Abstract
Reactivation from latency results in transmission of neurotropic herpesviruses from the nervous system to body surfaces, referred to as anterograde axonal trafficking. The virus-encoded protein pUS9 promotes axonal dissemination by sorting virus particles into axons, but whether it is also an effector of fast axonal transport within axons is unknown. To determine the role of pUS9 in anterograde trafficking, we analyzed the axonal transport of pseudorabies virus in the presence and absence of pUS9.
TEXT
All herpesviruses cycle through periods of active and latent infection, with members of the Alphaherpesvirinae subfamily typically establishing latency in neurons of the peripheral nervous system. Retrograde transport results in entry into the nervous system, transmitting particles from neuron terminals to sensory and autonomic ganglia. Reactivation from the latent state results in newly assembled viral particles traveling from the ganglia to sites of innervation at body surfaces by anterograde axonal trafficking (1). The resulting infections include presentations such as herpes labialis (herpes simplex virus 1 [HSV-1]) and shingles (varicella-zoster virus [VZV]). These viruses also encompass veterinary pathogens, including the well-studied pseudorabies virus (PRV) that serves as a model for severe neuroinvasive infections (2).
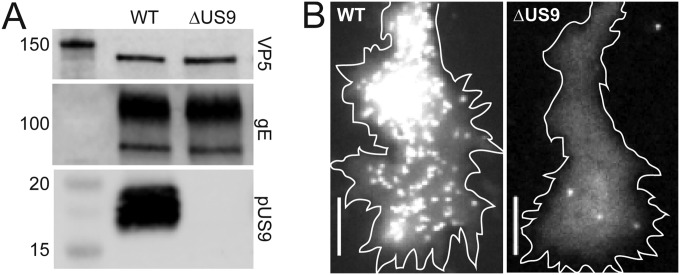
Anterograde axonal trafficking consists of two steps. Cargoes, including virus proteins, are sorted into the axon from the neuronal cell body (3). Once in the axon, the cargo moves to the distal axon terminal by microtubule-dependent fast axonal transport (4). The best characterized effector of herpesvirus axonal trafficking is the type II transmembrane protein pUS9 (5–11). PRV lacking pUS9 enters the nervous system by retrograde axon transport but, following replication in neurons, is attenuated for anterograde trafficking both in animals and in neuronal cell culture (9, 10, 12, 13). This defect is attributed to a decrease in viral particle sorting to axons, but whether pUS9 is an effector of fast axonal transport is unknown (14, 15). The presence of viral particles in axons and transmission of infection to cells at distal terminals indicate that rare anterograde trafficking events occur, but the scarcity of these events has precluded their analysis (9, 14). To determine whether pUS9 contributes to PRV fast axonal transport, we examined the transport of wild-type (WT) and ΔUS9 PRV particles that encode red fluorescent capsids (16). The fluorescent ΔUS9 mutant used in these studies was confirmed by restriction enzyme digest, sequencing across the deletion junction, absence of pUS9 expression, lack of viral particle accumulation at axon terminals resulting from anterograde transport in culture, and inability to spread by anterograde transportation within the rat visual system following intravitreal eye injection (Fig. 1 and Table 1) (17–21).
FIG 1.
Characterization of WT or ΔUS9 PRV used in this study. (A) Lysates of PK15 cells infected with either WT or ΔUS9 PRV were collected, run through SDS-PAGE, and transferred to a membrane as previously described (20). The membrane was cut at the 25-kDa marker. The upper half of the membrane was probed with an anti-VP5 monoclonal antibody (clone 3C10) and then with an anti-gE tail polyclonal antibody, and the lower half of the membrane was probed with an anti-US9 polyclonal antibody; all antibodies were used at a dilution of 1:1,000. The blot shown is representative of the results; n = 2. All antibodies were gifts from Lynn Enquist. (B) Dissociated DRGs were infected with 5 × 10^6 PFU of either WT or ΔUS9 PRV and imaged at 15 to 18 h.p.i. (21, 30). Representative images of capsid accumulation at axon growth cones are shown. Scale bars = 5 μm.
TABLE 1.
Viruses used in this study
| Virus | Reporter tag | Mutation | No. of rats with visual circuit transmission/total no. of ratsc | No. of transport events in avian DRG sensory neurons |
|
|---|---|---|---|---|---|
| Retrograde | Anterograde | ||||
| PRV-GS4284a | UL25/mCherry | None | 5/5 | 288 | 38 |
| PRV-GS5469 | UL25/mCherry | ΔUS9b | 0/5 | 192 | 35 |
PRV-GS4284 was described previously (16).
A stop codon was inserted after the start ATG, and codons 2 to 72 were deleted, thus removing all internal in-frame ATG codons.
Following intravitreal injection of WT or mutant virus into the eyes of rats, the number of rats exhibiting virus fluorescence in retinorecipient regions of the brain 48 h.p.i. (either lateral geniculate nucleus or superior colliculus) was determined (19).
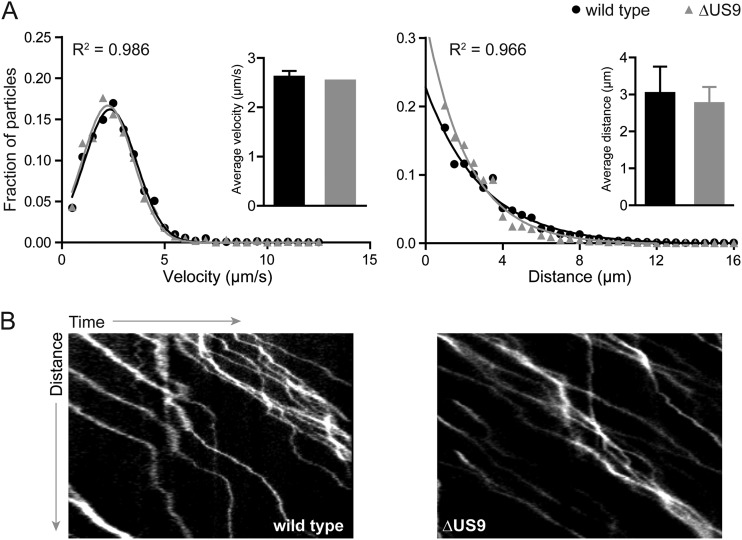
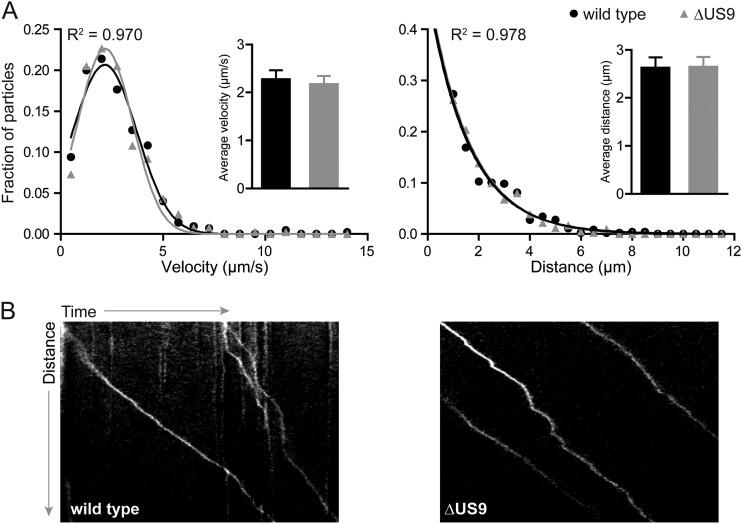
While envelope proteins, such as pUS9, are not expected to be effectors of the retrograde axon transport that occurs upon entry into nerve endings, an analysis was performed to confirm that this initial stage of neuronal infection was unperturbed (22, 23). Explants of avian dorsal root ganglion (DRG) sensory neurons were cultured and infected ex vivo. Individual viral particles were imaged at 10 frames/s during the first hour postinfection (h.p.i.), and the kinetics of axonal transport were measured for particles moving more than 0.5 μm using the kymograph function in the MetaMorph software package (Molecular Devices) as described previously (n > 190) (Table 1) (21, 24–26). As expected, the WT and ΔUS9 viruses had equivalent retrograde transport profiles based on run lengths and run velocities (Fig. 2). Because pUS9 supports the sorting of viral particles from soma to axons following replication (5–11), examining its subsequent role in fast axonal transport of particles to axon terminals was made difficult by the low frequency of these events (Fig. 1B; note the reduced number of viral particles accumulated at axon terminals following replication). We accomplished this analysis by extensive imaging of ΔUS9 PRV infections in isolated neurons in low-density cultures to capture a minimum of 30 transport events that were unambiguously moving away from an infected neuronal soma. Although WT events were far more frequent, an equivalent number were included for the comparative analysis (Table 1). The kinetics of ΔUS9 PRV microtubule-based anterograde axonal transport were indistinguishable from the transport kinetics of the wild type (Fig. 3). These results indicate that the well-described reduction in anterograde spread noted for ΔUS9 PRV cannot be directly attributed to a disruption of the fundamental ability of viral particles to engage in microtubule-based transport within axons (10, 14, 27).
FIG 2.
Retrograde transport of WT or ΔUS9 particles during initial infection of primary sensory neurons. E8-E10 chick DRG explants were infected with ∼1 × 10^7 PFU of either wild-type or ΔUS9 PRV, and incoming particles were imaged between 15 and 60 min postinfection based on red fluorescence emissions from the UL25/mCherry capsid tags. More than 190 particles each of WT and mutant virus were tracked. (A) Gaussian (velocity) or decaying exponential (run length) curves were fit to histograms by nonlinear regression; curve-fitting produced R2 values of >0.96. Insets show average velocities (left) and run lengths (right); error bars indicate standard deviations. (B) Representative kymographs of retrograde-moving WT or ΔUS9 particles.
FIG 3.
Anterograde transport of WT or ΔUS9 particles following replication in primary sensory neurons. Dissociated E8-E10 chick DRG cultures were infected with ∼5 × 10^6 PFU of either wild-type or ΔUS9 PRV, and egressing particles were imaged from 10 to 13 h.p.i. More than 30 particles each of WT and mutant virus were tracked in isolated axons that were unambiguously contiguous with a neuronal soma, thereby allowing for a clear assessment that all transport included in the analysis was anterograde directed. (A) Gaussian (velocity) or decaying exponential (run length) curves were fit to histograms by nonlinear regression; curve-fitting produced R2 values of >0.97. Insets show average velocities (left) and run lengths (right); error bars indicate standard deviations. (B) Representative kymographs of anterograde-moving WT or ΔUS9 particles.
The finding that PRV pUS9 interacts with KIF1A, a kinesin motor involved in the fast anterograde axonal transport of presynaptic vesicles (4), suggested a direct role for pUS9 in fast axonal transport (15). However, while a mutant pUS9 (Y49-50A) that does not interact with KIF1A (15) restricts the anterograde trafficking of capsids in axons, pUS9 (Y49-50A) itself remains competent for anterograde transport (14). Based on the current findings, an explanation for this perplexing observation can be offered: the pUS9-KIF1A interaction is required for sorting capsid-containing viral particles into axons but is dispensable for subsequent fast axonal transport within axons. Consistent with this, a protein that can sort into the axon independently from pUS9 and capsids, gM, also does not require pUS9 for axonal transport (28). Therefore, in the absence of pUS9, fewer viral particles are sent down axons due to inefficient sorting from the soma, but the probability that each individual particle will reach the axon terminal, as assessed by transport dynamics, remains unchanged. Inherent in this conclusion is the prediction that ΔUS9 viruses should traverse long distances in axons to spread across anterograde neural circuits in vivo, albeit at reduced frequency due to inefficient sorting.
In apparent contradiction to this prediction, pUS9 is often regarded as an essential determinant for the anterograde trafficking of PRV. The principle evidence in support of this idea comes from the complete lack of anterograde transneuronal transmission from the retina to retinorecipient regions of the brain following infection of rats with ΔUS9 PRV, a finding that was reproduced with the fluorescent viruses used in this study (Table 1) (10). However, this conclusion is tempered by the finding that HSV remains competent to traverse this visual circuit in the absence of pUS9 in mice, although at reduced efficiency (5). More importantly, other neuronal infection models do not support the conclusion that PRV lacking pUS9 does not participate in anterograde axon trafficking and transmission. Whereas an anterograde-deficient strain of PRV (PRV Bartha) does not spread in anterograde circuits within the brain (29), ΔUS9 PRV does so effectively, although not as robustly as wild-type PRV (10). Additionally, in cultures of primary neurons infected with PRV (9) or HSV-1 (7) or in cultures of neuronlike cells infected with HSV-1 (6), pUS9 is an enhancer of anterograde transmission but again is not essential for this process. Therefore, the inability of ΔUS9 PRV to traverse the rat anterograde visual circuitry may be the manifestation of a reduced ability to sort particles into axons, combined with the finite probability of a particle traveling the entire length of these long axons to reach the presynaptic terminals in the brain.
In conclusion, this study provides a kinetic characterization of PRV axonal transport in the absence of the pUS9 anterograde trafficking effector. Deletion of US9 in PRV reduced the number of capsid-containing viral particles sorted into axons but did not directly impair the subsequent step of fast axonal transport for those particles making it past the sorting barrier. The results indicate that the effector function of pUS9 in promoting anterograde axonal trafficking lies in initial sorting of particles to axons and not in fast axonal transport of particles from the proximal axon to terminals.
ACKNOWLEDGMENTS
This work was supported by NIH grants R01 AI056346 to G.A.S. and R01 NS077003 to G.E.P. and P.J.S. G.R.D was supported by the training program in the Cellular and Molecular Basis of Disease from the National Institutes of Health (grant T32 GM08061).
We thank Anne Fischer for excellent technical assistance.
REFERENCES
- 1.Smith G. 2012. Herpesvirus transport to the nervous system and back again. Annu Rev Microbiol 66:153–176. doi: 10.1146/annurev-micro-092611-150051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pomeranz LE, Reynolds AE, Hengartner CJ. 2005. Molecular biology of pseudorabies virus: impact on neurovirology and veterinary medicine. Microbiol Mol Biol Rev 69:462–500. doi: 10.1128/MMBR.69.3.462-500.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song AH, Wang D, Chen G, Li Y, Luo J, Duan S, Poo MM. 2009. A selective filter for cytoplasmic transport at the axon initial segment. Cell 136:1148–1160. doi: 10.1016/j.cell.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 4.Hirokawa N, Niwa S, Tanaka Y. 2010. Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron 68:610–638. doi: 10.1016/j.neuron.2010.09.039. [DOI] [PubMed] [Google Scholar]
- 5.McGraw HM, Awasthi S, Wojcechowskyj JA, Friedman HM. 2009. Anterograde spread of herpes simplex virus type 1 requires glycoprotein E and glycoprotein I but not Us9. J Virol 83:8315–8326. doi: 10.1128/JVI.00633-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snyder A, Polcicova K, Johnson DC. 2008. Herpes simplex virus gE/gI and US9 proteins promote transport of both capsids and virion glycoproteins in neuronal axons. J Virol 82:10613–10624. doi: 10.1128/JVI.01241-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howard PW, Howard TL, Johnson DC. 2013. Herpes simplex virus membrane proteins gE/gI and US9 act cooperatively to promote transport of capsids and glycoproteins from neuron cell bodies into initial axon segments. J Virol 87:403–414. doi: 10.1128/JVI.02465-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LaVail JH, Tauscher AN, Sucher A, Harrabi O, Brandimarti R. 2007. Viral regulation of the long distance axonal transport of herpes simplex virus nucleocapsid. Neuroscience 146:974–985. doi: 10.1016/j.neuroscience.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ch'ng TH, Enquist LW. 2005. Neuron-to-cell spread of pseudorabies virus in a compartmented neuronal culture system. J Virol 79:10875–10889. doi: 10.1128/JVI.79.17.10875-10889.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brideau AD, Card JP, Enquist LW. 2000. Role of pseudorabies virus Us9, a type II membrane protein, in infection of tissue culture cells and the rat nervous system. J Virol 74:834–845. doi: 10.1128/JVI.74.2.834-845.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polcicova K, Biswas PS, Banerjee K, Wisner TW, Rouse BT, Johnson DC. 2005. Herpes keratitis in the absence of anterograde transport of virus from sensory ganglia to the cornea. Proc Natl Acad Sci U S A 102:11462–11467. doi: 10.1073/pnas.0503230102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klopfleisch R, Klupp BG, Fuchs W, Kopp M, Teifke JP, Mettenleiter TC. 2006. Influence of pseudorabies virus proteins on neuroinvasion and neurovirulence in mice. J Virol 80:5571–5576. doi: 10.1128/JVI.02589-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brittle EE, Reynolds AE, Enquist LW. 2004. Two modes of pseudorabies virus neuroinvasion and lethality in mice. J Virol 78:12951–12963. doi: 10.1128/JVI.78.23.12951-12963.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor MP, Kramer T, Lyman MG, Kratchmarov R, Enquist LW. 2012. Visualization of an alphaherpesvirus membrane protein that is essential for anterograde axonal spread of infection in neurons. mBio 3(2):00063-12. doi: 10.1128/mBio.00063-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramer T, Greco TM, Taylor MP, Ambrosini AE, Cristea IM, Enquist LW. 2012. Kinesin-3 mediates axonal sorting and directional transport of alphaherpesvirus particles in neurons. Cell Host Microbe 12:806–814. doi: 10.1016/j.chom.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bohannon KP, Sollars PJ, Pickard GE, Smith GA. 2012. Fusion of a fluorescent protein to the pUL25 minor capsid protein of pseudorabies virus allows live-cell capsid imaging with negligible impact on infection. J Gen Virol 93(Pt 1):124–129. doi: 10.1099/vir.0.036145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Card JP, Whealy ME, Robbins AK, Moore RY, Enquist LW. 1991. Two alpha-herpesvirus strains are transported differentially in the rodent visual system. Neuron 6:957–969. doi: 10.1016/0896-6273(91)90236-S. [DOI] [PubMed] [Google Scholar]
- 18.Pickard GE, Smeraski CA, Tomlinson CC, Banfield BW, Kaufman J, Wilcox CL, Enquist LW, Sollars PJ. 2002. Intravitreal injection of the attenuated pseudorabies virus PRV Bartha results in infection of the hamster suprachiasmatic nucleus only by retrograde transsynaptic transport via autonomic circuits. J Neurosci 22:2701–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JI, Sollars PJ, Baver SB, Pickard GE, Leelawong M, Smith GA. 2009. A herpesvirus encoded deubiquitinase is a novel neuroinvasive determinant. PLoS Pathog 5:e1000387. doi: 10.1371/journal.ppat.1000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith GA, Enquist LW. 2000. A self-recombining bacterial artificial chromosome and its application for analysis of herpesvirus pathogenesis. Proc Natl Acad Sci U S A 97:4873–4878. doi: 10.1073/pnas.080502497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith GA, Gross SP, Enquist LW. 2001. Herpesviruses use bidirectional fast-axonal transport to spread in sensory neurons. Proc Natl Acad Sci U S A 98:3466–3470. doi: 10.1073/pnas.061029798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicola AV, Hou J, Major EO, Straus SE. 2005. Herpes simplex virus type 1 enters human epidermal keratinocytes, but not neurons, via a pH-dependent endocytic pathway. J Virol 79:7609–7616. doi: 10.1128/JVI.79.12.7609-7616.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maurer UE, Sodeik B, Grunewald K. 2008. Native 3D intermediates of membrane fusion in herpes simplex virus 1 entry. Proc Natl Acad Sci U S A 105:10559–10564. doi: 10.1073/pnas.0801674105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith GA, Pomeranz L, Gross SP, Enquist LW. 2004. Local modulation of plus-end transport targets herpesvirus entry and egress in sensory axons. Proc Natl Acad Sci U S A 101:16034–16039. doi: 10.1073/pnas.0404686101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antinone SE, Smith GA. 2010. Retrograde axon transport of herpes simplex virus and pseudorabies virus: a live-cell comparative analysis. J Virol 84:1504–1512. doi: 10.1128/JVI.02029-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zaichick SV, Bohannon KP, Hughes A, Sollars PJ, Pickard GE, Smith GA. 2013. The herpesvirus VP1/2 protein is an effector of dynein-mediated capsid transport and neuroinvasion. Cell Host Microbe 13:193–203. doi: 10.1016/j.chom.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyman MG, Feierbach B, Curanovic D, Bisher M, Enquist LW. 2007. Pseudorabies virus Us9 directs axonal sorting of viral capsids. J Virol 81:11363–11371. doi: 10.1128/JVI.01281-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kratchmarov R, Enquist LW, Taylor MP. 2015. Axonal sorting and transport of the pseudorabies virus glycoprotein gM independent of Us9. J Virol. 89:6511–6514. doi: 10.1128/JVI.00625-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Card JP, Levitt P, Enquist LW. 1998. Different patterns of neuronal infection after intracerebral injection of two strains of pseudorabies virus. J Virol 72:4434–4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antinone SE, Zaichick SV, Smith GA. 2010. Resolving the assembly state of herpes simplex virus during axon transport by live-cell imaging. J Virol 84:13019–13030. doi: 10.1128/JVI.01296-10. [DOI] [PMC free article] [PubMed] [Google Scholar]