ABSTRACT
The antibody response to proteins may be modulated by the presence of preexisting antigen-specific antibodies and the formation of immune complexes (ICs). Effects such as a general increase or decrease of the response as well as epitope-specific phenomena have been described. In this study, we investigated influences of IC immunization on the fine specificity of antibody responses in a structurally well-defined system, using the envelope (E) protein of tick-borne encephalitis (TBE) virus as an immunogen. TBE virus occurs in Europe and Asia and—together with the yellow fever, dengue, West Nile, and Japanese encephalitis viruses—represents one of the major human-pathogenic flaviviruses. Mice were immunized with a dimeric soluble form of E (sE) alone or in complex with monoclonal antibodies specific for each of the three domains of E, and the antibody response induced by these ICs was compared to that seen after immunization with sE alone. Immunoassays using recombinant domains and domain combinations of TBE virus sE as well as the distantly related West Nile virus sE allowed the dissection and quantification of antibody subsets present in postimmunization sera, thus generating fine-specificity patterns of the polyclonal responses. There were substantially different responses with two of the ICs, and the differences could be mechanistically related to (i) epitope shielding and (ii) antibody-mediated structural changes leading to dissociation of the sE dimer. The phenomena described may also be relevant for polyclonal responses upon secondary infections and/or booster immunizations and may affect antibody responses in an individual-specific way.
IMPORTANCE Infections with flaviviruses such as yellow fever, dengue, Japanese encephalitis, West Nile, and tick-borne encephalitis (TBE) viruses pose substantial public health problems in different parts of the world. Antibodies to viral envelope protein E induced by natural infection or vaccination were shown to confer protection from disease. Such antibodies can target different epitopes in E protein, and the fine specificities of polyclonal responses can differ between individuals. We conducted a mouse immunization study with TBE E protein alone or complexed to monoclonal antibodies specific for each of the three protein domains. We demonstrated that phenomena such as epitope shielding and antibody-induced structural changes can profoundly influence the fine specificity of antibody responses to the same immunogen. The study thus provided important new information on the potential immunomodulatory role of preexisting antibodies in a flavivirus system that can be relevant for understanding individual-specific factors influencing antibody responses in sequential flavivirus infections and/or immunizations.
INTRODUCTION
Several mosquito- and tick-transmitted flaviviruses are important human pathogens and have a substantial public health impact in countries of endemicity (1). These include the yellow fever (YF), dengue (DEN), West Nile (WN), Japanese encephalitis (JE), and tick-borne encephalitis (TBE) viruses, all of which also carry the potential to emerge in new, previously unaffected areas (2–6). Flaviviruses have an envelope (E) protein that is oriented parallel to the viral membrane and which forms a herringbone-like icosahedral shell at the surface of mature virions. As revealed by X-ray crystallography of soluble forms of E (sE) and cryo-electron microscopy (cryo-EM) of whole virions, the basic building block of the icosahedral viral envelope protein lattice is an antiparallel E dimer, with each monomer consisting of three distinct structural domains (DI, DII, and DIII; Fig. 1). Because of its essential functions in virus entry (7–9), E is the main target of the virus-neutralizing antibodies (Abs) that are responsible for conferring long-lasting immunity after infection or vaccination (10). As revealed by many studies performed with monoclonal antibodies (MAbs) and polyclonal antibodies, each of the three E domains can induce neutralizing antibodies (reviewed in reference 1), but the dominance of antibodies to different domains in anti-E responses appears to be strongly affected by species-specific as well as virus-specific factors. Antibodies to DIII contribute strongly to the neutralizing response in mice but not in humans, and these observations were made for both mosquito-borne and tick-borne flaviviruses (reviewed in references 11 and 12). In addition to such species-dependent phenomena, differences in immunodominance between different flaviviruses were also observed. In human dengue virus infections, for instance, cross-reactive antibodies directed to the conserved fusion peptide (FP) (Fig. 1) make up a substantial portion of the total antibody response (13, 14), whereas this site is not comparably dominant in the response to TBE virus infection or to TBE and YF virus vaccination (15, 16). Differences in the stability of E complexes at the virion surface, the extent of proteolytic maturation cleavage of the second envelope glycoprotein (prM) present in immature virions (reviewed in reference 17), and the dynamics of epitope exposure by viral “breathing” phenomena (18–23) may be responsible for such effects.
FIG 1.
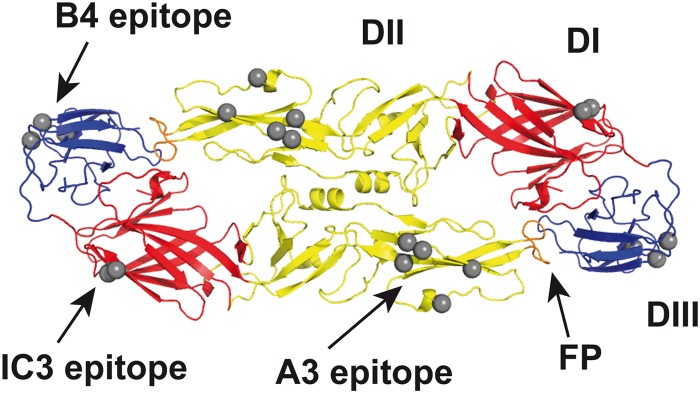
Ribbon diagram of the soluble E protein dimer of TBE virus (Protein Data Bank [PDB] code 1SVB; top view) with the location of epitopes of MAbs used in this study. Structural domains of sE are colored in red (DI), yellow (DII), and blue (DIII) and the fusion peptide (FP) in orange. Gray spheres indicate Cα atoms of residues involved in the binding of MAbs IC3, A3, and B4, as determined by the use of virus escape mutants (76, 77) and engineered recombinant E mutants (52).
Because E is the target of potently neutralizing antibodies, soluble or particulate immunogens of this protein have been evaluated as experimental flavivirus vaccines (reviewed in references 24 and 25). The use of antibody-antigen immune complexes (ICs) has been proposed as a means for improving the immune responses to immunogens from a variety of resources (26–35), but no such studies have yet been performed with flavivirus antigens. We have therefore conducted a model immunization study in mice and investigated the outcome of antibody responses to the TBE virus E protein in complex with monoclonal antibodies (MAbs) specific for each of the three domains of E. Potentially, the presence of preexisting antigen-specific antibodies and the formation of ICs can have a variety of indirect, non-antigen-specific effects, primarily mediated by the interaction of the Fc parts with Fc receptors that are present on innate and adaptive immune cells (reviewed in references 36 and 37). Such interactions may lead to an enhancement or a decrease of the overall antibody responses, depending on the specific situation of infection or immunization, the nature of the antigen, and the characteristics of the antibodies in the ICs (27, 29, 38–41). The potential beneficial nature of such general antibody-mediated effects may be a means for designing more-effective immunization protocols, e.g., to overcome impaired germinal center responses in the elderly (42), or for developing therapeutic vaccines (43). In addition, however, antibodies in ICs can also exert epitope-specific effects and modulate the specificity of the antibodies induced. Mechanisms such as epitope shielding or antibody-mediated conformational changes have been proposed to explain the phenomena observed (28, 38, 44, 45).
The immunomodulatory effect of ICs on the specificity of antibody responses is difficult to measure because of the large composite of antibody populations present in postimmunization sera. In our mouse immunization study, we therefore combined knowledge of the atomic structure of the TBE virus sE protein and of the binding sites of MAbs to obtain information on epitope-specific effects in the induction of antibodies upon IC immunization in a structurally defined system. For dissecting the specificities of antibody populations induced, we exploited previously established immunoassays using recombinant domains and domain combinations of E protein as well as a heterologous flavivirus E protein that allowed us to quantify distinct antibody subsets in polyclonal sera (15, 46). Using ICs with MAbs specific for each of the three E domains, we found neither an enhancing nor a decreasing effect on the overall response but demonstrated a specific modulation of the fine specificity of antibodies induced by two of the ICs. The mechanisms underlying these effects were different and were identified as epitope shielding in the case of the DIII-specific antibody and as antibody-mediated dissociation of the E dimer in the case of the DII-specific antibody.
MATERIALS AND METHODS
Production and purification of TBE virus.
Production of highly purified infectious virus was carried out essentially as described in reference 47. In brief, primary chicken embryo cells were infected with TBE virus strain Neudörfl (GenBank accession no. U27495). The cell supernatant was harvested 48 h postinfection and concentrated by ultracentrifugation, and the virus was purified by rate-zonal centrifugation followed by equilibrium sucrose density gradient centrifugation.
Expression and purification of recombinant proteins. (i) TBE virus sE, DI, and DIDII and WN virus sE.
Recombinant TBE virus sE (amino acid [aa] 1 to 400), DI (aa 1 to 52 plus 8-Gly linker plus aa 137 to 192 plus 8-Gly linker plus aa 285 to 302), and DIDII (aa 1 to 302) proteins were derived from TBE virus strain Neudoerfl and WN virus sE (aa 1 to 400) protein from strain New York 99 (GenBank accession number AF196835). All antigens were expressed in Schneider 2 (S2) cells with an enterokinase cleavage site and a double Strep (Trp-Ser-His-Pro-Gln-Phe-Glu-Lys) tag, using the pT389 vector (kindly provided by T. Krey and F. Rey, Institut Pasteur, France), as described previously (15, 16, 46). All Strep-tagged proteins were affinity purified using Strep-Tactin columns (IBA), according to the manufacturer's protocol.
SE for immunization was produced without a tag by introducing a stop codon after aa 400 of the sE sequence and purified by immunoaffinity chromatography using TBE virus-specific MAb B4.
(ii) DIII-TR fusion proteins: wt and lateral-ridge mut.
For the expression of DIII (aa 302 to 398) from TBE virus strain Neudoerfl, Escherichia coli strain BL21 and the pET 32a Xa/LIC vector (Novagen) were used. DIIIs (wild type [wt] and mutant [mut]) were produced as fusion proteins with thioredoxin (TR) carrying a C-terminal His tag and purified via Ni2+ affinity chromatography as described previously (48). The DIII-TR lateral-ridge mutant (amino acid substitutions S309A and K333E) was constructed in analogy to the WN virus DIII-lateral-ridge mutant (49) and has already been described in detail in reference 46.
(iii) The isolated DIII (containing a His tag) was produced by proteolytic cleavage performed with factor Xa of the TR fusion partner from DIII-TR as described previously for WN virus DIII (48).
Production and purification of MAbs and Fab fragments.
Mabs IC3 (IgG2b), A3 (IgG1), and B4 (IgG1) (50, 51) have specific TBE virus-neutralizing activities of 3.5 μg/ml, 23.3 μg/ml, and 70.9 μg/ml (the concentrations of each MAb at which 50% neutralization is achieved [NT50]), respectively (52). They were purified from serum-free hybridoma cell culture supernatants using protein A or G Sepharose High Performance columns (GE Healthcare Life Sciences), according to the manufacturer's instructions. Fab fragments were generated from purified MAbs by papain cleavage as described previously (53) and were purified by ion-exchange chromatography followed by gel filtration.
Quality control of immunogens and recombinant proteins.
The purity of recombinant proteins and MAbs was controlled by Agilent 2100 bioanalyzer electrophoresis and/or 15% SDS-PAGE. The oligomeric structures of recombinant sE proteins of TBE and WN virus have been determined by cross-linking and sedimentation analyses in previous studies (15, 48), confirming TBE virus sE to be a dimer and WN virus sE to be a monomer. The correct folding of recombinant proteins has been demonstrated in previous studies (15, 46).
Preparation of ICs for immunization.
ICs were prepared by mixing the TBE virus sE protein and MAb IC3, A3, or B4 in a molar ratio of 1:5 in phosphate-buffered saline (pH 7.4) and incubating for 1 h at 37°C. One IC vaccination dose contained 5 μg sE and 37.5 μg of the respective MAb.
Mouse immunization.
Mouse experiments were performed in strict accordance with the guidelines of the Federation of European Laboratory Animal Science Associations (FELASA) and Austrian federal law. The study was approved by the ethics committee of the Medical University of Vienna and the Austrian Federal Ministry of Science and Research (permit number BMWF-66.009/0237-II/3b/2011).
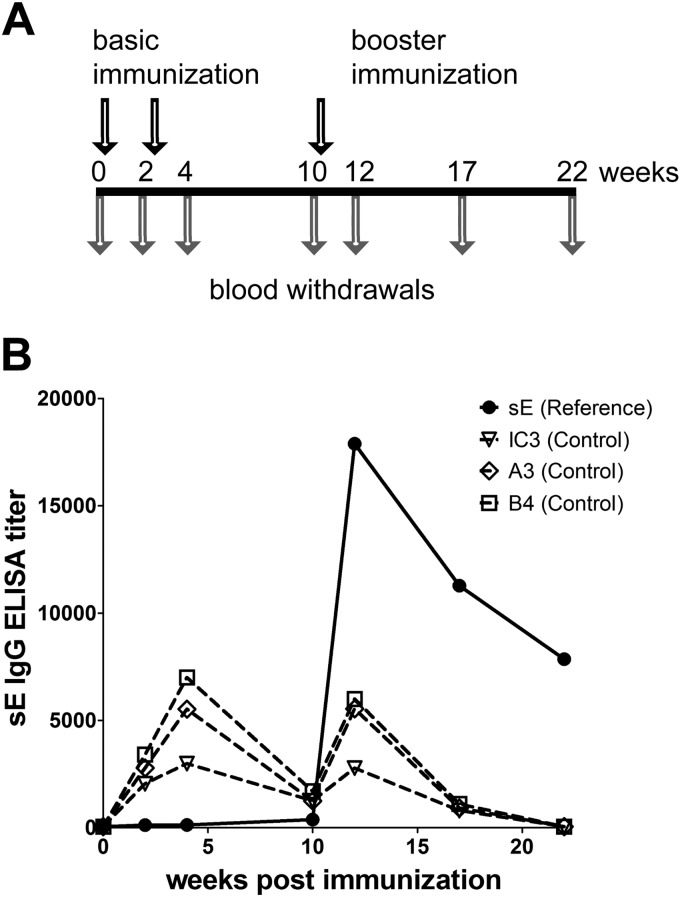
Groups of five C57BL/6 mice (Charles River Laboratories, Sulzfeld, Germany) were immunized intraperitoneally with 100 μl/dose/mouse of the IC twice with an interval of 14 days between immunizations and received a booster immunization 8 weeks after the last vaccination (Fig. 2A). The reference group was immunized with the sE protein alone, whereas control groups received only the respective MAb in the same amount and concentration as used in the IC immunization. Blood samples were taken from the tail vein at different time points.
FIG 2.
Immunization schedule, antibody response induced by sE alone (reference group), and detection of passively administered MAbs (control groups). (A) Immunization schedule. C57BL/6 mice (five mice per group) were immunized three times (arrows above time line) intraperitoneally (i.p.) with sE alone, with sE in complex with MAb IC3, A3, or B4, or with MAbs alone. Blood samples were taken at different time points (arrows below time line). (B) Results of ELISAs (using sE as an antigen) of serum pools from the sE-immunized reference group (solid line) and from control groups with passively administered MAbs (dashed lines) at different time points after immunization.
IgG–enzyme-linked immunosorbent assay (ELISA) for serum analysis. (i) sE, DI, DIDII, and virion ELISA.
Recombinant proteins (sE, DI, and DIDII) (50 ng/well) and 25 ng/well of purified whole TBE virus in carbonate buffer (pH 9.6) were coated overnight at 4°C onto Maxisorp 96-well plates (Nunc) and untreated 96-well microtiter plates (Nunc), respectively. Threefold serial dilutions of the mouse sera, starting at a dilution of 1:100, were added, and the reaction mixture was incubated for 1 h at 37°C. Bound antibodies were then detected using peroxidase-labeled sheep anti-mouse IgG conjugate or peroxidase-labeled rabbit anti-mouse IgG conjugate as described in reference 54. Absorbance values were determined at 490 nm. As negative controls, eight sera from naive mice were included in all tests to determine cutoff values. These were defined as the mean absorbance values for negative controls plus 3 standard deviations, as recommended in reference 55. This cutoff was used for titer calculations by curve fitting using a four-parameter logistic regression with GraphPad Prism 5 software (GraphPad Software Inc., San Diego, CA). Reactivities of sera with absorbance values below the cutoff were assigned a titer value of 50 (50% of the serum starting dilution). At least three independent experiments were performed for each serum to calculate geometric mean titers.
(ii) DIII ELISA (48).
A rabbit anti-His tag antibody (50 ng/well; QED Biosciences) in carbonate buffer (pH 9.6) was coated onto 96-well Maxisorp microtiter plates (Nunc) overnight at 4°C. Then, 50 ng/well of the isolated DIII was added to the plates and the reaction mixture was incubated for 1 h at 37°C. Serial dilutions of mouse sera were applied as described above, and bound antibodies were detected using a peroxidase-labeled goat anti-mouse IgG conjugate (Pierce). Titer calculations were performed as described above.
(iii) DIII-TR (wt and mut) ELISA.
DIII-TR (wt or mut; each 50 ng/well) in carbonate buffer (pH 9.6) was coated onto Maxisorp 96-well plates (Nunc) overnight at 4°C. Serial dilutions of the mouse sera were added as described above, and bound antibodies were detected using peroxidase-labeled sheep anti-mouse IgG conjugate. Titer calculation was performed as described above. For the determination of the percentage of DIII-lateral-ridge-specific antibodies of the total DIII antibody response, the following formula was used: 100 − [(titer DIII-TR mut/titer DIII-TR wt) × 100]. Statistical analyses were performed using unpaired t tests, and differences were considered significant when the P values were less than 0.05.
Chemical cross-linking.
SE, MAbs, Fab fragments, and ICs were prepared in TAN buffer (50 mM triethanolamine [TEA], 100 mM NaCl; pH 8.0) and incubated for 1 h at 37°C. Chemical cross-linking was performed essentially as described previously (56), using 10 mM dimethyl suberimidate (DMS; Pierce). Cross-linking was stopped by the addition of ethanolamine to reach a final concentration of 10 mM. Proteins were precipitated with trichloroacetic acid (TCA), subjected to SDS-PAGE using 5% polyacrylamide gels under nonreducing conditions as described in reference 57, and stained with Coomassie blue R-250.
Statistical analyses of ELISA ratios.
Ratios of titers obtained with each of the different ELISA antigens were calculated relative to those determined with TBE virus sE. Log ELISA titers were analyzed by the use of a general linear model with log sE titers used as the offset values. Differences from the reference group values were tested by linear contrasts with Bonferroni correction and were considered significant at P values below 0.05.
RESULTS
Mouse immunization.
To study the effect of defined antibodies in ICs on the specificity of antibody responses to the antigen, we immunized mice (C57BL/6) with the purified dimeric TBE virus E protein either alone or as a preformed complex with each of three purified E-specific neutralizing MAbs. These recognize epitopes in DI (MAb IC3), DII (MAb A3), and DIII (MAb B4) of E (Fig. 1) in the context of the virus particle and react also with the dimeric form of sE, as well as with the isolated DI (MAb IC3), the isolated DIII (MAb B4), and the isolated monomeric domain combination of DI plus DII (DIDII) (MAb A3) (15).
With each of these immunogens, groups of five mice were immunized twice with an interval of 2 weeks between immunizations followed by a booster immunization 8 weeks after the second immunization (Fig. 2A). In addition—to assess the persistence of passively transferred antibodies in the blood, which could have interfered with the analysis of immunization-induced antibodies—we used control groups of mice administered each of the MAbs without antigen at the same amount and concentration as in the ICs. Blood samples were taken at several time points as indicated in Fig. 2A, and the ELISA results (using sE as an antigen) obtained with the sE-immunized reference group and the MAb control groups are shown in Fig. 2B. The antibody response induced by sE peaked at week 12 (2 weeks after the booster) and then declined. The passively administered antibodies, however, became undetectable only at week 22, and we therefore performed all further analyses with samples of sE- and IC-immunized mice obtained at this later time point.
Immunization with ICs does not change the extent of the overall antibody response.
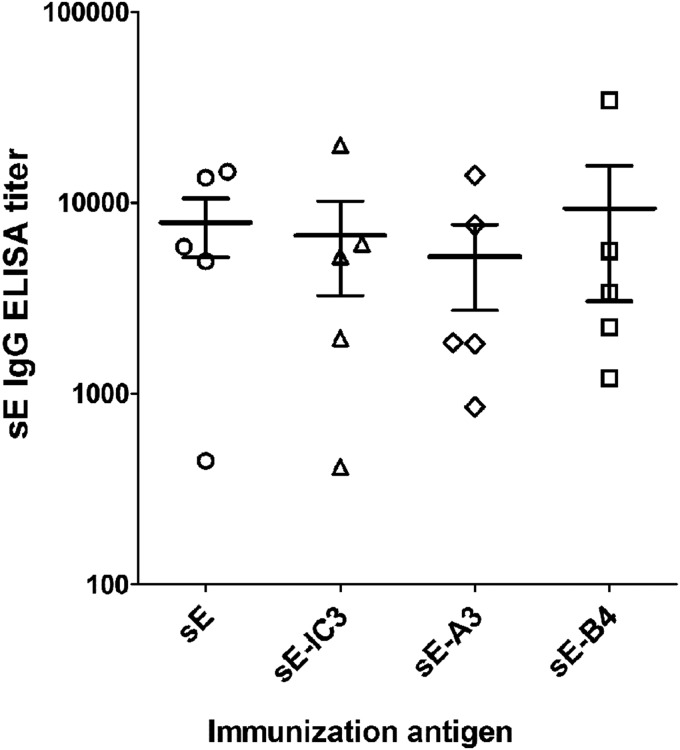
Single serum samples from all individual mice immunized with sE alone, as well as with the ICs sE-IC3, sE-A3, and sE-B4, taken at week 22 postimmunization, were tested for specifically induced antibodies in ELISA using sE as an antigen. The titers obtained for each single serum as well as their means are displayed in Fig. 3. Despite substantial differences in the results determined among the individual mice, no significant differences in the mean serum titers were observed.
FIG 3.
sE-specific antibody response. ELISA titers (using sE as an antigen) of serum samples of each mouse immunized with sE, sE-IC3, sE-A3, and sE-B4 were determined. The horizontal lines show the mean titers, and the error bars represent the standard errors of the means.
Effect on the fine specificities of antibody responses.
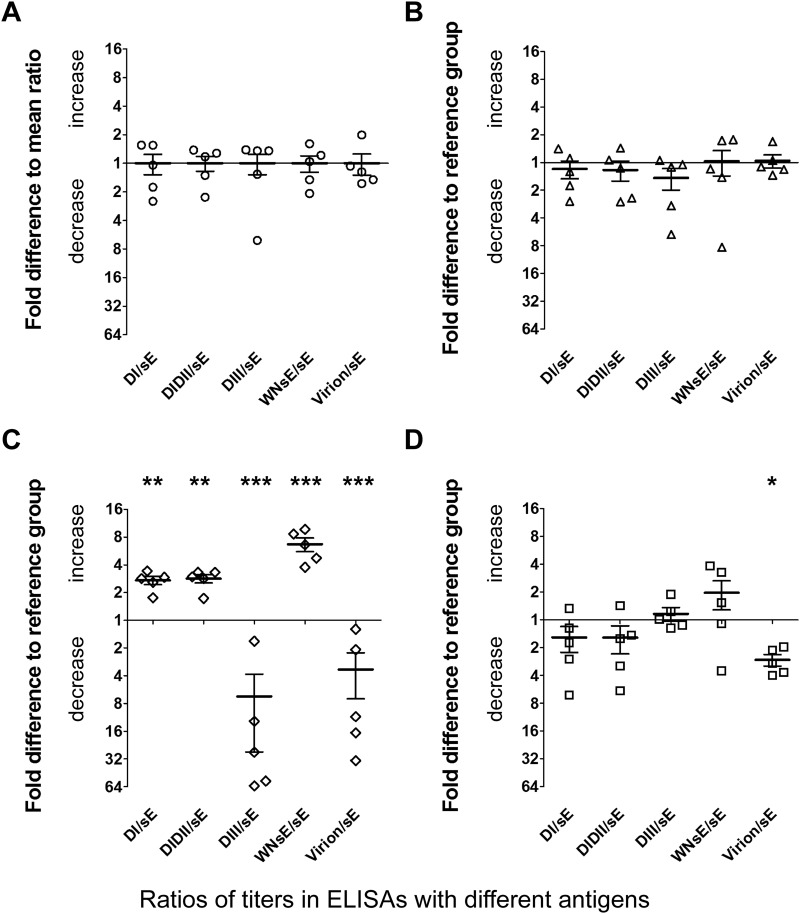
To find out possible differences in the specificities of antibody populations in the polyclonal postimmunization sera, we performed ELISAs with TBE virus sE and substructures thereof (DI, DIDII, and DIII) and with purified whole TBE virus as well as with the heterologous sE of WN virus for determining the extent of cross-reactive antibodies. For all sera, we calculated the ratios of titers obtained in the different ELISAs to the titers obtained in the TBE virus sE ELISA. The mean values of these ratios obtained for the reference group (mice immunized with sE only) were set to a value of 1 (Fig. 4A), and all results are displayed as fold differences relative to the mean values (Fig. 4).
FIG 4.
Fine-specificity patterns of the antibody responses induced by sE and different ICs. Titer ratios of single sera (i.e., titers in ELISAs with different antigens relative to the titers in sE-ELISA), displayed as fold increase or decrease relative to the mean ratios of the reference group, were determined. (A) sE-immunized reference group. (B) IC sE-IC3-immunized group. (C) IC sE-A3-immunized group. (D) IC sE-B4-immunized group. Error bars indicate the standard errors of the means. Black stars indicate statistically significant differences from the reference group values (general linear model with Bonferroni-corrected P values; *, P < 0.05; **, P < 0.01; ***, P < 0.001).
No significant differences in the fine specificities of the antibody responses were found after immunization with the IC sE-IC3 (Fig. 4B), in contrast to the results determined for the other two groups, which displayed substantial deviations from the pattern of the reference group (Fig. 4C and D). In the case of the sE-B4-immunized group, the ratio of virion reactivity to sE reactivity was significantly lower than in the sE-immunized (reference) group. For the other parameters, no significant differences were found (Fig. 4D). After immunization with the IC sE-A3, however, all fine-specificity parameters differed significantly and were either increased or decreased in comparison to the reference group parameters (Fig. 4C).
Evidence for epitope shielding by MAb B4.
We hypothesized that the reduced serum reactivity with virion relative to sE found with sera of IC sE-B4-immunized mice (Fig. 4D) could have been the result of epitope shielding by MAb B4. In the context of the virion, this epitope is part of the most exposed region of DIII (the DIII lateral ridge) (49, 58, 59), whereas other parts of DIII are at least partially buried at the virion surface. In contrast, in the soluble form of E and the ICs thereof used for immunization, such surfaces are accessible and can measurably induce antibodies in ELISAs using sE or DIII as the antigen. A selective reduction of B4 epitope-specific antibodies due to epitope shielding could therefore potentially be revealed by a lower virion/sE ELISA titer ratio, without affecting the DIII/sE ELISA titer ratio, as shown (Fig. 4D).
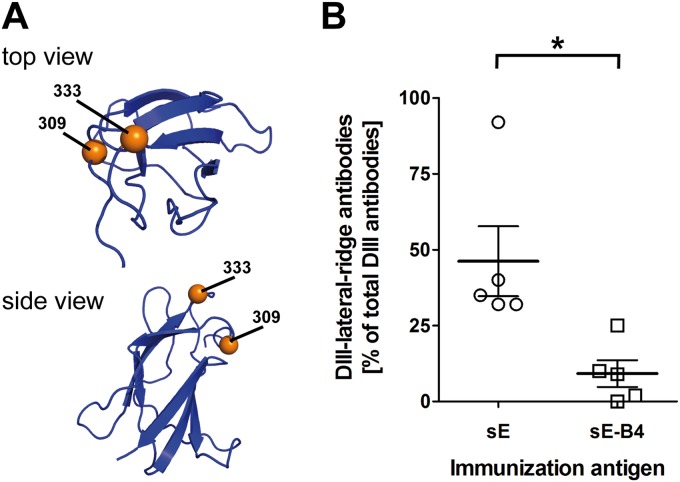
To test our hypothesis, we analyzed the sera of the IC sE-B4-immunized group as well as the sera of the sE-immunized reference group in an ELISA using wild-type DIII and a mutant DIII (Fig. 5A) as antigens. The latter contains two amino acid substitutions at the lateral ridge of DIII that completely abolish binding of MAb B4 (46). The titers obtained with the mutant and the wt DIII were used to calculate the percentage of DIII-lateral-ridge antibodies, as described in Materials and Methods, and the results are shown in Fig. 5B. Consistent with the presumed epitope shielding by MAb B4, the proportion of DIII-lateral-ridge antibodies was significantly lower in the sE-B4-immunized mice than in the reference group.
FIG 5.
(A) Ribbon diagram of DIII (PDB code 1SVB) in top and side views. The DIII mutant contains amino acid substitutions at positions 309 and 333, indicated as orange spheres. (B) Antibody response to the DIII-lateral-ridge epitope. Percentages of DIII-lateral-ridge ELISA antibodies in sera of mice immunized with sE or the IC sE-B4. The black star indicates a statistically significant difference between the values for the two groups (unpaired t test; *, P < 0.05).
Evidence for antibody-mediated conformational changes by MAb A3.
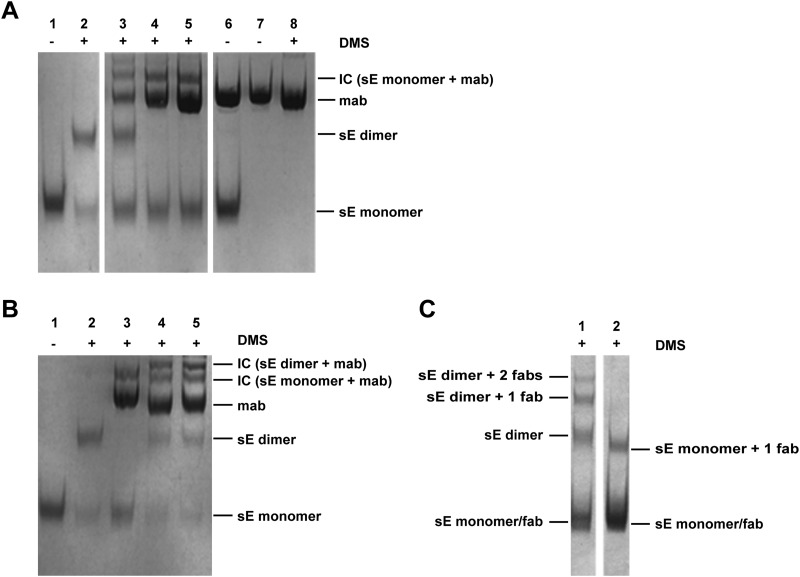
The differences in the ELISA reactivity patterns obtained with sera from IC sE-A3-immunized mice and sera from the reference group patterns were quite dramatic, and all parameters tested differed significantly (Fig. 4C). Antibody responses to all three domains of E were affected (increase of the ELISA titer ratios for DI/sE and DIDII/sE and decrease of that for DIII/sE), substantially more cross-reactive antibodies were induced (WN sE/TBE sE ELISA titer ratio), and a lower virion/sE ELISA titer ratio of the response was observed. These data argued against a simple shielding effect as described for MAb B4 but suggested a possible antibody-mediated structural change in the immunogen. We therefore analyzed the oligomeric structure of sE in the absence and presence of different concentrations of MAb A3, using chemical cross-linking and SDS-PAGE. The results are shown in Fig. 6A. Corresponding to its dimeric structure and previous work (60, 61), cross-linking of sE yielded bands of E monomers (∼50 kDa) and dimers (∼100 kDa) (Fig. 6A, lane 2). In the presence of MAb A3, however, a concentration-dependent disappearance of the E dimer band was observed, consistent with the dissociation of the E dimer. The appearance of a band of ∼200 kDa indicated cross-linking of sE monomers with the MAb (Fig. 6A, lanes 3 to 5). Control experiments with MAbs B4 and IC3 (Fig. 6B) did not provide evidence for such an antibody-induced dissociation of the sE dimer. The patterns obtained with these MAbs (Fig. 6B, lanes 4 and 5) differed substantially from that obtained with MAb A3 (Fig. 6B, lane 3). Specifically, sE dimer bands were present as well as an additional band at ∼250 kDa, corresponding in molecular mass to a cross-linked complex of the sE dimer and the MAb.
FIG 6.
Chemical cross-linking (DMS) and SDS-PAGE of sE and ICs. Protein bands corresponding to MAbs and monomeric and dimeric forms of sE as well as complexes formed between the monomeric or the dimeric form of sE and MAbs/Fab fragments are labeled. (A) Cross-linking of sE in the presence of different concentrations of MAb A3. Lane 1, sE—not cross-linked; lane 2, sE—cross-linked; lanes 3 to 5, sE cross-linked in the presence of increasing concentrations of MAb A3 (molar ratios of MAb to sE, 1:1, 3:1, and 5:1); lanes 6 to 8, controls with MAb A3 (lane 6, sE plus A3—not cross-linked; lane 7, A3—not cross-linked; lane 8, A3—cross-linked). (B) Cross-linking of sE in the presence of MAbs A3, IC3, and B4. Lane 1, sE—not cross-linked; lane 2, sE—cross-linked; lane 3, sE cross-linked in the presence of MAb A3; lane 4, sE cross-linked in the presence of MAb IC3; lane 5, sE cross-linked in the presence of MAb B4. The molar ratio of MAb to sE was 3:1 in all cases. (C) Cross-linking of sE in the presence of Fab fragments B4 and A3. Lane 1, sE cross-linked in the presence of Fab fragment B4; lane 2, sE cross-linked in the presence of Fab fragment A3. The molar ratio of Fab fragment to sE was 5:1 in both cases.
To test whether the dissociation effect mediated by MAb A3 required bivalent binding to sE, we conducted a similar experiment with the A3 Fab fragment and—as a control—the Fab fragment of the nondissociating MAb B4 (Fig. 6C). The bands observed with Fab fragment B4 (Fig. 6C, lane 1) corresponded in molecular masses to (i) sE monomers and the Fab fragment (both ∼50 kDa), (ii) sE dimers (∼100 kDa), (iii) a complex of sE dimers with one B4 Fab fragment (∼150 kDa), and (iv) a complex of sE dimers with two B4 Fab fragment molecules (∼200 kDa). A different cross-linking pattern was obtained with the Fab fragment of the dissociating MAb A3 (Fig. 6C, lane 2). Except for a band corresponding in molecular mass to an sE monomer-Fab fragment complex (∼100 kDa), none of the higher-molecular-mass complexes were found after exposure of sE to Fab fragment A3. This pattern is consistent with an A3 Fab fragment-mediated dissociation of sE dimers, similar to that shown with the whole antibody.
DISCUSSION
In this immunization study, we demonstrated epitope-specific effects in the modulation of the fine specificity of polyclonal antibody responses to the TBE virus E protein, when applied as ICs with two specific MAbs which have the same IgG subclass (IgG1). The most dramatic alteration of this response was observed after immunization with an IC containing a MAb directed against DII (MAb A3; Fig. 4C). The change in a physical property of the immunogen, i.e., the dissociation of the E dimer by this MAb (Fig. 6), provides a logical explanation for the altered specificity patterns observed. Since the same dissociating effect was also shown with the corresponding Fab fragment, possible influences of bivalent binding or Fc-mediated interactions can be excluded in this case. The MAb A3 epitope is located on the finger-like structure of DII, involving residues of the b β-sheet and the bc-loop next to the fusion peptide (FP) (60). The specificity pattern observed is fully compatible with the antibody-mediated exposure of the FP which is highly conserved and largely buried in the context of the E dimer by interactions with a groove provided by DI and DIII (Fig. 1). By the dissociation of the dimer, increased interactions of the FP with B cell receptors become possible, consistent with higher proportions of antibodies recognizing DII that are broadly flavivirus cross-reactive (Fig. 4C). Similar epitope-specific antibody-mediated structural changes have also been described for other viruses (62–64). In the case of HIV, they were implicated in the enhanced antigenicity of neutralizing antigenic sites involving the V3 loop of gp 120 after immunization with ICs containing the HIV gp 120 (28, 65).
The demonstration of TBE E dimer dissociation by MAb A3 also provides a late explanation for enhancement phenomena observed in competitive MAb binding studies used for epitope mapping (53). In the case of TBE virus, these analyses were conducted with both whole antibodies and Fab fragments and had shown that MAb A3 not only strongly increased the affinity of a broadly flavivirus cross-reactive antibody (MAb A1) but—as revealed by Scatchard analyses—also increased the number of binding sites, fully compatible with a MAb A3 antibody-mediated dissociation of E and concomitant exposure of the FP containing the MAb A1 epitope. Since the latter analyses were performed with purified virions and not with isolated sEs, it can be concluded that MAb A3 dissociates E dimers also in the context of whole virions.
The phenomena observed with flavivirus E-antibody complexes, such as the enhancement of antibody binding or the modulation of the specificity of antibody responses after IC immunization, have to be seen in the context of the dynamic nature of proteins and protein complexes and their oscillation between different conformational states that can be stabilized by new interaction partners. The flavivirus E protein may be especially prone to such dynamic changes because of the flexibility of junctions between its domains (66) allowing adoption of a variety of conformations that enable the structural changes and oligomeric rearrangements occurring during virus maturation, egress, and entry.
Antibody-mediated reorganizations of the oligomeric structure of E have been structurally defined by cryo-EM analyses of whole dengue viruses in complex with Fab fragments (21). This study showed that the binding of antibodies can shift the equilibrium structure of E at the virion surface to ensembles that are strikingly different from those observed in the absence of antibodies. The exposure of seemingly cryptic antigenic sites by breathing phenomena can also have strong implications for the mechanisms of antibody-mediated virus neutralization (18, 20, 67–69), and antibody-induced disruption of dimer contacts between the FP in one monomer and its accommodating pocket in the second monomer by a dengue E DIII-specific antibody has been proposed as an explanation for its neutralizing activity (19). It is presently unknown whether the dissociating effect of MAb A3 is due to antibody-mediated conformational changes that weaken E-dimer interactions or due to the fixation of a monomeric state present in a dimer-monomer equilibrium.
The immunomodulatory effects observed with MAb B4 are most likely due to epitope shielding. Compared to those of MAb A3, these effects were comparably subtle and reached statistical significance only with a single parameter, the ratio of virion-reactive antibodies to sE-reactive antibodies (Fig. 4D). Together with the lacking capacity of MAb B4 to dissociate the E dimer, these data argue against dramatic antibody-mediated structural changes in the immunogen. Analyses with mutants of the exposed lateral-ridge epitope in DIII (the binding site of MAb B4; Fig. 5) rather suggest that the shift of antibody specificity is due to the shielding of the epitope in the immunogen, thus restricting accessibility for the B cell receptor to this specific site without impairing the induction of antibodies to other accessible antigenic sites in DIII. Such interactions can explain the relative reduction of levels of virion-reactive antibodies compared to sE-reactive antibodies in sera after IC sE-B4 vaccination, because—in contrast to epitopes at the lateral ridge of DIII—substantial parts of DIII are largely buried in the context of the closed virion shell but are accessible in the sE dimer. The masking of B cell epitopes has been proposed as a mechanism by which maternal antibodies influence infant vaccine responses (70) and exert determinant-specific modulatory effects on the specificity of antibody responses (71–74).
It is possible that mechanisms like antibody-induced conformational changes and epitope shielding also play a biological role in a polyclonal situation by changing viral surface structures and the accessibility of certain epitopes. Such effects could influence the antibody response in sequential flavivirus infections or immunizations and—as a consequence—virus neutralization or antibody-dependent enhancement of infection. Specifically, an increase in the levels of cross-reactive, weakly neutralizing antibodies could impair antibody-mediated protection and favor pathological consequences of IC formation, similarly to those described by Watanabe et al. (75) in a mouse model. In this context, it is important that the compositions of antibody populations in sera from different individuals and the immune dominances of certain antibody populations can differ substantially (15, 16), and epitope-specific effects may therefore differ from individual to individual. Such aspects can be addressed in future studies to clarify their relevance in the complex and diverse settings of antibody populations in polyclonal sera.
ACKNOWLEDGMENTS
We thank Andrea Reiter and Walter Holzer for their excellent technical assistance.
This work was supported by the Austrian Science Fund FWF (projects P25265-B21 and APW01212FW).
REFERENCES
- 1.Pierson T, Diamond M. 2013. Flaviviruses. In Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Racaniello VR, Roizman B (ed), Fields virology, 6th ed (electronic) Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 2.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature 496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Sabatino D, Bruno R, Sauro F, Danzetta ML, Cito F, Iannetti S, Narcisi V, De Massis F, Calistri P. 2014. Epidemiology of West Nile disease in Europe and in the Mediterranean Basin from 2009 to 2013. Biomed Res Int 2014:907852. doi: 10.1155/2014/907852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Süss J. 2011. Tick-borne encephalitis 2010: epidemiology, risk areas, and virus strains in Europe and Asia—an overview. Ticks Tick Borne Dis 2:2–15. doi: 10.1016/j.ttbdis.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Hanna JN, Ritchie SA, Phillips DA, Lee JM, Hills SL, van den Hurk AF, Pyke AT, Johansen CA, Mackenzie JS. 1999. Japanese encephalitis in north Queensland, Australia, 1998. Med J Aust 170:533–536. [DOI] [PubMed] [Google Scholar]
- 6.Gardner CL, Ryman KD. 2010. Yellow fever: a reemerging threat. Clin Lab Med 30:237–260. doi: 10.1016/j.cll.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaufmann B, Rossmann MG. 2011. Molecular mechanisms involved in the early steps of flavivirus cell entry. Microbes Infect 13:1–9. doi: 10.1016/j.micinf.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pierson TC, Kielian M. 2013. Flaviviruses: braking the entering. Curr Opin Virol 3:3–12. doi: 10.1016/j.coviro.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smit JM, Moesker B, Rodenhuis-Zybert I, Wilschut J. 2011. Flavivirus cell entry and membrane fusion. Viruses 3:160–171. doi: 10.3390/v3020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pierson TC, Fremont DH, Kuhn RJ, Diamond MS. 2008. Structural insights into the mechanisms of antibody-mediated neutralization of flavivirus infection: implications for vaccine development. Cell Host Microbe 4:229–238. doi: 10.1016/j.chom.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heinz FX, Stiasny K. 2012. Flaviviruses and their antigenic structure. J Clin Virol 55:289–295. doi: 10.1016/j.jcv.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 12.Dowd KA, Pierson TC. 2011. Antibody-mediated neutralization of flaviviruses: a reductionist view. Virology 411:306–315. doi: 10.1016/j.virol.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beltramello M, Williams KL, Simmons CP, Macagno A, Simonelli L, Quyen NT, Sukupolvi-Petty S, Navarro-Sanchez E, Young PR, de Silva AM, Rey FA, Varani L, Whitehead SS, Diamond MS, Harris E, Lanzavecchia A, Sallusto F. 2010. The human immune response to Dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host Microbe 8:271–283. doi: 10.1016/j.chom.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai CY, Tsai WY, Lin SR, Kao CL, Hu HP, King CC, Wu HC, Chang GJ, Wang WK. 2008. Antibodies to envelope glycoprotein of dengue virus during the natural course of infection are predominantly cross-reactive and recognize epitopes containing highly conserved residues at the fusion loop of domain II. J Virol 82:6631–6643. doi: 10.1128/JVI.00316-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jarmer J, Zlatkovic J, Tsouchnikas G, Vratskikh O, Strauss J, Aberle JH, Chmelik V, Kundi M, Stiasny K, Heinz FX. 24 September 2014. Variation of the specificity of the human antibody responses after tick-borne encephalitis virus infection and vaccination. J Virol doi: 10.1128/JVI.02086-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vratskikh O, Stiasny K, Zlatkovic J, Tsouchnikas G, Jarmer J, Karrer U, Roggendorf M, Roggendorf H, Allwinn R, Heinz FX. 2013. Dissection of antibody specificities induced by yellow fever vaccination. PLoS Pathog 9:e1003458. doi: 10.1371/journal.ppat.1003458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pierson TC, Diamond MS. 2012. Degrees of maturity: the complex structure and biology of flaviviruses. Curr Opin Virol 2:168–175. doi: 10.1016/j.coviro.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Austin SK, Dowd KA, Shrestha B, Nelson CA, Edeling MA, Johnson S, Pierson TC, Diamond MS, Fremont DH. 2012. Structural basis of differential neutralization of DENV-1 genotypes by an antibody that recognizes a cryptic epitope. PLoS Pathog 8:e1002930. doi: 10.1371/journal.ppat.1002930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cockburn JJ, Navarro Sanchez ME, Fretes N, Urvoas A, Staropoli I, Kikuti CM, Coffey LL, Arenzana Seisdedos F, Bedouelle H, Rey FA. 2012. Mechanism of dengue virus broad cross-neutralization by a monoclonal antibody. Structure 20:303–314. doi: 10.1016/j.str.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Dowd KA, Jost CA, Durbin AP, Whitehead SS, Pierson TC. 2011. A dynamic landscape for antibody binding modulates antibody-mediated neutralization of West Nile virus. PLoS Pathog 7:e1002111. doi: 10.1371/journal.ppat.1002111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lok SM, Kostyuchenko V, Nybakken GE, Holdaway HA, Battisti AJ, Sukupolvi-Petty S, Sedlak D, Fremont DH, Chipman PR, Roehrig JT, Diamond MS, Kuhn RJ, Rossmann MG. 2008. Binding of a neutralizing antibody to dengue virus alters the arrangement of surface glycoproteins. Nat Struct Mol Biol 15:312–317. doi: 10.1038/nsmb.1382. [DOI] [PubMed] [Google Scholar]
- 22.Midgley CM, Flanagan A, Tran HB, Dejnirattisai W, Chawansuntati K, Jumnainsong A, Wongwiwat W, Duangchinda T, Mongkolsapaya J, Grimes JM, Screaton GR. 2012. Structural analysis of a dengue cross-reactive antibody complexed with envelope domain III reveals the molecular basis of cross-reactivity. J Immunol 188:4971–4979. doi: 10.4049/jimmunol.1200227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pierson TC, Kuhn RJ. 2012. Capturing a virus while it catches its breath. Structure 20:200–202. doi: 10.1016/j.str.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heinz FX, Stiasny K. 2012. Flaviviruses and flavivirus vaccines. Vaccine 30:4301–4306. doi: 10.1016/j.vaccine.2011.09.114. [DOI] [PubMed] [Google Scholar]
- 25.Pierson TC, Diamond MS. 2014. Vaccine development as a means to control dengue virus pathogenesis: do we know enough? Annu Rev Virol 1:375–398. doi: 10.1146/annurev-virology-031413-085453. [DOI] [PubMed] [Google Scholar]
- 26.Gosselin EJ, Wardwell K, Gosselin DR, Alter N, Fisher JL, Guyre PM. 1992. Enhanced antigen presentation using human Fc gamma receptor (monocyte/macrophage)-specific immunogens. J Immunol 149:3477–3481. [PubMed] [Google Scholar]
- 27.Manca F, Fenoglio D, Li Pira G, Kunkl A, Celada F. 1991. Effect of antigen/antibody ratio on macrophage uptake, processing, and presentation to T cells of antigen complexed with polyclonal antibodies. J Exp Med 173:37–48. doi: 10.1084/jem.173.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hioe CE, Visciano ML, Kumar R, Liu J, Mack EA, Simon RE, Levy DN, Tuen M. 2009. The use of immune complex vaccines to enhance antibody responses against neutralizing epitopes on HIV-1 envelope gp120. Vaccine 28:352–360. doi: 10.1016/j.vaccine.2009.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brady LJ. 2005. Antibody-mediated immunomodulation: a strategy to improve host responses against microbial antigens. Infect Immun 73:671–678. doi: 10.1128/IAI.73.2.671-678.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Celis E, Chang TW. 1984. Antibodies to hepatitis B surface antigen potentiate the response of human T lymphocyte clones to the same antigen. Science 224:297–299. doi: 10.1126/science.6231724. [DOI] [PubMed] [Google Scholar]
- 31.Abdel-Motal UM, Wigglesworth K, Galili U. 2009. Mechanism for increased immunogenicity of vaccines that form in vivo immune complexes with the natural anti-Gal antibody. Vaccine 27:3072–3082. doi: 10.1016/j.vaccine.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 32.Goins CL, Chappell CP, Shashidharamurthy R, Selvaraj P, Jacob J. 2010. Immune complex-mediated enhancement of secondary antibody responses. J Immunol 184:6293–6298. doi: 10.4049/jimmunol.0902530. [DOI] [PubMed] [Google Scholar]
- 33.Houston WE, Kremer RJ, Crabbs CL, Spertzel RO. 1977. Inactivated Venezuelan equine encephalomyelitis virus vaccine complexed with specific antibody: enhanced primary immune response and altered pattern of antibody class elicited. J Infect Dis 135:600–610. doi: 10.1093/infdis/135.4.600. [DOI] [PubMed] [Google Scholar]
- 34.Stäger S, Alexander J, Kirby AC, Botto M, Rooijen NV, Smith DF, Brombacher F, Kaye PM. 2003. Natural antibodies and complement are endogenous adjuvants for vaccine-induced CD8+ T-cell responses. Nat Med 9:1287–1292. doi: 10.1038/nm933. [DOI] [PubMed] [Google Scholar]
- 35.Villinger F, Mayne AE, Bostik P, Mori K, Jensen PE, Ahmed R, Ansari AA. 2003. Evidence for antibody-mediated enhancement of simian immunodeficiency virus (SIV) Gag antigen processing and cross presentation in SIV-infected rhesus macaques. J Virol 77:10–24. doi: 10.1128/JVI.77.1.10-24.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heyman B. 2003. Feedback regulation by IgG antibodies. Immunol Lett 88:157–161. doi: 10.1016/S0165-2478(03)00078-6. [DOI] [PubMed] [Google Scholar]
- 37.Nimmerjahn F, Ravetch JV. 2010. Antibody-mediated modulation of immune responses. Immunol Rev 236:265–275. doi: 10.1111/j.1600-065X.2010.00910.x. [DOI] [PubMed] [Google Scholar]
- 38.Brady LJ, van Tilburg ML, Alford CE, McArthur WP. 2000. Monoclonal antibody-mediated modulation of the humoral immune response against mucosally applied Streptococcus mutans. Infect Immun 68:1796–1805. doi: 10.1128/IAI.68.4.1796-1805.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caulfield MJ, Shaffer D. 1987. Immunoregulation by antigen/antibody complexes. I. Specific immunosuppression induced in vivo with immune complexes formed in antibody excess. J Immunol 138:3680–3683. [PubMed] [Google Scholar]
- 40.Coulie PG, Van Snick J. 1985. Enhancement of IgG anti-carrier responses by IgG2 anti-hapten antibodies in mice. Eur J Immunol 15:793–798. doi: 10.1002/eji.1830150810. [DOI] [PubMed] [Google Scholar]
- 41.Wiersma EJ, Coulie PG, Heyman B. 1989. Dual immunoregulatory effects of monoclonal IgG-antibodies: suppression and enhancement of the antibody response. Scand J Immunol 29:439–448. doi: 10.1111/j.1365-3083.1989.tb01143.x. [DOI] [PubMed] [Google Scholar]
- 42.Zheng B, Switzer K, Marinova E, Wansley D, Han S. 2007. Correction of age-associated deficiency in germinal center response by immunization with immune complexes. Clin Immunol 124:131–137. doi: 10.1016/j.clim.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 43.Yao X, Zheng B, Zhou J, Xu DZ, Zhao K, Sun SH, Yuan ZH, Wen YM. 2007. Therapeutic effect of hepatitis B surface antigen-antibody complex is associated with cytolytic and non-cytolytic immune responses in hepatitis B patients. Vaccine 25:1771–1779. doi: 10.1016/j.vaccine.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 44.Bouige P, Iscaki S, Cosson A, Pillot J. 1996. Molecular analysis of the modulatory factors of the response to HBsAg in mice as an approach to HBV vaccine enhancement. FEMS Immunol Med Microbiol 13:71–79. doi: 10.1111/j.1574-695X.1996.tb00218.x. [DOI] [PubMed] [Google Scholar]
- 45.Karlsson MC, Wernersson S, Diaz de Stahl T, Gustavsson S, Heyman B. 1999. Efficient IgG-mediated suppression of primary antibody responses in Fcgamma receptor-deficient mice. Proc Natl Acad Sci U S A 96:2244–2249. doi: 10.1073/pnas.96.5.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zlatkovic J, Tsouchnikas G, Jarmer J, Koessl C, Stiasny K, Heinz FX. 2013. Aluminum hydroxide influences not only the extent but also the fine specificity and functional activity of antibody responses to tick-borne encephalitis virus in mice. J Virol 87:12187–12195. doi: 10.1128/JVI.01690-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heinz FX, Kunz C. 1981. Homogeneity of the structural glycoprotein from European isolates of tick-borne encephalitis virus: comparison with other flaviviruses. J Gen Virol 57:263–274. doi: 10.1099/0022-1317-57-2-263. [DOI] [PubMed] [Google Scholar]
- 48.Zlatkovic J, Stiasny K, Heinz FX. 2011. Immunodominance and functional activities of antibody responses to inactivated West Nile virus and recombinant subunit vaccines in mice. J Virol 85:1994–2003. doi: 10.1128/JVI.01886-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oliphant T, Nybakken GE, Austin SK, Xu Q, Bramson J, Loeb M, Throsby M, Fremont DH, Pierson TC, Diamond MS. 2007. Induction of epitope-specific neutralizing antibodies against West Nile virus. J Virol 81:11828–11839. doi: 10.1128/JVI.00643-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heinz FX, Berger R, Tuma W, Kunz C. 1983. A topological and functional model of epitopes on the structural glycoprotein of tick-borne encephalitis virus defined by monoclonal antibodies. Virology 126:525–537. doi: 10.1016/S0042-6822(83)80010-5. [DOI] [PubMed] [Google Scholar]
- 51.Holzmann H, Stiasny K, York H, Dorner F, Kunz C, Heinz FX. 1995. Tick-borne encephalitis virus envelope protein E-specific monoclonal antibodies for the study of low pH-induced conformational changes and immature virions. Arch Virol 140:213–221. doi: 10.1007/BF01309857. [DOI] [PubMed] [Google Scholar]
- 52.Kiermayr S, Stiasny K, Heinz FX. 2009. Impact of quaternary organization on the antigenic structure of the tick-borne encephalitis virus envelope glycoprotein E. J Virol 83:8482–8491. doi: 10.1128/JVI.00660-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heinz FX, Mandl C, Berger R, Tuma W, Kunz C. 1984. Antibody-induced conformational changes result in enhanced avidity of antibodies to different antigenic sites on the tick-borne encephalitis virus glycoprotein. Virology 133:25–34. doi: 10.1016/0042-6822(84)90422-7. [DOI] [PubMed] [Google Scholar]
- 54.Heinz FX, Berger R, Majdic O, Knapp W, Kunz C. 1982. Monoclonal antibodies to the structural glycoprotein of tick-borne encephalitis virus. Infect Immun 37:869–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Frey A, Di Canzio J, Zurakowski D. 1998. A statistically defined endpoint titer determination method for immunoassays. J Immunol Methods 221:35–41. doi: 10.1016/S0022-1759(98)00170-7. [DOI] [PubMed] [Google Scholar]
- 56.Allison SL, Schalich J, Stiasny K, Mandl CW, Kunz C, Heinz FX. 1995. Oligomeric rearrangement of tick-borne encephalitis virus envelope proteins induced by an acidic pH. J Virol 69:695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maizel JV. 1971. 5-Polyacrylamide gel electrophoresis of viral proteins. Methods Virol 5:179–246. doi: 10.1016/B978-0-12-470205-9.50011-3. [DOI] [Google Scholar]
- 58.Nybakken GE, Oliphant T, Johnson S, Burke S, Diamond MS, Fremont DH. 2005. Structural basis of West Nile virus neutralization by a therapeutic antibody. Nature 437:764–769. doi: 10.1038/nature03956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oliphant T, Engle M, Nybakken GE, Doane C, Johnson S, Huang L, Gorlatov S, Mehlhop E, Marri A, Chung KM, Ebel GD, Kramer LD, Fremont DH, Diamond MS. 2005. Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nat Med 11:522–530. doi: 10.1038/nm1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rey FA, Heinz FX, Mandl C, Kunz C, Harrison SC. 1995. The envelope glycoprotein from tick-borne encephalitis virus at 2 A resolution. Nature 375:291–298. doi: 10.1038/375291a0. [DOI] [PubMed] [Google Scholar]
- 61.Heinz FX, Mandl CW, Holzmann H, Kunz C, Harris BA, Rey F, Harrison SC. 1991. The flavivirus envelope protein E: isolation of a soluble form from tick-borne encephalitis virus and its crystallization. J Virol 65:5579–5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clegg JC, Chanas AC, Gould EA. 1983. Conformational changes in Sindbis virus E1 glycoprotein induced by monoclonal antibody binding. J Gen Virol 64:1121–1126. doi: 10.1099/0022-1317-64-5-1121. [DOI] [PubMed] [Google Scholar]
- 63.Koromyslova AD, Hansman GS. 2015. Nanobody binding to a conserved epitope promotes norovirus particle disassembly. J Virol 89:2718–2730. doi: 10.1128/JVI.03176-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Poignard P, Fouts T, Naniche D, Moore JP, Sattentau QJ. 1996. Neutralizing antibodies to human immunodeficiency virus type-1 gp120 induce envelope glycoprotein subunit dissociation. J Exp Med 183:473–484. doi: 10.1084/jem.183.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kumar R, Tuen M, Li H, Tse DB, Hioe CE. 2011. Improving immunogenicity of HIV-1 envelope gp120 by glycan removal and immune complex formation. Vaccine 29:9064–9074. doi: 10.1016/j.vaccine.2011.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Y, Zhang W, Ogata S, Clements D, Strauss JH, Baker TS, Kuhn RJ, Rossmann MG. 2004. Conformational changes of the flavivirus E glycoprotein. Structure 12:1607–1618. doi: 10.1016/j.str.2004.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dowd KA, Mukherjee S, Kuhn RJ, Pierson TC. 2014. Combined effects of the structural heterogeneity and dynamics of flaviviruses on antibody recognition. J Virol 88:11726–11737. doi: 10.1128/JVI.01140-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fibriansah G, Ng TS, Kostyuchenko VA, Lee J, Lee S, Wang J, Lok SM. 2013. Structural changes in dengue virus when exposed to a temperature of 37 degrees C. J Virol 87:7585–7592. doi: 10.1128/JVI.00757-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fibriansah G, Tan JL, Smith SA, de Alwis AR, Ng TS, Kostyuchenko VA, Ibarra KD, Wang J, Harris E, de Silva A, Crowe JE Jr, Lok SM. 2014. A potent anti-dengue human antibody preferentially recognizes the conformation of E protein monomers assembled on the virus surface. EMBO Mol Med 6:358–371. doi: 10.1002/emmm.201303404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Siegrist CA. 2003. Mechanisms by which maternal antibodies influence infant vaccine responses: review of hypotheses and definition of main determinants. Vaccine 21:3406–3412. doi: 10.1016/S0264-410X(03)00342-6. [DOI] [PubMed] [Google Scholar]
- 71.Jelonek MT, Maskrey JL, Steimer KS, Potts BJ, Higgins KW, Keller MA. 1996. Maternal monoclonal antibody to the V3 loop alters specificity of the response to a human immunodeficiency virus vaccine. J Infect Dis 174:866–869. doi: 10.1093/infdis/174.4.866. [DOI] [PubMed] [Google Scholar]
- 72.Kurikka S, Olander RM, Eskola J, Kayhty H. 1996. Passively acquired anti-tetanus and anti-Haemophilus antibodies and the response to Haemophilus influenzae type b-tetanus toxoid conjugate vaccine in infancy. Pediatr Infect Dis J 15:530–535. doi: 10.1097/00006454-199606000-00011. [DOI] [PubMed] [Google Scholar]
- 73.Nohynek H, Gustafsson L, Capeding MR, Kayhty H, Olander RM, Pascualk L, Ruutu P. 1999. Effect of transplacentally acquired tetanus antibodies on the antibody responses to Haemophilus influenzae type b-tetanus toxoid conjugate and tetanus toxoid vaccines in Filipino infants. Pediatr Infect Dis J 18:25–30. doi: 10.1097/00006454-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 74.Panpitpat C, Thisyakorn U, Chotpitayasunondh T, Furer E, Que JU, Hasler T, Cryz SJ Jr. 2000. Elevated levels of maternal anti-tetanus toxin antibodies do not suppress the immune response to a Haemophilus influenzae type b polyribosylphosphate-tetanus toxoid conjugate vaccine. Bull World Health Organ 78:364–371. [PMC free article] [PubMed] [Google Scholar]
- 75.Watanabe S, Chan KW, Wang J, Rivino L, Lok SM, Vasudevan SG. 18 March 2015. Dengue virus infection with highly neutralizing levels of cross-reactive antibodies causes acute lethal small intestinal pathology without a high level of viremia in mice. J Virol doi: 10.1128/JVI.00216-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mandl CW, Guirakhoo F, Holzmann H, Heinz FX, Kunz C. 1989. Antigenic structure of the flavivirus envelope protein E at the molecular level, using tick-borne encephalitis virus as a model. J Virol 63:564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Holzmann H, Stiasny K, Ecker M, Kunz C, Heinz FX. 1997. Characterization of monoclonal antibody-escape mutants of tick-borne encephalitis virus with reduced neuroinvasiveness in mice. J Gen Virol 78(Pt 1):31–37. [DOI] [PubMed] [Google Scholar]