ABSTRACT
Natural killer (NK) cells provide a first line of defense against infection via the production of antiviral cytokines and direct lysis of target cells. Cytokines such as interleukin 12 (IL-12) and IL-18 are critical regulators of NK cell activation, but much remains to be learned about how cytokines interact to regulate NK cell function. Here, we have examined cytokine-mediated activation of NK cells during infection with two natural mouse pathogens, lymphocytic choriomeningitis virus (LCMV) and murine cytomegalovirus (MCMV). Using a systematic screen of 1,849 cytokine pairs, we identified the most potent combinations capable of eliciting gamma interferon (IFN-γ) production in NK cells. We observed that NK cell responses to cytokine stimulation were reduced 8 days after acute LCMV infection but recovered to preinfection levels by 60 days postinfection. In contrast, during MCMV infection, NK cell responses to cytokines remained robust at all time points examined. Ly49H-positive (Ly49H+) NK cells recognizing viral ligand m157 showed preferential proliferation during early MCMV infection. A population of these cells was still detected beyond 60 days postinfection, but these divided cells did not demonstrate enhanced IFN-γ production in response to innate cytokine stimulation. Instead, the maturation state of the NK cells (as determined by CD11b or CD27 surface phenotype) was predictive of responsiveness to cytokines, regardless of Ly49H expression. These results help define cytokine interactions that regulate NK cell activation and highlight variations in NK cell function during two unrelated viral infections.
IMPORTANCE Natural killer cells play an important role in immunity to many viral infections. From an initial screen of 1,849 cytokine pairs, we identified the most stimulatory cytokine combinations capable of inducing IFN-γ production by NK cells. Ly49H+ NK cells, which can be directly activated by MCMV protein m157, preferentially proliferated during MCMV infection but did not show enhanced IFN-γ production following direct ex vivo cytokine stimulation. Instead, mature CD11b+ and/or CD27+ NK cells responded similarly to innate cytokine stimulation regardless of Ly49H expression. Collectively, our data provide a better foundation for understanding cytokine-mediated NK cell activation during viral infection.
INTRODUCTION
Natural killer (NK) cells are a group of lymphocytes that contribute to early innate immune responses against a wide array of pathogens and some cancers (1–3). NK cells exert their effects via the production of antiviral and immunoregulatory cytokines and also through cytotoxic activity and direct lysis of target cells (4–6). Moreover, they can play a key role in immunoregulation, and they have been reported to either promote or limit adaptive immune responses to viral infections (7–12). NK cells are a heterogeneous population and progress through several developmental stages. These maturation states can be identified by cell surface expression of CD11b and CD27 and are associated with variations in NK cell functional capabilities (13, 14). Throughout their lifetime, activation of NK cells can be regulated by a broad array of cytokines, microbial ligands, or molecules on the surfaces of target cells that interact with both activating and inhibitory receptors on the NK cell surface (15–17). Several cytokines, particularly interleukin 12 (IL-12) and IL-18, are known to trigger gamma interferon (IFN-γ) production by NK cells in a potently synergistic manner (18–20). However, the interactions between many other cytokines are less well defined. Given the wide array of distinct inflammatory environments that may arise during infection or coinfection with different pathogens, more thorough knowledge of cytokine interactions will be key to understanding regulation of NK cell functions.
Two of the most well-characterized mouse models of antiviral immunity are lymphocytic choriomeningitis virus (LCMV) and murine cytomegalovirus (MCMV) infection. These viruses induce distinct cytokine profiles and also share several key differences in their NK cell-mediated immune responses with LCMV being a relatively resistant to NK cells and MCMV being sensitive to NK cells (7). Although NK cells become activated and exhibit cytotoxicity during LCMV infection, they may not be essential for protection (16, 21, 22) but could be involved in modifying subsequent antiviral T cell responses and act as a “rheostat-like” regulator of host immunity (7). In contrast, NK cells play a critical role in the control of MCMV infection. In C57BL/6 mice, which are resistant to MCMV infection, up to 50% of NK cells express the activating Ly49H receptor (23–25). This allows the NK cells to specifically recognize viral protein m157, expressed on the surfaces of MCMV-infected cells, which is essential for efficient control of the infection (7, 26–29). Therefore, MCMV infection offers the opportunity to examine NK cells that are not only activated by cytokines but also can respond directly to a defined viral ligand.
Here, we have examined the effects of 43 murine cytokines tested individually or in pairs to determine their relative ability to activate NK cells directly ex vivo. Moreover, we examined the effects of the top 10 most stimulatory cytokine pairs identified in this screen on NK cells during infection with two natural mouse pathogens, LCMV and MCMV. We found that cytokine-induced production of IFN-γ by NK cells was reduced during early LCMV infection but returned to levels comparable to those observed in uninfected mice by 60 days postinfection. This inhibition was specific to IFN-γ expression, as upregulation of membrane-bound CD69 and CD25 molecules following cytokine exposure was not dramatically affected. During MCMV infection, NK cell IFN-γ responses to cytokine stimulation 7 days or >60 days postinfection were comparable to those of naive mice. MCMV-specific Ly49H-positive (Ly49H+) NK cells preferentially proliferated during early MCMV infection, as determined by bromodeoxyuridine (BrdU) incorporation. BrdU+ Ly49H+ NK cells were still detected >60 days postinfection, but they did not exhibit enhanced responsiveness to cytokine stimulation. These results help characterize key features of NK cell responsiveness to innate cytokines throughout the course of model virus infection and may be useful targets for future therapeutic interventions.
MATERIALS AND METHODS
Mice and viral infections.
Female BALB/c and C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were infected at 6 to 12 weeks of age via intraperitoneal injection of 2 × 105 PFU LCMV-Armstrong or 5 × 104 PFU MCMV (WT-BAC MW97.01, derived from the Smith strain). All animal experiments were reviewed and approved by the Oregon Health and Science University Institutional Animal Care and Use Committee.
Reagents.
Recombinant murine cytokines (certified endotoxin free) were purchased from R&D Systems (Minneapolis, MN). Cytokines examined included IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-9, IL-10, IL-11, IL-12, IL-13, IL-15, IL-17, IL-18, IL-19, IL-20, IL-21, IL-23, IL-27, IL-28, IL-33, tumor necrosis factor alpha (TNF-α), TNF-β, CD27 ligand (CD27L), CD40L, CD137L, TNF-related apoptosis-inducing ligand (TRAIL), TNF-related activation-induced cytokine (TRANCE), TNF-related weak inducer of apoptosis (TWEAK), B cell activating factor (BAFF), LIGHT (homologous to lymphotoxin, exhibits inducible expression and competes with herpes simplex virus glycoprotein D for binding to herpesvirus entry mediator, a receptor expressed on T lymphocytes), TNF-like cytokine 1A (TL1A), glucocorticoid-induced TNF receptor-related protein ligand (GITRL), transforming growth factor α (TGF-α), TGF-β, vascular endothelial growth factor (VEGF), nerve growth factor (NGF), macrophage colony-stimulating factor (M-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), IFN-α, IFN-β, and IFN-γ. Anti-CD8α (53-6.7), anti-CD27 (LG.7F9), anti-BrdU (B44), and anti-IFN-γ (XMG1.2) were purchased from BD Pharmingen. Anti-CD25 (PC61) was purchased from BioLegend (San Diego, CA), and anti-CD3 (17A2), anti-Ly49H (3D10), anti-CD69 (H1.2F3), anti-CD11b (M1/70), and anti-CD49b (DX5) were purchased from eBioscience (San Diego, CA). Aqua cell viability stain was purchased from Invitrogen (Carlsbad, CA). Bromodeoxyuridine (BrdU) was purchased from Sigma-Aldrich (St. Louis, MO) and administered once daily by intraperitoneal injection (1 mg) on days 3 to 6 postinfection.
Stimulations and staining.
Spleens were pressed through 70-μm nylon filters to create single-cell suspensions and depleted of red blood cells by NH4Cl lysis prior to direct ex vivo stimulation. For the initial screen of 1,849 cytokine pairs, all cytokines were used at a final concentration of 100 ng/ml to maximize potential IFN-γ production. The top 10 most stimulatory cytokine combinations were then used at 10 ng/ml for subsequent experiments as indicated. Cells were stimulated with cytokines for 6 h in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 20 mM HEPES, penicillin-streptomycin, and l-glutamine at 37°C and 6% CO2. To assess cytokine production during stimulation, brefeldin A (2 μg/ml) (Sigma-Aldrich, St. Louis, MO) was added during the final hour of incubation. Cells were stained overnight at 4°C with Aqua (to identify live cells) and antibodies to cell surface markers in phosphate-buffered saline (PBS) plus 1% fetal calf serum (FCS) with 0.1 mg/ml mouse IgG (Sigma-Aldrich) and 1 μg/ml anti-CD16/32 (2.4G2; Fc block). For intracellular staining, cells were washed, fixed with 2% formaldehyde in PBS, permeabilized with Permwash (0.1% saponin [Sigma-Aldrich], 0.1% NaN3 [Sigma-Aldrich], and 2% FBS in PBS) and stained for intracellular cytokines for 1 h. For detection of BrdU incorporation, formaldehyde-fixed cells were treated with Permwash plus 10% dimethyl sulfoxide (DMSO) for 10 min, fixed again with 2% formaldehyde, and treated with DNase (300 μg/ml in PBS; Sigma-Aldrich) for 1 h at 37°C prior to intracellular staining. Data were acquired on an LSR Fortessa flow cytometer (BD, San Jose, CA) and analyzed using FlowJo software (Treestar, Ashland, OR).
Statistical analysis.
A two-tailed Student's t test was used to evaluate the statistical significance of differences between groups. A P value of <0.05 was considered significant.
RESULTS
Cytokine-mediated activation of NK cells before and after acute LCMV infection.
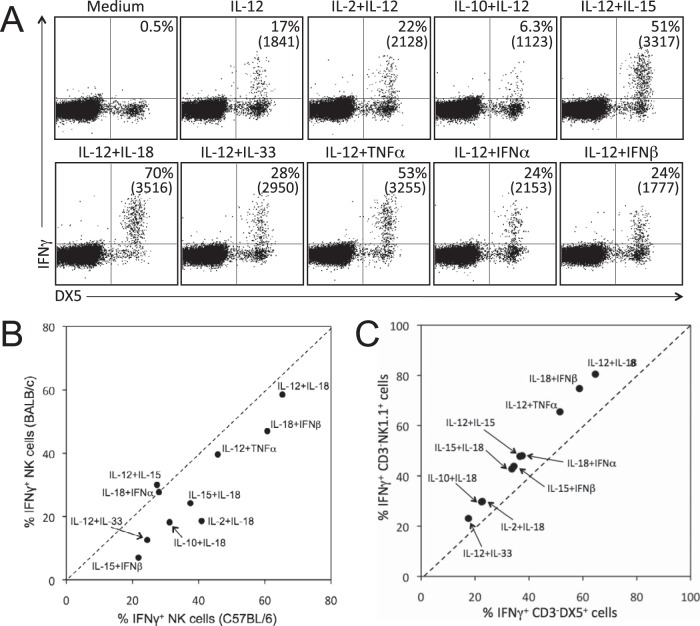
NK cell function can be regulated by a variety of cytokines. IL-12 is the prototypical innate cytokine capable of activating NK cells, and it acts synergistically with several other cytokines to elicit the production of IFN-γ. Mouse splenocytes were stimulated directly ex vivo with an array of individual and paired cytokines for 6 h, and production of the antiviral cytokine IFN-γ was measured to assess NK cell activation (Fig. 1). IL-12 alone induced IFN-γ expression in ∼17% of DX5-positive (DX5+) CD3− NK cells (Fig. 1A). When IL-12 was paired with IL-10, IFN-γ production was reduced by more than half (Fig. 1A), similar to our observations in CD8+ T cells (30). In contrast, when IL-12 was paired with IL-15, IL-18, IL-33, TNF-α, IFN-α, or IFN-β, there was synergistic enhancement of IFN-γ production, with up to 70% of NK cells becoming IFN-γ+ after exposure to the most potent cytokine pair identified, IL-12 plus IL-18 (Fig. 1A and Table 1). The mean fluorescence intensity (MFI) of IFN-γ staining correlates with the amount of IFN-γ that is secreted in vitro (30), and we found that cytokine combinations that triggered the highest frequency of IFN-γ+ cells also typically elicited the most IFN-γ production as well (Fig. 1A and data not shown). To verify that our observations were not mouse strain specific, we tested a representative panel of cytokine pairs in naive BALB/c and C57BL/6 mice (Fig. 1B). In general, we found that NK cells from either mouse strain responded similarly to the cytokine combinations tested and showed the same hierarchy of cytokine-based activation. However, there was slightly higher cytokine responsiveness in NK cells from C57BL/6 mice compared to BALB/c mice for 8 of the 10 cytokine combinations examined. Since NK cells from C57BL/6 mice express both DX5 and NK1.1, we compared the relative responsiveness of the CD3− DX5+ and CD3− NK1.1+ populations to cytokine stimulation (Fig. 1C). Following cytokine exposure, the CD3− DX5+ population demonstrated a modestly lower frequency of cells producing IFN-γ compared to the CD3− NK1.1+ population. On average, the DX5+ NK cell response to each cytokine combination reached 79% ± 2% of that observed among the NK1.1+ population. However, the overall hierarchy of cytokine-induced IFN-γ production by these two NK cell populations remained essentially the same regardless of which NK cell marker was used.
FIG 1.
Activation of NK cells following exposure to innate cytokines. Splenocytes from naive mice were stimulated in vitro with the indicated cytokines (10 ng/ml). At 6 h, IFN-γ production was assessed by intracellular cytokine staining and flow cytometry. (A) NK cells from BALB/c mice produce IFN-γ following exposure to a variety of cytokine combinations. Dot plots were gated on live, CD3− cells, and the number in the top right quadrant of each dot plot represents the percentage of CD3− DX5+ cells producing IFN-γ in response to the indicated cytokine combination. The mean fluorescence intensities (MFI) of IFN-γ+ cells are shown in parentheses. (B) NK cells from BALB/c and C57BL/6 mice exhibit a similar hierarchy of IFN-γ responses to cytokine stimulation. Each data point represents the percentage of CD3− DX5+ NK cells from BALB/c or C57BL/6 mice that produced IFN-γ in response to the indicated cytokine combination and is the average of 4 to 6 mice. (C) Cytokine-induced IFN-γ production by CD3− DX5+ cells and CD3− NK1.1+ cells in C57BL/6 mice. Data points represent the frequency of IFN-γ+ cells within each population following exposure to the indicated cytokine pair and are the averages of 8 mice.
TABLE 1.
Summary of cytokine combinations that trigger IFN-γ production by NK cellsa
| Naive mice |
LCMV-infected mice |
||||
|---|---|---|---|---|---|
| Day 8 postinfection |
>60 days postinfection |
||||
| Treatment | % IFN-γ+ NK cells | Treatment | % IFN-γ+ NK cells | Treatment | % IFN-γ+ NK cells |
| IL-12 + IL-18 | 65 | IL-12 + IL-18 | 33 | IL-12 + IL-18 | 58 |
| IL-18 + IFN-β | 61 | IL-18 + IFN-β | 25 | IL-18 + IFN-β | 49 |
| IL-12 + TNF-α | 46 | IL-12 + TNF-α | 18 | IL-12 + TNF-α | 38 |
| IL-12 + IL-15 | 41 | IL-12 + IL-15 | 17 | IL-18 + IFN-α | 35 |
| IL-18 + IFN-α | 38 | IL-2 + IL-18 | 17 | IL-12 + IL-15 | 35 |
| IL-15 + IL-18 | 31 | IL-15 + IL-18 | 12 | IL-15 + IL-18 | 31 |
| IL-2 + IL-18 | 28 | IL-18 + IFN-α | 11 | IL-2 + IL-18 | 27 |
| IL-10 + IL-18 | 27 | IL-10 + IL-18 | 9.1 | IL-10 + IL-18 | 25 |
| IL-12 + IL-33 | 24 | TNF-α + IFN-β | 8.9 | IL-18 + TNF-α | 22 |
| IL-15 + IFN-β | 22 | IL-15 + IFN-β | 7.4 | IL-12 + IL-33 | 21 |
| IL-12 | 12 | IL-12 | 2.3 | IL-18 | 14 |
| IL-18 | 9.9 | IL-18 | 2.2 | IL-12 | 7.7 |
| IFN-β | 5.2 | IFN-β | 1.9 | IFN-β | 3.2 |
| IL-15 | 4.4 | IL-15 | 1.1 | IL-33 | 2.9 |
| TNF-α | 1.5 | TNF-α | 0.8 | TNF-α | 2.1 |
| IFN-α | 1.4 | IL-33 | 0.6 | IL-15 | 2.1 |
| IL-33 | 0.9 | IL-2 | 0.6 | IFN-α | 0.9 |
| IL-10 | 0.4 | IFN-α | 0.2 | IL-10 | 0.8 |
| IL-2 | 0.2 | IL-10 | 0.2 | IL-2 | 0.7 |
The top 10 cytokine combinations (10 ng/ml) that triggered IFN-γ production by NK cells from naive mice or LCMV-infected mice 8 days or >60 days postinfection were ranked according to the percentage of live CD3− DX5+ NK cells producing IFN-γ. Spontaneous production of IFN-γ in medium-only controls was typically <1%, and this background was subtracted prior to preparing the table. Results represent the averages of six mice per group.
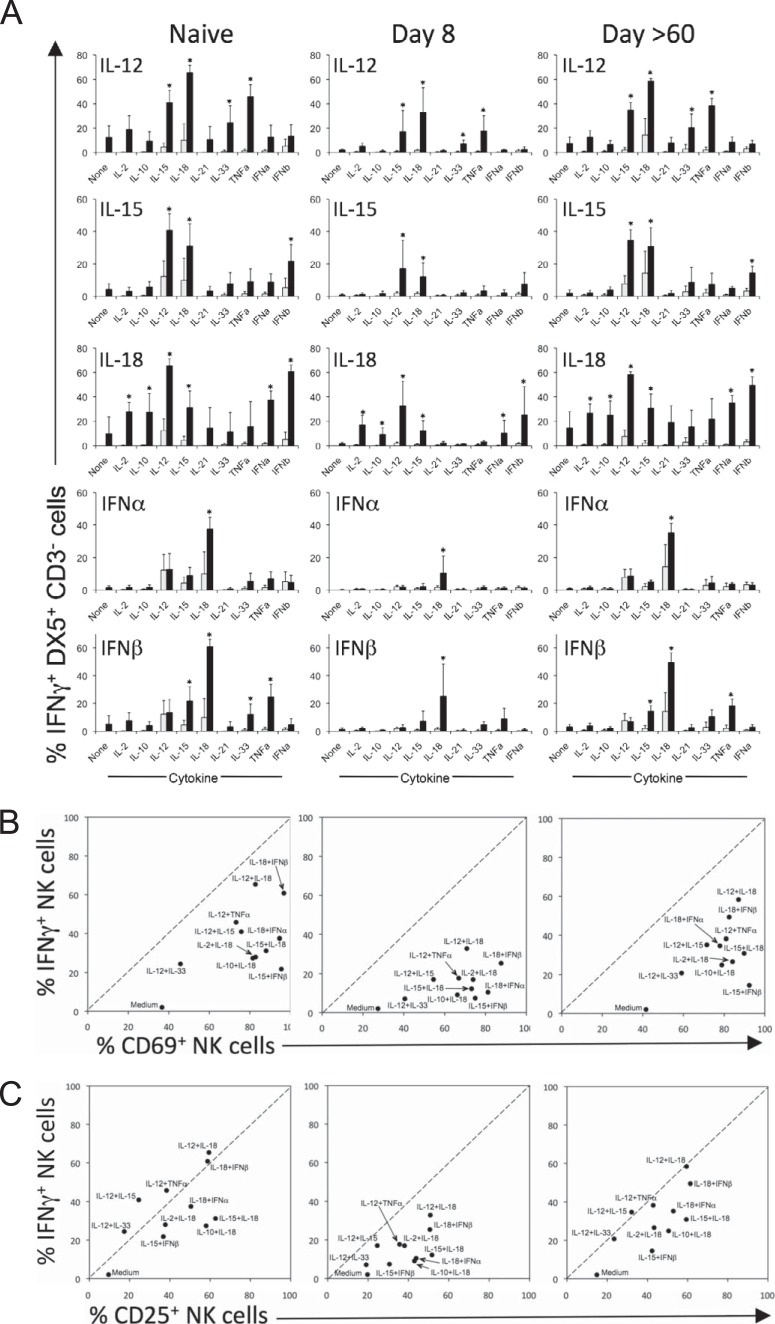
To further define the interactions among cytokines that regulate NK cell activation, we conducted a comprehensive analysis in which 43 commercially available cytokines were tested individually at a high concentration (100 ng/ml) and in all possible pairwise combinations (i.e., 1,849 cytokine pairs) for their ability to induce IFN-γ production by NK cells from LCMV-infected mice (8 days or >60 days postinfection; data not shown). The top 10 most stimulatory cytokine pairs identified in this initial screen comprised 10 individual cytokines, and pairwise combinations of these cytokines were tested at 10 ng/ml in naive or LCMV-infected mice (Table 1 and Fig. 2A). As expected, IL-12 plus IL-18 was the most potent cytokine combination, leading to IFN-γ production by 65 to 70% of NK cells in naive mice. IL-12 alone induced IFN-γ production in approximately 5 to 24% of NK cells and led to synergistic IFN-γ production when paired with IL-15, IL-33, or TNF-α. IL-15 is best known for its role in cell proliferation and homeostasis, but it is also a potent IFN-γ inducer for NK cells when paired with IL-12, IL-18, or IFN-β. Similar to IL-12, IL-18 acted synergistically with a wide array of cytokines, but it displayed a different synergistic profile. IL-18 enhanced IFN-γ production by NK cells when paired with IL-2, IL-10, IL-12, IL-15, IFN-α, or IFN-β, but not IL-33 or TNF-α. IFN-β enhanced IFN-γ production when paired with IL-15, IL-33, TNF-α, and especially IL-18. IFN-β and IFN-α showed similar patterns of synergies, though in each instance, IFN-β led to higher levels of IFN-γ production than IFN-α. Interestingly, while the overall pattern and hierarchy of cytokine synergies observed 8 days postinfection were similar to those of naive mice, the absolute frequency of NK cells producing IFN-γ in response to each cytokine combination was dramatically reduced (e.g., 65% IFN-γ+ versus 33% IFN-γ+ for IL-12 plus IL-18 in naive and day 8 LCMV-infected mice, respectively). Previous studies (21) have also found that acute LCMV infection reduces IL-12 responsiveness of splenic NK cells in vivo, further supporting these direct ex vivo results. However, by 60 days postinfection, cytokine responsiveness had returned to a level comparable to that observed in naive animals (Table 1 and Fig. 2).
FIG 2.
Cytokine-mediated activation of NK cells during LCMV infection. Splenocytes from uninfected (naive) or LCMV-infected (8 days or >60 days postinfection) BALB/c mice were stimulated directly ex vivo with the indicated cytokine combinations at 10 ng/ml for 6 h. (A) IFN-γ production by NK cells following exposure to innate cytokines. Light gray bars represent IFN-γ responses to the unpartnered individual cytokines indicated on the x axis, and the corresponding black bars represent IFN-γ responses to each cytokine in combination with the cytokine indicated at the top of each panel. Spontaneous production of IFN-γ in medium-only controls was typically <1%, and this background was subtracted prior to preparing the graphs. IFN-γ responses to each cytokine pair were compared to responses after stimulation with the individual cytokines using a two-tailed t test. Cytokine pairs that induced NK cell responses that were significantly different (P < 0.05) from both responses to the individual cytokines within the pair are indicated with an asterisk. (B) Differential induction of cell surface CD69 expression and IFN-γ expression in NK cells. (C) Cytokine-mediated regulation of CD25 expression on CD3− DX5+ NK cells. Each data point is the average of 4 to 6 mice per group.
Differential regulation of CD69 or CD25 and IFN-γ expression by NK cells.
In addition to producing IFN-γ, activated NK cells upregulate several immunomodulatory molecules such as CD69 and CD25 on their cell surface in response to cytokine stimulation. These molecules are important because CD69 expression influences lymphocyte migration (31, 32) and CD25 regulates cellular proliferation (33–35). Notably, while CD69 was upregulated on a high percentage of NK cells following cytokine stimulation, only a subset of these cells produced IFN-γ (Fig. 2B). For example, while more than 90% of NK cells from naive mice exposed to IL-15 plus IFN-β become activated as measured by CD69 expression, only 20% of these cells produce IFN-γ. CD25 upregulation was more closely matched with IFN-γ production for most cytokine pairs (Fig. 2C). Moreover, while IFN-γ production following cytokine stimulation was reduced 8 days after LCMV infection, upregulation of CD69 and CD25 was not inhibited, indicating differential regulation of these secreted and surface-bound proteins at this stage of the antiviral immune response.
Ly49H+ NK cells proliferate during MCMV infection but do not exhibit enhanced cytokine responsiveness.
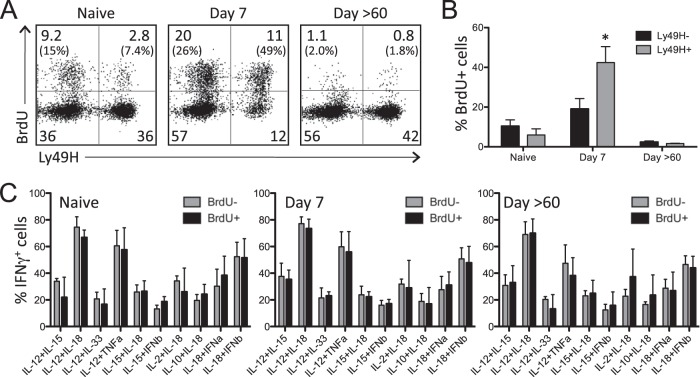
During MCMV infection of C57BL/6 mice, NK cells expressing Ly49H are activated in a receptor-specific manner by viral protein m157 (23, 24). Although typically classified as part of the innate immune system, recent studies have indicated that NK cells may display some characteristics traditionally associated with adaptive immunity, such as receptor-specific proliferation and the generation of long-lived “memory” cells with enhanced responsiveness to secondary exposure (36–42). To examine this further, BrdU was administered to naive mice and MCMV-infected mice (days 3 to 6 postinfection) to assess proliferation of Ly49H+ NK cells (i.e., responsive to the MCMV m157-activating ligand) and Ly49H− NK cells. Splenocytes were examined 7 or >60 days postinfection, and BrdU incorporation by Ly49H+ and Ly49H− NK cells was measured by flow cytometry (Fig. 3A and B). Naive mice displayed a basal level of NK cell proliferation, with 15% of Ly49H− cells and 7.4% of Ly49H+ cells staining positive for BrdU. Seven days after MCMV infection, there was a dramatic increase in the proliferation of Ly49H+ cells, with nearly 50% of these cells becoming BrdU+ compared to 26% BrdU+ Ly49H− NK cells. Interestingly, there was an increase in apoptotic Ly49H+ NK cells observed 7 days postinfection (data not shown) and an overall reduced frequency of Ly49H+ NK cells at this time point (Fig. 3A), indicating dynamic changes in NK cell proliferation and turnover. By 60 days postinfection, a population of the NK cells that proliferated during early MCMV infection was readily detected, with approximately 2% of Ly49H+ NK cells remaining BrdU+ at this later time point. To determine whether the Ly49H+ NK cells that proliferated during the early stages of MCMV infection exhibit enhanced responsiveness to inflammatory cytokine stimulation, they were stimulated directly ex vivo with each of the top 10 most stimulatory cytokine combinations (Table 1), and IFN-γ was measured by flow cytometry (Fig. 3C). When BrdU+ Ly49H+ cells were compared to BrdU− Ly49H+ cells, no significant difference in IFN-γ production was detected at any of the time points examined. These data demonstrate that while Ly49H+ NK cells preferentially proliferate during the early stages of MCMV infection, they do not become more responsive to cytokine-mediated stimulation during the memory phase of the antiviral immune response.
FIG 3.
BrdU incorporation and cytokine-induced IFN-γ production by Ly49H+ NK cells. Mice were infected with MCMV and given BrdU daily for 3 to 6 days postinfection. Uninfected mice received BrdU daily for 4 days prior to necropsy. (A) Representative flow cytometry dot plots showing BrdU incorporation by DX5+ CD3− NK cells from naive and MCMV-infected mice. Numbers represent the percentage of cells in each quadrant, and numbers in parentheses represent the percentages of Ly49H− or Ly49H+ cells that are BrdU+. (B) Comparison of BrdU incorporation by Ly49H− and Ly49H+ NK cells before and during MCMV infection. Significant increase in BrdU+ Ly49H+ cells at 7 days postinfection (P < 0.05) is indicated with an asterisk. (C) IFN-γ production by BrdU+ and BrdU− Ly49H+ NK cells following direct ex vivo exposure to innate cytokines. Splenocytes from naive or MCMV-infected mice were stimulated with the indicated cytokine combinations, and IFN-γ expression by NK cells was assessed by flow cytometry. Values are averages ± standard deviations (SD) (error bars) for the groups of mice (four mice per group).
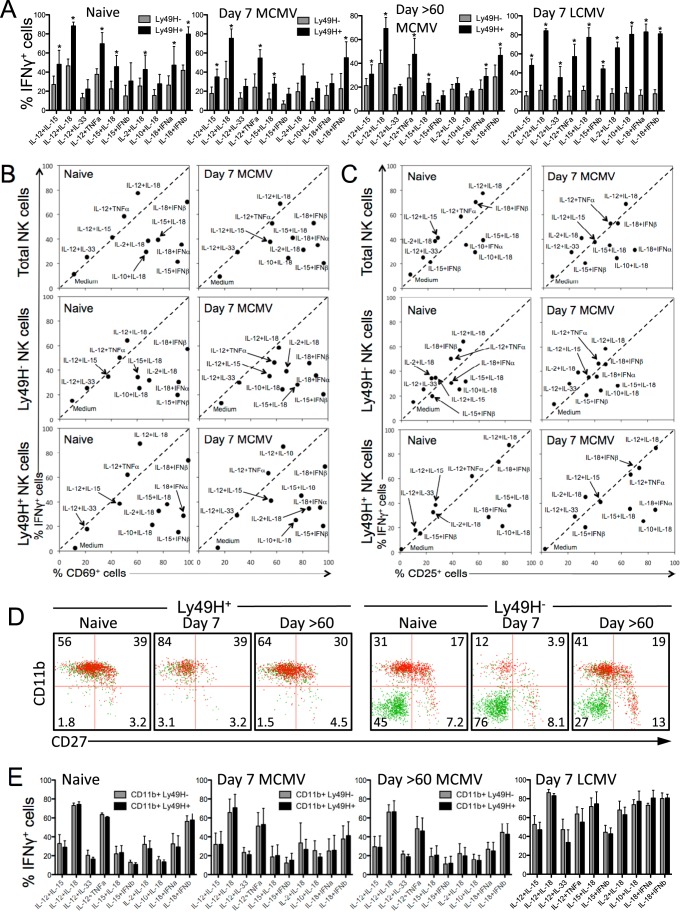
IFN-γ responses by Ly49H+ and Ly49H− NK cells during MCMV and LCMV infection.
We compared cytokine-induced IFN-γ production by “virus-specific” Ly49H+ NK cells and Ly49H− NK cells that lack the receptor for this key MCMV viral ligand. NK cells from naive mice, MCMV-infected mice 7 or >60 days postinfection, or LCMV-infected mice day 7 postinfection were stimulated directly ex vivo with the indicated cytokine pairs, and IFN-γ production was measured by flow cytometry (Fig. 4A). Ly49H+ cells consistently displayed a higher percentage of IFN-γ+ cells than Ly49H− NK cells in response to the 10 cytokine pairs that were tested in naive or virus-infected mice.
FIG 4.
Cytokine-mediated IFN-γ production and cell surface phenotype of Ly49H+ and Ly49H− NK cells during viral infection. (A) IFN-γ production by Ly49H+ and Ly49H− NK cells following exposure to innate cytokines. Splenocytes from naive, MCMV-infected, or LCMV-infected mice were stimulated directly ex vivo with the indicated cytokines (10 ng/ml) for 6 h. IFN-γ responses of Ly49H− and Ly49H+ NK cells to each cytokine pair were compared using a two-tailed t test. Cytokine pairs that induced IFN-γ responses that were significantly different (P < 0.05) in Ly49H− and Ly49H+ NK cells are indicated with an asterisk. (B) Cytokine-mediated induction of CD69 surface expression on NK cells. Each data point represents the percentage of total, Ly49H−, or Ly49H+ CD3− DX5+ cells expressing CD69 or IFN-γ following exposure to the indicated cytokine pair. (C) CD25 and IFN-γ expression by total CD3− DX5+ NK cells, Ly49H− CD3− DX5+, or Ly49H+ CD3− DX5+ NK cells following cytokine exposure. (D) Surface phenotype and IFN-γ production by Ly49H+ and Ly49H− NK cells from naive and MCMV-infected mice. Dot plots are pregated on live CD3− DX5+ cells, and IFN-γ+ and IFN-γ− cells are represented by red and green dots, respectively. (E) CD11b+ Ly49H+ and CD11b+ Ly49H− NK cell populations display matched responses to cytokine stimulation. Dot plots were gated on CD11b+ NK cells prior to analysis of IFN-γ production by Ly49H+ and Ly49H− subsets. Values are averages ± SD for the groups (four mice per group).
Direct ex vivo cytokine stimulation led to substantial upregulation of both CD69 (Fig. 4B) and CD25 (Fig. 4C) by total NK cells as well as both Ly49H+ and Ly49H− NK cells. Although there were generally fewer IFN-γ+ NK cells among the Ly49H− population compared to their Ly49H+ counterparts, each NK cell population responded similarly whether they were obtained from naive mice or MCMV-infected mice. In contrast to our observations in BALB/c mice in which CD69+ or CD25+ NK cells outnumbered their IFN-γ+ counterparts (Fig. 2), 4 cytokine combinations involving IL-12 induced IFN-γ and CD69 expression at equal frequencies (Fig. 4B). Cytokine combinations that involved IL-18 or IFN-α/β, on the other hand, triggered higher frequencies of CD69+ NK cells in comparison to IFN-γ+ NK cells. Cytokine-mediated CD25 upregulation was more variable, but there was a slight trend toward preferential upregulation of CD25 expression over IFN-γ production among NK cells stimulated with cytokine combinations involving IL-18 (Fig. 4C). Seven days after LCMV infection, NK cells from C57BL/6 mice were similar to those found in LCMV-infected BALB/c mice (Fig. 2) and showed a reduction in IFN-γ responses compared to naive mice (data not shown). However, this preferential downregulation of IFN-γ responses was not observed on day 7 after MCMV infection among any of the NK cell populations studied (Fig. 4B and C). It is unclear whether this difference is related to LCMV being relatively resistant to NK cells, whereas MCMV is considered to be more sensitive to NK cells (7).
NK cells can exist in several maturation states that are associated with variations in their effector function capabilities (13, 14, 43). Therefore, we examined CD27 and CD11b expression on cytokine-responsive Ly49H+ and Ly49H− NK cells to further define the NK cell population involved with IFN-γ production in response to innate cytokine exposure. Ly49H+ NK cells typically displayed a more mature surface phenotype than Ly49H− NK cells, with 85% of Ly49H+ cells expressing CD11b (compared to 48% CD11b+ Ly49H− cells). At 7 days postinfection, an even higher percentage of Ly49H+ NK cells were CD11b+ (95%), and the majority (84%) were CD11b+ CD27−, representing the surface phenotype associated with the most potent effector function (13). In contrast, only 16% of Ly49H− NK cells were CD11b+. By 60 days postinfection, the phenotypes of both the Ly49H+ and Ly49H− populations had returned to a profile similar to that observed in naive animals. The lower responsiveness of the total Ly49H− cell population, particularly at 7 days postinfection (Fig. 4A) was largely due to the high percentage of CD11b− CD27− NK cells within the Ly49H− population that were almost completely unresponsive to cytokine stimulation (Fig. 4D). To verify that CD11b expression was the determining factor in predicting NK cell responsiveness to inflammatory cytokines, the NK cell population was first gated on CD11b+ cells and then divided into Ly49H+ and Ly49H− subpopulations (Fig. 4E). When only mature, CD11b+ NK cells were compared, there was no longer any significant difference between Ly49H+ and Ly49H− NK cells in terms of IFN-γ responses to the top 10 most stimulatory cytokine combinations. Variation in the distribution of CD11b+ and CD11b− populations also explains the differences observed between the CD3− DX5+ and CD3− NK1.1+ NK cells in naive C57BL/6 mice shown in Fig. 1C. Following stimulation with each of the top 10 cytokine combinations, the average frequency of IFN-γ+ CD3− DX5+ cells was 79% ± 2% of the frequency of the IFN-γ+ cells observed among the CD3− NK1.1+ population (Fig. 1C). However, this appears to be due to differences in CD11b expression among these NK cell populations. When only CD11b+ NK cells were examined, the IFN-γ responses of the CD11b+ CD3− DX5+ cells were essentially identical (99.8% ± 0.7%) to the CD11b+ CD3− NK1.1+ responses. Collectively, our data indicate that the underlying maturation state of the cell (i.e., CD11b+ and/or CD27+), rather than the expression of a specific receptor (i.e., Ly49H), is more closely associated with enhanced IFN-γ responses to cytokine-mediated NK cell activation.
DISCUSSION
Cytokines play an essential role in regulating NK cell activation and are likely to be involved with effective innate antiviral immune responses. Since different pathogens induce unique cytokine profiles during the course of infection, innate immune cells may be exposed to a vast array of cytokine combinations that work in a combinatorial fashion to regulate cellular functions such as proliferation, cytotoxicity, and IFN-γ production. We have previously performed a comprehensive screen of 1,849 cytokine pairs that regulate innate activation of virus-specific CD8+ T cells in an antigen-independent manner (30). Here, we provide an analysis of cytokine-mediated NK cell activation. In addition to identifying the most stimulatory cytokine combinations capable of inducing IFN-γ production in NK cells, we demonstrated differences in NK cell functionality at different stages of LCMV and MCMV infection. Moreover, although we found that Ly49H+ “memory” NK cells that arose during MCMV infection did not show enhanced responsiveness to cytokine stimulation, we identified CD11b+ NK cells as the most responsive subset to cytokine-mediated activation regardless of Ly49H expression profile.
Several striking similarities emerged between cytokine combinations that regulate CD8+ T cells and NK cells. For example, the combination of IL-12 plus IL-18 remains the most potent activating cytokine pair for both CD8+ T cells and NK cells. For both cell types, IL-12 acted synergistically with a variety of cytokines to induce IFN-γ production, including IL-33, an IL-1 family member that has only recently been recognized for its immune activating capabilities but was originally classified as a Th2-promoting cytokine (44, 45). Moreover, 8 of the top 10 most stimulatory cytokine combinations were the same for NK cells and CD8+ T cells, and all 10 of these combinations utilize either IL-12 or IL-18. In other words, although IL-12 and IL-18 represent only ∼5% of the 43 cytokines examined, they are involved in 100% of the 10 most stimulatory cytokine combinations for NK cell-mediated IFN-γ production (Table 1). It is remarkable that from the >1,800 cytokine combinations tested, only IL-12 and IL-18 form the foundation for cytokine-mediated NK cell activation. Interestingly, the responsiveness of NK cells and CD8+ T cells to cytokine stimulation evolve quite differently over the course of LCMV infection. Eight days after LCMV infection, NK cell-mediated IFN-γ responses to inflammatory cytokines were reduced, and this is in contrast to LCMV-specific CD8+ T cells that were highly responsive to cytokine stimulation at this time point (30). Similar to observations of virus-specific CD8+ T cells (30, 46, 47), several cytokine combinations induced CD69 and/or CD25 upregulation in a majority of NK cells (e.g., IL-18 plus IFN-β) but only triggered IFN-γ production in a more limited subset of the NK cells. These differences were more pronounced in NK cells from BALB/c mice (Fig. 2B and C) than those from C57BL/6 mice (Fig. 4B and C). This indicates that although NK cells are becoming activated by cytokine exposure, their production of the antiviral and immunoregulatory cytokine IFN-γ is restrained in certain circumstances. Such differential regulation of secreted proteins versus cell surface proteins could potentially be an important mechanism for reducing immunopathology during infection while still maintaining or enhancing an effective immune response.
Although NK cells have traditionally been classified as part of the innate immune system, recent studies suggest that they may also exhibit some characteristics associated with adaptive immunity. For example, a population of memory-like NK cells that arise during MCMV infection has been described (38, 41, 42, 48). Much like CD8+ T cells, Ly49H+ NK cells can undergo ligand-specific proliferation, followed by a contraction phase with some cells surviving to become long-lived cells that persist beyond 70 days postinfection. These cells are protective against subsequent MCMV challenge in an adoptive transfer model and show enhanced IFN-γ production in response to anti-Ly49H or anti-NK1.1 stimulation (48), but their innate responses to cytokine-mediated activation have not been fully characterized. We found that Ly49H+ NK cells preferentially proliferated during the early course of MCMV infection (Fig. 3), and a long-lived population of BrdU+ Ly49H+ NK cells was still detected beyond 60 days postinfection. However, the BrdU+ Ly49H+ NK cells did not show a functional advantage over BrdU− Ly49H+ cells with respect to their production of IFN-γ upon exposure to cytokine stimulation (Fig. 3C). This is in contrast to the enhanced responsiveness of Ly49H+ memory NK cells to stimulation through the Ly49H receptor following MCMV infection (48). This suggests that memory NK cells may exert some degree of specificity in their recall responses by mounting distinct responses to cell surface ligands versus secreted cytokines. A similar phenomenon has been observed in CD8 T cells. Effector and memory CD8+ T cells regulate their responses to peptide antigen on the surface of an infected cell differently than when activated by soluble innate cytokines, both in terms of the cytokines they produce (e.g., TNF-α and IL-2 are produced upon exposure to peptide antigen, but not IL-12 plus IL-18) and their sensitivity to regulatory control by inhibitory cytokines (47, 49). Given the array of different cell surface receptors that govern NK cell activation (e.g., cytokine receptors, Ly49H, and other activating and inhibitory receptors), it is possible that similar mechanisms exist for NK cells to fine-tune their responses to their local microenvironment.
Notably, expression of Ly49H alone was not predictive of cytokine responsiveness. It has previously been demonstrated that CD11b and CD27 expression can be used to segregate NK cells into functionally distinct subsets, with cells expressing high levels of CD11b being the most mature in terms of IFN-γ production and cytolytic activity (13, 43). Although Ly49H+ NK cells appeared to be more responsive to cytokine stimulation than Ly49H− NK cells, when these cell populations were first normalized to contain only CD11b+ cells, then there were no longer any differences in innate responses to cytokine exposure (Fig. 4E). Therefore, the differences in IFN-γ responses by Ly49H+ and Ly49H− cells appear to be related to differences in the distribution of functionally mature CD11b+ and/or CD27+ NK cells within these two populations (Fig. 4D). While expression of Ly49H alone may not predict cytokine responsiveness, it is possible that stimulation through the Ly49H receptor could be involved in the maturation process or in the rescue of activated NK cells during viral infection, resulting in a higher proportion of CD11b+ cells within the Ly49H+ population. In this way, Ly49H may be involved with influencing cytokine responsiveness in an indirect manner, but more studies are needed to determine whether this is indeed the case. Collectively, our data help to define cytokine interactions, phenotypes, and cellular characteristics that govern NK cell activation during the course of viral infection. This information may be useful for future development of NK cell-based therapeutics and/or manipulation of the NK cell “rheostat” that serves to balance antiviral immune responses (7, 8).
ACKNOWLEDGMENTS
This work was supported by National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), grants U01 AI082196 and R01 AI054458 (to M.K.S.) and Oregon National Primate Research Center grant 8P51 OD011092-53 (to M.K.S.).
REFERENCES
- 1.Lodoen MB, Lanier LL. 2006. Natural killer cells as an initial defense against pathogens. Curr Opin Immunol 18:391–398. doi: 10.1016/j.coi.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.French AR, Yokoyama WM. 2003. Natural killer cells and viral infections. Curr Opin Immunol 15:45–51. doi: 10.1016/S095279150200002X. [DOI] [PubMed] [Google Scholar]
- 3.Wu J, Lanier LL. 2003. Natural killer cells and cancer. Adv Cancer Res 90:127–156. doi: 10.1016/S0065-230X(03)90004-2. [DOI] [PubMed] [Google Scholar]
- 4.Biron CA. 2012. Yet another role for natural killer cells: cytotoxicity in immune regulation and viral persistence. Proc Natl Acad Sci U S A 109:1814–1815. doi: 10.1073/pnas.1120528109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loh J, Chu DT, O'Guin AK, Yokoyama WM, Virgin HW IV. 2005. Natural killer cells utilize both perforin and gamma interferon to regulate murine cytomegalovirus infection in the spleen and liver. J Virol 79:661–667. doi: 10.1128/JVI.79.1.661-667.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vidal SM, Khakoo SI, Biron CA. 2011. Natural killer cell responses during viral infections: flexibility and conditioning of innate immunity by experience. Curr Opin Virol 1:497–512. doi: 10.1016/j.coviro.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Welsh RM, Waggoner SN. 2013. NK cells controlling virus-specific T cells: rheostats for acute vs. persistent infections. Virology 435:37–45. doi: 10.1016/j.virol.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waggoner SN, Cornberg M, Selin LK, Welsh RM. 2012. Natural killer cells act as rheostats modulating antiviral T cells. Nature 481:394–398. doi: 10.1038/nature10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitrovic M, Arapovic J, Jordan S, Fodil-Cornu N, Ebert S, Vidal SM, Krmpotic A, Reddehase MJ, Jonjic S. 2012. The NK cell response to mouse cytomegalovirus infection affects the level and kinetics of the early CD8+ T-cell response. J Virol 86:2165–2175. doi: 10.1128/JVI.06042-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robbins SH, Bessou G, Cornillon A, Zucchini N, Rupp B, Ruzsics Z, Sacher T, Tomasello E, Vivier E, Koszinowski UH, Dalod M. 2007. Natural killer cells promote early CD8 T cell responses against cytomegalovirus. PLoS Pathog 3:e123. doi: 10.1371/journal.ppat.0030123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrews DM, Estcourt MJ, Andoniou CE, Wikstrom ME, Khong A, Voigt V, Fleming P, Tabarias H, Hill GR, van der Most RG, Scalzo AA, Smyth MJ, Degli-Esposti MA. 2010. Innate immunity defines the capacity of antiviral T cells to limit persistent infection. J Exp Med 207:1333–1343. doi: 10.1084/jem.20091193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andoniou CE, Andrews DM, Degli-Esposti MA. 2006. Natural killer cells in viral infection: more than just killers. Immunol Rev 214:239–250. doi: 10.1111/j.1600-065X.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- 13.Chiossone L, Chaix J, Fuseri N, Roth C, Vivier E, Walzer T. 2009. Maturation of mouse NK cells is a 4-stage developmental program. Blood 113:5488–5496. doi: 10.1182/blood-2008-10-187179. [DOI] [PubMed] [Google Scholar]
- 14.Fu B, Wang F, Sun R, Ling B, Tian Z, Wei H. 2011. CD11b and CD27 reflect distinct population and functional specialization in human natural killer cells. Immunology 133:350–359. doi: 10.1111/j.1365-2567.2011.03446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pegram HJ, Andrews DM, Smyth MJ, Darcy PK, Kershaw MH. 2011. Activating and inhibitory receptors of natural killer cells. Immunol Cell Biol 89:216–224. doi: 10.1038/icb.2010.78. [DOI] [PubMed] [Google Scholar]
- 16.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. 1999. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol 17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 17.Lanier LL. 2008. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol 9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakahira M, Ahn HJ, Park WR, Gao P, Tomura M, Park CS, Hamaoka T, Ohta T, Kurimoto M, Fujiwara H. 2002. Synergy of IL-12 and IL-18 for IFN-gamma gene expression: IL-12-induced STAT4 contributes to IFN-gamma promoter activation by up-regulating the binding activity of IL-18-induced activator protein 1. J Immunol 168:1146–1153. doi: 10.4049/jimmunol.168.3.1146. [DOI] [PubMed] [Google Scholar]
- 19.Gherardi MM, Ramirez JC, Esteban M. 2003. IL-12 and IL-18 act in synergy to clear vaccinia virus infection: involvement of innate and adaptive components of the immune system. J Gen Virol 84:1961–1972. doi: 10.1099/vir.0.19120-0. [DOI] [PubMed] [Google Scholar]
- 20.Orange JS, Biron CA. 1996. An absolute and restricted requirement for IL-12 in natural killer cell IFN-gamma production and antiviral defense. Studies of natural killer and T cell responses in contrasting viral infections. J Immunol 156:1138–1142. [PubMed] [Google Scholar]
- 21.Pien GC, Biron CA. 2000. Compartmental differences in NK cell responsiveness to IL-12 during lymphocytic choriomeningitis virus infection. J Immunol 164:994–1001. doi: 10.4049/jimmunol.164.2.994. [DOI] [PubMed] [Google Scholar]
- 22.Welsh RM, Dundon PL, Eynon EE, Brubaker JO, Koo GC, O'Donnell CL. 1990. Demonstration of the antiviral role of natural killer cells in vivo with a natural killer cell-specific monoclonal antibody (NK 1.1). Nat Immun Cell Growth Regul 9:112–120. [PubMed] [Google Scholar]
- 23.Smith HR, Heusel JW, Mehta IK, Kim S, Dorner BG, Naidenko OV, Iizuka K, Furukawa H, Beckman DL, Pingel JT, Scalzo AA, Fremont DH, Yokoyama WM. 2002. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc Natl Acad Sci U S A 99:8826–8831. doi: 10.1073/pnas.092258599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. 2002. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science 296:1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- 25.Dokun AO, Kim S, Smith HR, Kang HS, Chu DT, Yokoyama WM. 2001. Specific and nonspecific NK cell activation during virus infection. Nat Immunol 2:951–956. doi: 10.1038/ni714. [DOI] [PubMed] [Google Scholar]
- 26.Cheng TP, French AR, Plougastel BF, Pingel JT, Orihuela MM, Buller ML, Yokoyama WM. 2008. Ly49h is necessary for genetic resistance to murine cytomegalovirus. Immunogenetics 60:565–573. doi: 10.1007/s00251-008-0313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vidal SM, Lanier LL. 2006. NK cell recognition of mouse cytomegalovirus-infected cells. Curr Top Microbiol Immunol 298:183–206. doi: 10.1007/3-540-27743-9_10. [DOI] [PubMed] [Google Scholar]
- 28.Brown MG, Dokun AO, Heusel JW, Smith HR, Beckman DL, Blattenberger EA, Dubbelde CE, Stone LR, Scalzo AA, Yokoyama WM. 2001. Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science 292:934–937. doi: 10.1126/science.1060042. [DOI] [PubMed] [Google Scholar]
- 29.Pyzik M, Gendron-Pontbriand EM, Vidal SM. 2011. The impact of Ly49-NK cell-dependent recognition of MCMV infection on innate and adaptive immune responses. J Biomed Biotechnol 2011:641702. doi: 10.1155/2011/641702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freeman BE, Hammarlund E, Raue HP, Slifka MK. 2012. Regulation of innate CD8+ T-cell activation mediated by cytokines. Proc Natl Acad Sci U S A 109:9971–9976. doi: 10.1073/pnas.1203543109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. 2006. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature 440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 32.Feng C, Woodside KJ, Vance BA, El-Khoury D, Canelles M, Lee J, Gress R, Fowlkes BJ, Shores EW, Love PE. 2002. A potential role for CD69 in thymocyte emigration. Int Immunol 14:535–544. doi: 10.1093/intimm/dxf020. [DOI] [PubMed] [Google Scholar]
- 33.Clausen J, Vergeiner B, Enk M, Petzer AL, Gastl G, Gunsilius E. 2003. Functional significance of the activation-associated receptors CD25 and CD69 on human NK-cells and NK-like T-cells. Immunobiology 207:85–93. doi: 10.1078/0171-2985-00219. [DOI] [PubMed] [Google Scholar]
- 34.Lee SH, Fragoso MF, Biron CA. 2012. A novel mechanism bridging innate and adaptive immunity: IL-12 induction of CD25 to form high-affinity IL-2 receptors on NK cells. J Immunol 189:2712–2716. doi: 10.4049/jimmunol.1201528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biron CA, Young HA, Kasaian MT. 1990. Interleukin 2-induced proliferation of murine natural killer cells in vivo. J Exp Med 171:173–188. doi: 10.1084/jem.171.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marcus A, Raulet DH. 2013. Evidence for natural killer cell memory. Curr Biol 23:R817–R820. doi: 10.1016/j.cub.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. 2011. Innate or adaptive immunity? The example of natural killer cells. Science 331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun JC, Beilke JN, Lanier LL. 2010. Immune memory redefined: characterizing the longevity of natural killer cells. Immunol Rev 236:83–94. doi: 10.1111/j.1600-065X.2010.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun JC, Lanier LL. 2009. Natural killer cells remember: an evolutionary bridge between innate and adaptive immunity? Eur J Immunol 39:2059–2064. doi: 10.1002/eji.200939435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. 2009. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A 106:1915–1919. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun JC, Lopez-Verges S, Kim CC, DeRisi JL, Lanier LL. 2011. NK cells and immune “memory”. J Immunol 186:1891–1897. doi: 10.4049/jimmunol.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun JC, Lanier LL. 2011. NK cell development, homeostasis and function: parallels with CD8+ T cells. Nat Rev Immunol 11:645–657. doi: 10.1038/nri3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim S, Iizuka K, Kang HS, Dokun A, French AR, Greco S, Yokoyama WM. 2002. In vivo developmental stages in murine natural killer cell maturation. Nat Immunol 3:523–528. doi: 10.1038/ni796. [DOI] [PubMed] [Google Scholar]
- 44.Liew FY, Pitman NI, McInnes IB. 2010. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol 10:103–110. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]
- 45.Smithgall MD, Comeau MR, Yoon BR, Kaufman D, Armitage R, Smith DE. 2008. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol 20:1019–1030. doi: 10.1093/intimm/dxn060. [DOI] [PubMed] [Google Scholar]
- 46.Suarez-Ramirez JE, Tarrio ML, Kim K, Demers DA, Biron CA. 2014. CD8 T cells in innate immune responses: using STAT4-dependent but antigen-independent pathways to gamma interferon during viral infection. mBio 5>(5):e01978-14. doi: 10.1128/mBio.01978-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Freeman BE, Meyer C, Slifka MK. 2014. Anti-inflammatory cytokines directly inhibit innate but not adaptive CD8+ T cell functions. J Virol 88:7474–7484. doi: 10.1128/JVI.00658-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun JC, Beilke JN, Lanier LL. 2009. Adaptive immune features of natural killer cells. Nature 457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beadling C, Slifka MK. 2005. Differential regulation of virus-specific T-cell effector functions following activation by peptide or innate cytokines. Blood 105:1179–1186. doi: 10.1182/blood-2004-07-2833. [DOI] [PubMed] [Google Scholar]