (Krajnak et al., 2006), and maintained at 37°C. Arteries were pressurized to 60 mm Hg and allowed to equilibrate for at least 1 h. The chamber buffer was then changed and vasoconstricting factors were added in half-log increments. Changes in the internal diameters of arteries were measured when arteries stabilized (approximately 5 min between applications of the agent) using an XC-ST30 video camera mounted on a Nikon T1-SM inverted microscope, a video dimension analyzer (Catamount Research and Development), and Data-Q Instruments software (Akron, OH). Re-dilation in response to ACh applied in half-log increments was measured in PE-constricted arteries and changes in the internal diameter were determined as described above. Dose-response curves to the various vasoactive factors were generated by averaging dose-dependent responses within a group and using GraphPad (Prism 5.1; San Diego, CA).
Statistical Analyses
Data from rats exposed to a single bout versus two bouts of vibration were analyzed separately. Separate comparisons were also made from samples collected after varying lengths of recovery. Mean concentrations of NOx (pm/μg protein) were calculated for each group and then analyzed using one-way analysis of variance (ANOVA). Pairwise comparisons were made using Student's t-test. The percent change in vascular diameter from baseline was calculated after the application of each dose of UK14304 or ACh. The average percent change in diameter at each dose was calculated and analyzed using two-way repeated-measures ANOVA (vibrated or control vs. dose). Significant interactions were made using oneway repeated-measures ANOVA, and Student's t-test for pairwise comparisons. All data were analyzed using JMP (version 10, SAS Institute, Inc., Cary, NC). Significant differences were those with p < .05.
RESULTS
One-Day Recovery
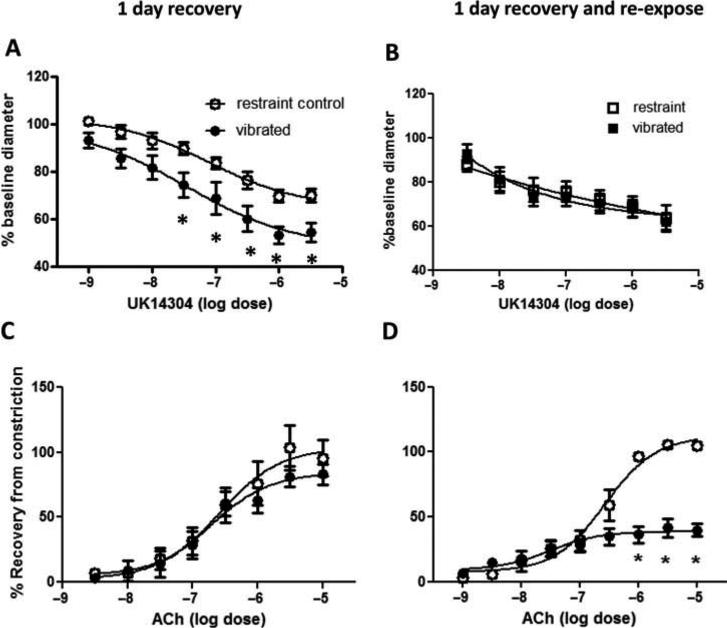
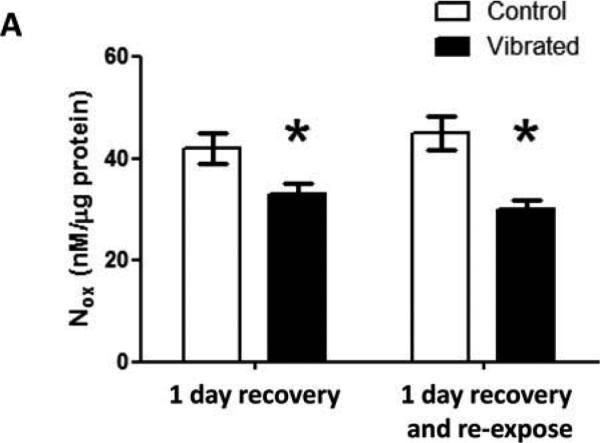
The baseline diameters of arteries in rats from the four different groups were not different (mean diameter ± SEM; 1-d recovery: control 320.57 ± 20.79, vibrated 352.71 ± 19.61; 1-d recovery and reexposure: control 357.71 ± 13.59, vibrated 363.43 ± 20.22). However, after 1 d of recovery, arteries from vibrated rats displayed a significant increase in sensitivity to UK14304-mediated vasoconstriction. ACh-induced re-dilation was similar in arteries from both groups of rats (Figures 1A and 1C, respectively) Arteries from rats re-exposed to vibration after 1 d of recovery did not show a marked change in responsiveness to UK14304, but did display a reduced responsiveness to ACh-mediated re-dilation (Figures 1B and 1D, respectively). Changes in vascular responsiveness were associated with reductions in vascular NOx concentrations (Figure 2).
FIGURE 1.
Ventral tail arteries collected from vibrated rats were more sensitive to UK14304-induced vasoconstriction than control rats after 1 d of recovery (A), but not after recovery and reexposure to a second bout of vibration (B). Arteries from vibrated rats did not display changes in ACh-mediated redilation after 1 d of recovery (C), but a decrease in sensitivity ACh-induced redilation was apparent in arteries from rat reexposed to vibration after 1 d of recovery (D). Asterisk indicates significant difference from control (p < .05).
FIGURE 2.
Vascular NOx concentrations were lower in arteries from vibrated than control-rats under both exposure conditions. Asterisk indicates significant difference from control (p < .05).
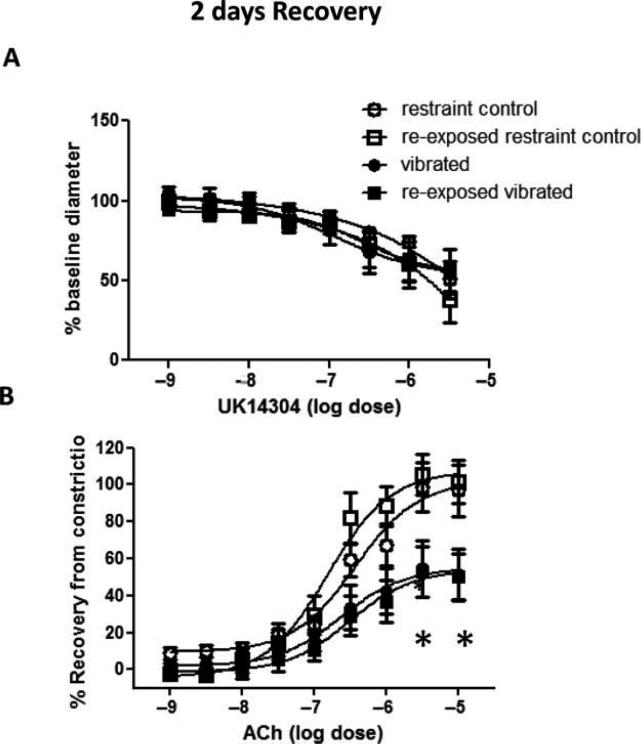
Two-Day Recovery
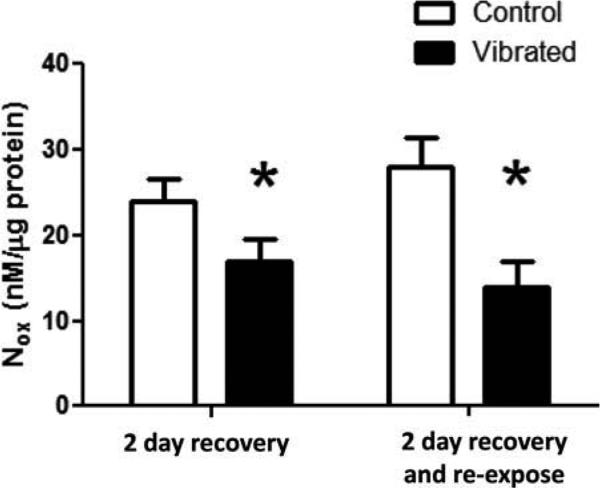
After 2 d of recovery, there was no marked change in UK14304-induced vasoconstriction in arteries from rats exposed to vibration (Figure 3A). However, the reduced responsiveness to ACh-induced re-dilation was maintained in all vibration-exposed arteries (Figure 3B). NOx concentrations also were lower in arteries collected from rats exposed to vibration than control rats (Figure 4).
FIGURE 3.
The vasoconstriction induced by UK14304 was similar in arteries from control and vibrated rats after 2 d of recovery (A). However, arteries from vibrated rats under both exposure conditions still displayed a reduced sensitivity to ACh-induced re-dilation (B). Asterisk indicates significant difference from control (p < .05).
FIGURE 4.
After 2 d of recovery, vascular NOx concentrations were lower in arteries from vibrated than in control rats under both exposure conditions. Asterisk indicates significant difference from control (p < .05).
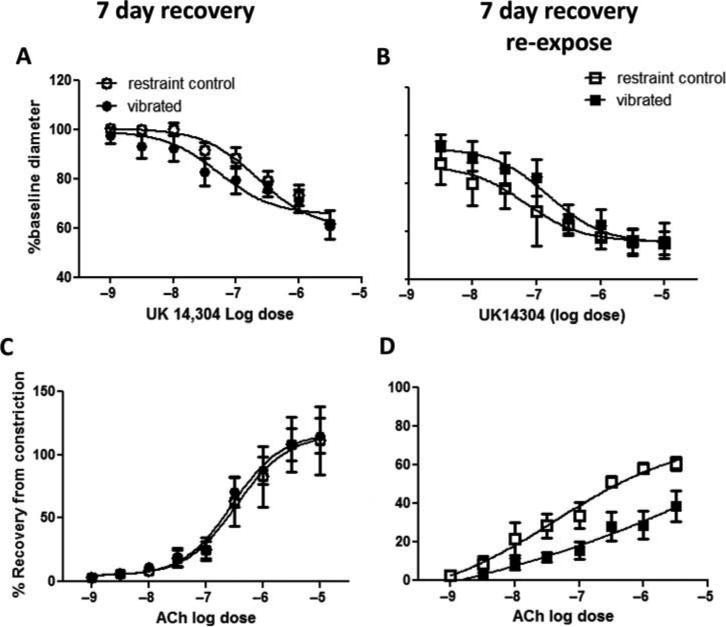
Seven-Day Recovery
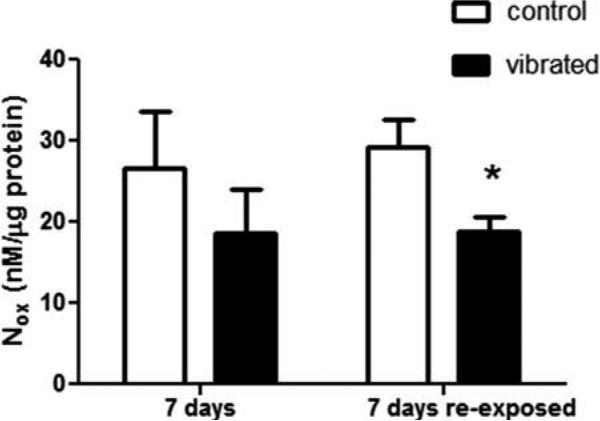
Both UK14304-induced vasoconstriction and ACh-induced vasodilation were similar in control and vibrated rats after 7 d of recovery (Figures 5A and 5C, respectively). There also were no changes in vascular NOx concentrations (Figure 6). Although re-exposure to vibration after 7 d of recovery did not markedly affect UK14304-induced vasoconstriction, there was a resultant decreased responsiveness to ACh-mediated re-dilation (Figure 5B and 5D, respectively). This change in responsiveness to ACh was associated with a reduction in vascular NOx concentrations in revibrated arteries (Figure 6).
FIGURE 5.
UK14304-induced vasoconstriction was similar in control and vibrated rats after 7 d of recovery (A), and after recovery and reexposure to a second bout of vibration (B). However, arteries from rats reexposed to vibration displayed a reduced sensitivity to ACh-induced re-dilation (D). Asterisk indicates significant difference from control (p < .05).
FIGURE 6.
Vascular NOx concentrations were reduced in rats that were re-exposed to vibration after 7 d of recovery.
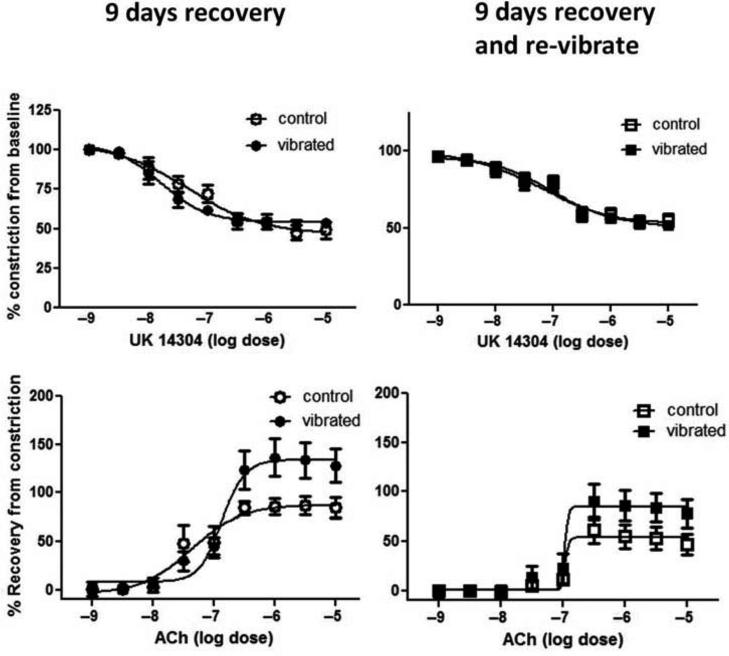
Nine-Day Recovery
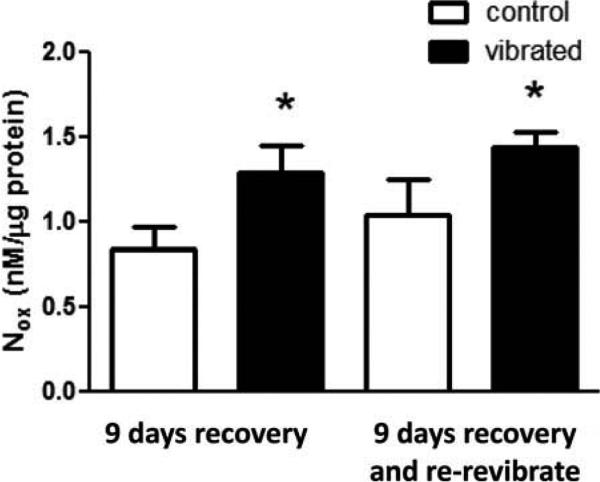
Arteries examined from animals following 9 d of recovery or 9 d of recovery and vibration did not display significant changes in UK14304-induced vasoconstriction (Figures 7A and 7B, respectively). Although there appeared to be an enhanced responsiveness to ACh-induced re-dilation after 9 d of recovery or recovery and vibration, the dose-dependent changes in artery diameter were not significant (Figure 7C and 7D, respectively). However, vascular NOx concentrations were increased in arteries from both vibrated groups after 9 d of recovery (Figure 8).
FIGURE 7.
Vasoconstriction in response to UK14304 was similar in control, vibrated, and revibrated arteries after 9 d of recovery (A and B). Although arteries from vibrated and revibrated rats appeared to be more sensitive than control rats to ACH-induced re-dilation, there were no significant differences (C and D).
FIGURE 8.
Vascular NOx concentrations were greater in arteries from vibrated and re-vibrated rats than from controls after 9 d of recovery (p < .05).
DISCUSSION
Implementing work-rotation schedules may reduce a workers exposure to hand-transmitted vibration, potentially reducing the risk of developing VWF. However, this prevention strategy may require more workers being trained to do a specific job, and the exposure of additional workers to vibration. Determining the length of time it takes for physiological systems to recover from exposure may allow employers to optimize the use of this prevention strategy and minimize some additional complications that the use of this strategy could introduce. Although human and animal studies examined the immediate recovery of blood flow to the fingers after a single exposure to vibration (Bovenzi et al., 2004; Curry et al., 2005), the studies presented here assessed vascular physiology to determine recovery from exposure to a single bout of vibration. These studies also examined how vascular physiology is altered by a subsequent vibration challenge. Our results suggest that it takes up to 9 d for vascular responses to display a full recovery after exposure to a single bout of vibration.
After 1 d of recovery, vibrated vessels displayed an increased responsiveness to α2C-adrenoreceptor mediated vasoconstriction that was no longer apparent after 2 d of recovery. α2C-Adrenoreceptors are intracellular receptors that translocate from the endoplasmic reticulum to the cell membrane under conditions that enhance oxidative stress (Hughes et al., 2009) . Thus, it is not surprising that increased-sensitivity to UK1430 is transient.
Endothelial-mediated vasodilation was also affected by vibration. Arteries from vibration-exposed rats displayed a decreased responsiveness to ACh-induced re-dilation. ACh induces vasodilation by acting on endothelial cells to stimulate NO release. In these studies, the diminished responsiveness of arteries to ACh was associated with changes in NOx concentrations, suggesting that cellular NO concentrations were also reduced. These findings are consistent with results of studies performed on arteries collected from rat paws that show that alterations in response to vasodilating factors are the result of elevations in ROS levels (Hughes et al., 2009). The increased production of ROS may lower vascular oxygen available for NO synthesis or synthesis of tetrahydrobiopterin, a cofactor needed for NO production, thereby resulting in a decrease in available NO (Hughes et al., 2009; Kotsonis et al., 1999). Studies previously demonstrated that vibration did not markedly affect vascular responsiveness to the NO mimetic S-nitroso-N-acetylpenicillamine (Hughes et al., 2009; Krajnak et al., 2009). Thus, it seems likely that reductions in sensitivity ACh-induced re-dilation are the result of a decrease in available NO.
In rats allowed to recover, vascular responses to ACh returned to control levels after 7 d. However, arteries from rats that were re-exposed to vibration after a 7-d recovery period displayed a diminished responsiveness to ACh-induced re-dilation immediately following the exposure and reduction in vascular NOx concentrations. These findings demonstrate that although basal vascular function returned to control levels after 7 d of recovery, vasular responses are still altered after a challenge.
Re-dilation in response to ACh was also at control levels after 9 d of recovery. In fact, arteries from the vibrated rats appeared to display a greater maximal response to ACh. Rats reexposed to vibration displayed a similar pattern, but the maximal response in the vibrated and control arteries was not significantly different. This increased responsiveness to ACh-induced vasodilation may be the result of an elevation in vascular NO concentrations in vibrated arteries. Other studies examining the effects of single exposure to vibration demonstrated that prolonged constriction induced by vibration resulted in pinching of the endothelial cells and injury (Govindaraju et al., 2006; Krajnak et al., 2006). It is possible that during the repair process, endothelial cells become more resilient and alter their responses to vibration to protect blood vessels from injury that may be produced by exposure to additional bouts of vibration.
Under the International Standards Organization standard 5349-1, which suggests exposure limits for workers using vibrating hand tools, workers should not be exposed to more than 4 h of vibration at 125 Hz in a single day. Thus, the exposure used in this study represents the maximal exposure a worker could receive if the worker were using a tool with a dominant frequency of 125 Hz. Epidemiological and experimental studies in humans suggest that the risk of developing VWF is greatest at frequencies between 100 and 300 Hz (Dong et al., 2008). The risk of developing VWF seems to be reduced in humans exposed to dominant frequencies between 30 and 60 Hz (Dong et al., 2008). Vibration-induced changes in arterial and sensorineural function display similar relationships between the frequency of the exposure and changes indicative of vascular and sensorineural dysfunction (Krajnak et al., 2010, 2012). Thus, it is possible that if workers rotate to tasks where tools have a dominant frequency between 30 and 60 Hz, or if they perform tasks where they are not exposed to vibration, their risk of developing VWF will be reduced.
CONCLUSIONS
These data demonstrated that work rotation schedules may be a reasonable approach that can be used to reduce worker exposure to hand-transmitted vibration and the negative effects on vascular function. Additional studies examining recovery of vascular function in humans after exposure to a single bout of vibration and repeated bouts of vibration at frequencies between 30 and 60 Hz may provide more detailed data on the length of time it takes arteries to recover in humans after exposure to a single bout of vibration. These data may help managers and workers determine whether it is feasible to employ work rotation schedules as a means of reducing vibration exposure and the potential risk of developing VWF.
Footnotes
DISCLAIMER
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
REFERENCES
- Bovenzi M, Welsh AJ, Griffin MJ. Acute effects of continuous and intermittent vibration on finger circulation. Int. Arch. Occup. Environ. Health. 2004;77:255–263. doi: 10.1007/s00420-004-0507-4. [DOI] [PubMed] [Google Scholar]
- Curry BD, Govindaraju SR, Bain JL, Zhang LL, Yan JG, Matloub HS, Riley DA. Evidence for frequency-dependent arterial damage in vibrated rat tails. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2005;284:511–521. doi: 10.1002/ar.a.20186. [DOI] [PubMed] [Google Scholar]
- Dong JH, Dong RG, Rakheja S, Welcome DE, McDowell TW, Wu JZ. A method for analyzing absorbed power distribution in the hand and arm substructures when operating vibrating tools. J. Sound Vibration. 2008;311:1286–1309. [Google Scholar]
- Govindaraju SR, Curry BD, Bain JL, Riley DA. Comparison of continuous and intermittent vibration effects on rat-tail artery and nerve. Muscle Nerve. 2006;34:197–204. doi: 10.1002/mus.20578. [DOI] [PubMed] [Google Scholar]
- Hughes JM, Wirth O, Krajnak K, Miller R, Flavahan S, Berkowitz DE, Welcome D, Flavahan NA. Increased oxidant activity mediates vascular dysfunction in vibration injury. J. Pharmacol. Exp. Ther. 2009;328:223–230. doi: 10.1124/jpet.108.144618. [DOI] [PubMed] [Google Scholar]
- Kotsonis P, Frey A, Frohlich LG, Hofmann H, Reif A, Wink DA, Feelisch M, Schmidt HHHW. Autoinhibition of neuronal nitric oxide synthase: Distinct effects of reactive nitrogen and oxygen species on enzyme activity. Biochem. J. 1999;340:745–752. [PMC free article] [PubMed] [Google Scholar]
- Krajnak K, Dong RG, Flavahan S, Welcome DE, Flavahan NA. Acute vibration increases α2c-adrenergic smooth muscle constriction and alters thermosensitivity of cutaneous arteries. J. Appl. Physiol. 2006;100:1230–1237. doi: 10.1152/japplphysiol.00761.2005. [DOI] [PubMed] [Google Scholar]
- Krajnak K, Miller GR, Johnson C, Waugh S, Kashon ML. Frequency-dependent effects of vibration on peripheral nerves and sensory nerve function in a rat model of hand-arm vibration syndrome. J. Occup. Environ. Med. 2012;54:1010–1016. doi: 10.1097/JOM.0b013e318255ba74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajnak K, Miller GR, Waugh S, Johnson C, Li S, Kashon ML. Characterization of frequency-dependent response of the vascular system to reptitive vibration. J. Occup. Environ. Med. 2010;52:584–594. doi: 10.1097/JOM.0b013e3181e12b1f. [DOI] [PubMed] [Google Scholar]
- Krajnak K, Waugh S, Johnson C, Miller R, Kiedrowski M. Vibration disrupts vascular function in a model of metabolic syndrome. Ind. Health. 2009;47:533–542. doi: 10.2486/indhealth.47.533. [DOI] [PubMed] [Google Scholar]
- Pelmear PL. Raynaud's phenomenon of occupational origin. Occup. Health (Lond.) 1971;23:39–44. [PubMed] [Google Scholar]
- Pelmear PL. Vibration white finger. Occup. Health (Lond.) 1974;26:307–310. [PubMed] [Google Scholar]
- Pyykko I, Gemne G. Patho-physiological aspects of peripheral circulatory disorders in the vibration syndrome. Scand. J. Work Environ. Health. 1987;13:313–316. doi: 10.5271/sjweh.2046. [DOI] [PubMed] [Google Scholar]
- Welcome DE, Krajnak K, Kashon ML, Dong RG. An investigation on the biodyamic foundation of a rat tail model. J. Eng. Med. (Proc. Inst. Mech. Eng. Part H) 2008;222:1127–1141. doi: 10.1243/09544119JEIM419. [DOI] [PubMed] [Google Scholar]